Mitigation of disease and browsing impacts, and translocation, supports post-fire threatened flora recovery
Sarah Barrett A , Colin J. Yates B , Rebecca Dillon B , Megan Dilly A , Ben Varcoe A , Darcy Martin A , Bayley Castlehow A and Carl R. Gosper
B , Megan Dilly A , Ben Varcoe A , Darcy Martin A , Bayley Castlehow A and Carl R. Gosper  B *
B *
A
B
Abstract
For plant species that have evolved in fire-prone environments, declines after wildfires are often driven by the combination of fire and other threatening processes. Mitigating the impacts of these threatening processes can sometimes effectively support post-fire population recovery.
We test the effectiveness of: (1) phosphite application to mitigate Phytophthora dieback; (2) fencing to exclude browsing by mammalian herbivores; and (3) translocation to sites where threats can be practically managed, for conservation of threatened flora affected by wildfires in 2018 and 2019 in the Stirling Range (Koi Kyeunu-ruff), south-western Australia.
Survival of Phytophthora-susceptible flora was compared in repeatedly sampled plots from prior to and after wildfire and ± recurrent phosphite application. Survival and growth of browsing-susceptible flora was compared post-fire in fenced and control plots. Survival, growth and flowering was compared between wild populations recruiting after wildfire and translocated populations.
Phosphite application increased survival of most Phytophthora-susceptible flora. Fencing led to greater growth and often increased survival. Translocated populations, with supplemental water, had greater growth rates and earlier flowering than wild populations, and a non-significant trend for higher survival.
These findings provide strong evidence supporting continuation of phosphite application, herbivore exclusion and translocation for post-fire recovery of the threatened flora of the Stirling Range.
With increasing wildfire extent, frequency and impact across the globe, successful management of non-fire threats will be crucial for post-fire conservation of threatened flora, with the approaches proving effective in this study likely to have conservation value elsewhere.
Keywords: exclusion fencing, fire regime, herbivore, phosphite, Phytophthora, recruitment, seed production area, Southwest Australian Floristic Regionxs.
Introduction
Wildfires are increasing in extent, frequency and severity across the globe, driven by patterns of land use and ongoing climatic warming and drying affecting ignitions and vegetation flammability (Halofsky et al. 2020; Canadell et al. 2021). Changes in fire regimes, such as altered fire interval, season or intensity, have large impacts on plant populations (Keeley et al. 1999; Tulloch et al. 2016; Miller et al. 2019; Auld et al. 2022). However, in ecosystems that have evolved with recurrent fire, it is often the combination of fire and the occurrence of other threats that drives plant declines (Barrett and Yates 2015; Gallagher et al. 2022; Auld et al. 2022). For example, in a recent assessment of the impacts of the unprecedented 2019–2020 fire season on Australian plant populations and ecological communities, Gallagher et al. (2022) and Keith et al. (2022) identified a range of threats additional to those related to fire regime that acted cumulatively or compounded fire effects to potentially cause declines in plant populations and ecological community condition. These threats included biotic processes such as disease, herbivory and weed invasion, abiotic processes such as pre- or post-fire drought and erosion, and anthropogenic disturbances such as pre- or post-fire logging and increased access for recreation. Gallagher et al. (2022) and Keith et al. (2022) concluded that post-fire recovery of plant populations needs a strong emphasis on mitigating the threats that compound fire impacts.
Large areas (~54,000 ha) of the Stirling Range National Park (~115,000 ha; SRNP) in south-western Australia burnt in wildfires in 2018 and 2019. The Stirling Range (Koi Kyeunu-ruff) is a hotspot of plant diversity in the Southwest Australian Floristic Region (SWAFR), supporting more than 1500 plant taxa and a high degree of short-range endemism, including >80 taxa found only within the National Park (Department of Conservation and Land Management (DCLM) 1999; Gioia and Hopper 2017). This floristic richness and endemism are associated with unique climatic and geomorphological conditions, with the range being an ancient quartzite feature that has resisted weathering to form the highest peaks in the region (Department of Conservation and Land Management (DCLM) 1999; Barrett and Yates 2015; Gosper et al. 2021a). Threatened flora are strongly represented in the Stirling Range, with 26 nationally listed threatened species affected by the 2018–2019 wildfires, along with one threatened ecological community (Barrett and Yates 2015; Gosper et al. 2021a; Keith et al. 2022). A specific threat syndrome (i.e. numerous taxa susceptible to the same set of conditions; Burgman et al. 2007) is associated with flora and vegetation decline in the Stirling Range: shared susceptibility to Phytophthora dieback and short fire intervals, compounded by post-fire herbivory (Barrett et al. 2008; Barrett and Yates 2015; Rathbone and Barrett 2017). Additionally, ongoing climatic warming and drying (Delworth and Zeng 2014) may be magnifying the impact of these threats by imposing growth, recruitment and reproductive limitations (Barrett et al. 2008; Barrett and Yates 2015; Keppel et al. 2017; Gosper et al. 2021b, 2022), but empirical evidence for Stirling Range flora to date is limited.
Phytophthora dieback causing plant decline and mortality through infection by pathogenic water moulds, in particular the introduced Phytophthora cinnamomi Rands, is a major threat to the flora of south-western Australia (Shearer et al. 2007; Barrett et al. 2008). An estimated 36% of plant species in the SRNP are susceptible to P. cinnamomi infection, with the degree of susceptibility varying between and within plant lineages (Shearer et al. 2004a). Over two-thirds of the SRNP is regarded as infested with the pathogen (Grant and Barrett 2003). Significantly, Phytophthora impact increases post-fire, likely associated with increased soil temperatures and changed hydrology in the post-fire environment favouring growth and dispersal of the pathogen among less-resilient root structures in recruiting plants (Moore 2005; Moore et al. 2015). Obligate seeders are prevalent in the Stirling Range (Keith et al. 2002), rendering the flora vulnerable to fire intervals shorter than the species’ maturation periods. Lower mean annual temperature and lower gross primary productivity, associated with higher elevation and rocky environments, such as the montane portions of the Stirling Range, are factors associated with longer juvenile periods in slow-maturing serotinous obligate seeders (Gosper et al. 2022). Studies modelling maturation time together with empirical documentation of juvenile periods, population declines and extinctions, indicate that fire intervals exceeding 20 years minimise risks of population extinctions in the slow-maturing serotinous obligate seeders of the Stirling Range (Barrett and Yates 2015; Gosper et al. 2022). Browsing of post-fire recruits and resprouts by mammalian herbivores can significantly limit rates of post-fire (re-)establishment (Rathbone and Barrett 2017).
Several management approaches have demonstrated value in mitigating the impacts of threats that compound the effects of wildfires. Application of the fungicide phosphite can reduce disease expression and mortality in Phytophthora-sensitive flora (Shearer et al. 2004b; Barrett and Rathbone 2018; Boulle et al. 2023). Installation of herbivore-exclusion fencing can lead to greater plant reproduction and growth rates (Rathbone and Barrett 2017). Establishment of new populations through translocations to locations that are largely free of the prevailing threats is a commonly used conservation action, and is particularly promoted for montane species to mitigate climate change impacts (Thomas 2011; Silcock et al. 2019). However, uncertainty remains over whether threat mitigation measures are effective after all fires, irrespective of fire-event effects such as post-fire weather and fire extent, and whether effectiveness is maintained over the medium to long term. In addition to some phytotoxicity effects at high phosphite application rates (Tynan et al. 2001), there are concerns over the implications of recurrent phosphite application, related to changes in interspecific competitive interactions or increases in phosphorus toxicity arising through increases in soil phosphorus (Lambers et al. 2013). Proteaceae, with their inability to down-regulate phosphorus uptake, which has arisen through evolution for nutrient acquisition on impoverished soils, may be particularly affected (Standish et al. 2007; Lambers et al. 2013), although field evidence for detrimental effects of phosphite application has not become apparent (Boulle et al. 2023). Fire scale and spatial arrangement of burnt patches and unburnt refuges has a substantial bearing on the persistence, recolonisation and browsing intensity of herbivorous animals, and hence exposure of post-fire recruits to high browsing pressure likely varies between fire events (Knight and Holt 2005; Bowman et al. 2021). Translocation success varies widely, presumably reflecting a range of species-, site-, approach- and event-linked idiosyncrasies (Godefroid et al. 2011; Silcock et al. 2019). In the SWAFR, where short-range endemism, high rates of species turnover and edaphic specialisation are typical (Cowling et al. 1994; Yates et al. 2019; Gosper et al. 2021a), translocations may prove particularly challenging.
In this study, we test the effectiveness of three widely applied post-fire threat mitigation approaches in mediating improved survival and/or growth of threatened flora affected by wildfires in the Stirling Range in 2018 and 2019, to inform future threat mitigation investment in the Stirling Range and elsewhere where similar threats apply. Specifically, we test whether:
Extended phosphite application over the period 2002–2022, including after recent wildfires, increases survival and population density relative to untreated habitat;
Installation of herbivore-exclusion fencing increases survival and growth relative to unfenced areas after recent wildfires; and
Initial survival, growth and flowering of translocated populations in locations free of Phytophthora, with mammalian herbivores excluded and with supplemental summer watering, exceeds those of wild populations recovering after recent fires where these threats are likely present, although with phosphite, rabbit control and fencing threat mitigation applied in many cases.
Materials and methods
Effect of phosphite treatment on survival
Three study sites were selected to investigate the effect of aerial application of the fungicide phosphite on the survival of three Phytophthora-susceptible species (Banksia anatona (A.S.George) A.R.Mast & K.R.Thiele, Daviesia glossosema Crisp and Daviesia pseudaphylla Crisp; all critically endangered under both the federal Environment Protection and Biodiversity Conservation Act 1999 and the Western Australian Biodiversity Conservation Act 2016). The sites were south of Bluff Knoll, with a recent fire history of being burnt in 2000 and 2018. Each site included an area that had been sprayed with phosphite annually to biennially at 6–24 kg ha−1 since 2002, and post-2018 at the reduced rate of 6 kg ha−1 in autumn 2020, 2021 and 2022, as part of operational phosphite applications to areas of up to 50 ha. The study sites were located within a landscape comprised of a mosaic of P. cinnamomi-infested and non-infested vegetation.
In the Daviesia populations, ten 5 × 5 m plots were established, five within and five outside phosphite-treated habitat. The total number of live individuals within the plots were counted, mostly annually, between 2002 and 2022 (year n = 17 over a 21 year period). In the B. anatona population, there were seven plots, five treated and two non-sprayed controls; individuals were counted in all plots annually from 2002 to 2018 and post-fire in spring 2018 (year n = 18 over a 17 year period, with pre- and post-fire data in 2018).
At the B. anatona and D. glossosema populations, the effect of phosphite on the survival of these species as well as additional Phytophthora-susceptible species (Banksia sphaerocarpa R.Br. var. sphaerocarpa, Hakea cucullata R.Br., Jacksonia calycina Domin and Lambertia ericifolia R.Br.) was further investigated. This study used a factorial design of two treatments (± phosphite, status as either diseased or healthy in 2015). Thirty-two 10 × 10 m plots were established (Barrett and Rathbone 2018) and individuals of each species were counted in 2015 and 2022, one set of 16 plots with B. anatona, and another set with the remaining five species. Thus, for each species, phosphite treatment and disease status were represented at two levels, to derive four treatment groups as follows, each with four replicate plots: (1) phosphite-treated + healthy; (2) phosphite-treated + diseased; (3) no-phosphite + healthy; (4) no-phosphite + diseased. Disease status was confirmed by visual field interpretation and laboratory analysis of samples in 2015.
Effect of herbivore exclusion on survival and growth
Three study sites were selected to investigate the effect of excluding mammalian herbivores on the growth and survival of three species after the autumn 2018 fire. Two sites were located on the upper slopes of Bluff Knoll (both with Darwinia collina C.A.Gardner (critically endangered), one also with Latrobea colophon Chappill & C.F.Wilkins (critically endangered)), with fences installed in 2020. In addition, one site was established south of Bluff Knoll with B. anatona where fences were installed in 2019. Fences were constructed with heavy galvanised netting (3 cm aperture) at a minimum height of 80 cm and supported with steel star pickets. A netting skirt, 25 cm in width, was fitted along the base of the fence to prevent herbivores from digging underneath. For D. collina, there were three fenced and three non-fenced (Location 1), and two fenced and two non-fenced (Location 2), 5 × 5 m plots. Latrobea colophon was sampled at the same Location 1 as D. collina in three fenced and three non-fenced plots, four of which overlapped with plots sampled for D. collina. Within each plot, ten seedlings were randomly selected and tagged with a unique number and their survival and height (cm) recorded in 2020, 2021 and 2022 (total n = 100 for D. collina, n = 60 for L. colophon). For B. anatona, two sets of four fenced and four non-fenced 10 × 10 m plots were established, one set in habitat with a previous 27-year fire interval prior to 2018 and burnt twice since 1990, the other set with an 18-year fire interval and burnt three times since 1990. Within each plot, 15 seedlings (one plot with 16) were randomly selected, tagged and their survival and height recorded in 2019, 2020, 2021 and 2022 (total n = 241). Individuals that were not able to be located in the final 2022 count were assumed to have died and these individuals were excluded from analysis of height increment (leaving n = 78 for D. collina, n = 23 for L. colophon and n = 194 for B. anatona).
Survival, growth and flowering of translocated and wild populations
Two translocation sites (seed production areas) were established for 13 Stirling Range threatened flora taxa at two undisclosed Phytophthora-free locations outside of the SRNP. Plants for translocation were germinated in winter to spring 2020 from previously collected seed stored in the Western Australian Seed Centre (Kensington) and grown under nursery conditions for approximately 9 months. Both translocation sites represent novel habitat for all species and were selected on characteristics considered to be critical for their survival, in particular, the absence of Phytophthora dieback, adequate rainfall, suitable soil types, suitable vegetation cover and levels of vegetation competition. Each site, approximately 1.5 ha in area, was fenced to exclude mammalian herbivores. In May to June 2021, seedlings were planted, individually labelled with permanent tags and reticulation installed with automatic watering set to deliver approximately 1 L of water weekly to each plant during the summer months (December to March) in 2021–2022 and 2022–2023. At the time of planting and in 2022 and 2023, survival and height were measured, along with presence of flowering for most species. In wild populations, a suite of monitoring was established for the same flora taxa after fires in 2018 and 2019; this included survival, growth and flowering of individuals within monitoring plots as well as for tagged individuals, with data collected annually.
Survival/flowering was calculated as the proportion of initial individuals that remained alive/flowered at the final measurement per species and was calculated at a population level. Again, individuals in wild populations that were not able to be located in the final count were assumed to have died. Height increment per individual over the monitoring period was the unit of analysis for plant growth.
Statistical analysis
The effect of phosphite treatment on plant density of B. anatona, D. glossosema and D. pseudaphylla in 5 × 5 m plots was tested with a repeated-measures ANOVA, with the repeated measure of year for the approximately 20 years of time series data. The effect of phosphite treatment and disease status in 2015 on plant density of B. anatona, B. sphaerocarpa, D. glossosema, H. cucullata, J. calycina and L. ericifolia in 10 × 10 m plots was tested with a repeated-measures ANOVA, with the repeated measure of year for 2015 and 2022 (after burning in 2018) samples.
The effect of herbivore-exclusion fencing on growth of B. anatona was tested with an ANOVA with factors of ± fencing, recent fire history (two levels – previous interval 27 and 18 years and two and three fires since 1990 respectively), the interaction of fencing × recent fire history, and plot nested in fencing × recent fire history. For survival of B. anatona, ANOVA factors were ± fencing, recent fire history and their interaction. For growth of D. collina, ANOVA factors were ± fencing, location, fencing × location and plot nested in fencing × location. For survival of D. collina, ANOVA factors were ± fencing, location (two locations) and their interaction. As there were very low rates of survival in unfenced L. colophon plots, increment was not analysed; survival between treatments was tested with a t-test.
For comparisons of plant survival, growth and flowering between translocated and wild populations, each of the two translocation sites and wild populations recruiting after the 2018 and 2019 fires were considered four different ‘treatments’. Comparisons were made at the same effective plant age (~2.5–3 years old; 2018 fire – final measurements summer 2020–2021, 2019 fire – final measurements summer 2022–2023, both translocations – final measurements summer 2022–2023). As individual species were not represented in all treatments (Table 1), differences in survival was analysed by including data from all species, using a one-way ANOVA with a single factor of Treatment. Flowering was not statistically analysed due to the complete absence of flowering in some treatments. Growth increment was tested separately in each species that was monitored in at least one translocation and one wild population, using a one-way ANOVA with a single factor of Treatment, with significant differences further examined with post-hoc pair-wise Newman–Keuls comparisons. Square-root transformation was required to meet analysis assumptions for height increment of B. anatona, B. montana (A.S.George) A.R.Mast & K.R.Thiele, Lambertia fairallii Keighery and L. colophon. All statistical analyses were conducted in Statistica 7.1 (https://docs.tibco.com/products/tibco-statistica-14-1-0).
| Species | 2018 fire | 2019 fire | Translocation A | Translocation B | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | L | F | Ht (cm) | n | L | F | Ht (cm) | n | L | F | Ht (cm) | n | L | F | Ht (cm) | ||
| Andersonia axillifloraA | 1.00 | 0 | nm | 1.00 | 0 | nm | 0.88 | 0 | nm | ||||||||
| Banksia anatona *** | 0.95 | 0 | nm | 24 | 0.70 | 0 | 24.0 ± 2.2c | 69 | 0.99 | 30.4 | 124.8 ± 4.7a | 53 | 0.79 | 39.0 | 48.6 ± 4.3b | ||
| B. brownii *** | 29 | 0.58 | 0 | 35.4 ± 2.4d | 109 | 0.76 | 0 | 66.9 ± 2.8c | 85 | 1.00 | 4.7 | 115.3 ± 3.3a | 50 | 1.00 | 0 | 81.3 ± 4.6b | |
| B. montana *** | 37 | 0.74 | 0 | 16.2 ± 1.9c | 0.03 | 0 | nm | 26 | 0.95 | 44.4 | 96.2 ± 4.9a | 50 | 0.90 | 19.2 | 60.5 ± 2.2b | ||
| Darwinia collina *** | 0.83 | 0 | nm | 10 | 1.00 | 0 | 2.5 ± 2.0b | 70 | 0.92 | 32.9 | 31.6 ± 1.1a | ||||||
| Daviesia glossosemaA | 0.54 | nm | nm | 0.70 | nm | nm | 0.67 | nm | nm | ||||||||
| D. obovata *** | 0.84 | 0 | nm | 20 | 1.00 | 0 | 64.6 ± 4.9a | 77 | 0.98 | 7.8 | 68.5 ± 2.8a | 58 | 0.48 | 0 | 33.0 ± 2.4b | ||
| D. pseudaphyllaA | 0.49 | nm | nm | 0.97 | nm | nm | 0.75 | nm | nm | ||||||||
| Gastrolobium luteifoliumA | 0.90 | 0 | nm | 0.94 | 0 | nm | |||||||||||
| G. vestitumA | 0.36 | 0 | nm | 0.98 | 58.9 | nm | |||||||||||
| Lambertia fairallii *** | 25 | 0.83 | 0 | 8.9 ± 0.7c | 10 | 1.00 | 100 | 72.3 ± 4.5a | 11 | 0.73 | 63.6 | 22.4 ± 3.5b | |||||
| Latrobea colophon *** | 13 | 0.43 | nm | 8.9 ± 2.1b | 99 | 0.99 | nm | 35.2 ± 1.8a | 92 | 0.66 | nm | 14.8 ± 1.2b | |||||
| Leucopogon gnaphalioidesA | 1.00 | 0 | nm | 0.75 | 0 | nm | 1.00 | 0 | nm | ||||||||
| Mean survivalns | 0.74 | 0.70 | 0.94 | 0.81 | |||||||||||||
Period of monitoring was to an effective age of ~3 years.
n, number of initial individuals monitored for height (Ht); L, proportion survival; F, percentage having flowered; nm, not measured.
Height: ***, P < 0.001; **, P < 0.01; *, P < 0.05; superscript lowercase letters indicate differences in pair-wise comparisons; Anot included in height analyses as height was not measured.
Survival: ns, not significant.
Results
Effects of phosphite treatment
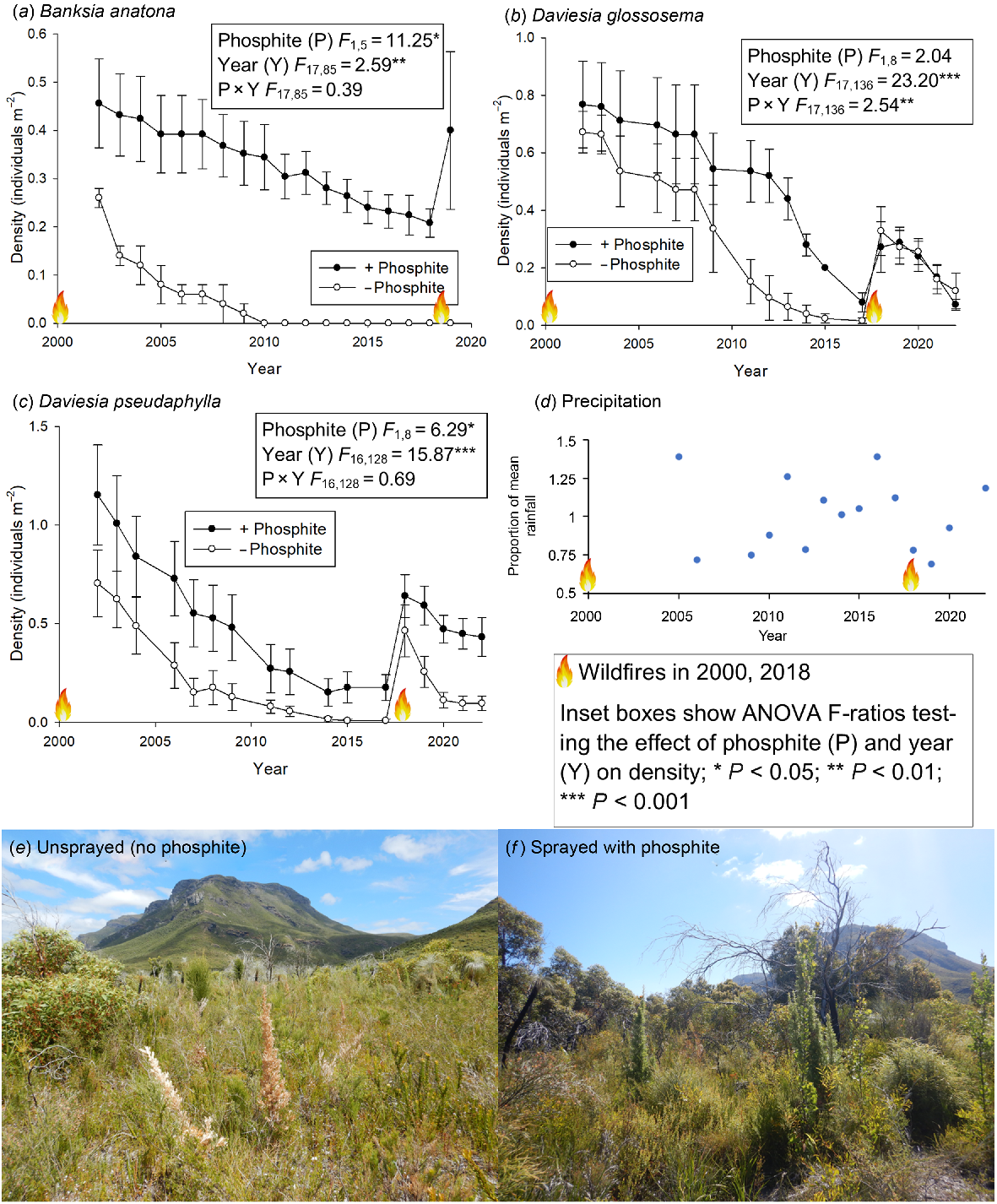
For all three species in the 5 × 5 m plots, there was an effect on plant density of the repeated measure of year – not unexpected, given density-dependent thinning over time and a germination-stimulating fire in the time sequence (Fig. 1). Two species (B. anatona and D. pseudaphylla) showed a significant effect of phosphite treatment, although caution is needed in interpreting this finding. Both species had higher density in phosphite-treated plots from the start of monitoring, which suggests site differences or differential impacts of Phytophthora prior to the commencement of monitoring and phosphite treatment. The initial differences in density were maintained over the course of the experiment, although in the case of B. anatona, no individuals survived through the 2000–2017 inter-fire interval in non-sprayed plots, and no recruits established after the 2018 wildfire. Banksia anatona has thus declined to local extinction in non-sprayed plots. Daviesia pseudaphylla density declined with time after the 2000 wildfire in both phosphite-treated and non-sprayed plots, with some germination and recruitment across both treatments after the 2018 wildfire. However, the trajectory of decline in density in non-sprayed plots after 2018 indicates that this species may soon be lost from the above-ground vegetation in these plots. Daviesia glossosema showed a phosphite × year interaction, which suggests that the effect of phosphite treatment was not consistent across all years. There was a more rapid decline in D. glossosema density over the period 2000–2018 in non-sprayed plots; however, recruitment was similar after the 2018 wildfire in both treatments and there was no evidence of divergence between treatments over the years of post-fire data available to date. The density of recruiting D. glossosema after the 2018 fire appeared much lower in both treatments than in the same plots after the 2000 fire.
Effects of phosphite spraying to mitigate the impact of Phytophthora on the density (mean ± s.e.) of threatened flora in 5 × 5 m plots, in the context of stand-replacement wildfires and annual precipitation: (a) Banksia anatona; (b) Daviesia glossosema; and (c) Daviesia pseudaphylla. (d) The proportion of mean annual precipitation received per year over the study period at Amelup (~10 km north-west of the study area; Bureau of Meteorology station number 10502, https://reg.bom.gov.au/climate/data/), noting that the years 2001–2004, 2007 and 2008 had incomplete records and are not shown, and that precipitation at this location should be viewed as a rough approximation of precipitation of the study area on montane portions of the Stirling Range. Example photographs (S. Barrett) of B. anatona habitat without (e) and with (f) recurrent phosphite spraying.
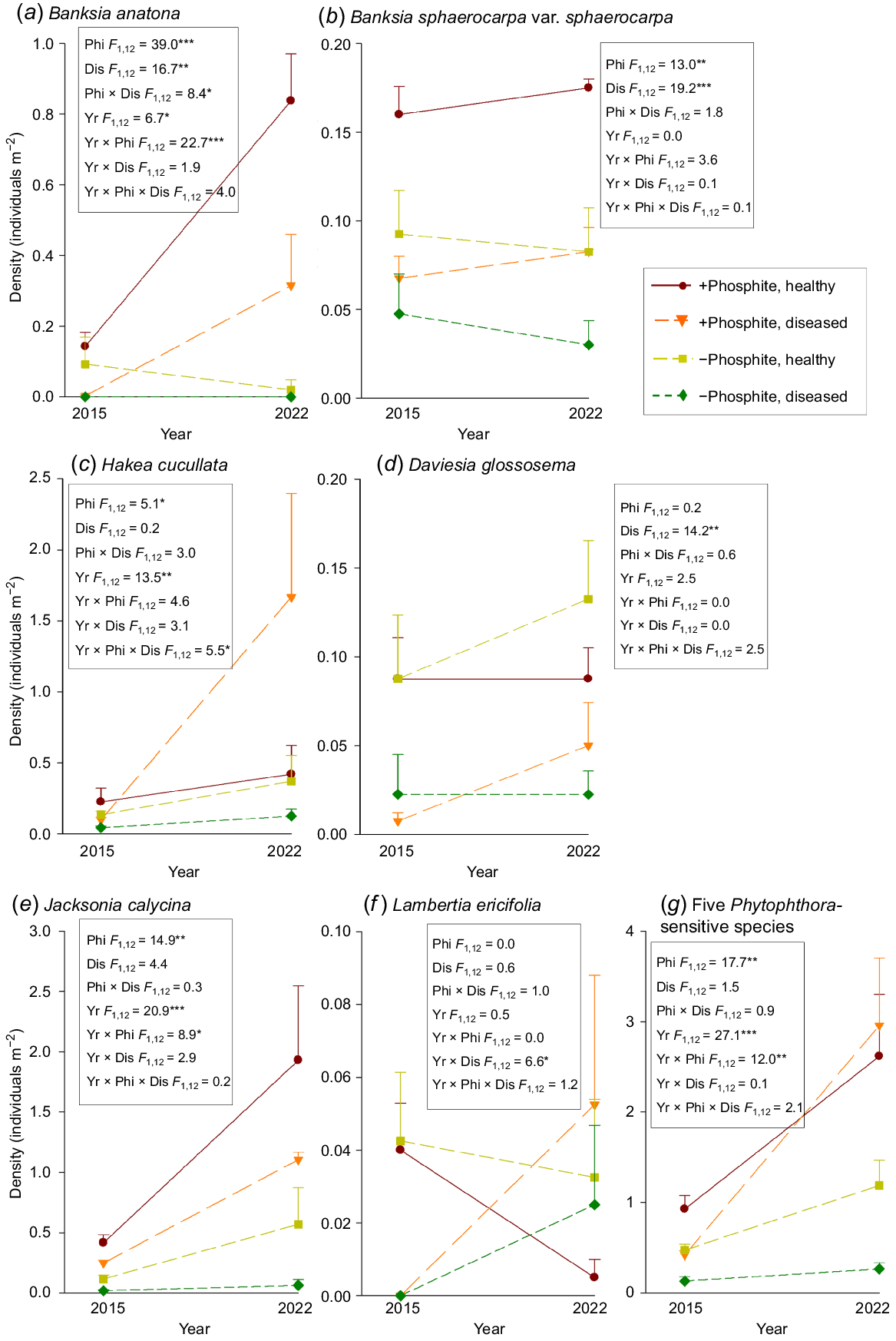
In the 10 × 10 m plots, which included treatments of ± phosphite application and ± Phytophthora presence at experiment commencement in 2015, with a fire between samples in 2015 and 2022, phosphite spraying emerged as the most important overall determinant of density of Phytophthora-sensitive flora (Fig. 2). Phosphite spraying had a significant main effect on density of B. sphaerocarpa, a significant interactive effect with year for B. anatona and J. calycina, and a significant interactive effect with year and disease for H. cucullata. In all significant interactions, pair-wise comparisons showed that phosphite-sprayed plots in 2022, and diseased + phosphite in 2022, respectively, had higher density than other fungicide treatment and year combinations. Disease had significant main effects on density of B. sphaerocarpa, a significant interaction with phosphite application for B. anatona, and was the only factor affecting D. glossosema, with putatively healthy plots having greater density. Effects of treatments on the density of L. ericifolia showed a significant year and disease interaction, but unexpectedly, with greater declines in density by 2022 in plots with Phytophthora absence in 2015. Aggregated density of the five species sampled concurrently on plots (all except B. anatona), showed a significant interaction between phosphite application and year, with higher density in phosphite-sprayed plots in 2022.
Effects of phosphite treatment (± phosphite) to mitigate the impact of Phytophthora, Phytophthora presence at the start of sampling in 2015 (healthy/diseased), and sampling year (2015 vs. 2022, including the effect of being burnt in 2018), on the density (mean ± s.e.) of Phytophthora-sensitive flora in 10 × 10 m plots in Stirling Range National Park: (a) Banksia anatona; (b) Banksia sphaerocarpa var. sphaerocarpa; (c) Hakea cucullata; (d) Daviesia glossosema; (e) Jacksonia calycina; (f) Lambertia ericifolia; and (g) aggregated density of five Phytophthora-sensitive species (B. sphaerocarpa, H. cucullata, D. glossosema, J. calycina, L. ericifolia) sampled concurrently on the same plots. Inset boxes show ANOVA F-ratios testing the effect of phosphite (Phi), disease (Dis) and year (Yr) on density; * P < 0.05; ** P < 0.01; *** P < 0.001.
Effects of herbivore exclusion on growth increment and survival
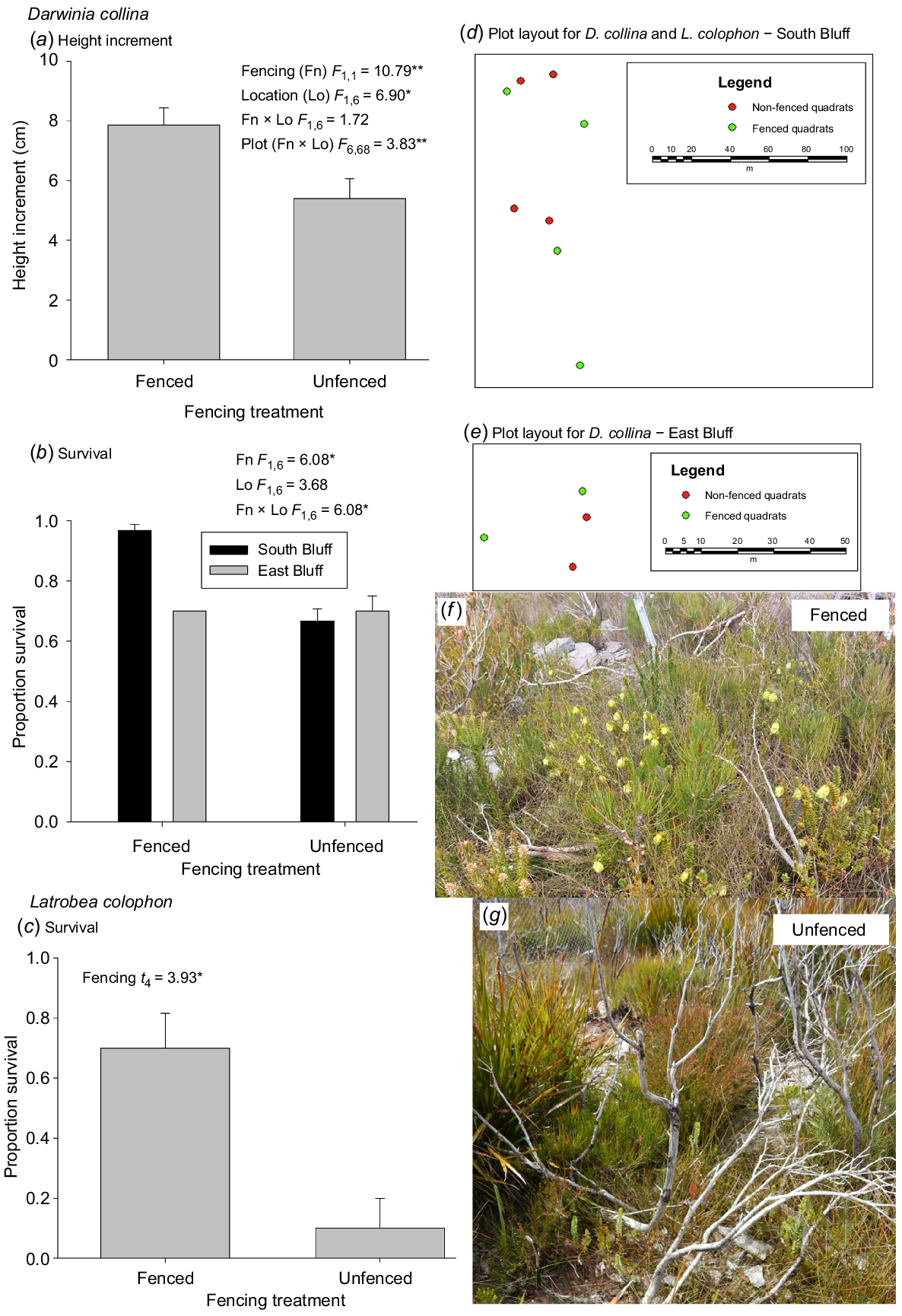
For both D. collina and B. anatona, plants fenced to exclude mammalian herbivores had greater growth increment than unfenced plants (Figs 3 and 4). For L. colophon, survival was so low in unfenced plots the increment data were not suitable for statistical analysis. For B. anatona where there was replication across two fire histories with differences in the number of fires and fire interval prior to the last fire in 2018, there was no effect of fire history on increment after the most recent fire. For D. collina where there was replication across two locations, growth increment after the most recent fire differed between locations. In both species, there was a significant plot effect.
Effects of fencing to exclude mammalian herbivores on the growth and survival (mean ± s.e.) of threatened flora recruiting after recent fire: (a) height increment of Darwinia collina; (b) survival of D. collina; (c) survival of Latrobea colophon. Inset boxes show statistical analysis results; * P < 0.05; ** P < 0.01; *** P < 0.001. Plot layout at South Bluff (d) and East Bluff (e) shows interspersion of fencing treatments. Example photographs (S. Barrett) of D. collina (yellow flowers) habitat inside (f) and outside (g) herbivore-exclusion fencing (visible to top left of (g)) in 2022.
Effects of fencing to exclude mammalian herbivores and fire history on the growth and survival (mean ± s.e.) of Banksia anatona recruiting after recent fire: (a) height increment; (b) survival. Inset boxes show statistical analysis results; * P < 0.05; ** P < 0.01; *** P < 0.001. Plot layout (c) shows interspersion of fencing treatments and spatial distribution of the two fire histories.
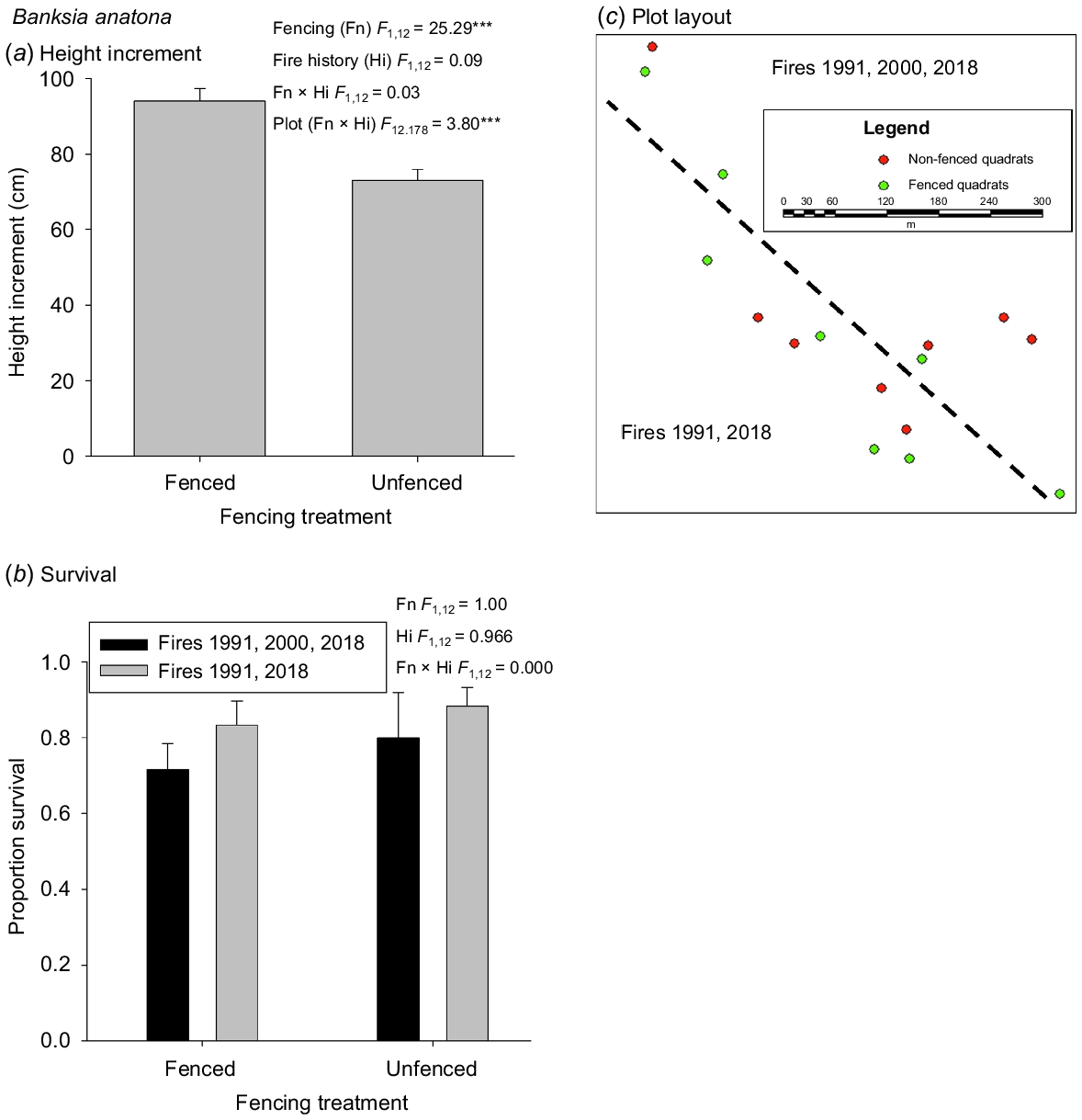
There was no evidence for differences in survival in B. anatona with fencing, nor with fire history (Fig. 4). In D. collina, there was a significant fencing × location interaction, in which the fenced plots at one location had significantly higher survival than the other fencing × location combinations (Fig. 3). Survival of L. colophon was substantially greater in fenced plots.
Survival, growth and flowering in wild and translocated populations
Mean proportion survival for threatened flora seedlings at ~3 years after germination did not differ between fire year (wild populations) and translocation site ‘treatments’ – wild populations after the 2018 fire, wild populations after the 2019 fire and translocation site A and B (F3,37 = 2.47; P = 0.08) (Table 1), noting that there was a trend for higher survival in translocations overall and particularly in some species, e.g. B. brownii R.Br., B. montana and D. pseudaphylla. Flowering was only recorded in translocated populations for the species assessed. Height increments differed between fire year and translocation site treatments for all species measured. There was a common pattern in pair-wise comparisons, with growth greatest at translocation site A (not significantly different from the 2019 fire in one case), then translocation site B, and least in the wild populations (Table 1).
Discussion
The post-fire threat mitigation and recovery actions examined here, which were applied after wildfires in 2018 and 2019 that affected a broad suite of threatened flora in the Stirling Range National Park in south-western Australia, all showed evidence of being effective in supporting post-fire flora recovery compared to controls. These findings provide empirical support for conservation managers to continue implementation of phosphite application to mitigate Phytophthora dieback, fencing to reduce mammalian herbivory, and translocation to seed production areas where threats can be managed. Continued implementation of these actions is recommended over the current inter-fire period, after future fires, and after fires elsewhere where similar threats operate (e.g. South Africa; Paap et al. 2023). Other research has shown that attention to fire regimes, particularly avoiding short fire intervals (<~25 years for the montane Stirling Range; Barrett and Yates 2015; Gosper et al. 2022), will also be important for the in situ conservation of the rich Stirling Range flora.
There was variation between species in the strength of survival responses to phosphite application to mitigate Phytophthora dieback. As only positive or neutral responses to phosphite were recorded, this research builds on previous studies demonstrating the importance for flora conservation of applying phosphite for Phytophthora mitigation (Shearer et al. 2004b; Barrett and Rathbone 2018; Boulle et al. 2023), notwithstanding potential risks of recurrent application for ecosystem phosphorus (Lambers et al. 2013). Although we concur with previous studies that have recommended research into new methods for Phytophthora control (Lambers et al. 2013; Boulle et al. 2023), in the absence of such new methods, phosphite application remains the best option for conservation of Phytophthora-susceptible flora in the Stirling Range and elsewhere. Operational phosphite spraying applied to areas ranging from 2.5 to 50 ha has been implemented in the Stirling Range since 2002, but costs are a significant factor; currently estimated at ~AU$300 ha−1.
Many of the plant species monitored in the phosphite plots showed an increase in density after the 2018 and 2019 wildfires compared to the pre-fire population (Fig. 2) – which is the expected response for obligate-seeder flora where fire acts as a recruitment trigger. However, for two (both Daviesia) of the three species monitored annually over the period 2002–2022, there was strong evidence that peak post-fire population density after the most recent fire was substantially lower than after the previous fire in 2000 (Fig. 1), irrespective of phosphite treatment. Prior fire interval would seem an unlikely explanation for this pattern, as the 18-year interval was greater than the interval preceding the 2000 fire and an interval of such length should be sufficient for development of an adequate soil-stored seed bank given local environmental conditions (Gosper et al. 2022). We speculate that dry post-fire conditions may have reduced post-fire recruitment success (Lamont et al. 1991), as 2018 and 2019 had rainfall much lower than average (Fig. 1d), or the phosphite treatment may not have been as effective as expected in mitigating Phytophthora impacts. Gradual and ongoing decline in plant abundance over decades, even though mitigated in phosphite-sprayed Daviesia plots, may still result in species decline.
One species stood out as showing an aberrant response to Phytophthora presence, for which no explanation is readily apparent. Lambertia ericifolia had greater declines in density by 2022 in plots with Phytophthora absence in 2015.
Fencing had positive or neutral effects on survival and growth of the flora sampled, which is consistent with previous studies (Rathbone and Barrett 2017). The fencing was designed to exclude mammalian herbivores, which in the Stirling Range are mainly the threatened indigenous quokka (Setonix brachyurus Quoy & Gaimard) and invasive rabbit (Oryctolagus cuniculus L.). Ongoing measurements will determine whether fencing also contributes to increased reproductive output and what effects fencing has on plant competitive interactions over longer periods after fire. However, over the period assessed to date, no weeds have been detected in fenced (or unfenced) plots and there has been no indication that any native species are becoming problematically dominant.
No significant difference was detected in seedling survival rates between wild populations (in either fire year) and translocated populations (in either location). However, at a species level, there were substantial declines in seedling survival in wild populations of B. montana and B. brownii compared with translocated populations, even with threat management. Further, based on local long-term monitoring data and population trends after the 2018 and 2019 fires, gradual and ongoing declines in wild populations may be anticipated. Whether the rate of decline in the wild populations exceeds that of the translocations over the long term, as expected, remains to be seen, although population declines over time are also typical of translocations (Silcock et al. 2019).
As comparisons of survival between translocated and wild populations are rarely reported (Menges et al. 2016; Reiter et al. 2016), it is difficult to put these results in context. Some studies have found no evidence for differences in seedling survival rates (Menges et al. 2016), whereas others have reported higher survival in translocated populations (Colas et al. 2008; Ward et al. 2022). Survival may be expected to be higher in translocated populations, through translocations usually having less exposure to threats, seedlings being spaced at a lower density to reduce competition and often, as in this case, receiving supplemental watering. Alternatively, wild populations have potential advantages in having evolved in their specific biotic, fire disturbance, geological and climate context, which may manifest in improved survival compared to translocations (e.g. Janissen et al. 2021). Differences in seedling survival between translocated and wild populations appeared to be greater for some species than others, perhaps reflecting differences amongst species in the habitat suitability of the translocation sites (Albrecht and Long 2019).
Higher seedling growth was generally recorded at translocation sites, and at one of the translocation sites in particular. Similarly, reproduction has already occurred in many species at the translocation sites, in some instances up to 7 years earlier than expected in the wild (Barrett et al. 2009; Gosper et al. 2022), providing an otherwise unavailable opportunity to collect seed in the short term for banking or other recovery actions. The translocation with greater growth (and greater flowering in some cases) was in the lowlands, in contrast to the montane natural range of all species and the other translocation site. Although a number of factors may be involved, the higher growth at the lowland translocation is consistent with more rapid maturation of south-western Australian serotinous obligate-seeder flora in locations with higher site productivity and higher mean annual temperature (Gosper et al. 2022). The short-term higher growth, considerably shorter juvenile periods and similar survival in the lowland translocation suggests that, with supplemental watering, montane Stirling Range flora may have greater climatic tolerances than their currently restricted montane niche would suggest. With ongoing warming and drying predicted in south-western Australia (Delworth and Zeng 2014), tolerance to warmer and drier conditions than currently experienced will be required if these species are to persist in situ and is worthy of further investigation.
In conclusion, this study highlights the positive effects of threat mitigation on the post-fire persistence of the threatened flora of the Stirling Range. Recovery actions of phosphite application, herbivore exclusion and establishment of seed production areas to support future in situ recovery efforts, have all combined to improve the trajectories of some of Western Australia’s most threatened species and provide insights that may be relevant to other areas experiencing similar threat syndromes in Australia and beyond.
Data availability
The data that support this study will be shared upon reasonable request to the corresponding author.
Declaration of funding
Parts of this work were funded by the Department of Climate Change, Energy, the Environment and Water, Commonwealth Government of Australia, through the Wildlife and Habitat Bushfire Recovery and Environment Restoration Fund programs.
Acknowledgements
The authors thank staff from the Department of Biodiversity, Conservation and Attractions (DBCA), including Andrew Crawford from the Western Australian Seed Centre, Leonie Monks and Michelle Boulle for assistance with the translocations, Greg Freebury for implementation of phosphite program, and numerous other DBCA employees past and present who have assisted with data collection and implementation.
References
Albrecht MA, Long QG (2019) Habitat suitability and herbivores determine reintroduction success of an endangered legume. Plant Diversity 41, 109-117.
| Crossref | Google Scholar | PubMed |
Auld TD, Keith DA, Gallagher RV, Tozer M, Ooi MKJ, Le Breton T, Allen S, Yates C, van Leeuwen S, Williams RJ, Mackenzie BDE (2022) Frameworks for identifying priority plants and ecosystems most impacted by major fires. Australian Journal of Botany 70, 455-493.
| Crossref | Google Scholar |
Barrett S, Rathbone D (2018) Long-term phosphite application maintains species assemblages, richness and structure of plant communities invaded by Phytophthora cinnamomi. Austral Ecology 43, 360-374.
| Crossref | Google Scholar |
Barrett S, Yates CJ (2015) Risks to a mountain summit ecosystem with endemic biota in southwestern Australia. Austral Ecology 40, 423-432.
| Crossref | Google Scholar |
Barrett S, Shearer BL, Crane CE, Cochrane A (2008) An extinction-risk assessment tool for flora threatened by Phytophthora cinnamomi. Australian Journal of Botany 56, 477-486.
| Crossref | Google Scholar |
Boulle M, Stewart BA, Barrett S (2023) To spray or not to spray: impact of phosphite spraying for Phytophthora cinnamomi control on Proteaceae species in southwestern Australia. Conservation Science and Practice 5, e12903.
| Crossref | Google Scholar |
Bowman DMJS, French BJ, Williamson GJ, Prior LD (2021) Fire, herbivores and the management of temperate Eucalyptus savanna in Tasmania: introducing the Beaufront fire – mammalian herbivore field experiment. Ecological Management & Restoration 22, 140-151.
| Crossref | Google Scholar |
Burgman MA, Keith D, Hopper SD, Widyatmoko D, Drill C (2007) Threat syndromes and conservation of the Australian flora. Biological Conservation 134, 73-82.
| Crossref | Google Scholar |
Canadell JG, Meyer CP, Cook GD, Dowdy A, Briggs PR, Knauer J, Pepler A, Haverd V (2021) Multi-decadal increase of forest burned area in Australia is linked to climate change. Nature Communications 12, 6921.
| Crossref | Google Scholar | PubMed |
Colas B, Kirchner F, Riba M, Olivieri I, Mignot A, Imbert E, Beltrame C, Carbonell D, Fréville H (2008) Restoration demography: a 10-year demographic comparison between introduced and natural populations of endemic Centaurea corymbosa (Asteraceae). Journal of Applied Ecology 45, 1468-1476.
| Crossref | Google Scholar |
Cowling RM, Witkowski ETF, Milewski AV, Newbey KR (1994) Taxonomic, edaphic and biological aspects of narrow plant endemism on matched sites in Mediterranean South Africa and Australia. Journal of Biogeography 21, 651-664.
| Crossref | Google Scholar |
Delworth TL, Zeng F (2014) Regional rainfall decline in Australia attributed to anthropogenic greenhouse gases and ozone levels. Nature Geosciences 7, 583-587.
| Crossref | Google Scholar |
Gallagher RV, Allen SP, Mackenzie BDE, Keith DA, Nolan RH, Rumpff L, Gosper CR, Pegg G, van Leeuwen S, Ooi MKJ, Yates CJ, Merow C, Williams RJ, Nikolopoulos EI, Beaumont LJ, Auld TD (2022) An integrated approach to assessing abiotic and biotic threats to post-fire plant species recovery: lessons from the 2019–2020 Australian fire season. Global Ecology and Biogeography 31, 2056-2069.
| Crossref | Google Scholar |
Gioia P, Hopper SD (2017) A new phytogeographic map for the Southwest Australian Floristic Region after an exceptional decade of collection and discovery. Botanical Journal of the Linnean Society 184, 1-15.
| Crossref | Google Scholar |
Godefroid S, Piazza C, Rossi G, Buord S, Stevens A-D, Aguraiuja R, Cowell C, Weekley CW, Vogg G, Iriondo JM, Johnson I, Dixon B, Gordon D, Magnanon S, Valentin B, Bjureke K, Koopman R, Vicens M, Virevaire M, Vanderborght T (2011) How successful are plant species reintroductions? Biological Conservation 144, 672-682.
| Crossref | Google Scholar |
Gosper CR, Coates DJ, Hopper SD, Byrne M, Yates CJ (2021a) The role of landscape history in the distribution and conservation of threatened flora in the Southwest Australian Floristic Region. Biological Journal of the Linnean Society 133, 394-410.
| Crossref | Google Scholar |
Gosper CR, Kinloch J, Coates DJ, Byrne M, Pitt G, Yates CJ (2021b) Differential exposure and susceptibility to threats based on evolutionary history: how OCBIL theory informs flora conservation. Biological Journal of the Linnean Society 133, 373-393.
| Crossref | Google Scholar |
Gosper CR, Miller BP, Gallagher RV, Kinloch J, van Dongen R, Adams E, Barrett S, Cochrane A, Comer S, McCaw L, Miller RG, Prober SM, Yates CJ (2022) Mapping risk to plant populations from short fire intervals via relationships between maturation period and environmental productivity. Plant Ecology 223, 769-787.
| Crossref | Google Scholar |
Halofsky JE, Peterson DL, Harvey BJ (2020) Changing wildfire, changing forests: the effects of climate change on fire regimes and vegetation in the Pacific Northwest, USA. Fire Ecology 16, 4.
| Crossref | Google Scholar |
Janissen B, French G, Selby-Pham J, Lawrie AC, Huynh T (2021) Differences in emergence and flowering in wild, re-introduced and translocated populations of an endangered terrestrial orchid and the influences of climate and orchid mycorrhizal abundance. Australian Journal of Botany 69, 9-20.
| Crossref | Google Scholar |
Keeley JE, Ne’eman G, Fotheringham CJ (1999) Immaturity risk in a fire-dependent pine. Journal of Mediterranean Ecology 1, 41-48.
| Google Scholar |
Keith DA, Allen SP, Gallagher RV, Mackenzie BDE, Auld TD, Barrett S, Buchan A, English V, Gosper C, Kelly D, McIllwee A, Melrose RT, Miller BP, Neldner VJ, Simpson CC, Tolsma AD, Rogers D, van Leeuwen S, White MD, Yates CJ, Tozer MG (2022) Fire-related threats and transformational change in Australian ecosystems. Global Ecology and Biogeography 31, 2070-2084.
| Crossref | Google Scholar |
Keppel G, Robinson TP, Wardell-Johnson GW, Yates CJ, Van Niel KP, Byrne M, Schut AGT (2017) A low-altitude mountain range as an important refugium for two narrow endemics in the Southwest Australian Floristic Region biodiversity hotspot. Annals of Botany 119, 289-300.
| Crossref | Google Scholar | PubMed |
Knight TM, Holt RD (2005) Fire generates spatial gradients in herbivory: an example from a Florida sandhill ecosystem. Ecology 86, 587-593.
| Crossref | Google Scholar |
Lambers H, Ahmedi I, Berkowitz O, Dunne C, Finnegan PM, Hardy GESJ, Jost R, Laliberté E, Pearse SJ, Teste FP (2013) Phosphorus nutrition of phosphorus-sensitive Australian native plants: threats to plant communities in a global biodiversity hotspot. Conservation Physiology 1, cot010.
| Crossref | Google Scholar |
Lamont BB, Connell SW, Bergl SM (1991) Seed bank and population dynamics of Banksia cuneata: the role of time, fire, and moisture. Botanical Gazette 152, 114-122.
| Crossref | Google Scholar |
Menges ES, Smith SA, Weekley CW (2016) Adaptive introductions: how multiple experiments and comparisons to wild populations provide insights into requirements for long-term introduction success of an endangered shrub. Plant Diversity 38, 238-246.
| Crossref | Google Scholar | PubMed |
Miller RG, Tangney R, Enright NJ, Fontaine JB, Merritt DJ, Ooi MKJ, Ruthrof KX, Miller BP (2019) Mechanisms of fire seasonality effects on plant populations. Trends in Ecology & Evolution 34, 1104-1117.
| Crossref | Google Scholar | PubMed |
Moore N, Barrett S, Howard K, Craig MD, Bowen B, Shearer B, Hardy G (2015) Time since fire and average fire interval are the best predictors of Phytophthora cinnamomi activity in heathlands of south-western Australia. Australian Journal of Botany 62, 587-593.
| Crossref | Google Scholar |
Paap T, Nndanduleni M, Wingfield M (2023) A critically endangered Proteaceae in the Cape Florisitic Region threatened by an invasive pathogen. Bothalia, African Biodiversity & Conservation 53, a6.
| Crossref | Google Scholar |
Rathbone DA, Barrett S (2017) Vertebrate browsing impacts in a threatened montane plant community and implications for management. Ecological Management and Restoration 18, 164-171.
| Crossref | Google Scholar |
Reiter N, Whitfield J, Pollard G, Bedggood W, Argall M, Dixon K, Davis B, Swarts N (2016) Orchid re-introductions: an evaluation of success and ecological considerations using key comparative studies from Australia. Plant Ecology 217, 81-95.
| Crossref | Google Scholar |
Shearer BL, Crane CE, Cochrane A (2004a) Quantification of the susceptibility of the native flora of the South-West Botanical Province, Western Australia, to Phytophthora cinnamomi. Australian Journal of Botany 52, 435-443.
| Crossref | Google Scholar |
Shearer BL, Crane CE, Fairman RG (2004b) Phosphite reduces disease extension of a Phytophthora cinnamomi front in Banksia woodland, even after fire. Australasian Plant Pathology 33, 249-254.
| Crossref | Google Scholar |
Shearer BL, Crane CE, Barrett S, Cochrane A (2007) Phytophthora cinnamomi invasion, a major threatening process to conservation of flora diversity in the South-west Botanical Province of Western Australia. Australian Journal of Botany 55, 225-238.
| Crossref | Google Scholar |
Silcock JL, Simmons CL, Monks L, Dillon R, Reiter N, Jusaitis M, Vesk PA, Byrne M, Coates DJ (2019) Threatened plant translocation in Australia: a review. Biological Conservation 236, 211-222.
| Crossref | Google Scholar |
Standish RJ, Stokes BA, Tibbett M, Hobbs RJ (2007) Seedling response to phosphate addition and inoculation with arbuscular mycorrhizas and the implications for old-field restoration in Western Australia. Environmental and Experimental Botany 61, 58-65.
| Crossref | Google Scholar |
Thomas CD (2011) Translocation of species, climate change, and the end of trying to recreate past ecological communities. Trends in Ecology & Evolution 26, 216-221.
| Crossref | Google Scholar | PubMed |
Tulloch AIT, Pichancourt J-B, Gosper CR, Sanders A, Chadès I (2016) Fire management strategies to maintain species population processes in a fragmented landscape of fire-interval extremes. Ecological Applications 26, 2175-2189.
| Crossref | Google Scholar | PubMed |
Tynan KM, Wilkinson CJ, Holmes JM, Dell B, Colquhoun IJ, McComb JA, Hardy GESJ (2001) The long-term ability of phosphite to control Phytophthora cinnamomi in two native plant communities of Western Australia. Australian Journal of Botany 49, 761-770.
| Crossref | Google Scholar |
Ward SG, Menges ES, Charton KT, Gonsiska PA, Peterson CL (2022) Using demographic findings to compare wild and translocated populations of Florida goldenaster (Chrysopsis floridana) in west-central Florida, U.S.A. Restoration Ecology 30, e13577.
| Crossref | Google Scholar |
Yates CJ, Robinson T, Wardell-Johnson GW, Keppel G, Hopper SD, Schut AGT, Byrne M (2019) High species diversity and turnover in granite inselberg floras highlight the need for a conservation strategy protecting many outcrops. Ecology and Evolution 9, 7660-7675.
| Crossref | Google Scholar | PubMed |


