Impacts of hanging method and high pre-rigor temperature and duration on quality attributes of ovine muscles
Y. H. B. Kim A E F , M. Kerr B , G. Geesink C and R. D. Warner DA AgResearch Ltd, Ruakura Research Centre, Hamilton, New Zealand.
B Department of Primary Industries, 600 Sneydes Road, Werribee, Vic. 3030, Australia.
C University of New England, Armidale, NSW 2351, Australia.
D CSIRO Animal, Food and Health Sciences, 671 Sneydes Road, Werribee, Vic. 3030, Australia.
E Present address: Purdue University, Department of Animal Sciences, Muscle Biology Lab, West Lafayette, IN 47907, USA.
F Corresponding author. Email: bradkim@purdue.edu
Animal Production Science 54(4) 414-421 https://doi.org/10.1071/AN13309
Submitted: 22 July 2013 Accepted: 21 January 2014 Published: 28 February 2014
Journal Compilation © CSIRO Publishing 2014 Open Access CC BY-NC-ND
Abstract
This study evaluated effects of high pre-rigor temperature and duration and suspension of lamb sides on quality traits and protein denaturation in two muscles [semimembranosus (SM) and longissimus thoracis et lumborum (LTL)]. Twenty-four lamb carcasses, within each of 3 slaughter days, were used to assign eight carcasses to one of four pre-rigor temperature treatments: chilled at 2°C directly after slaughter, or held at 37°C in water for 1.5, 3.0 or 4.5 h before transfer to a 2°C chiller. At ~15 min post slaughter, one side of each carcass was suspended from the Achilles tendon, whereas the other side was suspended by the aitch bone and the leg tied down to the ribs. The sides subjected to aitch bone hanging had an increased sarcomere length in the SM, but decreased sarcomere length in the LTL. For the LTL, the time of exposure to high pre-rigor temperature had a significant effect on measures of protein denaturation and related meat quality traits such as purge and colour, although tenderness (shear force) after 2 days of aging was not affected. For the SM, the high temperature treatment also resulted in increase in measures of protein denaturation and thus negatively influenced meat quality traits such as purge, colour and shear force after aging. However, these effects on purge and shear force in the SM were significantly mitigated by the aitchbone hanging treatment. The results of the present experiment indicate that pre-rigor aitchbone hanging of muscles can counteract the negative effects of high pre-rigor temperature on both water loss and meat tenderness.
Introduction
Pre-rigor processing (particularly chilling) has a substantial impact on meat quality attributes (Savell et al. 2005). Decreased meat tenderness and increased water loss (purge and/or drip) can result from chilling either too rapidly (cold-shortening), or too slowly (heat-toughening) in relation to the rate of pH decline (Locker and Hagyard 1963; Marsh et al. 1987; Jaime et al. 1992; Devine et al. 2002). Although it has been controversial whether high temperature-induced toughening is primarily due to heat-shortening or limited post-mortem proteolysis as a result of protein denaturation, adverse impacts of high pre-rigor temperature have been generally reported by many investigators (Marsh et al. 1981; Lee and Ashmore 1985; Devine et al. 1999; Geesink et al. 2000; Kim et al. 2010, 2012; Rosenvold and Wiklund 2011). However, several studies have found that high temperature-induced toughening conditions, in fact, may increase meat tenderness early post mortem due to the temperature-dependent activity of proteolytic enzymes (mainly µ-calpain) for myofibrillar protein degradation (Hwang et al. 2004; Bekhit et al. 2007; Thomson et al. 2008). Thus, high temperature-induced toughening conditions could be considered as a sensible strategy to improve meat tenderness if the meat is not to be aged for extended periods (Thomson et al. 2008). Prevention against the toughening effect of shortening under high pre-rigor temperatures can be achieved by alternative carcass suspension (tenderstretching, superstretching, etc.), or by tightly wrapping pre-rigor excised muscles to restrain shortening (Rosenvold et al. 2008; Warner et al. 2014b). In contrast, Kim et al. (2012) observed no significant protective effect against toughening by restraining muscles exposed to high pre-rigor temperatures.
Conflicting results with regard to high pre-rigor temperature and meat tenderness could be attributed to different pre-rigor conditions such as temperature, exposure duration and/or different muscles used in the studies. Therefore, it would be reasonable to question if (1) different exposure duration time to high pre-rigor temperature could affect the extent of protein denaturation and/or sarcomere length and subsequently meat quality attributes, particularly meat tenderness and (2) applying stretching to a carcass by tying the leg to the ribs could improve meat tenderness by either preventing high temperature-induced shortening or by providing synergistic impacts with high pre-rigor temperature on meat tenderness development for different muscles. Hence, the aim of this experiment was to investigate the effect of duration of high temperature-induced toughening conditions and hanging method (stretching) on water-holding capacity, protein denaturation, sarcomere length and tenderness of two muscles in the lamb carcass.
Methodology
Animals and treatments
The experiment was conducted using 3 slaughter days and a total of 24 lambs (6-month-old second cross; Border Leicester/Merino ewes × Dorset ram; n = 8 lambs per slaughter day). Lambs were slaughtered by head-only electrical stunning, while restrained in a V-Restrainer, followed by exsanguination and low voltage electrical stimulation was applied at 1 min post slaughter using a rectal probe and electrode clip applied to the stick wound. A square bipolar wave form was applied, providing 157-mA peak-to-peak (28–33V) with a frequency of 14 Hz for a duration of 60 s. One side was then suspended from the tailbone and the other side from the aitchbone. For the side suspended by the aitchbone, the hindleg was tightly pulled down proximally, (closer to the ribs) and tied into the ventral (underside of body) surface, thus resulting in a stretching action being applied to the leg muscles; see photo in Warner et al. (2014b) Then a temperature conditioning treatment was applied being: (i) Long/37°C – carcass placed in a 37°C vat of water for 4.5 h and then placed in a 2°C chiller (same conditions as Warner et al. (2014b), (ii) Medium/37°C – carcass placed in a 37°C vat of water for 3 h and then placed in a 2°C chiller, (iii) Short/37°C – carcass placed in a 37°C vat of water for 1.5 h and then placed in a 2°C chiller and (iv) Conventional 2°C – carcass placed into 2°C chiller where the carcass was placed directly into a chiller at 2°C after leaving the slaughter floor.
Allocation of lambs, carcasses and sides to treatments
Lambs were weighed at 1 week before the start of treatments, stratified on liveweight and within liveweight strata randomly allocated to 1 of 3 slaughter days and to two blocks within slaughter days, to ensure even liveweight distribution. The four treatments were randomly allocated within blocks and one side of each carcass was randomly allocated a stretch treatment, with the other side receiving the opposite stretch treatment.
Post-slaughter measurements and sampling
The pH and temperature fall in the longissimus thoracis et lumborum (LTL) (13th rib) and semimembranosus (SM) muscles were measured at 30 min post slaughter and hourly until 6 h post slaughter. Muscle pH and temperature was measured using a WP-80 pH meter with attached temperature probe and Ionode IJ44 electrode (TPS Pty Ltd, Brisbane, Qld, Australia) inserted into the muscle. Calibration occurred at room temperature using pH 4.00 and 7.00 buffers and temperature compensation was used during measurement. Muscle pH and temperature was recorded in duplicate and the average of the two values, at each time point was used in analysis. The hot carcass weight and fat depth (GR, total tissue thickness at the 12th rib, 110 mm from the midline) of each carcass were also measured.
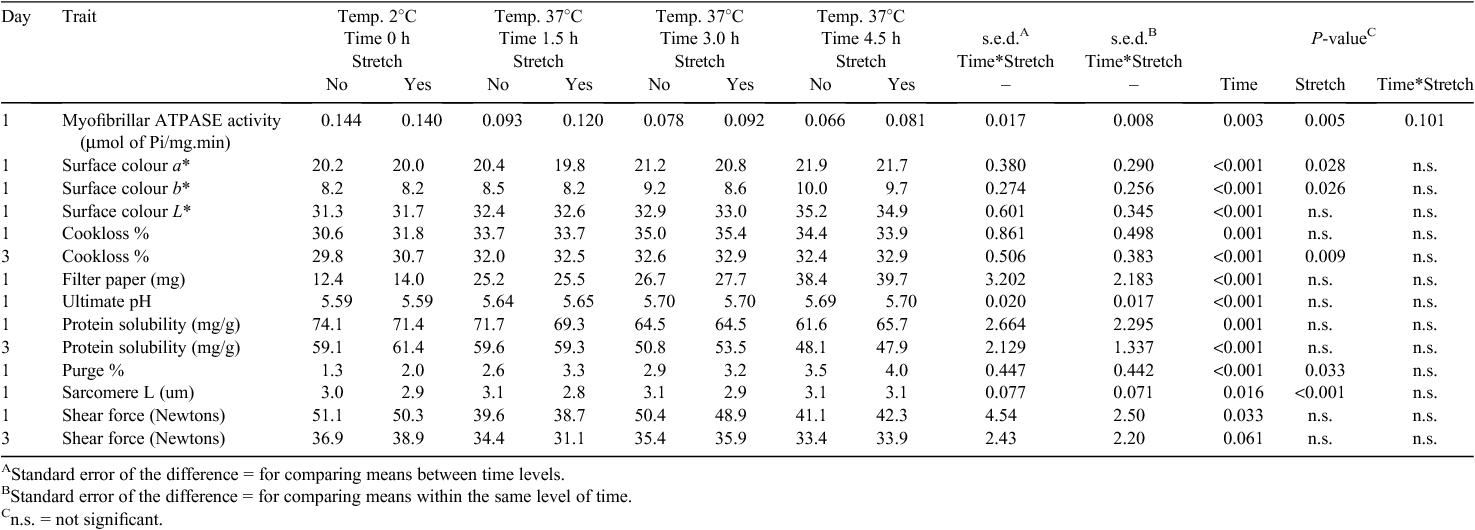
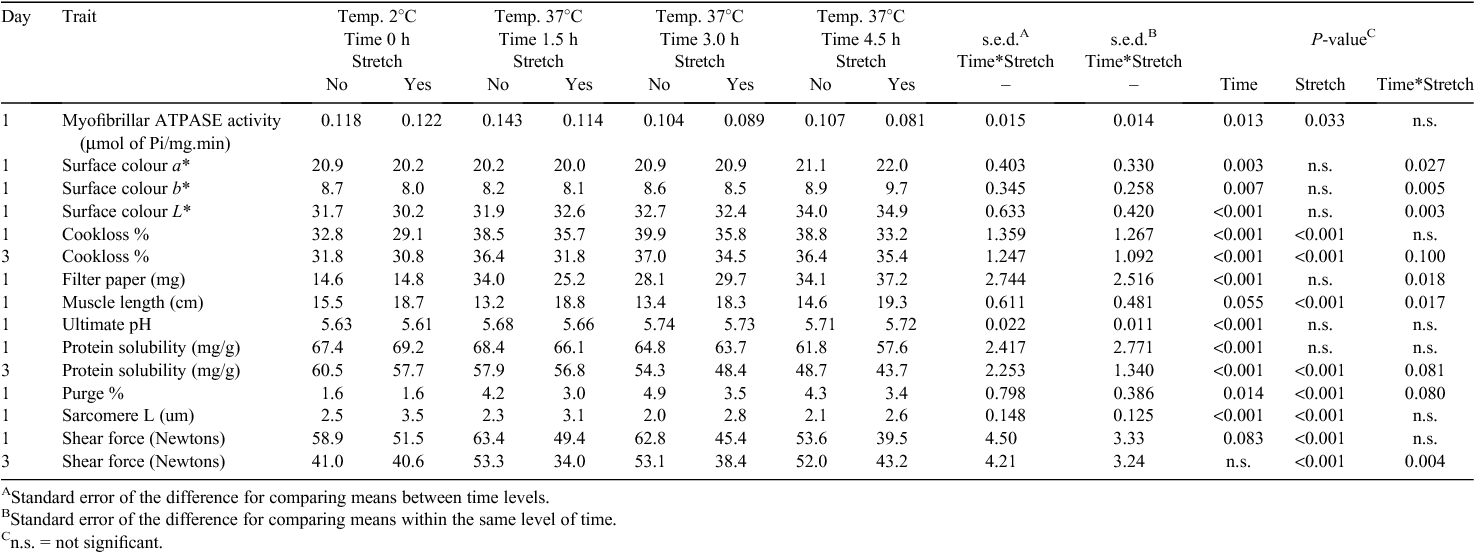
At 24 h post slaughter, the SM and LTL muscles were removed from both sides of the carcass. The length of the SM muscle was recorded. The muscles from each side were used to measure surface colour, surface exudate and ultimate pH. Also, a 2-g sample was removed for myofibril purification for myofibrillar ATPase activity, duplicate 1-g samples for sarcoplasmic protein solubility, ~80 g sample for Warner–Bratzler peak shear force and cooking loss and triplicate 2 by 1-cm2 samples for sarcomere length. A 200-g sample for aging was weighed before packing, then vacuum packed and stored at 2°C for 2 days.
After 2 days of aging in a vacuum bag, the muscles were removed from the bags, the purge loss, sarcoplasmic protein solubility, Warner–Bratzler peak shear force and cook loss were measured.
Measurements and analyses
The sarcomere length was determined using a helium-neon laser diffraction unit (custom built by the University of New England, Armidale). While still frozen a thin slice of muscle, cut parallel with the fibres, was placed between two microscope slides and squeezed flat before measuring the distance of light band diffraction in the muscle samples and converted to sarcomere length as shown below.
Sarcomere length (µm) = 0.635/sin [arctan(d/75) Ruddick and Richards (1975)]. Sarcomere lengths were recorded for 10 different myofibrils and the average was used for analysis.
On the day of boning, ~2 g of muscle was used to isolate myofibrils, for subsequent myofibrillar ATPase assay, using the procedure of Warner et al. (1997). In brief, muscle was excised and added to 15 mL of rigor buffer (RB) (10 mM imidazole, 75 mMKCl, 2 mM EGTA, 2 mM MgCl2, 2 mM NaN3, pH 7.2), cut finely with scissors, homogenised on ice, using a Polytron and also a Dounce with a B-pestle then centrifuged for 20 min at 4000g at 4°C. The pellet was resuspended in RB, and Dounce homogenised diluted, filtered and then centrifuged at 4000g at 4°C. The final suspension was prepared in 15 mL of RB with 50% glycerol and 1 mM dithiothreotol, and subsequently stored at −80°C.
Samples for the measurement of cook loss and shear force were trimmed of excess fat and blocks of 67 ± 2 g were prepared. The samples were placed in plastic bags and cooked at 70°C for 30 min in a water bath and cooled under running water for 30 min. The samples were dried with a paper towel and weighed to determine cook loss. Cooking loss is calculated as the difference in weight before, and subsequent to cooking, and expressed as a percent of initial weight before cooking. After measurement of cook loss the samples were cut (n = 8–10) into 4 by 1-cm2 pieces parallel to the muscle fibres. Tenderness was measured using a Warner–Bratzler shearing device fitted to a Lloyd texture analyser (Model LRX, Lloyd Instruments, Hampshire, UK) installed with a V-shaped cutting blade that sheared down through the samples. The cross head speed of the analyser was 300 mm/min. Shear force was measured in Newtons.
For the purge loss measurement, the weight of meat samples was collected before and after vacuum packaging (Day 3 post mortem) by drying the surface of the meat with paper towelling, after removal from the bags. The purge loss was calculated as the difference in the weight before and after aging and expressed as a percent of initial weight.
Colour was measured at Days 1 and 3 post mortem with a Minolta chromameter (model CR-200, Minolta, Ramsey, NJ, USA), 2° standard observer, D65 lighting and 8-mm aperture in the measuring head set on the L*, a* and b* system after leaving the muscle to bloom at ~4°C for 30 min. An average of three readings at different locations on the sample surface was recorded.
Sarcoplasmic protein solubility was determined in duplicate for each sample using a modified method described by Warner et al. (1997). A 1.0 ± 0.2-g sample was homogenised in 10 mL of ice-cold 0.025 M potassium phosphate, pH 7.2 using 3 by 4-s bursts. The samples were left to stand overnight at 2°C and centrifuged at 1500g for 20 min at 4°C and the supernatant was decanted. Protein concentration (mg/g tissue wet weight) of the supernatant was determined by the Biuret method (Gornall et al. 1949) using bovine serum albumin (BSA) as the standard.
Myofibrillar ATPase activity was used to measure myosin denaturation, as previously described by Greaser et al. (1969) and Warner et al. (1997). The myofibril suspension was removed from the freezer, washed free of glycerol by four dilution and centrifugation cycles (11 500g for 15 s) with rigor buffer plus 1 mg/mL BSA and 1 mM DTT. After the final re-suspension in RB/BSA/DTT, the protein concentration was determined using the Biuret assay. Calcium-activated myofibrillar ATPase activity was determined using a total protein concentration of 0.1 mg/mL in RB/BSA/DTT plus 2.2 mM CaC12. In each case, ATPase activity was determined at 22°C in triplicate 0.2-mL samples. The reaction was initiated using 5 µL of 0.2M ATP and was terminated after 5 min using 20 µL of ice-cold 25% trichloroacetic acid (TCA). A blank was included for each sample, which had TCA added before addition of ATP. After centrifuging the samples at 11 500g for 15 s at 4°C to precipitate the denatured protein, 0.1 mL of sample was used to determine inorganic phosphate production by the malachite green assay described in Carter and Karl (1982). Standard curves were prepared using 0.65 mM K2HPO4 (Sigma No. 661–9) and results were expressed as µ-mole of phosphate liberated per mg of protein per min.
Statistical analyses
The data was analysed using ANOVA in GenStat (GenStat Committee 2008) with the treatments being time and hanging method, allowing for interactions and a blocking structure accounting for slaughter day, block within slaughter day, carcass and side (slaughter day/block/carcass/side). Means for the main effects and their interactions were separated (F-test, P < 0.05) by using standard error of the difference.
Results
The average hot carcass weight was 19.8 kg (s.d. = 1.89) and the average GR fat depth was 11.9 mm (s.d. = 2.52).
pH and temperature decline rates
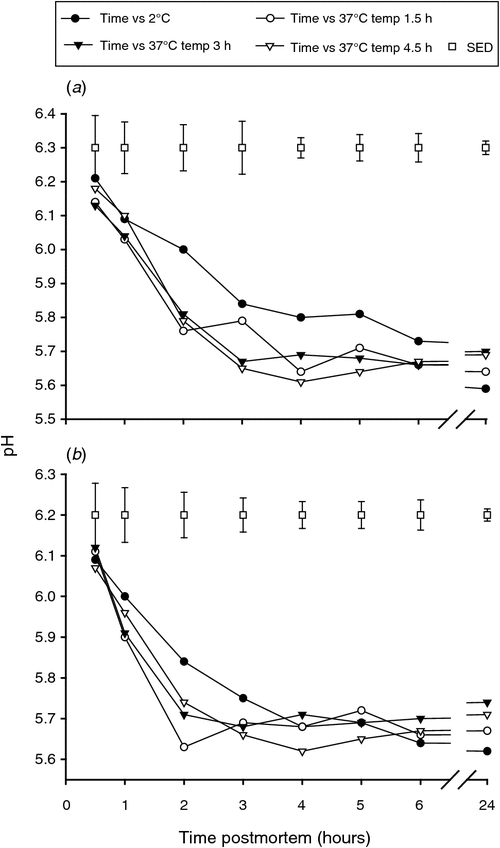
The initial pH was similar for all the muscles (P > 0.05), but a more rapid pH decrease in the muscles from the carcasses placed at 37°C was observed particularly up to 2 h post slaughter regardless of different conditioning time compared with the muscles from the carcasses placed at 2°C (Fig. 1a, b). This result is as expected since it is well established that the pH declines faster at higher temperatures.
|
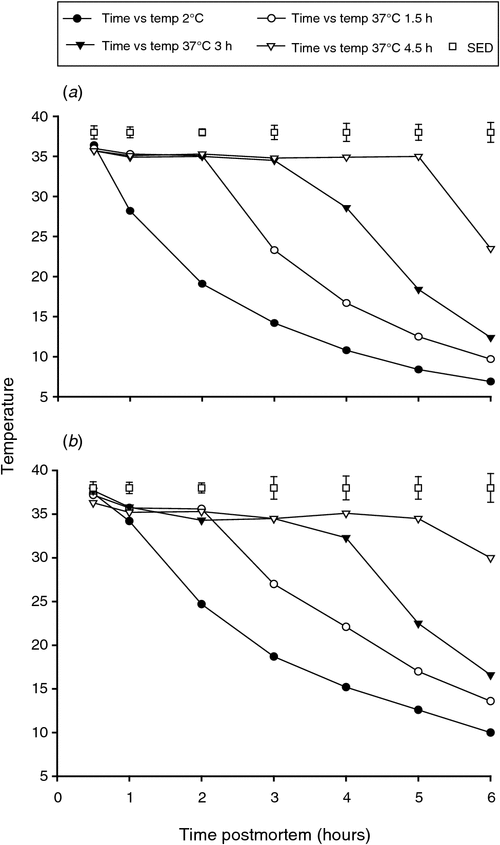
The temperature decline rates were consistent with the conditioning treatment that had been applied to the muscles (Fig. 2a, b). At all subsequent time points post slaughter, the temperature was lower (P < 0.05) for the non-conditioned muscles (placed at 2°C) than for all other temperature conditioning treatments (placed at 37°C). For each treatment, the rate of temperature fall while in the 2°C chiller was faster for the LTL than the SM muscle (Fig. 2a, b).
|
Sarcomere and muscle length
The aitchbone hanging treatment resulted in reduced sarcomere lengths in the LTL (Table 1; P < 0.001), but produced a 33% increase in muscle length and a 35% increase in sarcomere length of the SM (Table 2; P < 0.001). The longer exposure time at 37°C resulted in slightly longer sarcomeres in the LTL and shorter sarcomeres in the SM (Tables 1 and 2: P < 0.05 for both). With respect to the relation between pre-rigor temperature and shortening, the present data indicated the occurrence of heat-shortening in the SM muscle, but not in the LTL, from the carcasses placed at 37°C with various exposure times. There did not appear to be any cold-shortening in the muscles from the carcasses placed at 2°C. In contrast, there was a slight trend of decreasing sarcomere length of the SM with increasing the high temperature exposure time compared with the muscle from the non-conditioned treatment.
Shear force
Different responses to the conditioning treatments were observed in the shear force values of the muscles (Tables 1 and 2). Placing the carcasses at high pre-rigor temperature did not result in an adverse effect on shear force values of the LTL throughout the limited storage period (P > 0.05). In fact, the LTL from the carcasses placed at 37°C for 1.5 h had the numerically lowest shear force value compared with the LTL from other treatments. At 1 day post slaughter, conditioning for 4.5 h resulted in intermediate shear force values and no conditioning or conditioning for 3 h produced the highest shear force values of the LTL. Over all treatments, shear force of the SM was not significantly affected by the pre-rigor holding temperature, but at 1 day post mortem a trend towards lower shear force values with an increase in the high temperature exposure time was observed (P = 0.083; Table 2).
Applying aitchbone hanging did not influence shear force values of the LTL (P > 0.05) regardless of the different pre-rigor temperature and time conditions. However, for the SM muscle, lower shear force values at 1 day post slaughter were observed (P < 0.05) for aitchbone-hung muscles than for Achilles-hung muscles. At 3 days post slaughter, the non-conditioned SM had similar (P > 0.05) shear force values between aitchbone- and Achilles-hung treatments, whereas the positive stretching impact from the aitchbone hang was maintained in the SM from the carcasses exposed to 37°C for various times. In addition, no significant aging effect was observed in the shear force values of the SM temperature conditioned for 4.5 h regardless of the hang treatment.
Protein denaturation
High pre-rigor temperature induced more protein denaturation as indicated by lower protein solubility and myofibrillar ATPase activity (Tables 1 and 2). In general, with increasing time at high pre-rigor temperature, both protein solubility and myofibrillar ATPase activity decreased for both muscles (P < 0.05).
Aitchbone hanging did not affect protein solubility of the muscles (P > 0.05), but myofibrillar ATPase activity was affected by hanging method (P < 0.05), although a different response was observed for each muscle. Higher myofibrillar ATPase activity was observed in the LTL from the aitchbone-hung side, whereas the opposite was observed for the SM (Tables 1 and 2).
Water-holding capacity and colour
For both muscles, high temperature conditioning resulted in increased (P < 0.05) cook loss, surface exudate and purge. For the LTL, surface exudate and purge loss were the highest when carcasses were held at 37°C for 4.5 h. Aitchbone hanging did not significantly impact on water-holding capacity of the LTL undergoing the different temperature/time conditions. In fact, increased purge loss with aitchbone hanging was observed in the LTL (P < 0.05). However, stretching due to aitchbone hanging resulted in more positive impacts on the SM by decreasing cook loss and purge loss (P < 0.05), although the amount of surface exudates was not affected by stretching (P > 0.05).
Increased lightness values (higher L*) were observed from the muscles from the carcasses placed at 37°C (Tables 1 and 2; P < 0.05). Particularly, with increasing pre-rigor exposure time to 37°C, gradual increase in lightness of the muscles was found. Aitchbone hanging did not affect the lightness values of the muscles. In comparison to the lightness, a* (redness) and b* (yellowness) values were not substantially influenced by the treatments, although there were some statistically significant results found in these colour attributes.
Discussion
Results pertaining to the SM muscle generally showed clear effects of both the high temperature conditioning time and the hang treatment. The aitchbone hanging resulted in an increased muscle and sarcomere length, but did not completely prevent the effects of heat-shortening conditions, as evidenced by a decrease in sarcomere length with the duration of heat-shortening conditions. The impact of muscle shortening on shear force values of the SM was evident with a significant interaction between shear force and sarcomere length found at both 1 and 3 days post mortem. The interaction between the stretch treatment and the duration of high temperature conditioning for shear force after 3 days of aging indicates that the aging response in the SM from normally chilled carcasses was sufficient to compensate for the differences in contraction status, but that the aging response in high temperature conditioned SM was hampered by the treatment.
Results regarding myofibrillar ATPase activity support the theory posed by Offer (1991) stating that myosin bound to actin is less susceptible to denaturation than unbound myosin with both the stretch resulting from aitchbone hanging and the duration of high temperature conditioning having significant effects. However, a general measure of protein denaturation (protein solubility) was only significantly affected by the duration of high temperature conditioning. Similarly, meat quality traits directly affected by protein denaturation, i.e. those reflecting water-binding capacity at the muscle surface (colour and the filter paper test), were only significantly affected by the duration of high temperature conditioning. On the other hand, whole muscle or cut measures of water-binding capacity (cooking loss and purge) were affected by both the hang method and the duration of high temperature conditioning.
The aitchbone hanging treatment had a negligible effect on the sarcomere length of the LTL. Since the aitchbone hanging treatment was applied to one side of the intact carcasses, the stretching treatment was probably affecting the length of the LTL of both carcass sides. Results for the LTL of both carcass sides can therefore be discussed concerning the effects of duration of high temperature pre-rigor conditions on meat quality traits while preventing heat-shortening per se. Considering the results from this perspective, it is clear that the duration of high temperature pre-rigor conditions affects traits reflecting protein denaturation, such as, myofibrillar ATPase activity and protein solubility, and directly relates to meat quality traits such as meat colour and water-holding capacity. The results regarding shear force for the LTL appear to confirm that high temperature conditioning promotes tenderisation early post mortem, with high temperature-conditioned muscles being generally more tender at 1 day post mortem than the immediately chilled muscles. After 3 days of aging the difference in shear force in the LTL was no longer present, but neither were there any indications of a heat-toughening effect. The lack of a heat-toughening effect may be partly explained by the limited aging period (3 days). However, the lack of an effect of duration of high temperature conditioning on shear force at 3 days, despite a clear effect on measures of protein denaturation, argues against this explanation. A more convincing explanation would be that the impact of post-mortem proteolysis on tenderness of stretched muscles is rather limited. This is supported by the results reported by Wheeler et al. (2000) showing that a sarcomere length beyond ~2 µm, results in a uniform level of tenderness between different muscles despite a clearly documented difference in aging response between different muscles. In the present study, the sarcomere lengths of both the LTL and the SM appear to be rather long, but Bouton et al. (1975) found stretched samples produced sarcomere lengths of >3.0 µm. Taking the likely overestimation of sarcomere length into account, the stretching treatment appears to have prevented an adverse effect of heat-toughening conditions on tenderness by limiting the importance of post-mortem proteolysis for tenderness development.
In general, the results regarding meat quality traits of the SM suggest that even a rather limited delay in chilling can result in significant effects with regard to yield (purge and cooking loss) and tenderness (shear force at Day 3). These effects were mitigated by the stretching treatment. Heat-toughening conditions are usually not common in modern sheep abattoirs, although they have been found in sheep carcasses in Australia (Pearce et al. 2010) and the UK (Matthews 2011). Heat-induced toughening is certainly known to be prevalent in beef abattoirs, particularly in carcasses from grain-fed cattle (Warner et al. 2014a). The present results indicate that pre-rigor stretching can have an important protective effect against the negative effects of high pre-rigor temperatures on meat quality traits.
Acknowledgements
The funding provided by Meat Livestock Australia is gratefully acknowledged as well as the technical assistance provided by Levent Can, Jeremy Cottrell and Paul Weston and biometrical assistance of Kym Butler. The research was approved by the Victorian Institute of Animal Science Animal Ethics Committee.
References
Bekhit AED, Farouk MM, Cassidy L, Gilbert KV (2007) Effects of rigor temperature and electrical stimulation on venison quality. Meat Science 75, 564–574.| Effects of rigor temperature and electrical stimulation on venison quality.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3Mbns1amtg%3D%3D&md5=1c089d2e2c53750300287af5f86e53f0CAS |
Bouton PE, Harris PV, Shorthose WR (1975) Possible relationships between shear, tensile, and adhesion properties of meat and meat structure. Journal of Texture Studies 6, 297–314.
| Possible relationships between shear, tensile, and adhesion properties of meat and meat structure.Crossref | GoogleScholarGoogle Scholar |
Carter SG, Karl DW (1982) Inorganic phosphate assay with malachite green: an improvement and evaluation. Journal of Biochemical and Biophysical Methods 7, 7–13.
| Inorganic phosphate assay with malachite green: an improvement and evaluation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL3sXkvFykug%3D%3D&md5=f5faa8b4566e41fd2980b917e35eeba8CAS | 7153458PubMed |
Devine CE, Wahlgren NM, Tornberg E (1999) Effect of rigor temperature on muscle shortening and tenderisation of restrained and unrestrained beef M. longissimus thoracicus et lumborum. Meat Science 51, 61–72.
| Effect of rigor temperature on muscle shortening and tenderisation of restrained and unrestrained beef M. longissimus thoracicus et lumborum.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3MbnsVWqtA%3D%3D&md5=0fa670a2c0be3f3e48dbef00926751beCAS | 22061537PubMed |
Devine CE, Payne SR, Peachey BM, Lowe TE, Ingram JR, Cook CJ (2002) High and low rigor temperature effects on sheep meat tenderness and ageing. Meat Science 60, 141–146.
| High and low rigor temperature effects on sheep meat tenderness and ageing.Crossref | GoogleScholarGoogle Scholar | 22063237PubMed |
Geesink GH, Bekhit AD, Bickerstaffe R (2000) Rigor temperature and meat quality characteristics of lamb longissimus muscle. Journal of Animal Science 78, 2842–2848.
GenStat Committee (2008) ‘Genstat for Windows.’ 11th edn. (VSN International: Hertfordshire, UK)
Gornall AG, Bardawill CJ, David MM (1949) Determination of serum protein by means of biuret reaction. The Journal of Biological Chemistry 177, 751
Greaser ML, Cassens RG, Briskey EJ, Hoekstra WG (1969) Post-mortem changes in subcelllular fractions from normal and pale, soft, exudative porcine muscle. 2. Electron microscopy. Journal of Food Science 34, 125–132.
| Post-mortem changes in subcelllular fractions from normal and pale, soft, exudative porcine muscle. 2. Electron microscopy.Crossref | GoogleScholarGoogle Scholar |
Hwang IH, Park BY, Cho SH, Lee JM (2004) Effects of muscle shortening and proteolysis on Warner-Bratzler shear force in beef longissimus and semitendinosus. Meat Science 68, 497–505.
| Effects of muscle shortening and proteolysis on Warner-Bratzler shear force in beef longissimus and semitendinosus.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXlsVSmu7o%3D&md5=47c3afc94e61d28200f7ed6c2a3e3db9CAS | 22062419PubMed |
Jaime I, Beltrán JA, Ceña P, López-Lorenzo P, Roncalés P (1992) Tenderisation of lamb meat: effect of rapid postmortem temperature drop on muscle conditioning and aging. Meat Science 32, 357–366.
| Tenderisation of lamb meat: effect of rapid postmortem temperature drop on muscle conditioning and aging.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3MbntlSqtQ%3D%3D&md5=89c3c4e2c797083706a0d3bba594d11dCAS | 22059887PubMed |
Kim YH, Lonergan SM, Huff-Lonergan E (2010) Protein denaturing conditions in beef deep semimembranosus muscle results in limited µ-calpain activation and protein degradation. Meat Science 86, 883–887.
| Protein denaturing conditions in beef deep semimembranosus muscle results in limited µ-calpain activation and protein degradation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhtFCrurnE&md5=4becf077926d7fe23677f72d367918a4CAS | 20674187PubMed |
Kim YHB, Stuart A, Nygaard G, Rosenvold K (2012) High pre rigor temperature limits the ageing potential of beef that is not completely overcome by electrical stimulation and muscle restraining. Meat Science 91, 62–68.
| High pre rigor temperature limits the ageing potential of beef that is not completely overcome by electrical stimulation and muscle restraining.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XjtVCmsLg%3D&md5=1f902cbe6afe74ff48f6d5b2ac116177CAS |
Lee YB, Ashmore CR (1985) Effect of early postmortem temperature on beef tenderness. Journal of Animal Science 60, 1588–1596.
Locker RH, Hagyard CJ (1963) A cold shortening effect in beef muscles. Journal of the Science of Food and Agriculture 14, 787–793.
| A cold shortening effect in beef muscles.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaF2cXlsFOiug%3D%3D&md5=ebcef65dee72d68932c06451127cd7e1CAS |
Marsh BB, Lochner JV, Takahashi G, Kragness DD (1981) Effects of early post-mortem pH and temperature on beef tenderness. Meat Science 5, 479–483.
| Effects of early post-mortem pH and temperature on beef tenderness.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3MbmvVGmsg%3D%3D&md5=79ed9b14424f73b0dd7be364e1a8c99dCAS | 22054608PubMed |
Marsh BB, Ringkob TP, Russell RL, Swartz DR, Pagel LA (1987) Effects of early-postmortem glycolytic rate on beef tenderness. Meat Science 21, 241–248.
| Effects of early-postmortem glycolytic rate on beef tenderness.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3MbmvVymtA%3D%3D&md5=21c2e1f7a8e8a703443160d9a9db41daCAS | 22055055PubMed |
Matthews K (2011) Evaluation of the pH fall in English lamb abattoirs against the Meat Standards Australia pH/temperature window, Report to EBLEX. Available at http://www.eblex.org.uk/wp/wp-content/uploads/2013/04/evaluationofthephfallinenglishlambabattoirs_271011-final-report.pdf [Verified 6 February 2014]
Offer G (1991) Modelling of the formation of pale, soft and exudative meat: Effects of chilling regime and rate and extent of glycolysis. Meat Science 30, 157–184.
| Modelling of the formation of pale, soft and exudative meat: Effects of chilling regime and rate and extent of glycolysis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3MXmslygu7Y%3D&md5=ab78c2132e3b69fa280d2bb8d1e81744CAS | 22061833PubMed |
Pearce KL, Van De Ven R, Mudford CR, Warner RD, Hocking-Edwards JE, Pethick DW, Hopkins DL (2010) Case studies demonstrating the optimisation of medium voltage electrical stimulation of lamb carcases. Animal Production Science 50, 1107–1114.
| Case studies demonstrating the optimisation of medium voltage electrical stimulation of lamb carcases.Crossref | GoogleScholarGoogle Scholar |
Rosenvold K, Wiklund E (2011) Retail colour display life of chilled lamb as affected by processing conditions and storage temperature. Meat Science 88, 354–360.
| Retail colour display life of chilled lamb as affected by processing conditions and storage temperature.Crossref | GoogleScholarGoogle Scholar | 21316158PubMed |
Rosenvold K, North M, Devine C, Micklander E, Hansen P, Dobbie P, Wells R (2008) The protective effect of electrical stimulation and wrapping on beef tenderness at high pre rigor temperatures. Meat Science 79, 299–306.
| The protective effect of electrical stimulation and wrapping on beef tenderness at high pre rigor temperatures.Crossref | GoogleScholarGoogle Scholar | 22062758PubMed |
Ruddick JE, Richards JF (1975) Comparison of sarcomere length measurement of cooked chicken pectoralis muscle by laser diffraction and oil immersion microscopy. Journal of Food Science 40, 500–501.
| Comparison of sarcomere length measurement of cooked chicken pectoralis muscle by laser diffraction and oil immersion microscopy.Crossref | GoogleScholarGoogle Scholar |
Savell JW, Mueller SL, Baird BE (2005) The chilling of carcasses. Meat Science 70, 449–459.
| The chilling of carcasses.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3Mbns1Sisg%3D%3D&md5=9ca9d80413e7acb630edeb637dd809bfCAS | 22063744PubMed |
Thomson KL, Gardner GE, Simmons N, Thompson JM (2008) Length of exposure to high post-rigor temperatures affects the tenderisation of the beef M. longissmus dorsi. Australian Journal of Experimental Agriculture 48, 1442–1450.
| Length of exposure to high post-rigor temperatures affects the tenderisation of the beef M. longissmus dorsi.Crossref | GoogleScholarGoogle Scholar |
Warner RD, Kauffman RG, Greaser ML (1997) Muscle protein changes post mortem in relation to pork quality traits. Meat Science 45, 339–352.
| Muscle protein changes post mortem in relation to pork quality traits.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXisFyrtrY%3D&md5=7a2e794968870801cd62ddf945b9145dCAS | 22061472PubMed |
Warner RD, Dunshea FR, Gutzke D, Lau J, Kearney G (2014a) Factors influencing the incidence of high rigor temperature in beef carcasses in Australia. Animal Production Science 54, 363–374.
| Factors influencing the incidence of high rigor temperature in beef carcasses in Australia.Crossref | GoogleScholarGoogle Scholar |
Warner RD, Kerr M, Kim YHB, Geesink G (2014b) Pre-rigor carcass stretching counteracts the negative effects of high rigor temperature on tenderness and water-holding capacity – using lamb muscles as a model. Animal Production Science 54, 494–503.
| Pre-rigor carcass stretching counteracts the negative effects of high rigor temperature on tenderness and water-holding capacity – using lamb muscles as a model.Crossref | GoogleScholarGoogle Scholar |
Wheeler TL, Shackelford SD, Koohmaraie M (2000) Variation in proteolysis, sarcomere length, collagen content, and tenderness among major pork muscles. Journal of Animal Science 78, 958–965.