Genetic parameters for methane emissions in Australian sheep measured in portable accumulation chambers in grazing and controlled environments
P. K. Wahinya A * , V. H. Oddy B C , S. Dominik
A * , V. H. Oddy B C , S. Dominik  B D , D. J. Brown
B D , D. J. Brown  A , C. A. Macleay E , B. Paganoni
A , C. A. Macleay E , B. Paganoni  E , A. N. Thompson F , A. J. Donaldson C , K. Austin C , M. Cameron C and J. H. J. van der Werf
E , A. N. Thompson F , A. J. Donaldson C , K. Austin C , M. Cameron C and J. H. J. van der Werf  B
B
A Animal Genetics & Breeding Unit, University of New England, Armidale, NSW 2351, Australia.
B School of Environmental and Rural Science, University of New England, Armidale, NSW 2351, Australia.
C NSW Department of Primary Industries, Livestock Industries Centre, University of New England, Armidale, NSW 2351, Australia.
D CSIRO Agriculture and Food, Armidale, NSW 2350, Australia.
E Department of Primary Industries and Regional Development, 1 Verschuer Place, Bunbury, WA 6230, Australia.
F Centre for Animal Production and Health, Murdoch University, 90 South Street, Murdoch, WA 6150, Australia.
Animal Production Science 62(9) 818-827 https://doi.org/10.1071/AN21270
Submitted: 17 May 2021 Accepted: 11 February 2022 Published: 10 March 2022
© 2022 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Context: Genotype by environment interaction or sire re-ranking between measurements of methane emission in different environments or from using different measurement protocols can affect the efficiency of selection strategies to abate methane emission.
Aim: This study tested the hypothesis that measurements of methane emission from grazing sheep under field conditions, where the feed intake is unknown, are genetically correlated to measurements in a controlled environment where feed intake is known.
Methods: Data on emission of methane and carbon dioxide and uptake of oxygen were measured using portable accumulation chambers from 499 animals in a controlled environment in New South Wales and 1382 animals in a grazing environment in Western Australia were analysed. Genetic linkage between both environments was provided by 140 sires with progeny in both environments. Multi-variate animal models were used to estimate genetic parameters for the three gas traits corrected for liveweight. Genetic groups were fitted in the models to account for breed differences. Genetic correlations between the field and controlled environments for the three traits were estimated using bivariate models.
Key results: Animals in the controlled environment had higher methane emission compared to the animals in the field environment (37.0 ± s.d 9.3 and 35.3 ± s.d 9.4 for two protocols vs 12.9 ± s.d 5.1 and 14.6 ± s.d 4.8 mL/min for lambs and ewes (±s.d); P < 0.05) but carbon dioxide emission and oxygen uptake did not significantly differ. The heritability estimates for methane emission, carbon dioxide emission and oxygen uptake were 0.15, 0.06 and 0.11 for the controlled environment and 0.17, 0.27 and 0.35 for the field environment. The repeatability for the traits in the controlled environment ranged from 0.51 to 0.59 and from 0.24 to 0.38 in the field environment. Genetic correlations were high (0.85–0.99) but with high standard errors.
Conclusion: Methane emission phenotypes measured using portable accumulation chambers in grazing sheep can be used in genetic evaluation to estimate breeding values for genetic improvement of emission related traits. The combined measurement protocol-environment did not lead to re-ranking of sires.
Implication: These results suggest that both phenotypes could be used in selection for reduced methane emission in grazing sheep. However, this needs to be consolidated using a larger number of animals and sires with larger progeny groups in different environments.
Keywords: enteric emission, feed intake, grazing environment, heritability, measurement of methane emission, portable accumulation chamber, sheep, repeatability.
Introduction
Methane emission from livestock is increasingly becoming a societal concern, due to its effect on global warming (Boucher et al. 2009) and the financial implication for producers if a carbon price were applied to livestock products (Alcock and Hegarty 2011; Browne et al. 2011). Enteric emissions from livestock account for 73% of the total agriculture emissions and grazing sheep contribute 20% of the enteric fermentation emissions in Australia (Department of the Environment and Energy 2019). Emissions are projected to increase due to an increasing demand for livestock products. Australia has dedicated substantial resources to abate enteric methane emission in livestock. Several strategies have been suggested to reduce gas emissions involving either feeding practices and dietary additives or long-term strategies such as genetic improvement (Smith et al. 2008; Eckard et al. 2010). Previous studies have reported a modest heritability for methane emission adjusted for liveweight per head in Australian sheep (Goopy et al. 2016; Paganoni et al. 2017; Robinson et al. 2020) and New Zealand (Pinares-Patiño et al. 2013; Jonker et al. 2018; Rowe et al. 2019), so that it would be possible to reduce emission by selection. For direct selection on the trait, a practical measurement protocol needs to be established to obtain phenotypic information at scale on selection candidates, or on animals in a reference population that could be used for genomic prediction of breeding value.
Different methods and measurement protocols exist to measure phenotypes on methane emission from sheep. Closed-circuit respiration chambers (Blaxter and Clapperton 1965) and portable accumulation chambers (PAC) have been used to measure emission in controlled and field conditions respectively (Goopy et al. 2011; Goopy et al. 2016; Bond et al. 2019; Robinson et al. 2020). A measurement protocol to be adopted by breeders or in large scale phenotyping of reference populations for the purpose of genetic evaluation should be cost effective and practical whilst being predictive of lifetime rate of emission of an animal (Robinson et al. 2015). Under similar management conditions, PAC has been recommended as an effective, low-cost way to measure methane. The method is highly correlated with phenotypes obtained from respiration chambers (Robinson et al. 2020). However, the correlation between the different protocols used with PACs across environments needs to be evaluated as per Robinson et al. (2020).
Production of methane is determined by the amount and composition of feed eaten, the feeding schedule before measurement and the digesta flow rate from the rumen (Blaxter and Clapperton 1965; Pinares-Patiño et al. 2003; Goopy et al. 2014). These variables are typically not known during unsupervised recording of methane production under field conditions. Methane production measured under pasture grazing conditions would be potentially cheap and relatively practical, however measurement of feed intake is expensive and difficult for grazing animals. Also, measuring feed intake before testing for methane emission could increase stress levels in sheep due to isolation and confinement which disrupts their feeding behaviour and hence feed intake (Llonch et al. 2016). This poses the question whether methane measurement under grazing conditions could be a valid assessment of variation in methane production.
Genetic correlations between PAC measurement of methane emission under an environment with known feed intake and measurements under grazing conditions are important to establish whether these traits are genetically similar and whether sire rankings remain the same between the two measurement conditions. In addition to measuring methane emission, PACs can be used to simultaneously measure carbon dioxide production and oxygen uptake. Carbon dioxide and oxygen measured in PAC have been reported to be more heritable and have a higher genetic correlation with feed intake than methane emission for sheep at post-weaning, hogget and adult ages (Paganoni et al. 2017). These traits can consequently be cheaply measured in sheep using PAC and could be used as a proxy for feed intake. The objective of the study was to compare genetic parameters for methane emission, carbon dioxide emission and oxygen uptake measured using PACs in multiple breeds under different feeding protocols and environments, with controlled feed intake in New South Wales (NSW) vs field measurement with unknown feed intake in Western Australia (WA). Genetic correlations for the three traits were estimated between the two locations to evaluate whether sires’ estimated breeding values for these traits are re-ranked between the two feeding protocols and environments.
Materials and methods
Data
All protocols were approved by the University of New England Animal Ethics Committee (Approval AEC 15-021) and the Department of Primary Industries and Regional Development of Western Australia (WA) (Approval AEC 4-14-10). The climatic conditions, diet and measurement protocols, however, varied as described in detail below. The main difference in the measurement protocols was that in New South Wales (NSW), gas measurements were taken using PACs in a controlled environment where feed intake was known. In WA, the animals were measured under grazing conditions and the amount of feed intake was not known. In both locations, the liveweight of the animals was measured. The PACs and devices used to measure the concentration of methane, carbon dioxide and oxygen were as described by Goopy et al. (2016).
Controlled environment
In NSW, a total of 510 ewes from the Sheep Cooperative Research Centre Information Nucleus flock (van der Werf et al. 2010) kept at the University of New England’s Kirby Research station, in Armidale (30°28′S, 151°40′E), Australia, were measured in seven batches (groups of animals measured around the same time) between April 2015 and March 2016. The ewes were born between 2007 and 2013 so they were aged 2–8 years of age at the time of measurement. Armidale has a temperate to cool climate with warm summers and summer dominant rainfall. Ewes were tested multiple times using different measurement protocols. Animals were either measured immediately after coming off feed (PAC0) or 1 h after coming off feed (PAC1). Prior to the commencement of the trial ewes were first placed in individual pens, and habituated for at least 1 week for the PAC0 measurement, and at least 3 weeks for the PAC1 measurement. The diet included equal parts of lucerne chaff and cereal hay (9.6 MJ metabolisable energy (ME) per kg dry matter (DM), 33.3% acid detergent fibre, 52.2% neutral detergent fibre and 140 g crude protein per kg DM) at 1.5 (Batches 1 and 2) or 1.6 (Batches 3–7) times their maintenance requirement, calculated from the weight of the animals before transport (Robinson et al. 2020). Feed was provided daily at 08:00 hours. Repeat measurements for both PAC0 and PAC1 were spaced 2 weeks apart and methane, carbon dioxide and oxygen were measured as described in Oddy et al. (2019). In some instances, ewes were measured more than twice. Repeat measures of 58 animals were removed because they were recorded multiple times on the same day. After removing outlier phenotypes, records from 499 animals were retained. Preparatory analysis of the data established that the phenotypic correlation between PAC0 and PAC1 measurement protocols ranged from 0.87 to 1.00, therefore measurements by the two protocols were deemed to describe the same trait and data were combined for further investigation.
Grazing environment
The WA data included measurements of 1385 ewes and ewe lambs from the Sheep Cooperative Research Centre Information Nucleus flock at the Great Southern Agricultural Research Institute, Department of Agriculture and Food at Katanning (33°41′S, 117°35′E). The climate at Katanning is Mediterranean with mild winters and hot dry summers with 70–80% of the rainfall occurring between May and October. Animals were measured in six batches with three cohorts (Lambs, Ewe 1 and Ewe 2) of animals measured twice between 2014 and 2015. Ewe 1 (290) and Ewe 2 (464) cohorts included ewes born between 2007 and 2013, while Lambs (249) were female offspring of Ewe 1 and 2 born in 2014. All lambs were born between April and June 2014 and weaned mid-October while the ewes were born between July and August of their respective birth years. Each cohort was measured twice, approximately 1 month apart. To provide some linkage between measurements some animals were measured repeatedly across days and cohorts. Each cohort of sheep grazed (dry pasture 7.0–7.7 MJ ME/kg of DM, 43.5% acid detergent fibre and 72.9% neutral detergent fibre) together for 1 month prior to testing and were supplemented if there was insufficient pasture for liveweight maintenance. Lambs were supplemented with 100 g of lupins (14.1 MJ ME per kg DM, 21.3% acid detergent, 34.7% neutral detergent fibre and 317 g crude protein per kg DM) per day between the first and second measurement. The Ewe 1 cohort was supplementary fed with hay (8.2 MJ of ME per kg DM, 34.1% acid detergent fibre and 64.9% neutral detergent fibre and 56 g crude protein per kg DM) in February and March 2015 while the Ewe 2 cohort sheep grazed with 1500 kg DM per hectare of spring pasture and were not supplementary fed.
On the day of measurement, ewes were tested in up to 10 runs per day with up to 24 ewes per run. Animals were mustered into a holding paddock the day prior to measurement, weighed at 06:00 hours on the day of measurement and then draughted into two groups, one measured in the morning and the other in the afternoon. The afternoon group was returned to pasture before measurement. Ewes were off-pasture for no longer than 4 h, but at least 1 hour prior to methane measurement. A few ewes were measured more than twice and repeat measurements of animals that were measured multiple times on the same day were removed.
The first cohort of sheep measured were the lambs in November and December of 2014 (6–8 weeks post-weaning). Each day between 6 and 8 runs with 16–20 lambs per run were conducted. Measurement time ranged from 33 to 64 min. The Ewe 1 cohort was a mixture of pregnant and dry ewes measured in February and March 2015. Of the total of 594 ewes that were joined prior to measuring methane, 327 ewes were pregnant (first trimester) with singles, 167 with twins and 100 were dry. Each day ewes were tested in 2–9 runs with 20–24 animals per run. Most ewes were measured twice across the two measurement periods, with a small number that was only measured once or 3–5 times. The measurement time for the ewes ranged between 25 and 74 min. The Ewe 2 cohort had dry ewes measured in September and October of 2015. Each day 7–10 runs were conducted with 20–24 animals in each run with the measurement time ranging between 37 and 44 min. After removing phenotypic outliers across the whole dataset, records from 1382 animals were retained.
Statistical analyses
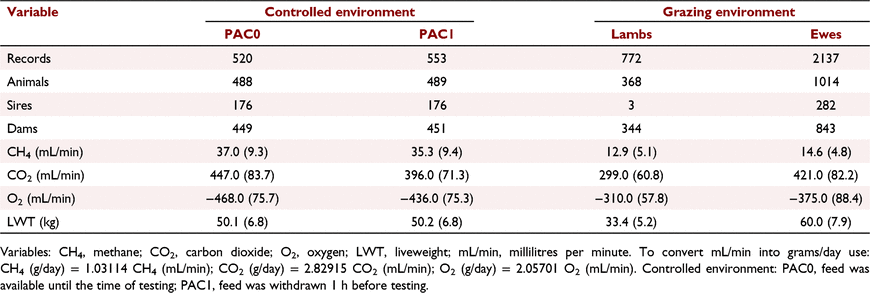
A summary of the data is provided in Table 1. The systematic effects used to model the three traits are described in Table 2. The fixed effects included: PAC protocol used (PAC0 or PAC1), batch, PAC chamber, birth year, test day and time of the day in the controlled environment and batch, PAC chamber, birth year, test day, time of the day, position of the PAC chamber in the experimental layout and pregnancy status in the grazing environment data. Only the significant fixed effects (described above) were fitted in the final model. Liveweight was fitted as a quadratic covariate in both flocks to account for the non-linear relationship between the liveweight and the three gas measurement traits as a proxy for feed intake. An interaction term between test day and time of the day was also fitted in both controlled and grazing environment datasets. The effect of rear type and the interaction term between age and liveweight were not significant for any of the gas traits and therefore, were not included in the model. Random effects for all the traits under the controlled and grazing environments included animal genetic effect to estimate the additive genetic variance and permanent environmental effect of the animal to account for correlations between repeated records, along with residual error. The amount of variation explained by year of birth, batch, chamber, date of measurement, PAC method, position of the chamber and pregnancy status (Table 3) were subsequently estimated by fitting these effects as random instead of fixed as described above. These estimates on the systematic environmental effects are important to inform the control of these effects in future experiments.

|

|
The statistical analyses was carried out in ASReml (Gilmour et al. 2015) to estimate (co)variances for methane emission, carbon dioxide emission and oxygen uptake. The pedigree for the animals in the controlled environment included 2649 animals over 18 generations, with 1012 sires, 1123 dams, 556 sires of sires, 462 sires of dams, 398 dams of sires, 276 dams of dams and three genetic groups. To account for variation in breeds the base animals were classified into maternal, terminal and Merino genetic groups. The sire breeds included pure-bred Merino, Border Leicester, Dorset Horn, Dorset, Texel, White Suffolk, Dohne, composite maternal whereas the dams were either pure-bred Merino or a Border Leicester × Merino cross. In the grazing environment dataset, the pedigree included 4396 animals over 19 generations, with 1317 sires, 2002 dams, 646 sires of sires, 752 sires of dams, 469 dams of sires, 662 dams of dams and three genetic groups as described above. The gas measurements (y) from animal j were modelled as:

where y is the vector of trait observations for methane emission, carbon dioxide emission and oxygen uptake; b is the fixed effect vector; u is the vector of random animal effects; g is the vector of fixed genetic group effects; pe is the vector containing random permanent environmental effects of animal and e the vector of random residual effects. The matrices X, Z1 and Z2 are incidence matrices that link observations to levels of fixed effects, additive genetic effects and permanent environmental effects, respectively. Q is a matrix of genetic groups allocating animals with unknown pedigree to either maternal, terminal or Merino populations. Trivariate models between the three gas traits were also used to estimate correlations between the gas traits within the controlled and grazing environments. Genetic correlations for methane emission, carbon dioxide emission and oxygen uptake between the controlled and grazing environments were estimated using both animal and sire models while fitting the significant fixed effects within each environment and genetic groups as described above with the residual covariance set to zero. A substantial number of sires (140) provided a genetic link between the two environments.
Results
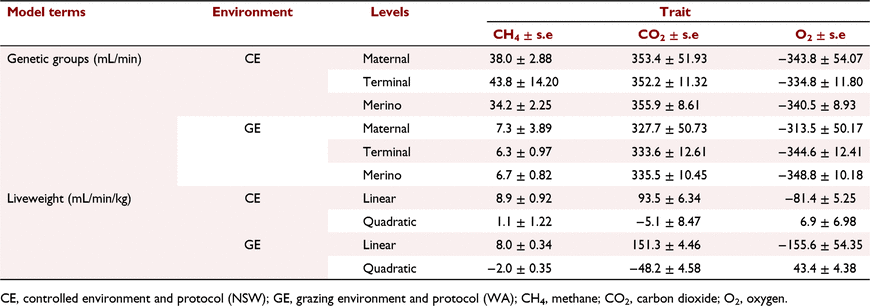
Methane emission, carbon dioxide emission and oxygen uptake were on average higher for sheep in a controlled environment than under grazing conditions (Table 1). Ewes in the controlled environment were on average 50.2 kg (34.4–74.5) while in the grazing environment the ewes were 60 kg (36.0–90.2) and the lambs 33.4 kg (17.6–66.5). In the controlled environments, sheep consumed on average 1.4 kg of feed on the day before measurement and 0.9 kg on the day of measurement. The genetic group solutions and liveweight coefficients for the three traits in the controlled and grazing environments are shown in Table 4. The genetic group solutions for the three gas traits were not significantly different within the environments. For both environments, positive linear coefficients were estimated for liveweight on methane and carbon dioxide emission and negative for oxygen uptake. The quadratic coefficients in the controlled environment were not significantly different from zero. In the grazing environment, quadratic terms for methane emission (−2.0) and carbon dioxide emission (−48.2) were negative whereas they were positive for oxygen uptake (43.4).
|
Overall, there were larger phenotypic variances for all three gas traits in the controlled than in the grazing environment as shown in Table 5. The estimated genetic variances and heritabilities for the three gas traits were not significantly different from zero in the controlled environment. The heritabilities for methane emission, carbon dioxide emission and oxygen uptake in the grazing environment were 0.17 ± s.e 0.05, 0.26 ± s.e 0.05 and 0.32 ± s.e 0.05, respectively. Methane emission (0.59 ± s.e 0.03 vs 0.23 ± s.e 0.03), carbon dioxide emission (0.51 ± s.e 0.03 vs 0.35 ± s.e 0.03) and oxygen uptake (0.53 ± s.e 0.05 vs 0.38 ± s.e 0.02) were more repeatable in the controlled compared to the grazing environment. The estimates of the genetic correlations between methane and the other traits in the controlled environment were not reliable due to the high standard errors. However, the phenotypic correlations between the three traits followed the same trend in both environments but correlations were generally higher in the controlled environment. Positive phenotypic correlations were estimated between methane emission and carbon dioxide (0.52 ± s.e 0.02 and 0.73 ± s.e 0.02). Both methane emission (−0.41 ± s.e 0.02 and −0.67 ± s.e 0.02) and carbon dioxide emission (−0.83 ± s.e 0.01 and −0.92 ± s.e 0.01) had negative phenotypic correlations with oxygen uptake. In the grazing environment, high positive genetic correlations were found between methane emission and carbon dioxide emission (0.77 ± s.e 0.09) whereas strong negative genetic correlations were estimated between methane emission and oxygen uptake (−0.67 ± s.e 0.11) and between carbon dioxide emission and oxygen uptake (−0.99 ± s.e 0.01).
Apart from the additive and permanent environmental variance, the effects of batch, date of measurement and the interaction between date and time of day explained more variation than the other effects. Although chamber, year of birth, pregnancy status and the position of the PAC chamber were significant, they explained the lowest proportion of the phenotypic variance for all the three gas traits in both environments. Batch, date and time of measurement was associated with higher variation in the grazing compared to controlled environment.
The animal and sire models estimated similar values for the genetic correlations as shown in Table 6. High genetic correlations were estimated for methane emission, carbon dioxide emission and oxygen uptake estimated between the controlled and grazing environments. However, these correlations should be interpreted with caution due to the high standard errors. Common sires had on average 2.68 (a range of 1–9) and 3.75 (a range of 1–13) progeny in the controlled and grazing environments, respectively. Although they are not shown here the additive, permanent environment and residual variances were similar to what was estimated using the trivariate models in this study.

|
Discussion
This study estimated genetic parameters for methane emission, carbon dioxide emission and oxygen uptake traits measured using PACs in a field grazing environment and the genetic association with measurements in a controlled environment where feed intake was known and controlled. Although the dataset used in this study was not large, the results indicate that PAC measurements for methane emission from sheep under grazing and controlled environments are heritable, and suggest that the three traits are genetically correlated. There would appear to be potential for commercial industry application of PAC measurements on grazing sheep for genetic evaluations to abate methane emission through selection. In the design of this study the measurement protocols were confounded with location and Armidale and Katanning constitute significant environmental differences in terms of temperature, humidity and feed availability. However, the high genetic correlations (acknowledging the high standard errors) between the two recording environments are reassuring in the sense that neither the natural environment nor the measurement protocol result in a large re-ranking of breeding values for emissions traits. This gives some confidence that PAC measurement of emission traits are relatively robust across such protocols as well as environment.
The methane emissions quantified in the grazing environment (14.1 mL/min) were similar to those reported for grazing sheep in New Zealand using PACs (12.6 mL/min) (Jonker et al. 2018). Methane emission in the controlled environment (36.1 mL/min) were also the same as those measured by Robinson et al. (2020) and Dominik et al. (2017) using PACs. The lower methane emissions in the grazing environment are principally due to decreased feed intake during the period immediately prior to measurement. This is accounted for in part by the fixed effects of test day and test time. The effect of disruption to feed intake has been observed in a controlled environment as higher phenotypic variance for feed intake on the day of measurement compared to the day before measurement (Robinson et al. 2020). In addition, animals remainedoff feed for at least 1–4 h on the test day in the grazing environment while the ewes in the controlled environment were habituated prior to measurement with a fixed amount of feed based on their maintenance requirements derived from their liveweight. In addition, a lower ME concentration in the diet was available for the animals in the grazing environment compared to the controlled environment. The average ewe liveweight was not significantly different between environments and, therefore, ewes’ carbon dioxide emission and oxygen uptake measurements were not significantly different between the grazing and controlled environments’ measurements. However, in the grazing environment, lambs were lighter compared to ewes and therefore had lower but not significantly different carbon dioxide emission and oxygen intake. Records on these systematic effects that influence the phenotypes are therefore important to ensure unbiased genetic evaluation.
In both the controlled and grazing environments, a kilogram increase in liveweight can be associated with a higher methane and carbon dioxide emission. A non-linear relationship however, exists between the three gas traits and liveweight based on the quadratic regression coefficients estimated in this study. We hypothesise that the negative quadratic coefficients in the grazing environment could be due to a reduction in feed intake per unit weight. The value of using liveweight to account for feed intake needs to be explored further since it does not account for all the variation due to feed intake. A model fitting liveweight and feed intake on the day of measurement for the controlled environment (results not presented) reduced the phenotypic variance for methane emission by 60%. After accounting for liveweight and feed intake the remaining variation still has a heritable component (h2 = 0.12 ± s.e 0.11). Carbon dioxide emission has also been recommended as a proxy for feed intake and feed efficiency in beef cattle (Herd et al. 2016; Arthur et al. 2018; Renand et al. 2019). Fitting liveweight and carbon dioxide as covariates in the grazing environment, reduced the phenotypic variance of methane emission by 53% and 27% for the controlled and grazing environments respectively. This indicates that in the absence of information on feed intake an alternative would be to use both liveweight and carbon dioxide.
The estimated genetic group solutions were not significantly different for all the three traits suggesting that there are no significant differences in methane emission, carbon dioxide emission and oxygen uptake between maternal, terminal and Merino breeds. Since the genetic group solutions were not significantly different producers and breeders can focus on genetic selection within breed to reduce methane emission.
Heritability and repeatability
This study builds onto the existing research findings that PAC measurements on methane emission in the grazing and controlled environments are heritable (Robinson et al. 2014, 2020; Goopy et al. 2016; Jonker et al. 2018). Selection strategies can therefore utilise the available genetic variation to reduce methane emission from grazing sheep in Australia in the longer term. The heritabilities of carbon dioxide emission (0.01–0.22) and oxygen uptake (0.08–0.38) estimated in this study are within the range of what is reported in other studies (Paganoni et al. 2017). It was difficult to accurately separate the animal additive and permanent environmental variance (especially in the controlled environment) with the current small data set, and so, reflecting the high standard errors, these heritability estimates should be interpreted with caution.
The shorter interval between the repeated measurements in the controlled environment led to significantly higher repeatabilities compared to a month interval in the grazing environment. Elsewhere, a repeatability of 0.55 has been reported for methane emission measure in respiration chambers with a 10–15-day interval and as high as 0.94 for measurements on consecutive days (Pinares-Patiño et al. 2013). The moderate repeatabilities also align with what was estimated for Merino ewes in a controlled environment (0.17–0.40) (Dominik and Oddy 2015), lambs and ewes grazing pastures (0.33–0.55) in New Zealand (Jonker et al. 2018), ewe lambs measured for 17 days in a controlled environment (0.36) in Ireland (O’Connor et al. 2021) and the phenotypic correlation (0.59) between two PAC measurement protocols in a controlled environment in Australia (Robinson et al. 2020). Moderate repeatabilities for methane emission (0.23 and 0.59), carbon dioxide emission (0.34 and 0.51) and oxygen uptake (0.38 and 0.53) in both environments suggest that there is benefit in having repeated measurements for genetic evaluation to rank animals for selection. In fact, the heritability based on n measurements is equal to heritability/[repeatability + (1 − repeatability)/n], based on two measurements, the heritability would increase by 33% for repeatability = 0.5 whereas the increase would be only 5% for repeatability = 0.9. However, for the purpose of building reference populations for genomic selection, it is more beneficial to measure more animals rather than multiple measurements per animal. Additionally, an optimal measurement protocol also needs to be evaluated to find a balance between cost and accuracy for routine genetic evaluation of methane emission to aid in selection and genetic improvement. This is in consideration of the high cost involved in recording the gas phenotypes.
Correlations within the environments
Positive genetic and phenotypic correlations between methane and carbon dioxide emission show that sheep with high methane emission also emit higher levels of carbon dioxide and consume more oxygen. These correlations are consistent with previous estimates for Australian sheep (Paganoni et al. 2017). Correlated response in methane emission could also be achieved by selecting for animals with a genetic potential for lower carbon dioxide emission. Since a positive genetic correlation is reported between carbon dioxide emission and feed intake, inclusion of the emission traits and production traits in the selection index may improve feed efficiency without compromising productivity (Paganoni et al. 2017; Robinson et al. 2020). High genetic correlations between PAC methane emission and feed intake (86–95%) also suggest that reducing methane and feed intake relative to production or liveweight is more effective than reducing methane relative to feed intake to reduce methane emission without compromising on production (Robinson et al. 2020). The high genetic correlations between carbon dioxide emission and oxygen uptake (−0.99 and −0.82) indicate that the two traits are the same genetically. Despite the high standard errors, the negative correlations were expected since carbon dioxide is exhaled (positive values) from the inhaled oxygen (negative values) from the lungs.
Correlations between the environments
In this study, strong genetic correlations (0.85–0.99) were estimated between methane emission, carbon dioxide emission and oxygen uptake in sheep measured in different locations and using different measurement protocols (field and controlled). This provides an indication that selection for methane emission, carbon dioxide emission and oxygen uptake could lead to a similar result, whether based on PAC measurements in a controlled environment or in a grazing environment. However, the genetic evaluation models need to account for possible differences in mean and variance that may result from differences in management and measurement protocols in the separate environments. The trait measurements should also be expressed in the same units and link sires included in the analysis. The slightly lower genetic correlations between methane emission in both environments show that methane (0.85) is more sensitive to the measurement protocol and environment compared to carbon dioxide emission (0.93–0.97) and oxygen uptake (0.98–0.99). These results need to be verified using a large dataset before implementation for routine genetic evaluation due to the high standard errors. Future studies should also consider evaluating the genetic correlation between grazing environment methane emission in multiple environments because the measurement protocols were confounded with the environment in this study.
Conclusions
Measurements of methane emission, carbon dioxide emission and oxygen uptake in the field using PACs can be used for genetic improvement. Although the values were associated with high standard errors, positive and favourable genetic correlations were estimated between methane emission, carbon dioxide emission and oxygen uptake measured in PACs in field and controlled environments. Methane emission phenotypes measured using PACs in a field environment and in controlled environments can therefore be used in genetic evaluation to estimate breeding values for genetic improvement of emission related traits.
Data availability
The data that support this study cannot be publicly shared due to ethical or privacy reasons and may be shared upon reasonable request to the corresponding author if appropriate.
Conflicts of interest
The authors declare no conflicts of interest.
Declaration of funding
This research was funded by Meat and Livestock Australia and the Australian Commonwealth Government (Project 119400343).
Acknowledgements
The authors acknowledge The University of Western Australia, University of New England, Kirby Research station and Katanning Research Facility, Meat and Livestock Australia Limited and the Commonwealth Government of Australia through the Department of Agriculture and Water Resources, Filling the Research Gaps Program for their support.
References
Alcock DJ, Hegarty RS (2011) Potential effects of animal management and genetic improvement on enteric methane emissions, emissions intensity and productivity of sheep enterprises at Cowra, Australia. Animal Feed Science and Technology 166–167, 749–760.| Potential effects of animal management and genetic improvement on enteric methane emissions, emissions intensity and productivity of sheep enterprises at Cowra, Australia.Crossref | GoogleScholarGoogle Scholar |
Arthur PF, Bird-Gardiner T, Barchia IM, Donoghue KA, Herd RM (2018) Relationships among carbon dioxide, feed intake, and feed efficiency traits in ad libitum fed beef cattle. Journal of Animal Science 96, 4859–4867.
| Relationships among carbon dioxide, feed intake, and feed efficiency traits in ad libitum fed beef cattle.Crossref | GoogleScholarGoogle Scholar | 30060045PubMed |
Blaxter KL, Clapperton JL (1965) Prediction of the amount of methane produced by ruminants. British Journal of Nutrition 19, 511–522.
| Prediction of the amount of methane produced by ruminants.Crossref | GoogleScholarGoogle Scholar |
Bond JJ, Cameron M, Donaldson AJ, Austin KL, Harden S, Robinson DL, Oddy VH (2019) Aspects of digestive function in sheep related to phenotypic variation in methane emissions. Animal Production Science 59, 55
| Aspects of digestive function in sheep related to phenotypic variation in methane emissions.Crossref | GoogleScholarGoogle Scholar |
Boucher O, Friedlingstein P, Collins B, Shine KP (2009) The indirect global warming potential and global temperature change potential due to methane oxidation. Environmental Research Letters 4, 044007
| The indirect global warming potential and global temperature change potential due to methane oxidation.Crossref | GoogleScholarGoogle Scholar |
Browne NA, Eckard RJ, Behrendt R, Kingwell RS (2011) A comparative analysis of on-farm greenhouse gas emissions from agricultural enterprises in south eastern Australia. Animal Feed Science and Technology 166–167, 641–652.
| A comparative analysis of on-farm greenhouse gas emissions from agricultural enterprises in south eastern Australia.Crossref | GoogleScholarGoogle Scholar |
Department of the Environment and Energy (2019) Australia’s emissions projections 2019, Commonwealth of Australia 2019. Available at https://www.industry.gov.au/sites/default/files/2020-07/australias-emissions-projections-2019-report.pdf
Dominik S, Oddy V (2015) Repeatabilities for methane emissions in Merino ewes on pasture across different ages. In ‘Proceedings of the 21st conference of the Association for the Advancement of Animal Breeding and Genetics (AAABG), 28–30 September 2015, Lorne, Victoria, Australia’. vol. 21, pp. 110–113
Dominik S, Robinson D, Donaldson A, Cameron M, Austin K, Oddy V (2017) Relationship between feed intake, energy expenditure and methane emissions: implications for genetic evaluation. In ‘Proceedings of the 22nd conference of the Association for the Advancement of Animal Breeding and Genetics (AAABG), 2–5 July 2017, Townsville, Queensland, Australia’. pp. 65–68
Eckard RJ, Grainger C, de Klein CAM (2010) Options for the abatement of methane and nitrous oxide from ruminant production: a review. Livestock Science 130, 47–56.
| Options for the abatement of methane and nitrous oxide from ruminant production: a review.Crossref | GoogleScholarGoogle Scholar |
Gilmour A, Gogel B, Cullis B, Welham S, Thompson R (2015) ‘ASReml user guide release 4.1 structural specification’. (VSN international ltd: Hemel Hempstead)
Goopy JP, Woodgate R, Donaldson A, Robinson DL, Hegarty RS (2011) Validation of a short-term methane measurement using portable static chambers to estimate daily methane production in sheep. Animal Feed Science and Technology 166-167, 219–226.
| Validation of a short-term methane measurement using portable static chambers to estimate daily methane production in sheep.Crossref | GoogleScholarGoogle Scholar |
Goopy JP, Donaldson A, Hegarty R, Vercoe PE, Haynes F, Barnett M, Oddy VH (2014) Low-methane yield sheep have smaller rumens and shorter rumen retention time. British Journal of Nutrition 111, 578–585.
| Low-methane yield sheep have smaller rumens and shorter rumen retention time.Crossref | GoogleScholarGoogle Scholar |
Goopy JP, Robinson DL, Woodgate RT, Donaldson AJ, Oddy VH, Vercoe PE, Hegarty RS (2016) Estimates of repeatability and heritability of methane production in sheep using portable accumulation chambers. Animal Production Science 56, 116–122.
| Estimates of repeatability and heritability of methane production in sheep using portable accumulation chambers.Crossref | GoogleScholarGoogle Scholar |
Herd RM, Velazco JI, Arthur PF, Hegarty RS (2016) Proxies to adjust methane production rate of beef cattle when the quantity of feed consumed is unknown. Animal Production Science 56, 231–237.
| Proxies to adjust methane production rate of beef cattle when the quantity of feed consumed is unknown.Crossref | GoogleScholarGoogle Scholar |
Jonker A, Hickey SM, Rowe SJ, Janssen PH, Shackell GH, Elmes S, Bain WE, Wing J, Greer GJ, Bryson B, Maclean S, Dodds KG, Pinares-Patiño CS, Young EA, Knowler K, Pickering NK, McEwan JC (2018) Genetic parameters of methane emissions determined using portable accumulation chambers in lambs and ewes grazing pasture and genetic correlations with emissions determined in respiration chambers. Journal of Animal Science 96, 3031–3042.
| Genetic parameters of methane emissions determined using portable accumulation chambers in lambs and ewes grazing pasture and genetic correlations with emissions determined in respiration chambers.Crossref | GoogleScholarGoogle Scholar | 29741677PubMed |
Llonch P, Troy SM, Duthie C-A, Somarriba M, Rooke J, Haskell MJ, Roehe R, Turner SP (2016) Changes in feed intake during isolation stress in respiration chambers may impact methane emissions assessment. Animal Production Science 58, 1011–1016.
| Changes in feed intake during isolation stress in respiration chambers may impact methane emissions assessment.Crossref | GoogleScholarGoogle Scholar |
O’Connor E, McGovern FM, Byrne DT, Boland TM, Dunne E, McHugh N (2021) Repeatability of gaseous measurements across consecutive days in sheep using portable accumulation chambers. Journal of Animal Science 99, skab288
| Repeatability of gaseous measurements across consecutive days in sheep using portable accumulation chambers.Crossref | GoogleScholarGoogle Scholar | 34637520PubMed |
Oddy VH, Donaldson AJ, Cameron M, Bond J, Dominik S, Robinson DL (2019) Variation in methane production over time and physiological state in sheep. Animal Production Science 59, 441–448.
| Variation in methane production over time and physiological state in sheep.Crossref | GoogleScholarGoogle Scholar |
Paganoni B, Rose G, Macleay C, Jones C, Brown DJ, Kearney G, Ferguson M, Thompson AN (2017) More feed efficient sheep produce less methane and carbon dioxide when eating high-quality pellets. Journal of Animal Science 95, 3839–3850.
| More feed efficient sheep produce less methane and carbon dioxide when eating high-quality pellets.Crossref | GoogleScholarGoogle Scholar | 28992015PubMed |
Pinares-Patiño CS, Ulyatt MJ, Lassey KR, Barry TN, Holmes CW (2003) Persistence of differences between sheep in methane emission under generous grazing conditions. The Journal of Agricultural Science 140, 227–233.
| Persistence of differences between sheep in methane emission under generous grazing conditions.Crossref | GoogleScholarGoogle Scholar |
Pinares-Patiño CS, Hickey SM, Young EA, Dodds KG, Maclean S, Molano G, Sandoval E, Kjestrup H, Harland R, Hunt C, Pickering NK, McEwan JC (2013) Heritability estimates of methane emissions from sheep. Animal 7, 316–321.
| Heritability estimates of methane emissions from sheep.Crossref | GoogleScholarGoogle Scholar | 23739473PubMed |
Renand G, Vinet A, Decruyenaere V, Maupetit D, Dozias D (2019) Methane and carbon dioxide emission of beef heifers in relation with growth and feed efficiency. Animals 9, 1136
| Methane and carbon dioxide emission of beef heifers in relation with growth and feed efficiency.Crossref | GoogleScholarGoogle Scholar |
Robinson DL, Goopy JP, Hegarty RS, Oddy VH, Thompson AN, Toovey AF, Macleay CA, Briegal JR, Woodgate RT, Donaldson AJ, Vercoe PE (2014) Genetic and environmental variation in methane emissions of sheep at pasture. Journal of Animal Science 92, 4349–4363.
| Genetic and environmental variation in methane emissions of sheep at pasture.Crossref | GoogleScholarGoogle Scholar | 25149329PubMed |
Robinson DL, Goopy JP, Hegarty RS, Oddy VH (2015) Comparison of repeated measurements of methane production in sheep over 5 years and a range of measurement protocols. Journal of Animal Science 93, 4637–4650.
| Comparison of repeated measurements of methane production in sheep over 5 years and a range of measurement protocols.Crossref | GoogleScholarGoogle Scholar | 26523556PubMed |
Robinson DL, Dominik S, Donaldson AJ, Oddy VH (2020) Repeatabilities, heritabilities and correlations of methane and feed intake of sheep in respiration and portable chambers. Animal Production Science 60, 880–892.
| Repeatabilities, heritabilities and correlations of methane and feed intake of sheep in respiration and portable chambers.Crossref | GoogleScholarGoogle Scholar |
Rowe S, Hickey S, Jonker A, Hess M, Janssen P, Johnson P, Bryson B, Knowler K, Pinares-Patino C, Bain W (2019) Selection for divergent methane yield in New Zealand sheep: a ten-year perspective. In ‘Proceedings of the 23rd conference of the Association of Advancement in Animal Breeding and Genetics (AAABG), 27 October–1 November 2019, Armidale, Australia’. vol 23, pp. 306–309
Smith P, Martino D, Cai Z, Gwary D, Janzen H, Kumar P, McCarl B, Ogle S, O’Mara F, Rice C, Scholes B, Sirotenko O, Howden M, McAllister T, Pan G, Romanenkov V, Schneider U, Towprayoon S, Wattenbach M, Smith J (2008) Greenhouse gas mitigation in agriculture. Philosophical Transactions of the Royal Society B: Biological Sciences 363, 789–813.
| Greenhouse gas mitigation in agriculture.Crossref | GoogleScholarGoogle Scholar |
van der Werf JHJ, Kinghorn BP, Banks RG (2010) Design and role of an information nucleus in sheep breeding programs. Animal Production Science 50, 998–1003.
| Design and role of an information nucleus in sheep breeding programs.Crossref | GoogleScholarGoogle Scholar |