The susceptibility of rare and threatened NSW species to the root-rot pathogen Phytophthora cinnamomi: 2. The identification of species requiring protection or further research
Keith L. McDougall A C * and Edward C. Y. Liew B
A C * and Edward C. Y. Liew B
A
B
C Present address: Department of Environment and Genetics, La Trobe University, Bundoora, Vic., Australia. Email: keith.mcdougall@latrobe.edu.au
Abstract
The response of most native plant species in New South Wales (NSW) to infection by the oomycete pathogen Phytophthora cinnamomi Rands is unknown, which makes decisions about disease management difficult.
We aim to improve knowledge about the potential threat from P. cinnamomi by testing a further 32 threatened species for their response to the pathogen and developing a method for prioritising management and susceptibility testing.
Susceptibility to infection and host response were evaluated in glasshouse experiments where the pathogen was introduced to pots containing the threatened species, and the results were compared with control uninoculated pots. Our prioritisation used modelled habitat suitability for P. cinnamomi, proximity to known P. cinnamomi occurrences, and numbers of plant species populations at least 1 km apart to rank 928 rare and threatened plant species native to NSW for either management or susceptibility testing.
Phytophthora cinnamomi was re-isolated from the roots of 10 of the 32 species assessed, most of which also showed significant mortality or disease symptoms. Darwinia peduncularis B.G.Briggs, Hibbertia circinata K.L.McDougall & G.T.Wright, Isopogon fletcheri F.Muell., Phebalium speciosum I.Telford, Pultenaea baeuerlenii F.Muell. and Pultenaea parrisiae J.D.Briggs & Crisp were the most severely affected species. The effect of P. cinnamomi is known for only 63 rare and threatened species in NSW. The Greater Sydney region is a hotspot for rare and threatened plant species with a high priority for susceptibility testing.
The prognosis in the wild for rare and threatened plant species affected by P. cinnamomi depends on (1) habitat suitability for the pathogen, with subalpine and arid-zone species unlikely to be affected, (2) the number of unaffected populations, with two severely affected species that occur only on infested sites (Hibbertia circinata and Prostanthera marifolia R.Br.) facing extinction in the near future and (3) climate, with some species (e.g. Pomaderris delicata N.G.Walsh & Coates) apparently affected only in unusually wet years. Further susceptibility testing of rare and threatened species is required. This should be supported by taxonomic studies of genera (e.g. Hibbertia, Pultenaea) commonly affected by the pathogen.
Many more plant species in NSW are likely to be severely affected by P. cinnamomi than currently known, and may require active management of the disease for their long-term survival.
Keywords: climate change, dieback, disease management, extinction risk, flood inoculation, pathogen susceptibility, prioritisation, threatened species.
Introduction
In the first paper in this series (Wan et al. 2019), we investigated the susceptibility of 16 New South Wales (NSW) threatened plant species to the pathogen Phytophthora cinnamomi Rands in glasshouse inoculation tests; seven species were found to be infected under the conditions of the tests, three of which developed severe disease leading to mortality. We also outlined the research identified at that time for a better understanding of the threat of the pathogen and how the threat might be mitigated. This included further susceptibility testing of threatened species, prioritising those with only one or a few populations.
The oomycete P. cinnamomi invades plant root systems under moist conditions, leading to the necrosis of plant tissue; plant death may follow, especially if there are other stressors present (Cahill et al. 2008). Not all plant species are affected and some may be affected only under unusual climatic conditions. Species in the families Dilleniaceae, Ericaceae, Fabaceae, Proteaceae and Xanthorrheaceae are disproportionately affected (Cahill et al. 2008) and this is reflected in the 86 of 120 threatened taxa from these families, which are regarded as being at risk from P. cinnamomi (Commonwealth of Australia 2018).
The disease triangle is a conceptual model in plant pathology that describes the disease process (Scholthof 2007). For disease to occur, there must be three elements (i.e. the apices of the triangle), namely, a pathogen, a susceptible host, and a suitable environment for them to co-occur in. Without any one of these, there cannot be disease. Although simplistic, it is useful for assessing the risk of disease. For instance, Rigg et al. (2018) found that Phebalium squamulosum subsp. alpinum (Benth.) Paul G.Wilson is a susceptible host to both Phytophthora cinnamomi and P. cambivora (Petri) Buisman. However, it grows in environments unsuitable to P. cinnamomi and so there is no risk of disease and the disease triangle cannot be complete for this species pair. Conversely, the cold environments suitable for P. squamulosum are also suitable for P. cambivora and disease may result from their co-occurrence, although this has apparently not happened yet. The possibility of disease for a plant species and a pathogen when they co-occur (i.e. a complete disease triangle) does not necessarily mean that mortality will occur or that there will be a detrimental effect on the plant population; this information is vital for plant species management. For this, a fourth apex might be added, the axis of which is proportional to the likelihood of mortality; the more two-dimensional is the tetrahedron, the less likely is a detrimental effect as a result of disease. With adequate knowledge about habitat suitability for a pathogen and its hosts (the disease triangle), and the severity of disease caused following infection (the fourth axis), it should be possible to prioritise resources for plant species management when the pathogen is present (e.g. ex situ conservation and chemical treatment) or absent (e.g. quarantine).
In the case of P. cinnamomi, habitat suitability has been evaluated using spatial modelling based on climate and known occurrences (e.g. Burgess et al. 2019, McDougall and Liew 2020). In NSW, the pathogen tends to occur in areas with >500 mm of annual rainfall and >10°C mean annual temperature (McDougall and Liew 2020). Habitat suitability for plant species can be assessed from the rapidly growing data availability on species locations in platforms such as the Atlas of Living Australia (https://www.ala.org.au).
The severity of disease from P. cinnamomi is known for very few plant species in NSW. In some cases, observations of poor plant health have led to the isolation of P. cinnamomi from the roots of symptomatic plants (e.g. McDougall and Summerell 2003). Although this is evidence that the pathogen can colonise the roots of such plants, it does not prove that it was the cause of disease. Proof requires Koch’s postulates to be fulfilled (e.g. Grimes 2006); i.e. the pathogen must be isolated from a diseased host and grown in pure culture, an isolate from the culture must again cause the disease when inoculated into a healthy plant and the same pathogen must then be re-isolated. Obtaining such proof in the field by re-introducing a pathogen to healthy plants will rarely be permissible. Causal proof of disease is therefore typically demonstrated under controlled conditions in a glasshouse. Replication of test plants and tests will then allow an evaluation of disease severity, when compared with tests on species of known disease severity, and the fulfillment of Koch’s postulates. However, the outcomes of glasshouse tests may not reflect plant responses in the wild where host or pathogen may be advantaged (e.g. by additional pressures on the host such as drought and insect herbivory or suppression of pathogen activity by soil properties). They may also create environmental conditions unlikely to occur for the species pair in the wild (as was the case for Phebalium squamulosum and P. cinnamomi in the glasshouse tests of Rigg et al. 2018) or test plant life stages either affected or not affected in the wild (e.g. seedlings compared with mature plants; Cahill et al. 2008). In the case of rare and threatened plant species, the propagation of large numbers of replicates is usually problematic, limiting replication and the ability to repeat tests. Despite its shortcomings, glasshouse susceptibility testing has been an important component of conservation management of the threat from P. cinnamomi in many parts of Australia (e.g. Tasmania: Barker and Wardlaw 1995; Western Australia: Shearer et al. 2013; South Australia: Kueh et al. 2012; New South Wales: Wan et al. 2019; Victoria: Reiter et al. 2004). Together with field pathogen sampling and observations of plant health, the results of glasshouse tests have enabled the timely allocation of resources for protecting the most at risk species from P. cinnamomi. Glasshouse testing is currently the only feasible approach for evaluating disease severity in rare and threatened species.
For the majority of plant species in NSW, glasshouse tests are required to determine their response to the presence of P. cinnamomi; i.e. whether they can be a host, whether they develop disease when infected and whether they will ultimately die. With potentially more than 4000 native species to test, prioritisation will be required. Our own glasshouse tests have selected from species listed under threatened species legislation as these are already recognised as being at risk of extinction. However, this approach may neglect species that are likely to be at risk in the future. Species occurring in areas highly suited to P. cinnamomi that have few populations, which are all proximal to known infestations, are likely to be at highest risk if they are found to be severely affected; these species should be given priority in testing. Similarly, for species already known to be severely affected by P. cinnamomi, those occurring in habitat suitable for P. cinnamomi, which have few populations proximal to known infestations, should be given priority for management.
In this paper, we present the results of additional glasshouse testing of NSW threatened species for susceptibility to P. cinnamomi. Using modelled habitat suitability for P. cinnamomi, plant species population parameters (numbers of populations and extent of occurrence), and proximity to known P. cinnamomi occurrences, we assign priority for susceptibility testing and disease management of NSW threatened and spatially restricted plant species within the modelled area.
Materials and methods
Species response
Between 2019 and 2022, we tested 32 species listed as threatened under the NSW Biodiversity Conservation Act 2016, and occurring predominantly on the coast and tablelands of eastern NSW, for susceptibility to P. cinnamomi infection (Table 1), Dracophyllum macranthum was listed as vulnerable at the time of testing in 2020, but delisted in 2021. Our intention, as stated in Wan et al. (2019), was to give preference to species occurring in one to a few populations, but species selection was largely governed by availability of plant material for propagation. All plants were grown at Australian National Botanic Gardens, Canberra, and the Australian Botanic Gardens, Mount Annan; Banksia conferta, Dillwynia tenuifolia, Dracophyllum macranthum, Isopogon fletcheri and Pultenaea aristata were grown from seed, whereas all other species were grown from cuttings. The plants were grown in propagation stock-tubes (5 cm × 5 cm × 12 cm), except for Grevillea acanthifolia subsp. paludosa and Prostanthera stricta, which were in larger tubes (8 cm × 8 cm × 18 cm) because of their larger size. The potting mix consisted of composted grade pine bark, coconut fibre, and propagation sand (Australian Standard AS3743). It had water-holding capacity of 45–55% and an air-filled porosity of 16–20% (pH 5.6–6.3). Nutrient solution (50 mL of half strength Earth Care Seasol seaweed concentrate) was applied to all plants once, a month prior to inoculation. Plants were delivered to the experimental glasshouse at PlantClinic, Royal Botanic Garden Sydney in early April of each year of testing, and the pots placed into large plastic tubs (2 m × 1 m) where they were acclimatised to the glasshouse conditions for about 3 months prior to inoculation in late June. The experiment was conducted in a temperature-controlled glasshouse. The internal temperature control was set at 23°C, with the temperature ranging from 12 to 26°C. Plants were watered every couple of days, except during flooding. The treatment groups were negative control and P. cinnamomi flood inoculation.
| Species | Status | Distribution | Year of test | |
|---|---|---|---|---|
| Banksia conferta A.S.George | CE | NC | 2019 | |
| Callistemon purpurascens S.M.Douglas & S.David | CE | CT | 2019 | |
| Correa baeuerlenii F.Muell. | V | SC | 2019 | |
| Correa lawrenceana var. genoensis Paul G.Wilson | E | ST | 2020 | |
| Darwinia peduncularis B.G.Briggs | V | CC, CT | 2020 | |
| Dillwynia tenuifolia DC. | V | CC, CT, NC | 2019 | |
| Dracophyllum macranthum E.A.Br. & N.Streiber | Not listed | NC | 2020 | |
| Epacris purpurascens Sims var. purpurascens | V | CC | 2019 | |
| Grevillea acanthifolia subsp. paludosa Makinson & Albr. | E | ST | 2019 | |
| Grevillea guthrieana Olde & Marriott | E | NC | 2020 | |
| Grevillea mollis Olde & Molyneux | E | NT | 2019 | |
| Grevillea obtusiflora R.Br. subsp. obtusiflora | E | CT | 2020 | |
| Grevillea shiressii Blakely | V | CC | 2020 | |
| Hakea archaeoides W.R.Barker | V | NC | 2020 | |
| Haloragodendron lucasii (Maiden & Betche) Orchard | E | CC | 2020 | |
| Hibbertia circinata K.L.McDougall & G.T.Wright | CE | SC | 2019/22 | |
| Hibbertia puberula subsp. glabrescens Toelken | CE | CC | 2019 | |
| Isopogon fletcheri F.Muell. | V | CT | 2019/20 | |
| Leionema ralstonii (F.Muell.) Paul G.Wilson | V | SC | 2019 | |
| Persoonia acerosa Sieber ex Schult. & Schult.f. | V | CC/CT | 2020 | |
| Persoonia hindii P.H.Weston & L.A.S.Johnson | E | CT | 2020 | |
| Phebalium speciosum I.Telford | CE | NC | 2022 | |
| Prostanthera askania B.J.Conn | E | CC | 2020 | |
| Prostanthera palustris B.J.Conn | V | NC | 2020 | |
| Prostanthera staurophylla F.Muell. | E | NT | 2020 | |
| Prostanthera stricta R.T.Baker | V | CT, CWS | 2019 | |
| Pultenaea aristata Sieber ex DC. | V | CC | 2019 | |
| Pultenaea baeuerlenii F.Muell. | V | ST | 2020 | |
| Pultenaea parrisiae J.D.Briggs & Crisp | V | ST | 2019 | |
| Rhodamnia rubescens (Benth.) Miq. | CE | CT, CC, NC, NT, SC | 2020 | |
| Rhodomyrtus psidioides (G.Don) Benth. | CE | CC, NC | 2020 | |
| Zieria murphyi Blakely | V | CT, SC | 2022 |
Phebalium squamulosum ssp. alpinum was found to be highly susceptible to P. cinnamomi (and clearly symptomatic when infected) in previous tests under similar glasshouse conditions (Rigg et al. 2018; Wan et al. 2019), and so was chosen as a positive control.
Pathogen cultivation and inoculation
The culturing conditions followed those of Wan et al. (2019). The P. cinnamomi isolate (RBG W1324) was used as the pathogen in the greenhouse experiment. To ensure its virulence, it was first revitalised and cultured from long-term storage, and passed through lupin seedling bait (Lupinus angustifolius) in a baiting cylinder containing sterilised soil, deionised water and the culture (as 1 cm2 agar plugs). After 10 days, infected lupin stem and root sections were plated onto V8 agar plates (20% clarified Campbell’s V8 juice, pH 6.5–7.5 adjusted with calcium carbonate). The cultures were incubated in the dark at 24°C for 4 days prior to subculturing onto more plates. The subcultures were incubated for 2 weeks prior to inoculating the inoculum mix, which consisted of seed (millet and bran) and propagation sand (2:1 vol:vol) blended consistently. This mix was autoclaved in polycarbonate jars (Nalgene 500 mL with 120 mm closure, filled to half full and moistened with deionised water). Six 1 cm2 plugs of the culture were placed in each autoclaved polycarbonate jar and incubated for 3 weeks. During this period, the inoculum was aseptically stirred where necessary to ensure consistency of growth throughout the mix. Blank V8 plugs were added to the mix for the negative control.
Inoculation and susceptibility assessments
Plants were inoculated in late July or early August (depending on the year of test) by inserting the inoculum mix into the sides of pots, making up to ~20% of the pot volume. A higher volume of inoculum was thus added for the species in larger pots. Contact with water stimulates the production of sporangia and zoospores that may mobilise to the growing points of the roots (Hardham 2005). Flooding was introduced 3 days after inoculation for 48 h once a week for 2 weeks. This was achieved by filling the tubs with deionised water to 3 cm below the soil surface. Plants were raised above the flood level by placing on elevation platforms after each flooding. After the initial flooding, the plants were watered every 2–3 days and monitored for disease symptoms and mortality.
Re-isolation of the pathogen from roots was attempted for any plants that died during the experiment and for all remaining plants when the experiment was concluded in November, about 14 weeks after inoculation. Roots were washed and examined for lesions and disease. Four to six sections (3 cm long) of roots that appeared lesioned were excised, surface-sterilised through tissue paper by using 70% ethyl alcohol and plated onto V8-PSM plates (Phytophthora-selective medium: V8 basal medium amended with the antibiotics hymexazol at 0.004% v/v, rifampicin at 0.004%, pimaricin at 0.0004% v/v). Where there were no obvious lesions, random selections of roots from each plant were plated. Isolation plates were incubated at 23°C for 5 days, after which any growth on the agar was examined under the microscope for confirmation of the pathogen.
The response of test species to the presence of P. cinnamomi was assessed by comparing treated and control plants within a species for (1) mortality (the relative percentage of plants surviving at the end of the test), and (2) disease. Disease was scored 0 where a plant had no symptoms, 0.25 where a plant had lesioned roots, but P. cinnamomi was not re-isolated, 0.5 where a plant had rotten roots, but P. cinnamomi was not re-isolated, and 1 for a plant from which P. cinnamomi was re-isolated. The disease scores were summed for each species and scaled between 0 and 100, depending on the number of plants tested.
Comparisons of mortality and disease between the inoculated treatment and non-inoculated controls were made for each species by using Pearson’s chi-squared tests. The tests were performed using R (ver. 4.2.3; R Core Team 2023). P-values were simulated using Monte-Carlo tests by random sampling of 2000 contingency tables with set marginals. A P-value of <0.05 was considered to be statistically significant, whereas P ≥ 0.05 and <0.1 was regarded as near significant.
Prioritising species for susceptibility testing and management
Location data for 418 plant species with extant populations listed as threatened under the NSW Biodiversity Conservation Act 2016 and occurring predominantly within the area of P. cinnamomi-modelled habitat were extracted from the Atlas of Living Australia (ALA; https://www.ala.org.au) between 22 March and 5 April 2023. The location data for an additional 714 species from eastern NSW with fewer than 100 NSW records in the ALA were also extracted. A species was then excluded from the data if (1) it occurred on Lord Howe Island, because habitat there was not modelled for P. cinnamomi suitability by McDougall and Liew (2020), (2) occurred in saline habitat because such habitat is unsuitable for P. cinnamomi, (3) it was an epiphyte or lithophyte, which are unlikely to come into contact with the pathogen, and (4) occurred commonly in other States. The final data set contained 928 rare and threatened taxa (including subspecies; Supplementary material S1).
Geographic outliers (isolated records at the edge of range greater than 1° beyond the majority of records and outside the accepted range of a species as indicated in the Flora of New South Wales (https://plantnet.rbgsyd.nsw.gov.au/floraonline.htm) were removed from the data. Data were then disaggregated for each species to one record/km2 to remove duplicates and reduce spatial bias (following the approach used by McDougall and Liew 2020).
Prioritisation for susceptibility testing and management was based on the following three variables:
Spatial data from McDougall and Liew (2020) were plotted onto the open-source geographic information system software QGIS ver. 3.22.0 (https://www.qgis.org/en/site/) and used to obtain P. cinnamomi habitat suitability for each species record. Records in western NSW (not modelled by McDougall and Liew 2020) were assigned a habitat suitability of zero. This is consistent with modelling of broader habitat suitability (Burgess et al. 2019), which has shown that P. cinnamomi is unlikely to survive in arid environments. Mean habitat suitability (Prioritisation category A) was then calculated for each species as a value between 0 (unsuitable) and 1 (highly suitable).
A projected P. cinnamomi habitat suitability value for 2070 (A2070) was obtained for each record of species known to be susceptible using the modelled habitat suitability layer of McDougall and Liew (2020) under the 8.5 rcp climate scenario, and values averaged for each species.
Abundance was assessed as the number of records of a species after disaggregation (i.e. the number of records at least 1 km apart); species with fewer than 10 records were assigned a value of 10. An abundance score (B) was then calculated as 1/(log10 abundance), giving a value between 0 and 1.
Spatial P. cinnamomi occurrence data from McDougall and Liew (2020) were plotted onto QGIS ver. 3.22.0 and used to obtain the distance of each plant record from the nearest P. cinnamomi record. The 95 percentile of distances to P. cinnamomi records was then calculated for each species to represent proximity; species with 95 percentile proximity less than 10 km were assigned a value of 10. A proximity score (C) was calculated as 1/(log10 proximity), giving a value between 0 and 1.
A priority score was then calculated for each species as A × B × C × 100, giving a value between 0 and 100.
Results
Species response
Of the 32 threatened species tested for susceptibility to P. cinnamomi, five species (Darwinia peduncularis, Hibbertia circinata, Isopogon fletcheri, Phebalium speciosum and Pultenaea baeuerlenii) had significantly (or near significantly, P < 0.1) more deaths and re-isolations for inoculated plants than for control uninoculated plants, as determined by chi-squared tests (Table 2). Four species (Correa lawrenceana var. genoensis, Dracophyllum macranthum, Grevillea obtusiflora subsp. obtusiflora and Haloragodendron lucasii) had significantly (or near significantly, P < 0.1) more re-isolations for inoculated plants than for uninoculated plants, but not significantly (P > 0.1) more deaths. Correa baeuerlenii and Pultenaea parrisiae had significantly (P < 0.05) more deaths in inoculated pots than in uninoculated control pots, but not significantly more re-isolations in inoculated pots, with no re-isolations in the case of Correa baeuerlenii and only two of six for Pultenaea parrisiae. Overall, P. cinnamomi was re-isolated from the roots of 10 of the 32 species assessed. The control species, Phebalium squamulosum subsp. alpinum, was highly susceptible to infection and readily killed (comparing inoculated and uninoculated controls), confirming that the results of this and previous tests are comparable.
| Species | nC | nI | Mortality | Re-isolation | |||
|---|---|---|---|---|---|---|---|
| Control | Inoculated | Control | Inoculated | ||||
| Banksia conferta | 10 | 10 | 0 | 0 | 0 | 0 | |
| Callistemon purpurascens | 6 | 6 | 0 | 1 | 0 | 0 | |
| Correa baeuerlenii | 10 | 10 | 0 | 5* | 0 | 0 | |
| Correa lawrenceana var. genoensis | 10 | 9 | 0 | 0 | 0 | 5* | |
| Darwinia peduncularis | 9 | 9 | 0 | 5* | 0 | 4^ | |
| Dillwynia tenuifolia | 10 | 10 | 1 | 5 | 0 | 0 | |
| Dracophyllum macranthum | 10 | 10 | 0 | 2 | 0 | 8*** | |
| Epacris purpurascens var. purpurascens | 9 | 9 | 0 | 3 | 0 | 0 | |
| Grevillea acanthifolia subsp. paludosa | 10 | 10 | 0 | 0 | 0 | 0 | |
| Grevillea guthrieana | 10 | 10 | 2 | 4 | 0 | 0 | |
| Grevillea mollis | 8 | 8 | 0 | 0 | 0 | 0 | |
| Grevillea obtusiflora subsp. obtusiflora | 10 | 10 | 0 | 2 | 0 | 4^ | |
| Grevillea shiressii | 10 | 10 | 0 | 0 | 0 | 0 | |
| Hakea archaeoides | 10 | 10 | 0 | 0 | 0 | 0 | |
| Haloragodendron lucasii | 10 | 10 | 0 | 0 | 0 | 7** | |
| Hibbertia circinataA | 18 | 19 | 1 | 17*** | 0 | 12*** | |
| Hibbertia puberula subsp. glabrescens | 6 | 6 | 0 | 2 | 0 | 0 | |
| Isopogon fletcheriA | 14 | 13 | 1 | 8* | 0 | 11*** | |
| Persoonia acerosa | 10 | 10 | 0 | 0 | 0 | 0 | |
| Persoonia hindii | 10 | 10 | 0 | 0 | 0 | 0 | |
| Phebalium speciosum | 10 | 10 | 0 | 9*** | 0 | 8*** | |
| Phebalium squamulosum subsp. alpinumA | 20 | 20 | 2 | 18*** | 0 | 17*** | |
| Prostanthera askania | 10 | 10 | 0 | 0 | 0 | 0 | |
| Prostanthera palustris | 10 | 10 | 0 | 0 | 0 | 0 | |
| Prostanthera staurophylla | 10 | 10 | 0 | 0 | 0 | 0 | |
| Prostanthera stricta | 10 | 10 | 0 | 0 | 0 | 0 | |
| Pultenaea aristata | 5 | 6 | 1 | 4 | 0 | 0 | |
| Pultenaea baeuerlenii | 10 | 10 | 0 | 7*** | 0 | 6** | |
| Pultenaea parrisiae | 5 | 6 | 0 | 6** | 0 | 2 | |
| Rhodamnia rubescens | 10 | 10 | 0 | 0 | 0 | 0 | |
| Rhodomyrtus psidioides | 10 | 10 | 0 | 0 | 0 | 0 | |
| Zieria murphyi | 10 | 10 | 0 | 1 | 0 | 0 | |
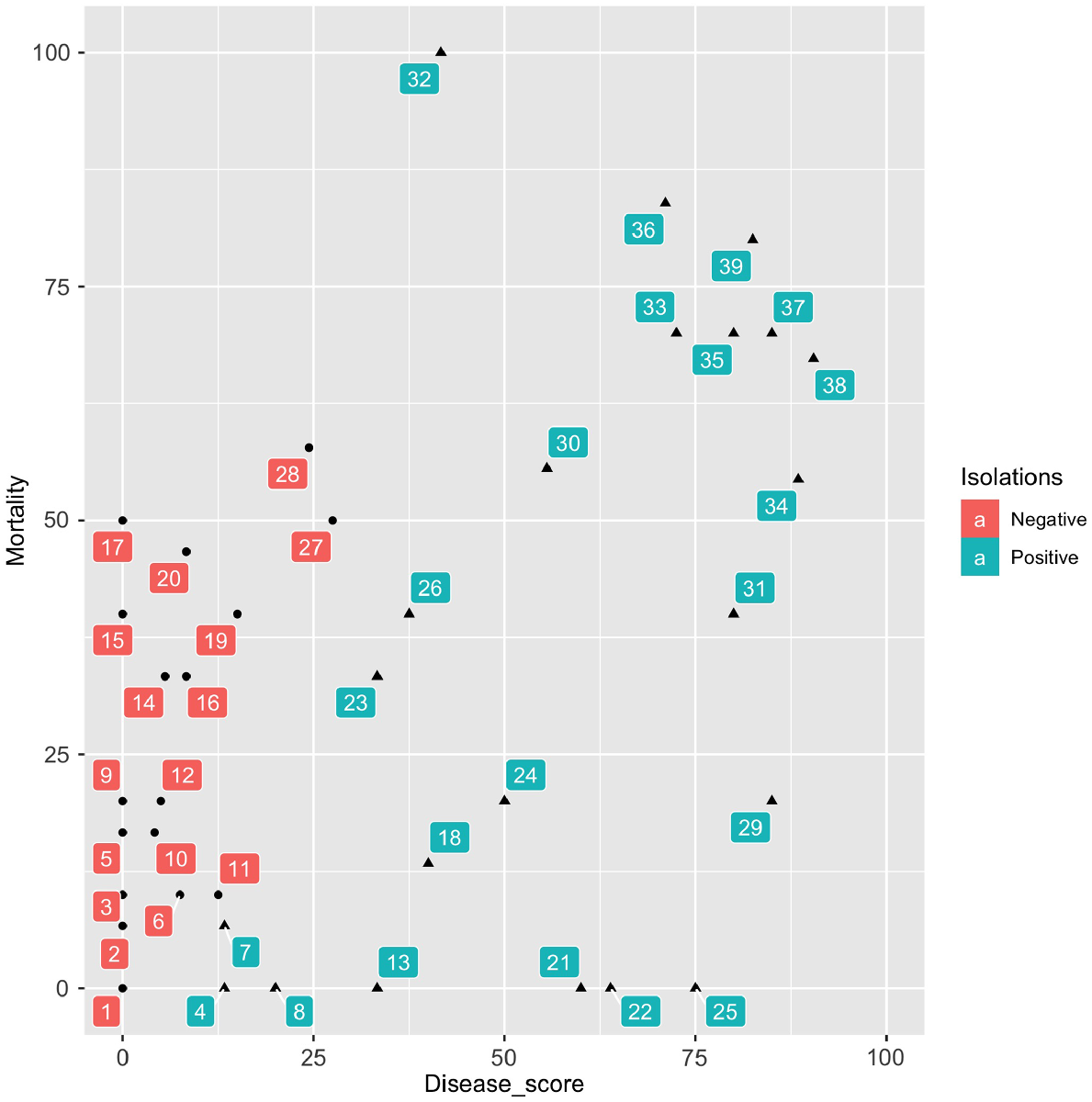
Plotting mortality and disease scores for the current and previous inoculation tests conducted at the RBG Sydney (Rigg et al. 2018; Wan et al. 2019), 58 species have been tested and P. cinnamomi has been re-isolated from 22 of them (Fig. 1).
Mortality and disease scores for 58 tested species. Mortality is the percentage of treated plants that died minus the percentage of control plants that died. Disease is the mean score for treated plants minus the mean score for control plants (scaled between 0 and 100 for the numbers of plants tested) where a plant with no symptoms was scored 0, a plant with lesioned roots was scored 0.25, a plant with rotten roots was scored 0.5 and a plant from which P. cinnamomi was re-isolated was scored 1. Acacia terminalis subsp. Eastern Sydney, Banksia conferta, B. vincentia, Boronia deanei, Callitris oblonga subsp. corangensis, Grevillea acanthifolia subsp. paludosa, G. mollis, G. shiressii, Hakea archaeoides, Orites lancifolius, Persoonia acerosa, P. hindii, Prostanthera askania, P. palustris, P. staurophylla, P. stricta, Rhodamnia rubescens, Rhodomyrtus psidiodes, Zieria baeuerlenii, Z. murphyi; 2. Kunzea muelleri; 3. Grevillea renwickiana; 4. Prostanthera cuneata; 5. Callistemon purpurascens; 6. Eucalyptus parvula; 7. Epacris petrophila; 8. Banksia serrata; 9. Grevillea guthrieana; 10. Leionema ralstonii; 11. Pimelea bracteata; 12. Asterolasia buxifolia; 13. Podocarpus lawrencei; 14. Epacris purpurascens; 15. Dillwynia tenuifolia; 16. Hibbertia puberula; 17. Correa baeuerlenii; 18. Oxylobium ellipticum; 19. Hibbertia spanantha; 20. Pultenaea aristata; 21. Grevillea victoriae subsp. nivalis; 22. Correa lawrenceana var. genoensis; 23. Nematolepis ovatifolium; 24. Grevillea obtusifolia subsp. obtusifolia; 25. Haloragodendron lucasii; 26. Pomaderris delicata; 27. Phebalium bifidum; 28. Plinthanthesis rodwayi; 29. Dracophyllum macranthum; 30. Darwinia peduncularis; 31. Nematolepis rhytidophylla; 32. Pultenaea parrisiae; 33. P. baeuerlenii; 34. Isopogon fletcheri; 35. Prostanthera marifolia; 36. Hibbertia circinata; 37. Pultenaea praecipua subsp. praecipua; 38. Phebalium squamulosum subsp. alpinum; 39. P. speciosum. Phytophthora cinnamomi was re-isolated from 22 species (indicated as Positive).
Prioritising species for susceptibility testing and management
Of 100 taxa with the highest scores for prioritising susceptibility testing (ranging in score from 66 to 100), there were multiple species of Asterolasia (2 taxa), Boronia (2), Deyeuxia (2), Epacris (7), Eucalyptus (5), Euphrasia (2), Genoplesium (5), Grevillea (5), Hibbertia (15), Leionema (2), Leptospermum (3), Pomaderris (2), Prasophyllum (3), Pterostylis (3), Thismia (2), Zieria (6) (Supplementary material S1). More than half of these top 100 ranked species (52 species) are not listed as threatened under the NSW Biodiversity Conservation Act 2016.
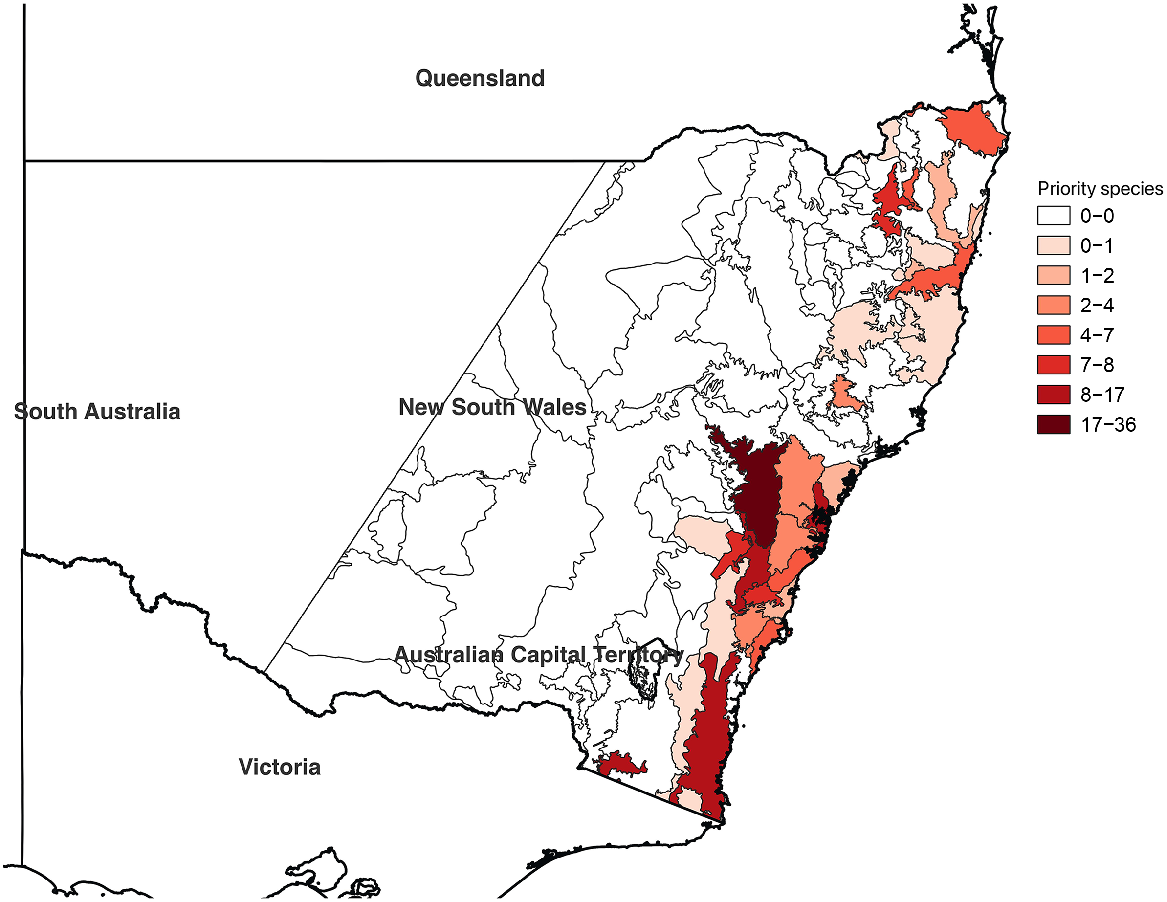
The highest 100 ranked taxa were not evenly distributed across the area of modelled Phytophthora cinnamomi habitat suitability (Fig. 2), with more than one-third of the taxa occurring in the Blue Mountains area (Burragorang, Kanangra and Wollemi Interim Biogeographic Regionalisation for Australia version 7 (IBRA 7) subregions). Other IBRA 7 subregions containing more than five species were Coffs Harbour and Escarpment, Jervis, Moss Vale, Northeast Forest Lands (Gibralter Range), Pittwater, Scenic Rim (mostly Border Ranges National Park), South East Coastal Ranges, Sydney Cataract and Washpool. These areas represent the co-occurrence of high habitat suitability for P. cinnamomi and many taxa of limited distribution.
Numbers of species in the 100 highest-priority species for susceptibility testing in Interim Biogeographic Regionalisation for Australia version 7 (IBRA 7) subregions. Wollemi IBRA 7 subregion contains by far the most priority species (36). The delimited area within NSW is that modelled by McDougall and Liew (2020) for Phytophthora cinnamomi habitat suitability.
Of the 33 rare and threatened species in NSW known to be moderately to severely affected by P. cinnamomi, the pathogen is present in all populations of four species, present in some populations of 12 species, and thought to be absent in all populations of three species; for the remaining 14 species, the status of P. cinnamomi in populations is unknown (Table 3). The priority score for management of these species ranged from two (Phebalium squamulosum subsp. alpinum, which is very low priority) to 97 (Isopogon fletcheri, which is very high priority). Three severely affected species had a priority score of >80 (Hibbertia circinata, Isopogon fletcheri and Prostanthera marifolia). The change in priority score by 2070, solely on the basis of the predicted change in habitat suitability for P. cinnamomi, was less than 10 for most species. The priority score for two species is predicted to increase by more than 10 (Pultenaea parrisiae, severely affected; Plinthanthesis rodwayi, moderately affected), whereas the score for six species will decrease by more than 10; indeed, two of those species (Phebalium speciosum, severely affected; Phebalium bifidum, moderately affected) are predicted to have extremely low scores by 2070.
| Scientific name | Effect | NSW status | Priority score | Δ Score (2070) | |
|---|---|---|---|---|---|
| P. cinnamomi present in all populations | |||||
| Boronia imlayensis Duretto | Mod. | CE | 91 | −1 | |
| Hibbertia circinata | Severe | CE | 91 | −1 | |
| Prostanthera marifolia R.Br. | Severe | CE | 89 | −3 | |
| Pomaderris delicata N.G.Walsh& Coates | Mod. | CE | 45 | +2 | |
| P. cinnamomi present in some populations | |||||
| Wollemia nobilis W.G.Jones, K.D.Hill & J.M.Allen | Severe | CE | 78 | −9 | |
| Grevillea irrasa subsp. irrasa Makinson | Severe | E | 69 | −7 | |
| Prostanthera walteri F.Muell. | Severe | V | 66 | +5 | |
| Pultenaea baeuerlenii | Severe | V | 65 | +9 | |
| Plinthanthesis rodwayi (C.E.Hubb.) S.T.Blake | Mod. | CE | 55 | +16 | |
| Pultenaea parrisiae | Severe | V | 52 | +15 | |
| Pultenaea benthamii F.Muell. | Severe | E | 40 | +4 | |
| Epacris purpurascens var. purpurascens | Mod. | V | 37 | −6 | |
| Persoonia mollis subsp. budawangensis S.Krauss & L.A.S.Johnson | Severe | V | 36 | +5 | |
| Tasmannia purpurascens (Vickery) A.C.Sm. | Severe | V | 32 | +2 | |
| Pultenaea pycnocephala F.Muell. ex Benth. | Severe | V | 24 | −5 | |
| Tetratheca subaphylla Benth. | Severe | V | 20 | +4 | |
| P. cinnamomi thought to be absent in all populations | |||||
| Nematolepis rhytidophylla (Albr.& N.G.Walsh) Paul G.Wilson | Severe | V | 58 | −4 | |
| Nematolepis ovatifolia (F.Muell.) Paul G.Wilson | Mod. | V | 3 | +5 | |
| Phebalium squamulosum subsp. alpinum | Severe | V | 2 | +5 | |
| P. cinnamomi status unknown | |||||
| Isopogon fletcheri | Severe | V | 97 | −8 | |
| Haloragodendron lucasii | Mod. | E | 92 | +1 | |
| Hibbertia spanantha Toelken & A.F.Rob. | Mod. | CE | 88 | −23 | |
| Hibbertia puberula subsp. glabrescens | Mod. | CE | 76 | −15 | |
| Dracophyllum macranthum | Severe | E | 72 | −4 | |
| Correa lawrenceana var. genoensis | Mod. | E | 61 | +1 | |
| Pultenaea praecipua M.A.M.Renner & P.H.Weston subsp. praecipua | Severe | CE | 58 | +3 | |
| Darwinia peduncularis | Severe | V | 54 | −8 | |
| Pultenaea aristata | Mod. | V | 38 | −3 | |
| Phebalium speciosum | Severe | CE | 30 | −28 | |
| Correa baeuerlenii | Mod. | V | 28 | −1 | |
| Phebalium bifidum P.H.Weston& M.J.Turton | Mod. | E | 27 | −21 | |
| Grevillea obtusiflora subsp. obtusiflora | Mod. | E | 22 | −12 | |
| Dillwynia tenuifolia | Mod. | V | 19 | −14 |
The NSW status refers to species listed under the Biodiversity Conservation Act 2016: V, Vulnerable; E, Endangered; CE, Critically Endangered; a putative status for unlisted species based on extent of occurrence alone is underlined. Priority score is based on habitat suitability for P. cinnamomi, number of populations and proximity of plant species records to known infestations and ranked from 0 (lowest priority) to 100 (highest possible priority). Δ Score (2070) refers to the absolute change in priority score by 2070, assuming that only habitat suitability for P. cinnamomi changes.
Discussion
From our glasshouse susceptibility tests (Rigg et al. 2018; Wan et al. 2019; this paper), we have extracted P. cinnamomi from the roots of 22 plant species occurring in NSW, from 58 rare and threatened species tested. In all, 7 of the 36 species from which P. cinnamomi was not re-isolated had significantly greater mortality of inoculated plants than of control plants. Phytophthora cinnamomi can clearly have an effect on some NSW species, but the results were somewhat ambiguous, with effects ranging from negligible to severe, and from infected and rarely killed to often killed but rarely infected. How then should the results of these tests be interpreted and used in a management context?
Apart from species with habitat constraints on P. cinnamomi effects (e.g. P. squamulosum subsp. alpinum, which occurs in cold habitats unsuitable for the pathogen), a precautionary approach should be taken for species found to be severely affected in glasshouse inoculation tests; such species should be assumed to be severely affected in the wild, with the results serving as a trigger for field investigations of plant health and the necessity of pathogen hygiene. We identified 10 severely affected species, with high levels of re-isolation and mortality in glasshouse tests. Phytophthora cinnamomi has also been isolated from the roots of two of these species in the wild, namely Hibbertia circinata and Pultenaea baeuerlenii, and, in the case of the former at least, it appears to be driving that species towards extinction (McDougall et al. 2023).
For species that had moderate to high levels of re-isolation but little or no mortality, they either tolerate infection or the tests were not given enough time for poor plant health and mortality to develop. Tolerance of infection by P. cinnamomi has been demonstrated in many Australian plant species (e.g. some grasses, Phillips and Weste 1984; annual forbs in Western Australia, Crone et al. 2013). Tolerance may occur through cell compartmentalisation, which limits pathogen movement in plant roots (Cahill et al. 1989). Disease management will generally not be required for tolerant species. However, without closer study, it will not be possible to distinguish between tolerance and insufficient time for disease development and, so, again, a precautionary approach to management is warranted for these species. In addition, species that are capable of being infected but rarely show symptoms may be adversely affected when other stressors are present in wild populations (e.g. insect herbivores, other pathogens, extreme climate events).
Species in the glasshouse tests that had significantly high mortality but no apparent infection by P. cinnamomi may still be susceptible to infection and should be treated as such for management purposes. Wan et al. (2019) suggested that the pathogen may have already left the host by the time re-isolation was attempted in two test species in glasshouse tests (Plinthanthesis rodwayi and Phebalium bifidum), which had very few roots remaining to test. In the current study, we tried to avoid this by attempting re-isolation as soon as plants died; however, one species (Correa baeuerlenii) still had significant mortality but no re-isolations. Only apparently lesioned parts of roots were plated out for the re-isolations and, so, it is possible that infected areas were missed if the lesions were not obvious. The entire root system should be plated out if possible.
For species in our glasshouse tests that had no re-isolations and no significant mortality, disease management in wild populations will rarely be required. The conditions in the glasshouse were ideal for the pathogen, with initial flooding and proximity to plant roots of only a few millimetres; with few or no effects, the species are probably effectively resistant to infection.
Across all of our glasshouse susceptibility tests (Rigg et al. 2018; Wan et al. 2019; this paper), 10 of 58 species (17%) were severely affected by P. cinnamomi. This is very close to the 14% of species in south-western Western Australia regarded by Shearer et al. (2004) as highly susceptible (i.e. severely affected) in the wild. The NSW sample may be small but it does suggest that the effect of this pathogen on the State’s flora will not be insubstantial and may be comparable to that in other jurisdictions.
Our prioritisation of NSW rare and threatened species for susceptibility testing is not meant to be prescriptive and there is no prioritisation score, below which testing is not warranted. It is also unlikely to be free from error because of spatial errors in ALA data. However, it should help identify species potentially at most risk if they are severely affected by P. cinnamomi, and enable appropriate management to be implemented. Availability of test plants will often affect species choice. Indeed, propagation techniques for some species (e.g. of the genera Thismia and Euphrasia, both containing highly ranked species) are unknown. In addition, the appearance of symptomatic plants in the field may increase the urgency of testing. However, it is clear that some parts of NSW should be the focus of susceptibility testing because they are where the habitat is most suitable for P. cinnamomi and where numerous rare plant species are found. Most notable of these is the Blue Mountains area, including the Burragorang, Kanangra and Wollemi IBRA 7 subregions. The discovery through glasshouse testing of species severely affected by P. cinnamomi may make species eligible for threatened species listing; 52 of the 100 highest ranked species for testing are not listed as threatened, but have extent of occurrences that meet Criterion B1 for IUCN assessment (IUCN Standards and Petitions Committee (2022).
In total, 15 of the 100 highest-ranked species for susceptibility testing were in the genus Hibbertia. This in part reflects the recent recognition of distinct taxa with narrow ranges from within widespread species aggregates (e.g. Toelken and Miller 2012; McDougall et al. 2018b). Indeed, one of the species tested for susceptibility in this paper, Hibbertia circinata, was until recently included in the widespread species H. linearis; H. circinata is highly susceptible to infection and under a high risk of extinction because its only population is at a site infested with P. cinnamomi (McDougall et al. 2023). The same may be true for the genus Pultenaea. Pultenaea praecipua subsp. praecipua (previously P. sp. Genowlan Point) was one of eight new Pultenaea taxa recently described in the Blue Mountains (Renner et al. 2022). It was found to be severely affected by P. cinnamomi in a glasshouse study (Wan et al. 2019); none of the other seven new taxa has been tested for susceptibility to P. cinnamomi. This highlights the importance of plant taxonomic research in Phytophthora management.
As with our prioritisation of species for susceptibility testing, there is no empirical score for species of known susceptibility below which management is not warranted and some lowly ranked species may be at risk under unusual climatic events. This is partly because the habitat suitability model for P. cinnamomi (Category A) used mean climatic conditions (McDougall and Liew 2020). If P. cinnamomi can survive in marginal habitat, extreme weather events may periodically make conditions favourable for disease development. Extreme rainfall events are thought to have influenced episodic disease in eucalypts in eastern Victoria (Tregonning and Fagg 1984). This appears also to be the case for the critically endangered shrub Pomaderris delicata. Its priority score was low because its Phytophthora habitat suitability was only 0.45, indicating marginal habitat. Pomaderris delicata was found by Wan et al. (2019) to be susceptible to infection but rarely showed symptoms in glasshouse tests. There has been an active program of enhancement planting for this species, following a population crash from an unknown cause in 2012 (McDougall et al. 2018a). Deaths of mature plants were again observed in 2022 and the pathogen was re-isolated from roots of symptomatic plants in the wild (Rob Armstrong, NSW Department of Planning and Environment, pers. comm.). Mortality in the natural population may be linked to La Niña weather events, bringing above-average rainfall to the populations and increasing suitability for the pathogen. Annual rainfall at nearby Goulburn in 2010, 2021 and 2022 (La Niña years) was the highest in the period from 1972. Prophylactic treatment with phosphite of plants of susceptible species in marginal habitat for P. cinnamomi where the pathogen is present may be required when a La Niña year is forecast.
For species with a high priority score, which have a moderate to severe response to infection, resource allocation and the type of management undertaken will depend on whether P. cinnamomi is already present and, especially, whether it is present in all populations. Phytophthora cinnamomi is present in or close to all populations of Hibbertia circinata, Boronia imlayensis, Prostanthera marifolia and Pomaderris delicata. Treatment of plants with phosphite has been found to provide some plant species with a defence to infection (e.g. Hardy et al. 2001) and will be appropriate if the chemical does not have phytotoxic effects. Ex situ conservation (e.g. the storage of genetic material or establishment of live collections in botanic gardens) is likely to be important. If treatment of plants in situ fails, the identification of Phytophthora-free refugia may be required so that protectable populations can be established.
Phytophthora cinnamomi is present in some populations of the following species severely affected by the pathogen: Prostanthera walteri, Wollemia nobilis, Plinthanthesis rodwayi, Pultenaea baeuerlenii, P. benthamii, P. parrisiae, Persoonia mollis subsp. budawangensis and Grevillea irrasa subsp. irrasa. The management response for these species may depend on the number of populations unaffected. For highly restricted species, the response will be much the same as for species with all populations infested, except that uninfested populations, if confirmed to be free of the pathogen, will be the refugia for these species, and hygiene to prevent the introduction of P. cinnamomi will be critical. Hygiene will be especially important for Nematolepis rhytidophylla, which is known only from one population in a remote mountain plateau of south-eastern NSW; limited soil sampling in the vicinity has so far not located the pathogen. The co-existence of other threats may make management of P. cinnamomi more difficult. In the case of W. nobilis, a second Phytophthora species (P. multivora P.M. Scott & T. Jung) is also present at one site and known to be pathogenic (Puno et al. 2015).
Soil sampling is required in populations of Isopogon fletcheri, Hibbertia puberula subsp. glabrescens, Haloragodendron lucasii, Hibbertia spanantha, Pultenaea praecipua subsp. praecipua, Dracophyllum macranthum, Correa lawrenceana var. genoensis, Darwinia peduncularis and Phebalium speciosum to determine whether P. cinnamomi is present; monitoring for disease symptoms is also recommended. Management options will depend on the outcomes of these surveys. Curiously, Dracophyllum macranthum was delisted during the course of our glasshouse susceptibility tests because it was found to be more abundant than first thought (Bell and Sims 2018). Its high susceptibility to infection may necessitate a re-evaluation of its conservation status if the pathogen is found in the population, although its lithophytic habitat may provide protection from P. cinnamomi.
Similarly, monitoring plant health in highly ranked species thought to be unaffected by P. cinnamomi is recommended in case the pathogen has a greater effect in the field than in the glasshouse. This is especially important for Eucalyptus imlayensis, only known from a single population of 17 individuals (Simmons et al. 2023) within a site infested with P. cinnamomi. In a glasshouse test (E. C. Y. Liew and K. L. McDougall, unpubl. data), plants did not develop symptoms and P. cinnamomi was not re-isolated from roots. The consequences of disease from P. cinnamomi in the wild population, perhaps under unusual climatic conditions, could be catastrophic.
The modelled effect of climate change on habitat suitability for P. cinnamomi indicates that there will be little change in risk for high-priority species known to be affected by the pathogen, but it is possible that climate extremes (e.g. drought, intense rainfall events) will exacerbate disease regardless of what happens to mean conditions. A possible exception is Phebalium speciosum, which occurs in marginal habitat for P. cinnamomi now (mean habitat score = 0.44), but is predicted to occur in habitat highly unfavourable to P. cinnamomi in 2070 (mean habitat score = 0.02). The questions will be that if P. cinnamomi is already present in populations of Phebalium speciosum, can the host survive for long enough to benefit from declining habitat quality for P. cinnamomi and, will the habitat still be suitable for Phebalium speciosum in 2070? An important caveat to these predictions of change is that they consider only suitability of sites for P. cinnamomi and not whether the pathogen is closer to threatened species populations in 2070 or whether some populations have become extinct (variables B and C in our prioritisation score).
Local extinction of the rare and threatened species we highlight as being severely affected by P. cinnamomi is unlikely to lead to catastrophic ecosystem decline because they are not ecosystem dominants or keystone species. However, loss of these species may have less obvious, yet significant, consequences. For instance, co-extinctions may occur where there are narrow plant–insect mutualisms (Moir et al. 2010); such close associations have been identified for moth pollinators of some Boronia species (e.g. Duretto et al. 2023). There may also be lost opportunities associated with extinction; for example, some endophytic fungi of Wollemia nobilis have medicinal properties (Strobel et al. 1997; Staniek et al. 2010).
Conclusions
Woinarski et al. (2021) identified factors responsible for uncertainty in threatened species listing and management. These included inadequate taxonomy and poor knowledge of threats. We have highlighted how both of these are important in evaluating the threat from P. cinnamomi and prioritising management responses, and indeed how they are inter-linked. Until the taxonomy of some apparently widespread taxa (e.g. Hibbertia and Pultenaea spp.) is resolved, the threat from P. cinnamomi cannot be adequately assessed. A precautionary response is recommended until knowledge about susceptibility and risk is quantified for more species. This should involve assuming that P. cinnamomi is absent from populations of highly ranked species of unknown susceptibility, which tend to occur in localised areas highly suitable for the pathogen. Hygiene for these species should be implemented where possible; this might include limiting access by vehicles and people to dry periods, and implementing effective boot-cleaning facilities for bushwalkers (Liew et al. 2023). Greater protection and management of refuge areas as recommended by Woinarski et al. (2021) will buy time for many species until research provides the knowledge needed for targeted species management, as will management of P. cinnamomi at the landscape level.
Data availability
The raw data for the species prioritisation are provided in Supplementary material S1.
Declaration of funding
Funding for this project was provided by the NSW Department of Environment and Planning as part of the Saving Our Species (SoS) program.
Author contributions
KLM prepared a first draft of the paper and analysed the data; ECYL maintained the plants in the glasshouse, inoculated pots and plated out roots for re-isolation; KLM and ECYL contributed to the preparation of the final paper.
Acknowledgements
Thank you to Maureen Phelan for the help with planting, general plant maintenance, inoculation and harvesting and for staff at the Australian National Botanic Gardens and National Botanic Garden for their expertise in growing the plants.
References
Barker PC, Wardlaw TJ (1995) Susceptibility of selected Tasmanian rare plants to Phytophthora cinnamomi. Australian Journal of Botany 43, 379-386.
| Crossref | Google Scholar |
Bell S, Sims R (2018) Extensive populations of Dracophyllum macranthum (Ericaceae) in Coorabakh National Park suggest a review of threat status. Australasian Plant Conservation 27(2), 11-14.
| Crossref | Google Scholar |
Burgess TI, McDougall KL, Scott PM, Hardy GESt, Garnas J (2019) Predictors of Phytophthora diversity and community composition in natural areas across diverse Australian ecoregions. Ecography 42, 565-577.
| Crossref | Google Scholar |
Cahill D, Legge N, Grant B, Weste G (1989) Cellular and histological changes induced by P. cinnamomi in a group of plant species ranging from fully susceptible to fully resistant. Phytopathology 79, 417-424.
| Crossref | Google Scholar |
Cahill DM, Rookes JE, Wilson BA, Gibson L, McDougall KL (2008) Turner review no. 17. Phytophthora cinnamomi and Australia's biodiversity: impacts, predictions and progress towards control. Australian Journal of Botany 56, 279-310.
| Crossref | Google Scholar |
Crone M, McComb JA, O’Brien PA, Hardy GESJ (2013) Annual and herbaceous perennial native Australian plant species are symptomless hosts of Phytophthora cinnamomi in the Eucalyptus marginata (jarrah) forest of Western Australia. Plant Pathology 62, 1057-1062.
| Crossref | Google Scholar |
Duretto MF, Heslewood MM, Bayly MJ (2023) A molecular phylogeny of Boronia (Rutaceae): placement of enigmatic taxa and a revised infrageneric classification. Australian Systematic Botany 36, 81-106.
| Crossref | Google Scholar |
Grimes DJ (2006) Koch’s Postulates—then and now. Microbe 1, 223-228.
| Google Scholar |
Hardham AR (2005) Phytophthora cinnamomi. Molecular Plant Pathology 6, 589-604.
| Crossref | Google Scholar | PubMed |
Hardy GESJ, Barrett S, Shearer BL (2001) The future of phosphite as a fungicide to control the soilborne plant pathogen Phytophthora cinnamomi in natural ecosystems. Australasian Plant Pathology 30, 133-139.
| Crossref | Google Scholar |
IUCN Standards and Petitions Committee (2022) Guidelines for Using the IUCN Red List Categories and Criteria. Version 15. Prepared by the Standards and Petitions Committee of the IUCN Species Survival Commission, Gland, Switzerland. Available at https://www.iucnredlist.org/documents/RedListGuidelines.pdf
Kueh KH, McKay SF, Facelli E, Facelli JM, Velzeboer RMA, Able AJ, Scott ES (2012) Response of selected South Australian native plant species to Phytophthora cinnamomi. Plant Pathology 61, 1165-1178.
| Crossref | Google Scholar |
Liew ECY, Phelan M, McDougall KL (2023) The efficacy of a range of hygiene measures for boot cleaning to protect natural vegetation from Phytophthora cinnamomi. Scientific Reports 13, 5825.
| Crossref | Google Scholar | PubMed |
McDougall KL, Liew ECY (2020) Quantifying the distribution and threat of Phytophthora cinnamomi in New South Wales: implications for its management in natural vegetation. Cunninghamia 20, 153-181.
| Google Scholar |
McDougall KL, Summerell BA (2003) The impact of Phytophthora cinnamomi on the flora and vegetation of New South Wales – a re-appraisal. In ‘Phytophthora in Forests and Natural Ecosystems. 2nd International IUFRO Working Party 7.02.09 Meeting’, Albany, Western Australia, October 2001. (Eds. JA McComb, GESJ Hardy, IC Tommerup) pp. 49–56. (Murdoch University Print: Murdoch, WA, Australia)
McDougall KL, Armstrong R, McAuliffe J, Taylor D (2018a) Threatened plant translocation case study: Pomaderris delicata (Delicate Pomaderris), Rhamnaceae. Australasian Plant Conservation 27, 10-12.
| Google Scholar |
McDougall KL, Wright GT, Walsh NG (2018b) Hibbertia circinata (Dilleniaceae: subgen. Hibbertia), a new species from south-eastern New South Wales. Telopea 21, 39-44.
| Crossref | Google Scholar |
McDougall KL, Wright GT, Bredell PM, James EA, Simmons L (2023) Mount Imlay – an island of floristic significance on the brink. Cunninghamia 23, 1-9.
| Google Scholar |
Moir ML, Vesk PA, Brennan KEC, Keith DA, Hughes L, McCarthy MA (2010) Current constraints and future directions in estimating coextinction. Conservation Biology 24, 682-690.
| Crossref | Google Scholar | PubMed |
Phillips D, Weste G (1984) Field resistance in three native monocotyledon species that colonize indigenous sclerophyll forest after invasion by Phytophthora cinnamomi. Australian Journal of Botany 32, 339-352.
| Crossref | Google Scholar |
Puno VI, Laurence MH, Guest DI, Liew ECY (2015) Detection of Phytophthora multivora in the Wollemi Pine site and pathogenicity to Wollemia nobilis. Australasian Plant Pathology 44, 205-215.
| Crossref | Google Scholar |
R Core Team (2023) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Available at https://www.R-project.org/
Reiter N, Weste G, Guest D (2004) The risk of extinction resulting from disease caused by Phytophthora cinnamomi to endangered, vulnerable or rare plant species endemic to the Grampians, Western Victoria. Australian Journal of Botany 52, 425-433.
| Crossref | Google Scholar |
Renner MAM, Barrett RL, Clarke S, Clugston JAR, Wilson TC, Weston PH (2022) Morphological and molecular evidence refute a broad circumscription for Pultenaea glabra (Fabaceae: Mirbelieae), with implications for taxonomy, biogeography, and conservation. Australian Systematic Botany 35, 225-277.
| Crossref | Google Scholar |
Rigg JL, McDougall KL, Liew ECY (2018) Susceptibility of nine alpine species to the root rot pathogens Phytophthora cinnamomi and P. cambivora. Australasian Plant Pathology 47, 351-356.
| Crossref | Google Scholar |
Scholthof KBG (2007) The disease triangle: pathogens, the environment and society. Nature Reviews Microbiology 5, 152-156.
| Crossref | Google Scholar | PubMed |
Shearer BL, Crane CE, Cochrane A (2004) Quantification of the susceptibility of the native flora of the South-West Botanical Province, Western Australia, to Phytophthora cinnamomi. Australian Journal of Botany 52, 435-443.
| Crossref | Google Scholar |
Shearer BL, Crane CE, Cochrane JA, Dunne CP (2013) Variation in susceptibility of threatened flora to Phytophthora cinnamomi. Australasian Plant Pathology 42, 491-502.
| Crossref | Google Scholar |
Simmons L, Wright GT, McDougall KL, James EA (2023) Conservation and genomic diversity of a rare tree, Eucalyptus imlayensis (Myrtaceae), regenerating after wildfire. Muelleria 41, 17-28.
| Google Scholar |
Staniek A, Woerdenbag HJ, Kayser O (2010) Screening the endophytic flora of Wollemia nobilis for alternative paclitaxel sources. Journal of Plant Interactions 5, 189-195.
| Crossref | Google Scholar |
Strobel GA, Hess WM, Li JY, Ford E, Sears J, Sidhu RS, Summerell B (1997) Pestalotiopsis guepinii, a taxol-producing endophyte of the Wollemi pine, Wollemia nobilis. Australian Journal of Botany 45, 1073-1082.
| Crossref | Google Scholar |
Toelken HR, Miller RT (2012) Notes on Hibbertia (Dilleniaceae) 8. Seven new species, a new combination and four new subspecies from subgen. Hemistemma, mainly from the central coast of New South Wales. Journal of the Adelaide Botanic Garden 25, 71-96.
| Google Scholar |
Tregonning KC, Fagg PC (1984) Seasonal rainfall and Eucalyptus dieback epidemics associated with Phytophthora cinnamomi in Gippsland, Victoria. Australian Forest Research 14, 219-234.
| Google Scholar |
Wan JSH, McDougall KL, Liew ECY (2019) The susceptibility of rare and threatened NSW species to the root-rot pathogen Phytophthora cinnamomi: 1. Initial testing and identification of key research questions. Australian Journal of Botany 67, 510-516.
| Crossref | Google Scholar |


