Characterising the woody vegetation in contrasting habitat types in the lower Fitzroy River, Western Australia
Fiona L. Freestone A § , Caroline A. Canham A § * , Samantha A. Setterfield A , Michael M. Douglas A , Leah S. Beesley A and Robyn C. Loomes B
A § * , Samantha A. Setterfield A , Michael M. Douglas A , Leah S. Beesley A and Robyn C. Loomes B
A School of Agriculture and Environment, The University of Western Australia, Crawley, WA 6009, Australia.
B Department of Water and Environmental Regulation, Government of Western Australia, Joondalup, WA 6027, Australia.
Australian Journal of Botany 70(6) 421-431 https://doi.org/10.1071/BT22039
Submitted: 13 April 2022 Accepted: 4 August 2022 Published: 14 September 2022
© 2022 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Context: Riverine systems consist of distinct habitats along a landscape gradient and characterising the composition and structure of vegetation in these habitats can support environmental water-management decisions. However, in many regions, including northern Australia, there is a paucity of hydro-ecological data.
Aims: We aimed to characterise the species composition and the structure of riparian and floodplain woody vegetation of the lower Fitzroy River.
Methods: We surveyed woody vegetation in different habitats within the riparian zone and floodplain. Multivariate analysis was used to assess differences in the composition of riparian woody species among the four habitat types and univariate analysis was used to compare vegetation structure, recruitment, and environmental variables among habitats.
Key results: The composition and the physical structure of woody species differed among habitat types of the lower Fitzroy River, indicating a zonation of riparian and floodplain vegetation in response to fluvial processes and water availability. The floodplain was characterised by sparsely distributed Eucalyptus microtheca and a sparse (∼30%) canopy cover. In contrast, the riverbank habitat type had very large trees (mean basal area = 0.26 m2), with a dense canopy cover (∼80%) and was dominated by Melaleuca argentea, M. leucadendra and Barringtonia acutangula. Both the top of bank and off-channel wetlands represent a more intermediary environment, characterised by greater species richness and greater seedling recruitment.
Conclusions: Identifying these habitat types and characterising their physical and biological properties, such as the relationship between flooding and the composition of woody species, provides a framework to assist the management of large floodplain river systems.
Keywords: ecohydrology, environmental water requirements, floodplain, riparian, riparian trees, semi-arid ecosystem, tropical ecosystem, water management.
Introduction
Freshwater systems, including rivers, floodplains and associated riparian vegetation, support high biodiversity and provide critical ecosystem services (Riis et al. 2020). They are also some of the most threatened ecosystems in the world (Tockner and Stanford 2002; Davidson 2014; Albert et al. 2021). A major contributing threat is modification of flow regime that alters the timing, frequency, duration and extent of flows, such as through extraction of water for consumption and agricultural purposes (Dudgeon et al. 2006; Flitcroft et al. 2019). Changes to the natural flow regime can alter the assemblage and physical structure of riparian plant communities (White and Stromberg 2011). Water management that aims to protect vegetation includes consideration of the relationship between flows and biota; however, the development of quantitative relationships requires a high degree of hydrological and ecological information that is not available for many river systems (Poff and Zimmerman 2010). Instead, habitats along a landscape or hydrological gradient may be investigated, providing an indirect link between hydrology and vegetation characteristics (Stromberg and Merritt 2016).
The organising role of flow on the abiotic and biotic characteristics of riparian and floodplain vegetation is well documented (Naiman and Décamps 1997). Many rivers, particularly those with large, wide floodplains, display distinct natural zonation of vegetation along a hydrological gradient associated with position in the landscape, from the banks of the river channel to the edge of the floodplain (Casanova and Brock 2000; Deane et al. 2017; Jansson et al. 2019). Habitats closest to the main channel are often characterised by high-velocity flows and long periods of inundation, and are dominated by species that tolerate these hydrological stressors, including true rheophytes (Bejarano et al. 2011). In contrast, floodplain habitats do not remain inundated for as long, and may experience peiods of drought in water-limited environments. Thus, the floodplain habitats are often dominated by species that withstand drier conditions, yet may still require semi-regular flooding for reproduction (Capon et al. 2016), with flood flows found to increase seed production and dispersal and aid germination and seedling establishment (Greet et al. 2022). Identifying the different habitats within the landscape and characterising the species and vegetation structure that they support can assist with determining how changes in water flows may affect riparian and floodplain vegetation (Merritt et al. 2010). However, in many regions there is limited information about the distribution of riparian and floodplain plants in relation to hydrology. Improving our understanding of these ecosystems is essential to inform water policy and resource development (Dudgeon et al. 2006).
Northern Australia is one region where there is limited hydro-ecological information, but water-resource development is a federal government priority (Douglas et al. 2005, 2019; DIIS 2015). The Fitzroy River in the Kimberley region of north-western Australia (Fig. 1) is one such system under increasing pressure for the development of freshwater resources (DIIS 2015; Petheram et al. 2018). The Fitzroy River is a long (>700 km) river, with an extensive floodplain of up to 15 km wide (Taylor 1998). With only 56% of the world’s long rivers (500–1000 km) being identified as free-flowing (Grill et al. 2019), the Fitzroy River has global significance as a largely unregulated system. The natural flow regime of the Fitzroy River supports many significant environmental, cultural, and economic values (Douglas et al. 2019; Poelina et al. 2019). For example the river supports sawfish (Pristis pristis) that are listed as Vulnerable under the Environment Protection and Biodiversity Conservation Act (EPBC Act 1999), and populations of barramundi (Lates calcarifer) and catfish (Neoarius graeffei), which are important species for customary hunting (Jackson et al. 2012). The riparian vegetation of the Fitzroy River provides habitat for terrestrial animals, including riparian specialist the purple-crowned fairy wren (Malurus coronatus coronatus), which is listed as Endangered under the EPBC Act (EPBC Act 1999; Skroblin and Legge 2010). Flow alteration can affect these values. As part of a large transdisciplinary project, Douglas et al. (2019) completed a literature review and developed a hydro–socio–ecological (HSE) model for the Fitzroy River. The model identified important habitats within the riverine system of the lower Fitzroy River, including the riparian zone, floodplain and off-channel wetlands. Highlighting these different habitat types assists with identifying their distinct biota and biological processes, as well as the flow components required to maintain them. The HSE model emphasised the cultural values of riparian vegetation, and the importance of protecting flow components to support ecological and cultural values. However, the review also highlighted a deficiency of ecological data to support water-management decisions, with very little information on the distribution and physical structure of riparian woody vegetation in relation to river flows (Douglas et al. 2019).
|
|
The primary aim of this study was to describe the species composition and the structure of riparian and floodplain woody vegetation of the lower Fitzroy River. On the basis of a review of the literature (Douglas et al. 2019) and field observations, four broad habitat types were identified along a hydrological gradient from the banks of the lower Fitzroy River up on to the floodplain (Fig. 1). These habitat types were termed riverbank, top of bank, floodplain, and off-channel wetlands. It was expected that each habitat would support a different combination of woody plant species, reflecting plant water requirements. It was also expected that vegetation stand structural parameters, such as canopy cover, diameter at breast height (DBH), and indicators of seedling recruitment would differ among habitat types. This study will inform water-management decisions by characterising woody vegetation for each habitat type in the riparian zone and floodplain.
Materials and methods
Study area
The Fitzroy River catchment, covering approximately 90 000 km2, is located on the southern edge of the tropics in northern Western Australia (Fig. 1). The catchment has a wet–dry climate, with 93% of rain falling in the wet season (November–April) (Pusey and Kath 2015). There is also high inter-annual variability, with a median annual rainfall of 791 mm and a range between 397 mm and 1294 mm from 1991 to 2019. Maximum temperatures frequently exceed 40°C in the wet season, with relative humidity as high as 90%. Maximum winter temperatures reach 30°C and rarely drop below 10°C overnight (Curtin Aero Gauge, −17.58S, 123.83E; retrieved from www.bom.gov.au/data). There is a naturally large variability in river discharge among years, with annual discharge at Fitzroy Crossing ranging between 468 and 19 972 GL year−1, with a median of 4232 GL year−1 (years 1984–2020 at the Fitzroy Crossing gauge (AWRC 802055); retrieved from www.bom.gov.au/waterdata/).
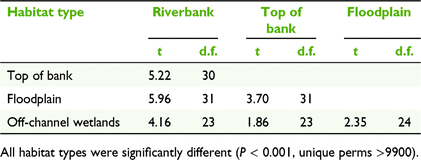
The study area focussed on the lower Fitzroy River, spanning approximately 300 km from the town of Fitzroy Crossing to the bridge at Willare (−17.73S, 123.64SE). There are distinct habitat types within the riverine environment of the lower Fitzroy River, which reflect fluvial processes (Fig. 2). The main river channel has steep banks that are generally ∼15 m wide and with an elevation of ∼5 m above the dry-season water level (Table 1). The riverbank habitat type is exposed to high-velocity flows and long periods of inundation during the wet season. The top of the bank refers to the areas adjacent to the riverbank where the land is generally flat (Table 1). Continuing away from the riverbanks, the lower Fitzroy River has a large floodplain, extending up to 15 km from the main river channel. The floodplain is inundated in large floods and vegetation is distinct from the surrounding savanna. Within the floodplain there are ‘wetlands’ that retain water in most years, which include depressions, floodplain wetlands and flood runner channels that flow from the main channel before overbank flooding (Table 1).
Vegetation data
Surveys were undertaken during the dry season in northern Australia, between July and September 2018. Site selection was stratified on the basis of their landscape position to sample the four broad habitat types: riverbank, top of bank, floodplain and off-channel wetlands (Table 1, Fig. 2). In total, 58 sites were selected in collaboration with Traditional Owners from Walalakoo, Yi-martuwarra and Gooniyandi native title groups, on the basis of cultural significance and accessibility, and Traditional Owners and Indigenous rangers assisted with data collection.
At each site, a 10 m × 40 m quadrat was placed within the identified habitat type. The location of the river was noted and riverbank, top of bank and floodplain quadrats ran parallel to the river. Wetland quadrats were placed in the tree line on the top of banks, running parallel with the waterbody. All woody species within the 10 m × 40 m quadrat were recorded and identified to species level. Taxonomy followed Wheeler et al. (1992) and Dixon (2007) and naming conventions and weed status followed FloraBase, the Western Australian Flora website (www.florabase.dpaw.wa.gov.au).
Canopy cover was assessed using a sampling pole and line point-intercept method at one hundred points around the perimeter of each site (i.e. one point every metre around 10 m × 40 m site). Canopy cover was recorded as either present or absent by using a GRS Densitometer attached to the pole facing upwards. Diameter at breast height (DBH) was measured for each tree (>1.5 m tall). Measurements of DBH were summed for multi-stemmed trees (e.g. such as Barringtonia acutangula (L.) Gaertn.) and total DBH for each tree was classified into 10 cm categories (11th category: DBH 100+ cm). Recruitment was assessed by counting individual tree seedlings, classified as small (<0.5 m tall) or large (0.5–1.5 m tall), within each 10 m × 40 m site.
Environmental variables
Nine environmental variables were assessed to help characterise the physical environment of the different habitat types and to assess the relationship with woody species composition; distance from the estuary (km), distance from the main channel (km), the number of days inundated during a small flood, the number of days inundated during a large flood, soil type (% clay), mean annual rainfall (mm), mean maximum temperature (°C), mean pan evaporation (mm) and fire history. Hydrological conditions were assessed using the two flood-duration variables (small and large flood). The smaller flood year (2013) represents regular flood events, which occur annually, with a 1 in 1 annual exceedance probability (AEP), overbank flows for 55 days, and maximum discharge of 69 110 mL day−1 at the Fitzroy Barrage gauge (AWRC 802003; Supplemenatary material Fig. S1). The larger flood year (2000) had a 1 in 11 AEP, with overbank flows for 119 days and a substantially larger maximum daily discharge of 759 049 mL day−1 at the Fitzroy Barrage gauge. Inundation variables were predicted from a river-discharge model (Hughes et al. 2017) and a two-dimensional hydrodynamic model provided by the Commonwealth Scientific and Industrial Research Organisation (CSIRO) through the Northern Australia Water Resource Assessment program (Karim et al. 2018). The output from the hydrodynamic model was a raster dataset (30 m × 30 m pixel resolution) of predicted daily water levels across the study area, which was converted to total number of days of inundation for each pixel. The number of days of inundation was extracted from the centre of each site (i.e. 10 m × 40 m vegetation quadrats) for the large (2000) and small (2013) flood years as model input variables.
Soil type was characterised by collecting one sample from the top 20 cm at each site and using field texture analysis (as per (McDonald et al. 1998) to estimate percentage clay content. Climatic variables were long-term average rainfall and average monthly maximum temperature, which were extracted for each site from gridded spatial datasets from the Australian Bureau of Meteorology (retrieved from www.bom.gov.au/climate/data-services/maps.shtml). Fire history was assessed using a remotely sensed raster product, which described the fraction of years burnt between 2005 and 2015 (Pintor et al. 2019). Values for environmental variables at each site are provided in Supplemantary material Table S1.
Data analysis
Multivariate methods were used to assess differences in the composition of riparian woody species among the four habitat types. Analyses were completed in Primer (ver. 7, Primer-E Ltd, Plymouth, UK) with the PERMANOVA+ add-on (Anderson et al. 2008). Species associations were visualised using non-metric multidimensional scaling (nMDS) ordination using Bray–Curtis dissimilarities of species composition and abundance, which was fourth-root transformed. Permutational multivariate analysis of variance (PERMANOVA) was used to test for differences in composition among the different habitat types (one-way hypothesis test), again by using Bray–Curtis dissimilarities. Similarity percentage (SIMPER) was used to determine species contributions to within-group similarity for each habitat type, and dissimilarities between habitat types, on a pairwise basis. Using SIMPER to assess within-group similarity defines species that are typical for each habitat type, following Clarke and Gorley (2015). Values for environmental variables at each site are provided in Table S1.
The relationship between species composition (fourth root-transformed count data) and environmental variables was assessed using the BIOENV procedure, a non-parametric method that generates Spearman rank correlations (ρ) between a biological (dis)similarity matrix and an environmental (dis)similarity matrix (Clarke and Gorley 2015). The significance of rank correlations (ρ) were determined using a global BEST test, a non-parametric version of a Mantel test (Clarke and Gorley 2015). Species abundance data as a Bray–Curtis resemblance matrix was compared with normalised environmental data as a resemblance matrix by using Euclidean distance. Prior to analysis, the correlation between environmental variables was assessed using Draftsman plots and Pearson’s r, and correlated variables (r2 > 0.7) were excluded from the BIOENV analysis. Variables excluded were inundation in 2000, pan evaporation and mean maximum temperature.
Univariate methods were used to assess the relationship between environmental variables and vegetation structure and recruitment. These analyses were performed in R (v. 3.6.1, R Foundation for Statistical Computing, Vienna, Austria, 2019). An assessment of the homogeneity of variance using Levene’s test and the normality of data using Shapiro–Wilks, as well as a visual assessment of Q-Q plots indicated that data did not meet the assumptions of ANOVA. Therefore, the non-parametric Kruskal–Wallis test with Tukey–Kramer–Nemenyi pairwise test with Bonferroni adjustment was used to assess differences among habitat types for duration of inundation, soil clay content, canopy cover, basal area and number of seedlings.
Results
Species composition
In total, 1872 individual trees greater than 1.5 m tall were recorded, encompassing 32 species. The most abundant species, with more than 200 individuals, were B. acutangula, Atalaya hemiglauca (F.Muell.) Benth, Eucalyptus microtheca F.Muell., and E. camaldulensis Dehnh. (Table S2). Species were from 13 families, with Myrtaceae being the most common (10 species) and Fabaceae and Moraceae the second-most common (five species each). Almost half the total species recorded were found in only one habitat type (15 species), and only three species were found across all habitats; E. camaldulensis, E. microtheca and Terminalia platyphylla F.Muell. (Table S2).
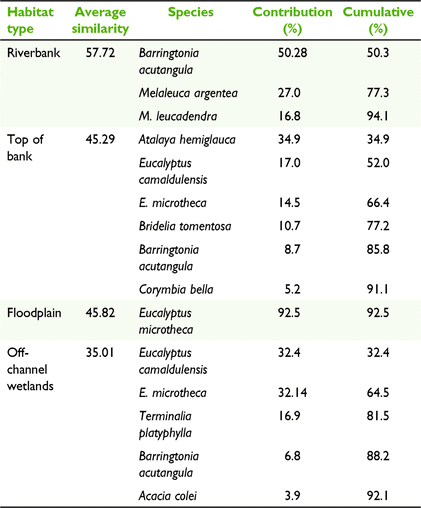
The composition of tree species differed among habitat types (Fig. 3, Table 2), with different species being associated with each habitat type (PERMANOVA, P < 0.001, unique perms >9900). The floodplain and riverbank had the greatest dissimilarity (average dissimilarity of 98.26; Table S3), indicating the largest disparity in species composition between habitat types. This difference was primarily driven by B. acutangula, E. microtheca, Melaleuca argentea W.Fitzg. and M. leucadendra (L.) L., which had a cumulative contribution of 68% of the total dissimilarity between these habitat types (Table S3). The floodplain was dominated by E. microtheca, which occurred at 82% of the sites surveyed in this habitat type (Table S2) and contributed 92.5% to the average within-group similarity of 45.8 (Table 3). In contrast, the riverbank habitat type was characterised by B. acutangula, M. argentea, and M. leucadendra, with these three species accounting for 94.1% of the average within group similarity of 57.72 (Table 3).
|
|
Top of bank was the most diverse habitat type and it took six species to describe 91.1% of the average within-group similarity of 45.29 (Table 3). A. hemiglauca, E. camaldulensis, E. microtheca and Bridelia tomentosa Blume had the greatest contribution to describing this habitat type (Table 3). Off-channel wetlands were dominated by E. microtheca and E. camaldulensis, with both species occurring at 78% of wetland sites and with a cumulative contribution of 64.5% to average similarity. T. platyphylla was also common at wetlands, occurring at 56% of sites (Table S2).
Species composition and environmental variables
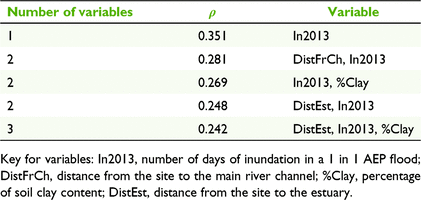
Duration of inundation (as assessed with the small flood year, 2013) was the environmental variable with the highest correlation with woody species composition, although the correlation was not strong (BIOENV, ρ = 0.351; Table 4). Riverbanks and tops of banks had the longest duration of inundation, being inundated for approximately 100 days in the large flood year (2000), and 53 and 34 days respectively in the small flood year (Kruskal–Wallis, P = 0.44; Table 1). Floodplains and off-channel wetlands had a similar duration of inundation for both flood variables, and a shorter duration of inundation compared with riverbanks and tops of banks. In the small flood year, floodplains and off-channel wetlands were inundated for an average of 6 days, or 51 and 61 days respectively during a large flood (Table 1). In the small flood (2013), 15 (of 17) floodplain sites and eight (of nine) off-channel wetlands were not inundated at all. In the large flood (2000), four floodplain and two off-channel wetlands were not inundated. Distance from the main river channel and soil clay content were also weakly correlated with woody species composition, and, combined with inundation, generated a Spearman’s rank correlation of 0.269 (Table 4).
|
The other environmental variables assessed using the BIOENV procedure were not strongly correlated with the species resemblance matrix, indicating that the distance from estuary (which was correlated with rainfall, temperature and pan evaporation) and fire frequency did not have a strong influence on woody species composition (Table 4).
Vegetation structure and recruitment
Canopy cover was greatest for the riverbank habitat type, with a mean cover of 79%, and was smallest for the floodplain at ∼28% (Kruskal–Wallis, P < 0.05). Tops of banks and off-channel wetlands had a smaller canopy cover than did riverbanks, but larger than the floodplain, with a cover of ∼65% (Fig. 4b).
Of the 1872 trees measured, 47 had a DBH greater than 100 cm, 21 of which were M. argentea. The largest tree was a M. argentea at a riverbank site, which had a DBH of 288 cm. The majority of large trees (83%) were found at riverbank sites, which had 39 of the total of 47 trees recorded with a DBH greater than 100 cm (Fig. 5). In contrast, most floodplain trees were <20 cm in DBH, with only five trees with a DBH of >50 cm. The largest tree recorded on the floodplain was E. microtheca, which measured 79 cm in DBH. Site mean DBH was greatest on the riverbanks (0.26 m2) and was smallest at the top of bank (0.04 m2) and floodplain (0.03 m2) habitat types (Fig. 4b).
The number of small seedlings (<0.5 m tall) was highly variable among replicate sites (Fig. 6a), and there was generally more variability among sites than among habitat types. The riverbank had very few small seedlings (approximately fewer than five individuals per plot). In contrast, the off-channel wetland habitat type had 53 (s.e. = 36) small seedlings; however, the large standard error indicates the patchiness of recruitment and large differences among replicate sites, i.e. a large number of small seedlings were recorded at two sites (343 at one site and 145 at another). Three top of bank sites also had a high number of small seedlings (i.e. >100) compared with the mean value of 72 for this habitat type. Large seedlings (0.5–1.5 m tall) were most common at top of bank and off-channel wetland habitat types, but again there were large standard errors for this variable (Fig. 6b).
Discussion
Identifying and describing the features of riverine habitats provides indirect evidence of the relationship between water flows and the assemblage and physical structure of riparian vegetation. In data-limited environments, this critical information is required to assist with environmental water-management decisions. In the lower Fitzroy River, the composition of woody species and the physical structure of vegetation differed among habitat types, indicating a zonation of riparian and floodplain vegetation in response to fluvial processes and water availability. The floodplain habitat type was characterised by sparsely distributed E. microtheca with a sparse canopy cover (<30%). In contrast, the riverbank habitat type had very large trees, with a dense canopy cover (∼80%) and was dominated by M. argentea, M. leucadendra and B. acutangula. Both tops of banks and off-channel wetlands represent a more intermediary environment, characterised by greater species richness and greater recruitment of seedlings. Identifying and characterising these different habitat types is useful for the management of riparian vegetation, with each corresponding to different flow needs to maintain vegetation.
Riverbanks are the direct interface between the terrestrial environment and the main river channel, and in tropical wet–dry climates they are exposed to short but significant floods with high-velocity flows and long periods of inundation. The B. acutangula and Melaleuca trees that dominate the riverbanks of the lower Fitzroy River are likely to be physiologically adapted to withstand submergence in fast-flowing water (Tran et al. 2013; Canham et al. 2021a), with evidence of significant adventitious root development by the Melaleuca species (Fig. S2). Although there are large trees along the riverbanks, there was little evidence of recruitment, with very few seedlings being found on the riverbanks. Our findings are similar to those of Pettit and Froend (2001), who observed that along the Ord River (also in the Kimberley region) large flood flows inhibited the establishment of seedlings, with recruitment being limited to riverbends where water movement is slower. The current study had similar observations, with seedlings noted on recently deposited sand on the riverbed in riverbends, although these areas were outside the study area and not formally captured in the dataset. Fluvial disturbance is the dominant force shaping the vegetation of the riverbank habitat type, with species being adapted to withstand flooding, and high-velocity flows that limit seedling recruitment.
The top of the bank habitat type is a transitional zone between the scouring flows of the main river channel and the greater floodplain. The top of the bank supported more seedling recruitment and had a greater number of woody species than did the riverbank habitat. B. acutangula was common between both the riverbank and the top of the bank, but other species that may be less tolerant to high-velocity flows, such as E. camaldulensis and E. microtheca, were more common at tops of banks. The highest occurrence of A. hemiglauca was in the top of bank habitat, but interestingly the species is found throughout northern and central Australia in a range of habitats, from rivers and creeks to savanna (Brock 2001). Our findings indicated that in the lower Fitzroy River, A. hemiglauca had a greater recruitment success on the tops of banks, which may be related to greater water availability than in the surrounding landscape. In addition to high water availability, slower-velocity flows are likely to allow greater recruitment, supporting a higher species richness than in the riverbank. A shallow alluvial water table on the top of the banks may also support species with higher water-use requirements (Canham et al. 2021b). Top of the bank vegetation may be susceptible to changes in flow that reduce overbank flooding, owing to a reduction in inundation and reduced recharge of the alluvial aquifer.
The extensive floodplain of the lower Fitzroy River was dominated by sparsely distributed E. microtheca, with small trees and limited canopy cover. Although there have been few studies of this species in the Kimberley, evidence from south-eastern Australia indicates that the closely related Eucalyptus coolabah requires a sequence of flood years for seedling establishment (Roberts and Marston 2011). Floods facilitate recruitment of seedlings on the floodplain, moving seed across the landscape and providing high soil moisture, which can promote the establishment of riparian and floodplain species, which may not otherwise be possible in this semi-arid environment (Good et al. 2017). The long periods of limited water availability punctuated by periods of inundation provide suitable conditions for the establishment and survival of E. microtheca, which dominate this habitat type.
Wetlands occurring within the distributary channels and low-lying areas of the floodplain form an important part of the landscape of the lower Fitzroy River, providing locations of greater water availability than in the adjacent dry floodplain. Off-channel wetlands supported a similar species richness as that found for the top of the banks, with similar species being present, including E. camaldulensis and T. platyphylla. Vegetation structure also differed from the surrounding floodplain environment, with greater canopy cover and larger trees. These areas of denser vegetation may provide habitat for terrestrial fauna, such as bats (Blakey et al. 2017) or reptiles and amphibians (Semlitsch and Bodie 2003) in a large floodplain, many kilometres away from the main river channel. The higher water availability of wetlands supports recruitment of woody species, with evidence of seedlings in this habitat type. There is currently limited data available on the ecology of off-channel wetlands in the Fitzroy River; however, they are likely to be vulnerable to hydrological changes that disconnect them from the main river channel. Also, although not a focus of the current study, vegetation along riverbanks and wetlands is threatened by grazing and trampling, as cattle congregate around water sources (Pusey and Kath 2015). Future research is encouraged to assess the impact of grazing pressure and to determine appropriate management of livestock in the riparian zone.
Hydrological changes that alter riparian and floodplain vegetation may also affect cultural values and should be taken into consideration as part of water-planning processes (Douglas et al. 2019). The riparian vegetation of the Fitzroy River has significant cultural values for the indigenous people of the lower Fitzroy River. Indigenous knowledge has described the zonation of riparian vegetation, recognising the importance of flows for plant species associated with freshwater places. Milgin et al. (2019) used the phrase ‘wila (freshwater) jakoo (belong to) baloo (plants)’ to describe the plant species that belong to freshwater places in the Nyikina language. These plants are distinct from birrajakoo baloo, or plants that belong to the country, which refers to all plants on country, including both savanna and freshwater species (Milgin et al. 2019). In the current study, the description of riparian and floodplain vegetation as being in zones, or habitat types, has similarities to traditional ecological knowledge, and drawing on parallels between the two knowledge systems can assist with the sharing of knowledge and the management of riparian and floodplain vegetation.
Conclusions
Identifying and characterising habitat types within a large floodplain river system, such as the lower Fitzroy River, is a critical early step in providing a framework to water managers. In the data-poor system of the Fitzroy River, we have identified the common woody species and where in the landscape they are likely to be found. We have also described differences in the recruitment of woody species and the physical structure of vegetation according to habitat type. Water managers are encouraged to consider how changes to the flow regime may affect landscape units differentially, so as to help protect riparian and floodplain vegetation across the river and floodplain landscape.
Supplementary material
Supplementary material is available online.
Data availability
Data are available through the University of Western Australia’s research repository (https://research-repository.uwa.edu.au/en/datasets/).
Conflicts of interest
No conflicts of interest has been declared by the authors.
Declaration of funding
This project was supported through funding from the Australian Government’s National Environmental Science Program through the Northern Australia Environmental Resources Hub.
Acknowledgements
We gratefully acknowledge the Walalakoo, Yanunijarra and Gooniyandi Prescribed Bodies Corporate for providing us with permission to undertake research on country. We thank Traditional Owners and Indigenous rangers for accompanying us on country, sharing knowledge and assisting with surveys. We also thank pastoral managers of Myroodah, Mount Anderson, Jubilee Downs, Millijidee and Gogo Stations for access to study sites. We acknowledge the Kimberley Land Council, particularly Karen Dayman, for project facilitation. We thank staff at the Government of Western Australia’s Department of Water and Environmental Regulation for providing advice on hydrological data and water-take scenarios and the Commonwealth Scientific and Industrial Research Organisation for running the MIKE21 model and making outputs available. We thank Anna Pintor for providing fire-history data. Thanks go to the Department of Biodiversity, Conservation and Attractions and the Department of Primary Industries and Regional Development, the Western Australian herbarium and to Kevin Kenneally for assistance with plant identification.
References
Albert JS, Destouni G, Duke-Sylvester SM, Magurran AE, Oberdorff T, Reis RE, Winemiller KO, Ripple WJ (2021) Scientists’ warning to humanity on the freshwater biodiversity crisis. Ambio 50, 85–94.| Scientists’ warning to humanity on the freshwater biodiversity crisis.Crossref | GoogleScholarGoogle Scholar |
Anderson MJ, Gorley RN, Clarke KR (2008) ‘Permanova+ for Primer: guide to software and statistical methods.’ (Primer-E Ltd: Plymouth, UK)
Bejarano DM, Nilsson C, González Del Tánago M, Marchamalo M (2011) Responses of riparian trees and shrubs to flow regulation along a boreal stream in northern Sweden. Freshwater Biology 56, 853–866.
| Responses of riparian trees and shrubs to flow regulation along a boreal stream in northern Sweden.Crossref | GoogleScholarGoogle Scholar |
Blakey RV, Kingsford RT, Law BS, Stoklosa J (2017) Floodplain habitat is disproportionately important for bats in a large river basin. Biological Conservation 215, 1–10.
| Floodplain habitat is disproportionately important for bats in a large river basin.Crossref | GoogleScholarGoogle Scholar |
Brock J (2001) ‘Native plants of Northern Australia.’ (Reed New Holland: Sydney, NSW, Australia)
Canham CA, Beesley LS, Gwinn DC, Douglas MM, Setterfield SA, Freestone FL, Pusey BJ, Loomes RC (2021a) Predicting the occurrence of riparian woody species to inform environmental water policies in an Australian tropical river. Freshwater Biology 66, 2251–2263.
| Predicting the occurrence of riparian woody species to inform environmental water policies in an Australian tropical river.Crossref | GoogleScholarGoogle Scholar |
Canham CA, Duvert C, Beesley LS, Douglas MM, Setterfield SA, Freestone FL, Clohessy S, Loomes RC (2021b) The use of regional and alluvial groundwater by riparian trees in the wet–dry tropics of northern Australia. Hydrological Processes 35, e14180
| The use of regional and alluvial groundwater by riparian trees in the wet–dry tropics of northern Australia.Crossref | GoogleScholarGoogle Scholar |
Capon S, James C, George A (2016) Riverine trees and shrubs. In ‘Vegetation of Australian riverine landscapes’. (Eds S Capon, C James, M Reid) pp. 119–141. (CSIRO Publishing: Melbourne, Vic., Australia)
Casanova MT, Brock MA (2000) How do depth, duration and frequency of flooding influence the establishment of wetland plant communities? Plant Ecology 147, 237–250.
| How do depth, duration and frequency of flooding influence the establishment of wetland plant communities?Crossref | GoogleScholarGoogle Scholar |
Clarke KR, Gorley RN (2015) ‘PRIMER v7: user manual/tutorial.’ (PRIMER-E Ltd: Devon, UK)
Davidson NC (2014) How much wetland has the world lost? Long-term and recent trends in global wetland area. Marine and Freshwater Research 65, 934–941.
| How much wetland has the world lost? Long-term and recent trends in global wetland area.Crossref | GoogleScholarGoogle Scholar |
Deane DC, Nicol JM, Gehrig SL, Harding C, Aldridge KT, Goodman AM, Brookes JD (2017) Hydrological-niche models predict water plant functional group distributions in diverse wetland types. Ecological Applications 27, 1351–1364.
| Hydrological-niche models predict water plant functional group distributions in diverse wetland types.Crossref | GoogleScholarGoogle Scholar |
DIIS (2015) Our north, our future: white paper on developing northern Australia. (Department of Industry, Innovation and Science, Commonwealth Government of Australia: Canberra, ACT, Australia)
Dixon DJ (2007) Ficus carpentariensis – a new sandpaper fig for northern Australia and a revision of the F. opposita complex (Moraceae: Ficus subg. Ficus sect. Sycidium inrormal group F. copiosa). Nuytsia 16, 269–284.
Douglas MM, Bunn SE, Davies PM (2005) River and wetland food webs in Australia’s wet–dry tropics: general principles and implications for management. Marine and Freshwater Research 56, 329–342.
| River and wetland food webs in Australia’s wet–dry tropics: general principles and implications for management.Crossref | GoogleScholarGoogle Scholar |
Douglas MM, Jackson S, Canham CA, Laborde S, Beesley L, Kennard MJ, Pusey BJ, Loomes R, Setterfield SA (2019) Conceptualizing hydro–socio–ecological relationships to enable more integrated and inclusive water allocation planning. One Earth 1, 361–373.
| Conceptualizing hydro–socio–ecological relationships to enable more integrated and inclusive water allocation planning.Crossref | GoogleScholarGoogle Scholar |
Dudgeon D, Arthington AH, Gessner MO, et al. (2006) Freshwater biodiversity: importance, threats, status and conservation challenges. Biological Reviews 81, 163–182.
| Freshwater biodiversity: importance, threats, status and conservation challenges.Crossref | GoogleScholarGoogle Scholar |
EPBC Act (1999) Environment Protection and Biodiversity Conservation Act (EPBC) 1999. Available at https://www.legislation.gov.au/Details/C2016C00777
Flitcroft R, Cooperman MS, Harrison IJ, Juffe-Bignoli D, Boon PJ (2019) Theory and practice to conserve freshwater biodiversity in the Anthropocene. Aquatic Conservation: Marine and Freshwater Ecosystems 29, 1013–1021.
| Theory and practice to conserve freshwater biodiversity in the Anthropocene.Crossref | GoogleScholarGoogle Scholar |
Good M, Smith R, Pettit N (2017) Forest and woodlands of Australia’s rivers and floodplains. In ‘Australian vegetation’. 3rd edn. (Ed. DA Keith) pp. 516–543. (Cambridge University Press: Cambridge, UK)
Greet J, Fischer S, Walsh CJ, Sammonds MJ, Catford JA (2022) Restored river-floodplain connectivity promotes riparian tree maintenance and recruitment. Forest Ecology and Management 506, 119952
| Restored river-floodplain connectivity promotes riparian tree maintenance and recruitment.Crossref | GoogleScholarGoogle Scholar |
Grill G, Lehner B, Thieme M, et al. (2019) Mapping the world’s free-flowing rivers. Nature 569, 215–221.
| Mapping the world’s free-flowing rivers.Crossref | GoogleScholarGoogle Scholar |
Hughes J, Yang A, Wang B, Marvanek S, Carlin L, Seo L, Petheram C, Vaze J (2017) Calibration of river system and landscape models for the Fitzroy, Darwin and Mitchell catchments. A technical report to the Australian Government from the CSIRO Northern Australia Water Resource Assessment, part of the National Water Infrastructure Development Fund: Water Resource Assessments. CSIRO.
Jackson S, Finn M, Featherston P (2012) Aquatic resource use by indigenous Australians in two tropical river catchments: the Fitzroy River and Daly River. Human Ecology 40, 893–908.
| Aquatic resource use by indigenous Australians in two tropical river catchments: the Fitzroy River and Daly River.Crossref | GoogleScholarGoogle Scholar |
Jansson R, Ström L, Nilsson C (2019) Smaller future floods imply less habitat for riparian plants along a boreal river. Ecological Applications 29, e01977
| Smaller future floods imply less habitat for riparian plants along a boreal river.Crossref | GoogleScholarGoogle Scholar |
Karim F, Peña-Arancibia J, Ticehurst C, et al. (2018) Floodplain inundation mapping and modelling for the Fitzroy River, Darwin and Mitchell catchments. A technical report to the Australian Government from the CSIRO Northern Australia Water Resource Assessment, part of the National Water Infrastructure Development Fund: Water Resource Assessments. CSIRO Land and Water.
McDonald RC, Isbell RF, Speight JG, Walker J, Hopkins MS (1998) ‘Australian soil and land survey field handbook.’ (Australian Collaborative Land Evaluation Program, Canberra: Canberra, ACT, Australia)
Merritt DM, Scott ML, LeRoy Poff N, Auble GT, Lytle DA (2010) Theory, methods and tools for determining environmental flows for riparian vegetation: riparian vegetation-flow response guilds. Freshwater Biology 55, 206–225.
| Theory, methods and tools for determining environmental flows for riparian vegetation: riparian vegetation-flow response guilds.Crossref | GoogleScholarGoogle Scholar |
Milgin A, Nardea L, Grey H, Laborde S, Jackson S (2019) Birr inmany jada Warloongaryi nganka Woonyoomboo-ni (The source of life, story and Law in Nyikina Country, as shared by Woonyoomboo). Walalakoo Aboriginal Corporation in partnership with the National Environmental Science Program. Available at https://nesplandscapes.edu.au/publications/nyikina-seasonal-calendar/
Naiman RJ, Décamps H (1997) The ecology of interfaces: riparian zones. Annual Review of Ecology and Systematics 28, 621–658.
| The ecology of interfaces: riparian zones.Crossref | GoogleScholarGoogle Scholar |
Petheram C, Bruce C, Chilcott C, Tetreault Campbell S, Watson I (2018) Chapter 1: preamble. Water resource assessment for the Fitzroy catchment. A report to the Australian Government from the CSIRO Northern Australia Water Resource Assessment, part of the National Water Infrastructure Development Fund: Water Resource Assessments. CSIRO. pp. 1–15. https://doi.org/
| Crossref |
Pettit NE, Froend RH (2001) Variability in flood disturbance and the impact on riparian tree recruitment in two contrasting river systems. Wetlands Ecology and Management 9, 13–25.
| Variability in flood disturbance and the impact on riparian tree recruitment in two contrasting river systems.Crossref | GoogleScholarGoogle Scholar |
Pintor A, Kennard M, Álvarez-Romero JG, Hernandez S (2019) ‘Prioritising threatened species and threatening processes across northern Australia: user guide for data.’ (James Cook University: Townsville, Qld, Australia)
Poelina A, Taylor KS, Perdrisat I (2019) Martuwarra Fitzroy River Council: an Indigenous cultural approach to collaborative water governance. Australasian Journal of Environmental Management 26, 236–254.
| Martuwarra Fitzroy River Council: an Indigenous cultural approach to collaborative water governance.Crossref | GoogleScholarGoogle Scholar |
Poff NL, Zimmerman JKH (2010) Ecological responses to altered flow regimes: a literature review to inform the science and management of environmental flows. Freshwater Biology 55, 194–205.
| Ecological responses to altered flow regimes: a literature review to inform the science and management of environmental flows.Crossref | GoogleScholarGoogle Scholar |
Pusey BJ, Kath J (2015) ‘Environmental water management in the Fitzroy River Valley: information availability, knowledge gaps and research needs.’ (The University of Western Australia and the Western Australian Department of Water: WA, Australia)
Riis T, Kelly-Quinn M, Aguiar FC, et al. (2020) Global overview of ecosystem services provided by riparian vegetation. BioScience 70, 501–514.
| Global overview of ecosystem services provided by riparian vegetation.Crossref | GoogleScholarGoogle Scholar |
Roberts J, Marston F (2011) ‘Water regime for wetland and floodplain plants. A source book for the Murray Darling basin.’ (National Water Commission: Canberra, ACT, Australia)
Semlitsch RD, Bodie JR (2003) Biological criteria for buffer zones around wetlands and riparian habitats for amphibians and reptiles. Conservation Biology 17, 1219–1228.
| Biological criteria for buffer zones around wetlands and riparian habitats for amphibians and reptiles.Crossref | GoogleScholarGoogle Scholar |
Skroblin A, Legge S (2010) The distribution and status of the western subspecies of the Purple-crowned Fairy-wren (Malurus coronatus coronatus). Emu - Austral Ornithology 110, 339–347.
| The distribution and status of the western subspecies of the Purple-crowned Fairy-wren (Malurus coronatus coronatus).Crossref | GoogleScholarGoogle Scholar |
Stromberg JC, Merritt DM (2016) Riparian plant guilds of ephemeral, intermittent and perennial rivers. Freshwater Biology 61, 1259–1275.
| Riparian plant guilds of ephemeral, intermittent and perennial rivers.Crossref | GoogleScholarGoogle Scholar |
Taylor CFH (1998) Geomorphology of the Fitzroy River In ‘Limnology of the Fitzroy River, Western Australia: a technical workshop’. (Eds A Storey, L Beesley) (Edith Cowan University: WA, Australia)
Tockner K, Stanford JA (2002) Riverine flood plains: present state and future trends. Environmental Conservation 29, 308–330.
| Riverine flood plains: present state and future trends.Crossref | GoogleScholarGoogle Scholar |
Tran DB, Dargusch P, Moss P, Hoang TV (2013) An assessment of potential responses of Melaleuca genus to global climate change. Mitigation and Adaptation Strategies for Global Change 18, 851–867.
| An assessment of potential responses of Melaleuca genus to global climate change.Crossref | GoogleScholarGoogle Scholar |
Wheeler JR, Rye BL, Koch BL, Wilson AJG (1992) ‘Flora of the Kimberley region.’ (Western Australian Herbarium, Department of Conservation and Land Management: Perth, WA, Australia)
White JM, Stromberg JC (2011) Resilience, restoration, and riparian ecosystems: case study of a dryland, urban river. Restoration Ecology 19, 101–111.
| Resilience, restoration, and riparian ecosystems: case study of a dryland, urban river.Crossref | GoogleScholarGoogle Scholar |
§ Co-first authors



