Measurement of corticosterone in the plasma, eggs and faeces of laying hens
Joanna M. Engel A , Paul H. Hemsworth A , Kym L. Butler A B and Alan J. Tilbrook
A , Kym L. Butler A B and Alan J. Tilbrook  C *
C *
A Animal Welfare Science Centre, Faculty of Veterinary and Agricultural Sciences, The University of Melbourne, Parkville, Vic. 3010, Australia.
B Biometrics Team, Agriculture Victoria Research, Department of Jobs, Precincts and Regions, Hamilton, Vic. 3300, Australia.
C Centre for Animal Science, Queensland Alliance for Agriculture and Food Innovation and the School of Veterinary Science, The University of Queensland, St Lucia, Qld 4072, Australia.
Animal Production Science 62(9) 828-835 https://doi.org/10.1071/AN21535
Submitted: 13 October 2021 Accepted: 23 February 2022 Published: 26 April 2022
© 2022 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Context: Stress responses in chickens are commonly assessed from measurements of corticosterone in blood, but there is an increasing research effort to develop non-blood means of assessing the activity of the hypothalamo–pituitary (HPA) axis. It is common to measure corticosterone in the eggs and faeces.
Aims: We extended previous work by undertaking a study of caged laying hens comparing basal concentrations of corticosterone in plasma, faeces, egg albumen and egg yolk on a between-cage basis. We tested the hypothesis that there are positive relationships between corticosterone in plasma and corticosterone in each of the other matrices.
Methods: Blood samples were collected from each bird at a single point in time. In Experiment 1, these comparisons (between plasma concentrations of corticosterone on Day 1 and egg albumen, egg yolk and faecal concentrations of corticosterone on Days 1, 3 and 4 of the study) were made for hens of two ages under basal conditions, whereas, in Experiment 2, the comparisons (between plasma concentrations of corticosterone on Day 3 and egg albumen, egg yolk and faecal concentrations of corticosterone on Days 1 and 2 of the study) were made for hens housed at different space allowances with and without access to a nest box. The birds without a nest box had not had experience with a nest box prior to sampling.
Key results: There was a statistically significant (P = 0.012), but limited, positive relationship between plasma and egg albumen concentrations of corticosterone under basal conditions in Experiment 2. There were no other statistically significant (P > 0.05) relationships in either experiment. These results suggest that measures of corticosterone in the albumen, yolk and faeces of laying hens are unlikely to be robust predictors of basal concentrations of corticosterone in the blood.
Conclusions: Although there was some indication that concentrations of corticosterone in albumen may be related to concentrations in blood under basal conditions, based on all the results, this suggestion is made cautiously.
Implications: More comprehensive research is required to establish if measures of corticosterone in egg components and faeces are related to chronic basal activity of the hypothalamo–pituitary axis in laying hens. There is also a need to understand the impact of corticosterone on production, reproduction and welfare in hens from measures in both blood and non-blood matrices.
Keywords: corticosterone, egg, excreta, faeces, laying hen, plasma, stress.
Introduction
There is increasing interest in using non-blood measures to assess stress in livestock, including laying hens. In blood, the most commonly measured substances to assess stress are the glucocorticoids, which are indicative of the activity of the hypothalamo–pituitary adrenal (HPA) axis (Selye 1946; Sapolsky et al. 2000). Glucocorticoids have also been measured in a variety of non-blood matrices in many species with variable relationships to concentrations in blood (Möstl et al. 2005; Lane 2006; Burnard et al. 2017; Tilbrook and Ralph 2017; Tilbrook et al. 2018; Weaver et al. 2021). Development of non-blood measures of glucocorticoids have commonly been undertaken in the belief that they are non-invasive and, thus, reduce the likelihood of the sampling procedure stimulating the HPA axis, which may confound the results (Möstl et al. 2005; Lane 2006; Burnard et al. 2017; Tilbrook and Ralph 2017). Nonetheless, it is possible to develop procedures to sample blood where animals are habituated such that the HPA axis is not activated (Ralph and Tilbrook 2016; Tilbrook and Ralph 2017). Even if the blood sampling procedure does activate the HPA axis, the effect is unlikely be seen in that sample, because it takes from minutes to hours for glucocorticoids to be synthesised, be measurable in the blood and to act (Sapolsky et al. 2000; Ralph and Tilbrook 2016; Tilbrook and Ralph 2017).
Nevertheless, it remains useful to develop the ability to assess stress responses by measuring glucocorticoids in matrices other than blood, especially in situations when assessment of stress responses is needed on a group of animals basis. These situations include experimental examination of management options of animals (such as diets, pen design, level of human contact, genetics), in which the experimental unit will usually be a group of hens. Another situation is the commercial monitoring of the overall stress status of animals in a farm, or portion of a farm. In these situations, the use of matrices, such as faeces or eggs, avoids the labour resource of handling individual animals, a major cost saving. Indeed, the use of other matrices involving faeces or eggs might be practically difficult on an individual animals basis in many experimental and commercial situations, and, thus, the assessment of the efficacy of different matrices is most practically useful on a group basis. Also, in terms of laying hens, the use of other matrices, such as faeces, egg albumen or egg yolk, could be necessary when the impact of the sampling procedure on the HPA axis cannot be controlled. Other matrices could also be advantageous when it is necessary to determine the activity of the HPA axis over an extended period. When glucocorticoids are measured in matrices other than blood, it is important to appreciate the time between synthesis of glucocorticoids and their deposition in the matrices.
In laying hens, the principal glucocorticoid, corticosterone, has been measured in eggs (Rettenbacher et al. 2005; Downing and Bryden 2008; Cook et al. 2009; Singh et al. 2009) and faeces (Dehnhard et al. 2003; Rettenbacher et al. 2004; Möstl et al. 2005; Cook et al. 2009). Comparisons with blood concentrations have led to inconsistent outcomes. Corticosterone concentrations have been measured in both yolk and albumen following administration of adrenocorticotrophic hormone (ACTH) and infusion of corticosterone (Rettenbacher et al. 2005). Whereas there was no effect of ACTH treatment, after administration of radio-labelled corticosterone, peak levels were measured in the albumen and outermost layer of the yolk after 1 day, whereas the highest levels of corticosterone appeared in the innermost layers of the yolk 4–6 days later (Rettenbacher et al. 2005). On an individual hen basis, a positive correlation (r = 0.87) was found between plasma and albumen corticosterone concentrations 1 day after hens were given injections of different doses of corticosterone that led to plasma concentrations ranging from ∼1–40 ng/mL in different hens (Downing and Bryden 2008). However, cursory examination of the results in this paper (Fig. 1 of the paper) suggests only a weak relationship between hens injected with the same dose of corticosterone. Corticosterone was measured in the yolk and albumen of eggs of hens of different strains in floor pens and cages (Singh et al. 2009). There were some differences between strains, and there were age effects such that there were higher concentrations of corticosterone in the egg components at 22 weeks than at 45 weeks. Concentrations of corticosterone in yolk were higher in hens in cages than in floor pens, whereas the concentrations in albumen were higher in hens from floor pens (Singh et al. 2009). No blood samples were collected in this study. In another study, where corticosterone was compared in serum, egg albumen and yolk, on a between-hen basis, there were several positive correlations under basal conditions, but not following stimulation with ACTH (Cook et al. 2009b). This led the authors to question the usefulness of concentrations of corticosterone in albumen and yolk as biomarkers of stress-induced activity of the HPA axis (Cook et al. 2009b). Concentrations of metabolites of corticosterone in faeces of hens increased following injection of ACTH with a similar albeit less pronounced pattern of increase in plasma corticosterone following ACTH (Dehnhard et al. 2003).
Although there is evidence that it is possible to measure corticosterone in eggs and faeces of hens, the relevance in terms of assessing stress remains unclear, because the results have been variable both within and between studies. Furthermore, the relative usefulness of eggs compared with faeces as non-blood measures of stress are unknown, because there have been no direct comparisons of corticosterone in blood, eggs and faeces in the one study. Therefore, we have extended this area of research to include measurement of corticosterone in plasma, faeces, egg albumen and egg yolk in laying hens of different ages, under basal conditions and when housed in adulthood under different space allowances with and without access to a nest box. As we were examining basal conditions, we did not impose any stress treatments. We examined the hypothesis that on a between-cage of hen basis, in laying hens, there are positive relationships between corticosterone in blood, and measures of corticosterone in faeces, egg albumen and egg yolk when these non-blood measures are taken on the same day or different days to the plasma sample.
Materials and methods
Animal experimentation ethics approval was obtained for all procedures from the Animal Ethics Committee at the University of Melbourne. The procedures in this experiment were conducted in accordance with the Australian Prevention of Cruelty to Animals Act 1986 and the National Health and Medical Research Council/Commonwealth Scientific and Industrial Research Organisation/Australian Animal Commission ‘Australian practice for the care and use of animals for scientific purposes’.
Animals and housing
Experiment 1
Laying hens (n = 154) of the Hy-Line Brown breed were housed in cages of seven or eight birds with space allowance of 550–625 cm2/bird within two commercial poultry sheds, at the same commercial poultry farm, in Kinglake West (Victoria, Australia; 37°28′S, 145°14′E). The age of the hens was 34 weeks in one shed and 47 weeks in the other shed. Each shed consisted of several aisles of adjacent cages. Within each shed, five cages were selected near the middle of an aisle and five were selected from near the end of an aisle. Within each shed, different aisles were used for the five ‘near middle’ cages, and different aisles were used for the five ‘near end’ cages. Hens were exposed to 14.5 h of light in the shed housing 34-week-old hens and 16 h in the shed housing 47-week-old hens. Hens were fed a formulated layer diet based on 95 g feed consumed/hen per day (formulated and mixed by the farm to have 15% crude protein), with feed delivered up to four times each day.
Experiment 2
Experiment 2 was designed to jointly examine the effects of space allowance during rearing (7–16 weeks of age), space allowance during adulthood (16–34 weeks of age) and nest box access during adulthood on the welfare of caged laying hens. The hens without a nest box had not had experience with nest boxes prior to sampling. Full details of this aspect of the study have been previously reported in Engel et al. (2019). Within each of four time replicates, during adulthood, there was one cage of six hens in each of eight factorial (two rearing space allowances (315 and 945 cm2 per bird) × 2 adult space allowances (542 and 1648 cm2 per bird) × 2 nest box conditions (presence vs absence)) combinations. Hens were Hy-line Brown pullets that were sourced, at 7 weeks of age, from a commercial poultry farm in Victoria, Australia. The experiment was performed at an agricultural research facility, with non-treatment conditions (diet, lighting, temperature etc.) chosen to approximate commercial production. As reported by Engel et al. (2019), there were no effects of the treatments during rearing or adulthood on plasma, egg albumen and yolk. and fecal corticosterone concentrations at 26 and 27 weeks of age.
Sample collection and preparation
Experiment 1
In each shed, blood samples (3 mL) were collected at a single point in time via the wing vein from the birds on the first day of collection (Day 1 of collection) from 12:00–15:30 hours using a 4.5-mL heparin-coated syringe with a 23-G needle. All samples were taken within 2 min of the hen being removed from her home cage to avoid an acute stress response due to capture and handling, influencing basal plasma cortisol concentrations (Broom and Johnson 1993). Hens were then placed in a holding crate until all birds from the same cage had been sampled. The order of selecting pens for blood sampling was randomised.
On Day 1, faecal and egg samples were taken over 24 h, 06:00–06:00 hours (Table 1). Sampling then ceased for 1 day (Day 2). Egg and excreta were then collected over 24 h for the following 2 days (Days 3 and 4).
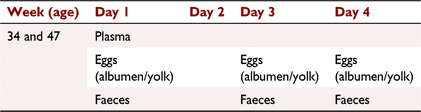
|
Plasma was harvested from the blood by centrifugation. Eggs were separated into the albumen and yolk. Faecal samples were placed in a drying oven at 60°C for 48 h. For each of the three 24-h sample periods, plasma, albumen, yolk and faecal samples were pooled for each cage, and then stored at –20°C until extraction and analysis.
Experiment 2
Blood (3 mL) was collected at a single point in time from the wing vein of each bird, using a 4.5-mL heparin-coated syringe with a 23-G needle, at 12:00 hours on a single occasion at 26 weeks of age and on a single occasion at 27 weeks of age (Table 2). All samples were taken within 2 min of the hen being removed from her home cage. After centrifugation, the samples were pooled within the cage and over the 2 days, to produce a single combined sample for each cage. The plasma was then stored at –20°C until extracted and analysed. As in Experiment 1, all samples were taken within 2 min of the hen being removed from her home cage.
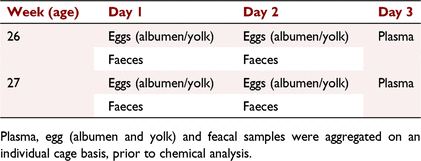
|
All unbroken eggs were collected from each cage over the 2 days prior to blood collection at 26 and 27 weeks of age (Table 2). Eggs were separated into the albumen and yolk, and a sample was retained (4–6 g and 10–12 g for the yolk and albumen, respectively). Albumen and yolk samples were pooled per cage and then pooled again for the four collection days (2 days in each of week 26 and 27). They were then frozen at –20°C until sent for extraction and analysis.
Excreta from each cage were also collected over the 2 days prior to baseline blood sampling at 26 and 27 weeks of age. Collection occurred over three 1-h periods on each day. At this time, manure belts were cleared of excreta and greaseproof paper was placed under each cage. At the end of each hour, excreta were collected into an aluminium container. At the end of the day, samples were placed in a drying oven at 60°C for 48 h. Once dry, samples were ground. Samples for each cage were pooled over the four sampling days (2 days in each week 26 and 27) and frozen at –20°C until extraction and analysis.
Sample analysis
Experiment 1 and time replicates 1 and 2 from Experiment 2
Plasma samples were not extracted. The assay used was a Corticosterone HS Enzyme Immunoassay (IDS, Boldon, UK). The sensitivity for the assay was 0.17 ng/mL with and intra-assay variations of 7–15%.
Egg yolk was extracted following accepted methodologies (Cook et al. 2009b). Briefly, egg yolk (0.5 g) was added to 1 mL of distilled water and vortexed until mixed. The mixture was extracted with 3 mL hexane:diether (30:70 v/v), vortexed and left to settle before being snap frozen in an ethanol/dry ice bath. The supernatant was collected, dried and 1 mL of ethanol added, before being frozen at –20°C overnight. The samples were centrifuged the next day, and the supernatant removed and dried once more before being suspended in phosphate-buffered saline (PBS) and analysed. Egg albumen was extracted following published methodologies (Downing and Bryden 2008). Briefly, egg albumen (5 g) was added to 5 mL of distilled water. These were mixed, and 0.5 g of the mixture was extracted with 4 mL of diethyl ether, shaken for 10 min and then frozen at –80°C. The supernatant was then collected and dried. The samples were suspended in PBS and analysed. Ground, dried excreta were extracted using validated methodologies (Wasser et al. 1994). Briefly, ground, dried excreta (0.1 g) were extracted with 1 mL of 80% methanol. The samples were then vortexed for 30 min and centrifuged. The supernatant was dried and then suspended in PBS for analysis. The assay used was a Corticosterone HS Enzyme Immunoassay (IDS). The sensitivity of the assay was 0.17 ng/mL with an intra-assay variation of 7–15%.
Time replicates 3 and 4 from Experiment 2
The extraction procedure for replicates 3 and 4 in Experiment 2 differed from those used in Experiment 1 and time replicates 1 and 2 of Experiment 2, due to the use of a different chemistry laboratory. For the samples analysed in replicates 3 and 4, plasma samples were extracted following the methods described by Downing and Bryden (2008). Egg yolk was extracted using the protocol described for laboratory 1. The only exceptions were that 0.1 g of yolk was added to 0.5 mL of distilled water and the ethanol mixture was frozen at –80°C overnight. Egg albumen samples were extracted following the same procedures as the laboratory used for Experiment 1 and time replicates 1 and 2, except that 0.5 g of albumen was mixed with 1 mL of distilled water. Ground, dried excreta were extracted following the methodologies described by Brown et al. (1994). Further extraction details from replicates 3 and 4 of this experiment are presented in Engel et al. (2019).
The radioimmunoassay included one standard curve and unknown extracted samples in duplicate. The standard curve included triplicate tubes for total counts (and non-specific binding, nine replicates of the zero standard, three replicates of each standard and six replicates each of two quality control pools containing 0.71 and 2.22 ng/mL, which were used to estimate the intra-assay coefficients of variation (5% and 5.7%).
A total of 100 μL of H3-corticosterone tracer (1,2,6,7-H3; Amersham Biosciences, Sydney, NSW, Australia) and 150 μL first antibody (B3-163; Endocrine Sciences, Calabasas, CA, USA) were added to tubes containing extracted samples, and 150 μL of buffer was added to the non-specific binding tubes. Tubes were vortexed and incubated at 4°C for 24 h. On Day 2, normal rabbit serum (100 μL, 1:800) was added, followed by 100 μL second antibody (anti-rabbit serum; 1:60 in PBS). The tubes were mixed and incubated overnight at 4°C. On Day 3, 1 mL of 6% polyethylene glycol (PEG 6000) in PBS was added to all tubes (except total counts). The tubes were centrifuged in a refrigerated (5°C) centrifuge at 1500g for 25 min, the supernatant aspirated and the pellet was redissolved in 500 μL of HCL (0.05 M). The solution was dispensed into counting vials and then mixed with 2 mL of scintillant (Starcint; Packard Chemicals Operations). The vials were capped, shaken and left in the dark for 2 h before counting in a liquid scintillation counter (Packard Tri Carb 1500). The sensitivity of the assay was 0.1 ng/mL, with an intra-assay variation of 5–5.7%.
Statistical analysis
Experiment 1
Basal plasma corticosterone was related to each other corticosterone measurement using parallel linear regression with different intercepts for the shed with 34-week-old hens and the shed with 47-week-old hens. Additionally, an effect of position in aisle (‘near middle’ or ‘near end’) was examined by adding a two-level factor for this term in the model for each corticosterone measurement. In all analyses, the unit of analysis was all hens in a cage.
Experiment 2
Basal plasma corticosterone was linearly related to each other corticosterone measurement by fitting restricted maximum likelihood models that also included a fixed effect of time replicate and random effects for the blocking terms of the experimental design (namely, experimental half within time replicate during rearing stage, shed row within half during adult stage and banks of cages within half during adult stage; Engel et al. 2019). P-values for each response were calculated using Wald F-tests. As there were large effects of time replicate on basal plasma corticosterone, the results are presented as parallel linear responses for the four replicates. Data were analysed using Genstat (VSN International, 14th edition).
Results
Experiment 1
There was no evidence (P > 0.1) of a position in aisle effect for any of the analyses. The results of the parallel regressions are shown in Fig. 1. There was no evidence of a relationship between plasma corticosterone concentrations on Day 1, and any of albumen, yolk and faecal corticosterone concentrations on Days 1, 3 and 4 (P > 0.1).
Experiment 2
Within each time replicate, there was a relationship (P = 0.012) between plasma concentrations of corticosterone on Day 3, and egg albumen corticosterone concentrations on Days 1 and 2 (Fig. 2). However, pen means for plasma differed by up to 0.75 ng/mL from the predicted value of plasma obtained from egg albumen. This compares with the range of pen means, within time replicates being 1.5 ng/mL.
No relationships between plasma corticosterone concentration on Day 3 and the other measurements of corticosterone on Days 1 and 2 were found (P > 0.1; Fig. 2).
Discussion
The results of these experiments do not provide robust support for the contention that measurements of corticosterone in eggs and faeces will allow assessment of the basal activity of the HPA axis for groups of laying hens in cages. The results of Experiment 1, where no relationship between plasma corticosterone on Day 1 and corticosterone in any of the other matrices on Days 1, 3 and 4 was detected, suggest that measures of corticosterone in albumen, yolk and faeces do not reflect the basal activity of the HPA axis in the laying hens assessed from samples taken at a single point in time. The only result that contradicted these findings was a positive significant relationship between basal plasma on Day 3 and albumen concentrations of corticosterone on Days 1 and 2 in Experiment 2. It is tempting to suggest that this finding provides limited support for the usefulness of measuring corticosterone in egg albumen to evaluate plasma measures. Nevertheless, this is a reserved suggestion, because the overwhelming outcome from both experiments is that the concentrations in albumen, yolk and faeces are unreliable predictors of the average basal activity of the HPA axis in cages of hens. The variability that our study has highlighted sits well with the variability in the literature (see Introduction), and it underscores how difficult it is to develop non-blood measures of endocrine activity in poultry.
The positive relationship between basal plasma and albumen concentrations of corticosterone in Experiment 2 is similar to other studies (Rettenbacher et al. 2005; Downing and Bryden 2008). Nevertheless, as indicated above, there were no other relationships in either experiment, emphasising a lack of reliability in using these non-blood measures to assess the activity of the HPA axis. We also found that plasma concentrations of corticosterone in laying hens do not consistently reflect the concentrations in the liver, heart and skeletal muscle (Ralph et al. 2015), again highlighting the difficulty in establishing relationships between blood and non-blood measures of glucocorticoids in hens. It is possible that the differences between the experiments in the relationship between the concentrations of corticosterone in plasma and albumen was due, at least in part, to the difference between the two experiments in the timing of the blood sampling, and the sampling in eggs and faeces. In Experiment 1, the blood sample was collected prior to the eggs and faeces, whereas, in Experiment 2, the blood sample was collected after the eggs and faeces. This study aimed at understanding the relationships in concentrations of corticosterone between blood, egg and faeces under basal conditions, therefore, it is important to consider that the handling of birds to collect blood may stimulate an acute stress response (see Introduction). Any possible effect of acute activation of the HPA axis would have been avoided in Experiment 2, making it likely to be more representative of basal activity of the HPA axis than Experiment 1. Nevertheless, this suggestion is based on the collection of blood samples at a single point in time. Although it is common to collect blood samples at a single point in time in poultry research, there are limitations to interpretations of the activity of the HPA axis based on this sampling regimen (see below). Further investigation is required with more frequent collection of blood at varying times relative to the collection of eggs and faeces.
The scale of measurement (e.g. between individual hens vs between cages of hens vs between hen farms etc.) can, in principle, affect the relationships between measurements. Our study examined relationships on a between-cage of hens basis, which is different to most previous studies that examined relationships on a between-hen basis. Faecal and egg corticosterone measurements are likely to be most practically useful on the scale we used, or larger scales, because these are the scales in which individual blood sampling becomes more difficult. Nevertheless, our results are in accord with the previous work. This is unsurprising, as different relationships at a cage scale to an individual hen scale would indicate that there were systematic environmental (including social) differences between cages or systematic genetic differences between the hens of different cages that affect either (1) the relationship between plasma corticosterone and the level of corticosterone in faeces or eggs or (2) the average level of corticosterone in different matrices.
Research to date has largely focused on acute activity of the HPA axis rather than prolonged or chronic activity of the HPA axis. The current experiments were not designed to evaluate the basal activity of the HPA axis over an extended period. As indicated, we took blood samples at a single point in time in both experiments. A blood sample at a single point in time may not be sufficient to elucidate the pattern of synthesis of corticosterone, and may not account for diurnal activity of the HPA axis, which is known to occur in hens (Beuving and Vonder 1977). The concentrations of corticosterone or its metabolites in the eggs and faeces are likely to be the result of accumulation over an extended period (Goymann 2005). With respect to albumen, it has been suggested that concentrations of corticosterone represent the general period during which the albumen is laid down in the egg (Etches 1996). There is no doubt that corticosterone can migrate to the egg. This has been clearly shown in experiments where corticosterone has been administered to hens (Rettenbacher et al. 2005; Downing and Bryden 2008). Nevertheless, it is difficult to interpret these experiments from a physiological perspective, and the time of accumulation of corticosterone is such that it is unlikely to be related to plasma concentrations in a blood sample collected at a single point in time, because the accumulation occurs over days (see Introduction). It is possible that corticosterone measures in egg, in particular, and perhaps in faeces, represent activity of the HPA axis over an extended time, similarly to glucocorticoids in hair and wool in mammals (Burnard et al. 2017). Hitherto, the research has not been conducted to test this hypothesis in laying hens.
Although discussion of the sampling method and time is appropriate when interpreting these results, it is also important to appreciate the complexity of the HPA axis and how measures of glucocorticoids, whether in blood, eggs or faeces, can help in the assessment of the activity of stress systems, such as the HPA axis. Furthermore, it is imperative to consider the impact of the stress responses on the hen when relating stress to production, reproduction and welfare (Tilbrook and Ralph 2017; Tilbrook et al. 2018). We have provided insight into the inputs that activate the HPA axis (Ralph and Tilbrook 2016), and into the importance of the effects of corticosterone in tissue at the site of action in laying hens (Ralph et al. 2015). Although there is evidence in both laying hens and broilers that physical handling can affect egg production (Hemsworth and Edwards 2020), currently, our knowledge in laying hens is not adequately advanced to understand the impact of the activity of the HPA axis from peripheral measures of corticosterone alone. Furthermore, interpreting the consequences of the actions of glucocorticoids from peripheral measures is complicated, because these hormones can have different physiological and behavioural effects in different circumstances and at different times (Tilbrook and Ralph 2017). Systematic studies are required to establish how peripheral measures of corticosterone under certain circumstances relate to the impact of stress on laying hens. This has been successfully achieved in female sheep with respect to the impact of glucocorticoids on reproduction (Ralph et al. 2016), and is now required in laying hens for the impact of stress on production, reproduction and welfare.
In conclusion, measures of corticosterone in albumen, yolk and faeces of laying hens are not robust predictors of plasma concentrations of corticosterone when using a sample of blood collected at a single point in time. Although there is some indication that concentrations of corticosterone in albumen may be related to concentrations in blood under basal conditions, this relationship is not definitive. There is a need for research that includes taking more frequent blood samples at varying times relative to the collection of matrices, such as egg and faeces. Importantly, research has not been conducted to establish if these non-blood measures are related to chronic basal activity of the HPA. In addition to the need for research to resolve this, there is a need to understand how peripheral measures of corticosterone in laying hens can provide knowledge about the impact of stress on production, reproduction and welfare of laying hens.
Data availability
The data that support this study will be shared upon reasonable request to the corresponding author.
Conflicts of interest
The authors declare no conflicts of interest. Alan Tilbrook is an Associate Editor of Animal Production Science, but was blinded from the peer-review process for this paper.
Declaration of funding
This project was supported by the Australian Egg Corporation Limited (Australian Eggs).
Acknowledgements
The authors acknowledge the advice and support from Philip Szepe and Professor Tina Widowski.
References
Beuving G, Vonder GA (1977) Daily rhythm of corticosterone in laying hens and the influence of egg laying. Reproduction 51, 169–173.| Daily rhythm of corticosterone in laying hens and the influence of egg laying.Crossref | GoogleScholarGoogle Scholar |
Broom DM, Johnson KG (1993) ‘Stress and animal welfare.’ Vol. 993. (Chapman & Hall: London, UK; Melbourne)
Brown JL, Wasser SK, Wildt DE, Graham LH (1994) Comparative aspects of steroid hormone metabolism and ovarian activity in felids, measured noninvasively in feces. Biology of Reproduction 51, 776–786.
| Comparative aspects of steroid hormone metabolism and ovarian activity in felids, measured noninvasively in feces.Crossref | GoogleScholarGoogle Scholar | 7819459PubMed |
Burnard C, Ralph CR, Hynd P, Edwards JH, Tilbrook AJ (2017) Hair cortisol and its potential value as a physiological measure of stress response in human and non-human animals. Animal Production Science 57, 401–414.
| Hair cortisol and its potential value as a physiological measure of stress response in human and non-human animals.Crossref | GoogleScholarGoogle Scholar |
Cook NJ, Renema R, Wilkinson C, Schaefer AL (2009) Comparisons among serum, egg albumin and yolk concentrations of corticosterone as biomarkers of basal and stimulated adrenocortical activity of laying hens. British Poultry Science 50, 620–633.
| Comparisons among serum, egg albumin and yolk concentrations of corticosterone as biomarkers of basal and stimulated adrenocortical activity of laying hens.Crossref | GoogleScholarGoogle Scholar | 19904642PubMed |
Dehnhard M, Schreer A, Krone O, Jewgenow K, Krause M, Grossmann R (2003) Measurement of plasma corticosterone and fecal glucocorticoid metabolites in the chicken (Gallus domesticus), the great cormorant (Phalacrocorax carbo), and the goshawk (Accipiter gentilis). General and Comparative Endocrinology 131, 345–352.
| Measurement of plasma corticosterone and fecal glucocorticoid metabolites in the chicken (Gallus domesticus), the great cormorant (Phalacrocorax carbo), and the goshawk (Accipiter gentilis).Crossref | GoogleScholarGoogle Scholar | 12714017PubMed |
Downing JA, Bryden WL (2008) Determination of corticosterone concentrations in egg albumen: a non-invasive indicator of stress in laying hens. Physiology & Behavior 95, 381–387.
| Determination of corticosterone concentrations in egg albumen: a non-invasive indicator of stress in laying hens.Crossref | GoogleScholarGoogle Scholar |
Engel JM, Widowski TM, Tilbrook AJ, Butler KL, Hemsworth PH (2019) The effects of floor space and nest box access on the physiology and behavior of caged laying hens. Poultry Science 98, 533–547.
| The effects of floor space and nest box access on the physiology and behavior of caged laying hens.Crossref | GoogleScholarGoogle Scholar | 30165652PubMed |
Etches RJ (1996) Egg formation. In ‘Reproduction in poultry’. (Ed. RJ Etches) pp. 167–207. (CAB international: Wallingford, UK)
Goymann W (2005) Noninvasive monitoring of hormones in bird droppings: physiological validation, sampling, extraction, sex differences, and the influence of diet on hormone metabolite levels. Annals of the New York Academy of Sciences 1046, 35–53.
| Noninvasive monitoring of hormones in bird droppings: physiological validation, sampling, extraction, sex differences, and the influence of diet on hormone metabolite levels.Crossref | GoogleScholarGoogle Scholar | 16055842PubMed |
Hemsworth PH, Edwards LE (2020) Natural behaviours, their drivers and their implications for laying hen welfare. Animal Production Science 61, 915–930.
| Natural behaviours, their drivers and their implications for laying hen welfare.Crossref | GoogleScholarGoogle Scholar |
Lane J (2006) Can non-invasive glucocorticoid measures be used as reliable indicators of stress in animals? Animal Welfare 15, 331–342.
Möstl E, Rettenbacher S, Palme R (2005) Measurement of corticosterone metabolites in birds’ droppings: an analytical approach. Annals of the New York Academy of Sciences 1046, 17–34.
| Measurement of corticosterone metabolites in birds’ droppings: an analytical approach.Crossref | GoogleScholarGoogle Scholar | 16055841PubMed |
Ralph CR, Tilbrook AJ (2016) INVITED REVIEW: The usefulness of measuring glucocorticoids for assessing animal welfare. Journal of Animal Science 94, 457–470.
| INVITED REVIEW: The usefulness of measuring glucocorticoids for assessing animal welfare.Crossref | GoogleScholarGoogle Scholar | 27065116PubMed |
Ralph CR, Hemsworth PH, Leury BJ, Tilbrook AJ (2015) Relationship between plasma and tissue corticosterone in laying hens (Gallus gallus domesticus): implications for stress physiology and animal welfare. Domestic Animal Endocrinology 50, 72–82.
| Relationship between plasma and tissue corticosterone in laying hens (Gallus gallus domesticus): implications for stress physiology and animal welfare.Crossref | GoogleScholarGoogle Scholar | 25447882PubMed |
Ralph CR, Lehman MN, Goodman RL, Tilbrook AJ (2016) Impact of psychosocial stress on gonadotrophins and sexual behaviour in females: role for cortisol? Reproduction 152, R1–R14.
| Impact of psychosocial stress on gonadotrophins and sexual behaviour in females: role for cortisol?Crossref | GoogleScholarGoogle Scholar | 27069009PubMed |
Rettenbacher S, Möstl E, Hackl R, Ghareeb K, Palme R (2004) Measurement of corticosterone metabolites in chicken droppings. British Poultry Science 45, 704–711.
| Measurement of corticosterone metabolites in chicken droppings.Crossref | GoogleScholarGoogle Scholar | 15623226PubMed |
Rettenbacher S, Möstl E, Hackl R, Palme R (2005) Corticosterone in chicken eggs. Annals of the New York Academy of Sciences 1046, 193–203.
| Corticosterone in chicken eggs.Crossref | GoogleScholarGoogle Scholar | 16055852PubMed |
Sapolsky RM, Romero LM, Munck AU (2000) How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocrine Reviews 21, 55–89.
| How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions.Crossref | GoogleScholarGoogle Scholar | 10696570PubMed |
Selye H (1946) The general adaptation syndrome and the diseases of adaptation. The Journal of Clinical Endocrinology 6, 117–230.
| The general adaptation syndrome and the diseases of adaptation.Crossref | GoogleScholarGoogle Scholar |
Singh R, Cook N, Cheng KM, Silversides FG (2009) Invasive and noninvasive measurement of stress in laying hens kept in conventional cages and in floor pens. Poultry Science 88, 1346–1351.
| Invasive and noninvasive measurement of stress in laying hens kept in conventional cages and in floor pens.Crossref | GoogleScholarGoogle Scholar | 19531702PubMed |
Tilbrook AJ, Ralph CR (2017) Hormones, stress and the welfare of animals. Animal Production Science 58, 408–415.
| Hormones, stress and the welfare of animals.Crossref | GoogleScholarGoogle Scholar |
Tilbrook AJ, Barekatain R, Ralph CR (2018) Insights into assessment of the welfare of laying hens in Australia. In ‘29th annual Australian poultry science symposium’, 4–7 February 2018, Sydney, NSW, Australia. (World Poultry Science Association, Australian Branch)
Wasser SK, Monfort SL, Southers J, Wildt DE (1994) Excretion rates and metabolites of oestradiol and progesterone in baboon (Papio cynocephalus cynocephalus) faeces. Reproduction 101, 213–220.
| Excretion rates and metabolites of oestradiol and progesterone in baboon (Papio cynocephalus cynocephalus) faeces.Crossref | GoogleScholarGoogle Scholar |
Weaver SJ, Hynd PI, Ralph CR, Hocking Edwards JE, Burnard C, Narayan E, Tilbrook AJ (2021) Chronic elevation of plasma cortisol causes differential expression of predominating glucocorticoid in plasma, saliva, fecal, and wool matrices in sheep. Domestic Animal Endocrinology 74, 106503
| Chronic elevation of plasma cortisol causes differential expression of predominating glucocorticoid in plasma, saliva, fecal, and wool matrices in sheep.Crossref | GoogleScholarGoogle Scholar | 32846373PubMed |


