Improving beef meat colour scores at carcass grading
J. M. Hughes A D , G. Kearney B and R. D. Warner CA CSIRO Animal, Food and Health Sciences, Level 1, Block 10, 39 Kessels Road, Coopers Plains, Qld 4108, Australia.
B 36 Paynes Road, Hamilton, Vic. 3300, Australia.
C CSIRO Animal, Food and Health Sciences, 671 Sneydes Road, Werribee, Vic. 3030, Australia.
D Corresponding author. Email: joanne.hughes@csiro.au
Animal Production Science 54(4) 422-429 https://doi.org/10.1071/AN13454
Submitted: 31 October 2013 Accepted: 10 January 2014 Published: 21 February 2014
Journal Compilation © CSIRO Publishing 2014 Open Access CC BY-NC-ND
Abstract
Unacceptable meat colour scores at the time of carcass grading are associated with reduced meat quality and consumer rejection. We hypothesised that the meat colour at carcass grading would be influenced by the pH and temperature decline post slaughter, as these would be determined by animal and processing factors. Beef carcasses (n = 1512) at seven Australian processing plants were assessed, at grading, for the meat colour of the M. longissimus thoracis. Statistical modelling determined the animal, carcass and processing factors contributing to the meat colour score at carcass grading. The occurrence of unacceptably dark meat dropped from 8 to 3% when the time of grading was increased from 14 to 31 h post slaughter (P < 0.01). A high temperature at pH 6 (rigor temperature), high final pH (pHF), pasture feeding and older animals were associated with dark M. longissimus thoracis at carcass grading (P < 0.05 for all). Less than 30% of carcasses with non-compliant pHF displayed a dark non-compliant meat colour >3, indicative of an opportunity to determine the mechanism behind this pH-induced colour development and thus reduce the incidence of non-compliance. It is recommended that when there is a high occurrence of carcasses with a dark meat colour >3 that the time from slaughter to grading is checked to ensure carcasses are in full rigor at the grading point. This will assist in minimising economic penalties due to dark-coloured carcasses. Finally, animal factors, such as maturity and feeding regime also had a considerable impact on the meat colour at carcass grading.
Additional keywords: dark cutting, feeding regime, maturity, myoglobin.
Introduction
Beef meat colour is an important attribute for visual appearance and consumer acceptability. In Australia during 2012–13, Meat Standards Australia (MSA) reported the main reason for carcasses not meeting specification was due to unfavourably dark meat colour (>3) and/or a high pH (pH >5.70), with 5.3% carcasses failing for one of these reasons or a combination of both (Meat and Livestock Australia Limited 2013). The cost to the industry of dark meat colour, based on MSA-graded carcasses, is estimated to be ~$400 per affected carcass (industry sources) and to be in the region of $35 million/annum for MSA carcasses alone. As MSA carcasses are approximately only 24% of the beef carcasses in Australia, the cost to the national beef industry is much higher. Consequently, there is a need to minimise the incidence of beef carcasses failing colour specification. Dark and light meat colours are associated with the unfavourable conditions known as dark-cutting and heat-induced toughening, respectively. Both of these quality conditions negatively impact water-holding capacity, visual appearance, shelf-life and eating quality aspects such as tenderness (Purchas and Aungsupakorn 1993; Ferguson et al. 1999; Kim et al. 2014; Warner et al. 2014b). It is well known that pre-slaughter stress in cattle depletes glycogen reserves and is associated with a high pH of the meat, particularly in animals on poor nutritional feeding regimes before slaughter (Lawrie 1958; Knee et al. 2007). In comparison, heavier grain-fed animals can be exposed to post-slaughter processing, which can generate unfavourable high temperature, low pH conditions in the muscle (Warner et al. 2014a).
The surface colour of meat is largely determined by the concentration and chemical status of myoglobin, and depth of the myoglobin layers [see Faustman and Cassens (1990) for a review] and also by the changes in the structure of the muscle post mortem, which is much less known and understood. During early post-slaughter pH decline of the muscle, there is evidence of structural alterations and drip channel formation (Heffron and Hegarty 1974; Bertram et al. 2004), which could result in differences in absorbance, reflectance and oxygen penetration into the muscle. As the muscle enters rigor, there can be a reduction of 14–16% in muscle fibre diameter and consequently an increase in extracellular space (Heffron and Hegarty 1974). In addition, myofibrils can undergo shrinkage, creating the opportunity for drip formation and loss (Diesbourg et al. 1988; Bertram et al. 2004). The magnitude of these changes is a pH-dependent process and is a primary driver in the change in colour of muscle post mortem. A limited pH decline results in a high pH (pH >5.7) and a dark colour at grading, as opposed to a full pH decline to 5.4–5.5, which results in a bright cherry-red colour at grading (Murray 1989). Also, the rate of this pH decline is influential in the process, as the temperature of the muscle is important (Hamm 1961; Renerre 1990). A rapid post-mortem metabolism (fast pH fall) can result in denaturing conditions, resulting in changes in light scattering and also loss of sarcoplasmic proteins such as myoglobin from muscle (Swatland 2008) and, hence, a paler colour. For these reasons, we hypothesised that the meat colour at grading would be influenced by the pH and temperature decline post slaughter, as these would be determined by animal and processing factors. Although the influences of animal and processing factors on beef meat colour have been reported, the factors relevant to Australia under modern processing conditions need to be known. Therefore, the aim of this study was to test the factors which could contribute to beef meat colour at grading, in Australian beef processing plants. From this information we can look to optimise beef meat colour and, hence, supply information to industry to improve the meat colour at grading.
Methods
Animals and treatments
Data from 1512 beef carcasses were obtained from seven beef processing plants across five states in Australia. This study was a component of a larger study described by Warner et al. (2014a). In brief, data were collected from 103 mobs of cattle over a total of 28 days from March to June 2006 (autumn and winter months). Data on pre-slaughter feeding were collected and comprised a finishing feed type of either milk (n = 58), pasture (n = 364) or grain (n = 1090). Animals were allocated into one of four categories; vealer, young/prime, cow/ox or grain. Each category was determined by the plant, depending upon dentition, feeding regime and carcass weight. ‘Vealers’ had no erupted permanent incisor teeth and their carcasses weighed no more than 170 kg (still suckling the cow but have access to pasture). Cattle categorised as ‘young/prime’ were animals that were grazing pasture pre-slaughter and had 0–4 permanent incisors. Cattle categorised as ‘cow/ox’ were grazing pasture pre-slaughter and had 6–8 permanent incisors. The animals in the ‘grain’ category were grain-fed (in a feedlot) and had 0–4 permanent incisors. Within the ‘grain’ category the range of ‘days on feed’ was between 60 and 350 days. No bulls, exhibiting secondary sexual characteristics, were included in the study.
Animals were stunned using a captive bolt pistol, and were either immobilised (n = 904) or directly shackled by the hind limb immediately before exsanguination without immobilisation (n = 568). The time of exsanguination was recorded and defined as the time of slaughter. Some animals (n = 309) were also exposed to electrical stimulation and almost all animals had a rigidity probe (n = 1409 animals) attached to the carcass during hide pulling. The durations of electrical inputs at the immobiliser, electrical stimulation and hide puller were recorded (seconds). Carcasses were then halved, weighed (hot carcass weight), the gender (female or castrate) was recorded and the fat depth (mm) was measured at the P8 site on the rump of the carcass (Meat and Livestock Australia Limited 2010). The time of entry into the chiller was also recorded.
Temperature and pH decline
After placement in the chiller, two pH, temperature and time measurements were recorded in the M. longissimus lumborum in proximity to the 2nd to 5th lumbar vertebrae on individual carcasses. Where possible, the second measurement was timed to ensure the muscle pH was below 6.0. For each measurement, care was taken to place the pH meter into a fresh incision rather than just using the site where the last measurement was recorded. A Jenco TPS pH meter (WP-80), with a polypropylene spear-type gel electrode (Ionode IJ 44) and temperature probe (part no. 121247), all manufactured and purchased from TPS Pty Ltd, Springwood, Qld, Australia, were used to measure pH and temperature. The pH meter and electrode were calibrated at ambient temperature using buffers of 4.00 and 7.00 and checked regularly for re-calibration. After overnight chilling, a pH was measured at the time of grading, which occurred 14–31 h post slaughter defined as the final pH (pHF). The temperature at which a carcass passed through pH 6 was defined as the temperature at pH 6 (rigor temperature) and the calculation is described in (Warner et al. 2014a).
Grading assessments
Carcass sides were quartered at the 4th/5th ribs (vealer only) or between the 10th/11th, 11th/12th or 12th/13th ribs. The exposed M. longissimus thoracis was allowed to bloom for a minimum of 120 min before colour assessment. Assessment was carried out with the aid of light from a torch (1400–3000 lx, 12-V, 20-W diachronic glass-faced low voltage, tungsten halogen lamp, part number: 01-E710A2, Emroth Technologies Pty Ltd, Kenmore, Qld, Australia) held at an angle of ~45° to the muscle surface and at a distance of 30–45 cm from the meat surface. Meat colour was subjectively scored on a scale from 1 (split into 1A, 1B, 1C) to 7 using AUS-MEAT colour chips by a qualified assessor according to the AUS-MEAT chiller assessment language (AUS-MEAT 2005). Meat colour was considered to be paler at the lower end of the scale (meat colour scores 1A, 1B and 1C) and unacceptably dark at the higher end of the scale (meat colour scores >3). MSA classifies carcasses as dark-cutting when the meat colour score is >3 and/or has an ultimate pH >5.70. The time of assessment was recorded and was between 14 and 31 h post slaughter with M. longissimus thoracis temperatures 7.4 ± 3.3°C. Graders also measured the size (cm2) of the exposed M. longissimus thoracis and defined this as eye muscle area (EMA).
Statistical analyses
Due to the minimal numbers of muscle colour grades 5 (n = 40) and 6 (n = 18), these grades were pooled. For simplicity of illustration, meat colour scores >3 were pooled for tables and graphs. The method of generalised linear model with a multinomial distribution and logit link function was used to analyse the data for the dependent variable meat colour score. A total of 15 explanatory variates were tested in the initial model; 10 quantitative and 5 qualitative. The initial analysis included the terms; plant, mob, gender, category, days on feed (nested within grain category), time from slaughter to chiller, time from slaughter to colour grading, temperature at pH 6, pHF, hot carcass weight, EMA, P8 fat depth, duration of immobiliser, duration of stimulator and duration of rigidity probe and relevant interactions. Grader was tested and found to be confounded with plant and thus could not be included in the model. Extensive examination of mob was undertaken but it was removed for several reasons, but mainly for parsimonious reasons. Terms were only included in the final model if they were statistically significant (P < 0.05). All statistical analyses were performed using Genstat (2008). Plant was included in the model in order to adjust for its effect, but the results are not presented as the focus of the study was on finding influencing factors, not on finding differences between plants.
Using the significant terms within the meat colour model, the predicted percent of each meat colour score was calculated. The rate of change between meat colour scores was calculated by dividing the predicted percentage difference of two meat colour score groups by the actual time (hours) interval.
Results
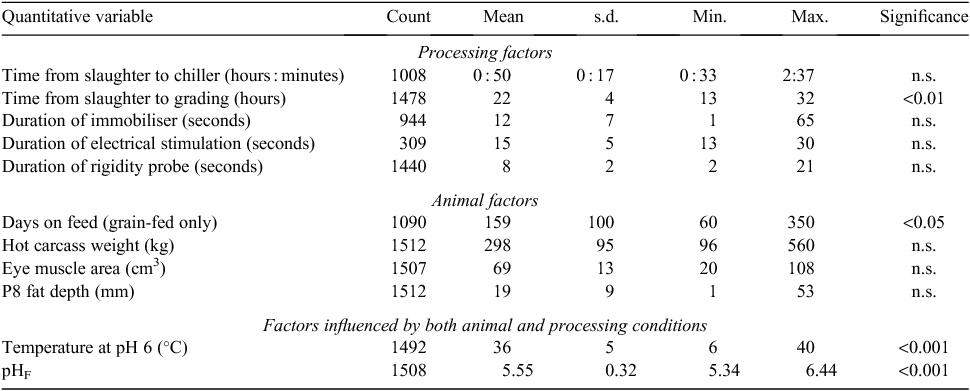
A summary of all the quantitative and qualitative variates tested are given in Tables 1 and 2, respectively. From all the explanatory variables tested, six variables were found to be significant (P < 0.05; Tables 1 and 2). These were; plant, time from slaughter to carcass grading, temperature at pH 6, pHF, category and ‘days on feed’ within the grain-fed category. The remaining variables (the time from slaughter to chiller, hot carcass weight, EMA, P8 fat depth, gender, duration of immobiliser, electrical stimulation or rigidity probe) and relevant interactions were found to have no effect on the meat colour score (P > 0.05). From all the carcasses measured, 10% had a meat colour score >3 and would be considered non-compliant, using the MSA grading system (Meat and Livestock Australia Limited 2010).
|
Time from slaughter to grading
The time from slaughter to grading influenced the meat colour score (P < 0.01; Table 1) and was highly variable (14–31 h). The predicted percent of each meat colour score over the time of grading is presented in Table 3. Carcasses graded early (14 h post slaughter) showed a higher incidence of meat colour scores >3 (8%), compared with carcasses graded at 31 h post slaughter, which showed fewer meat colour scores >3 (3%).
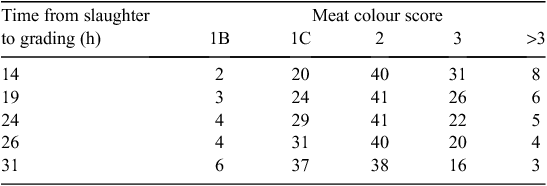
|
Carcasses graded at 14 h post slaughter had 22% light meat colour scores (1B/1C) whereas those carcasses graded at 31 h post slaughter had 43% light meat colour scores (1B/1C). The majority of the carcasses (75%) graded after 31 h post slaughter were of meat colour 1C or 2, whereas if carcasses were graded after only 14 h post slaughter, the majority (71%) were meat colour scores 2 or 3, indicative of a darker appearance.
The rate change in the occurrence of meat colour scores also appeared to vary between 14 and 31 h and for the purpose of presentation has been segregated into four groups (Table 4). Between 14 and 19 h post slaughter the rate of decrease in occurrence of meat colours >3 was largest (0.4%/h), whereas between 24 and 31 h post slaughter only 0.2%/h changed from having meat colour >3 to <3. In addition, more carcasses per hour moved into the 1B or 1C meat colour in the 26–31-h post-slaughter window, compared with the 14–19-h post-slaughter window. Thus, more dark colours (>3) become lighter from 14 to 19 h and more intermediate meat colours (>1C) become lighter (1B or 1C) from 26 to 31 h post slaughter.
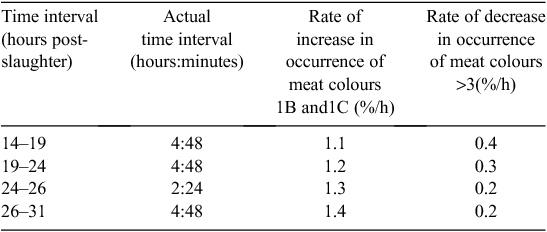
|
Temperature at pH 6
The temperature of the muscle during the development of rigor, as measured by temperature at pH 6, had an effect on the meat colour score (P < 0.001; Table 1). The range in temperature at pH 6 was from 15 to 40°C and the effect on the predicted meat colour score is shown in Fig. 1. High temperature at pH 6 carcasses (40°C) displayed the lightest colours, with 38% of carcasses being graded as either 1B or 1C and the fewest dark colours, with only 4% having a meat colour score >3. This temperature also generated the lowest percentage (59%) of carcasses with a meat colour of 2 or 3. In comparison, a low temperature at pH 6 (15°C) displayed the highest percentage (18%) of dark meat colour scores >3 and smallest percent (10%) of lighter meat colour scores 1B and 1C. The largest percent (73%) of meat colour scores 2 or 3, were observed at 15 and 25°C, but at 25°C there were only 10% with meat colour score >3, meaning there were 8% fewer dark-coloured carcasses.
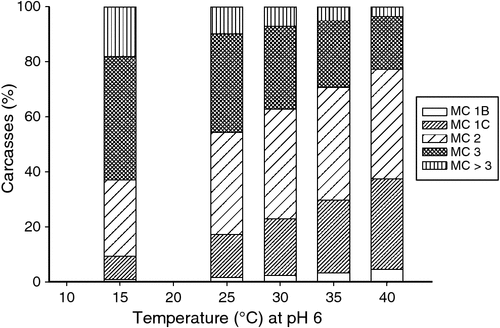
|
Final pH
The pHF in the M. longissimus thoracis at grading influenced the meat colour score (P < 0.001; Table 1). Carcasses with a higher pHF had a higher incidence of darker meat colour scores (Fig. 2). In those carcasses having pHF above the dark-cutting reference point, the percent of meat colour scores >3 increased, with pHF values of 5.8, 6.0 and 6.2 having 28, 74 and 96%, respectively. In comparison, those carcasses with a lower pHF of 5.4 and 5.6 had only 1 and 5% of meat colour scores >3. It is interesting to note, that almost half (47%) of carcasses with a pHF of 5.80 had a meat colour score of 3. But, generally, a darker meat colour score was typically observed when the pHF of the carcass was high.
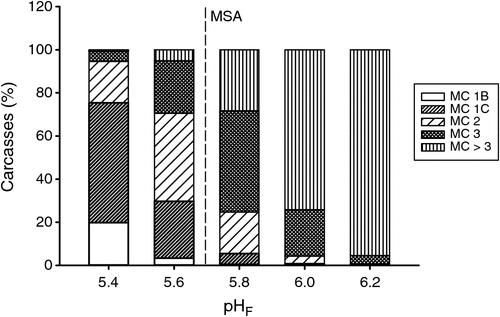
|
Carcasses with a low pHF (5.4) had the highest percent of lighter meat colours; with colour score 1B and 1C occurring in 20 and 56% carcasses at this pHF, respectively. As the pHF increased, the lighter meat colours declined, with no meat colour 1B and 6% of meat colours 1C being observed in carcasses with a pHF >5.7. However, over 70% of carcasses with a pHF of 5.8 had a meat colour score <4 and this group had the highest incidence of meat colour score 3, which composed 47% of all carcasses in the group. Thus, a pHF~5.8 or greater did not correlate well with dark meat colours.
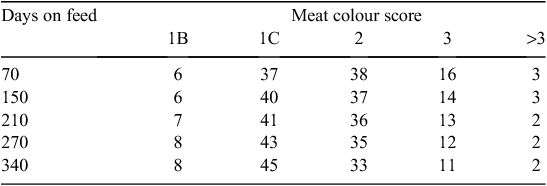
Category and feeding regime of animal
The category of the animal, and the ‘days on feed’ within the grain-fed category, influenced the meat colour at grading (P < 0.001, Table 2 and P < 0.05, Table 1, respectively), and are summarised in Fig. 3 and Table 5. The younger animals (vealer category) displayed the largest percent (54%) of pale meat colour scores 1B (9%) and 1C (45%) and the lowest percent of meat colour scores >3 (2%). In comparison, the older animals, cows and ox, displayed a very high percent of darker meat colour scores >3 (33%).
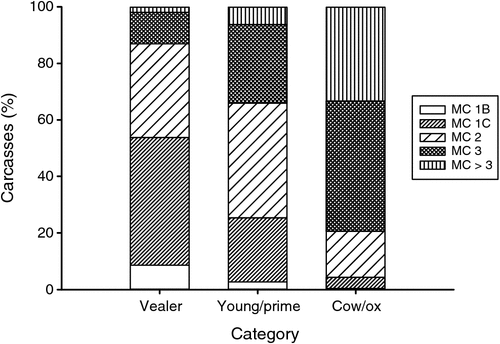
|
|
Darker meat colour scores >3 were twice as prevalent in the young/prime (pasture-fed) category (6%) compared with the grain categories (2–3%). The grain categories displayed a large percentage (43–53%) of light meat colour scores 1B and 1C. Thus, approximately half of all grain-fed cattle had a light meat colour and these values were comparable to the vealer category. In comparison, the young/prime category had only 26% of carcasses with a meat colour 1B or 1C, but had the most number of carcasses graded as meat colour 2 or 3 (68%).
Discussion
Influence of time from slaughter to grading on the occurrence of ‘dark-coloured’ meat
In our study, delaying the grading time from 14 to 31 h post slaughter reduced the occurrence of ‘unacceptably’ dark-coloured meat from 8 to 3%, almost a 3-fold reduction. Thus we can accept the hypothesis that the time post slaughter when grading occurs influences the meat colour score at grading. This is not a novel finding as Murray (1989) reported that the incidence of dark-coloured beef was 3 times higher at 15–18 h than at 23–26 h post stunning in the Canadian beef industry. While this phenomenon is well known worldwide, the AUS-MEAT chiller assessment requirements state that a non-stimulated carcass cannot be assessed until 18 h post slaughter (Food Science Australia 2004). Furthermore, AUS-MEAT states that a carcass that has received full stimulation can be assessed at 8 h post slaughter (Food Science Australia 2004). Our results show that even 14 h is too early to grade beef carcasses for meat colour. Thus it is recommended when grading a carcass after ~14 h post slaughter and observing a dark meat colour score >3, the time from slaughter to grading could be lengthened to achieve the ultimate pH and therefore minimise economic penalties to that particular carcass.
Influence of time from slaughter to grading on lightness of meat and rate of colour change
The rate of meat colour change from dark colour scores (>3) to lighter meat colour scores (1B or 1C) was apparent between 14 and 31 h. The % dark colour scores (>3) decreases and the % pale colour score increases from 14 to 19 h and between 26 and 31 h post slaughter, intermediate meat colour scores (>1C) decrease and pale colour scores (1B or 1C) increase. Thus, the muscle appears to become lighter during this early post-slaughter period and is consistent with other findings (Young et al. 1999). The mechanisms which are primarily responsible for the lightening effect observed are not currently known. However, it is believed that it could be due to the progression of the pH decline and the effect this would have on both the structure and the myoglobin status of the muscle.
A proposed mechanism for lighter meat colour scores
During the early post-slaughter period, the pH of the muscle usually falls from 7 to ~5.4, which coincides with a reduction in muscle fibre diameter, increase in extracellular space, myofibrillar shrinkage and drip formation (Heffron and Hegarty 1974; Offer and Cousins 1992; Bertram et al. 2004). Consequently, there is an increase in light penetrating or being transmitted into the structure, and light can be either absorbed by pigments such as myoglobin or scattered by the structural elements. The deeper the light is transmitted into the structure, the higher the absorption by myoglobin and the less light is scattered, hence the darker the appearance (Swatland 2004). This is a pH-dependent process, with high pH muscles having swollen fibres and proportionately less light scattering, whereas muscles with a lower pH undergo more myofibrillar and fibre shrinkage and consequently scatter more light (Swatland 2008). Furthermore, if denaturing conditions occur, denaturation of the myosin head results in further shrinkage in the myofibrillar lattice (Offer 1991).The magnitude of these structural changes would depend on the progress of the pH decline and thus would be time dependent (Diesbourg et al. 1988; Offer and Cousins 1992). In our study, a longer time from slaughter to meat colour grading may provide the muscle with more time to undergo shrinkage of these structural elements compared with those graded earlier. Thus, a greater time between slaughter and grading allows for further progression of the pH decline (and more time allowed for shrinkage of the structural elements), which should correspond to the development of lightness observed at the surface of the meat. Additionally, as this shrinkage occurs, the looser structure could allow for an increase in oxygen diffusion and subsequent pigment oxygenation, promoting red oxymyoglobin formation (Lawrie 1958; MacDougall 1982; Young and West 2001). In comparison, muscles graded earlier would have undergone less shrinkage and consequently appear darker due to the higher density of the lattice. These pH-dependent changes in the structure and pigment occurring during the early post-slaughter period are believed to be contributory to the lightening of the meat colour score over the time period investigated. Further research is required to validate these theories.
Temperature at pH 6 effects
A higher carcass temperature at pH 6 was associated with a higher incidence of lighter meat colours. High temperatures at rigor can result in reduced tenderness and a failure to age (Kim et al. 2014; Warner et al. 2014b). This can generate a condition known as heat-induced toughening and can create denaturing conditions for the muscle proteins and lead to aggregation of sarcoplasmic proteins and precipitation onto the surface of myofibrillar proteins (Bendall and Wismer-Pedersen 1962). This lowers the ability of the proteins to bind water molecules, generating drip and increased light scattering (Bendall and Wismer-Pedersen 1962; Kim et al. 2014). In comparison, muscles going into rigor at lower temperatures had a higher incidence of darker meat colours and have been associated with a thinner red oxymyoglobin layer (Renerre 1990). In addition, the rate of these structural changes within the muscle and variations in metabolic activity between carcasses could also be influential to the incidence of meat colour at grading. Hence, the time to reach pH 6 could be confounded with the temperature at pH 6, and would need to be further investigated. For this study, we focussed purely on the effect of temperature at pH 6 and the relationship to meat colour at grading. In short, a high temperature (40°C) at pH 6.0 generated the largest percent of light meat colours 1B and 1C (38%) and a low temperature (15°C) generated the largest percent of darker meat colours >3 (18%), therefore conditions should be created to minimise such extremities. From the data, the temperature for generating the largest percent of meat colours 2 and 3 and minimal meat colours >3 was 25°C and therefore plants should target a pH 6.0 around this carcass temperature. This is also a suitable temperature for meat tenderness (Geesink et al. 2000).
Final pH
As discussed above, obtaining an optimal pH decline in the muscle is desirable and so is achieving a pHF which is neither too high nor low. The relationship of pH and meat colour interaction is widely researched, with detrimental effects of pre-slaughter stress on utilisation of energy stores and consequently formation of meat with a high pHF being documented (Lawrie 1958; Wismer-Pedersen 1959). A high pHF is associated with tightly packed muscle fibres, reduced light scattering and only a thin layer of red oxymyoglobin on the surface of the meat (Renerre 1990). In comparison, those carcasses with a low pHF (5.4) have a larger percent of lighter colour scores 1B and 1C (76%) in our data, most likely due to the acidic environment created in the muscle causing denaturation of structural proteins and visualised as having a lighter meat colour that is more exudative (Kim et al. 2014). As discussed previously, there appears to be an effect of the rate of pH decline and the time post slaughter in which changes to the structure of the muscle occur which determine the colour observed at grading. The results of this study highlight the inconsistencies that occur between high pH meat and dark meat colours of the carcass. Consequently, an opportunity exists to determine the mechanism of this pH-induced light scattering effect in order to understand ways to reduce the incidence of non-compliant carcasses.
Animal factors
Finally, animal factors such as maturity and feeding regime also affected the meat colour score at grading. Vealers and grain-fed animals had ~50% of the lighter 1B and 1C meat colour scores and older animals had the highest incidence of dark cutting (33%). Muscles from older animals are well known to exhibit darker coloured meat (Page et al. 2001; Mlynek and Gulinski 2007; McGilchrist et al. 2012). This reduction in lightness is most likely to be caused by an increase in myoglobin concentration and lower numbers of large ‘white’ fast glycolytic fibres and more ‘red’ slow oxidative fibres (Moon et al. 2006; Mlynek and Gulinski 2007). Together, this age-induced transition to a slower, more oxidative condition of the muscle could be contributory to the darker meat colour observed.
Furthermore, pasture-fed animals had a lower percent (26%) of lighter meat colours compared with grain (43–53%) and usually have higher muscle concentrations of antioxidants, such as tocopherol and ascorbic acid, and thus have higher myoglobin colour stability and lower levels of lipid and protein oxidation (Descalzo and Sancho 2008). This could minimise protein denaturation, thus preserving the native structure and function of the proteins, thereby improving colour stability and in this study is favourable to intermediate colour scores 2 and 3. Additionally, depending upon seasonal variation of pasture and pre-slaughter animal treatment, these animals could have depleted glycogen stores, which is associated with an increase in dark cutting (Knee et al. 2007). In comparison, grain feeding can increase the glycogen and fat content of the animals, resulting in a thicker subcutaneous fat layer (increasing P8 fat depth), a higher core body temperature (Jacob et al. 2014) and thus more insulation during the carcass chilling. Although hot carcass weight and P8 fat depth were not significant within the meat colour model, as the temperature at pH 6 increased, so too did the occurrence of lighter meat colours. Also, grain-fed animals may be less prone to stressful circumstances due to exposure and habituation to new environments, transportation and human interaction. Therefore, animal specific pre-slaughter factors, such as animal handling, maturity and feeding regime can also impact the meat colour at carcass grading.
Conclusion
A longer time from slaughter to grading was found to result in a lighter meat colour. Muscles graded at 14 h post slaughter had a dark-cutting incidence (MSA colour score >3) of 8%, whereas those graded at 31 h post slaughter had an incidence of 3%. We postulate this was due to structural shrinkage of the muscle fibres and myofibrils, which increases the light scattering properties of the muscle. Also, a high temperature at pH 6 was associated with lighter colour at grading and an optimal temperature around 25°C was recommended to minimise meat colour issues such as dark-cutting and heat-induced toughening. Less than 30% of carcasses with non-compliant pHF displayed a dark non-compliant meat colour score >3, indicative of an opportunity to determine the mechanism behind this pH-induced colour development and thus to reduce the incidence of non-compliance. From these results, when grading a carcass after ~14 h post slaughter and observing a dark meat colour score >3, it is recommended that the time from slaughter to grading could be lengthened to minimise economic penalties to that particular carcass. Finally, animal specific pre-slaughter factors, such as animal handling, maturity and feeding regime can impact meat colour observed at carcass grading.
Acknowledgements
The assistance of Janine Lau, Rob Strachan and Linden Cowper and funding from Meat Livestock Australia and Australian Meat Processors Corporation is gratefully acknowledged.
References
AUS-MEAT (2005) ‘Handbook of Australian meat. International red meat manual.’ (AUS-MEAT, Ltd: South Brisbane)Bendall JR, Wismer-Pedersen J (1962) Some properties of the fibrillar proteins of normal and watery pork muscle. Journal of Food Science 27, 144–159.
| Some properties of the fibrillar proteins of normal and watery pork muscle.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaF2cXhvFaitQ%3D%3D&md5=a99c7acf6b55c139a20c889b21fea461CAS |
Bertram HC, Schafer A, Rosenvold K, Andersen H (2004) Physical changes of significance for early post mortem water distribution in porcine M. longissimus. Meat Science 66, 915–924.
| Physical changes of significance for early post mortem water distribution in porcine M. longissimus.Crossref | GoogleScholarGoogle Scholar | 22061025PubMed |
Descalzo AM, Sancho AM (2008) A review of natural antioxidants and their effects on oxidative status, odor and quality of fresh beef produced in Argentina. Meat Science 79, 423–436.
| A review of natural antioxidants and their effects on oxidative status, odor and quality of fresh beef produced in Argentina.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXmsVGisLg%3D&md5=17ae7d368911f58563e1b9cd82a8b1a7CAS | 22062902PubMed |
Diesbourg L, Swatland HJ, Millman BM (1988) X-ray diffraction measurements of postmortem changes in the myofilament lattice of pork. Journal of Animal Science 66, 1048–1054.
Faustman C, Cassens RG (1990) The biochemical basis for discoloration in fresh meat: a review. Journal of Muscle Foods 1, 217–243.
| The biochemical basis for discoloration in fresh meat: a review.Crossref | GoogleScholarGoogle Scholar |
Ferguson DM, Thompson JM, Polkinghorne R (1999) Meat standards Australia, a ‘PACCP’ based beef grading scheme for consumers. 3) PACCP requirements which apply to carcass processing. Proceedings of the 45th International Congress of Meat Science and Technology 1, 18–19.
Food Science Australia (2004) Visual assessment of meat colour and marbling – Meat Technology Update 2/04. Food Science Australia, Brisbane, Qld.
Geesink GH, Bekhit AD, Bickerstaffe R (2000) Rigor temperature and meat quality characteristics of lamb longissimus muscle. Journal of Animal Science 78, 2842–2848.
Genstat (2008) ‘Genstat.’ (VSN International Limited: Hempstead, UK)
Hamm R (1961) Biochemistry of meat hydration. In ‘Advances in food research. Volume 10’. (Eds CO Chichester, EM Mrak) pp. 355–463. (Academic Press: Kulmbach, Germany)
Heffron JJA, Hegarty PVJ (1974) Evidence for a relationship between ATP hydrolysis and changes in extracellular space and fibre diameter during rigor development in skeletal muscle. Comparative Biochemistry and Physiology. Part A, Physiology 49, 43–55.
| Evidence for a relationship between ATP hydrolysis and changes in extracellular space and fibre diameter during rigor development in skeletal muscle.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaE2MXlsFejtw%3D%3D&md5=049baa7c5fb404062bb86c905188e41fCAS |
Jacob RH, Surridge VSM, Beatty DT, Gardner GE, Warner RD (2014) Grain feeding increases core body temperature of beef cattle. Animal Production Science 54, 444–449.
| Grain feeding increases core body temperature of beef cattle.Crossref | GoogleScholarGoogle Scholar |
Kim YHB, Warner RD, Rosenvold K (2014) Influence of high pre-rigor temperature and fast pH fall on muscle proteins and meat quality: a review. Animal Production Science 54, 375–395.
| Influence of high pre-rigor temperature and fast pH fall on muscle proteins and meat quality: a review.Crossref | GoogleScholarGoogle Scholar |
Knee BW, Cummins LJ, Walker PJ, Kearney G, Warner RD (2007) Reducing dark-cutting in pasture-fed beef steers by high-energy supplementation. Australian Journal of Experimental Agriculture 47, 1277–1283.
| Reducing dark-cutting in pasture-fed beef steers by high-energy supplementation.Crossref | GoogleScholarGoogle Scholar |
Lawrie RA (1958) Physiological stress in relation to dark-cutting beef. Journal of the Science of Food and Agriculture 9, 721–727.
| Physiological stress in relation to dark-cutting beef.Crossref | GoogleScholarGoogle Scholar |
MacDougall DB (1982) Changes in the colour and opacity of meat. Food Chemistry 9, 75–88.
| Changes in the colour and opacity of meat.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL38Xls1Wisrk%3D&md5=5df65721491a09fdcb31d22030350e99CAS |
McGilchrist P, Alston CL, Gardner GE, Thomson KL, Pethick DW (2012) Beef carcasses with larger eye muscle areas, lower ossification scores and improved nutrition have a lower incidence of dark cutting. Meat Science 92, 474–480.
| Beef carcasses with larger eye muscle areas, lower ossification scores and improved nutrition have a lower incidence of dark cutting.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC38jjsVKhuw%3D%3D&md5=fe9793a8548011feaa3267536b957463CAS | 22717222PubMed |
Meat and Livestock Australia Limited (2010) ‘Meat Standards Australia beef information kit.’ (MLA: Sydney)
Meat and Livestock Australia Limited (2013) ‘2012–2013 Annual outcomes report.’ (MLA: Sydney)
Mlynek K, Gulinski P (2007) The effect of growth rate and age at slaughter on dressing percentage and color, pH48 and microstructure of longissimus dorsi muscle in Black-and-White (BW) bulls vs commercial crossbreds of BW with beef breeds. Animal Science Papers and Reports 25, 65–71.
Moon SS, Yang HS, Park GB, Joo ST (2006) The relationship of physiological maturity and marbling judged according to Korean grading system to meat quality traits of Hanwoo beef females. Meat Science 74, 516–521.
| The relationship of physiological maturity and marbling judged according to Korean grading system to meat quality traits of Hanwoo beef females.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3MbnsFCgtw%3D%3D&md5=2d383d8ab7510cd75cb68d194e84f1e3CAS | 22063056PubMed |
Murray AC (1989) Factors affecting beef color at time of grading. Canadian Journal of Animal Science 69, 347–355.
| Factors affecting beef color at time of grading.Crossref | GoogleScholarGoogle Scholar |
Offer G (1991) Modelling of the formation of pale, soft and exudative meat: effects of chilling regime and rate and extent of glycolysis. Meat Science 30, 157–184.
| Modelling of the formation of pale, soft and exudative meat: effects of chilling regime and rate and extent of glycolysis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3MXmslygu7Y%3D&md5=ab78c2132e3b69fa280d2bb8d1e81744CAS | 22061833PubMed |
Offer G, Cousins T (1992) The mechanism of drip production: formation of two compartments of extracellular space in muscle post mortem. Journal of the Science of Food and Agriculture 58, 107–116.
| The mechanism of drip production: formation of two compartments of extracellular space in muscle post mortem.Crossref | GoogleScholarGoogle Scholar |
Page JK, Wulf DM, Schwotzer TR (2001) A survey of beef muscle color and pH. Journal of Animal Science 79, 678–687.
Purchas RW, Aungsupakorn R (1993) Further investigations into the relationship between ultimate pH and tenderness for beef samples from bulls and steers. Meat Science 34, 163–178.
| Further investigations into the relationship between ultimate pH and tenderness for beef samples from bulls and steers.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3sXksVOqsL0%3D&md5=d32768ebd3b63090e0b29ed548ec9569CAS | 22060661PubMed |
Renerre M (1990) Factors involved in the discoloration of beef meat. International Journal of Food Science & Technology 25, 613–630.
| Factors involved in the discoloration of beef meat.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3MXhsVWlu7w%3D&md5=5af244a77842cf8f4c84807542aea917CAS |
Swatland HJ (2004) Progress in understanding the paleness of meat with a low pH. South African Journal of Animal Science 34, 1–7.
| Progress in understanding the paleness of meat with a low pH.Crossref | GoogleScholarGoogle Scholar |
Swatland HJ (2008) How pH causes paleness or darkness in chicken breast meat. Meat Science 80, 396–400.
| How pH causes paleness or darkness in chicken breast meat.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhtVagu7zN&md5=104473857379df63bfc3b11cc8730e81CAS | 22063345PubMed |
Warner RD, Dunshea FR, Gutzke D, Lau J, Kearney G (2014a) Factors influencing the incidence of high rigor temperature in beef carcasses in Australia. Animal Production Science 54, 363–374.
| Factors influencing the incidence of high rigor temperature in beef carcasses in Australia.Crossref | GoogleScholarGoogle Scholar |
Warner RD, Thompson JM, Polkinghorne R, Gutzke D, Kearney GA (2014b) A consumer sensory study of the influence of rigor temperature on eating quality and ageing potential of beef striploin and rump. Animal Production Science 54, 396–406.
| A consumer sensory study of the influence of rigor temperature on eating quality and ageing potential of beef striploin and rump.Crossref | GoogleScholarGoogle Scholar |
Wismer-Pedersen J (1959) Quality of pork in relation to rate of pH change post mortem. Food Research 24, 711–727.
| Quality of pork in relation to rate of pH change post mortem.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaF3cXktVSnuw%3D%3D&md5=6196c808e37308ce5a3ea2b7b0a8b73fCAS |
Young, OA, West, J (2001) Meat colour. In ‘Meat science and applications’. (Eds YH Hui, WK Nip, RW Rogers, OA Young) pp. 39–69. (Marcel Dekker, Inc.: New York)
Young OA, Priolo A, Simmons NJ, West J (1999) Effects of rigor attainment temperature on meat blooming and colour on display. Meat Science 52, 47–56.
| Effects of rigor attainment temperature on meat blooming and colour on display.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3MbnsVKrtQ%3D%3D&md5=d1de6dac70818dd6b018f515b7b1e176CAS | 22062142PubMed |