HFIP-assisted Brønsted acid catalysed synthesis of furan derivatives
Bonnie Pu A and Thanh Vinh Nguyen A *
A *
A School of Chemistry, University of New South Wales, Sydney, NSW 2052, Australia.
Australian Journal of Chemistry 76(1) 58-62 https://doi.org/10.1071/CH22212
Submitted: 28 September 2022 Accepted: 16 November 2022 Published: 18 January 2023
© 2023 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
The furan framework is ubiquitous in naturally occurring compounds, and furan-containing structures are also key intermediates to many industrially important chemicals and materials. There have been reports of numerous methods to synthesise furans, however most of them use transition metal catalysts or Brønsted acid catalysts under harsh conditions. This work describes the development of a new non-metal Brønsted acid catalytic method for the synthesis of 2-aryl-3-carboxylate ester furans and 2,3-diaryl furans from ynone substrates. The method was shown to be efficient under very mild reaction conditions with up to 94% product yields.
Keywords: Brønsted acid catalysis, catalysis, cyclization reaction, furan, HFIP, hydrogen‐bonding, metal‐free, solvent effect.
Introduction
The furan framework is ubiquitous in naturally occurring compounds,[1] and furan-containing structures are also key intermediates to many industrially important chemicals and materials.[2] Many furan derivatives exhibit bioactivity and have been used as anti-infective, anti-tumour, cardiovascular and anti-inflammatory agents. Examples of furan-containing pharmaceuticals that are currently on the market include Nitrofurantoin®, an antibiotic, and Amiodarone®, a potent antiarrhythmic (Fig. 1).[3] Industrially, furan derivatives can also be found in a range of pesticides, and they can be polymerised to form chemically resistant resins and lacquers. An example of a binder resin which has recently been developed is the polyfurfuryl alcohol resin (Fig. 1), which can be made from biomass, a renewable source.[4]

|
Although there are many naturally occurring furan compounds, it is important to develop new synthetic methods to access useful naturally occurring furans on a larger scale, as well as synthetic structural analogues. The development of new methods may also allow the design and synthesis of specific furan frameworks with suitable structural features for niche applications. Classical approaches to furan synthesis include Paal–Knorr and Feist–Bénary synthesis (Fig. 2a), and they are still being used in modern synthetic chemistry. The main disadvantage of the Paal–Knorr synthesis of furans is the difficulty of synthesising reaction substrates,[5] while the key issue with Feist–Bénary is reaction efficiency. An increasingly popular approach to synthesising furan derivatives in the past decade is the intramolecular cyclisation of ynone substrates, which have ketone and alkyne functionalities at 1,4-positions. This 4-pentyne-1-one backbone structure can cyclise intramolecularly through a 5-exo-dig manner to form substituted furans with functionalisation possible on the C2, C3 and C5 positions (Fig. 2b). This reaction does not occur spontaneously, but a wide range of catalysts can be used to facilitate the reaction via different mechanisms. In recent years, numerous metal-based Lewis acid catalysts have been used to promote this reaction. Most notable catalysts include FeCl3 as reported by Golonka and Schindler[6] and Bi(OTf)3 as developed by Chang and co-workers.[7,8] However, these methods associate with the issue of trace metal impurities after product purification, while non-metal catalysts have been rarely used.[9,10] The development of novel synthetic methods to access furans using organic catalytic systems is thus attractive as they may be able to overcome the limitations of established pathways.

|
Results and discussion
Inspired by our previous work on metal-free cyclisation reactions[9,11–15] especially the I2-catalysed furan synthesis[9] and the HFIP-assisted carbonyl-olefin metathesis reaction,[14] we decided to investigate the catalytic activity of para-toluenesulfonic acid 1 (pTSA)/1,1,1,3,3,3-hexafluoroisopropanol (HFIP) in the promotion of the intramolecular cyclisation reactions of ynones to form furan derivatives. pTSA, also denoted as TsOH, is a relatively strong organic Brønsted acid that is commonly used in synthetic laboratories due to its excellent solubility in polar solvents and bench-stability, as it is a solid at room temperature.[16,17] These properties, in addition to its inexpensive cost, ready availability and low toxicity, allow pTSA to be a good homogenous reaction promoter in organic synthesis. HFIP is a highly polar solvent capable of hydrogen bonding and cation stabilisation with low nucleophilicity.[18] It has a low boiling point of 59°C, which allows it to be easily removed under reduced pressure or through distillation, where it can potentially be reused. HFIP has become increasingly popular in organic synthesis as it can increase reaction rate and product yield and promote otherwise unfavourable reactions due to the substrate’s electronic effects.[19] It is often used in conjunction with Lewis or Brønsted acid catalysts, and works that explore the mechanism of the reaction suggest that the HFIP activates the catalyst by creating hydrogen bonds with the catalyst and substrate (Fig. 3).[19]

|
Initial screening reactions on four model substrates 2a–d with pTSA in more conventional lab solvents such as toluene, THF, MeCN, DCM or DCE were quickly confirmed to be ineffective, which was consistent with the literature.[6] Gratifyingly, our test reactions with the pTSA/HFIP catalytic system showed > 50% conversion for all four substrates when heated. These reaction mixtures were relatively clean with only trace amounts of unwanted by-products. The reaction was subsequently optimised using 2d as the model substrate and HFIP as solvent. This substrate was chosen as it was easy to synthesise in large batches, unlike substrates 2a–c (see the Supporting Information for details). After an extensive optimisation study, we concluded that the reaction was most efficient with 10 mol% of pTSA catalyst in 300 μL HFIP per mmol of substrate, or 3.3 M concentration, at ambient temperature with reaction time ~24 h. Some notable variations from optimal conditions are presented in Table 1, while the full optimisation study can be seen in pages S3–S7 of the Supporting Information.

|
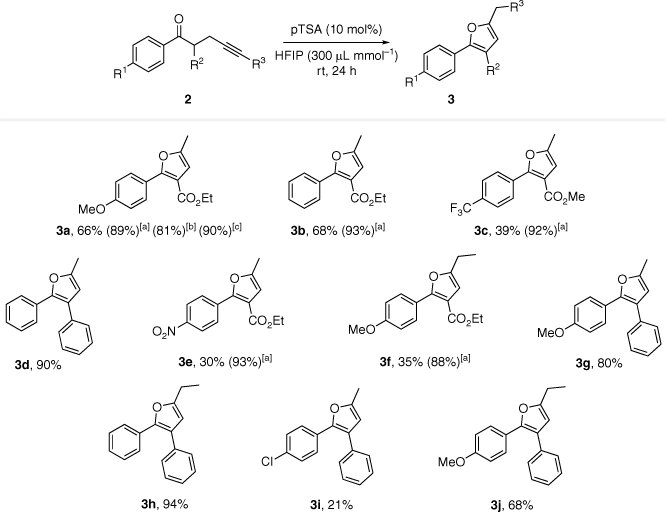
Having the optimal conditions in hand, we applied these to a range of ynone substrates. Most of them worked well to form furan products using our newly developed protocol (Scheme 1). Product purification was also straightforward as no reaction workup was required and reaction mixtures were generally simple with minimal by-product formation. The reaction substrates were well separated from the products by column chromatography due to the difference in hydrogen bonding capabilities of the carbonyl and furan functionalities, as well as the lack of enolisation in the products.
Some more challenging substrates required modification of the optimal conditions to obtain better efficiencies. Reaction yields were found to be relatively low for α-carboxyloxy ynone substrates as optimisation was actually completed on an α-aryl ynone substrate (2d). This decrease in yield was caused by low substrate-to-product conversion, and therefore we found that increasing the reaction time or temperature would lead to an increased reaction yield. Indeed, the variation of reaction conditions with substrate 2a showed that 3a could be obtained in 90% yield by either leaving the reaction mixture for 5 days at room temperature or by heating the reaction mixture to 50°C for 24 h. The reaction was therefore repeated for other α-carboxyl ynone substrates at 50°C with excellent results.
Our product yields were comparable to yields from established methods in the literature, as Golonka and Schindler achieved yields of 94 and 95% for 3a and 3b respectively in their work.[6] Their reaction conditions were similar, with 10 mol% FeCl3 catalyst and 48 h at room temperature. Similarly, our yields for 2,3-diaryl furans were comparable to yields achieved by Chan et al. as they produced 3d with 88% yield.[7] Unfortunately most of the other substrates they used were substituted on the C3 aryl so further product yield comparisons were not possible.
The comparison of room temperature α-carboxyloxy product yields (3a–c, e, f) highlight the impact of the R1 substituent. Yields of substrates with electron donating or neutral groups (3a: 66%, 3b: 68%) were consistently higher than those with electron withdrawing groups (3c: 39%, 3e: 30%). This was also evident in α-aryl substrates (3d: 90%, 3g: 80% vs 3i: 21%). From this, we can propose that either electron withdrawing moieties deactivated the substrate which led to higher activation barriers as mentioned before, or the mechanism proceeds through a carbocation intermediate that is more stabilised with neutral/electron-donating substituents. The second theory is plausible as the carbocation intermediate was well documented in the literature.
Similarly, as mentioned before, comparisons of room temperature yields of 3a/3g, 3b/3d, and 3f/3j which differ only by the R2 group show that substrates with the phenyl group have significantly higher yields than substrates with the ester group (14–33% difference). From this, we can see that the electronic character of R1 and R2 groups impact reaction yields in a similar manner, and therefore, they may be acting on the same part of the molecule. Alternatively, this could be justified individually as the phenyl group may be more useful in stabilising the intermediate than the ester group, as it is available for bonding interactions, which may occur within or between aryl structures on molecules. The rigidity of the phenyl ring may also assist product formation more compared to the ester as the ester may have to expend energy to move into a favourable conformation.
Based on all these insights, and mechanistic discussions provided in the literature for similar acid-catalysed reactions,[9,14] a hypothesised reaction mechanism is proposed in Scheme 2. In this hypothesised mechanism, HFIP acts as a highly ionising and strong hydrogen-bonding solvent which would coordinate to pTSA and render it a much stronger Brønsted acid. At the same time, it favours the enol form 2′ of the substrate. Protonation of 2′ leads to the formation of carbocation intermediate 4, which cyclises in 5-exo-dig fashion to give intermediate 5. Aromatisation of 5, most likely via a proton transfer step, affords product 3.

|
Conclusion
We have developed a new metal-free catalytic system with pTSA in HFIP which can efficiently cyclise ynone substrates to access a range of 2-aryl-3-carboxylate ester furans and 2,3-diaryl furans with high yields of up to 94%. The reaction was also shown to be viable under ambient conditions for electron rich 2,3-diaryl furans.
Supplementary material
The Supporting Information is available free of charge and includes experimental details and spectroscopic data for all products. Supplementary material is available online.
Data availability
The data that support this study are available in the article and accompanying online supplementary material.
Conflicts of interest
There is no conflict of interest to declare.
Declaration of funding
This work was funded by the Australian Research Council (grant FT180100260 and DP200100063 to T. V. N.).
Author contributions
T. V. N. conceived the ideas and designed the project. B. P. carried out all experimental work. The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
References
[1] Kwiecień H, Wodnicka A. 5.3 - Five-Membered Ring Systems: Furans and Benzofurans. In Gribble GW, Joule JA, editors. Progress in Heterocyclic Chemistry. Vol. 31. Elsevier; 2020. pp. 281–323.[2] FN Peters Jr, Industrial Uses of Furans. Ind Eng Chem 1939, 31, 178.
| Industrial Uses of Furans.Crossref | GoogleScholarGoogle Scholar |
[3] R Banerjee, K Hks, M Banerjee, Medicinal significance of furan derivatives: A Review. Int J Res Phytochem Pharmacol 2015, 5, 48.
[4] R Marefat Seyedlar, M Imani, SM Mirabedini, Bio-based furan coatings: adhesion, mechanical and thermal properties. Polym Bull 2021, 78, 577.
| Bio-based furan coatings: adhesion, mechanical and thermal properties.Crossref | GoogleScholarGoogle Scholar |
[5] V Amarnath, K Amarnath, Intermediates in the Paal-Knorr Synthesis of Furans. J Org Chem 1995, 60, 301.
| Intermediates in the Paal-Knorr Synthesis of Furans.Crossref | GoogleScholarGoogle Scholar |
[6] AN Golonka, CS Schindler, Iron(III) chloride-catalyzed synthesis of 3-carboxy-2,5-disubstituted furans from γ-alkynyl aryl- and alkylketones. Tetrahedron 2017, 73, 4109.
| Iron(III) chloride-catalyzed synthesis of 3-carboxy-2,5-disubstituted furans from γ-alkynyl aryl- and alkylketones.Crossref | GoogleScholarGoogle Scholar |
[7] C-K Chan, Y-C Chen, Y-L Chen, M-Y Chang, Synthesis of substituted phenanthrofurans. Tetrahedron 2015, 71, 9187.
| Synthesis of substituted phenanthrofurans.Crossref | GoogleScholarGoogle Scholar |
[8] M-Y Chang, Y-C Cheng, Y-J Lu, Bi(OTf)3-Mediated Cycloisomerization of γ-Alkynyl Arylketones: Application to the Synthesis of Substituted Furans. Org Lett 2015, 17, 1264.
| Bi(OTf)3-Mediated Cycloisomerization of γ-Alkynyl Arylketones: Application to the Synthesis of Substituted Furans.Crossref | GoogleScholarGoogle Scholar |
[9] DP Pace, R Robidas, UPN Tran, CY Legault, TV Nguyen, Iodine-Catalyzed Synthesis of Substituted Furans and Pyrans: Reaction Scope and Mechanistic Insights. J Org Chem 2021, 86, 8154.
| Iodine-Catalyzed Synthesis of Substituted Furans and Pyrans: Reaction Scope and Mechanistic Insights.Crossref | GoogleScholarGoogle Scholar |
[10] Y Peng, J Luo, Q Feng, Q Tang, Understanding the Scope of Feist–Bénary Furan Synthesis: Chemoselectivity and Diastereoselectivity of the Reaction Between α-Halo Ketones and β-Dicarbonyl Compounds. Eur J Org Chem 2016, 2016, 5169.
| Understanding the Scope of Feist–Bénary Furan Synthesis: Chemoselectivity and Diastereoselectivity of the Reaction Between α-Halo Ketones and β-Dicarbonyl Compounds.Crossref | GoogleScholarGoogle Scholar |
[11] UPN Tran, G Oss, DP Pace, J Ho, TV Nguyen, Tropylium-promoted carbonyl-olefin metathesis reactions. Chem Sci 2018, 9, 5145.
| Tropylium-promoted carbonyl-olefin metathesis reactions.Crossref | GoogleScholarGoogle Scholar |
[12] G Oss, TV Nguyen, Iodonium-Catalyzed Carbonyl–Olefin Metathesis Reactions. Synlett 2019, 30, 1966.
| Iodonium-Catalyzed Carbonyl–Olefin Metathesis Reactions.Crossref | GoogleScholarGoogle Scholar |
[13] UPN Tran, G Oss, M Breugst, et al. Carbonyl–Olefin Metathesis Catalyzed by Molecular Iodine. ACS Catal 2019, 9, 912.
| Carbonyl–Olefin Metathesis Catalyzed by Molecular Iodine.Crossref | GoogleScholarGoogle Scholar |
[14] T Anh To, C Pei, RM Koenigs, T Vinh Nguyen, Hydrogen Bonding Networks Enable Bronsted Acid-Catalyzed Carbonyl-Olefin Metathesis. Angew Chem Int Ed 2022, 61, e202117366.
| Hydrogen Bonding Networks Enable Bronsted Acid-Catalyzed Carbonyl-Olefin Metathesis.Crossref | GoogleScholarGoogle Scholar |
[15] K Omoregbee, KNH Luc, AH Dinh, TV Nguyen, Tropylium-promoted prenylation reactions of phenols in continuous flow. J Flow Chem 2020, 10, 161.
| Tropylium-promoted prenylation reactions of phenols in continuous flow.Crossref | GoogleScholarGoogle Scholar |
[16] B Baghernejad, Application of p-toluenesulfonic Acid (PTSA) in Organic Synthesis. Curr Org Chem 2011, 15, 3091.
| Application of p-toluenesulfonic Acid (PTSA) in Organic Synthesis.Crossref | GoogleScholarGoogle Scholar |
[17] H Sharghi, P Shiri, M Aberi, An overview on recent advances in the synthesis of sulfonated organic materials, sulfonated silica materials, and sulfonated carbon materials and their catalytic applications in chemical processes. Beilstein J Org Chem 2018, 14, 2745.
| An overview on recent advances in the synthesis of sulfonated organic materials, sulfonated silica materials, and sulfonated carbon materials and their catalytic applications in chemical processes.Crossref | GoogleScholarGoogle Scholar |
[18] I Colomer, AER Chamberlain, MB Haughey, TJ Donohoe, Hexafluoroisopropanol as a highly versatile solvent. Nat Rev Chem 2017, 1, 0088.
| Hexafluoroisopropanol as a highly versatile solvent.Crossref | GoogleScholarGoogle Scholar |
[19] V Pozhydaiev, M Power, V Gandon, J Moran, D Lebœuf, Exploiting hexafluoroisopropanol (HFIP) in Lewis and Brønsted acid-catalyzed reactions. Chem Commun 2020, 56, 11548.
| Exploiting hexafluoroisopropanol (HFIP) in Lewis and Brønsted acid-catalyzed reactions.Crossref | GoogleScholarGoogle Scholar |