Sequential infections by 32 isolates of Phoma medicaginis increase production of phytoestrogens in Medicago polymorpha var. brevispina
Mahtab Omidvari A , Gavin R. Flematti B , Ming Pei You A , Payman Abbaszadeh-Dahaji C and Martin J. Barbetti A *
A *
A School of Agriculture and Environment and the UWA Institute of Agriculture, University of Western Australia, 35 Stirling Highway, Crawley, WA 6009, Australia.
B School of Molecular Sciences, University of Western Australia, 35 Stirling Highway, Crawley, WA 6009, Australia.
C Department of Soil Sciences, Faculty of Agricultural Science, Vali-e-Asr University of Rafsanjan, Rafsanjan, Iran.
Crop & Pasture Science 73(12) 1367-1384 https://doi.org/10.1071/CP22098
Submitted: 22 March 2022 Accepted: 29 May 2022 Published: 13 July 2022
© 2022 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Context: Studies of Phoma black stem and leaf spot disease (caused by Phoma medicaginis) in annual medics (Medicago spp.) normally involve a ‘once-only’ inoculation not reflecting multiple pathogen infection and phytoestrogen production cycles in the field. Phytoestrogen production by plants can result in lower ovulation rates in grazing animals.
Aims: We aimed to determine whether sequential infections by P. medicaginis increase production of phytoestrogens in annual medics, and to measure the genetic diversity of isolates.
Methods: In a greenhouse experiment, pathogenicity and virulence were investigated across 32 isolates of P. medicaginis following one, two or three rounds of inoculation of M. polymorpha var. brevispina. Production of the phytoestrogens coumestrol and 4′-O-methyl coumestrol was measured, and correlation with disease parameters assessed. DNA sequencing using ITS, β-tubulin, calmodulin and P. medicaginis-specific EFNI-1α was applied for phylogenetic analysis of isolates from Western Australia and elsewhere.
Key results: Across isolates, highest leaf disease incidence was 76%, petiole disease incidence 61%, leaf disease severity 52% and petiole disease severity 53%. Stem coumestrol content range was 45–1247 mg kg−1, and 4′-O-methyl coumestrol 0–344 mg kg−1. All measures were highest after three rounds of inoculation. Overall, there was a positive correlation of leaf disease incidence with coumestrol content (P < 0.05) and of both leaf and petiole disease incidence with 4′-O-methylcoumestrol content (P < 0.01, P < 0.05, respectively). Phylogenetic analysis revealed a high degree of genetic similarity among Western Australian isolates, generally grouping into a single separate cluster across the four markers, and genetically distinct from isolates sourced outside Australia.
Conclusions: Leaf disease incidence was the best discriminating disease parameter for coumestrol and 4′-O-methylcoumestrol content. Western Australian isolates of P. medicaginis were genetically similar and unique, possibly due to geographic separation.
Implications: The study emphasised the importance of sequential inoculations when screening annual Medicago genotypes towards developing cultivars with superior disease resistance and enhanced animal reproductive outcomes.
Keywords: 4′-O-methylcoumestrol, annual medic, annual Medicago spp, coumestrol, genetic variation, phoma black stem and leaf spot, Phoma medicaginis, phytoestrogen.
Introduction
For decades there has been strong interest in annual Medicago species as high-quality forage legumes (Chatterton and Chatterton 1996). They are highly valued for animal production (Lüscher et al. 2014) and play a critical role in dryland farming systems (Barbetti et al. 2006). Phoma black stem and leaf spot (caused by Phoma medicaginis) is a damaging disease on both annual and perennial Medicago species in temperate and Mediterranean regions (Lamprecht and Knox-Davies 1984). It greatly reduces seed and herbage yield and, where severe, can cause incomplete defoliation and premature death in highly susceptible annual Medicago cultivars (Barbetti 1989b, 1995a; Barbetti and Fang 1991; Barbetti and Nichols 1991; Ellwood et al. 2006b). Phoma medicaginis has a wide host range (e.g. Lamprecht and Knox-Davies 1984), also infecting Pisum and Trifolium species (Barbetti and Khan 1987). Classification of Phoma species can be challenging (Fatehi et al. 2003), particularly when based on morphology, where the major distinction from Ascochyta species has long been conidial size and aseptate conidia (Boerema and Bollen 1975); however, this has been resolved with the use of molecular sequencing (e.g. Kamphuis et al. 2008, 2012).
First disease symptoms are brown to black spots on leaves, stems and petioles, and as disease progresses, these coalesce causing chlorosis and finally abscission of leaflets and stem collapse (Barbetti 1987, 1989b). Phoma medicaginis can also cause significant root disease on annual Medicago species (Barbetti 1989a), and a rot complex in both crowns (Rodriguez and Leath 1992) and roots (Rodriguez 2005) of lucerne (Medicago sativa). In Western Australia, surveys showed it to be widely associated with root disease in annual Medicago species (You et al. 2000). The disease is particularly severe during wet weather conditions in spring when conidia from pycnidia within lesions are readily spread by rain splash (Barbetti 1987, 1989b; Barbetti et al. 2006; Ellwood et al. 2006a). Rising spring temperatures also foster the disease (Barbetti 1987, 1991), and in annual Medicago species, disease is most severe at day/night temperatures of 21°C/16°C, followed by 18°C/13°C, and least severe at 15°C/10°C (Barbetti 1987).
Phoma medicaginis infection stimulates production of phytoestrogens in annual Medicago species (Barbetti 1991, 1995a, 2007; Barbetti and Nichols 1991; Barbetti et al. 2006, 2020). Fields et al. (2018) reported that lucerne inoculated with Stemphylium vesicarium contained 169 ± 25.1 mg kg−1 of coumestrol compared with 3.4 ± 0.84 mg kg−1 in control plants. Phytoestrogens can provide some advantages to plants, such as protection against some fungal and bacterial pathogens (Wasserman et al. 2013) and against attack by pests (He and Dixon 2000; Deavours and Dixon 2005). However, they can result in lower ovulation rates in grazing animals, with consequent reduced reproduction or even infertility, particularly with sheep (Croker et al. 1994a, 1994b, 1999, 2005; Smith et al. 1979) but also with cattle (Barbetti et al. 2020).
These phytoestrogens are similar to the endogenous estrogens of mammals in terms of structure and function (Pierson and Ferkin 2015). The majority of phytoestrogens are phenolic compounds including isoflavones and coumestans, for which extensive research has been conducted. (Patisaul and Jefferson 2010). Phytoestrogen content in annual Medicago species can also increase in absence of plant disease (Francis and Millington 1965), for instance, in response to soil type (Francis and Millington 1971), superphosphate availability (Marshall and Parkin 1970) and stage of plant growth (Francis and Millington 1965). However, it is P. medicaginis that particularly stimulates elevated levels of phytoestrogens such as coumestrol (Barbetti and Fang 1991; Barbetti and Nichols 1991; Barbetti 2007) in annual Medicago species.
Although various management strategies such as close grazing (Barbetti 1989b; Barbetti et al. 2006, 2020) and application of fungicides (Barbetti 1983, 1989b) offer reduction of Phoma black stem and leaf spot, locating host resistance remains the most promising approach (Barbetti 1989b, 1990; Barbetti et al. 2020). In addition, our recent study (Omidvari et al. 2021) highlighted that the intrinsic ability of annual Medicago cultivars to produce phytoestrogens in the absence of the pathogen, and their comparative ability to produce phytoestrogens in the presence of P. medicaginis, were together critical in developing more resistant cultivars with improved reproductive outcomes for grazing animals. However, in that study, we utilised a ‘once-only’ pathogen inoculation, which contrasts with what happens naturally in the field. There is a need for studies that better epitomise the in-field, multiple infection cycles in relation to final disease incidence/severity and production cycles of coumestrol and 4′-O-methyl coumestrol.
We hypothesised that levels of both Phoma black stem and leaf spot and phytoestrogens would be higher after multiple infection cycles. Therefore, in the present study, we investigated differences across leaf and stem disease incidence and severity during and after multiple infection cycles of 32 isolates of P. medicaginis, and the relationship of these disease parameters with production of coumestrol and 4′-O-methyl coumestrol. We also examined phylogenetically informative gene regions to determine the diversity of local isolates of P. medicaginis and how these compared with isolates from elsewhere. The local isolates had previously been investigated for their virulence and effect on phytoestrogen production across three annual Medicago cultivars (M. truncatula cv. Cyprus, M. polymorpha var. brevispina cv. Serena, and M. murex cv. Zodiac) (Omidvari et al. 2021). The relative virulence of these isolates varied across different assessment parameters and their effects on phytoestrogen production were also different.
Materials and methods
Isolates of P. medicaginis
Thirty-two isolates of P. medicaginis collected from a range of annual Medicago species and cultivars in Western Australia were used. These were obtained from the Culture Collection at the Department of Primary Industries and Regional Development, Western Australia, as lyophilised cultures in glass ampules. Full isolate details are provided in Omidvari et al. (2021).
Inoculum preparation
Inoculum preparation was as described by Omidvari et al. (2021). Briefly, preserved isolates in lyophilised ampoules were subcultured onto Sanderson and Srb medium (Dhingra and Sinclair 1995) for 21 days at 25°C (±2°C) under 16 h/8 h light/dark. After 3 weeks, 5 mL sterile water was added to each plate and then conidia were harvested by rubbing the colony surface with a bent glass rod. Conidial concentration of each isolate was adjusted to 5 × 106 spores mL−1 by using a haemocytometer counting chamber, and the inoculum produced was always applied immediately in virulence tests (Chihaoui et al. 2015).
Virulence tests
The highly susceptible M. polymorpha var. brevispina (Barbetti 1990, 1995a, 1995b) was used to evaluate the virulence of different isolates across multiple inoculations. A single cultivar was chosen in order to avoid cultivar × isolate interactions as found in our earlier study (Omidvari et al. 2021). To remove any potential fungal seed contamination and to ensure high germination rates, seeds were surface-sterilised with 70% ethanol for 30 s, washed with sterile distilled water, and then scarified to break hardseed dormancy (when freshly ripened they also contain embryo dormancy) using sandpaper. Seeds were then transferred to Petri dishes lined with moist, sterile blotting paper. Imbibed seeds were transferred to water agar plates and incubated at 25°C in darkness until radicles emerged (Mhadhbi et al. 2005). Seeds were then transplanted into pots 6 cm by 6 cm by 14 cm, containing a portion of steam-pasteurised potting mix that consisted of 2.5 m3 fine composted pine bark, 1 m3 coco peat, 5 m3 brown river sand, 10 kg slow-release fertiliser Osmoform NXT (22 N:2.2 P2O5:9.1 K2O:1.2 Mg + trace elements; Everris International, Geldermalsen, the Netherlands), 10 kg dolomite (CalMag), 5 kg gypsum clay-breaker, 5 kg extra fine limestone, 4 kg iron heptasulfate and 1 kg iron chelate. Potting mix was pasteurised at 63°C for 45 min. Each pot was sown with four seeds at 5 mm depth and pots were maintained in a naturally lit greenhouse at 18°C (±4°C), watered daily with deionised water and allowed to drain to field capacity. Plants were fertilised weekly, according to manufacturer’s recommendation, with Thrive (Yates Australia, Sydney, NSW, Australia), containing macronutrients (25 N:5 P:8.8 K) and the full range of micronutrients needed for plant growth.
Plants were spray-inoculated with the conidial suspension of each isolate until run-off, using a hand-held and operated aerosol sprayer. Tween 20 (0.001%) was added to conidial suspensions to ensure that inoculation droplets remained attached to the plant. Control plants were also treated with 0.001% Tween 20®, but in sterile distilled water. Inoculated plants were then placed into clear plastic containers with lids, measuring 770 mm long, 570 mm wide and 475 mm high, used as inoculation/incubation chambers to maintain high humidity (40 pots per container). Walls and lids of incubation boxes were internally misted with sterile distilled water and box lids closed for 72 h post-inoculation to maintain high humidity (Ellwood et al. 2007). The whole experiment was fully repeated once.
Multiple inoculation timings and disease assessments
There were three inoculation timings. The first inoculation was when plants were 6 weeks of age, with assessments made in situ for the different disease parameters at 8 weeks of age (14 days post-inoculation (dpi)), and four plants from each isolate randomly picked for harvest a further 2 weeks later at 10 weeks of age (i.e. 28 dpi) for phytoestrogen assessments. The second and third inoculations were conducted when plants were 10 and 18 weeks old, respectively, with similar time intervals for disease assessments (14 dpi) and harvesting of plants for phytoestrogen analyses (28 dpi).
Four disease assessment parameters were used, as reported previously (Omidvari et al. 2021): (1) leaf disease incidence (%LDI), calculated as the percentage of leaves per pot showing disease symptoms; (2) leaf disease severity (%LDS), visually estimated as the leaf area diseased per pot as a percentage of the total leaf area; (3) petiole/stem disease incidence (%PDI), calculated as the percentage of petioles/stems per pot showing disease symptoms; (4) petiole disease severity (%PDS), visually estimated as the petiole/stem area diseased per pot as a percentage of the total petiole/stem area. All disease assessments were made by the same person throughout.
Phytoestrogen assessment
Solvents
Details of solvent use for phytoestrogen assessments are as provided previously (Omidvari et al. 2021). Coumestrol and 4′-O-methylcoumestrol standards were purchased from Sigma-Aldrich (St. Louis, MO, USA) and ALB Technology (Henderson, NV, USA), respectively, and used to prepare a calibration curve from 1 to 100 mg L−1 with nine concentrations for each standard. The relationship between peak area and concentration was linear, with correlation coefficient (r) >0.9997 for both coumestrol and 4′-O-methylcoumestrol. High-pressure liquid chromatography (HPLC)-grade methanol, HPLC-grade acetonitrile and HPLC-grade trifluoroacetic acid were purchased from Sigma-Aldrich. Water was obtained from a Milli-Q water purification unit (Millipore Australia). The stock standard solutions (100 μg mL−1) of coumestrol and 4′-O-methyl coumestrol were prepared in aqueous methanol (2:8 v/v) and stored in the dark at 4°C. A reference standard (10 μg mL−1) for HPLC retention times was prepared daily by dilution of the stock solutions with aqueous methanol (2:8 v/v).
Quantitation
For phytoestrogen assessment, plants sampled for each isolate or control treatment were harvested and bulked together into a single composite sample. Details of quantification of phytoestrogens used in phytoestrogen assessments are as provided by Omidvari et al. (2021). Three weeks after the final disease assessment, stem samples were harvested from all treatments for analysis of contents coumestrol and 4′-O-methyl coumestrol. Stem samples were used because diseased leaves and petioles had dehisced and because stems are a reliable indicator or phytoestrogen levels (Barbetti and Fang 1991). Samples were oven-dried at 70°C, and finely ground in two steps: first with a mechanical grinder that coarsely ground the stem material, and second by freezing with liquid nitrogen and finely grinding the material in a mortar and pestle. Ground samples (0.2 g) were extracted via methodology adapted from Fields et al. (2018) with 2 mL aqueous methanol (2:8 v/v) in glass sample vials (8 mL). All stem samples were hand-mixed for 30 s and put on an end-over-end mixer for 16 h at room temperature. Samples were filtered through glass fibre syringe filters, and supernatant (1 mL) was transferred into 2-mL glass auto-sampler vials. Extracted samples were analysed for coumestrol and 4′-O-methyl coumestrol content by HPLC. For comparative analysis, the extracts were spiked with 4-hydroxycoumarin (50 μg mL−1) as an internal standard and relative quantifications determined based on HPLC–diode array detector (DAD) peak areas that were normalised based on the area of the recovered internal standard peak. Retention times for coumestrol and 4′-O-methylcoumestrol were at 28.5 and 43.8 min, respectively. These two compounds were identified in the samples based on their relative retention times and absorbance spectra. The HPLC analyses were performed with an Agilent 1260 series instrument (Agilent Technologies, Santa Clara, CA, USA) equipped with a quaternary pump, auto injector and DAD. Separation was performed using an Apollo reversed phase C18 column (250 mm × 4.6 mm ID, 5 μm particle size; Grace Discovery Sciences, Bannockburn, IL, USA) at 25°C. The mobile phase consisted of solvent A 0.1% (v/v) trifluoroacetic acid in water, and solvent B acetonitrile. The elution gradient was as follows: 0–40 min, 20–50% B; 40–41 min, 50–50% B; 41–50 min, 50–100% B; and held at 100% B for 10 min. The flow rate set at 1 mL min−1 with the compounds detected at 254 nm and injection volume 20 μL. The column was re-equilibrated for 5 min between samples. Amounts of coumestrol and 4′-O-methylcoumestrol were quantified as mg kg−1 dry weight of plant material extracted.
In addition to the above, three separate subsamples were taken from each of the dried harvest materials from each isolate × timing of inoculation treatment (and similarly for control treatment), and separately assessed for amounts of coumestrol and 4′-O-methylcoumestrol. The mean of the values for three subsamples is presented as the coumestrol or 4′-O-methylcoumestrol value for each isolate, or for the control. Preliminary studies (data not presented) highlighted that three subsamples ensured a reliable and repeatable measure of the level of coumestrol or 4′-O-methylcoumestrol present.
Molecular identification
DNA extraction
Sample DNA was extracted from 2-week-old fungal cultures of 28 isolates previously identified as P. medicaginis growing on Sanderson and Srb medium. The remaining four isolates were referenced according to their already available molecular identification details in GenBank. A modified procedure of Cenis (1992) was used for DNA extraction. Briefly, fresh mycelial mat, ∼2 mm by 2 mm, was put into a 2-mL tube containing two ceramic beads (2 mm) and 300 μL extraction buffer (200 mM Tris–HCl pH 8.5, 250 mM NaCl, 25 mM EDTA, 0.5% w/v SDS). The mycelial mat was homogenised in a Precellys Evolution homogeniser (Bertin Technologies, Montigny-le-Bretonneux, France) at 2490g in two cycles of 60 s. Then, 3 M sodium acetate (150 μL, pH 5.2) was added and maintained at room temperature for 10 min. Tubes were then centrifuged at 16 816g for 10 min and the supernatant was transferred to a fresh 2-mL Eppendorf tube. An equal volume of isopropanol (∼450 μL) was added to the tube and maintained for at least 15 min at 22°C to precipitate the DNA. Precipitated DNA was pelleted by centrifuging at 16 816g for 15 min, then washed with 70% w/v ethanol, vacuum-dried for 10 min and resuspended in 50 μL Tris-EDTA buffer. DNA samples were held at 4°C until needed.
Polymerase chain reaction amplification and DNA sequencing
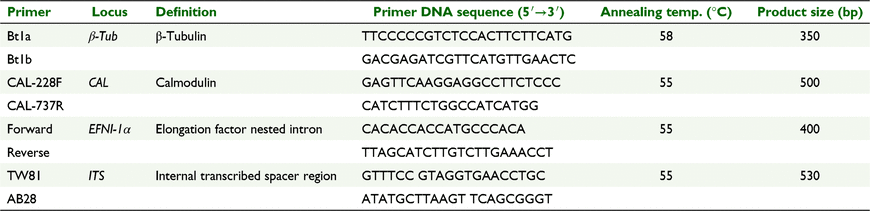
The internal transcribed spacer (ITS) ribosomal DNA (rDNA) region can be of limited value for resolution of closely related species or strains (Ellwood et al. 2006a); therefore, three additional phylogenetically informative regions including β-tubulin, calmodulin, and P. medicaginis-specific elongation factor nested intron (EFNI)-1α were examined for distinguishing isolates. A PCR-based assay was conducted using each primer set to amplify a DNA product of predicted size from P. medicaginis isolates (Table 1) (Ellwood et al. 2006a). Amplification was performed in a volume of 25 μL with 1 μL each primer (final concentration of each primer 10 pmol μL−1), 1 μL 10 ng DNA template, 12.5 μL DreamTaq Green PCR Master Mix (2×) (Thermo Fisher Scientific, Waltham, MA, USA), and 9.5 μL nuclease-free water. DNA amplifications were carried out in a 9600 Thermocycler (PerkinElmer, Waltham, MA, USA) programmed at 95°C for 8 min, followed by 35 cycles at 95°C for 15 s, then 55°C (58°C for β-tubulin; Table 1) for 20 s, then 72°C for 60 s, then a final elongation step of 72°C for 5 min. The DNA products of predicted size from P. medicaginis were visualised by electrophoresis on a 1% (w/v) agarose gel containing GelRed stain (diluted to 1/10 000) and viewing under UV light. PCR products were then sequenced by Macrogen, Seoul, South Korea.
|
Sequence alignment and phylogenetic analysis
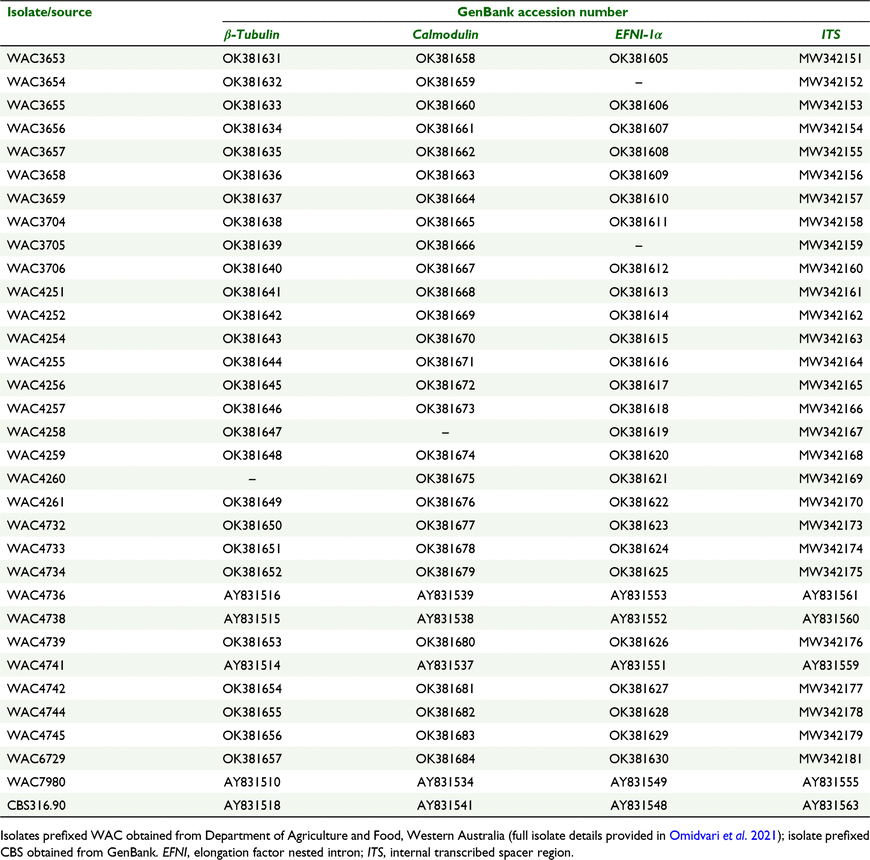
The P. medicaginis isolates in this study were initially compared with closely related species using ITS sequence data alone because those data were available in GenBank. Then additional phylogenetic trees were constructed including β-tubulin, calmodulin, P. medicaginis-specific EFNI-1α fragments. The 32 isolates used in the phylogenetic tree included 28 sequenced isolates from this study with the addition of another four isolates (WAC4736, WAC4738, WAC4741 and WAC7980) previously sequenced by Ellwood et al. (2006a) (see accession numbers in Table 2).
|
For ITS, we selected the most closely related GenBank ITS sequences from five P. medicaginis reference isolates in GenBank (AY504634, CBS316.90, DQ092494, KF181254 and MK765001) and, with our 32 ITS isolates’ sequence data, assembled a tree rooted to Phaeosphaeria nodorum (U77362). For the second tree, P. medicaginis isolate CBS316.90 from GenBank was included, with accession nos AY831518, AY831541, AY831548 and AY831563 (Table 2) relating to β-tubulin, calmodulin, P. medicaginis-specific EFNI-1α and ITS sequences, respectively. Sequence alignment and phylogenetic tree building were performed using Geneious v. 9.1.4 (Biomatters, San Diego, CA, USA). Sequence consensuses were used for BLAST in NCBI, and multiple alignment (alignment type, global alignment with free end gaps; cost matrix, 65% similarity (5.0/−4.0); gap open penalty, 12; gap extension penalty, 3) was used for phylogenetic tree building (genetic distance model, Tamura–Nei; tree building method, neighbour-joining tree; resampling method, bootstrap; random seed, 534 442; no. of replications, 100; support threshold, >50%).
Experimental design and statistical analyses
The whole greenhouse experiment was fully repeated once. There was one annual Medicago species (M. polymorpha var. brevispina), 32 P. medicaginis isolates and one control (mock inoculation), and three inoculation times and corresponding harvest times. The total number of pots in each experiment was 396, comprising cultivar (1) × isolates (33) × inoculation times (3) × replications (4), where 132 pots were inoculated once (at 6 weeks, harvested at 10 weeks), 132 pots were inoculated twice (at 6 and 10 weeks, and harvested at 14 weeks) and 132 pots were inoculated three times (at 6, 10 and 18 weeks and harvested at 22 weeks). Each experiment was arranged as a randomised block design using the ‘Generate a Standard Design’ function in GenStat 18.1 (18th Edition; VSNi, Hemel Hempstead, UK). Normality of data and homogeneity of the original and repeat experimental datasets were tested before conducting analyses. Data from the original and the repeat experimental datasets were not significantly different (P > 0.05) by t-test. Therefore, data from the original and repeat experiments were combined, and analysed as a single dataset. %LDI, %PDI, %LDS and %PDS were assessed. Fisher’s least significant differences (l.s.d.s) were used to indicate significant differences between treatments. Correlations among all of the disease parameters and between all of the disease parameters and phytoestrogens were computed by using the linear regression function of GenStat.
Results
Disease expression across P. medicaginis isolates
There were significant effects of P. medicaginis isolate on %LDI, %PDI, %LDS and %PDS (all P ≤ 0.001) in every round of inoculation, confirming variation for virulence across isolates of P. medicaginis as assessed across the different disease parameters. Control plants in all inoculation rounds remained disease-free (Table 3).
%LDI
For the first inoculation, the three most virulent isolates in terms of %LDI were WAC3704 (41%), WAC4744 (39%) and WAC4260 (38%) and three least virulent isolates were WAC3653, WAC4254 and WAC4741 (all 0%). For the second inoculation, isolates WAC4736 (38%), WAC4251 (37%) and WAC4254 (36%) were most virulent and WAC4741 (12%) was least virulent. For the third inoculation, most virulent isolates were WAC4255 (76%) and WAC4734 (75%) and least virulent was WAC4741 (43%) (Table 3).
%PDI
The most virulent isolates in the first inoculation as assessed by %PDI were WAC4744 (27%) and WAC4732 (26%) and the least were WAC3653, WAC3656, WAC4254, WAC4736, WAC4738 and WAC4741 (all 0%) and WAC3706 (2%). For the second inoculation, the most virulent isolates were WAC4736 (22.5%), WAC4259 (21.1%), WAC4260 (20%) and WAC4739 (19.9%), whereas least virulent were WAC4255 (2%), WAC4741 (2%) and WAC3653 (3%). In relation to the third inoculation, most virulent were WAC4732 (61%) and WAC4260 (58%) and least was WAC7980 (13%) (Table 3).
%LDS
For the first inoculation, the most virulent isolates with regard to %LDS were WAC4734 (41%), WAC6729 (39%) and WAC4745 (38%), whereas WAC3653, WAC4254 and WAC4741 rated nil LDS. For the second inoculation, the most virulent isolates were WAC4252 (45%) and WAC4254 (42.5%) and the least virulent were WAC3653 (5%), WAC4741 (5%), WAC3658 (7%) and WAC4742 (7%). For the third inoculation, most virulent were WAC4732 (52%) and WAC4736 (51%) and least virulent WAC6729 (12%) and WAC7980 (13%) (Table 3).
%PDS
For the first inoculation, most virulent isolates in terms of %PDS were WAC7980 (30%), WAC6729 (29%) and WAC4732 (28%), whereas WAC3653, WAC3656, WAC3706, WAC4254, WAC4736, WAC4738 and WAC4741 all rated no nil PDS. In the second inoculation, most virulent was WAC4736 (34%) and least virulent were WAC4255 (0%), WAC4741 (1%), WAC3653 (2%) and WAC3654 (2%), but these last four did not differ from the control plants. For the third inoculation, most virulent isolates were WAC4736 (53%), WAC4732 (52%) and WAC4258 (52%) and least virulent WAC4741 (8%) and WAC6729 (10%) (Table 3).
Disease expression across different rounds of inoculation
There were significant main effects of inoculation rounds on all disease parameters %LDI, %PDI, %LDS and %PDS (all P ≤ 0.001) (Table 3). The highest %LDI was recorded for the third inoculation (57.6%) and the lowest for the first inoculation (23.5%), with the second inoculation intermediate (26.6%). The highest, intermediate and lowest %PDI occurred for the third (41.6%), second (13.8%) and first (11.6%) inoculations, respectively. With regard to %LDS, the third inoculation had the highest rating (30.1%), followed by the second (22.2%) and first (17.5%) inoculations. Likewise, for %PDS, the third inoculation had the highest rating (27.3%), the second inoculation was intermediate (12.3%), and the first inoculation had the lowest (11.3%) (Table 3).
Disease expression interaction effects
There was a significant P. medicaginis isolate × inoculation round interaction effect on all disease parameters %LDI, %PDI, %LDS and %PDS (all P ≤ 0.001) (Table 3).
Leaf disease incidence (%LDI) was highest with isolates WAC4255 and WAC4734 in the third round of inoculation, whereas isolates WAC3653, WAC4254 and WAC4741 showed the lowest %LDI in the first round of inoculation (Table 3).
Petiole disease incidence (%PDI), was highest for isolates WAC4732 and WAC4260 in the third round of inoculation, whereas it was lowest for isolates WAC4736, WAC3653, WAC3656, WAC4254, WAC4738, WAC4741 and WAC3706 in the first round of inoculation and for isolates WAC4741, WAC4255 and WAC3653 in the second round of inoculation (Table 3).
Leaf disease severity (%LDS) was highest for isolates WAC4732, WAC4736 in the third round of inoculation, whereas it was lowest for isolates WAC3653, WAC4741 and WAC4254 in the first round of inoculation (Table 3).
Petiole/stem disease severity (%PDS) was highest for isolates WAC4736, WAC4732 and WAC4258 in the third round of inoculation, whereas WAC4736 was one of six isolates with the lowest %PDS in the first round of inoculation (Table 3).
Phytoestrogen expression across different rounds of inoculation
There were highly significant overall effects of inoculation round in terms of phytoestrogen expression (P ≤ 0.001) (Table 3). The highest level of coumestrol was for the third inoculation (658.3 mg kg−1 stem dry weight), and the lowest level for the first inoculation (262.5 mg kg−1), and the level for the second inoculation was intermediate (483.4 mg kg−1) (Fig. 1). Likewise, the highest level of 4′-O-methylcoumestrol was for third inoculation (183.2 mg kg−1), the lowest for the first inoculation (24.2 mg kg−1), and the level for the second inoculation was intermediate (65 mg kg−1) (Fig. 1).
Phytoestrogen expression across P. medicaginis isolates
Overall, Phoma black stem and leaf spot resulted in very high levels of both coumestrol and 4′-O-methyl coumestrol compared with levels in uninoculated control plants (Table 3).
For the first inoculation, levels of coumestrol produced ranged from 45 to 447 mg kg−1 and levels of 4′-O-methyl coumestrol ranged from 0 to 82 mg kg−1. The isolate that resulted in the highest level of coumestrol was WAC4258 (447 mg kg−1), and the lowest levels of coumestrol were caused by isolates WAC4732, WAC4741, WAC3706 and WAC4261 (96, 99, 104 and 116 mg kg−1, respectively). For the first inoculation, isolate WAC3657 resulted in the greatest production of 4′-O-methyl coumestrol (82 mg kg−1).
For the second inoculation, the greatest amount of coumestrol was produced in plants inoculated with isolates WAC7980 and WAC4744 (909 and 874 mg kg−1, respectively), and the least in plants inoculated with WAC4261 (166 mg kg−1). In relation to 4′-O-methylcoumestrol, the highest production resulted from isolates WAC4739, WAC4256, WAC4258, WAC3658 (135, 120, 119 and 112 mg kg−1, respectively).
For the third inoculation, the greatest amount of coumestrol resulted from inoculation with isolates WAC4739 and WAC4741 (1247 and 1166 mg kg−1, respectively). In terms of 4′-O-methylcoumestrol, isolates WAC3705, WAC3658 and WAC3704 (344, 284 and 268 mg kg−1, respectively) caused greatest production.
Phytoestrogen expression interaction effects
There was a significant P. medicaginis isolate × round of inoculation interaction effect on both coumestrol and 4′-O-methylcoumestrol production (P ≤ 0.001) (Table 3). Production of coumestrol was greatest for isolates WAC4739 and WAC4741 in the third round of inoculation, whereas nearly one-third of the isolates produced the lowest level of coumestrol in the first round of inoculation (Table 3). Production of 4′-O-methyl coumestrol was highest for isolates WAC3705, WAC3658 and WAC3704 in the third round of inoculation, whereas isolates WAC3706, WAC4261, WAC4732 and WAC4741 did not produce any 4′-O-methyl coumestrol in the first round of inoculation (Table 3).
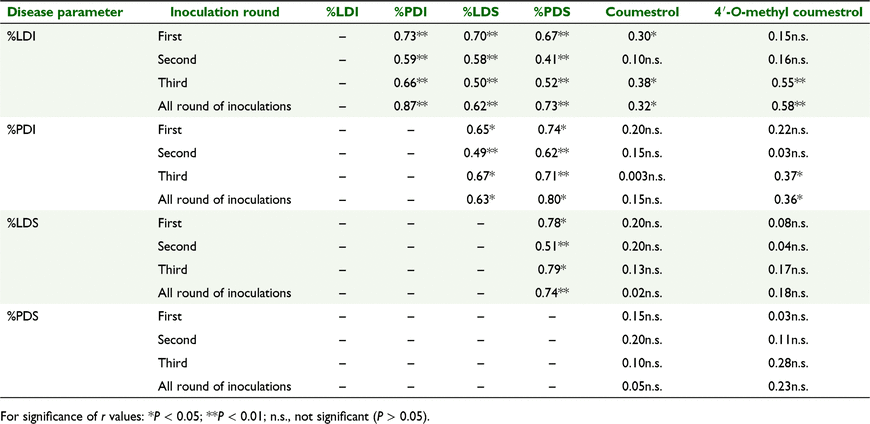
Correlation of phytoestrogen production with disease parameters
Overall, meaned across the three inoculations, there was a significant positive correlation of leaf disease incidence with coumestrol (P < 0.05), and of both leaf and petiole disease incidence with 4′-O-methyl coumestrol contents (P < 0.01 and P < 0.05, respectively). This was most evident in the first inoculation, where %LDI was significantly and positively correlated with production of coumestrol (P < 0.05), and third inoculation, where %LDI was significantly and positively correlated with coumestrol and 4′-O-methylcoumestrol (P < 0.05 and P < 0.01, respectively) and %PDI was correlated with 4′-O-methylcoumestrol production (P < 0.05).
Correlation between disease factors
Leaf disease incidence was significantly and positively correlated with %PDI, %LDS and %PDS in first, second and third inoculation rounds (all P < 0.001) and for all inoculation rounds grouped and meaned together (P < 0.001) (Table 4). %PDI was significantly and positively correlated with leaf disease severity %LDS and petiole disease severity (%PDS) in the first (P < 0.05 and P < 0.01), second (both P < 0.001) and third (P < 0.01 and P < 0.001 respectively) inoculation rounds. %LDS was significantly and positively correlated with %PDS in the first (P < 0.05), second (P < 0.001) and third (P < 0.05) inoculation rounds and for all inoculation rounds grouped and meaned together (P < 0.001) (Table 4).
Sequence alignment and phylogenetic analysis
ITS region
The P. medicaginis isolates were initially compared by using ITS sequence data alone because it provided the most readily available comparative data in GenBank. The phylogenetic tree constructed with 32 isolates of P. medicaginis as well as five accessions from GenBank (AY504634, KF181254, CBS316.90, MK765001 and DQ092494) separated into two clades (Fig. 2). The P. medicaginis isolates from GenBank clustered together to form Clade I. All isolates from the present study (including four P. medicaginis isolates previously sequenced by Ellwood et al. 2006a; shown in bold in Fig. 2) clustered together into Clade II. Generally, there was a very high degree of similarity for all isolates from Western Australia (Fig. 2). All sequences have been deposited in the GenBank database (see details in Table 2).
|
|
Concatenated sequencing of β-tubulin, calmodulin and EFNI-1α
Sequencing of EFNI-1α highlighted significant differences in length and sequence among P. medicaginis isolates and compared with other species. This dissimilarity inhibited sequence alignments with other species such that the phylogenetic tree using combined gene regions was solely constructed among P. medicaginis isolates. The tree of concatenated sequences of β-tubulin, calmodulin and EFNI-1α constructed for the 32 isolates of P. medicaginis and CBS316.90 retrieved from GenBank separated into two clades (Fig. 3). WAC3704 and WAC3706 clustered together in Clade I. All remaining isolates (including four isolates previously sequenced by Ellwood et al. 2006a) clustered together with CBS316.90 to form Clade II.
|
|
|
|
Acknowledgements
The authors thank Robert Creasy and Bill Piasini in the UWA Plant Growth Facilities for their technical assistance in plant growth facilities.
References
Barbetti MJ (1983) Fungal foliage diseases of pasture legumes. Journal of the Department of Agriculture, Western Australia 24, 10–12.Barbetti MJ (1984) Subterranean clover foliage fungi as root pathogens. Australasian Plant Pathology 13, 38–40.
| Subterranean clover foliage fungi as root pathogens.Crossref | GoogleScholarGoogle Scholar |
Barbetti MJ (1987) Effects of temperature and humidity on disease caused by Phoma medicaginis, resistance in some Medicago cultivars and the incidence of seed-borne inoculum. Australian Journal of Experimental Agriculture 27, 851–856.
| Effects of temperature and humidity on disease caused by Phoma medicaginis, resistance in some Medicago cultivars and the incidence of seed-borne inoculum.Crossref | GoogleScholarGoogle Scholar |
Barbetti MJ (1989a) Response of Medicago cultivars to fungal root pathogens associated with Trifolium subterraneum. Plant Protection Quarterly 4, 1–3.
Barbetti MJ (1989b) Strategies for control of Phoma black stem in annual Medicago species. Australian Journal of Experimental Agriculture 29, 635–640.
| Strategies for control of Phoma black stem in annual Medicago species.Crossref | GoogleScholarGoogle Scholar |
Barbetti MJ (1990) Resistance in annual Medicago species to Phoma medicaginis under controlled environment and field conditions. Australian Journal of Experimental Agriculture 30, 209–214.
| Resistance in annual Medicago species to Phoma medicaginis under controlled environment and field conditions.Crossref | GoogleScholarGoogle Scholar |
Barbetti MJ (1991) Effects of temperature and humidity on diseases caused by Phoma medicaginis and Leptosphaerulina trifolixs in lucerne (Medicago sativa). Plant Pathology 40, 296–301.
| Effects of temperature and humidity on diseases caused by Phoma medicaginis and Leptosphaerulina trifolixs in lucerne (Medicago sativa).Crossref | GoogleScholarGoogle Scholar |
Barbetti MJ (1995a) Relative resistance, associated yield losses and phyto-oestrogen production from fungal foliar diseases in new and old annual Medicago cultivars. Australian Journal of Agricultural Research 46, 441–450.
| Relative resistance, associated yield losses and phyto-oestrogen production from fungal foliar diseases in new and old annual Medicago cultivars.Crossref | GoogleScholarGoogle Scholar |
Barbetti MJ (1995b) Resistance in annual Medicago species to Phoma medicaginis and Leptosphaerulina trifolii under field conditions. Australian Journal of Experimental Agriculture 35, 209–214.
| Resistance in annual Medicago species to Phoma medicaginis and Leptosphaerulina trifolii under field conditions.Crossref | GoogleScholarGoogle Scholar |
Barbetti MJ (2007) Resistance in annual Medicago spp. to Phoma medicaginis and Leptosphaerulina trifolii and its relationship to induced production of a phyto-oestrogens. Plant Disease 91, 239–244.
| Resistance in annual Medicago spp. to Phoma medicaginis and Leptosphaerulina trifolii and its relationship to induced production of a phyto-oestrogens.Crossref | GoogleScholarGoogle Scholar | 30780554PubMed |
Barbetti MJ, Fang CS (1991) Relationship between Phoma black stem severity and herbage and seed yield and coumestrol content in three Medicago polymorpha var. brevispina cultivars. Australian Journal of Agricultural Research 42, 409–415.
| Relationship between Phoma black stem severity and herbage and seed yield and coumestrol content in three Medicago polymorpha var. brevispina cultivars.Crossref | GoogleScholarGoogle Scholar |
Barbetti MJ, Khan TN (1987) Cross-pathogenicity of Phoma medicaginis isolates from the genera Medicago, Pisum and Trifolium. Phytophylactica 19, 517–519.
Barbetti MJ, Nichols PGH (1991) Effect of Phoma medicaginis and Leptosphaerulina trifolii on herbage and seed yield and coumestrol content of annual Medicago species. Phytophylactica 23, 223–228.
Barbetti MJ, Riley IT, You MP, Li H, Sivasithamparam K (2006) The association of necrotrophic fungal pathogens and plant parasitic nematodes with the loss of productivity of annual medic-based pastures in Australia and options for their management. Australasian Plant Pathology 35, 691–706.
| The association of necrotrophic fungal pathogens and plant parasitic nematodes with the loss of productivity of annual medic-based pastures in Australia and options for their management.Crossref | GoogleScholarGoogle Scholar |
Barbetti MJ, You M, Jones RAC (2020) Medicago truncatula and other annual Medicago spp.: interactions with root and foliar fungal, oomycete, and viral pathogens. In ‘The model legume Medicago truncatula’. Chapter 5.2.1.1. (Eds FJ de Bruijn, DY Liu) pp. 293–306. (Wiley: Chichester, UK)
Boerema GH, Bollen GJ (1975) Conidiogenesis and conidial septation as differentiating criteria between Phoma and Ascochyta. Persoonia 8, 111–144.
Castell-Miller CV, Zeyen RJ, Samac DA (2007) Infection and development of Phoma medicaginis on moderately resistant and susceptible alfalfa genotypes. Canadian Journal of Plant Pathology 29, 290–298.
| Infection and development of Phoma medicaginis on moderately resistant and susceptible alfalfa genotypes.Crossref | GoogleScholarGoogle Scholar |
Cenis JL (1992) Rapid extraction of fungal DNA for PCR amplification. Nucleic Acids Research 20, 2380
| Rapid extraction of fungal DNA for PCR amplification.Crossref | GoogleScholarGoogle Scholar | 1594460PubMed |
Chatterton L, Chatterton B (1996) ‘Sustainable dryland farming: combining farmer innovation and medic pasture in a Mediterranean climate.’ (Cambridge University Press: Cambridge, UK)
Chihaoui S-A, Djébali N, Mrabet M, Barhoumi F, Mhamdi R, Mhadhbi H (2015) Phoma medicaginis colonizes Medicago truncatula root nodules and affects nitrogen fixation capacity. European Journal of Plant Pathology 141, 375–383.
| Phoma medicaginis colonizes Medicago truncatula root nodules and affects nitrogen fixation capacity.Crossref | GoogleScholarGoogle Scholar |
Croker KP, Barbetti MJ, Nichols PGH (1994a) Incidence of coumestrol in medic pastures in Western Australia. In ‘Proceedings of the Australian Society of Animal Production’. Vol. 20, p. 416. (Australian Society of Animal Production)
Croker K, Nichols PGH, Barbetti MJ, Adams N (1994b) Sheep infertility from pasture legumes. Farmnote No. 6/94. Department of Agriculture Western Australia, Perth, WA, Australia.
Croker K, Nichols PGH, Barbetti MJ, Adams N (1999) Sheep infertility from pasture legumes. Farmnote No. 79/99. Department of Agriculture Western Australia, Perth, WA, Australia.
Croker K, Nichols PGH, Barbetti MJ, Adams N (2005) Sheep infertility from pasture legumes. Farmnote No. 41/2005. Department of Agriculture Western Australia, Perth, WA, Australia.
Deavours BE, Dixon RA (2005) Metabolic engineering of isoflavonoid biosynthesis in alfalfa. Plant Physiology 138, 2245–2259.
| Metabolic engineering of isoflavonoid biosynthesis in alfalfa.Crossref | GoogleScholarGoogle Scholar | 16006598PubMed |
Deavours BE, Liu C-J, Naoumkina MA, Tang Y, Farag MA, Sumner LW, Noel JP, Dixon RA (2006) Functional analysis of members of the isoflavone and isoflavanone O-methyltransferase enzyme families from the model legume Medicago truncatula. Plant Molecular Biology 62, 715–733.
| Functional analysis of members of the isoflavone and isoflavanone O-methyltransferase enzyme families from the model legume Medicago truncatula.Crossref | GoogleScholarGoogle Scholar | 17001495PubMed |
Dhingra OD, Sinclair JB (1995) ‘Basic plant pathology methods.’ 2nd edn. p. 359. (CRC Press: Boca Raton, FL, USA)
Dixon RA (2001) Natural products and plant disease resistance. Nature 411, 843–847.
| Natural products and plant disease resistance.Crossref | GoogleScholarGoogle Scholar | 11459067PubMed |
Dixon RA, Achnine L, Kota P, Liu C-J, Reddy MSS, Wang L (2002) The phenylpropanoid pathway and plant defence: a genomics perspective. Molecular Plant Pathology 3, 371–390.
| The phenylpropanoid pathway and plant defence: a genomics perspective.Crossref | GoogleScholarGoogle Scholar | 20569344PubMed |
Djebali N (2013) Aggressiveness and host range of Phoma medicaginis isolated from Medicago species growing in Tunisia. Phytopathologia Mediterranea 52, 3–15.
Ellwood SR, Kamphuis LG, Oliver RP (2006a) Identification of sources of resistance to Phoma medicaginis isolates in Medicago truncatula SARDI core collection accessions, and multigene differentiation of isolates. Phytopathology 96, 1330–1336.
| Identification of sources of resistance to Phoma medicaginis isolates in Medicago truncatula SARDI core collection accessions, and multigene differentiation of isolates.Crossref | GoogleScholarGoogle Scholar | 18943665PubMed |
Ellwood SR, D’Souza NK, Kamphuis LG, Burgess TI, Nair RM, Oliver RP (2006b) SSR analysis of the Medicago truncatula SARDI core collection reveals substantial diversity and unusual genotype dispersal throughout the Mediterranean basin. Theoretical and Applied Genetics 112, 977–983.
| SSR analysis of the Medicago truncatula SARDI core collection reveals substantial diversity and unusual genotype dispersal throughout the Mediterranean basin.Crossref | GoogleScholarGoogle Scholar | 16402186PubMed |
Ellwood S, Kamphuis LG, Pfaff T, Oliver RP, Samac DA, Foster-Hartnett D, Tivoli B, Onfroy C, Moussart A, Villegas AM, Sillero JC, Rubiales D (2007) Inoculation and growth with foliar pathogenic fungi. In ‘The Medicago truncatula handbook’. (Eds U Mathesius, EP Journet, LW Sumner) pp. 1–14. (The Noble Foundation: Ardmore, OK USA)
Fatehi J, Bridge PD, Punithalingam E (2003) Molecular relatedness within the ‘Ascochyta pinodes–complex’. Mycopathologia 156, 317–327.
| Molecular relatedness within the ‘Ascochyta pinodes–complex’.Crossref | GoogleScholarGoogle Scholar | 14682458PubMed |
Fields RL, Barrell GK, Gash A, Zhao J, Moot DJ (2018) Alfalfa coumestrol content in response to development stage, fungi, aphids, and cultivar. Agronomy Journal 110, 910–921.
Francis CM, Millington AJ (1965) Wether bioassay of annual pasture legumes. IV. The oestrogenic activity of annual medic pastures. Australian Journal of Agricultural Research 16, 927–35.
| Wether bioassay of annual pasture legumes. IV. The oestrogenic activity of annual medic pastures.Crossref | GoogleScholarGoogle Scholar |
Francis CM, Millington AJ (1971) The presence of methylated coumestans in annual Medicago species: response to a fungal pathogen. Australian Journal of Agricultural Research 22, 75–80.
| The presence of methylated coumestans in annual Medicago species: response to a fungal pathogen.Crossref | GoogleScholarGoogle Scholar |
Fuchs A, Davidse LC, De Waard MA, De Wit PJGM (1983) Contemplations and speculations on novel approaches in the control of fungal plant diseases. Pesticide Science 14, 272–293.
| Contemplations and speculations on novel approaches in the control of fungal plant diseases.Crossref | GoogleScholarGoogle Scholar |
Hammond-Kosack KE, Parker JE (2003) Deciphering plant–pathogen communication: fresh perspectives for molecular resistance breeding. Current Opinion in Biotechnology 14, 177–193.
| Deciphering plant–pathogen communication: fresh perspectives for molecular resistance breeding.Crossref | GoogleScholarGoogle Scholar | 12732319PubMed |
He XZ, Dixon RA (2000) Genetic manipulation of isoflavone 7-O-methyltransferase enhances biosynthesis of 4′-O-methylated isoflavonoid phytoalexins and disease resistance in alfalfa. Plant Cell 12, 1689–1702.
| Genetic manipulation of isoflavone 7-O-methyltransferase enhances biosynthesis of 4′-O-methylated isoflavonoid phytoalexins and disease resistance in alfalfa.Crossref | GoogleScholarGoogle Scholar | 11006341PubMed |
Jayasiri SC, Hyde KD, Jones EBG, Jeewon R, Ariyawansa HA, Bhat JD, Camporesi E, Kang JC (2017) Taxonomy and multigene phylogenetic evaluation of novel species Boeremia and Epicoccum with new records of Ascochyta and Didymella in Didymellaceae. Mycosphere 8, 1080–1101.
| Taxonomy and multigene phylogenetic evaluation of novel species Boeremia and Epicoccum with new records of Ascochyta and Didymella in Didymellaceae.Crossref | GoogleScholarGoogle Scholar |
Kamphuis LG, Lichtenzveig J, Oliver RP, Ellwood SR (2008) Two alternative recessive quantitative trait loci influence resistance to spring black stem and leaf spot in Medicago truncatula. BMC Plant Biology 8, 30
| Two alternative recessive quantitative trait loci influence resistance to spring black stem and leaf spot in Medicago truncatula.Crossref | GoogleScholarGoogle Scholar | 18366746PubMed |
Kamphuis LG, Williams AH, Kuster H, Trengove RD, Singh KB, Oliver RP, Ellwood SR (2012) Phoma medicaginis stimulates the induction of the octadecanoid and phenylpropanoid pathways in Medicago truncatula. Molecular Plant Pathology 13, 593–603.
| Phoma medicaginis stimulates the induction of the octadecanoid and phenylpropanoid pathways in Medicago truncatula.Crossref | GoogleScholarGoogle Scholar | 22212347PubMed |
Kernkamp MF, Hemerick GA (1953) The relation of Ascochyta imperfecta to alfalfa seed production in Minnesota. Phytopathology 43, 378–383.
Lamprecht SC, Knox-Davies PS (1984) Preliminary survey of foliage diseases of annual Medicago spp. in South Africa. Phytophylactica 16, 177–183.
Leath KT (1990) Spring black stem and leaf spot. In ‘Compendium of alfalfa diseases’. (Eds DL Stuteville, DC Erwin) pp. 16–17. (American Phytopathological Society: St. Paul, MN, USA)
Lüscher A, Mueller-Harvey I, Soussana JF, Rees RM, Peyraud JL (2014) Potential of legume-based grassland–livestock systems in Europe: a review. Grass and Forage Science 69, 206–228.
| Potential of legume-based grassland–livestock systems in Europe: a review.Crossref | GoogleScholarGoogle Scholar | 26300574PubMed |
Marshall T, Parkin RJ (1970) Phosphate applications affect the coumestrol level of medics. Journal of the Department of Agriculture Western Australia, Series 4 11,
Mhadhbi H, Jebara M, Limam F, Huguet T, Elarbi Aouania M (2005) Interaction between Medicago truncatula lines and Sinorhizobium meliloti strains for symbiotic efficiency and nodule antioxidant activities. Physiologia Plantarum 124, 4–11.
| Interaction between Medicago truncatula lines and Sinorhizobium meliloti strains for symbiotic efficiency and nodule antioxidant activities.Crossref | GoogleScholarGoogle Scholar |
Omidvari M, Flematti G, You MP, Abbaszadeh-Dahaji P, Barbetti MJ (2021) Phoma medicaginis isolate differences determine disease severity and phytoestrogen production in annual Medicago spp. Plant Disease 105, 2851–2860.
| Phoma medicaginis isolate differences determine disease severity and phytoestrogen production in annual Medicago spp.Crossref | GoogleScholarGoogle Scholar | 33851866PubMed |
Omidvari M, Flematti G, You MP, Abbaszadeh-Dahaji P, Barbetti MJ (2022) Phoma black stem severity and phytoestrogen production in annual Medicago spp. is primarily determined by interaction of cultivar and pathogen isolate. Plant Pathology 71, 860–872.
| Phoma black stem severity and phytoestrogen production in annual Medicago spp. is primarily determined by interaction of cultivar and pathogen isolate.Crossref | GoogleScholarGoogle Scholar |
Patisaul HB, Jefferson W (2010) The pros and cons of phytoestrogens. Frontiers in Neuroendocrinology 31, 400–419.
| The pros and cons of phytoestrogens.Crossref | GoogleScholarGoogle Scholar | 20347861PubMed |
Pierson LM, Ferkin MH (2015) The impact of phytoestrogens on sexual behavior and cognition in rodents. Mammalian Biology 80, 148–154.
| The impact of phytoestrogens on sexual behavior and cognition in rodents.Crossref | GoogleScholarGoogle Scholar |
Reed KFM (2016) Fertility of herbivores consuming phytoestrogen-containing Medicago and Trifolium species. Agriculture 6, 35
| Fertility of herbivores consuming phytoestrogen-containing Medicago and Trifolium species.Crossref | GoogleScholarGoogle Scholar |
Reid RL (1990) ‘Manual of Australian agriculture.’ 5th edn. (Australian Institute of Agricultural Science: Sydney, Australia)
Rodriguez RdP (2005) Histological studies on the development of Phoma root rot of alfalfa. Journal of Agriculture 89, 251–262.
Rodriguez RDP, Leath KT (1992) Pathogenicity of Phoma medicaginis var. medicaginis to crowns of alfalfa. Plant Disease 76, 1237–1240.
| Pathogenicity of Phoma medicaginis var. medicaginis to crowns of alfalfa.Crossref | GoogleScholarGoogle Scholar |
Smith JF, Jagusch KT, Brunswick LFC, Kelly RW (1979) Coumestans in lucerne and ovulation in ewes. New Zealand Journal of Agricultural Research 22, 411–416.
| Coumestans in lucerne and ovulation in ewes.Crossref | GoogleScholarGoogle Scholar |
Tivoli B, Baranger A, Sivasithamparam K, Barbetti MJ (2006) Annual Medicago: from a model crop challenged by a spectrum of necrotrophic pathogens to a model plant to explore the nature of disease resistance. Annals of Botany 98, 1117–1128.
| Annual Medicago: from a model crop challenged by a spectrum of necrotrophic pathogens to a model plant to explore the nature of disease resistance.Crossref | GoogleScholarGoogle Scholar | 16803846PubMed |
Wasserman MD, Milton K, Chapman CA (2013) The roles of phytoestrogens in primate ecology and evolution. International Journal of Primatology 34, 861–878.
| The roles of phytoestrogens in primate ecology and evolution.Crossref | GoogleScholarGoogle Scholar |
You MP, Sivasithamparam K, Riley IT, Barbetti MJ (2000) The occurrence of root-infecting fungi and parasitic nematodes in annual Medicago spp. in Western Australian pastures. Australian Journal of Agricultural Research 51, 435–444.
| The occurrence of root-infecting fungi and parasitic nematodes in annual Medicago spp. in Western Australian pastures.Crossref | GoogleScholarGoogle Scholar |