Multiple lines of evidence infer centurial-scale habitat change and resilience in a threatened plant species at Mount Dangar, Hunter Valley, New South Wales
Stephen A. J. Bell A * , Phil Lamrock B , Heather A. Haines
A * , Phil Lamrock B , Heather A. Haines  C and Chris Turney C D E
C and Chris Turney C D E
A Conservation Science Research Group, School of Environmental and Life Sciences, University of Newcastle, University Drive, Callaghan, NSW 2308, Australia.
B Cormal Environmental, Mudgee, NSW 2850, Australia.
C School of Biological, Earth and Environmental Sciences, University of New South Wales, Sydney, NSW, Australia.
D Chronos 14Carbon-Cycle Facility, Mark Wainwright Analytical Centre, University of New South Wales, Sydney, NSW, Australia.
E Present address: Division of Research, University of Technology Sydney, Ultimo, NSW, Australia.
Australian Journal of Botany 70(6) 432-446 https://doi.org/10.1071/BT22036
Submitted: 1 April 2022 Accepted: 26 August 2022 Published: 28 September 2022
© 2022 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Context: Populations of the threatened plant Acacia dangarensis at Mount Dangar (Hunter Valley, New South Wales) may best be managed by recognising centurial, rather than decadal, change in habitat.
Aim: Multiple data sources have been used to explore the hypothesis that above-ground presence of A. dangarensis is driven by centurial-scale cycles in climate (wet–dry phases) and fire.
Methods: Current-day floristic composition is contrasted with that documented by pre- and post-1900 botanical explorers for A. dangarensis and the fire-sensitive Callitris glaucophylla. Examination of fire history, oral recollections, rainfall and specimen collection databases, and radiocarbon (14C) and dendrochronological analyses of A. dangarensis have been used to build an ecological history of Mount Dangar.
Key results: There is no evidence of A. dangarensis occurring on Mount Dangar between 1825 (the first documented exploration) and 1979 (the first collection). Furthermore, historical wet–dry cycles where sufficient fuel was likely to have accumulated to propagate fire (required for seed germination) infer that the species may have last germinated from the seed bank c. 1730, but senesced prior to 1825. Our results suggest that a major fire during the extremely dry Austral summer of 1957–1958 killed most of the then dominant C. glaucophylla individuals. This fire followed 7–10 years of well above-average rainfall, allowing sufficient fuels to accumulate for fire to heat the soil and again release Acacia seed from dormancy.
Conclusions: Long-term resilience in A. dangarensis is highlighted irrespective of fire irregularity and recurrent drought that have occurred over at least the past 195 years.
Implications: Centurial-scale cycles in climate and fire appear to drive above-ground presence in this species. When present, occasional fruiting events may be sufficient to maintain the seed bank until suitable climatic conditions again favour a major wildfire event and subsequent seedling recruitment.
Keywords: Acacia, centurial-scale habitat change, climate, dendrochronology, fire, radiocarbon dating, range-restricted endemic, resilience, threatened plants.
Introduction
Management of threatened species occasionally requires the initiation of actions and interventions before a complete understanding of life history is gained, and this precautionary approach may avert species extinction (Scheele et al. 2018). In some cases, prompt intervention is warranted (e.g. Mackenzie and Keith 2009; Cuneo et al. 2018), whereas in others, the magnitude of the ecological knowledge gap may be too great to justify action. For many taxa, there is little knowledge available to inform how, for example, intervention in germination triggers or pollinator networks may benefit the species (Williams 2006; Silcock et al. 2015; Scheele et al. 2018), or whether modification to habitat is required to improve above-ground presence and dispersal mechanisms (Török et al. 2020). But for species where available life-history data span only a fraction of their expected life span (which may be 100 years or more), there is a need for caution before implementing management practices. Many plant species operate on long cycles of boom or bust, relying on rare environmental events (such as fire, floods or high rainfall years) to trigger episodic germination (Kenny et al. 2017; Zimmer et al. 2017; Elliott et al. 2019; Miller et al. 2019). Long periods of dormancy are typical in these species, often involving persistent seed banks held in the canopy or the soil for decades or centuries (Long et al. 2015). Species occurring in landscapes that rarely experience appropriate germination triggers can subsequently be the subject of inappropriate management decisions (Scheele et al. 2018), which may be unnecessary and compromise the allocation of scant conservation resources (Burgman 2002; Bateman et al. 2017).
Improved understanding of how major environmental disturbance events interact with a species’ life history will ultimately benefit its conservation (Scheele et al. 2018; Elliott et al. 2019). Environmental drivers for ecological change, such as the incidence and severity of fire and drought and the variable time scales over which they may operate, can dictate extinction and new recruitment in plant populations (Keith 1996; Zhang et al. 2019). Fire is a well researched driver of ecosystem regulation and rejuvenation (McLauchlan et al. 2020), and when infrequent fire events are coupled with stresses imposed under drought conditions, plant species may disappear from the above-ground flora and habitats may be substantially modified (e.g. Tozer et al. 2021). Conversely, with the appropriate environmental triggers, new generations of these species may reappear after seeds are released from dormancy, often in areas from which they were previously unknown (e.g. Bell and Holzinger 2015), and there are numerous examples in the literature where species have re-appeared after long absences following a major fire event (e.g. Miles and Cameron 2007; Cheal 2010; Binns 2013). Some authors also stress that persistence of rare species in certain habitats is entirely dependent on the occurrence of these rare disturbance events (e.g. Elliott et al. 2019; Miller et al. 2019); yet, such a phenomenon is rarely acknowledged in the context of threatened species management.
For one site in the Hunter Valley region of New South Wales, we suspect that the interplay of centurial-scale fire, drought and rainfall is a major driver of ecological change in the above-ground presence of common and rare plant species. Mount Dangar, lying near the confluence of the Hunter and Goulburn rivers, is a dominant feature in the regional landscape. Like other peaks of unusual geology supporting rare species (e.g. Mount Buffalo in Victoria: Calder and Calder 1998; Walsh 1998; banded iron formations in Western Australia: Miller et al. 2019), several range-restricted plant species occur on or in the immediate vicinity of Mount Dangar. In recent years, the management of each of these has become a focus of the New South Wales (NSW) Government through their Saving our Species program, including the critically endangered and range-restricted endemic tree Acacia dangarensis. Although contemporary survey data have shown little changes in the distribution and abundance of this species over the short term (S. Bell and P. Lamrock, unpubl. data), gaining a more comprehensive understanding of the ecology of this long-lived species requires examination of data from multiple sources across longer time frames to guide future management.
This paper uses written (explorer and surveyor journals) and oral (local residents) evidence, supported by herbarium, radiocarbon (14C) and dendrochronological analyses, to assess population fluctuations of A. dangarensis because they relate to historical fire disturbance and climatic variability affecting Mount Dangar over the past 200 years. We acknowledge at the outset that combining conventional and unconventional data sources such as these may result in intuitive rather than analytical findings; however, this is often necessary to further understanding of plant species and ecosystem changes operating over century-scale life cycles (Lunt 2002, and other papers in this volume; Caseldine and Turney 2010; Hédl et al. 2021). On the basis of our findings, the current-day population of A. dangarensis is placed into ecological context, and we provide guidance for future management of this species on Mount Dangar, and potentially also for other long-lived species that occur in similar landforms or in areas with similar disturbance regimes.
Study area
Mount Dangar is a unique natural feature within Goulburn River National Park, located in the upper Hunter Valley of New South Wales, 150 km north-west of the major regional town of Newcastle (Fig. 1). It comprises a basalt peak rising to 673-m elevation above sea level, 340 m above the surrounding landscape otherwise dominated by sandstone hills and ridges of the Triassic Narrabeen series. Present-day vegetation on Mount Dangar is determined primarily by aspect and soil depth, and fire history. It is dominated on deeper basalt soils by A. dangarensis on southern and eastern slopes, together with less abundant Eucalyptus ‘albemol’ (a purported hybrid between E. albens and E. moluccana: McRae and Cooper 1985), Callitris glaucophylla, Ficus rubiginosa and Brachychiton populneus. The mid-storey is dominated by Notelaea microcarpa, but few other shrub species are evident. Ground-layer vegetation is herbaceous and grassy in nature, varying widely in response to local rainfall conditions. Common taxa include Nyssanthes diffusa, Solanum brownii, Rytidosperma racemosum var. racemosum, Paspalidium criniforme, Einadia hastata, Einadia nutans, Clematicissus opaca, Dysphania carinata and Euphorbia planiticola. During times of drought, such as occurred in 2017–2019, almost all of the ground-layer vegetation dies back or is consumed by herbivores. The dense stands of A. dangarensis on sheltered slopes are even aged (S. Bell and P. Lamrock, unpubl. data) and often occur to the exclusion of all other tree species, consistent with major episodic germination events of several other Acacia species (e.g. Hunter 2005; Prober et al. 2007; T. Tame, unpubl. data, 1981).
|
|
The much drier and rockier northern basalt slope supports vine thicket vegetation dominated by N. microcarpa with Alectryon oleifolius, Pandorea pandorana, Clematicissus opaca, Cayratia clematidea, Cynanchum viminale subsp. australe and Adiantum aethiopicum (floristic group 1 of Curran et al. 2008). Steep basalt scree slopes associated with this habitat occasionally support stands of Senecio linearifolius var. dangarensis and Abutilon tubulosum. Surrounding sandstone landscapes carry a range of species typical of such habitats (L. Hill, unpubl. data, 1999), most of which are rarely, if ever, present on the basalt. These include the trees Eucalyptus caleyi subsp. caleyi, Eucalyptus nubila, Corymbia trachyphloia subsp. amphistomatica, Eucalyptus dawsonii, Eucalyptus punctata, Eucalyptus crebra, Acacia doratoxylon, Acacia crassa subsp. crassa and Eucalyptus sparsifolia. The fire sensitive Callitris endlicheri is a common component of the slopes and ridges across Goulburn River National Park, alluding to the scarcity of major high-intensity fire in these landscapes.
The low elevation of the Great Dividing Range in the Hunter Valley facilitates the presence of more western elements in the vegetation (Di Virgilio et al. 2012), which in combination with a dry climate infers fire patterns more typical of western lands (i.e. rare high-severity fires and long inter-fire intervals). Fire ignitions leading to major fire events are, consequently, rare in Goulburn River National Park, and since records began in 1983, over 66% of the reserve has remained unburnt (Lamrock 2017). Lightning strikes are the main ignition sources, although relative to the nearby Wollemi National Park, these occur at a lower frequency. Low-rainfall conditions contribute to relative slow plant growth, and, hence, considerable time is required to develop sufficient fuel structure to promote large wildfires. On average, large wildfires in the reserve occur at intervals of ∼20 years (L. Menke, pers. comm.).
Materials and methods
Fire history
Complete post-settlement histories of fire events in any one region are rarely available, and, hence, for this study multiple sources were examined. Fire records held by the NSW National Parks and Wildlife Service (NPWS) for Mount Dangar (1979–1980 to the present) were reviewed, and Landsat imagery (https://landsatlook.usgs.gov/viewer.html; July 1972–October 1992) was examined to search for fire scarring of the vegetation, as has been done elsewhere (e.g. Milne 1986; Callister et al. 2016). A review of historical reports in local newspapers and other written sources (https://trove.nla.gov.au/newspaper/; search terms ‘fire’, ‘wildfire’, ‘Mt Dangar’, ‘Mount Dangar’ and nearby local settlements ‘Gungal’, ‘Denman’, ‘Sandy Hollow’), and oral recollections from long-time residents of the area were also undertaken.
To provide a physical on-ground indication of the last fire-induced recruitment event of A. dangarensis that might corroborate written and oral evidence, dating of senesced individuals was undertaken using radiocarbon (14C) and dendrochronological techniques. Two individuals, one from each of the southern and northern faces of Mount Dangar and representative of the size class of the majority of the population, were analysed for radiocarbon at the Chronos 14Carbon-Cycle facility at UNSW (four samples taken from each tree). These eight samples were chemically pre-treated following the base–acid–base–acid-bleaching (BABAB) methods outlined in Turney et al. (2021), modified to include three initial acetone washes of 30 min each at 55°C and the first base step performed as 30 min base washes at 75°C, rather than one single base wash left at room temperature overnight. These samples were heavily resinous; so, seven base washes were performed during this first base step to ensure all mobile resin material had been removed from the wood. The eight radiocarbon samples were then graphited, measured and analysed as per Turney et al. (2021). Additionally, dendrochronological analysis was undertaken in conjunction with radiocarbon dating, following the multi-technique approach to tree dating discussed in Haines et al. (2018). The two trees were sampled at multiple heights along the trunk with cross-section discs collected. For each cross-section, ring counting was conducted and ring structures analysed and compared across discs within each tree.
Rainfall and drought
Bureau of Meteorology (2022a, 2022b) climate data were examined to provide an outline of recurrent drought and rainfall patterns from the late 1800s until the present, to identify climatic cycles that may promote fire events. Few weather stations were recording data prior to 1900; hence, long-term patterns in rainfall variability and drought can be identified only by reference to documented trends. The Denman (Palace Street) weather station (Station number 61016; 105-m elevation; ∼20 km east-south-east of Mount Dangar) provided a useful proxy in the absence of more reliable data on rainfall at Mount Dangar; it has a near-continuous rainfall record of 131 years between January 1883 and September 2014 (October 2003 the only data gap). For the period October 2014 to December 2019, the Baerami (Old Dairy) weather station (Station number 61423; 205-m elevation; ∼5 km south of Mount Dangar) has been added into this dataset to provide a continuous 136-year record. To examine the interplay of climate and fire, ‘dry’ periods were defined as ≥3 consecutive years of below-average rainfall, and ‘wet’ periods were explored through analysis of moving average annual rainfall measured over consecutive intervals of 5–20 years. Identified wet periods were considered representative of the likely accumulation of plant growth material that might be sufficient to carry major fire.
Botanical explorers
Writings of early botanical explorers (pre-1900) are increasingly being called on to inform contemporary land management and re-create understandings of the vegetation prior to European settlement (e.g. Bowman and Panton 1993; Franco and Morgan 2007; Silcock et al. 2013; Green et al. 2020). To this end, a search of the literature was undertaken to review the ecological observations of explorers who traversed Mount Dangar. Post-1900, visitation by other more contemporary workers increased as European settlement expanded, although, unlike for the earlier explorers, few recorded their observations in any systematic way. As a proxy, examination of the plant collections contained within the Australia’s Virtual Herbarium database (AVH, see https://avh.chah.org.au/) was undertaken to glean information on post-1900 explorers, their collected specimens and habitat notes.
Results
Fire history
A review of NPWS fire records showed that no fires have occurred on Mount Dangar over the past 42 years, and although not all images were of a sufficient clarity to be certain, we could find no evidence of wildfire over the 1972–1992 period in Landsat imagery. Combined, data from these two modern-day sources imply that no fires have occurred across Mount Dangar over at least the past 49 years, since 1972. Historical written sources returned very few hints of wildfire prior to 1972. The Maitland Daily Mercury briefly reported on Tuesday 23 January 1906 (p. 3) that ‘a large bush fire in the vicinity of Mount Dangar, near Denman, was raging on Saturday night, and it is to be hoped did little damage to the holdings of settlers in the locality’. Meyer (1987) refers to a major bushfire that occurred in the Worondi and Gungal districts during the ‘New Year of 1905’, which, despite inconsistencies in year, may or may not represent this same blaze. These accounts represent the only documented evidence that Mount Dangar had potentially burnt. T. Tame (unpubl. data, 1981) briefly described a fire that began ‘in the vicinity of Sandy Hollow’ on the 2 December 1979, following 7 months of below-average rainfall, but there is no suggestion that it extended to Mount Dangar.
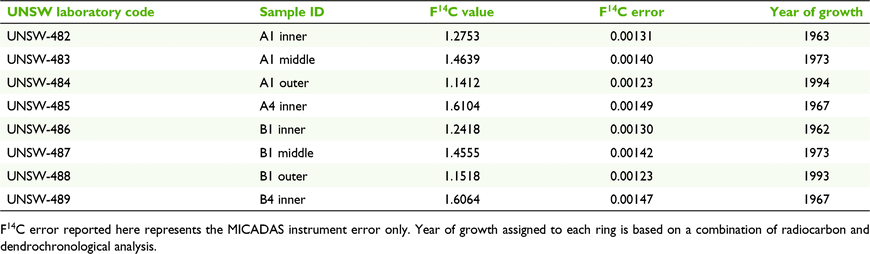
Fifth-generation resident of the settlement of Gungal, Clarise Judge, recalled a major fire event in November–December 1957 (while she was heavily pregnant) that burnt ‘everything’ between the Golden Highway at Gungal south to the Goulburn River. Mount Dangar lies within this area, and, although it cannot be definitively proven that Mount Dangar was burnt during this event, the implication is that it was. Corroborative evidence for this timeline was found in the results of 14C and dendrochronological analyses. The radiocarbon dates from all eight samples of A. dangarensis analysed are shown in Table 1, with samples collected from the lowest cross-sections (A1 and B1) as well from a cross-section mid-way up each tree (A4 and B4). For all four cross-sections, trees were sampled from the innermost ring, with additional samples taken from the lowest cross-sections from the outermost ring, as well as a ring midway between these points (Table 1, Fig. 2). Numerous false rings were noted; however, these rings did not appear around the entire circumference of the cross-sections. Additionally, true rings showed sharp boundaries throughout when viewed under magnification, but the false ring boundaries became blurred. This allowed for easy delineation between true and false rings, with the ring counts confirmed by the radiocarbon dates, and final ages assigned by combining the two methods. Comparing ring structures between the A1 and A4 samples as well the B1 and B4 samples also corroborated the ages assigned to the two trees. It was determined that Tree A presented rings from 1960 to 1996 and Tree B from 1962 to 1997. The innermost ring on Tree A and the outermost ring on Tree B are slightly different from the respective inner and outer dates given in Table 1 because of the poor quality in some rings (as seen in Fig. 2), which meant the ring sampled for radiocarbon analysis was selected a few rings away from the inner or outer ring. As the lowest cross-sections were sampled slightly off the ground and it takes time for a tree to commence growth after a fire, these results indicate that the fire of 1957–1958 was likely to be the source of seed germination for these specimens and the remainder of the population, given sampled trees were representative of the dominant size class.
|
|
|
Rainfall and drought
The Bureau of Meteorology (2022a, 2022b) reported that three major droughts have affected regional New South Wales over the past 125 years, namely, the ‘Federation Drought’ (1895–1903), the ‘World War II Drought’ (1937–1945) and the ‘Millennium Drought’ (2001–2010). These drought events have persisted for 8–9 years, and additionally several shorter intense droughts have also occurred across this period (e.g. 1914–1915, 1965–1967, 1982–1983). The most recent drought began in mid-2017 and it is equivalent to a major drought event on the long-term historical record (100 years).
For Mount Dangar, the combined long-term rainfall record at Denman (n = 131 years, 1998–2014) and Baerami (n = 5 years, 2014–2019) shows nine dry periods over the course of 136 years (Fig. 3). These periods occurred at more-or-less regular intervals but many included occasional good years (above-average rainfall). Dry periods occurred from the commencement of records in 1883–1888 (6 years, possibly longer), 1899–1907 (9 years), 1912–1923 (12 years), 1925–1929 (5 years), 1935–1944 (10 years), 1964–1967 (4 years), 1979–1983 (4 years), 2002–2006 (5 years) and 2017–2019 (3 years). Four of these dry periods coincided with the three major droughts identified as the Federation Drought, World War II Drought and Millennium Drought, but there have been several other very dry periods in the area around Mount Dangar, spanning between 3 and 12 years of duration. The very dry year of 1957 (291 mm, half of the long-term annual average) corresponds to the period recalled by Clarise Judge, and is supported by 14C and dendrochronology results, for the major fire that was likely to be burning across Mount Dangar.
Prior to ignition of fire in November 1957, the total rainfall for January to October 1957 was just 213 mm (36% of the annual average) and this was the driest January to October period for any year over 136 years in this locality (the next-lowest was 227 mm in 1918). For the 10 years preceding the very dry 1957, seven of those years were wet years with well above-average rainfall. This suggests that plant growth over the 1947–1956 period would have been prolific, leading to a build-up of fuels sufficient to carry a fire over Mount Dangar. Contemporary observations show that fuel build-up and decay happens rapidly on Mount Dangar in response to rainfall (S. Bell and P. Lamrock, unpubl. data), differing from surrounding sandstone landscapes where fuel loads are more constant. High fuel accumulation followed by rapid drying prior to decay, therefore, provides conditions more conducive to fire and detecting previous rainfall patterns that replicate this scenario may identify other potential fire opportunities.
Consequently, examination of average annual rainfall over periods of 5–20 years between 1884 and 1957 found the 8-year moving average to be the greatest immediately prior to this fire (1956), at 774 mm and 186 mm above the long-term average (588 mm). The next-highest were the 7-year (765 mm), 9-year (758 mm) and 10-year (740 mm) averages, suggesting that between 7 and 10 years of high rainfall was sufficient to accumulate the necessary fuel for the 1957 wildfire event to carry across the basalt. Plotting the 8-year moving annual average rainfall for all years to 2019 showed the highest peak in 1956, immediately preceding the very dry 1957 and fire igniting in November of that year. Fig. 3 shows that the combination of very low rainfall (<300 mm year−1) following a very wet preceding 8 years (>760 mm) has occurred only twice (1955, 1956) between 1890 and 2019, leading up to the 1957 fire event. Other wet periods have occurred, evidenced in the many peaks shown in Fig. 3, but these have not been followed by a very dry year where rainfall is less than half of the long-term annual average.
Botanical explorers
Pre-1900 explorers
Allan Cunningham, botanical explorer and collector, undertook four expeditions through the Hunter Valley between 1823 and 1827 (Lee 1925; Whitehead 2013, 2017a, 2017b). On 22 April 1825, Cunningham climbed Mount Dangar, likely becoming the first European to reach the summit, a feat possibly not repeated until R. T. Baker and W. M. Carne collected there in 1903. Cunningham’s observations and notes have provided a significant reference point on the vegetation of Mount Dangar at that time, and, with a viewing of the paths where he walked (recreated in Whitehead 2017a, 2017b), are even more valuable. Cunningham traversed north-east from his campsite on the Goulburn River to Mount Dangar summit, and then descended down its south-easterly flank back to the Goulburn River. Fig. 4 presents an interpretation of this likely route, on the basis of Whitehead (2017a) but augmented by our own experiences traversing Mount Dangar and surrounding hills, and including the current-day distribution of A. dangarensis (S. Bell and P. Lamrock, unpubl. data). On this trek, Cunningham made note of over 30 plant taxa and contrasted those occurring at lower and upper elevations (see Supplementary Table S1). He made the specific observation that there was little difference in vegetation between that occurring near the summit (on basalt) and that lower down (presumably on the sandstone ranges), stating that ‘In our ascent to the Summit of Mt. D. the vegetation differs nothing from that of the Callitris ranges below it…’ (unpublished ‘Journal of Allan Cunningham’s excursions, 1822–1832’, p. 35). Cunningham recorded only a single wattle species on that day (‘an Acacia with large falcate leaves and cylindrical spikes’), most likely to be Acacia crassa subsp. crassa (Fig. 5), which today is common on the sandstone hills; no Acacia was recorded from the summit. This is of particular interest, because current-day knowledge of the distribution of A. dangarensis on Mount Dangar shows that Cunningham walked through these areas on both his ascent and descent that day (see Fig. 4), and in 2022 this species dominates the slopes here in almost mono-specific stands (Fig. 5). Had A. dangarensis been present above-ground in 1825, it might be expected that Cunningham would have collected, or at the very least written about the presence of, this species.
|
|
Of interest among Cunningham’s other observations is a ‘Senecio sp. glaucous lanceolate leaves’ from the upper slopes, which can refer only to Senecio linearifolius var. dangarensis, a taxon that has been until recently known solely from Mount Dangar (Fig. 5). Cunningham also noted ‘Kennedia rubicunda or a new sp. rounded leaves’, which is almost certainly the vulnerable Kennedia retrorsa (Fig. 5) occurring on the sandstone slopes and gullies in the vicinity of Mount Dangar (Kennedia rubicunda is a more coastal species, not known from the mountain nor Goulburn River National Park; L. Hill, unpubl. data, 1999). The fact that Cunningham observed and noted these rare species is testament to his observational skills and ability to identify regional novelties.
Other pre-1900 explorers mention Mount Dangar in their writings but few with a botanical lean appeared to have climbed it. Ludwig Leichhardt explored much of the Hunter Valley in the 1840s, including the area around Wybong, Denman and Mount Dangar. He wrote on 18 April 1843 ‘I had found the Dodonaea pinnatifida [perhaps Dodonaea multijuga or Dodonaea boroniifolia] on similarly dry ground near Mt Dangar’ implying that he knew the country reasonably well (Darragh and Fensham 2013), but apparently did not climb it.
Post-1900 explorers
From 1900, there has been increased interest in botany and botanical exploration in the Hunter region, as in other parts of the country. An extract of all plant specimens from AVH undertaken in January 2022 for Mount Dangar and immediate surrounds shows that 28 botanists have collected there over a period of 115 years between 1903 and 2018 (see Supplementary Table S2). Several of these collectors were eminent botanists of their time (Supplementary Table S3), working in a period when there were many poorly explored regions and lodging many thousands of plant specimens in different herbaria. W. M. Carne and R. T. Baker were the first to collect in 1903, submitting just three taxa. Other collectors, such as J. L. Boorman in 1904, were fastidious observers and collected nearly 60 different taxa from the area. Boorman collected seven Acacia species (but not A. dangarensis) as well as other (now) threatened taxa, including Kennedia retrorsa, Pomaderris queenslandica and Senecio linearifolius var. dangarensis. Subsequent expeditions were undertaken by many other well-regarded botanists between 1908 and 2018 (see Table S2).
Acacia dangarensis was collected on Mount Dangar only from 1979 at its initial discovery, and it remains extant in 2022. No earlier observation records exist in the NSW Bionet Wildlife Atlas database (searched January 2022) for this species. Notes associated with the 1979 collections refer to dominant straight-stemmed trees 8–10 m in height, but trees of up to 18 m high now occur in some parts of the mountain.
Discussion
Allan Cunningham described the vegetation on Mount Dangar in 1825, with little specific observations of the dominant canopy species, yet such knowledge would have been particularly informative for threatened-species management today. However, he did observe that there was no obvious change from the Callitris-dominated sandstone ranges at lower elevations to the basalt slopes higher up. This was despite the distinction made between the two underlying geologies; ‘Mount Dangar is of a sandstone whereas its summit is a whin [=hard dark rock, basalt] of coarse fragments’. This suggests that at the time Cunningham was on Mount Dangar, much of it was characterised by Callitris. The upper slopes today support some individuals of very old C. glaucophylla (Fig. 6), and these predominantly occur on the exposed western end of the mountain but are scattered across other areas including within low forest of A. dangarensis. Drought in eastern NSW during 1823–1824 (Nicholls 1988; Fenby and Gergis 2013) and general dry conditions on the NSW central slopes between 1820 and 1828 (Freund et al. 2017) suggest that ground-layer vegetation may have been scant in 1825, which may explain the lack of distinction made by Cunningham between sandstone and basalt habitats. Our own observations on the effect of the recent 2017–2019 drought on ground vegetation at Mount Dangar would support this.
|
|
Low-stature box (Eucalyptus ‘albemol’) dominates the upper summit of Mount Dangar today, and scattered individuals occur across most slopes, yet Cunningham made little mention of these in his notes from 1825. Box eucalypts were one of the earliest recognisable trees in the first days of the colony, given their presence on the Cumberland Plain (Eucalyptus moluccana: Benson and Howell 2002) and dominance on the western slopes of NSW (e.g. numerous mentions in Cunningham’s 1823 journey from Bathurst to the Liverpool Plains; Field 1825). Even after leaving Mount Dangar and on the same journey northward in 1825, Cunningham made mention of box trees near the Liverpool Plains (Lee 1925). For the summit of Mount Dangar, Cunningham recorded only ‘a small eucalypti’ among a number of other shrubs and herbs, suggesting that eucalypts may have been insignificant there in 1825 (or perhaps he was unable to recall their identity when writing his notes that evening). The fact that he did not think it necessary to contrast the vegetation occurring on the basalt with that on the lower sandstone areas, as he did with the geology, provides strong circumstantial evidence that, in 1825, the two habitats appeared similar and were characterised by Callitris. This makes the apparent absence of A. dangarensis at that time, when it is so dominant today, particularly intriguing. We suspect that, in 1825, there was no stand-dominating A. dangarensis present on Mount Dangar, but that it lay in dormancy as seeds in the soil awaiting a fire event of sufficient intensity.
However, there remains the possibility that A. dangarensis was present above ground as scattered individuals in 1825 and was missed or overlooked by Cunningham, and that it persisted for subsequent decades in low abundance. Given our observations and analyses that the even-aged stands of trees now present on Mount Dangar germinated c. 1960, evidence for A. dangarensis existing prior to this should be found in the observations of earlier workers, but this is lacking; four separate collecting events (1903–1953; see Table S2) by eminent botanists did not report on or collect the species during this period. Prior to Cunningham’s visit, historical weather patterns suggest that conditions suitable for a fire-induced mass germination event last occurred between 1730 and 1760 (discussed later), although sporadic, intermittent germination from the seed bank may have occurred in the inter-fire interval after this period.
Fire, drought and major vegetation change
The interplay of fire and drought has shaped much of the vegetation in Australia (e.g. Sakaguchi et al. 2013; Zhang et al. 2017a, 2017b), including parts of the upper Hunter Valley (Tierney and Watson 2009; T. Tame, unpubl. data, 1981). This region receives between 500 and 800 mm of rainfall annually, and, as a consequence, most areas support an open vegetation with few areas of rainforest or wet sclerophyll forest (McRae and Cooper 1985; Peake 2006; L. Hill, unpubl. data, 1999). This suggests that wildfires are rarely contained by structural features of the vegetation, except where low-fuel basalt habitats limit fire spread under most conditions. Low rainfall and high temperatures throughout the year are unconducive to rapid plant growth, meaning that fuel build-up in these landscapes is a slow process. Irregular, but extreme, fire events, therefore present opportunities for major stand-altering disturbances to the vegetation, particularly for habitats dominated by slow growing and fire-sensitive Callitris species.
Stand-altering fire events might explain the differing habitat observed on Mount Dangar by Cunningham in 1825, compared with that present today. Denham et al. (2016), for example, documented changes to the vegetation following a major fire event in the Warrumbungle Ranges, in areas that had remained unburnt for at least 50 years. This region has a climate comparable to that in the upper Hunter Valley (rainfall 750 mm year−1, warm to hot summers and mild winters), and shares with it many plant species. Following extreme wildfire there in 2013, habitats dominated by both Eucalyptus and Callitris underwent considerable structural change, with severely burnt areas suffering nearly 100% Callitris death but eucalypts displaying considerably better survival. Any recovery of Callitris to pre-fire densities in these areas now relies on post-fire seedling emergence; however, in the interim, major habitat changes have occurred in areas where Callitris once dominated. Mature stands of C. glaucophylla are known to reduce fire potential in highly flammable environments, and fire-free intervals of at least 50 years are required to enable sufficient recovery to supress all but extreme fire events (Cohn et al. 2011). This implies considerable modification of the landscape post-fire, and recovering habitats will be shaped by subsequent climate and fire recurrence.
Fires in the Goulburn River valley are generally limited by slow plant growth and fuel accumulation, but when they do occur they tend to be major events (L. Menke, pers. comm.). The preponderance of C. endlicheri (another fire-sensitive species) across sandstone habitats of Goulburn River National Park supports the notion that major fire is rare, and historical and contemporary observations of fire events provide further evidence. For example, on two occasions Meyer (1987) discussed a major bushfire that occurred in 1905 at the foot of Mount Dangar and associated hills, but there is no indication that the mountain was affected by this fire, and observations by NPWS fire practitioners conducting hazard-reduction burns at the sandstone–basalt interface support this fire behaviour (Lamrock 2017). As evident on Fig. 3, 1905 was one of five very dry years where annual rainfall was less than half of the long-term average, but in this case the preceding 7–10-year rainfall amounts were insufficient to accumulate the fuel necessary for wildfire.
More recently, a major fire event beginning to the west of Goulburn River National Park in 2017 (the ‘Sir Ivan Doherty Fire’) ignited near Leadville and rapidly spread east. This blaze ultimately burnt through 55 324 ha of forest and grassland and was eventually brought under control only with a favourable weather change. Without this change in weather, it is feasible that this fire may have continued east right through the national park to Mount Dangar. This very dry 2017 (338 mm at Denman) was preceded by 10 years of mostly above-average rainfall approaching that seen in 1957, conducive to a major fire event. Hindsight now suggests that the Sir Ivan Doherty Fire may have been of the appropriate scale required to burn Mount Dangar, consuming the current cohort of A. dangarensis but breaking dormancy in the well stocked soil seed bank underneath.
Previous studies on the incidence of historical droughts and wet periods in Australia have provided strong corroborative evidence for the role of extreme fire and climate conditions on the ecology of Mount Dangar. The drought atlas for eastern Australia (Palmer et al. 2015) shows two major periods of excessive wet conditions over the past 500 years, one of these (1950s−1970s) corresponding to the period leading up to the postulated 1957–1958 fire event at Mount Dangar, and the second for the 1730–1760 period. A third less obvious wetter period also occurred between 1560 and 1580. These three periods in history, where several successive very wet years (in which to accumulate fuels) were followed by drought (to promulgate extreme wildfire) recur approximately over a 200-year cycle. As postulated here, high levels of plant growth promoted through the successive wet years of 1949–1956, followed by rapid curing of vegetation during the very dry 1957 created ideal conditions for a major wildfire. The devastating effects of severe short-term reductions in rainfall leading to catastrophic fire events have been noted elsewhere (e.g. the Black Thursday bushfires of 1851, Freund et al. 2017), and repetition of this pattern during earlier centuries may have similarly led to major fire events driven by wet–dry climate phases. Key canopy species including C. glaucophylla and A. dangarensis may, consequently, be operating in alternating cycles of dominance across the mountain. Under this scenario, continuing A. dangarensis senescence and fire absence may permit C. glaucophylla to re-emerge as the dominant species over the next century, with A. dangarensis dominance potentially returning during the 22nd century.
Links between wet–dry climatic cycles and major fire events have been identified in other habitats elsewhere in Australia. For vegetation dominated by Eucalyptus camaldulensis in the Murray River valley, Zhang et al. (2017b) found that fire risk was best explained by rainfall conditions occurring prior to the event. Similarly, Silcock et al. (2016) established that fire events in the mulga (Acacia aneura) lands of semi-arid Queensland were infrequent and occurred only after at least 2 years of above-average summer rainfall, arguing that fire is, consequently, feasible only a few times per century. Both of these scenarios are similar to our proposed hypothesis for Mount Dangar. In this case, average or above rainfall over a 7–10-year period is first required to enable sufficient growth and accumulation of plant material, followed by a rapid change to severe drought, to prepare fuel for burning and promulgate fire.
Implications for threatened species management
Rare episodic events shaping major vegetation change can have important implications on the management of threatened plant species (Lunt 2002; Elliott et al. 2019; Miller et al. 2019). For Mount Dangar, it appears from fire history and rainfall records and the writings of Cunningham that the nature of the mountain has changed dramatically within the past 200 years, with a major shift identified owing to wildfire in 1957–1958. We hypothesise that this fire event removed most of the existing C. glaucophylla-dominated woodland and replaced it with dense forests of Acacia dangarensis. Now listed as a critically endangered species because of inappropriate fire regimes, observed senescence and little recruitment (NSW Threatened Species Scientific Committee 2018), understanding the ecology of this species is paramount for the future management of Mount Dangar, particularly in the face of declining above-ground populations (Bell and Elliott 2013). Complicating this understanding is the presence of three other threatened species on or below the mountain (and several others in adjacent habitats). However, knowledge gained from historical collections for two of these suggests strong resilience to environmental stochasticity; both Senecio linearifolius var. dangarensis and Kennedia retrorsa, although not named as such at the time, were anecdotally noted from Mount Dangar by Cunningham in 1825, and have been recorded intermittently there ever since, despite fluctuating wet and dry periods, and the postulated 1957–1958 fire event. The third species, Lasiopetalum longistamineum, occurs in sheltered locations below the mountain and was first collected in 1904 and has also shown resilience through multiple drought periods.
For the structure-changing small tree A. dangarensis, it is telling that the first recorded collections of this taxon were not until 1979 (see Supplementary Table S4), despite the visits of Cunningham in 1825 and eight further botanists over nine visits between 1903 and 1973. Most of these botanists were keen observers and collectors and it seems unlikely that all of them overlooked the presence of what is now a community dominant tree on the southern and eastern flanks of Mount Dangar. In 1904, J. L. Boorman undertook a detailed survey of the mountain, collecting 57 different taxa, which included 7 other Acacia species, and the threatened Kennedia retrorsa, Pomaderris queenslandica and Senecio linearifolius var. dangarensis. The postulated fire event in 1957 falls between L. A. S. Johnson’s visit there in 1953 where no A. dangarensis was collected, and initial collections of the species by R. Coveny in 1979 (when plants would have been ∼17 years of age). Other collectors in the intervening period (H. Dorman in 1966, A. N. Rodd in 1966 and 1968, J. R. H. Phillips in 1968, G. L. Webster in 1973) collected predominantly from sandstone-based habitats at lower elevations and may not have fully explored A. dangarensis habitat (see Table S2).
If, as postulated, germination of the current cohort of A. dangarensis was triggered by the 1957–1958 wildfire event, trees would now be ∼60 years of age. Dendrochronology and 14C dating of two sample trees, representative of the dominant size class of the population, support this germination date. This implies the following two possible scenarios for germination of the current cohort of trees: either scarification of seed following the fire event may have triggered some germination between 1958 and 1961, these seedlings succumbing to water stress, desiccation or the intense grazing pressure that would have inevitably followed; or, no germination occurred until the very wet 1963 (>320 mm above average), at which point sufficient ground-layer vegetation had developed to ease both desiccation and grazing impacts. Several other long-lived Acacia species display similar episodic germination events in response to rare fire events. For example, populations of Acacia silvestris (Clayton-Greene and Wimbush 1988), Acacia binervia (Benson and McDougall 1996), Acacia blakei (Hunter 2005), Acacia chrysotricha (P. Richards, unpubl. data, 2011), Acacia wollarensis (Bell and Driscoll 2017) and Acacia covenyi (Tozer et al. 2021) are all thought or known to germinate post-fire and develop dominant long-lived stands maintained by fire on long fire-free cycles.
Long-term viability of soil-stored A. dangarensis seed, perhaps for up to 150 years, is central to our hypothesis of centurial habitat change at Mount Dangar. Resistance to desiccation of Acacia seed in habitats that, like Mount Dangar, rarely experience major disturbance events such as fire is evident in many species through their hard, impervious outer layers. Leino and Edqvist (2010) suggested that most Acacia following this strategy occur in dry climates, the seeds surviving in a dried state and thereby reducing the rate of desiccation. Long et al. (2015) noted that some hard-seeded species including those in the Fabaceae have reportedly persisted in soil for up to 1300 years, although these are likely to be the exception rather than the rule. They argued that persistence of any seed in the soil seed bank is dependent on its resistance to germination (absence of suitable growth conditions) or death (natural attrition), and on the prevailing conditions that are conducive to those fates. Ewart (1908) made the astute observation that hard-coated seeds such as those of Acacia distribute themselves in time rather than space. The impermeable coats of his ‘macrobiotic’ seeds (those remaining viable for 15 to >100 years), best exhibited in the Fabaceae, show no special means of dispersal, and that ‘the probable extreme duration of vitality for any known seed [of the Fabaceae] may be set between 150 and 250 years’. Research by Gilbert (1959) on community succession in Tasmanian wet sclerophyll forests extended this maximum longevity for Acacia seeds, documenting how large numbers of Acacia dealbata seedlings emerged across extensive areas following logging of mixed eucalypt forest that had not been disturbed for an estimated 350–500 years. Such observations imply that it is perfectly feasible for large numbers of A. dangarensis seed to remain viable in the dry environment of Mount Dangar for periods of 100–150 years.
However, we recognise that it is also plausible for occasional intermittent germination and persistence of A. dangarensis to have occurred without fire disturbance, leading to localised replenishment of the seed bank in some areas. For this to have played a major role in current-day distribution, it must have regularly occurred at high densities (100s of individuals) and across a widespread area (∼80–100 ha) to explain the large dominant stands of the species that are present today, and such a scenario is not reflected in historical collections from the area (no observations or collections between 1825 and the wildfire in 1957). Subsequent to release, Acacia seed is primarily dispersed by ants (Auld 1996), and as a group these vectors move seed over only short distances from the parent plant (<3 m: Gómez and Espadaler 2013). Seed therefore accumulates in the seed bank close to its parent, and for a seed bank covering 80–100 ha to develop and source the current cohort of trees, the species would have either (1) been highly prevalent and easily observed by botanists consistently over the past 200 years, or (2) undergone intermittent germination and seed-bank accumulation progressively across 80–100 ha and over several centuries to account for current day distribution. The first of these scenarios seems improbable, and the second, at the very least, confirms centurial seed longevity in this species; therefore, mass germination triggered by major fire is the most likely life strategy operating in this species.
Conclusions
Multiple lines of evidence (review of fire and climate records, dendrochronology and 14C dating, historical and contemporary writings, oral accounts, botanical collections and observations) have outlined the possible ecological history of Mount Dangar over the past 200 years. We postulate that the only major fire occurring on the mountain during this time frame was in the Austral summer of 1957–1958, which resulted in a stand-altering change to the vegetation by removing the likely dominant C. glaucophylla canopy and stimulating mass germination of A. dangarensis c. 1960. This fire occurred during an exceptionally dry year following 7–10 years of very wet conditions, a scenario that appears to repeat approximately every 200 years. Prior to this germination event, it is likely that A. dangarensis was not present in the above-ground flora in any great numbers and may have persisted only in the soil seed bank for ∼150 years (to c. 1800). The current cohort of Acacia individuals, at 60 years of age, are in a senescent phase and may persist only for another decade. However, provided that occasional flowering and fruiting of individuals continues to replenish the seed bank, there is little urgency to instigate recovery actions until perhaps 50 years after such replenishment has ceased (c. 2080). Acacia dangarensis assumes the model of a species compatible with centurial-scale disturbance events, typically in the form of extreme fire with high longevity of soil stored seed. Given the current age of individuals, the next wildfire event to affect Mount Dangar should be managed for promulgation rather than suppression, to release seed from dormancy and refresh above-ground populations. On the basis of history, such an event will occur only under extreme environmental conditions and may not eventuate for a considerable period of time.
Supplementary material
Supplementary material is available online.
Data availability
The data that support this study will be shared upon reasonable request to the corresponding author.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Declaration of funding
Data collected for this project was completed under contract to the first two authors as part of a wider project funded through the NSW Government Saving our Species initiative, supported in-kind by remaining authors.
Acknowledgements
Much of the work developed here has been accumulated through numerous visits to Mount Dangar and surrounds as part of the NSW Government’s Saving our Species initiative for threatened plant species. We are grateful for the opportunity to be involved in this work, and thank Lucas Grenadier, Lisa Menke, Greg Lowe, Berin Mackenzie, Luke Foster and Paul Hillier for assistance at various times, and pilots and crew from Park Air and Commercial Helicopters. Thanks also go to Tony Orchard for deciphering the journal writings of Allan Cunningham for his April 1825 period in the upper Hunter Valley.
References
Auld TD (1996) Ecology of the Fabaceae in the Sydney region: fire, ants and the soil seedbank. Cunninghamia 4, 531–552.Bateman PW, Pearlman P, Robertson P, Schultz B, Wardell-Johnson G (2017) Is the Biodiversity Conservation Act 2016 (WA) fit for purpose? Pacific Conservation Biology 23, 146–149.
| Is the Biodiversity Conservation Act 2016 (WA) fit for purpose?Crossref | GoogleScholarGoogle Scholar |
Bell SAJ, Driscoll C (2017) Acacia wollarensis (Fabaceae, Mimosoideae sect. Botrycephalae), a distinctive new species endemic to the Hunter Valley of New South Wales, Australia. Telopea 20, 125–136.
| Acacia wollarensis (Fabaceae, Mimosoideae sect. Botrycephalae), a distinctive new species endemic to the Hunter Valley of New South Wales, Australia.Crossref | GoogleScholarGoogle Scholar |
Bell S, Elliott M (2013) Preliminary results suggest fire is required to maintain Acacia dangarensis, a threatened single-population endemic from the Hunter Valley of NSW. Australasian Plant Conservation 22, 9–10.
Bell S, Holzinger B (2015) Wildfire reveals new populations of the endangered Commersonia rosea and Monotaxis macrophylla in northern Wollemi National Park, NSW. Australasian Plant Conservation 23, 2–4.
Benson D, Howell J (2002) Cumberland Plain Woodland ecology then and now: interpretations and implications from the work of Robert Brown and others. Cunninghamia 7, 631–650.
Benson D, McDougall L (1996) Ecology of Sydney plant species. Part 4: dicotyledon family Fabaceae. Cunninghamia 4, 553–752.
Binns D (2013) Euphrasia arguta: management of a species previously presumed extinct. Australasian Plant Conservation 21, 10–11.
Bowman DMJS, Panton WJ (1993) Decline of Callitris intratropica R. T. Baker and H. G. Smith in the Northern Territory: implications for pre- and post-European colonization fire regimes. Journal of Biogeography 20, 373–381.
| Decline of Callitris intratropica R. T. Baker and H. G. Smith in the Northern Territory: implications for pre- and post-European colonization fire regimes.Crossref | GoogleScholarGoogle Scholar |
Bureau of Meteorology (2022a) Previous droughts. Available at http://www.bom.gov.au/climate/drought/knowledge-centre/previous-droughts.shtml
Bureau of Meteorology (2022b) Climate data online. Available at http://www.bom.gov.au/climate/data/
Burgman MA (2002) Are listed threatened plant species actually at risk? Australian Journal of Botany 50, 1–13.
| Are listed threatened plant species actually at risk?Crossref | GoogleScholarGoogle Scholar |
Calder M, Calder J (1998) The scientific value of Mount Buffalo National Park. The Victorian Naturalist 115, 164–168.
Callister KE, Griffioen PA, Avitabile SC, Haslem A, Kelly LT, Kenny SA, Nimmo DG, Farnsworth LM, Taylor RS, Watson SJ, Bennett AF, Clarke MF (2016) Historical maps from modern images: using remote sensing to model and map century-long vegetation change in a fire-prone region. PLoS ONE 11, e0150808
| Historical maps from modern images: using remote sensing to model and map century-long vegetation change in a fire-prone region.Crossref | GoogleScholarGoogle Scholar |
Caseldine CJ, Turney C (2010) The bigger picture: towards integrating palaeoclimate and environmental data with a history of societal change. Journal of Quaternary Science 25, 88–93.
| The bigger picture: towards integrating palaeoclimate and environmental data with a history of societal change.Crossref | GoogleScholarGoogle Scholar |
Cheal D (2010) Velvet Thread-petal Stenopetalum velutinum rediscovery in Victoria. The Victorian Naturalist 127, 19–22.
Clayton-Greene KA, Wimbush DJ (1988) Acacia dry scrub communities in the Byadbo area of the Snowy Mountains. Cunninghamia 2, 9–24.
Cohn JS, Lunt ID, Ross KA, Bradstock RA (2011) How do slow-growing, fire-sensitive conifers survive in flammable eucalypt woodlands? Journal of Vegetation Science 22, 425–435.
| How do slow-growing, fire-sensitive conifers survive in flammable eucalypt woodlands?Crossref | GoogleScholarGoogle Scholar |
Cuneo P, Emery N, Errington G, Sherieff A (2018) Assisted run(a)way: translocation planning to secure the Bankstown Hibbertia. Australasian Plant Conservation: Journal of the Australian Network for Plant Conservation 27, 23–25.
Curran TJ, Clarke PJ, Bruhl JJ (2008) A broad typology of dry rainforests on the western slopes of New South Wales. Cunninghamia 10, 381–405.
Darragh TA, Fensham RJ (2013) The Leichhardt diaries: early travels in Australia during 1842–1844. Memoirs of the Queensland Museum: Cultural Heritage Series 7, 1–540.
Denham AJ, Vincent BE, Clarke PJ, Auld TD (2016) Responses of tree species to a severe fire indicate major structural change to Eucalyptus–Callitris forests. Plant Ecology 217, 617–629.
| Responses of tree species to a severe fire indicate major structural change to Eucalyptus–Callitris forests.Crossref | GoogleScholarGoogle Scholar |
Di Virgilio G, Laffan SW, Ebach MC (2012) Fine-scale quantification of floral and faunal breaks and their geographic correlates, with an example from south-eastern Australia. Journal of Biogeography 39, 1862–1876.
| Fine-scale quantification of floral and faunal breaks and their geographic correlates, with an example from south-eastern Australia.Crossref | GoogleScholarGoogle Scholar |
Elliott CP, Lewandrowski W, Miller BP, Barrett M, Turner SR (2019) Identifying germination opportunities for threatened plant species in episodic ecosystems by linking germination profiles with historic rainfall events. Australian Journal of Botany 67, 256–267.
| Identifying germination opportunities for threatened plant species in episodic ecosystems by linking germination profiles with historic rainfall events.Crossref | GoogleScholarGoogle Scholar |
Ewart AJ (1908) On the longevity of seeds. Proceedings of the Royal Society Victoria 21, 1–210.
Fenby C, Gergis J (2013) Rainfall variations in south-eastern Australia part 1: consolidating evidence from pre-instrumental documentary sources, 1788–1860. International Journal of Climatology 33, 2956–2972.
| Rainfall variations in south-eastern Australia part 1: consolidating evidence from pre-instrumental documentary sources, 1788–1860.Crossref | GoogleScholarGoogle Scholar |
Field B (1825) ‘Geographical memoirs on New South Wales by various hands Containing an account of the Surveyor General’s late expedition to two new ports; the discovery of Moreton Bay River, with the adventures for seven months there of two shipwrecked men; a route from Bathurst To Liverpool Plains: together with other papers on the aborigines, the geology, the botany, the timber, the astronomy, and the meteorology of New South Wales and Van Diemen’s Land.’ (John Murray: London, UK)
Franco JA, Morgan JW (2007) Using historical records, aerial photography and dendroecological methods to determine vegetation changes in a grassy woodland since European settlement. Australian Journal of Botany 55, 1–9.
| Using historical records, aerial photography and dendroecological methods to determine vegetation changes in a grassy woodland since European settlement.Crossref | GoogleScholarGoogle Scholar |
Freund M, Henley BJ, Karoly DJ, Allen KJ, Baker PJ (2017) Multi-century cool- and warm-season rainfall reconstructions for Australia’s major climatic regions. Climate of the Past 13, 1751–1770.
| Multi-century cool- and warm-season rainfall reconstructions for Australia’s major climatic regions.Crossref | GoogleScholarGoogle Scholar |
Gilbert JM (1959) Forest succession in the Florentine Valley, Tasmania. Papers and Proceedings of the Royal Society of Tasmania 93, 129–151. https://eprints.utas.edu.au/14083/
Gómez C, Espadaler X (2013) An update of the world survey of myrmecochorous dispersal distances. Ecography 36, 1193–1201.
| An update of the world survey of myrmecochorous dispersal distances.Crossref | GoogleScholarGoogle Scholar |
Green J, Mooney S, Hemmings F (2020) Reconstruction of the pre-European vegetation of Wolgan Valley, western Blue Mountains, NSW. Cunninghamia 20, 139–152.
Haines HA, Olley JM, English NB, Hua Q (2018) Anomalous ring identification in two Australian subtropical Araucariaceae species permits annual ring dating and growth-climate relationship development. Dendrochronologia 49, 16–28.
| Anomalous ring identification in two Australian subtropical Araucariaceae species permits annual ring dating and growth-climate relationship development.Crossref | GoogleScholarGoogle Scholar |
Hédl R, Cousins SAO, Decocq G, Szabó P, Wulf M (2021) The importance of history for understanding contemporary ecosystems: insights from vegetation science. Journal of Vegetation Science 32, e13048
| The importance of history for understanding contemporary ecosystems: insights from vegetation science.Crossref | GoogleScholarGoogle Scholar |
Hunter JT (2005) Floristics and distribution of Wattle Dry Sclerophyll Forests and Scrubs in north-eastern New South Wales. Cunninghamia 9, 317–323.
Keith D (1996) Fire-driven extinction of plant populations: a synthesis of theory and review of evidence from Australian vegetation. Proceedings of the Linnean Society of New South Wales 116, 37–78.
Kenny SA, Moxham C, Sutter G (2017) The response of rare floodplain plants to an environmental watering event at Hattah Lakes, Victoria. The Victorian Naturalist 134, 19–27.
Lamrock P (2017) Discussion Paper: Mt Dangar saving our species fire management of threatened species. Report to NSW National Parks and Wildlife Service, NSW, Australia.
Lee I (1925) ‘Early Explorers in Australia: from the log books and journals with maps and illustrations.’ (Methuen and Co. Ltd: London, UK) Available at http://www.artuccino.com/Allan_Cunningham/Ida_Lee/Index.html
Leino MW, Edqvist J (2010) Germination of 151-year old Acacia spp. seeds. Genetic Resources and Crop Evolution 57, 741–746.
| Germination of 151-year old Acacia spp. seeds.Crossref | GoogleScholarGoogle Scholar |
Long RL, Gorecki MJ, Renton M, Scott JK, Colville L, Goggin DE, Commander LE, Westcott DA, Cherry H, Finch-Savage WE (2015) The ecophysiology of seed persistence: a mechanistic view of the journey to germination or demise. Biological Reviews 90, 31–59.
| The ecophysiology of seed persistence: a mechanistic view of the journey to germination or demise.Crossref | GoogleScholarGoogle Scholar |
Lunt ID (2002) Grazed, burnt and cleared: how ecologists have studied century-scale vegetation changes in Australia. Australian Journal of Botany 50, 391–407.
| Grazed, burnt and cleared: how ecologists have studied century-scale vegetation changes in Australia.Crossref | GoogleScholarGoogle Scholar |
Mackenzie BDE, Keith DA (2009) Adaptive management in practice: conservation of a threatened plant population. Ecological Management & Restoration 10, S129–S135.
| Adaptive management in practice: conservation of a threatened plant population.Crossref | GoogleScholarGoogle Scholar |
McLauchlan KK, Higuera PE, Miesel J, Rogers BM, Schweitzer J, Shuman JK, Tepley AJ, Varner JM, Veblen TT, Adalsteinsson SA, Balch JK, Baker P, Batllori E, Bigio E, Brando P, Cattau M, Chipman ML, Coen J, Crandall R, Daniels L, Enright N, Gross WS, Harvey BJ, Hatten JA, Hermann S, Hewitt RE, Kobziar LN, Landesmann JB, Loranty MM, Maezumi SY, Mearns L, Moritz M, Myers JA, Pausas JG, Pellegrini AFA, Platt WJ, Roozeboom J, Safford H, Santos F, Scheller RM, Sherriff RL, Smith KG, Smith MD, Watts AC (2020) Fire as a fundamental ecological process: research advances and frontiers. Journal of Ecology 108, 2047–2069.
| Fire as a fundamental ecological process: research advances and frontiers.Crossref | GoogleScholarGoogle Scholar |
McRae RHD, Cooper MG (1985) Vegetation of the Merriwa area, New South Wales. Cunninghamia 1, 351–369.
Meyer GI (1987) ‘In the shadow of Mt Dangar.’ (Geoffrey I. Meyer: Muswellbrook, NSW, Australia)
Miles J, Cameron S (2007) Observations on the ecology and conservation status of Haloragis exalata subsp. exalata (Haloragaceae) in southern New South Wales. Cunninghamia 10, 263–272.
Miller BP, Symons DR, Barrett MD (2019) Persistence of rare species depends on rare events: demography, fire response and phenology of two plant species endemic to a semiarid Banded Iron Formation range. Australian Journal of Botany 67, 268–280.
| Persistence of rare species depends on rare events: demography, fire response and phenology of two plant species endemic to a semiarid Banded Iron Formation range.Crossref | GoogleScholarGoogle Scholar |
Milne AK (1986) The use of remote sensing in mapping and monitoring vegetational change associated with bushfire events in Eastern Australia. Geocarto International 1, 25–32.
| The use of remote sensing in mapping and monitoring vegetational change associated with bushfire events in Eastern Australia.Crossref | GoogleScholarGoogle Scholar |
Nicholls N (1988) More on early ENSOs: evidence from Australian Documentary Sources. Bulletin of the American Meteorological Society 69, 4–6.
| More on early ENSOs: evidence from Australian Documentary Sources.Crossref | GoogleScholarGoogle Scholar |
NSW Threatened Species Scientific Committee (2018) Acacia dangarensis, final determination to list as a critically endangered species. Available at https://www.environment.nsw.gov.au/topics/animals-and-plants/threatened-species/nsw-threatened-species-scientific-committee/determinations/final-determinations/2017-2018/acacia-dangarensis-a-small-tree-critically-endangered-species-listing
Palmer JG, Cook ER, Turney CSM, Allen K, Fenwick P, Cook BI, O’Donnell A, Lough J, Grierson P, Baker P (2015) Drought variability in the eastern Australia and New Zealand summer drought atlas (ANZDA, CE 1500–2012) modulated by the Interdecadal Pacific Oscillation. Environmental Research Letters 10, 124002
| Drought variability in the eastern Australia and New Zealand summer drought atlas (ANZDA, CE 1500–2012) modulated by the Interdecadal Pacific Oscillation.Crossref | GoogleScholarGoogle Scholar |
Peake TC (2006) The Vegetation of the Central Hunter Valley, New South Wales. A report on the findings of the Hunter Remnant Vegetation Project. Hunter–Central Rivers Catchment Management Authority, Paterson, NSW, Australia.
Prober SM, Thiele KR, Bramwell M (2007) Intense fires promote uncommon post-fire ephemerals in Currawang Acacia doratoxylon dry scrubs of Little River Gorge, East Gippsland. Victorian Naturalist 124, 320–331.
Sakaguchi S, Bowman DMJS, Prior LD, Crisp MD, Linde CC, Tsumura Y, Isagi Y (2013) Climate, not Aboriginal landscape burning, controlled the historical demography and distribution of fire-sensitive conifer populations across Australia. Proceedings of the Royal Society of London – B. Biological Sciences 280, 20132182
| Climate, not Aboriginal landscape burning, controlled the historical demography and distribution of fire-sensitive conifer populations across Australia.Crossref | GoogleScholarGoogle Scholar |
Scheele BC, Legge S, Armstrong DP, Copley P, Robinson N, Southwell D, Westgate MJ, Lindenmayer DB (2018) How to improve threatened species management: an Australian perspective. Journal of Environmental Management 223, 668–675.
| How to improve threatened species management: an Australian perspective.Crossref | GoogleScholarGoogle Scholar |
Silcock JL, Piddocke TP, Fensham RJ (2013) Illuminating the dawn of pastoralism: evaluating the record of European explorers to inform landscape change. Biological Conservation 159, 321–331.
| Illuminating the dawn of pastoralism: evaluating the record of European explorers to inform landscape change.Crossref | GoogleScholarGoogle Scholar |
Silcock JL, Healy AJ, Fensham RJ (2015) Lost in time and space: re-assessment of conservation status in an arid-zone flora through targeted field survey. Australian Journal of Botany 62, 674–688.
| Lost in time and space: re-assessment of conservation status in an arid-zone flora through targeted field survey.Crossref | GoogleScholarGoogle Scholar |
Silcock JL, Witt GB, Fensham RJ (2016) A 150-year fire history of mulga (Acacia aneura F. Muell. ex Benth.) dominated vegetation in semiarid Queensland, Australia. The Rangeland Journal 38, 391–415.
| A 150-year fire history of mulga (Acacia aneura F. Muell. ex Benth.) dominated vegetation in semiarid Queensland, Australia.Crossref | GoogleScholarGoogle Scholar |
Tierney DA, Watson P (2009) Fire and the vegetation of the Hunter Central Rivers CMA. A Hotspots Fire Project report. Nature Conservation Council, Sydney, NSW, Australia.
Török P, Bullock JM, Jiménez-Alfaro B, Sonkoly J (2020) The importance of dispersal and species establishment in vegetation dynamics and resilience. Journal of Vegetation Science 31, 935–942.
| The importance of dispersal and species establishment in vegetation dynamics and resilience.Crossref | GoogleScholarGoogle Scholar |
Tozer M, Ooi M, Simpson C, Wilkins K, Zylstra P (2021) How the severe fires of 2019-2020 promoted regeneration of the rare Bendethera shrublands. Australasian Plant Conservation 29, 12–15.
Turney C, Becerra-Valdivia L, Sookdeo A, Thomas ZA, Palmer J, Haines HA, Cadd H, Wacker L, Baker A, Andersen MS, Jacobsen G, Meredith K, Chinu K, Bollhalder S, Marjo C (2021) Radiocarbon protocols and first intercomparison results from the Chronos 14Carbon-Cycle Facility, University of New South Wales, Sydney, Australia. Radiocarbon 63, 1003–1023.
| Radiocarbon protocols and first intercomparison results from the Chronos 14Carbon-Cycle Facility, University of New South Wales, Sydney, Australia.Crossref | GoogleScholarGoogle Scholar |
Walsh N (1998) Mount Buffalo: botanical bridge or island? The Victorian Naturalist 115, 186–187.
Whitehead J (2013) Tracking and mapping the explorers Volume 4. Cunningham’s Pandora’s Pass. (The Allan Cunningham Project: Coonabarabran, NSW, Australia) Available at https://www.allancunninghambotanist1839.com/index.php/john-whitehead-books/#04-Pandoras-Pass
Whitehead J (2017a) Tracking and mapping the explorers Volume 5. Cunningham’s Expedition across the Liverpool Plains 1825. (The Allan Cunningham Project: Coonabarabran, NSW, Australia) Available at https://www.allancunninghambotanist1839.com/index.php/john-whitehead-books/#05-Liverpool-Plains-1825
Whitehead J (2017b) Tracking and mapping the explorers Volume 7. Cunningham’s Expedition to the Darling Downs 1827. (The Allan Cunningham Project: Coonabarabran, NSW, Australia) Available at https://www.allancunninghambotanist1839.com/index.php/john-whitehead-books/#07-Darling-Downs-1827
Williams PR (2006) Determining the management requirements of threatened plant species. Ecological Management & Restoration 7, 148–151.
| Determining the management requirements of threatened plant species.Crossref | GoogleScholarGoogle Scholar |
Zhang Y, Lim S, Sharples JJ (2017a) Wildfire occurrence patterns in ecoregions of New South Wales and Australian Capital Territory, Australia. Natural Hazards 87, 415–435.
| Wildfire occurrence patterns in ecoregions of New South Wales and Australian Capital Territory, Australia.Crossref | GoogleScholarGoogle Scholar |
Zhang Y, Lim S, Sharples JJ (2017b) Effects of climate on the size of wildfires in the Eucalyptus camaldulensis forests and the dry lands of the Riverina Bioregion, Australia. Forest Ecology and Management 401, 330–340.
| Effects of climate on the size of wildfires in the Eucalyptus camaldulensis forests and the dry lands of the Riverina Bioregion, Australia.Crossref | GoogleScholarGoogle Scholar |
Zhang F, Quan Q, Ma F, Tian D, Hoover DL, Zhou Q, Niu S (2019) When does extreme drought elicit extreme ecological responses? Journal of Ecology 107, 2553–2563.
| When does extreme drought elicit extreme ecological responses?Crossref | GoogleScholarGoogle Scholar |
Zimmer HC, Florentine SK, Enke R, Westbrooke M (2017) Rainfall and grazing: not the only barriers to arid-zone conifer recruitment. Australian Journal of Botany 65, 109–119.
| Rainfall and grazing: not the only barriers to arid-zone conifer recruitment.Crossref | GoogleScholarGoogle Scholar |


