A population viability analysis of K’gari (Fraser Island) wongari (dingoes)
Robert Appleby A * , Bradley P. Smith B , Darryl Jones A , Gabriel Conroy C and Linda Behrendorff D
B , Darryl Jones A , Gabriel Conroy C and Linda Behrendorff D
A
B
C
D
Abstract
Small, isolated populations such as those found on islands are at an increased risk of extinction. This includes K’gari (Fraser Island) wongari (dingoes). Although aspects of wongari ecology and behaviour are well documented, much about population dynamics remains uncertain. Even where relevant research has been conducted (e.g. population abundance), results remain equivocal. We conducted a population viability analysis (PVA) to investigate the influence of different abundance estimates, along with variable rates of mortality, carrying capacity, catastrophes and breeding success on extinction probability. In favourable, undisturbed conditions, modelling showed a high probability of population persistence over 50 and 100 years. Consistently high levels of mortality resulted in increased extinction probabilities, especially at low- and mid-level population sizes. Promiscuous breeding behaviour, higher female breeding success, and higher male availability reduced extinction probabilities. Our approach demonstrated the utility of population viability analysis for identifying important factors that meaningfully contribute to wongari extinction risk. However, inferences and actionable recommendations for managers were limited owing to a paucity of information for certain, critical parameters. Our findings highlighted the need for better data on wongari reproduction and mortality to help fill significant knowledge gaps required to accurately predict the long-term survival of this iconic population.
Keywords: abundance, breeding, canidae, catastrophes, conservation, extinction, mortality, survival.
Introduction
Small, geographically isolated populations, such as those commonly found on islands, are more susceptible to extinction processes than are more abundant and interconnected mainland populations (Frankham 1998; Eldridge et al. 1999; Brook et al. 2006; Traill et al. 2010). Such populations often have a variety of interacting geographical, genetic and demographic constraints, and vary in the relative impacts and intensity of stochastic events (Ginsberg et al. 1995; Woodroffe and Ginsberg 1998; Lacy 2000). An extreme example is that of the Isle Royale wolf (Canis lupus) population, which became effectively extinct in 2017 and required extensive genetic rescue in subsequent years (Hervey et al. 2021). Extinction risk might be further exacerbated by climate change (Urban 2015), through impacts such as sea-level change (see Gontz et al. 2015), severe weather events, fire and pathogens (Wardell-Johnson 2015).
K’gari (formerly known as Fraser Island) is home to an isolated population of free-ranging dingoes (wongari) (Family Canidae; either Canis familiaris following Jackson et al. 2017 or Canis dingo following Smith et al. 2019). This wongari population is both infamous, owing to negative human–wongari interactions, and a major tourist draw card, alongside being an important contributor to ecosystem processes on the island (Behrendorff et al. 2018a). The population also has a long-standing cultural connection with the island’s Traditional Owners and First Nations Peoples, the Butchulla (Behrendorff 2021; Conroy et al. 2021). Like many other aspects of dingo ecology and behaviour (Smith 2015b; Smith et al. 2019; Donfrancesco et al. 2023), the viability of the K’gari wongari population is debated.
Conroy et al. (2016a, 2021) provided evidence that genetic diversity in the K’gari wongari population is very low and is genetically different from mainland populations. Populations with higher genetic diversity are better-able to undergo the phenotypic and molecular changes necessary to survival, whereas lower-diversity populations trend towards extinction (Willi et al. 2006; Stiebens et al. 2013). Conroy et al. (2021) further reported that the K’gari wongari population had an effective population size of 25.7 individuals (sampling period: 1999–2014). This is well below generally recommended rules-of-thumb for even short-term population survival, let alone the many hundreds or thousands often recommended for long-term persistence (Reed et al. 2003; Traill et al. 2007; Traill et al. 2010; Brook et al. 2011; Flather et al. 2011; Jamieson et al. 2012; Frankham et al. 2013, 2014a, 2014b; but see Shoemaker et al. 2013 and a subsequent rebuttal from Reed and McCoy 2014). Even though Conroy et al. (2021) found little evidence of inbreeding, the molecular marker-based approach they initially used can underestimate inbreeding (Frankham 1998). A previous study by Cairns et al. (2018), which was based on a small sample, first detected inbreeding among K’gari wongari, whereas Leon-Apodaca et al. (2024) provided further evidence of inbreeding and a strong founder effect. There was also evidence that the population appears to be purging highly deleterious mutations (Leon-Apodaca et al. 2024). The most recent study by Miller et al. (2024) confirmed low genetic diversity, and declining genetic variation through genetic drift and inbreeding in the past two decades. Although the focus of the present study is on demographic and behavioural rather than genetic variables, such results highlight the potential precarity of the K’gari wongari population.
A particularly contentious element of wongari management relates to the lethal removal of individuals for risk management purposes (‘destruction(s)’ hereafter) (Thompson et al. 2003). For example, Allen et al. (2015) argued that at the rates of destruction witnessed at the time (an average of approximately five individuals per year), breeding success and population sustainability were unlikely to be negatively affected. They argued that this conclusion was supported by data showing that destructions usually targeted young males, and very rarely involved adult (breeding-capable) females. O’Neill et al. (2017) took a different view, arguing that destructions potentially kept the wongari population in a perpetual state of social disorder and dysfunction, in turn, leading to an on-going cycle of destructions and conflict. Such a cycle, they contended, could result in mortality rates that threaten the persistence of the population. However, no formal analysis of whether or how variable mortality might affect the population has been performed.
In this study, we explored the theoretical viability of the K’gari wongari population by using population viability analysis (PVA, see Banks 2004; Taylor and Goldingay 2012). Rather than attempt to produce functional predictions regarding population viability per se, our primary aim was to investigate which demographic and behavioural variables, and changes therein, exerted considerable influence on population viability.
Methods
Study site
K’gari is a sub-tropical, sand barrier island, located off the coast of Queensland, 288 km north of Brisbane, Australia. It has an area of approximately 1840 km2 and was inscribed onto the World Heritage List in 1992 (Walker et al. 2022). Native Title was granted to the Butchulla Peoples in 2014 (see Walker et al. 2022). The island National Park is co-managed by the Queensland Parks and Wildlife Service (QPWS), the Butchulla Aboriginal Corporation (BAC) and Butchulla Native Title Aboriginal Corporation (BNTAC).
Approximately 450,000 people visit K’gari annually (Waldron and McCallum 2021). Human–wongari conflict, occasionally involving serious human injury (in one case, a death), remains a highly publicised source of management concern (see Appleby et al. 2017; Tapply 2018). Rates of serious incidents peak annually during wongari mating and whelping seasons, but do not appear strongly correlated with human visitation rates (Appleby et al. 2017). K’gari wongari appear to have a wide and varied natural diet and the incidence of anthropogenic food sources in scats and stomach contents has reduced since management interventions were established in the early 2000s (e.g. limiting wongari access to anthropogenic waste; Behrendorff et al. 2016). Some ∼30,000 kg of marine carcasses wash up naturally along the island’s coastline each year, enough to meet the food requirements of approximately 5–10% of the island’s wongari population (Behrendorff et al. 2018b). The island occasionally experiences tropical and ex-tropical cyclones (Gontz et al. 2015) and the impacts of cyclones are predicted to increase in the future (Walker et al. 2022). Large fires can also occasionally occur and climate change is a recognised threat for ecosystems on the island, with temperatures predicted to increase by 1–2°C, 4–5% less rainfall and greater rates of evaporation (Walker et al. 2022).
Modelling
We used Vortex (ver. 10.3.3.0, https://scti.tools/vortex/; Lacy et al. 2018) for the PVA. A summary of the major inputs into Vortex that were fixed (i.e. did not change during any modelling) along with, where available, supporting sources are presented in Table 1.
| Parameter | Value | Sources | |
|---|---|---|---|
| Number of iterations | 500 | ||
| Extinction definition | Only 1 sex remains | ||
| Inbreeding depression | 6.29 (50) [default] | ||
| Environmental concordance: survival and reproduction | 0.5 [default] | ||
| Age first breeding (males) | 2 | ||
| Max. reproductive age (females, males) | 11, 9 | Behrendorff and Allen (2016) | |
| % male at birth | 53 | Corbett (2001) | |
| Age distribution for given population | Stable | ||
| Dispersal | Not included | ||
| State variables | Not included | ||
| Max. lifespan | 13 | Behrendorff and Allen (2016) | |
| Max. number of litters per year (per breeding pair) | 1 | Allen et al. (2015) | |
| Max. litter size | 10 | Allen et al. (2015), Smith (2015a, 2015b) | |
| Number of offspring per female (average) | 4.5 | Allen et al. (2015) | |
| Supplementation | Not included | ||
| Genetics | Not included |
Variable parameters
Parameters that varied in different modelling scenarios are provided in Table 2. All models (n = 43) were broadly grouped into the following six main categories: (1) abundance; (2) mortality; (3) territorial (or dispersal) ‘sinks’; (4) catastrophes; (5) carrying capacity; and (6) reproductive behaviour. However, most models involved a composite of interactions between parameters at various levels. Wherever possible, we used data derived directly from K’gari in choosing parameter values, but where necessary, we drew from the wider dingo literature. Where no data were available, values were simply manipulated by using what were regarded as reasonable possibilities to examine the impact on population survival (e.g. mortality, catastrophes, carrying capacity, female and male breeding success). More specific details are provided in subsequent sections.
| Scenario class | Model number | Population size | Mortality rates | Catastrophe | Carrying capacity | Mating system | Age at first breeding (females) | % Female breeding | % Male available | Harvest (cull) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Abundance (A) | 1 | 73 | Undisturbed | Nil | 300 | LTM | 2 | 50 | 50 | Nil | |
| A | 2 | 123 | Undisturbed | Nil | 300 | LTM | 2 | 50 | 50 | Nil | |
| A | 3 | 257 | Undisturbed | Nil | 300 | LTM | 2 | 50 | 50 | Nil | |
| A | 4 | 73 | Undisturbed | Nil | 300 | LTM | 2 | 50 | 50 | 1 at Year 0 | |
| A | 5 | 123 | Undisturbed | Nil | 300 | LTM | 2 | 50 | 50 | 1 at Year 0 | |
| A | 6 | 257 | Undisturbed | Nil | 300 | LTM | 2 | 50 | 50 | 1 at Year 0 | |
| Mortality (M) | 7 | 73 | B&D_unadj | Nil | 300 | LTM | 2 | 50 | 50 | 1 at Year 0 | |
| M | 8 | 123 | B&D_unadj | Nil | 300 | LTM | 2 | 50 | 50 | 1 at Year 0 | |
| M | 9 | 257 | B&D_unadj | Nil | 300 | LTM | 2 | 50 | 50 | 1 at Year 0 | |
| M | 10 | 73 | B&D_adj | Nil | 300 | LTM | 2 | 50 | 50 | 1 at Year 0 | |
| M | 11 | 123 | B&D_adj | Nil | 300 | LTM | 2 | 50 | 50 | 1 at Year 0 | |
| M | 12 | 257 | B&D_adj | Nil | 300 | LTM | 2 | 50 | 50 | 1 at Year 0 | |
| Territorial sink (TS) | 13 | 14 | Undisturbed | Nil | 300 | LTM | 2 | 50 | 50 | Nil | |
| TS | 14 | 14 | B&D_unadj | Nil | 300 | LTM | 2 | 50 | 50 | Nil | |
| Catastrophe (C) | 15 | 123 | Undisturbed | 1/20 years | 300 | LTM | 2 | 50 | 50 | 1 at Year 0 | |
| C | 16 | 123 | Undisturbed | 1/10 years | 300 | LTM | 2 | 50 | 50 | 1 at Year 0 | |
| C | 17 | 123 | B&D_unadj | 1/20 years | 300 | LTM | 2 | 50 | 50 | 1 at Year 0 | |
| C | 18 | 123 | B&D_unadj | 1/10 years | 300 | LTM | 2 | 50 | 50 | 1 at Year 0 | |
| Carrying capacity (CC) | 19 | 73 | Undisturbed | Nil | 80 | LTM | 2 | 50 | 50 | 1 at Year 0 | |
| CC | 20 | 123 | Undisturbed | Nil | 150 | LTM | 2 | 50 | 50 | 1 at Year 0 | |
| CC | 21 | 73 | B&D_unadj | Nil | 80 | LTM | 2 | 50 | 50 | 1 at Year 0 | |
| CC | 22 | 123 | B&D_unadj | Nil | 150 | LTM | 2 | 50 | 50 | 1 at Year 0 | |
| CC | 23 | 257 | B&D_unadj | Nil | 270 | LTM | 2 | 50 | 50 | 1 at Year 0 | |
| Reproductive behaviour (RB) | 24 | 73 | B&D_unadj | Nil | 300 | Poly | 2 | 50 | 50 | 1 at Year 0 | |
| RB | 25 | 123 | B&D_unadj | Nil | 300 | Poly | 2 | 50 | 50 | 1 at Year 0 | |
| RB | 26 | 73 | Undisturbed | Nil | 300 | LTM | 2 | 35 | 50 | 1 at Year 0 | |
| RB | 27 | 123 | Undisturbed | Nil | 300 | LTM | 2 | 35 | 50 | 1 at Year 0 | |
| RB | 28 | 73 | B&D_unadj | Nil | 300 | LTM | 2 | 35 | 50 | 1 at Year 0 | |
| RB | 29 | 123 | B&D_unadj | Nil | 300 | LTM | 2 | 35 | 50 | 1 at Year 0 | |
| RB | 30 | 257 | B&D_unadj | Nil | 300 | LTM | 2 | 35 | 50 | 1 at Year 0 | |
| RB | 31 | 73 | B&D_unadj | Nil | 300 | LTM | 2 | 65 | 50 | 1 at Year 0 | |
| RB | 32 | 123 | B&D_unadj | Nil | 300 | LTM | 2 | 65 | 50 | 1 at Year 0 | |
| RB | 33 | 257 | B&D_unadj | Nil | 300 | LTM | 2 | 65 | 50 | 1 at Year 0 | |
| RB | 34 | 73 | Undisturbed | Nil | 300 | LTM | 2 | 50 | 35 | 1 at Year 0 | |
| RB | 35 | 73 | B&D_unadj | Nil | 300 | LTM | 2 | 50 | 35 | 1 at Year 0 | |
| RB | 36 | 123 | B&D_unadj | Nil | 300 | LTM | 2 | 50 | 35 | 1 at Year 0 | |
| RB | 37 | 257 | B&D_unadj | Nil | 300 | LTM | 2 | 50 | 35 | 1 at Year 0 | |
| RB | 38 | 73 | B&D_unadj | Nil | 300 | LTM | 2 | 50 | 65 | 1 at Year 0 | |
| RB | 39 | 123 | B&D_unadj | Nil | 300 | LTM | 2 | 50 | 65 | 1 at Year 0 | |
| RB | 40 | 257 | B&D_unadj | Nil | 300 | LTM | 2 | 50 | 65 | 1 at Year 0 | |
| RB | 41 | 73 | B&D_unadj | Nil | 300 | LTM | 1 | 50 | 50 | 1 at Year 0 | |
| RB | 42 | 123 | B&D_unadj | Nil | 300 | LTM | 1 | 50 | 50 | 1 at Year 0 | |
| RB | 43 | 257 | B&D_unadj | Nil | 300 | LTM | 1 | 50 | 50 | 1 at Year 0 |
Supporting information for select parameters is available in subsequent sections below. B&D_unadj, Baxter and Davies (2013, 2018) unadjusted; LTM, long-term monogamy; Poly, polygynous.
Given current disparity in abundance estimates, we examined the effects of different abundance levels on population viability by using the lower (n = 73) and mid-range (n = 123) estimates from Conroy et al. (2016b), and an upper estimate (n = 257) from Allen et al. (2015). In this sense, modelling reflected something akin to a ‘meta’ range, from 73 to 257 individuals, with a credible point estimate of 123 individuals, similar to the point estimate in Appleby and Jones (2011; n = 130). All reported estimates have residual error, and all have respective ranges that overlap.
We used a sex ratio at birth that was slightly skewed towards males (53%) as observed by Corbett (2001) in mainland populations. Age distributions were considered to be stable over time. The maximum lifespan of both sexes was set at 13 years, with maximum breeding age set at 11 years for females and 9 years for males (Behrendorff and Allen 2016). ‘Broods’ (litters) were set to one per year following Allen et al. (2015), although they noted that multiple litters might occasionally occur, and the maximum number of offspring per litter was set to 10 pups (Allen et al. 2015; Smith 2015a, 2015b), with an average of 4.5 (Allen et al. 2015).
Dingo mating systems are generally not well understood although preferential mating/pairings have been observed in captive dingoes (Corbett 2001). Such observations, along with the fact that wongari are cooperative breeders (Allen et al. 2015), have previously led to an assumption of long-term monogamy as the primary mating strategy (Lord et al. 2013; Tatler et al. 2021). A study of wild dingoes in central Australia by Tatler et al. (2021) reported a mixture of mating strategies ranging from multi-year monogamy in a single breeding pair, but more commonly, promiscuity, including incestuous pairings. On K’gari, multi-year, long-term stable pairings have been observed (R. Appleby, unpubl. data; L. Behrendorff, unpubl. data), but promiscuity cannot be ruled out. We therefore examined the impact of both long-term monogamy and polygynous mating strategies on population survival.
Female breeding success in wongari is likely to be a particularly influential component of population viability over the long term (Allen et al. 2015), but can be a difficult variable to accurately establish. For instance, Thomson et al. (1992) observed that in north-western Australia, most females bred or cycled, regardless of age and year, finding that 10/11 females examined at post-mortem had bred in the previous year. Despite such high rates, observed numbers of litters for each territory averaged 1.1, suggesting that even if most females mated, litters may not be raised successfully. Allen et al. (2015) also suggested that, on average, one litter is raised per territory on K’gari.
Catling et al. (1992) studied dingo populations in central and south-eastern Australia and found that only 9% of ‘young’ (presumably juvenile and subadult) and 33% of adult females contained fetuses during the breeding season from May to August. Some 44% of wild adult females and 21% of young females were observed to be lactating between June and October in those study populations. Using data presented in Catling et al. (1992, table 2, p. 205), we tallied average observations of placental scarring observed across their central Australian study groups, finding that in flush years an average of 54.6% of females (a composite of adults and juveniles) had scarring, whereas in drought years, an average of only 33.66% of females had scarring recorded. The highest percentage of placental scarring observed in any given area was 63%.
Given what has been outlined above, we investigated the impacts of models at 35%, 50% and 65% female breeding success, largely following Catling et al. (1992), and an average of one litter per territory, following Allen et al. (2015). We found little information about male breeding success/availability; so, specified it to be either 50% or 80% for certain models.
Annual mortality rates can also be difficult to estimate accurately, particularly for pups, which would be expected to have a higher attrition rate than have older wongari under naturalistic conditions. However, Thomson et al. (1992) did not report overly high rates of mortality in observed litters in the Fortescue River region, Western Australia, with the mean number of pups at nine natal dens being 5.2 ± 0.4. At approximately 3 months of age, this mean dropped to 4.4 ± 0.3 (15 litters observed), and at 4–6 months of age, was 4.1 ± 0.3 (14 litters). Thomson et al. (1992) suggested that approximately one or two of an average of five juveniles (individuals between 0 and 1 year of age) will die annually. Here, we have chosen two of five, or 40% for both juvenile males and females, as a baseline rate.
During the 3 years (1977–1979) of Thomson et al. (1992) observations, in which large-scale poison baiting did not occur in their study site, overall mortality rates in observed (collared) dingo ranged from 14% to 21%, calculated as an annual index. Most deaths were from natural causes. Sample sizes ranged from 15 to 46 individuals, and did not include pup mortality. In Year 1, during which 16 animals were radio-tracked, no deaths occurred. Mange, not known to be widely present in the K’gari population, was recorded in the Thomson et al. (1992) study. Taken together, this suggests that rates of natural mortality in relatively undisturbed dingo populations are generally low.
Although mortality data from QPWS databases offer little information about natural causes of death (Corbett 2009) and cannot be compared or contrasted with information about artificial causes, they are still useful for assessing relative mortality rates across ages and sexes within a casual category (natural or artificial). Table 3 presents a summary of confirmed natural and artificial death records (n = 308, 2001–2021) of wongari extracted from a QPWS mortality database, for which there was information about the age and sex class of an individual. These data overlap with and extend beyond that presented in Allen et al. (2015).
| Age–sex class | Frequency (natural) | %Total (natural) | Frequency (artificial) | %Total (artificial) | |
|---|---|---|---|---|---|
| Adult female | 15 | 0.13 | 18 | 0.09 | |
| Adult male | 12 | 0.11 | 27 | 0.14 | |
| Subadult female | 8 | 0.07 | 25 | 0.13 | |
| Subadult male | 10 | 0.09 | 39 | 0.20 | |
| Juvenile female | 31 | 0.27 | 31 | 0.16 | |
| Juvenile male | 32 | 0.28 | 54 | 0.27 | |
| Pup female | 2 | 0.02 | 0 | 0.00 | |
| Pup male | 3 | 0.03 | 4 | 0.02 |
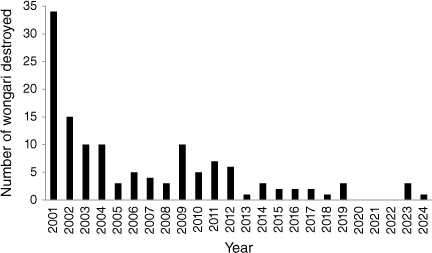
These records indicate that juvenile males and females are found dead from natural causes more frequently than other age and sex classes. Humane destructions accounted for the majority of artificial mortality, but the frequency of destructions has declined over the past two decades (Fig. 1) No destructions occurred between 2020 and 2022, rising to three in 2023 and one in 2024 (not shown).
There are also other forms of artificial mortality on K’gari such as vehicle strike, suspected illegal poisonings and illegal shooting (Queensland Parks and Wildlife Service (QPWS), K’gari, unpubl. data). Excluding sanctioned destructions, an average of 4 (±0.46, range = 1–9, 2001–2021) deaths from artificial causes are recorded annually. Between 2001 and 2021, a total of 62/422 (14.7%) of recorded deaths was confirmed from vehicle strike. The annual mean for confirmed strike events was 3.05 (±0.35, range = 1–6) deaths.
Baxter and Davies (2013, 2018; see also White 2021) provided the best-available information on mortality for subadult and adult wongari on K’gari. Of the 18 wongari fitted with GPS-tracking collars during the study, five died (~27.8%) within approximately a 10-month deployment period. One death was from destruction, one from a confirmed vehicle strike, one from a suspected vehicle strike, with the remaining two being of unknown causes. Table 4 provides details of mortality for each age and sex class calculated using data from Baxter and Davies (2013, 2018).
| Item | Age–sex class | ||||
|---|---|---|---|---|---|
| AF | AM | SF | SM | ||
| Total collared | 6 | 3 | 4 | 5 | |
| # Died | 2 | 1 | 0 | 2 | |
| # Died (%) | 0.33 | 0.33 | 0.00 | 0.40 | |
| Monthly rate | 0.03 | 0.03 | 0.00 | 0.04 | |
| Projected annual rate | 0.4 | 0.4 | NA | 0.48 | |
| Projected annual # | 2.4 | 1.2 | NA | 2.4 | |
The observed (unadjusted) percentages of deaths by age and sex were as follows: adult females, 33%; adult males, 33%; subadult females, 0% (defaulted to 20% in models); and subadult males, 40%. Adjusting for numbers of collared animals of each age and sex class, annual projections of percentage mortality by age and sex class were as follows: adult females, 40%; adult males, 40%; subadult females, NA (defaulted to 20% in models); subadult males, 48%.
In the absence of more detailed information, we have chosen an ‘undisturbed’ mortality range of 20% for both male and female subadult and adult classes, commensurate with the upper rate observed in Thomson et al. (1992). For certain models, we increased this to the ‘unadjusted’ or ‘adjusted’ rates informed by Baxter and Davies (2013, 2018). We do not specifically distinguish between natural and artificial causes, with the exception of a cull, which was used in most models as the only form of ‘Harvest’ (see section below).
Large-scale perturbations to the population are possible, through factors such as wild fire, or the introduction of a disease. For example, given the proximity of the mainland, it is possible that a disease such as canine parvovirus has occurred before (FIDO 2015), or could be introduced to island (FINIA 2017). We modelled catastrophes at a frequency of once per 10 and 20 years, at both an undisturbed and artificially inflated mortality rate (all models used n = 123 individuals). The severity of a given catastrophe was classified as having reduced the population in total by 50% (including the percentage of breeders).
Because increases in overall mortality rates were used in most models, the harvest parameter was limited to a single year at the very beginning of modelling periods (unless otherwise specified). This equated to removing 32 wongari, corresponding to the cull of 32 individuals within a period from late May to July 2001. Historical culls have also occurred and may have indeed affected population viability (Conroy et al. 2021), but were not included here because of scant, reliable details about sex and age classes of any wongari involved.
Results
Table 5 provides population survival probabilities for 43 Vortex models projected over 50 and 100 years for all models. Additionally, two related models (Models 13 and 14) were examined over a 20-year period. We generated a four-level categorical ranking of extinction risk (risk Level) based on survival probability, with the ‘highest risk’ (dark grey) being a ≤20% survival probability within either 50 or 100 years, followed by a ‘<50%’ survival probability (light grey), a ‘>50%’ survival probability and ‘lowest risk’ (≥80% survival probability) categories. In total, 15 models were classified as highest risk, with a further three models classified as the population having <50% survival probability. Of those 15 high-risk models, 11 were identified as having the highest extinction risk within 50 years (Table 5, bold values).
| Scenario class | Model number | Survival probability (20/50 years) | Survival probability (100 years) | Extinction risk level | |
|---|---|---|---|---|---|
| Abundance | 1 | 1.00 | 1.00 | Lowest | |
| Abundance | 2 | 1.00 | 1.00 | Lowest | |
| Abundance | 3 | 1.00 | 1.00 | Lowest | |
| Abundance | 4 | 1.00 | 1.00 | Lowest | |
| Abundance | 5 | 1.00 | 1.00 | Lowest | |
| Abundance | 6 | 1.00 | 1.00 | Lowest | |
| Mortality | 7 | 0.17 | 0.01 | Highest | |
| Mortality | 8 | 0.79 | 0.35 | <50 Surv. | |
| Mortality | 9 | 1.00 | 0.83 | Lowest | |
| Mortality | 10 | 0.00 | 0.00 | Highest | |
| Mortality | 11 | 0.01 | 0.00 | Highest | |
| Mortality | 12 | 0.18 | 0.00 | Highest | |
| Territorial sink | 13 | 0.94/0.81 | 0.71 | >50 Surv. | |
| Territorial sink | 14 | 0.25/0.00 | 0.00 | Highest | |
| Catastrophe | 15 | 0.99 | 0.96 | Lowest | |
| Catastrophe | 16 | 0.90 | 0.69 | >50 Surv. | |
| Catastrophe | 17 | 0.34 | 0.02 | Highest | |
| Catastrophe | 18 | 0.07 | 0.00 | Highest | |
| Carrying capacity | 19 | 0.99 | 0.96 | Lowest | |
| Carrying capacity | 20 | 1.00 | 1.00 | Lowest | |
| Carrying capacity | 21 | 0.09 | 0.00 | Highest | |
| Carrying capacity | 22 | 0.69 | 0.04 | Highest | |
| Carrying capacity | 23 | 0.99 | 0.79 | >50 Surv. | |
| Reproductive behaviour | 24 | 0.95 | 0.86 | Lowest | |
| Reproductive behaviour | 25 | 1.00 | 1.00 | Lowest | |
| Reproductive behaviour | 26 | 0.93 | 0.87 | Lowest | |
| Reproductive behaviour | 27 | 1.00 | 1.00 | Lowest | |
| Reproductive behaviour | 28 | 0.00 | 0.00 | Highest | |
| Reproductive behaviour | 29 | 0.08 | 0.00 | Highest | |
| Reproductive behaviour | 30 | 0.59 | 0.00 | Highest | |
| Reproductive behaviour | 31 | 0.58 | 0.28 | <50 Surv. | |
| Reproductive behaviour | 32 | 0.97 | 0.87 | Lowest | |
| Reproductive behaviour | 33 | 1.00 | 0.99 | Lowest | |
| Reproductive behaviour | 34 | 0.99 | 0.99 | Lowest | |
| Reproductive behaviour | 35 | 0.01 | 0.00 | Highest | |
| Reproductive behaviour | 36 | 0.10 | 0.00 | Highest | |
| Reproductive behaviour | 37 | 0.67 | 0.00 | Highest | |
| Reproductive behaviour | 38 | 0.56 | 0.34 | <50 Surv. | |
| Reproductive behaviour | 39 | 0.99 | 0.93 | Lowest | |
| Reproductive behaviour | 40 | 1.00 | 1.00 | Lowest | |
| Reproductive behaviour | 41 | 0.59 | 0.25 | <50 Surv. | |
| Reproductive behaviour | 42 | 0.97 | 0.84 | Lowest | |
| Reproductive behaviour | 43 | 1.00 | 0.98 | Lowest |
Abundance models
When ‘undisturbed’ levels of mortality were imposed, population survival was essentially certain (P = 1.0) for all three population abundance levels used here (n = 73, 123 and 257, Models 1–3, Table 5). The inclusion of a ‘harvest’ had no impact on survival (Models 4–6).
Mortality models
Introducing ‘unadjusted’ mortality levels (see ‘B&D_unadj’, Table 2) resulted in the highest risk of extinction for the population at the lowest abundance estimate (n = 73, Model 7). The model, set to n = 123 individuals (Model 8) experiencing the same unadjusted mortality levels, was predicted to have a <50% of survival, and the n = 257 model (Model 9) was predicted to have the lowest risk of extinction at 100 years. When ‘adjusted’ (see ‘B&D_adj’, Table 2) levels of mortality were introduced, models at all abundance levels (Models 10–12) exhibited the highest risk of extinction at 100 years (after rounding, all models P = 0.0).
Territorial sink models
Two hypothetical territorial ‘sink’ models were run, with the territorial population experiencing unadjusted levels of mortality (Model 14) predicted to have the highest risk of extinction at 50 and 100 year projections, and a 25% chance of survival after 20 years. In contrast, the territorial population experiencing undisturbed rates of mortality (Model 13) was predicted to have a >50% chance of survival after 100 years and a probability of 0.94 of survival after 20 years.
Catastrophe models
Four models with added catastrophes were produced all at the same population abundance (n = 123), two using undisturbed rates of mortality (Models 15 and 16), the former at a rate of one catastrophe each 20 years, the latter at a rate of one catastrophe each 10 years. Model 15 resulted in the lowest extinction risk and Model 16 had a >50% chance of survival at 100 years. When unadjusted mortality rates were introduced (Models 17 and 18), both models resulted in the highest category of risk regardless of catastrophe rate.
Carrying capacity models
Of the five models produced with variable levels of carrying capacity (Models 19–23), two models (Models 21 and 22) resulted in the highest category of extinction risk. Both models had unadjusted rates of mortality imposed, with Model 21 using an abundance estimate of n = 73 and Model 22 using an abundance estimate of n = 123. The same rate of mortality imposed when abundance was set at n = 257 resulted in a >50% chance of survival (Model 23).
Reproductive behaviour models
In total, 20 models were investigated using variable reproduction and reproductive behavioural parameters (Models 24–43). Six models (Models 28–30 and Models 35–37) resulted in the highest category of extinction risk. In contrast, when polygynous mating was specified for two models (Models 24 and 25), the lowest risk of extinction was reported, even though these models used low abundance levels (n = 73 and n = 123 respectively) and unadjusted rates of mortality were specified. When female breeding success was reduced from 50% to 35% (Models 28–30), the population was projected to go extinct with virtual certainty, regardless of abundance. The same was true when male availability was reduced from 50% to 35% (Models 35–37). When age at first breeding was set to 1 for females, one model (Model 41), set to lowest population abundance, resulted in a <50% chance of survival. Survival probability was greatest at higher abundance levels (Models 42 and 43).
Discussion
Our PVA has shown that certain combinations of favourable conditions, such as low levels of mortality, few catastrophes, high levels of carrying capacity and medium-to-high rates of breeding success for both males and females, resulted in a very high probability of medium-to-long term population survival. For example, even at the lowest estimate of population abundance (n = 73), at ‘undisturbed’ levels of mortality, population survival was essentially certain. This was the case even when the 2001 cull of 32 wongari was accounted for (Models 4–6 and beyond, Table 5).
Under such conditions, numerical recovery of a population towards maximum carrying capacity from even a small number of wongari is projected. This possibly mirrors the founding event of the island’s wongari population, whereby a small number of isolated founders gave rise to a larger population (Cardoso et al. 2009; Conroy et al. 2021; Leon-Apodaca et al. 2024) and dingoes more generally (Savolainen et al. 2004). Something similar may have also occurred following any large, historical culls or bottlenecks on the island (Petrie 1995; Conroy et al. 2021). However, such events hide a high potential cost in the form of genetic drift (Masel 2011). In turn, genetic diversity (Leon-Apodaca et al. 2024) and effective population size (Conroy et al. 2021) can decrease. Although numerical recovery is therefore theoretically feasible from even low levels, it is far from ideal from a conservation genetics standpoint and in terms of maximising adaptive potential (Frankham 1998).
Our analysis of two territory-sized ‘populations’ (Models 13 and 14, Table 5) respectively experiencing lower and higher rates of mortality was potentially informative. Wongari are a social canid that exhibit territoriality and unequal reproduction. In undisturbed territories, it would be expected that some young dingoes would survive, disperse and breed elsewhere, leading to higher overall genetic diversity. In territories experiencing sustained and consistently higher mortality, fewer or even no individuals may be available to disperse, possibly reducing overall genetic diversity. Miller et al. (2024) recently reported that no clear spatial aggregation was detected in the K’gari wongari population and suggested that this was likely to be because inter-pack breeding is common enough so that packs cannot be easily distinguished from one another. However, a low effective population size (Conroy et al. 2021; Miller et al. 2024) also shows that a subset of individuals disproportionately contributes to breeding and which individuals these are currently remains obscured. Miller et al. (2024) also found that allelic diversity in the population was reducing over time. Although the latter are likely to be primarily occurring because of factors such as genetic drift (Miller et al. 2024), we posit that territorial sinks could also play a role. Ultimately, whether or not territorial sinks exist on the island requires further, empirical investigation and could form a part of a larger investigation focussed on bridging current knowledge gaps.
Introducing sustained higher levels of annual mortality to models led to the highest chance of extinction when population abundance was set at n = 73 (Model 7), and a <50% chance of survival when population abundance was set at n = 123 (Model 8). Adjusting observed rates of mortality from Baxter and Davies (2013, 2018; see Methods) to an annual rate, Models 10–12 resulted in the highest risk of extinction, regardless of population size. In the case of models utilising the lowest and mid-level estimates of abundance, such mortality increases equated to a small number of additional wongari consistently dying each year. Thus, the persistent loss of animals above what we deemed to be an undisturbed level, particularly at low population abundance and rates of breeding success, could be enough for the population to begin trending towards extinction. We did not specify where any additional losses might arise, but could include destructions, vehicle strikes, deliberate killing by members of the public, disease or natural disasters. The rate of destructions for risk management purposes has generally decreased over the past two decades (Fig. 1; Behrendorff 2021), but rates of vehicle strikes remain persistent. Poisonings and other forms of illegal killing appear rare, but may increase extinction risk if population abundance is particularly low.
An important caveat here is that the mortality rates used during modelling were often speculative rather than realistically reflecting wongari mortality on K’gari. In the case of ‘undisturbed’ mortality rates, data were drawn from a dingo study in Western Australia, where conditions may have been markedly different from those on K’gari. The higher mortality rates used in many models, inspired in part from Baxter and Davies (2013, 2018), although informative from a theoretical or modelling impact standpoint, were obtained over a brief period (~10 months) and had very low levels of sex and age class representation. In turn, considerable caution is needed in interpreting any results that rely on these data beyond demonstrating the importance of particular parameters on theoretical survival probability. The same, general degree of caution is true for most of the data used here.
To our knowledge, no population-wide catastrophe-like event has occurred on K’gari in the past 20 years. The unpredictability of climate change coupled with the fact that visitors have been observed illegally bringing domestic dogs to the island (R. Appleby, pers. obs.; FINIA 2017), a possible vector for the introduction of diseases, raises the chances of catastrophic events occurring. Our modelling suggests that provided other forms of mortality are close to an ‘undisturbed’ level, abundance is high and breeding success is not limited, a catastrophic event once every 10–20 years is unlikely to impact population survival in the medium to long term, at least numerically speaking. However, if rates of mortality are higher, extinction risk rises, particularly at low-to-medium abundances. Here, carrying capacity may also play an important role, because in years where the population may be able to numerically recover from perturbations such as catastrophes, low carrying capacity may still be a major limiting factor.
Wongari reproductive behaviour is poorly understood at present, but being a social canid exhibiting territoriality (Corbett 2001; Smith 2015b) is likely to heavily influence breeding rates and success. Our modelling demonstrated just how influential such parameters are on population survival, with those models having low (35%) male or female availability/breeding success rates, all resulting in the highest probability of extinction, regardless of abundance. In contrast, higher breeding success greatly bolstered population survivability. For example, allowing younger females (Age 1) to breed resulted in marked improvements to longer-term survival (Models 42 and 43, and to a lesser extent, Model 41). The same was true when changing models from long-term monogamy to polygynous mating (Models 24 and 25). It is possible that such parameters are density dependent, and thus, change as the population size decreases/increases and/or approaches carrying capacity. Again, this highlights the important interplay that might exist among different variables.
Improving information used in wongari population viability modelling
So as to update information about population dynamics, rectify disparity in abundance estimates and fill current knowledge gaps relating to population survival, we recommend the initiation of a series of data collection projects. These should include an island-wide wongari population dynamics, breeding and dispersal study. Given the demonstrable importance in relation to population viability, more effort is required to establish credible information about wongari survival and mortality across all age classes, echoing Miller et al. (2024). Monitoring of key prey species and other food sources may help determine carrying capacity, and if and how this changes over time. Diseases represent potentially catastrophic threats to the population and should be monitored and abated wherever possible.
As our knowledge of important variables improves, it is imperative that this information be regularly assimilated into new PVA modelling to examine population survival probability and to subsequently inform management decisions. We encourage on-going discussions and collaboration among QPWS, First Nations Peoples, researchers and other stakeholders to continue exploring the most effective strategies to guide management for long-term persistence of this important wongari population.
Current modelling suggests that in favourable conditions relating to abundance, mortality, breeding success and catastrophes, the K’gari wongari population is likely to persist for at least the next 50–100 years from a demographic standpoint. Survival probability decreases, sometimes markedly, when two or more of these factors simultaneously remain less than favourable over a sustained period of time.
Data availability
Data files used to produce results are available by contacting the corresponding author via email.
Conflicts of interest
Linda Behrendorff works for the Queensland Parks and Wildlife Service. The remaining authors declare no conflicts of interest.
Declaration of funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Acknowledgements
We thank Queensland Parks and Wildlife Service for providing data. We are also grateful to Kylie Cairns, an anonymous reviewer, Ben Allen and the editing team.
References
Allen, B., Higginbottom, K., Bracks, J., Davies, N., and Baxter, G. (2015). Balancing dingo conservation with human safety on Fraser Island: the numerical and demographic effects of humane destruction of dingo. Australasian Journal of Environmental Management 22(2), 197-215.
| Crossref | Google Scholar |
Appleby, R., Mackie, J., Smith, B., Bernede, L., and Jones, D. (2017). Human–dingo interactions on Fraser Island: an analysis of serious incident reports. Australian Mammalogy 40(2), 146-156.
| Crossref | Google Scholar |
Banks, P. B. (2004). Population viability analysis in urban wildlife management: modelling management options for Sydney’s quarantined bandicoots. In ‘Urban Wildlife: more than meets the eye’. (Eds D. Lunney, S. Burgin.) pp. 63–69. (Royal Zoological Society: NSW, Australia) 10.7882/9780958608572
Baxter, G., and Davies, N. (2013). Tracking dingo on Fraser Island. School of Geography, Planning and Environmental Management, the University of Queensland. Available at https://parks.des.qld.gov.au/parks/fraser/pdf/wongari-trackingreport-2013.pdf
Baxter, G., and Davies, N. (2018). Movements of dingoes on K’gari-Fraser Island: implications for management. Australasian Journal of Environmental Management 25(1), 132-146.
| Crossref | Google Scholar |
Behrendorff, L. (2021). Best-practice dingo management: six lessons from K’gari (Fraser Island). Australian Zoologist 41(3), 521-533.
| Crossref | Google Scholar |
Behrendorff, L., and Allen, B. L. (2016). From den to dust: longevity of three dingoes (Canis lupus dingo) on Fraser Island (K’gari). Australian Mammalogy 38(2), 256-260.
| Crossref | Google Scholar |
Behrendorff, L., Leung, L. K. P., McKinnon, A., Hanger, J., Belonje, G., Tapply, J., Jones, D., and Allen, B. L. (2016). Insects for breakfast and whales for dinner: the diet and body condition of dingoes on Fraser Island (K’gari). Scientific Reports 6, 23469.
| Crossref | Google Scholar | PubMed |
Behrendorff, L., Belonje, G., and Allen, B. L. (2018a). Intraspecific killing behaviour of canids: how dingoes kill dingoes. Ethology Ecology and Evolution 30(1), 88-98.
| Crossref | Google Scholar |
Behrendorff, L., Leung, L. K. P., and Allen, B. L. (2018b). Utilisation of stranded marine fauna washed ashore on K’gari (Fraser Island), Australia, by dingoes. Australian Journal of Zoology 66(2), 128-138.
| Crossref | Google Scholar |
Brook, B. W., Traill, L. W., and Bradshaw, C. J. (2006). Minimum viable population sizes and global extinction risk are unrelated. Ecology Letters 9(4), 375-382.
| Crossref | Google Scholar | PubMed |
Brook, B. W., Bradshaw, C. J., Traill, L. W., and Frankham, R. (2011). Minimum viable population size: not magic, but necessary. Trends in Ecology and Evolution 26(12), 619-620.
| Crossref | Google Scholar | PubMed |
Cairns, K. M., Shannon, L. M., Koler-Matznick, J., Ballard, J. W. O., and Boyko, A. R. (2018). Elucidating biogeographical patterns in Australian native canids using genome wide SNPs. PLoS One 13, e0198754.
| Crossref | Google Scholar | PubMed |
Cardoso, M. J., Eldridge, M. D., Oakwood, M., Rankmore, B., Sherwin, W. B., and Firestone, K. B. (2009). Effects of founder events on the genetic variation of translocated island populations: implications for conservation management of the northern quoll. Conservation Genetics 10(6), 1719-1733.
| Crossref | Google Scholar |
Catling, P. C., Corbett, L. K., and Newsome, A. E. (1992). Reproduction in captive and wild dingo (Canis familiaris dingo) in temperate and arid environments of Australia. Wildlife Research 19(2), 195-209.
| Crossref | Google Scholar |
Conroy, G. C., Lamont, R. W., Bridges, L., Stephens, D., Wardell-Johnson, A., and Ogbourne, S. M. (2021). Conservation concerns associated with low genetic diversity for K’gari–Fraser Island dingoes. Scientific Reports 11(1), 9503.
| Crossref | Google Scholar | PubMed |
Donfrancesco, V., Allen, B. L., Appleby, R., Behrendorff, L., Conroy, G., Crowther, M. S., Dickman, C. R., Doherty, T., Fancourt, B. A., Gordon, C. E., Jackson, S. M., Johnson, C. N., Kennedy, M. S., Koungoulos, L., Letnic, M., Leung, L. K.-P., Mitchell, K. J., Nesbitt, B., Newsome, T., Pacioni, C., Phillip, J., Purcell, B. V., Ritchie, E. G., Smith, B. P., Stephens, D., Tatler, J., van Eeden, L. M., and Cairns, K. M. (2023). Understanding conflict among experts working on controversial species: A case study on the Australian dingo. Conservation Science and Practice 5(3), e12900.
| Crossref | Google Scholar |
Eldridge, M. D., King, J. M., Loupis, A. K., Spencer, P. B., Taylor, A. C., Pope, L. C., and Hall, G. P. (1999). Unprecedented low levels of genetic variation and inbreeding depression in an island population of the black‐footed rock‐wallaby. Conservation Biology 13(3), 531-541.
| Crossref | Google Scholar |
FIDO. (2015). Fighting Ferals on Fraser Island. Available at https://fido.org.au/fighting-ferals-on-fraser-island-2/ [accessed 5 November 2024]
FINIA (2017) Irresponsible Pet Owners Impact K’gari Wildlife. Available at https://finia.org.au/2017/11/05/irresponsible-pet-owners-impact-kgari-wildlife/ [accessed 23 December 2022].
Flather, C. H., Hayward, G. D., Beissinger, S. R., and Stephens, P. A. (2011). Minimum viable populations: is there a ‘magic number’ for conservation practitioners? Trends in Ecology and Evolution 26(6), 307-316.
| Crossref | Google Scholar | PubMed |
Frankham, R. (1998). Inbreeding and Extinction: Island Populations. Conservation Biology 12(3), 665-675.
| Crossref | Google Scholar |
Frankham, R., Brook, B. W., Bradshaw, C. J., Traill, L. W., and Spielman, D. (2013). 50/500 rule and minimum viable populations: response to Jamieson and Allendorf. Trends in Ecology and Evolution 28(4), 187-188.
| Crossref | Google Scholar | PubMed |
Frankham, R., Bradshaw, C. J., and Brook, B. W. (2014a). Genetics in conservation management: revised recommendations for the 50/500 rules, Red List criteria and population viability analyses. Biological Conservation 170, 56-63.
| Crossref | Google Scholar |
Franklin, I. R., Allendorf, F. W., and Jamieson, I. G. (2014b). The 50/500 rule is still valid – reply to Frankham et al. Biological Conservation 176, 284-285.
| Crossref | Google Scholar |
Ginsberg, J. R., Mace, G. M., and Albon, S. (1995). Local extinction in a small and declining population: wild dogs in the Serengeti. Proceedings of the Royal Society of London. Series B: Biological Sciences 262(1364), 221-228.
| Crossref | Google Scholar | PubMed |
Gontz, A. M., Moss, P. T., Sloss, C. R., Petherick, L. M., McCallum, A., and Shapland, F. (2015). Understanding past climate variation and environmental change for the future of an iconic landscape – K’gari Fraser Island, Queensland, Australia. Australasian Journal of Environmental Management 22(2), 105-123.
| Crossref | Google Scholar |
Hervey, S. D., Rutledge, L. Y., Patterson, B. R., Romanski, M. C., Vucetich, J. A., Belant, J. L., Beyer, D. E., Jr, Moore, S. A., and Brzeski, K. E. (2021). A first genetic assessment of the newly introduced Isle Royale gray wolves (Canis lupus). Conservation Genetics 22(6), 913-926.
| Crossref | Google Scholar |
Jackson, S. M., Groves, C. P., Fleming, P. J., Aplin, K. P., Eldridge, M. D., Gonzalez, , and Helgen, K. M. (2017). The Wayward Dog: Is the Australian native dog or Dingo a distinct species? Zootaxa 4317(2),.
| Crossref | Google Scholar |
Jamieson, I. G., and Allendorf, F. W. (2012). How does the 50/500 rule apply to MVPs? Trends in Ecology and Evolution 27(10), 578-584.
| Crossref | Google Scholar | PubMed |
Lacy, R. C. (2000). Considering threats to the viability of small populations using individual-based models. Ecological Bulletins 48, 39-51.
| Google Scholar |
Leon-Apodaca, A., Kumar, M., del Castillo, A., Conroy, G., Lamont, R. W., Ogbourne, S., Cairns, K., Borburgh, L., Behrendorff, L., Subramanian, S., and Szpiech, Z. A. (2024). Genomic consequences of isolation and inbreeding in an island dingo population. Genome Biology and Evolution 16(7), evae130.
| Crossref | Google Scholar | PubMed |
Lord, K., Feinstein, M., Smith, B., and Coppinger, R. (2013). Variation in reproductive traits of members of the genus Canis with special attention to the domestic dog (Canis familiaris). Behavioural Processes 92, 131-142.
| Crossref | Google Scholar | PubMed |
Masel, J. (2011). Genetic drift. Current Biology 21(20), R837-R838.
| Crossref | Google Scholar | PubMed |
Miller, S. M., Behrendorff, L., Allen, B. L., Andrew, R. L., Ballard, G., Ballard, J. W. O., et al. (2024). Isolation, small population size, and management influence inbreeding and reduced genetic variation in K’gari dingoes. Conservation Genetics 25(4), 955-971.
| Crossref | Google Scholar |
O’Neill, A. J., Cairns, K. M., Kaplan, G., and Healy, E. (2017). Managing dingoes on Fraser Island: culling, conflict, and an alternative. Pacific Conservation Biology 23(1), 4-14.
| Crossref | Google Scholar |
Reed, J. M., and McCoy, E. D. (2014). Relation of minimum viable population size to biology, time frame, and objective. Conservation Biology 28(3), 867-870.
| Crossref | Google Scholar | PubMed |
Reed, D. H., O’Grady, J. J., Brook, B. W., Ballou, J. D., and Frankham, R. (2003). Estimates of minimum viable population sizes for vertebrates and factors influencing those estimates. Biological Conservation 113(1), 23-34.
| Crossref | Google Scholar |
Savolainen, P., Leitner, T., Wilton, A. N., Matisoo-Smith, E., and Lundeberg, J. (2004). A detailed picture of the origin of the Australian dingo, obtained from the study of mitochondrial DNA. Proceedings of the National Academy of Sciences 101(33), 12387-12390.
| Crossref | Google Scholar | PubMed |
Shoemaker, K. T., Breisch, A. R., Jaycox, J. W., and Gibbs, J. P. (2013). Re-examining the minimum viable population concept for long‐lived species. Conservation Biology 27(3), 542-551.
| Crossref | Google Scholar | PubMed |
Smith, B. P., Cairns, K. M., Adams, J. W., Newsome, T. M., Fillios, M., Déaux, E. C., Parr, W. C. H., Letnic, M., Van Eeden, L. M., Appleby, R. G., Bradshaw, C. J. A., Savolainen, P., Ritchie, E. G., Nimmo, D. G., Archer-Lean, C., Greenville, A. C., Dickman, C. R., Watson, L., Moseby, K. E., Doherty, T. S., Wallach, A. D., Morrant, D. S., and Crowther, M. S. (2019). Taxonomic status of the Australian dingo: the case for Canis dingo Meyer, 1793. Zootaxa 4564(1), 4564.
| Crossref | Google Scholar | PubMed |
Stiebens, V. A., Merino, S. E., Roder, C., Chain, F. J., Lee, P. L., and Eizaguirre, C. (2013). Living on the edge: how philopatry maintains adaptive potential. Proceedings of the Royal Society B: Biological Sciences 280(1763), 20130305.
| Crossref | Google Scholar | PubMed |
Tapply, J. (2018). Contemporary dingo management on K’gari (Fraser Island, Great Sandy National Park) under the Queensland Parks and Wildlife Service. Australasian Journal of Environmental Management 25(1), 119-131.
| Crossref | Google Scholar |
Tatler, J., Prowse, T. A., Roshier, D. A., Cairns, K. M., and Cassey, P. (2021). Phenotypic variation and promiscuity in a wild population of pure dingoes (Canis dingo). Journal of Zoological Systematics and Evolutionary Research 59(1), 311-322.
| Crossref | Google Scholar |
Taylor, B. D., and Goldingay, R. L. (2012). Facilitated movement over major roads is required to minimise extinction risk in an urban metapopulation of a gliding mammal. Wildlife Research 39(8), 685-695.
| Crossref | Google Scholar |
Thomson, P., Rose, K., and Kok, N. (1992). Dingoes in North-Western Australia. V. Population Dynamics and Variation in the Social System. Wildlife Research 19(5), 509-603.
| Crossref | Google Scholar |
Thompson, J., Shirreffs, L., and McPhail, I. (2003). Dingoes on Fraser Island—Tourism Dream or Management Nightmar. Human Dimensions of Wildlife 8(1), 37-47.
| Crossref | Google Scholar |
Traill, L. W., Bradshaw, C. J., and Brook, B. W. (2007). Minimum viable population size: a meta-analysis of 30 years of published estimates. Biological Conservation 139(1–2), 159-166.
| Crossref | Google Scholar |
Traill, L. W., Brook, B. W., Frankham, R. R., and Bradshaw, C. J. (2010). Pragmatic population viability targets in a rapidly changing world. Biological Conservation 143(1), 28-34.
| Crossref | Google Scholar |
Urban, M. C. (2015). Accelerating extinction risk from climate change. Science 348(6234), 571-573.
| Crossref | Google Scholar | PubMed |
Waldron, R. P., and McCallum, A. B. (2021). A review of road infrastructure development and contemporary degradation on K’gari-Fraser Island. Australasian Journal of Environmental Management 28(2), 104-125.
| Crossref | Google Scholar |
Walker, K. E., Baldwin, C., Conroy, G. C., Applegate, G., Archer-Lean, C., Arthington, A. H., Behrendorff, L., Gilby, B. L., Hadwen, W., and Henderson, C. J. (2022). Ecological and Cultural Understanding as a Basis for Management of a Globally Significant Island Landscape. Coasts 2, 152-202.
| Crossref | Google Scholar |
Wardell-Johnson, A. (2015). Future of an icon: K’gari-Fraser Island, climate change and social expectations. Australasian Journal of Environmental Management 22(2), 91-104.
| Crossref | Google Scholar |
White, N. J. (2021). Spatio-temporal ecology of dangerous wildlife in a conservation setting, and implications for management of human–wildlife interactions: wongari (dingoes – Canis dingo) on K’gari (Fraser Island), Queensland, Australia. PhD Thesis, School of Earth and Environmental Sciences, The University of Queensland, St Lucia, Australia. 10.14264/4b330b6
Willi, Y., Van Buskirk, J., and Hoffmann, A. A. (2006). Limits to the adaptive potential of small populations. Annual Review of Ecology, Evolution, and Systematics 37, 433-458.
| Crossref | Google Scholar |
Woodroffe, R., and Ginsberg, J. R. (1998). Edge effects and the extinction of populations inside protected areas. Science 280(5372), 2126-2128.
| Crossref | Google Scholar | PubMed |