Evaluation of management options for climate-change adaptation of threatened species: a case study of a restricted orchid
Caitlin R. Rutherford A * , Andrew M. Rogers B , Suzanne M. Prober C , Erika M. Roper
A * , Andrew M. Rogers B , Suzanne M. Prober C , Erika M. Roper  D , Emma Cook E and April E. Reside A B F
D , Emma Cook E and April E. Reside A B F
A
B
C
D
E
F
Handling Editor: Noushka Reiter
Abstract
Global climate is changing rapidly, necessitating timely development of specific, actionable species conservation strategies that incorporate climate-change adaptation. Yet, detailed climate-change adaptation planning is noticeably absent from species management plans. This is problematic for restricted species, which often have greater extinction risk.
Focusing on the restricted and endangered Tarengo leek orchid (Prasophyllum petilum), we aimed to adapt and test a framework for producing strategies for its management under climate change.
We used expert elicitation to estimate the severity of threats and assess potential management actions to mitigate threat impacts. We created a conceptual model detailing ecology, threats and likely impacts of climate change on the species, including the interactions between components.
Although climate change-related threats will affect the species, the most severe threats were non-climate threats including grazing, weeds, and habitat degradation. Fire management was deemed highly beneficial but had low feasibility for some populations. Without management, experts estimated up to a 100% decrease in one P. petilum population, and up to 50% decrease if management remained unchanged.
Management actions with the highest benefit and feasibility addressed the non-climate threats, which, in turn, can give the species the best opportunity to withstand climate-change impacts. Experts highlighted the difficulty of addressing climate threats with such limited knowledge; therefore, further research was recommended.
This adapted framework enabled a structured analysis of threats, and informed selection of priority adaptation options. We recommend its use for other restricted species for efficient and robust decision-making in climate-change management.
Keywords: climate-change adaptation frameworks, climate-change resilience, controlled burns, expert elicitation, orchid, Prasophyllum, Tarengo leek orchid, threatened species management.
Introduction
Anthropogenic climate change is already affecting biodiversity from the genetic level through to populations and species (Scheffers et al. 2016) and is projected to become one of the most significant threats to biodiversity (Bellard et al. 2012; Thurman et al. 2020). There has been a recent increase in research assessing impacts of climate change on species, ecosystems, and processes (Foden et al. 2019); however, there is an overwhelming gap between the research and its practical implementation (Knight et al. 2008; Poiani et al. 2011; Dubois et al. 2020). Recommended climate-change actions in literature are often general (e.g. increase connectivity), broad-scale, covering large areas or multiple conservation features (Dawson et al. 2011; Pacifici et al. 2015; Reside et al. 2018), and lack in evidence for effectiveness (Prober et al. 2019). This can limit their usefulness for conservation practitioners designing and implementing the actions (Dubois et al. 2020).
Consequently, management and recovery plans often lack actionable strategies to mitigate climate risk to the target species or community (Heller and Zavaleta 2009; Hoeppner and Hughes 2019; Naujokaitis-Lewis et al. 2021). Interactions between climate change and non-climate threats, and uncertainty and variability in future climate trajectories, further compound the difficulty faced by managers trying to address climate threats (Lawler et al. 2015). The incorporation of climate-change considerations into conservation action plans is required because management decisions made now will affect the persistence of species into the future (Marcer et al. 2013).
The nature of species’ vulnerability to climate change varies widely; therefore, effective, specific strategies need to be developed to mitigate climate risk (Dawson et al. 2011). Only a small proportion of species management plans include explicit and thorough climate-change adaptation actions (e.g. Balzotti et al. 2016; Rudin-Bitterli et al. 2021). Researching specific climate-change impacts and adaptation can be time- and resource-consuming (Mawdsley et al. 2009), emphasising the need for a repeatable framework for rapid species assessment and decision-making. Some tools exist in the literature; for example, Oliver et al. (2012) used decision trees based on differences between a species current distribution and future projected climate space to test species’ adaptive capacity to climate change and recommend actions accordingly. Another tool, developed by Cross et al. (2012), provides a framework for developing management actions for climate-affected ecosystems, ecological functions, and individual species. These frameworks provide a method of bridging the research–implementation gap that can hinder effective species conservation (Dawson et al. 2011). What is lacking is a clear demonstration of the application of such methods in different ecological, management and knowledge contexts, particularly where knowledge of species is limited, as often occurs for rare and restricted species (Dunwiddie et al. 2016).
Restricted species, such as those with small, isolated populations or restricted environmental niches, are often at a higher risk of extinction (Beyer and Manica 2020; Staude et al. 2020). Restricted species can be less able to adapt to changes in climate, because of small populations, a restricted gene pool from which to evolve, and barriers to dispersal such as fragmented landscapes and limited seed-dispersal ability (Warren et al. 2013; Barber et al. 2016; Beyer and Manica 2020). Furthermore, the rapidity of climatic change can reduce the ability for species to adapt, further highlighting the urgency with which management decisions must be made (Jump and Penuelas 2005). Rare and restricted species are also often data deficient, owing to survey difficulty and cost, species elusivity, or inadequate knowledge of ecological requirements (Hamilton et al. 2015; Wang et al. 2015), further exacerbating the challenge of developing effective actions that will enable species to persist (Foden et al. 2019). Given these challenges, a tested process for assessing management actions for dealing with the impacts of climate change for restricted species is needed.
Species-focused climate-change plans that directly link climate-change impacts to adaptation actions are lacking in Australia (Hoeppner and Hughes 2019; Reynolds et al. 2021), despite the continuing track record of species decline and extinction, and species and ecosystem vulnerability to climate change (Hughes 2011; Woinarski et al. 2019; Bergstrom et al. 2021; Cresswell et al. 2021). With the projected intensification of climate change, managers are required to make timely decisions about threatened species management often at shorter timescales than field research can be conducted (Dunwiddie et al. 2016). This study addresses this urgent need by adapting and testing a process of incorporating climate-change adaptation into a conservation management plan and providing robust management recommendations for a restricted, threatened species.
The Orchidaceae family contains many restricted-range species with specialised relationships with pollinators and symbiotic fungi, making them highly susceptible to environmental perturbation and often necessitating an individual-species approach to management (Swarts and Dixon 2009; Wraith and Pickering 2019; Phillips et al. 2020). Worldwide, the Orchidaceae is the most diverse yet threatened group of flowering plants. As of 2019, approximately 10% (n = 184) of Australia’s orchid species are nationally listed as Threatened under the Environment Protection and Biodiversity Conservation Act 1999 (Cth) (DCCEEW 2024), with 21 species having climate change listed as a threat (Wraith and Pickering 2019). Forty of the listed species are within the genus Prasophyllum (DCCEEW 2023). This study focuses on the threatened Tarengo leek orchid (Prasophyllum petilum) of south-eastern Australia, which requires targeted actions to ensure persistence under the projected changes in climate (EPSD 2019; DAWE 2021).
The aims of this study were (1) to determine the relative severity of threats to P. petilum, (2) to identify management actions to address these threats, and (3) to predict the benefit and feasibility of undertaking these actions. We used expert elicitation to test these aims, and used the results to formulate a framework, modified from Cross et al. (2012), for incorporating climate-change adaptation into conservation management of restricted species. This work was undertaken in close collaboration with those responsible for implementing management for this species; therefore, the research was designed to establish feasible actions that will assist P. petilum in adapting to projected climatic changes and safeguard the existing populations in perpetuity, and can be readily implemented by our project partners. The resulting framework is designed to enable managers, particularly government planners, to build climate-change consideration into restricted-species management and planning.
Materials and methods
The framework
We used a modified ‘adaptation for conservation targets’ framework (Cross et al. 2012), to test the applicability of a structured, five-step framework for incorporating climate-change adaptation into species conservation management on a restricted species. First, we collated all published information on the focal-species ecology, existing threats, and potential threats from climate change to build a conceptual model detailing the interactions among these variables. Then, using established elicitation methods, we consulted experts to verify and gather further information on species ecology and threats, update the conceptual model to identify areas for targeted management (Smith et al. 2020) and assessed potential management actions to mitigate these threats (Supplementary Fig. S1).
The orchid P. petilum was chosen as a suitable focal species after discussion with ecologists from the Environment, Planning, and Sustainable Development Directorate of the Australian Capital Territory (ACT) Government. Prasophyllum petilum is listed as Endangered nationally (Environmental Protection and Biodiversity Conservation Act 1999), in the ACT (Nature Conservation Act 2014) and New South Wales (NSW; Biodiversity Conservation Act 2016). At the time of the design of this study, the ACT Action Plan was the only active plan to identify climate change as a threat to P. petilum (EPSD 2019); however, a Conservation Advice has since been released for P. petilum, also identifying climate change as a threat (DAWE 2021).
Prasophyllum petilum is a terrestrial orchid known to occur in small populations in the ACT and in eastern NSW (Fig. 1a). It has a short flowering period in late spring, remaining dormant as an underground tuber for up to 5 months during summer and autumn (Bell 2020; Fig. 2).
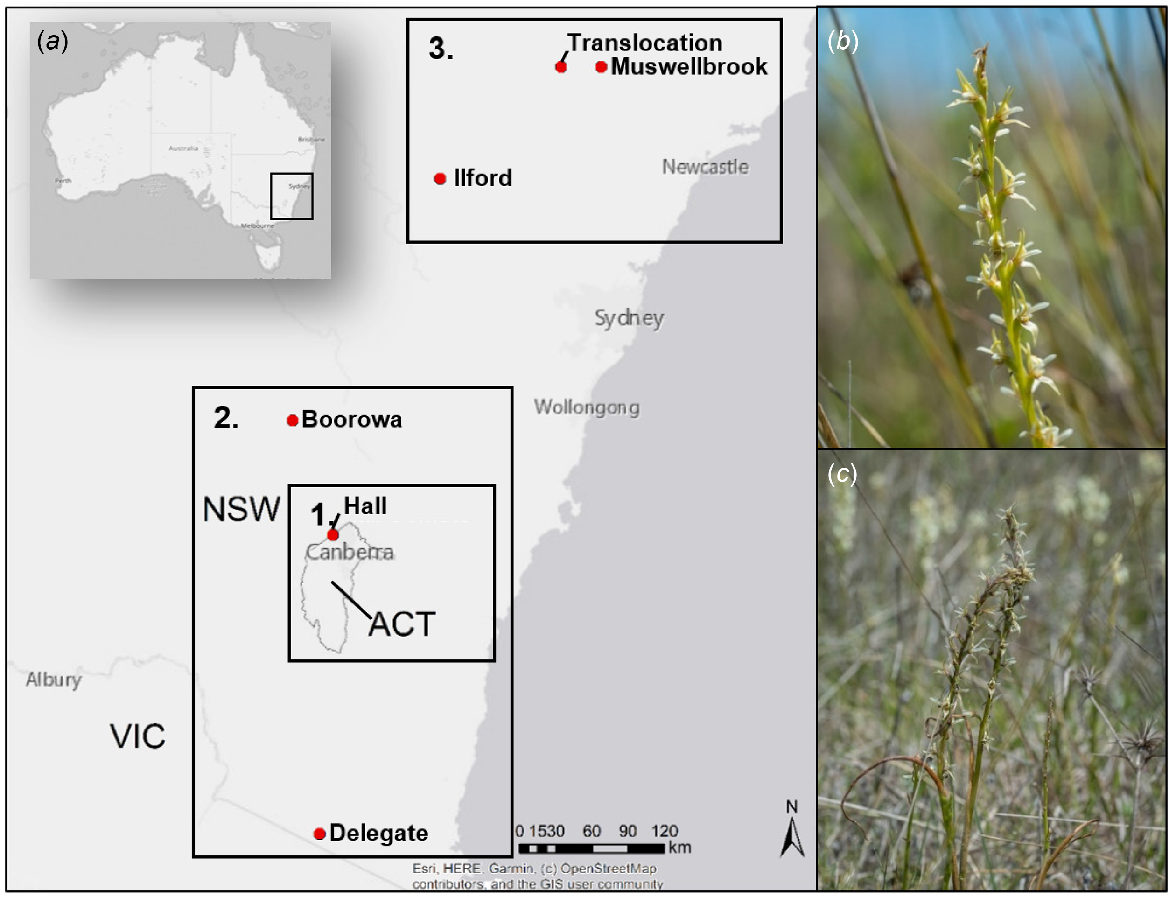
(a) Approximate location of known extant Prasophyllum petilum populations in NSW and the ACT, Australia. Locations are generalised to reduce threat of illegal collection (Wraith and Pickering 2019; OEH 2021a). Rectangles show the division of populations into three management regions: (1) ACT management region; (2) south-eastern NSW management region; and (3) northern NSW management region. (b, c) P. petilum (photos by E. Roper, 2021).
Prasophyllum petilum annual life cycle. Latitudinal variability in timing of life stages (e.g. dormancy may begin earlier in more northern populations than in the southern ones, and may occur for up to 5 months) is observed (DECCW 2010; Wilson et al. 2016; Bell 2020; OEH 2021a). OMF, orchid mycorrhizal fungi.
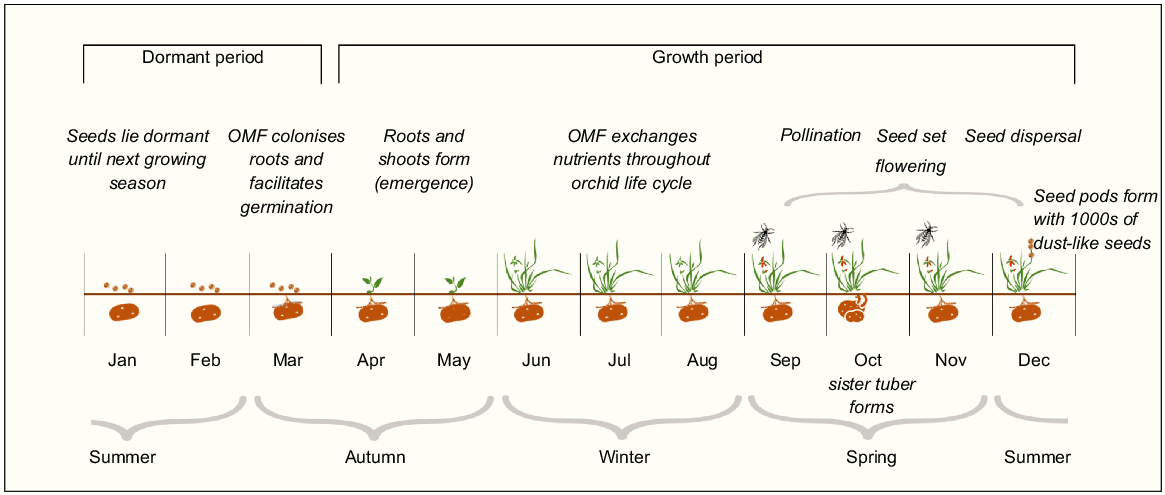
Individual plants can grow up to 30 cm above ground and produce up to 20 green or red–brown flowers on an inflorescence (Bishop 1996; Wilson et al. 2016; Fig. 1b, c). Some Prasophyllum spp. orchids have a nectar-rewarding pollination system (Bates 1984; Peakall 1989; Gaskett and Gallagher 2018) and can be pollinated by wasps, bees, and beetles (Jones 1988). Many orchids worldwide have only one primary pollinator; however, nectar-rewarding orchid species are more likely to be generalists (Ackerman et al. 2023). The number and distribution of species that pollinate P. petilum is unknown; however, the tricolour soldier beetle (Chauliognathus tricolour) has been incidentally observed pollinating P. petilum flowers at Boorowa (E. Roper, pers. comm.; Fig. S2). Prasophyllum spp. orchids also require orchid mycorrhizal fungi (OMF) (primarily Ceratobasidium spp.) for germination and exchange of carbon throughout the orchid’s lifetime (McQualter et al. 2007; EPSD 2019; Freestone et al. 2021; Fig. 2). The number of individual flowering plants within populations fluctuates yearly, because some individuals remain dormant for several consecutive years (Wilson et al. 2016). Prolonged dormancy may be the result of suboptimal environmental conditions, such as drought or prolonged absence of fire, causing depletion of energy stores, although the full suite of factors influencing annual emergence and flowering is still unknown (Coates et al. 2006; Bell 2020). The absence of flowering and density of surrounding vegetation can constrain detection and accurate population estimates (Bell 2019). Prasophyllum petilum persists in both open grassy woodland and grassland habitats of varying native and non-native species composition (Supplementary Table S1). Populations of the orchid are small and isolated and are subject to non-climate threats, including grazing from livestock and native and non-native herbivores, and competition with invasive weed species. Here we used the following management regions that reflect the different management populations to assess the threats and management options: (1) ACT, (2) south-eastern NSW, and (3) northern NSW management region (Fig. 1a; Table S1). The ACT population is a subset of the south-eastern NSW population, and this reflects the division of management responsibilities across jurisdictions.
Vulnerability to climate change is mostly assessed as a relative measure across an assemblage of species, comparing species’ different exposures, sensitivities, and adaptive capacities, often based on species’ traits (Williams et al. 2008; Foden et al. 2013; Lee et al. 2015; Reside et al. 2016; Dudley et al. 2019). On the basis of broad trait characterisations, P. petilum was designated a medium climate-change risk in relation to 324 other species considered (see Dudley et al. 2019 for full methodology). With this in mind, we assessed the relative severity of each threat to P. petilum.
A literature search to identify potential climate-change threats to P. petilum specifically identified limited information, so we broadened the search to include Prasophyllum spp. and Australian orchids generally. Rainfall is a likely influence on germination, emergence and flowering of orchids through its effect on the microenvironment’s soil moisture (Rasmussen et al. 2015; Bell 2020). Extreme heat can wilt emergent plants or prolong dormancy of some orchid species (Evans et al. 2020). Fire is an important consideration in eastern Australia, given the flammability of ecosystems, and the increasing incidence of severe ‘megafire’ events (Dowdy 2018). Fire events can be detrimental during the growth period of orchids by damaging emergent plants, affecting pollinator food sources and habitat, and damaging orchid mycorrhizal fungi (Jasinge et al. 2018; Roper 2021). Conversely, burns can be beneficial during the dormant period by stimulating flowering through nutrient enrichment or reducing vegetative competition (Coates et al. 2006). Therefore, to assess the exposure of P. petilum to these potential climate threats, we identified the changes relating to temperature, rainfall, and risk of severe fire-weather days likely to occur within the range of known occurrences of P. petilum populations by using the NSW and ACT Regional Climate Modelling (NARCliM).
Predicting climate-change impacts on a species presents a significant challenge owing to the inherent uncertainty and assumptions within the multiple models and projections available (Regan et al. 2002; Cross et al. 2012; Reside et al. 2018). At the time NARCliM was developed (2010–2014), the Special Report on Emissions Scenarios (SRES) Scenario A2 (high emissions, high population growth, independently operating nations) was deemed the most likely scenario on the basis of global emissions trajectories (Peters et al. 2013; OEH 2014). By overlaying generalised P. petilum population locations in ArcMap (ver. 10.7.1), we qualitatively assessed the magnitude of change of several climate-change variables across the P. petilum distribution for both near (2020–2039) and far future (2060–2079). Relevant climate variables were mean change in the number of hot days (≥35°C) per year, mean annual change in average temperature, mean annual change in maximum temperature, change in average rainfall in summer, autumn, winter and spring, and change in the number of severe fire-weather days (forest fire danger index (FFDI) ≥ 50). The magnitude of these variables for each region were taken from NARCliM projections, based on SRES Scenario A2 (OEH 2014).
Increases in all temperature variables were projected across all of P. petilum distribution, as well as small increases in frequency of the number of severe fire-weather days per year (i.e. between 0 and 1.5 more days per year with severe fire weather across the distribution) (Fig. S3, Table S2; McArthur 1967; Dowdy et al. 2019). Rainfall was projected to vary seasonally, with overall increases in summer and autumn rainfall and decreases in winter and spring rainfall in the ACT and south-eastern NSW regions. In contrast, the northern NSW management region was likely to experience increases in winter and spring rainfall in the far future (Table S2, Fig. S3).
Using the information collated from Steps 2 and 3, we created a conceptual model for P. petilum, which detailed the interactions among potential climate threats and the species ecology, associations, and non-climate threats (Cross et al. 2012). The model showed explicit links among components and detailed the complexity of numerous interacting processes and drivers (Margoluis et al. 2009). The model was iteratively updated as new information was obtained from expert knowledge.
Expert elicitation is increasingly used in conservation to complement empirical data and fill gaps in knowledge to help make decisions about complex systems in increasingly urgent timeframes (Carwardine et al. 2012; Martin et al. 2012; Reside et al. 2019). To obtain quantitative data from experts, we used a structured, modified Delphi process, similar to that described by Carwardine et al. (2012). The Delphi process is a method of gathering expert opinion and approaching consensus by discussion or mathematical aggregation, ensuring that experts are able to review the group answers and discuss reasons for variations in estimates (Kuhnert et al. 2010). Using this approach, two half-day workshops were conducted with relevant orchid experts. The first workshop determined the severity of threats to P. petilum, and the second identified and assessed management options for mitigation of those threats. These workshops each ran for 5 h and were conducted online, which allowed a broader range of experts to participate (Kuhnert et al. 2010). Experts were defined as those with knowledge of or experience with the species and its past or current management, including ecologists, land managers, and P. petilum researchers (Roy et al. 2020). We refined a list of relevant experts through discussion with key government contacts, through a literature search, and through other experts. Experts were first invited to the two workshops via email and were given the option to fill out the standardised elicitation questionnaire (Table S3) out-of-session if unable to attend.
Eight experts were provided a background and agenda document prior to the workshop, as well as a list of questions (Table S4). Each question corresponded to a different potential threat to P. petilum populations reflected by the conceptual model. The questions were divided into direct threats to populations and their influence on various components of the orchid life stages (e.g. flowering, recruitment), the symbiotic associations (orchid mycorrhizal fungi and pollinators), the microenvironment, and how climate change would influence current threats to P. petilum such as weed encroachment and habitat degradation (Table S4). The inclusion of non-climate threats, such as grazing and competition with weeds, was to capture the complex interactions among different components of the orchid life cycle and requirements, such as symbionts (Cross et al. 2012; Butt et al. 2016). After an initial discussion of threats, ecology, and assessment of background material (including the conceptual model), experts were asked to individually estimate the severity of each threat to the species by using the International Union for Conservation of Nature (IUCN) Threats Classification Scheme (ver. 3.2) (Table 1).
| Severity of threat to populations | |
|---|---|
| Unknown | |
| 0 – No declines | |
| 1 – Causing or likely to cause negligible declines in population | |
| 2 – Causing or likely to cause fluctuations | |
| 3 – Causing or likely to cause relatively slow but significant declines (<20% over 10 years) | |
| 4 – Causing or likely to cause rapid declines (20–30% over 10 years) | |
| 5 – Causing or likely to cause very rapid declines (>30% over 10 years) |
Experts were also asked to assign a value to reflect their certainty of each of their estimates. Certainty scores could range between 0.5 and 1, where 1 represented complete confidence in their severity estimate, and scores below 0.5 were considered no better than random estimates (Hemming et al. 2018). Certainty was incorporated into the conceptual model and informed where large knowledge gaps occurred (Carwardine et al. 2012). This first round of elicitation was a hybrid approach where four experts participated in the online workshop and four remotely completed the questionnaire only. When all questionnaire responses were recorded, we calculated the arithmetic mean and standard error of expert estimates of severity and their certainty, resulting in a single mean severity and certainty score for each threat. These were ranked from highest to lowest mean severity for both near and far future. Using the ‘wilcox.test’ function in R Studio (R Core Team 2024), a Wilcoxon’s signed-rank test was applied to the highest scoring threats in the far future to determine whether there was a statistically significant change in severity in the near future and far future. The preliminary anonymised and aggregated results were presented to the workshop participants for discussion, and a broad consensus of the highly ranked threats was reached.
The four experts who participated were all past or current managers of the species. All experts (n = 8, including those that participated in the first workshop and completed questionnaires outside of session), were emailed a summary document detailing the preliminary results of Workshop 1, including the list of threats ranked by severity and updated conceptual models, and were invited to comment on the results and provide any management insight. We facilitated an initial discussion of species objectives, which were decided to be in line with current manager objectives for the populations; to maintain or enhance P. petilum populations in the wild (DECCW 2010; EPSD 2019; OEH 2021b). Potential management options were then discussed to address the most severe threats identified in the updated conceptual model. To maximise resource efficiency, only actions that would address higher-severity threats (≥3) were analysed. We compiled an initial list of management options from existing plans for P. petilum and similar species and updated the list within the workshop as new actions were suggested by experts. Actions included those that directly addressed the changing local climatic conditions (supplementary watering), those that increased population persistence over the long term (translocation), ‘low-regret’ actions (weed control), and research actions to address knowledge gaps highlighted in Workshop 1 (Table S6).
Experts were asked to individually estimate the feasibility and benefit of each action for the three regions that they were familiar with (Fig. 1a; Table S7). Terminology and scoring methodology were defined prior to individual elicitation to ensure consistent interpretation of questions (Regan et al. 2002; Kuhnert et al. 2010; Martin et al. 2012; Fig. S4, Table S8). Using the ACT region as a representative region, experts were also asked to estimate the trajectory of P. petilum populations if current management continued and if all management ceased, scored using the benefit scale in Fig. S4. A paired, one-tailed Student’s t-test was applied using R (R Core Team 2024) to determine whether the difference in scores for the scenarios was statically significant.
Actions were assessed by taking the arithmetic mean of feasibility and benefit estimates for each region and were presented to the experts for further discussion.
Compliance with ethical standards
This study was exempt from human ethics approval; however, we completed this study according to the University of Queensland Human Research Ethics requirements for studies with low and negligible risk (LNR). Prior to participation in any elicitation, activities experts received a comprehensive Participant Information Sheet detailing the study background, objectives, elicitation process, data storage and anonymity, and returned a signed consent form.
Results
What are the most severe threats to Prasophyllum petilum?
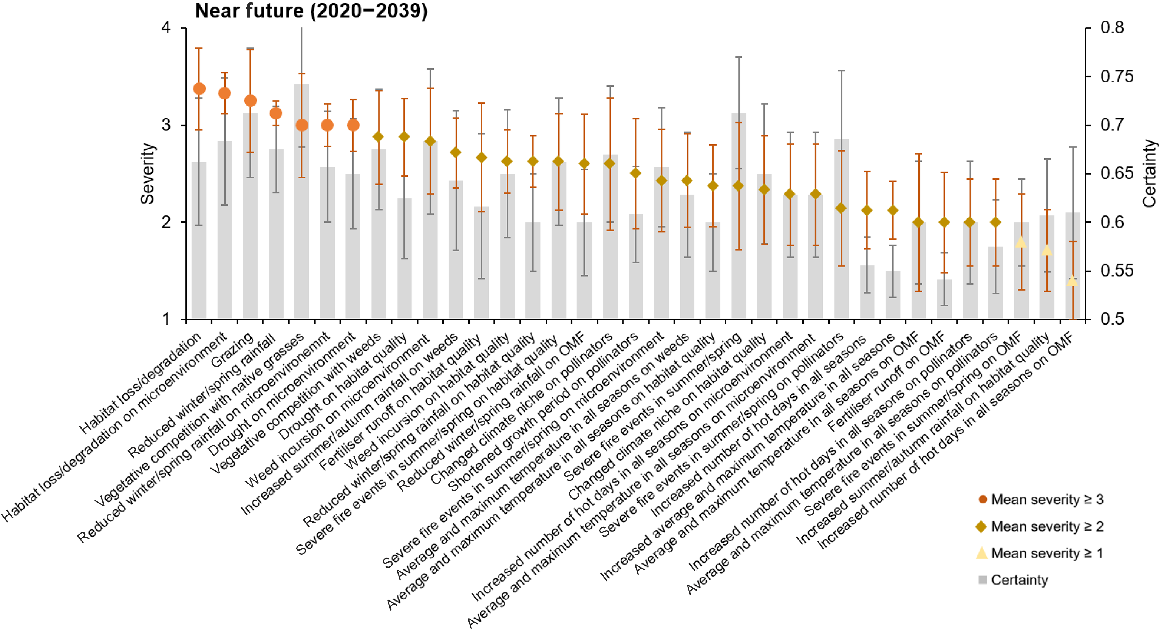
The majority of threats to P. petilum, for both near (71%) and far future (60%), were of only moderate severity (scores between 2 and 3, indicating that the threats were likely to cause fluctuations in the populations; Figs 3, 4). However, seven threats were identified as ‘likely to cause slow but significant declines’ in the near future, recording a mean severity score of ≥3 (Fig. 3). The most severe of these threats was habitat loss and degradation (mean severity score of 3.38 ± 0.42), as well as other non-climate threats of grazing (3.25 ± 0.53) and vegetative competition with native grasses (3 ± 0.53). The climate threats estimated to cause declines were related to changes in rainfall, specifically reduced winter/spring rainfall (3.13 ± 0.13). As well as direct impacts on populations, some threats were identified to cause decline indirectly through their effect on the microenvironment, including habitat loss and degradation (3.33 ± 0.21), drought (3 ± 0.27), and reduced winter and spring rainfall (3 ± 0.21). The mean severity score of all threats, except for vegetative competition with native grasses, had small increases from near to far future, resulting in more threats being ranked as ‘high severity’ (mean severity score of ≥3) (from 7 in near future to 13, in far future), indicating that threats will become more severe over time without management intervention (Figs 4, S5). These additional threats included vegetative competition with weeds (3.25 ± 0.31), the impact of increased summer/autumn rainfall on weeds (3.14 ± 0.46), and reduced winter/spring rainfall on the orchid mycorrhizal fungi (3.4 ± 0.4). Grazing (3.75 ± 0.37) and reduced winter and spring rainfall (3.75 ± 0.16) were the highest-ranked threats in the far future. Of the top 13 threats in far future, four increased significantly (P < 0.05) from their near-future estimates (Table S5).
Mean severity of threats to P. petilum in the near future (2020–2039; shown with points), with associated mean certainty (grey bars). Severity was on a scale between 1 (no change in population) and 5 (causing rapid population declines). Certainty was estimated between 0.5 (50% certain) and 1 (completely certain). Error bars represent standard errors of the mean (grey indicating certainty, orange indicating severity). A list of all threats can be found in Table S4.
Mean severity of threats to P. petilum in the far future (2060–2079; shown as points), with associated mean certainty (grey bars). Severity was on a scale between 1 (no change in population) and 5 (causing rapid population declines). Certainty was estimated between 0.5 (50% certain) and 1 (completely certain). Error bars represent standard errors of the mean (grey for certainty, orange for severity). A list of all threats can be found in Table S4.
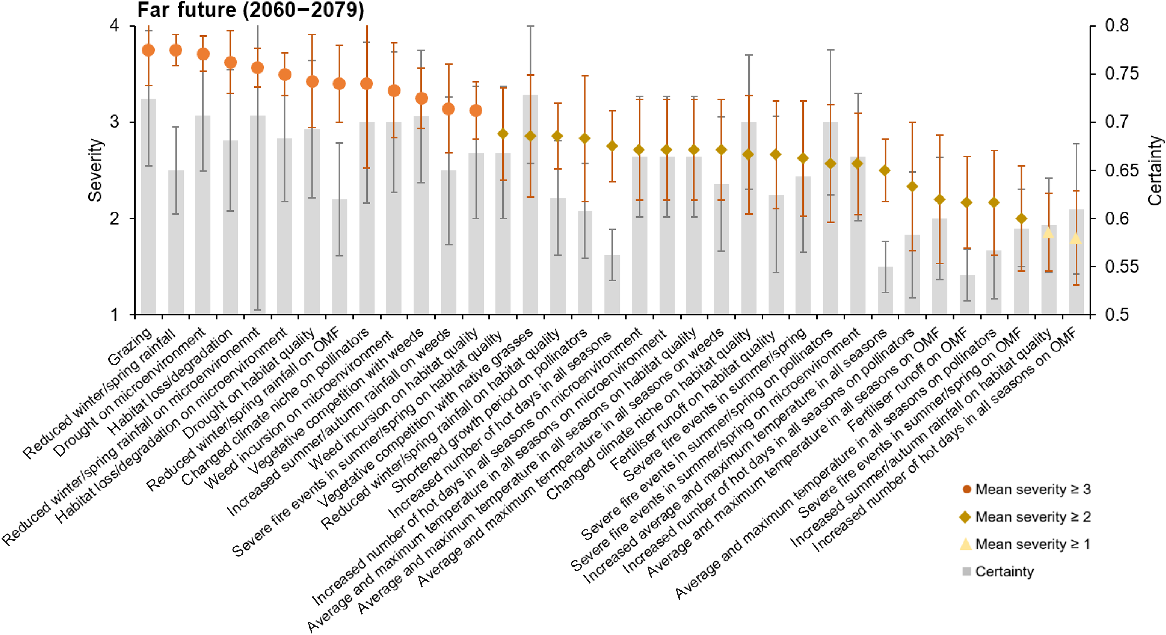
Experts’ certainty of threat estimates ranged between 0.54 (impact of fertiliser on orchid mycorrhizal fungi) and 0.74 (impact of vegetative competition with native grasses), although only 8% scored >0.7 in the near future, and 26% in the far future. Most certainty scores ranged between 0.6 and 0.7 (near future: 80%; far future: 54%). The lowest certainty was attributed to temperature-related threats and fertiliser runoff in both time intervals (Figs 3, 4).
Management options for P. petilum
As a representative population, experts estimated that in the ACT management region, P. petilum population size would decline considerably (estimates ranged between 75% and 100% decline) by the far future (2060–2079) if all management ceased. Experts also estimated that population decline will continue with current management (estimates ranged between 0% and 50% decline), although to a considerably less severe degree (P < 0.05; Table S9).
Actions to minimise population decline from climate and non-climate threats varied in their estimated benefit and feasibility across the three management regions (Table 2). Among the three highest-ranked benefit actions at each region were controlled burns (ACT management region: 126.67%; south-eastern NSW management region: 125%; northern NSW management region: 125%; Fig. 5). The highest-benefit actions in each region were those that directly affect population size by reducing competition from other plants and habitat degradation, such as controlled burns and habitat restoration (Table 2). Actions that varied in benefit among the regions included fencing and mowing regimes, reflecting the differences in the threats to each region. Actions that recorded both a high benefit and feasibility were mowing regime at Hall (ACT management region), grazing regime at Delegate (south-eastern NSW management region), and stakeholder liaison and community awareness (northern NSW management region) (Table 2).
| Management action | 1. ACT management region | 2. South-eastern NSW management region | 3. Northern NSW management region | ||||
|---|---|---|---|---|---|---|---|
| Benefit (%) | Feasibility | Benefit (%) | Feasibility | Benefit (%) | Feasibility | ||
| Supplementary watering | 58.33 | 0.33 | 0.25 | 0.13 | 0.25 | 0.13 | |
| Fencing/exclosures | −25.00 | 0.25 | 75.00 | 0.88 | −25.00 | 0.50 | |
| Caging | 60.00 | 0.25 | 25.00 | 0.50 | 25.00 | 0.50 | |
| Mowing regime | 150.00 | 0.88 | 37.50 | 0.63 | 75.00 | 0.35 | |
| Controlled burns | 126.67 | 0.25 | 125.00 | 0.85 | 125.00 | 0.63 | |
| Grazing regime | 62.50 | 0.00 | 62.50 | 0.93 | 12.50 | 0.45 | |
| Translocation | 73.33 | 0.58 | 22.50 | 0.38 | 22.50 | 0.38 | |
| Hand pollination | 106.67 | 0.42 | 10.00 | 0.20 | 10.00 | 0.20 | |
| Habitat restoration | 115.00 | 0.38 | 57.50 | 0.38 | 100.00 | 0.50 | |
| Stakeholder liaison | 68.33 | 0.90 | 57.50 | 0.93 | 100.00 | 0.93 | |
| Community awareness activities | 46.67 | 0.78 | 57.50 | 0.93 | 100.00 | 0.93 | |
| Assisted gene flow | 60.00 | 0.38 | 5.00 | 0.20 | 0.00 | 0.20 | |
| Species distribution modelling | 83.33 | 0.67 | 0.00 | 0.75 | 0.00 | 0.75 | |
| Ex situ climate threshold trials | 83.33 | 0.75 | 0.00 | 0.63 | 0.00 | 0.63 | |
| OMF distribution modelling | 100.00 | 0.75 | 0.00 | 0.50 | 0.00 | 0.50 | |
| Pollinator ecology and monitoring | 100.00 | 0.67 | 0.00 | 0.63 | 0.00 | 0.63 | |
| Climate and population monitoring | 50 | 0.85 | 0.00 | 0.93 | 0.00 | 0.93 | |
| Confirm ID of unverified populations | 100 | 0.92 | 0.00 | 0.88 | 0.00 | 0.88 | |
The top three most beneficial and feasible actions for each region are highlighted in grey.
Actions and the severe threats they address with associated feasibility (colour) and benefit (arrow size) in the short term for (a) ACT management region, (b) south-eastern NSW management region and (c) northern NSW management region. Threats include direct effects on populations and indirect effects on microenvironment, habitat, pollinators, and OMF as described in Figs S6–S9.
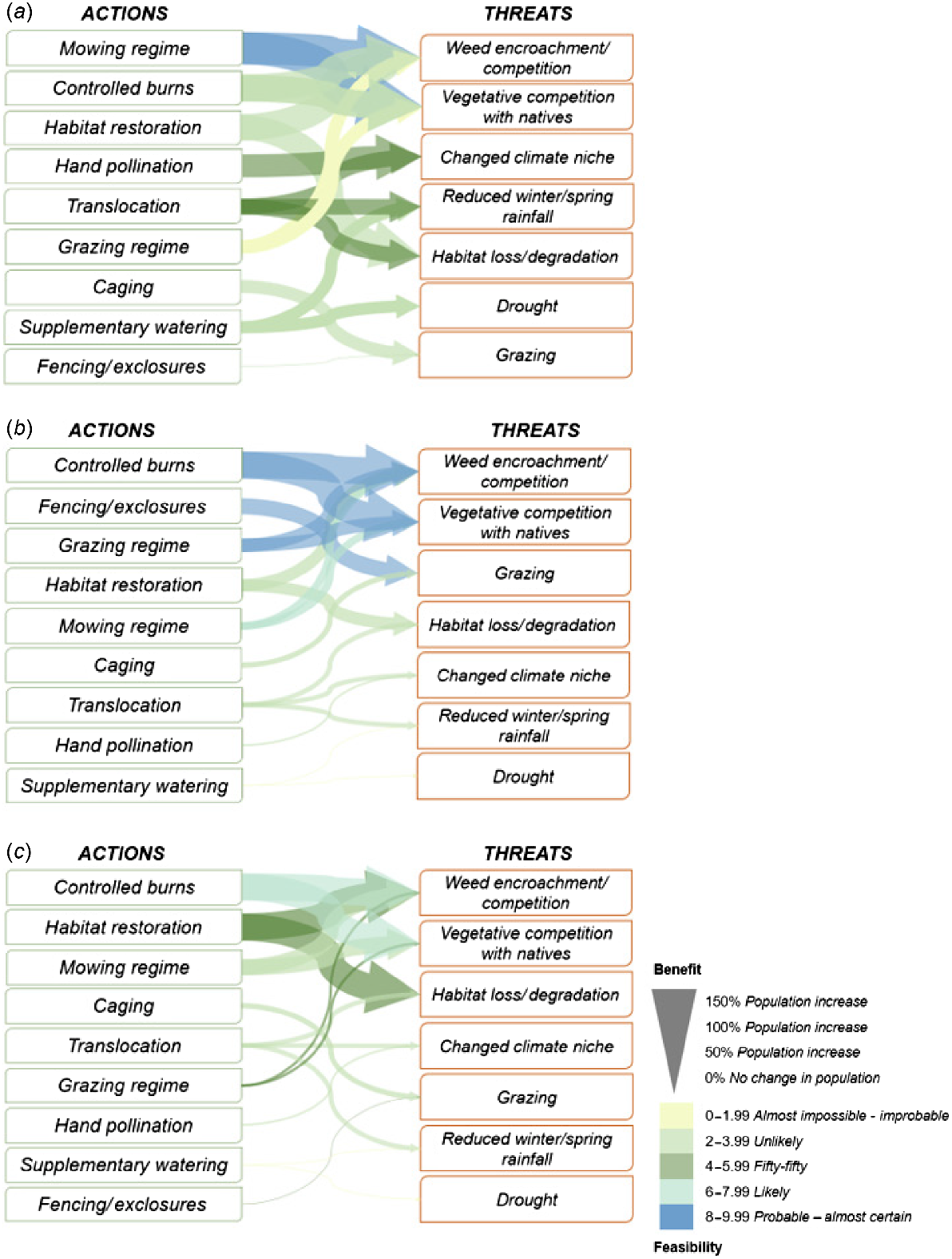
The suite of actions assessed included research actions and stakeholder and community liaison and communication. The research areas include distribution modelling of the species and associated mycorrhizal fungi, verifying species records, ex situ climate threshold trials, genetic studies, and research and monitoring of pollinators (Table 2). Climate-suitability modelling and confirming the taxonomy of unverified populations in all management regions were highly likely to be successfully implemented (between 0.85 and 0.93; Table 2). Experts also considered stakeholder and community liaison to be essential components of species management (between 57% and 100% beneficial), especially given the different tenures within each region. All research actions, except for genetic analysis for assisted gene flow were considered feasible (between 0.50 and 0.93; Table 2).
Discussion
Managers and conservation decision-makers face increasing need to incorporate climate-change adaptation into species management, and to identify beneficial and feasible management actions to specifically mitigate these threats (Cross et al. 2012; Reside et al. 2018). Drawing on the available knowledge, our study found that the most severe climate threats to P. petilum are likely to be resulting from changed rainfall. This corroborates the observations that P. petilum flowering and emergence are correlated with winter rainfall in the northern NSW management region, and thrive in moist microenvironments (Bell 2020). Experts indicated that too little was known about the species biology to accurately estimate its response to increased average and extreme temperatures. Similar knowledge gaps were identified regarding the impacts of climate change on pollinators and orchid mycorrhizal fungi, where some experts designated an ‘unknown’ response rather than a numeric score. Other knowledge gaps, including taxonomy, pollinators, and population estimates, were identified through discussion within the workshop. Although a broad consensus of severity of non-climate threats was reached among the workshop participants, important differences among populations were identified. For example, overgrazing was scored as a severe threat to all populations, but grazing type varied. Livestock grazing was present only at Delegate (south-eastern NSW management region), whereas grazing by sulfur-crested cockatoos (Cacatua galerita) was a significant threat at Hall (ACT management region), and other grazing (e.g. European rabbits, Oryctolagus cuniculus, and various macropod species) and invertebrate damage were observed at other populations.
Management recommendations
Addressing the non-climate threats is established as a key climate-change management approach, especially when climate impacts are uncertain, unknown, or difficult to manage directly (Mawdsley et al. 2009; Hagger et al. 2013; Prober et al. 2019). Given the modest negative trajectory of P. petilum that is likely under current management, and substantial estimated decline under a ‘no management’ regime, we recommend both continuing the broad strategy of addressing the region- or population-specific non-climate threats to increase resilience of the species to climate change (Prober et al. 2019) and implementing additional actions recommended from this study. As well as the maintenance of current actions, such as mowing in the ACT management region, grazing and planned burns in the south-eastern NSW management region, and stakeholder liaison in the form of a planning memorandum of understanding (MOU) in the northern NSW management region, we provided several further recommendations for each region, given the difference in threats and benefit and feasibility of actions across the three regions. At Hall (ACT management region) we recommended conducting a supplementary watering trial in drier-than-average years, to inform future management regimes in preparation for increased duration and intensity of droughts, which are predicted to occur in the near and far future (Kirono et al. 2020). Also notably, supplementary watering and translocation were the only actions listed that directly addressed the climate threats of reduced rainfall and drought. These actions scored low feasibility and only marginally greater than zero benefit at the south-eastern NSW and northern NSW management regions, but slightly higher for the ACT management region, where there is a single population. Within the south-eastern management region, fencing and grazing regimes are currently in place at Delegate, and stakeholder liaison is ongoing at Boorowa, where a grazing regime to reduce biomass could reduce competition with weeds and native grasses and promote growth. A controlled burn planned for Boorowa could inform its benefit for other populations. Continued long-term population monitoring, with the addition of research into population dynamics in relation to climatic conditions to determine site-specific climatic influences, is recommended. Within the northern NSW management region, stakeholder liaison could further promote feasibility of introducing a mowing regime and other actions, including planned management burns. Further liaison with mining and development industry was recommended to prevent habitat loss. Verifying species records of P. petilum in this region was also recommended to increase the robustness of distribution and climate suitability modelling.
Understanding feasibility in the socioecological context is crucial when establishing management plans. Controlled burns (particularly for biodiversity management) are important for managing threatened plants from grassy ecosystems (Coates et al. 2006), but can be challenging to implement in close proximity to human settlement (Florec et al. 2020). The complexities surrounding fire management have resulted in a high proportion of Australia’s threatened plants being threatened by altered fire regimes (Ward et al. 2021; DAWE 2022). Controlled burns were identified as highly beneficial for P. petilum for all three regions; and experts shared results of a burn trial in the south-eastern NSW management region, which showed a positive flowering response to fire, noting that this did not inform the impact of fire frequency. Yet, controlled burns scored low feasibility in the ACT management region because of the constraints of the land being managed as a cemetery. However, the benefit to the population could be high enough to warrant further investigation into overcoming barriers to implementation here (Carwardine et al. 2012). Prioritisation via cost–benefit analyses (e.g. Bottrill et al. 2008), although beyond the scope of this study, may help further reconcile the differences in benefit and feasibility of actions. The sharing of the unpublished results of the fire trial in discussion during the workshop informed the experts on their assessment of the benefit of fire, highlighting the value of the discussions between experts and managers across regions.
Experts agreed that although research actions would not directly mitigate assessed threats or increase population size, they were necessary to facilitate increased benefit and feasibility of other actions and contribute to the overall knowledge of the species and its climate-change risk (Carwardine et al. 2012; Lee et al. 2015; Dudley et al. 2019). Examples include clarifying the taxonomy by using genetic analysis and evaluating the potential value of assisted gene flow from northern to southern populations (Aitken and Whitlock 2013). At the time of this study, the Australian Institute of Botanic Science and the NSW Saving our Species Program were concurrently researching optimal thermal conditions for P. petilum germination (J. Wait, pers. comm.). Collaboration across institutions for research will better inform species climate-change response, and increase the certainty with which threat severity is estimated and management actions are prioritised.
Using the framework
This study further demonstrated the value of expert knowledge in conservation management planning where empirical data are lacking (Burgman et al. 2011; Martin et al. 2012; Mata et al. 2017). This approach, adapted from the framework of Cross et al. (2012), gathered and shared information not publicly available and quantified the severity of potential threats to a restricted species with highly specialised requirements. Species experts were able to share information that was aggregated in a transparent and repeatable way to support recommendations and decision-making (Mata et al. 2017). Workshop discussion further demonstrated the utility of engaging a range of experts from different jurisdictions (multiple state governments for cross-border management) and different institutions (government and academic institutions) to collaborate and communicate, especially where managers have limited time and funds to dedicate to single species (Taft et al. 2020).
We acknowledge some limitations of the chosen approach. The hybrid approach of the first elicitation (online workshop and out-of-session questionnaire) increased participation and reduced some logistical constraints (Kuhnert et al. 2010), but reduced the opportunity for knowledge sharing and discussion. Having only one round of elicitation for each stage (threats and management response) also reduced the ability of experts to revise their estimates on the basis of new information or further clarification of questions (Kuhnert et al. 2010; Martin et al. 2012). Even though concerted efforts were made to ensure clear terminology and phrasing (Martin et al. 2012), there were some instances of linguistic uncertainty (Reside et al. 2018). Future applications of this process should incorporate a second round of elicitation to address these limitations and minimise the forms of biases, such as group think and anchoring, often seen in elicitation (Morgan 2014; Hemming et al. 2018).
Experts also expressed some difficulty in assigning categories to determine threat, for example, the difference between a 20% and 30% decline (the difference between a score of 4 or 5), given the high uncertainty of climate change and lack of knowledge of P. petilum pollinator and orchid mycorrhizal fungi. Uncertainty around the manifestation of climate change at fine scales is common to climate-change adaptation studies. We attempted to account for climate variability by including extreme events, such as extreme hot days, as well as averages (Butt et al. 2016). Where climate-change impacts have yet to be observed, and corresponding mitigation actions yet to be trialled, uncertainty for the action benefit and feasibility will remain high. Managers’ confidence with actions was greater where successful implementation had been demonstrated, and this bias needs to be taken into account. Mechanistic modelling of climate suitability considering species requirements (i.e. obligate mycorrhizal fungi associations) could be explored and results incorporated within conceptual models and this framework to inform management decisions (e.g. Breiner et al. 2018; Tomlinson et al. 2020). The challenges of sparse occurrence, data deficiency and complex specialised requirements are shared by many other threatened species (Tingley et al. 2019; Ward et al. 2021). The framework demonstrated here addresses the challenges of individual species assessments and streamlines the process with a structured, rigorous process that can be easily followed by conservation practitioners for other species with similar conservation challenges.
To our knowledge, this is the first study to assess climate-change threats and corresponding management action priorities for a restricted orchid species in Australia. The recommendations from this study inform on ground management of the species and show the applicability of this framework to rare and restricted species with limited published information. This project also facilitated discussion between managers of the species and promoted knowledge sharing between experts. With the suggested improvements and further testing of the process with other threatened species, this framework could be an important tool for the rapid conservation planning for narrow-ranged and data-deficient Australian plant species under threat from climate change. This is an important step towards bridging the research–implementation gap (Knight et al. 2008; Cross et al. 2012) and ensuring managers have the best tools and knowledge available to make quick but informed decisions to conserve species and biodiversity in the face of a rapidly changing and intensifying climate.
Data availability
The data that support this study will be shared upon reasonable request to the corresponding author.
Author contributions
Study conception and design, material preparation, data collection and analysis were performed by Caitlin Rutherford, April Reside and Andrew Rogers. All authors contributed to the workshops. The first draft of the paper was written by Caitlin Rutherford and all authors commented on previous versions of the paper. All authors read and approved the final paper.
Acknowledgements
We acknowledge the invaluable contributions of the workshop participants as experts on orchid conservation. We thank the members from the Environment, Planning, and Sustainable Development Directorate of the ACT Government for their help in choosing a focal species. We also thank the reviewers for their insightful feedback on the manuscript.
References
Ackerman JD, Phillips RD, Tremblay RL, Karremans A, Reiter N, Peter CI, Bogarín D, Pérez-Escobar OA, Liu H (2023) Beyond the various contrivances by which orchids are pollinated: global patterns in orchid pollination biology. Botanical Journal of the Linnean Society 202, 295-324.
| Crossref | Google Scholar |
Aitken SN, Whitlock MC (2013) Assisted gene flow to facilitate local adaptation to climate change. Annual Review of Ecology, Evolution, and Systematics 44, 367-388.
| Crossref | Google Scholar |
Balzotti CS, Kitchen SG, McCarthy C (2016) Beyond the single species climate envelope: a multifaceted approach to mapping climate change vulnerability. Ecosphere 7, e01444.
| Crossref | Google Scholar |
Barber QE, Nielsen SE, Hamann A (2016) Assessing the vulnerability of rare plants using climate change velocity, habitat connectivity, and dispersal ability: a case study in Alberta, Canada. Regional Environmental Change 16, 1433-1441.
| Crossref | Google Scholar |
Bates R (1984) Pollination of Prasophyllum elatum R.Br. The Orchadian 8, 14-17.
| Google Scholar |
Bell SAJ (2019) Translocation ‘success’ is all about detection: experiences with two threatened orchids from the Hunter Valley of NSW. Australasian Plant Conservation: Journal of the Australian Network for Plant Conservation 28, 27-31.
| Crossref | Google Scholar |
Bell SAJ (2020) Translocation of threatened terrestrial orchids into non-mined and post-mined lands in the Hunter Valley of New South Wales, Australia. Restoration Ecology 28, 1396-1407.
| Crossref | Google Scholar |
Bellard C, Bertelsmeier C, Leadley P, Thuiller W, Courchamp F (2012) Impacts of climate change on the future of biodiversity. Ecology Letters 15, 365-377.
| Crossref | Google Scholar | PubMed |
Bergstrom DM, Wienecke BC, van den Hoff J, Hughes L, Lindenmayer DB, Ainsworth TD, Baker CM, Bland L, Bowman DM, Brooks ST, Canadell JG, et al. (2021) Combating ecosystem collapse from the tropics to the Antarctic. Global Change Biology 27, 1692-1703.
| Crossref | Google Scholar | PubMed |
Beyer RM, Manica A (2020) Historical and projected future range sizes of the world’s mammals, birds, and amphibians. Nature Communications 11, 5633.
| Crossref | Google Scholar |
Bottrill MC, Joseph LN, Carwardine J, Bode M, Cook C, Game ET, Grantham H, Kark S, Linke S, McDonald-Madden E, Pressey RL, Walker S, Wilson KA, Possingham HP (2008) Is conservation triage just smart decision making? Trends in Ecology & Evolution 23, 649-654.
| Crossref | Google Scholar | PubMed |
Breiner FT, Nobis MP, Bergamini A, Guisan A (2018) Optimizing ensembles of small models for predicting the distribution of species with few occurrences. Methods in Ecology and Evolution 9, 802-808.
| Crossref | Google Scholar |
Burgman MA, McBride M, Ashton R, Speirs-Bridge A, Flander L, Wintle B, Fidler F, Rumpff L, Twardy C (2011) Expert status and performance. PLoS ONE 6, e22998.
| Crossref | Google Scholar | PubMed |
Butt N, Possingham HP, De Los Rios C, Maggini R, Fuller RA, Maxwell SL, Watson JEM (2016) Challenges in assessing the vulnerability of species to climate change to inform conservation actions. Biological Conservation 199, 10-15.
| Crossref | Google Scholar |
Carwardine J, O’Connor T, Legge S, Mackey B, Possingham HP, Martin TG (2012) Prioritizing threat management for biodiversity conservation. Conservation Letters 5, 196-204.
| Crossref | Google Scholar |
Coates F, Lunt ID, Tremblay RL (2006) Effects of disturbance on population dynamics of the threatened orchid Prasophyllum correctum D.L.Jones and implications for grassland management in south-eastern Australia. Biological Conservation 129, 59-69.
| Crossref | Google Scholar |
Cresswell ID, Janke T, Johnston EL (2021) Australia state of the environment 2021: overview. (Commonwealth of Australia: Canberra, ACT, Australia) Available at https://soe.dcceew.gov.au/sites/default/files/2022-07/soe2021-overview.pdf [Verified 20 December 2021]
Cross MS, Zavaleta ES, Bachelet D, Brooks ML, Enquist CAF, Fleishman E, Graumlich LJ, Groves CR, Hannah L, Hansen L, Hayward G, Koopman M, Lawler JJ, Malcolm J, Nordgren J, Petersen B, Rowland EL, Scott D, Shafer SL, Shaw MR, Tabor GM (2012) The adaptation for conservation targets (ACT) framework: a tool for incorporating climate change into natural resource management. Environmental Management 50, 341-351.
| Crossref | Google Scholar | PubMed |
DAWE (2021) Conservation Advice for Prasophyllum petilum (Tarengo Leek Orchid). (Department of Agriculture, Water and the Environment: Canberra, ACT, Australia) Available at https://www.environment.gov.au/biodiversity/threatened/species/pubs/55144-conservation-advice-29092021.pdf [Verified 2 February 2024]
DAWE (2022) Fire regimes that cause declines in biodiversity as a key threatening process. (Department of Agriculture, Water and the Environment: Canberra, ACT, Australia) Available at https://www.dcceew.gov.au/environment/biodiversity/threatened/key-threatening-processes/fire-regimes-that-cause-declines-in-biodiversity [Verified 6 August 2024]
Dawson TP, Jackson ST, House JI, Prentice IC, Mace GM (2011) Beyond predictions: biodiversity conservation in a changing climate. Science 332, 53-58.
| Crossref | Google Scholar |
DCCEEW (2023) Species Profile and Threats Database, EPBC Act List of Threatened Flora. (Department of Climate Change, Energy, the Environment and Water: Canberra, ACT, Australia) Available at https://www.environment.gov.au/cgi-bin/sprat/public/publicthreatenedlist.pl?wanted=flora [Verified 2 February 2024]
DCCEEW (2024) Environment Protection and Biodiversity Conservation Act 1999 (Cth). (Department of Climate Change, Energy, the Environment and Water: Canberra, ACT, Australia) Available at http://www.environment.gov.au/epbc/index.html [Verified 14 October 2024]
DECCW (2010) National Recovery Plan for the Tarengo Leek Orchid Prasophyllum petilum. (Department of Environment, Climate Change and Water: Sydney, NSW, Australia) Available at https://www.dcceew.gov.au/sites/default/files/documents/prasophyllum-petilum-recovery-plan.pdf [Verified 1 August 2021]
Dowdy AJ (2018) Climatological variability of fire weather in Australia. Journal of Applied Meteorology and Climatology 57, 221-234.
| Crossref | Google Scholar |
Dowdy AJ, Ye H, Pepler A, Thatcher M, Osbrough SL, Evans JP, Di Virgilio G, McCarthy N (2019) Future changes in extreme weather and pyroconvection risk factors for Australian wildfires. Scientific Reports 9, 10073.
| Crossref | Google Scholar | PubMed |
Dubois NS, Gomez A, Carlson S, Russell D (2020) Bridging the research-implementation gap requires engagement from practitioners. Conservation Science and Practice 2, e134.
| Crossref | Google Scholar |
Dudley A, Butt N, Auld TD, Gallagher RV (2019) Using traits to assess threatened plant species response to climate change. Biodiversity and Conservation 28, 1905-1919.
| Crossref | Google Scholar |
Dunwiddie PW, Haan NL, Linders M, Bakker JD, Fimbel C, Thomas TB (2016) Intertwined fates: opportunities and challenges in the linked recovery of two rare species. Natural Areas Journal 36, 207-215.
| Crossref | Google Scholar |
EPSD (2019) ACT Native Woodland Conservation Strategy: Tarengo Leek Orchid Prasophyllum Petilum Action Plan. (Environment, Planning and Sustainable Development: Canberra, ACT, Australia) Available at https://www.environment.act.gov.au/__data/assets/pdf_file/0005/576527/Woodland-Conservation-Strategy-Tarengo-Leek-Orchid.pdf [Verified 1 August 2021]
Evans A, Janssens S, Jacquemyn H (2020) Impact of climate change on the distribution of four closely related Orchis (Orchidaceae) species. Diversity 12, 312.
| Crossref | Google Scholar |
Florec V, Burton M, Pannell D, Kelso J, Milne G (2020) Where to prescribe burn: the costs and benefits of prescribed burning close to houses. International Journal of Wildland Fire 29, 440-458.
| Crossref | Google Scholar |
Foden WB, Butchart SHM, Stuart SN, Vié JC, Akçakaya HR, Angulo A, DeVantier LM, Gutsche A, Turkak E, Cao L, Donner SD, Katariya V, Bernard R, Holland RA, Hughes AF, O’Hanlon SE, Garnett ST, Şekercioğlu Çagan H, Mace GM, Lavergne S (2013) Identifying the world’s most climate change vulnerable species: a systematic trait-based assessment of all birds, amphibians, and corals. PLoS ONE 8, e65427.
| Crossref | Google Scholar | PubMed |
Foden WB, Young BE, Akçakaya HR, Garcia RA, Hoffmann AA, Stein BA, Thomas CD, Wheatley CJ, Bickford D, Carr JA, Hole DG, Martin TG, Pacifici M, Pearce-Higgins JW, Platts PJ, Visconti P, Watson JEM, Huntley B (2019) Climate change vulnerability assessment of species. Wiley Interdisciplinary Reviews: Climate Change 10, e551.
| Crossref | Google Scholar |
Freestone MW, Swarts N, Reiter N, Tomlinson S, Sussmilch FS, Wright MW, Holmes GD, Phillips RD, Linde CC (2021) Continental-scale distribution and diversity of Ceratobasidium orchid mycorrhizal fungi in Australia. Annals of Botany 128, 329-343.
| Crossref | Google Scholar | PubMed |
Gaskett AC, Gallagher RV (2018) Orchid diversity: spatial and climatic patterns from herbarium records. Ecology and Evolution 8, 11235-11245.
| Crossref | Google Scholar | PubMed |
Hagger V, Fisher D, Schmidt S, Blomberg S (2013) Assessing the vulnerability of an assemblage of subtropical rainforest vertebrate species to climate change in south-east Queensland. Austral Ecology 38, 465-475.
| Crossref | Google Scholar |
Hamilton SH, Pollino CA, Jakeman AJ (2015) Habitat suitability modelling of rare species using bayesian networks: model evaluation under limited data. Ecological Modelling 299, 64-78.
| Crossref | Google Scholar |
Heller NE, Zavaleta ES (2009) Biodiversity management in the face of climate change: a review of 22 years of recommendations. Biological Conservation 142, 14-32.
| Crossref | Google Scholar |
Hemming V, Burgman MA, Hanea AM, McBride MF, Wintle BC (2018) A practical guide to structured expert elicitation using the IDEA protocol. Methods in Ecology and Evolution 9, 169-180.
| Crossref | Google Scholar |
Hoeppner JM, Hughes L (2019) Climate readiness of recovery plans for threatened Australian species. Conservation Biology 33, 534-542.
| Crossref | Google Scholar | PubMed |
Hughes L (2011) Climate change and Australia: key vulnerable regions. Regional Environmental Change 11, 189-195.
| Crossref | Google Scholar |
IUCN (2021) Threats Classification Scheme Version 3.2. International Union Conservation of Nature. Available at https://www.iucnredlist.org/resources/threat-classification-scheme [Verified 12 October 2021]
Jasinge NU, Huynh T, Lawrie AC (2018) Changes in orchid populations and endophytic fungi with rainfall and prescribed burning in Pterostylis revoluta in Victoria, Australia. Annals of Botany 121, 321-334.
| Crossref | Google Scholar | PubMed |
Jump AS, Penuelas J (2005) Running to stand still: adaptation and the response of plants to rapid climate change. Ecology Letters 8, 1010-1020.
| Crossref | Google Scholar | PubMed |
Kirono DGC, Round V, Heady C, Chiew FHS, Osbrough S (2020) Drought projections for Australia: updated results and analysis of model simulations. Weather and Climate Extremes 30, 100280.
| Crossref | Google Scholar |
Knight AT, Cowling RM, Rouget M, Balmford A, Lombard AT, Campbell BM (2008) Knowing but not doing: selecting priority conservation areas and the research–implementation gap. Conservation Biology 22, 610-617.
| Crossref | Google Scholar | PubMed |
Kuhnert PM, Martin TG, Griffiths SP (2010) A guide to eliciting and using expert knowledge in Bayesian ecological models. Ecology Letters 13, 900-914.
| Crossref | Google Scholar | PubMed |
Lawler J, Watson J, Game E (2015) Conservation in the face of climate change: recent developments. F1000Research 4, 1158.
| Crossref | Google Scholar |
Lee JR, Maggini R, Taylor MFJ, Fuller RA (2015) Mapping the drivers of climate change vulnerability for Australia’s threatened species. PLoS ONE 10, e0124766.
| Crossref | Google Scholar | PubMed |
Marcer A, Sáez L, Molowny-Horas R, Pons X, Pino J (2013) Using species distribution modelling to disentangle realised versus potential distributions for rare species conservation. Biological Conservation 166, 221-230.
| Crossref | Google Scholar |
Margoluis R, Stem C, Salafsky N, Brown M (2009) Using conceptual models as a planning and evaluation tool in conservation. Evaluation and Program Planning 32, 138-147.
| Crossref | Google Scholar | PubMed |
Martin TG, Burgman MA, Fidler F, Kuhnert PM, Low-Choy S, McBride M, Mengersen K (2012) Eliciting expert knowledge in conservation science. Conservation Biology 26, 29-38.
| Crossref | Google Scholar | PubMed |
Mata L, Garrard GE, Kutt AS, Wintle BC, Chee YE, Backstrom A, Bainbridge B, Urlus J, Brown GW, Tolsma AD, Yen AL, New TR, Bekessy SA (2017) Eliciting and integrating expert knowledge to assess the viability of the critically endangered golden sun-moth Synemon plana. Austral Ecology 42, 297-308.
| Crossref | Google Scholar |
Mawdsley JR, O’Malley R, Ojima DS (2009) A review of climate-change adaptation strategies for wildlife management and biodiversity conservation. Conservation Biology 23, 1080-1089.
| Crossref | Google Scholar | PubMed |
McQualter E, Cross R, McLean CB, Ladiges PY (2007) Prasophyllum and its associated mycorrhizal fungi. Lankesteriana International Journal on Orchidology 7, 497-501.
| Google Scholar |
Morgan MG (2014) Use (and abuse) of expert elicitation in support of decision making for public policy. Proceedings of the National Academy of Sciences 111, 7176–-7184.
| Crossref | Google Scholar |
Naujokaitis-Lewis I, Endicott S, Guezen J (2021) Treatment of climate change in extinction risk assessments and recovery plans for threatened species. Conservation Science and Practice 3, e450.
| Crossref | Google Scholar |
OEH (2014) New South Wales Climate change snapshot. (Office of Environment and Heritage: Sydney, NSW, Australia) Available at https://www.climatechange.environment.nsw.gov.au/sites/default/files/2021-06/NSW%20climate%20change%20snapshot.pdf [Verified 16 August 2021]
OEH (2021a) Tarengo Leek Orchid Profile. Office of Environment and Heritage NSW: Sydney, NSW, Australia) Available at https://threatenedspecies.bionet.nsw.gov.au/profile?id=10666 [Verified 16 August 2021]
OEH (2021b) Tarengo Leek Orchid (Prasophyllum petilum): Saving our Species strategy. (NSW Office of Environment and Heritage: Sydney, NSW, Australia) Available at https://www.environment.nsw.gov.au/savingourspeciesapp/project/224 [Verified 16 August 2021]
Oliver TH, Smithers RJ, Bailey S, Walmsley CA, Watts K (2012) A decision framework for considering climate change adaptation in biodiversity conservation planning. Journal of Applied Ecology 49, 1247-1255.
| Crossref | Google Scholar |
Pacifici M, Foden WB, Visconti P, Watson JEM, Butchart SHM, Kovacs KM, Scheffers BR, Hole DG, Martin TG, Akçakaya HR, Corlett RT, Huntley B, Bickford D, Carr JA, Hoffmann AA, Midgley GF, Pearce-Kelly P, Pearson RG, Williams SE, Willis SG, Young B, Rondinini C (2015) Assessing species vulnerability to climate change. Nature Climate Change 5, 215-224.
| Crossref | Google Scholar |
Peakall R (1989) A new technique for monitoring pollen flow in orchids. Oecologia 79, 361-365.
| Crossref | Google Scholar | PubMed |
Peters GP, Andrew RM, Boden T, Canadell JG, Ciais P, Le Quéré C, Marland G, Raupach MR, Wilson C (2013) The challenge to keep global warming below 2C. Nature Climate Change 3, 4-6.
| Crossref | Google Scholar |
Phillips RD, Reiter N, Peakall R (2020) Orchid Conservation: from theory to practice. Annals of Botany 126, 345-362.
| Crossref | Google Scholar | PubMed |
Poiani KA, Goldman RL, Hobson J, Hoekstra JM, Nelson KS (2011) Redesigning biodiversity conservation projects for climate change: examples from the field. Biodiversity and Conservation 20, 185-201.
| Crossref | Google Scholar |
Prober SM, Doerr VAJ, Broadhurst LM, Williams KJ, Dickson F (2019) Shifting the conservation paradigm: a synthesis of options for renovating nature under climate change. Ecological Monographs 89, 1-23.
| Crossref | Google Scholar |
R Core Team (2024) R: a language and environment for statistical computing. (R Foundation for Statistical Computing) Available at https://www.R-project.org/ [Verified 28 July 2024]
Rasmussen HN, Dixon KW, Jersáková J, Těšitelová T (2015) Germination and seedling establishment in orchids: a complex of requirements. Annals of Botany 116, 391.
| Crossref | Google Scholar | PubMed |
Regan HM, Colyvan M, Burgman MA (2002) A taxonomy and treatment of uncertainty for ecology and conservation biology. Ecological Applications 12, 618-628.
| Crossref | Google Scholar |
Reside AE, VanDerWal J, Garnett ST, Kutt AS (2016) Vulnerability of Australian tropical savanna birds to climate change. Austral Ecology 41, 106-116.
| Crossref | Google Scholar |
Reside AE, Butt N, Adams VM (2018) Adapting systematic conservation planning for climate change. Biodiversity and Conservation 27, 1-29.
| Crossref | Google Scholar |
Reside AE, Critchell K, Crayn DM, Goosem M, Goosem S, Hoskin CJ, Sydes T, Vanderduys EP, Pressey RL (2019) Beyond the model: expert knowledge improves predictions of species’ fates under climate change. Ecological Applications 29, e01824.
| Crossref | Google Scholar | PubMed |
Reynolds A, Deluca L, Gottschalk A, Gray K, Leonardi S, Picker I (2021) The climate gap: report on climate threat management for critically endangered species and ecological communities under the EPBC Act. (Green Law and Australian Conservation Foundation) Available at https://greenlawnetwork.org/wp-content/uploads/2021/12/The-Climate-Gap-GreenLaw-December-2021.pdf [Verified 20 December 2021]
Roper E (2021) Second year post-fire monitoring of the Endangered Superb Midge Orchid (Genoplesium superbum) in south-eastern NSW. Australasian Plant Conservation 30, 12-14.
| Crossref | Google Scholar |
Roy HE, Peyton JM, Booy O (2020) Guiding principles for utilizing social influence within expert-elicitation to inform conservation decision-making. Global Change Biology 26, 3181-3184.
| Crossref | Google Scholar | PubMed |
Rudin-Bitterli TS, Evans JP, Mitchell NJ (2021) Fitness consequences of targeted gene flow to counter impacts of drying climates on terrestrial-breeding frogs. Communications Biology 4, 1195.
| Crossref | Google Scholar |
Scheffers BR, de Meester L, Bridge TCL, Hoffmann AA, Pandolfi JM, Corlett RT, Butchart SHM, Pearce-Kelly P, Kovacs KM, Dudgeon D, Pacifici M, Rondinini C, Foden WB, Martin TG, Mora C, Bickford D, Watson JE (2016) The broad footprint of climate change from genes to biomes to people. Science 354, aaf7671.
| Crossref | Google Scholar |
Smith M, Jackson C, Palmer N, Palmer B (2020) A structured analysis of risk to important wildlife elements in three Australian Wildlife Conservancy sanctuaries. Ecological Management and Restoration 21, 42-50.
| Crossref | Google Scholar |
Staude IR, Navarro LM, Pereira HM (2020) Range size predicts the risk of local extinction from habitat loss. Global Ecology and Biogeography 29, 16-25.
| Crossref | Google Scholar |
Swarts ND, Dixon KW (2009) Terrestrial orchid conservation in the age of extinction. Annals of Botany 104, 543-556.
| Crossref | Google Scholar | PubMed |
Taft HR, McCoskey DN, Miller JM, Pearson SK, Coleman MA, Fletcher NK, Mittan CS, Meek MH, Barbosa S (2020) Research–management partnerships: an opportunity to integrate genetics in conservation actions. Conservation Science and Practice 2, e218.
| Crossref | Google Scholar |
Thurman LL, Stein BA, Beever EA, Foden W, Geange SR, Green N, Gross JE, Lawrence DJ, LeDee O, Olden JD, Thompson LM, Young BE (2020) Persist in place or shift in space? Evaluating the adaptive capacity of species to climate change. Frontiers in Ecology and the Environment 18, 520-528.
| Crossref | Google Scholar |
Tingley R, Macdonald SL, Mitchell NJ, Woinarski JCZ, Meiri S, Bowles P, Cox NA, Shea GM, Böhm M, Chanson J, Tognelli MF, et al. (2019) Geographic and taxonomic patterns of extinction risk in Australian squamates. Biological Conservation 238, 108203.
| Crossref | Google Scholar |
Tomlinson S, Lewandrowski W, Elliott CP, Miller BP, Turner SR (2020) High-resolution distribution modeling of a threatened short-range endemic plant informed by edaphic factors. Ecology and Evolution 10, 763-777.
| Crossref | Google Scholar | PubMed |
Wang HH, Wonkka CL, Treglia ML, Grant WE, Smeins FE, Rogers WE (2015) Species distribution modelling for conservation of an endangered endemic orchid. AoB PLANTS 7, plv039.
| Crossref | Google Scholar |
Ward M, Carwardine J, Yong CJ, Watson JEM, Silcock J, Taylor GS, Lintermans M, Gillespie GR, Garnett ST, Woinarski J, Tingley R, Fensham RJ, Hoskin CJ, Hines HB, Roberts JD, Kennard MJ, Harvey MS, Chapple DG, Reside AE (2021) A national-scale dataset for threats impacting Australia’s imperiled flora and fauna. Ecology and Evolution 11, 11749-11761.
| Crossref | Google Scholar | PubMed |
Warren R, Vanderwal J, Price J, Welbergen JA, Atkinson I, Ramirez-Villegas J, Osborn TJ, Jarvis A, Shoo LP, Williams SE, Lowe J (2013) Quantifying the benefit of early climate change mitigation in avoiding biodiversity loss. Nature Climate Change 3, 678-682.
| Crossref | Google Scholar |
Williams SE, Shoo LP, Isaac JL, Hoffmann AA, Langham G (2008) Towards an integrated framework for assessing the vulnerability of species to climate change. PLOS Biology 6, e325.
| Crossref | Google Scholar | PubMed |
Wilson N, Seddon J, Baines G (2016) Factors influencing the flowering of the Tarengo Leek Orchid (Prasophyllum petilum). Technical Report 36. (Environment, Planning and Sustainable Development Directorate, ACT Government: Canberra, ACT, Australia) Available at https://www.environment.act.gov.au/__data/assets/pdf_file/0018/1026342/TR36-Factors-influencing-the-flowering-of-the-Tarengo-Leek-Orchid.pdf [Verified 1 August 2021]
Woinarski JCZ, Braby MF, Burbidge AA, Coates D, Garnett ST, Fensham RJ, Legge SM, McKenzie NL, Silcock JL, Murphy BP (2019) Reading the black book: the number, timing, distribution and causes of listed extinctions in Australia. Biological Conservation 239, 108261.
| Crossref | Google Scholar |
Wraith J, Pickering C (2019) A continental scale analysis of threats to orchids. Biological Conservation 234, 7-17.
| Crossref | Google Scholar |


