A brief history of the northern quoll (Dasyurus hallucatus): a systematic review
Harry A. Moore A B J , Judy A. Dunlop A B C D , Chris J. Jolly A , Ella Kelly E , John C. Z. Woinarski F , Euan G. Ritchie G , Scott Burnett H , Stephen van Leeuwen I , Leonie E. Valentine B , Mitchell A. Cowan A and Dale G. Nimmo A
A B J , Judy A. Dunlop A B C D , Chris J. Jolly A , Ella Kelly E , John C. Z. Woinarski F , Euan G. Ritchie G , Scott Burnett H , Stephen van Leeuwen I , Leonie E. Valentine B , Mitchell A. Cowan A and Dale G. Nimmo A
A Institute for Land, Water and Society, School of Environmental Science, Charles Sturt University, Albury, NSW 2640, Australia.
B School of Biological Sciences, University of Western Australia, Crawley, WA 6009, Australia.
C Department of Biodiversity, Conservation and Attractions, Bentley Delivery Centre, Locked Bag 104, Perth, WA, Australia.
D Western Australian Feral Cat Working Group, Mandurah, WA 6210, Australia.
E Department of Environment, Land, Water and Planning, Nicholson Street, East Melbourne, Vic. 3002, Australia.
F Threatened Species Recovery Hub, National Environmental Science Program, Charles Darwin University, Darwin, NT 0810, Australia.
G Centre for Integrative Ecology and School of Life and Environmental Sciences, Deakin University, Burwood, Vic. 3125, Australia.
H PO Box 1219, Maleny, Qld 4552, Australia.
I School of Molecular and Life Sciences, Curtin University, Bentley, WA 6102, Australia.
J Corresponding author. Email: harryamos@live.com.au
Australian Mammalogy 44(2) 185-207 https://doi.org/10.1071/AM21002
Submitted: 14 January 2021 Accepted: 13 July 2021 Published: 26 August 2021
Journal Compilation © CSIRO 2022 Open Access CC BY-NC-ND
Abstract
In response to Australia’s current extinction crisis, substantial research efforts have been targeted towards some of the most imperilled species. One such species is the northern quoll (Dasyurus hallucatus), a marsupial predator that has recently suffered substantial declines in range and is now listed as Endangered. We conducted a systematic review of all literature relevant to the conservation and ecology of northern quolls. We reviewed 143 studies, including research articles, government and industry reports, theses, and books, and quantified research effort in terms of topic, location, and publication period. We then summarised research relevant to northern quoll taxonomy, genetics, distribution, habitat associations, diet, reproduction, movement, threats, management, and Indigenous knowledge. Research effort was higher between 2011 and 2020 than the previous four decades combined. Northern quolls in the Northern Territory were the most studied, followed by the Pilbara, the Kimberley, and Queensland populations. Most studies focused on northern quoll distribution and habitat, management, and threats – primarily cane toads, predation, and fire. We conclude with a non-exhaustive list of ten future research directions. If pursued, these future research directions should provide information critical to managing and conserving northern quolls.
Keywords: biodiversity conservation, dasyurids, Dasyurus hallucatus, Endangered species, extinction crisis, habitat management, imperilled species, mammal extinction, marsupial predator, northern quolls, research studies, systematic literature reviews.
Introduction
Biodiversity conservation and management is most effective when derived from a robust evidence base (Salafsky et al. 2019). Despite an ever growing of ecological information (Bornmann and Mutz 2015), there remains a considerable gap between the science of conservation biology and the on-ground implementation of evidence-based policy (Knight et al. 2008; Evans and Cvitanovic 2018; Rose et al. 2019). One reason for this is that conservation practitioners can struggle to access, interpret, and synthesise an increasingly dispersed and voluminous body of scientific literature (Pullin and Knight 2005). Similar problems are encountered by researchers, leading to repetition and redundancy in ecological science (Pulsford et al. 2016). Systematic literature reviews are one means of overcoming these issues by identifying, selecting, and appraising research on a predefined topic, drawing out evidence-based management implications and critical knowledge gaps for further research (Moher et al. 2009).
Australia’s contemporary mammal extinction rate is the globe’s highest (Woinarski et al. 2019a). Yet more Australian mammals are forecast to become extinct within the next two decades (Geyle et al. 2018). In response to this crisis, increased research effort has been directed towards Australian species that have suffered population contractions over the last 240 years (Fleming and Bateman 2016). One such species is the northern quoll (Dasyurus hallucatus) – a medium-sized marsupial predator (240–1120 g) endemic to northern Australia. Prior to European colonisation, northern quolls were distributed across much of northern Australia (Braithwaite and Griffiths 1994), but have since suffered substantial declines (Fig. 1) (Moore et al. 2019). As such, northern quolls are listed as Endangered both nationally (TSSC 2005) and globally (Oakwood et al. 2016).

|
Due to their ongoing and precipitous decline, much of the research focused on the ecology and conservation of northern quolls has occurred within the last two decades. However, accessing this research can be challenging, largely because it is spread across a diverse and dispersed literature (e.g. books, journals, government and consultant’s reports, and theses). To help overcome this barrier, we conducted a systematic review of all literature relevant to the ecology and conservation of northern quolls across their entire range.
The complexities of species conservation are such that multiple disciplinary approaches are often required to achieve meaningful progress (Dick et al. 2016). With this in mind, we take a deliberately broad approach to our review. We summarise research related to the northern quoll’s diet, distribution and habitat associations, genetics and taxonomy, reproduction, movement, and threats and management. We also summarise available Indigenous knowledge related to the northern quoll’s ecology. We identify and discuss knowledge gaps throughout and provide a non-exhaustive list of future research directions for the northern quoll. If applied, we believe these future research directions could provide knowledge critical to improving the conservation of northern quolls.
Materials and methods
Database compilation
We followed the approach used by Ashman et al. (2019) to collate relevant literature for this review. Three electronic databases were searched (Web of Science, Scopus and Google Scholar) on 17 August 2020 using the search terms ‘northern quoll’ OR ‘Dasyurus hallucatus’. The search was updated on 15 April 2021. Search terms were located in publication title, abstract, keywords and main text. Searches retrieved studies from a range of categories including peer-reviewed literature, MSc and PhD theses, government and publicly available industry reports, and government action and recovery plans. Studies were reviewed in a three-step process (Fig. 2). First, duplicates were removed, then titles and/or abstracts were screened to detect the terms ‘northern quoll’ or ‘Dasyurus hallucatus’, or a reference to a broader community of species that was likely to include northern quolls, for example, ‘tropical mammals of Australia’. Lastly, full texts were reviewed. Studies were excluded if they were not immediately relevant to the ecology and conservation of the species. Therefore, for example, studies that focused solely on the physiology or anatomy of northern quolls were removed unless the publication made explicit reference to the relevance of their findings to ecology or conservation of wild populations (e.g. predator-aversion trials conducted in captivity).

|
Data retrieved
For each of the 143 studies that were included in the final analysis, we recorded date of publication, study population(s) (if applicable), and study site(s) (if applicable). Study populations were adapted from Moore et al. (2019) and comprise four largely spatially segregated units: Queensland, Northern Territory, the Kimberley region of Western Australia, and the Pilbara region (including the neighbouring Little Sandy and Great Sandy Desert bioregions) of Western Australia. We also extracted home range estimates from studies that listed them. We pooled publication dates into six categories: prior to 1980, 1981–1990, 1991–2000, 2001–2010, 2011–2020, 2021–2030. We used decadal increments to categorise studies given they were fine enough to detect changes in research effort through time, but also coarse enough to capture a substantial number of studies within each increment. We then categorised studies into one or more of nine topics, adapted from Ashman et al. (2019):
taxonomy and genetics,
distribution, declines and habitat associations,
diet (prey choice, scat composition),
reproduction,
movement,
threats,
conservation management (direct management actions, legislation, action and recovery plans),
Indigenous knowledge, and
miscellaneous (methods, reviews, albinism).
Threats
For studies that included the topic ‘threats’, we categorised the threats examined within the publication according to threat categories adapted from the National Recovery Plan (Hill and Ward 2010). Threat categories adapted from the recovery plan included the introduced and toxic cane toad (Rhinella marina), feral cat (Felis catus) and dingo/dog (Canis spp.) predation, fire, grazing, habitat clearing, mining, disease, feral predator baiting, and vehicle strikes.
Topic summary and future research needs
We provide written summaries for all research topics included in the review except for ‘behaviour’, which is discussed within the management section, and ‘miscellaneous’, which is discussed throughout. Within summaries, we discuss important findings in the context of the ecology and conservation of northern quolls. We also identify and discuss knowledge gaps to highlight areas that may require future research. We finish by providing a non-exhaustive list of ten future research areas that could be useful to refine management action(s) to conserve northern quolls.
Results and discussion
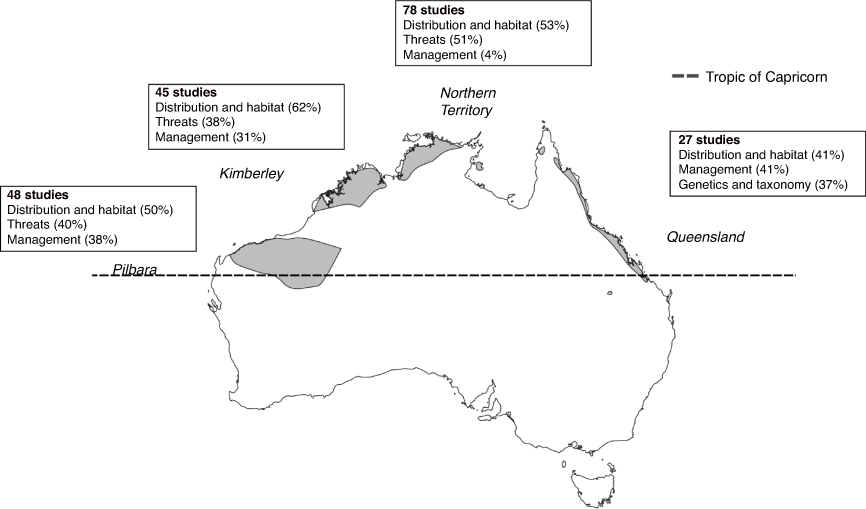
The temporal distribution of the 143 studies shows a substantial increase in research effort in recent years (Fig. 3) (Supplementary material S1). The earliest study included in our analysis was published from 1926 (Thomas 1926) and the most recent was published in May 2021 (Moore et al. 2021). Most studies were conducted between 2011 and 2020 (86), and few studies were conducted prior to 1981 (2) (Fig. 3). The region with the most studies was the Northern Territory (78), followed by the Pilbara (48), the Kimberley (45), and Queensland (27) (Fig. 4). We identified 18 studies which were at least partly laboratory based. Each of the nine study topics were represented at least once within studies included in this review (Fig. 5). It is important to note that we found a limited amount of grey literature in this review and, as such, a number of environmental impact assessments (and the implications of their findings) were likely missed.

|
|

|
Taxonomy and genetics
We identified 21 studies relevant to northern quoll taxonomy, covering all major populations. Northern quolls (Dasyurus hallucatus Gould 1842) were described by John Gould (1842) from two specimens collected at Port Essington in the Northern Territory. They are the smallest of six Dasyurus species: including the spotted-tailed quoll (D. maculatus), western quoll (D. geoffroii), eastern quoll (D. viverrinus), bronze quoll (D. spartacus), and New Guinean quoll (D. albopunctatus). The latter two species are only found in Papua New Guinea and Indonesia. The current range of the northern quoll overlaps with the current range of the northern subspecies of spotted tailed quoll (Dasyurus maculatus gracilis) in North Queensland. In the Pilbara, subfossil evidence suggests northern quolls overlap with the historic distribution of the western quoll (Baynes and McDowell 2010).
In 1926, northern quolls were separated into four subspecies, based largely on the width of the skull at the nasals: D. h. hallucatus (Northern Territory to central Queensland); D. h. predator (Cape York, Queensland); D. h. exilis (the Kimberley); and D. h. nesaeus (Groote Eylandt) (Thomas 1926). No Pilbara specimens were included in this examination. Although these subspecies are no longer recognised (Jackson et al. 2015), there is now genetic evidence to suggest northern quolls in each of the four major populations (Queensland, Northern Territory, the Kimberley and the Pilbara) do represent distinct lineages. Firestone et al. (2000) suggest northern quoll cytochrome b sequences from Queensland and the Northern Territory are at least as divergent as those between western quolls and the bronze quoll. Woolley et al. (2015) and Hohnen et al. (2016b) found that the Northern Territory and Kimberley northern quoll populations are genetically divergent, and How et al. (2009) found the same for Kimberley and Pilbara populations. The results of more recent morphological examinations are mixed: Umbrello (2018) found significant differences in skull size, dentition, and external characteristics between Queensland, Northern Territory, Kimberley, and Pilbara populations, whereas Viacava et al. (2020) found few consistent differences. Some island populations separated from the mainland by permanent sea channels (Bigge, Boongaree, Koolan), as well as less permanent channels (Dolphin Island) also appear genetically divergent from mainland populations (How et al. 2009; Spencer et al. 2017; Chan et al. 2020). However, this is not the case for Groote Eylandt quolls, which genetically align with the mainland Northern Territory population (Woolley et al. 2015).
The disjunct distribution of mainland northern quolls, accompanied by the genetic and morphological differences between populations, correspond to biogeographical barriers across northern Australia (Bowman et al. 2010). Separating the Queensland and Northern Territory populations is the Carpentaria Gap; a series of clay pans that limit dispersal between Cape York and the rest of the Australian monsoonal tropics for a range of taxa (Bowman et al. 2010). For example, the Carpentaria Gap separates a recently recognised species of glider, Petaurus ariel, from its sister species, Petaurus notatus (Cremona et al. 2020). Similarly, the gap between the Northern Territory and Kimberley populations aligns with the Ord Arid Intrusion, which divides sandstone blocks between Arnhem Land in the Northern Territory and the Kimberley (Bowman et al. 2010), and acts as a dispersal barrier for other species that use rocky habitat, such as rock-wallabies (Petrogale spp.) (Potter et al. 2012). The Kimberley and Pilbara populations of northern quoll are separated by the Great Sandy Desert – an extensive dune system often implicated in the isolation of both species and populations (Edwards et al. 2017).
The taxonomic status of the northern quoll may not yet be fully resolved. Lack of taxonomic clarity has previously hampered conservation efforts in other taxa (Peterson 2006; Pickett et al. 2020). In the case of the northern quoll, understanding if populations should be treated as distinct taxonomic units or are genetically similar enough to be intermixed is likely to have important management implications, particularly in relation to cross-population translocations (as discussed in the ‘Management’ section). As such, further clarification of the extent to which northern quoll populations differ both genetically and morphologically should be a priority for future research. Other research related to northern quoll genetics are discussed within the ‘Management’ section.
Distribution, habitat associations, and geographic range contraction
A total of 80 studies were included in the ‘Distribution, declines, and habitat associations’ topic, with the most focused on the Northern Territory population (n = 42), followed by the Kimberley (n = 28), Pilbara (n = 24), and Queensland populations (n = 11) (Fig. 5).
Prior to European colonisation, the geographic range of northern quolls incorporated much of northern Australia above the Tropic of Capricorn (90° South) and within 200 km of the coastline (but likely extended inward much further (Braithwaite and Griffiths 1994; Turpin and Bamford 2014) covering a total area of over 1.2 million km2. Average annual rainfall across this area varies substantially, ranging from 220 mm in the eastern extent of the Pilbara, to nearly 4500 mm in the Wet Tropics of northern Queensland (BOM 2020). Similarly, average maximum temperature in the warmest months ranges from 42.1°C in the Pilbara to 25.5°C in southern Queensland (BOM 2020). Northern quolls naturally occur on at least 32 islands, mostly off the Northern Territory and Kimberley (Supplementary material S2). Many of these are relatively free from human disturbance and lack introduced predators and/or cane toads (Woinarski et al. 2007; How et al. 2009). In the Northern Territory, northern quolls are particularly associated with large, remote, and rugged islands (Woinarski et al. 2007), although they are notably absent from two large Northern Territory islands – Bathurst (2600 km2) and Melville (5786 km2). In 2003 and 2017, northern quolls were translocated to three islands outside of their historical geographic range in the Northern Territory (Rankmore et al. 2008; Kelly 2018) (see ‘Management’ section).
Northern quolls occur across a broad range of habitat types including tropical and monsoonal rainforest, Eucalyptus woodlands, Eucalyptus open forests, lowland savanna, vine thickets, on beaches, and amongst human settlements (Pollock 1980; Begg 1981; Schmitt et al. 1989; Braithwaite and Griffiths 1994; Oakwood 1997), but appear most abundant in rugged and rocky landscapes, including rocky hills, patches of granite outcrops, boulder-strewn slopes, rocky creek lines, and gorges (Calaby 1973; McKenzie et al. 1975; Begg 1981; Kitchener et al. 1981; Schmitt et al. 1989; Braithwaite and Griffiths 1994; Oakwood 1997; Pollock 1999; Olds et al. 2016; Molloy et al. 2017; Ibbett et al. 2018; Moore et al. 2021) (Fig. 6). This preference may be due to rocky habitats holding permanent water and more food resources than surrounding habitats (Braithwaite and Griffiths 1994; Burnett 1997). However, evidence from some studies suggests diet is unlikely to be a key factor explaining the northern quolls preference for rocky habitat: Oakwood (1997) and Hernandez Santin (2017) found limited evidence that quoll selected rocky habitat based on differences in intrinsic dietary resources. Further, Thomas et al. (2021) found quolls occupying rocky habitat consumed a narrower range of prey resources than quolls that occurred in nearby savanna woodland, and also displayed lower body condition.
Another factor linking quolls to rocky habitats is the availability of shelter, particularly dens – small, enclosed spaces that northern quolls use as either short-term shelter sites (temporary den) or semi-permeant dwellings used to raise offspring (natal den). Northern quolls den in tree hollows (both alive and dead), termite mounds, logs, and goanna burrows (Oakwood 1997). In more arid regions in which trees and logs are scarce (e.g. the Pilbara), rocky crevices within granite boulder piles and rocky mesas are critical for providing both temporary and natal den sites for northern quolls (Cowan et al. 2020b). In the Pilbara, Cowan et al. (2020b) found rocky dens act as thermal refuges, being buffered from extreme external temperatures, which often exceeded safe temperatures for northern quolls (i.e. >36.5°C; Cooper and Withers 2010). Even in habitats where alternative den sites are available (e.g. tree hollows and logs), females selectively den in rocky habitat, and there is some evidence that females with home ranges comprised of more rocky habitat survive longer (Oakwood 1997). Rocky dens also provide refuge from predators, and there is evidence that feral cats and dingoes are less likely to occur in rugged, rocky habitats (Hernandez-Santin et al. 2016; Hohnen et al. 2016a).
Since European colonisation – and particularly within the past 50 years (Braithwaite and Griffiths 1994) – the geographic range of northern quolls has declined by at least 45.2% and the volume of their ecological niche has also declined substantially (Moore et al. 2019). Declines have been most severe in Queensland, where >400 000 km2 of former habitat is now unoccupied, constituting a range contraction of >75% (Moore et al. 2019). The Northern Territory is the second most affected population, which has experienced a 58% range contraction (115 024 km2), mostly from the more arid southern extent of their historic distribution (Braithwaite and Griffiths 1994; Ziembicki et al. 2013; Moore et al. 2019; von Takach et al. 2020). The Kimberley population has seen a 17% decline, equating to 25 986 km2 of lost range; (Moore et al. 2019), whereas the Pilbara – the most arid region currently supporting northern quoll populations – has so far seen little to no evidence of decline (Spencer et al. 2013; Moore et al. 2019).
These broadscale declines in geographic range arise through numerous local declines and extincitons that have been well documented. For example, Burnett and Zwar (2009) found no evidence to suggest northern quolls persist in the southern Mary River catchment north of Brisbane, despite the known occurrence of historic populations. Populations in Far North Queensland have also been subject to substantial decline. For example, Perry et al. (2015) and Burnett (1997) found northern quolls are largely absent from sizeable areas of Cape York Peninsula where they once occurred. In the Northern Territory, Ibbett et al. (2018) found northern quoll trap success in Kakadu National Park was significantly lower in 2002 than it was in 1980 (Begg 1981) and Woinarski et al. (2011b) found northern quoll abundance was lower at sites within Kakadu in 2007–2009 than it was in 2001–2004.
There are clear spatial patterns in the decline and persistence of northern quoll populations. In Queensland, the Northern Territory, and the Kimberly, topographically simple landscapes that receive low rainfall and are distant from permanent water have seen extremely severe declines (Burnett 1997; Pollock 1999; Woinarski et al. 2008; Radford et al. 2014; Moore et al. 2019), and areas that would have historically been marginal habitat have seen the greatest declines (Moore et al. 2019). Hence, extant populations tend to occur in topographically rugged areas with high annual rainfall (Moore et al. 2019). The extent of decline across populations corresponds with the length of time that the population has co-occurred with the introduced cane toad (see threat section). Persisting mainland populations of northern quolls now exist on the Central Mackay coast, Wet Tropics, Einasleigh Uplands and Cape York Peninsula bioregions in Queensland, the Arnhem Plateau, Darwin Coastal Plain, Daly Basin, Pine Creek bioregions, and Groote Eylandt in the Northern Territory, the Central and North Kimberley bioregions, Dampierland, and the Pilbara, Great Sandy and Little Sandy Deserts bioregions.
Diet
We identified 30 studies that examined the diet of the northern quoll, representing all study populations, with the most studies conducted in the Northern Territory. A large number of these studies (n = 14) focused on the consumption of cane toads, or baits designed to kill feral cats and dingos (n = 7) by quolls, and are discussed in the ‘threats’ section below. Northern quolls are omnivorous, opportunistic foragers that consume a range of invertebrate, vertebrate, and plant species. Research examining sex-based dietary difference is scarce; however, there is some evidence that females northern quoll consume less vertebrate prey items than males, probably because females are typically smaller in mass (Oakwood 1997).
Invertebrates are a dominant feature in the diets of northern quolls across all populations, with beetles (Coleoptera), grasshoppers (Orthoptera), ants (Hymenopterans) and spiders (Arachnida) appearing most frequently within scats (Dixon and Huxley 1985; Oakwood 1997; Pollock 1999; Radford 2012; Dunlop et al. 2017). Indigenous people from Arnhem Land in the Northern Territory also observed northern quolls feeding on wai (worms), grubs, and moths (Dixon and Huxley 1985). In Kakadu National Park (Northern Territory), Oakwood (1997) found invertebrate consumption peaked in the early dry season, coinciding with the arrival of juveniles into the population. In the Pilbara, invertebrate occurrence in the northern quoll diets decreases with the occurrence of rodents and plant material, potentially indicating invertebrates may be a staple food item, but not always preferred (Dunlop et al. 2017).
A diverse range of vertebrate prey also appear in the diet of northern quolls, including rodents (Melomys burtoni, Pseudomys delicatulus, Pseudomys hermannsbergensis, Rattus rattus, Rattus sordidus, Zyzomys argurus), rabbits (Oryctolagus cuniculus), other dasyurids (Dasykaluta rosamondae, Ningaui timeleyai, Pseudantechinus sp., Sminthopsis macroura, Sminthopsis youngsoni), gliders (Petaurus spp.), possums (Trichosurus vulpecula), bandicoots (Isoodon auratus, Isoodon macrourus), bats (Nyctophilus spp., Rhinonicteris aurantia), birds, lizards (Scincidae spp., Agamidae spp., Varanidae spp., Gekkonidae spp.), snakes, and frogs (Dixon and Huxley 1985; Oakwood 1997; Pollock 1999; Radford 2012; Dunlop et al. 2017). Larger mammals including kangaroos (Osphranter spp.), cows (Bos taurus), cats (Felis catus) and dogs/dingoes (Canis spp.) have also been recorded in northern quoll scats (Dunlop et al. 2017), presumably consumed as carrion. In the Kimberley, northern quolls consume a larger proportion of larger prey, such as golden bandicoots (Isoodon auratus), in recently burnt habitats, potentially because hunting this prey item is easier when vegetation cover is reduced (Radford 2012).
Plant material has been recorded in the diet of northern quolls in the Northern Territory (Oakwood 1997), the Kimberley (Radford 2012), and the Pilbara (Dunlop et al. 2017), and is typically comprised of fleshy fruits, seeds, and flowers. In the Northern Territory, Oakwood (1997) found fruits from wild grape plants (Ampellocissus acetose) were the most common plant material to appear in northern quoll diets, with peak consumption occurring in the late dry to early wet season. In the Pilbara, native figs (Ficus spp.) were the most frequently recorded plant group within northern quoll scats, occurring within 16.1% of total scats measured (Dunlop et al. 2017).
Reproduction
We identified 17 studies related to northern quoll reproduction. Northern quolls typically breed during the dry season – between June and July in Queensland, May and June in the Northern Territory, June to October in the Kimberley, and July to September in the Pilbara. Variation in the timing of breeding also occurs within populations and between years, mostly driven by variation in rainfall (Schmitt et al. 1989; Braithwaite and Griffiths 1994; Oakwood 2000). Male northern quolls are known to exhibit semelparity, a reproductive strategy where animals only breed once in their lifetime, characterised by increased levels of testosterone followed by rapid condition loss, with individuals rarely living longer than 11 months (Oakwood et al. 2001), although survival varies.
In the Northern Territory, most male quolls die within two weeks of mating (Dickman and Braithwaite 1992; Oakwood 2004a), although a small percentage may survive to a second breeding season (Begg 1981; Schmitt et al. 1989). Female survival is also very low in the Northern Territory (typically less than ~40% between years), although they can survive for up to 3 years (Braithwaite and Griffiths 1994; Oakwood 2000; Cremona et al. 2017b). In the Kimberley, Schmitt et al. (1989) found 4% of males and 37% of females survived to reach a second breeding season. In the Pilbara, just over 5% of males and 40% of females survive to a second breeding season (Hernandez-Santin et al. 2019). On Groote Eylandt, Heiniger et al. (2020) found 0% of males and 39.6% of females survived to their second year. They also found 8.7% of females survived to their third year.
A consequence of large annual die-offs in the adult population of northern quolls is that the likelihood of population persistence is heavily reliant on offspring survivorship. For example, in the Pilbara, an increase in mean juvenile mortality of 5% could potentially result in a 20% decline in overall population size (Moro et al. 2019). One strategy northern quolls may use to increase juvenile survival is polyandry, where females mate with multiple males to increase genetic diversity among offspring, conferring a group net fitness benefit. For example, a recent study found that 100% of examined northern quoll litters (n = 16) had young sired by multiple males, and in some litters, every offspring was fathered by a different male (Chan et al. 2020).
It is possible that risks associated with semelparity are likely counterbalanced by benefits derived from reduced competition between males and offspring in areas where resources are limited. As such, Cook (2010b) suggested male die off may be less pronounced in populations where resources are plentiful. However, more recent studies suggest this may not necessarily be the case: Heiniger et al. (2020) found complete semelparity was observed in northern quoll populations on Groote Eylandt where resources are sufficient to support high quoll densities. Further research is required to better understand the evolutionary drivers and consequences of semelparity in northern quolls.
Following mating, females undergo a gestation period of between 21 and 25 days before giving birth (Oakwood 2000). Mothers have between five and nine nipples (normally eight) (Begg 1981; Braithwaite and Griffiths 1994), and Chan et al. (2020) found that females on a small Pilbara island consistently had six nipples compared to eight on the adjacent mainland. Although up to 17 young can be born (Nelson and Gemmell 2003), nipple number determines the maximum number of young that can be carried after birth. Braithwaite and Griffiths (1994) found that, on average, females that lived closer to creek lines – areas that are more productive than surrounding habitat and likely more conducive to reproduction – had more nipples than females that lived further from creek lines. Once young are attached to a nipple, mortality over the following 3 months ranges from less than 2% (Oakwood 2000) to 86% (Braithwaite and Griffiths 1994). Young are deposited in dens at roughly two and a half months of age (Oakwood 2000), are independently foraging at 4 months, and are trappable by 5 months of age (Oakwood 1997). Young are weaned by 6 months and disperse shortly thereafter (Oakwood 2000).
We found little information regarding northern quoll reproduction in Queensland. For example, no research included in this review documented whether quolls in Queensland show evidence of complete or partial annual male die-off, as observed in other regions (Oakwood et al. 2001). Documenting the post-breeding survival rate of northern quolls in Queensland will allow managers to better estimate the risk of Queensland subpopulations becoming locally extinct.
Movement
We identified 12 studies that investigated the movement ecology of northern quolls, which were spread across all regions (Fig. 5). Seven of these studies included home range estimates, calculated using three different methods (delimiting supposed home range, minimum convex polygon, and 95% minimum convex polygon) (Table 1). Across all home range studies, a general trend appears to be that male home ranges are typically larger than female home ranges. This was especially apparent during the breeding season, when males move large distances in search of females. In the Northern Territory, Oakwood (2002) found that males occupied a much larger home range (84 ± 16 ha) than females (34.8 ± 6.4 ha). The difference was largest in the breeding season when males expanded their home range to seek mating opportunities (Oakwood 2002). These males also travelled further between dens (average = 1.9 km) than females (average = 1.2 km). Similarly, on Groote Eylandt, Heiniger et al. (2020) found the average home range of male northern quolls (215 ± 58.4 ha) to be four times larger than that of females (53.1 ± 38.8 ha), although this was largely due to male quolls expanding their home ranges by an average of 300% during the breeding season. Prior to the start of breeding season, average home range sizes were larger for females (79.0 ± 58.8 ha) than they were for males (72.9 ± 24.4 ha) (Heiniger et al. 2020).

|
In the Kimberley, (Cook 2010a) found home ranges were on average larger for males (64 ± 37 ha) than females (7 ± 2 ha). Maximum distance between dens was also greater for males (1.2 km) than females (0.4 km). Schmitt et al. (1989) found quolls moved further between successive trap locations in the breeding season (104 ± 99 m) when compared to the non-breeding season (61 ± 82 m). In the Pilbara, average male home range estimates were between 2.7 and 28.6 times larger than female home range estimates (Table 1). Similar to the Northern Territory and Kimberly populations, male quolls in the Pilbara appear to move further in the breeding season when compared to the non-breeding season (Schmitt et al. 1989; Oakwood 2002; Hernandez-Santin et al. 2021). Although we did not find any direct measurements of home range for northern quolls in Queensland, Burnett et al. (2013) provides a mean half maximum distance moved for 25 individuals (334.6 m), suggesting a crude circular home range estimate of 35 ha.
Lab-based studies investigating northern quoll locomotion have found northern quolls tend to sacrifice speed in favour of manoeuvrability in order to avoid making mistakes (Wynn et al. 2015; Amir Abdul Nasir et al. 2017). On Groote Eylandt, northern quolls with greater agility when moving around corners are more likely to survive their first 21 months of life than quolls that move slower around corners, potentially because they are better at avoiding predators, such as dingoes, feral cats, and birds of prey (Rew-Duffy et al. 2020).
Although male-biased dispersal is common in other dasyurids, evidence for this in northern quolls is limited (Oakwood 2002). Further research is required to better understand patterns in northern quoll dispersal. However, there is evidence that male northern quolls disperse further than females (Oakwood 2000) and male consecutive dens can be up to 4 km apart (Cook 2010b). In the Kimberley, genetic data suggests that habitats with higher annual rainfall and lower topographical ruggedness are likely to facilitate increased dispersal between subpopulations (Hohnen et al. 2016b).
Threats
A total of 70 studies included the topic ‘threats’, most of which were focused on northern quolls in the Northern Territory (n = 40), with substantially fewer studies focused on the Pilbara (n = 18), the Kimberley (n = 17) or Queensland (n = 9) (Fig. 7).

|
Cane toads
The most commonly investigated threat was cane toads (n = 22). In 1935, cane toads (Rhinella marina) were introduced to a research station near Cairns, Queensland, Australia (17°04′S, 145°47′E) (Lever 2001). From there, toads quickly expanded their distribution into other parts of Queensland. Cane toads first invaded the Northern Territory in the 1980s (Freeland and Martin 1985), reached Kakadu National Park in 2001 (Woinarski et al. 2002) and progressed through to Western Australia c. 2009.
Like some other native predators, northern quolls that attempt to consume cane toads rapidly succumb to their novel and potent defensive toxins (Shine 2010). Oakwood (2004b) found 31% of radio-tracked quoll mortalities in Kakadu were likely caused by cane toads, whereas O’Donnell et al. (2010) found 29%, and Jolly et al. (2018a) found 85% of toad-naïve quolls and 18% of toad-trained quolls died as a result of attempting to consume cane toads.
In far north Queensland, Burnett (1997) presented anecdotal evidence that cane toads were the cause of northern quoll extirpation between 1983 and 1995. Although northern quoll populations in the Northern Territory exhibited declines prior to toad arrival (Woinarski et al. 2001; Ziembicki et al. 2013; Ibbett et al. 2018), rapid declines, often to extirpation, followed the invasion front (Woinarski et al. 2010, 2011b). In the Kimberley, Indigo (2020) found northern quoll populations declined by 86–96% following toad arrival, despite these populations being repeatedly exposed to thiabendazole-laced cane toad sausages – a technique shown to elicit toad-aversion in captive quolls (see management section). Despite these declines, there is evidence that northern quolls can co-exist successfully with cane toads, for instance, in central Queensland, where toads and quolls have co-existed for over 80 years (Sabath et al. 1981). Although it has been confirmed that Queensland northern quolls (and likely all other populations of northern quolls) are not physiologically resistant to cane toad toxins (Ujvari et al. 2013), natural variation in quoll behavioural responses to toads may have made some quolls less vulnerable to toad related mortality than others (see ‘Management’ section).
Predation
Feral cats and dingoes are considered the most active predators to northern quolls across the majority of their range (Hill and Ward 2010), and this was reflected in the number of northern quoll studies that included reference to feral cat or dingo/dog predation (n = 15 and n = 21, respectively). Feral cats were introduced onto the Australian mainland with the arrival of the ‘first fleet’ of British colonists (Abbott 2008). As such, northern quolls have had limited evolutionary exposure to feral cats as predators and limited opportunity to adapt to feral cat predation risk (<240 years). By contrast, northern quolls have co-existed with dingoes across their historical range for up to 8000 years (Cairns and Wilton 2016; Zhang et al. 2020). This suggests the impacts of dingoes, and potentially their close relatives, domestic dogs, are unlikely to threaten the persistence of northern quolls on their own, but instead are amplified when acting in conjunction with other threats such as altered fire regimes, livestock grazing, habitat loss, and predation from feral cats (Geary et al. 2019b). Another introduced predator, the red fox (Vulpes vulpes), is also likely to depredate on northern quolls in some areas of their southern distribution (Cramer et al. 2016), although foxes are not present across the majority of the northern quoll range in northern Australia (Saunders et al. 2010).
In the Mackay Bowen region of Queensland, Pollock (1999) recorded eleven occurrences of quoll mortality as a result of domestic dogs and one record of predation by a black-headed python (Aspidites melanocephalus). This author also recorded predation by domestic or feral cats; but the number of quolls killed was not recorded. Cat predation on northern quolls in Queensland has also been recorded in the Rockhampton and Cape Upstart regions (Burnett pers. comm., 2020). In Kakadu National Park, Northern Territory, Oakwood (2000) tracked 9 of 15 radio-tracked quolls to their death as a result of predation (dingo = 4, feral cat = 2, owl = 1, king brown snake, Pseudechis australis = 1, olive python, Liasis olivaceus = 1). Cremona et al. (2017a) found three of four quolls tracked to their death likely died of dingo/dog predation. Similarly, Jolly et al. (2018a) found at least 7 of 19 quolls were killed by dingoes. However, it’s possible this number over represents the threat of dingoes, given that quolls in this case were breed on a predator free island, and thus were likely naïve to dingo predatory cues. In the Pilbara, Cowan et al. (2020a) found 6 of 41 collared northern quolls died as result of feral cat predation and two from dingo predation.
In a behavioural study, quolls from mainland Queensland were shown to recognise and avoid the scent of cats and dingoes, as did their captive born young, suggesting a genetic basis for predator recognition, including recognition of the introduced feral cat (Jolly et al. 2018b). However, quolls that had been translocated to Astell Island to conserve them against the impacts of cane toads appeared to have lost the ability to recognise dingoes and cats, after only 13 generations (Jolly et al. 2018b). The capacity of northern quolls to detect their predators could explain why Hernandez-Santin et al. (2016) found northern quolls avoided areas used by feral cats. The loss of antipredator traits observed on Astell Island may explain the role of dingo predation in the rapid extirpation of quolls during a reintroduction attempt (Jolly et al. 2018a). Unfortunately, any attempts to train quolls to recognise dingoes as predators in captivity failed to impart predator aversion on quolls prior to reintroduction (Jolly et al. 2020).
Fire
A large number of studies (n = 24) considered the impacts of fire on northern quolls. We found no studies that recorded evidence of northern quoll mortality as a direct result of fire; however, we did find evidence from several populations that fire can have negative impacts on northern quoll populations. In the Northern Territory, Begg (1981) found that fire delayed northern quoll breeding and reduced the mean number of young that left the pouch, Oakwood (1997) found 55% of female northern quolls perished soon after fire in Kakadu, and Kerle and Burgman (1984) found that, although northern quolls were common just after fire (<1 year), they declined in the following year. Corbett (2003) and Andersen et al. (2005) found northern quolls were more abundant at unburnt sites than burnt sites. Griffiths and Brook (2015) found modelled recruitment was 20% lower after late dry season fire.
In the Kimberley, Radford et al. (2015) found northern quolls were less abundant at sites with larger extents of habitat burnt within the previous year, high fire frequency, and a greater distance from unburnt patches. Ondei et al. (2021) found northern quolls were only detected at rainforest sites, which burn less frequently than adjacent savanna sites, where no northern quolls were detected. Finally, in the Pilbara, Hernandez-Santin et al. (2016) found northern quolls were negatively associated with habitat that had been recently burnt.
There are multiple mechanisms that could explain observed negative impacts of fire on northern quolls. It is possible that food may be scarce immediately following fire, which reduces overall habitat suitability. For example, in the Kimberley, Radford and Andersen (2012) found the total number of invertebrates – a key prey item for northern quoll – declined by 80–90% in the week following fire. However, this same study found invertebrates were rapidly restored following the first wet season after fire, and there was no significant difference in invertebrate numbers between pre- and post-fire sessions within a year (Radford 2012). This study also found quolls consumed higher proportions of vertebrate prey following small-scale fires, presumably because the simplified post-fire landscape facilitated improved predation success on these more energetically valuable prey items.
Another probable mechanism is that fire increases predation risk, as a result of reduced ground-layer vegetation cover (Kerle and Burgman 1984; Oakwood 1997). Interactions between fire and predation have previously been implicated in the decline of numerous Australian mammals (Woinarski et al. 2010; Leahy et al. 2016; Hradsky et al. 2017; McGregor et al. 2017), particularly for species like northern quolls that fall within a critical weight range (CWR) of species most vulnerable to predation from feral cats, foxes and dingoes (Woinarski 2015).
It is important to note that some studies have not found fire to have a negative impact on northern quolls. Woinarski et al. (2004) found northern quolls near Darwin in the Northern Territory were more common at sites burnt annually, compared to sites that were long unburnt. Similarly, von Takach et al. (2020) did not find fire frequency to be an important determinant of northern quoll niche contraction across the Top End of the Northern Territory. In the Kimberley, Cook (2010a) found fire had little impact on northern quoll home range size, even when an animal’s entire home range was burnt. These contrasting results highlight that the impact of fire on northern quolls is likely to be context-specific. This is further demonstrated by Radford et al. (2020), who found northern quolls declined under prescribed burning in woodland habitats, but increased under prescribed burning in sandstone habitat. Additional research is required to further investigate the context-specific impacts of fire on northern quoll populations across their range.
Grazing
Overgrazing by feral and managed livestock has long been implicated in the decline of Australia’s mammal fauna (Woinarski et al. 2011b). Here, we identified 11 studies which discuss the impacts of over-grazing on northern quolls. The primary mechanism by which over-grazing is thought to impact northern quolls is through the removal of ground-level vegetation, which likely exposes quolls to increased levels of predation (Oakwood 1997). Water-hole eutrophication and soil-erosion caused by livestock, as well as extensive tree removal by graziers, are also likely to impact northern quolls. Braithwaite and Griffiths (1994) suggest the combined impacts of grazing by feral ungulates are likely to have contributed to the decline of northern quolls across their range and similar assessments are also made elsewhere (Oakwood 1997; Woinarski et al. 2011b; Radford et al. 2015). However, it is important to note that quolls do appear to persist at some sites where heavy grazing occurs (Woinarski et al. 2008; Hill and Ward 2010; H. A. Moore, pers comms.).
Habitat clearing
We identified six studies that discussed the impact of habitat clearing on northern quoll populations. Habitat clearing has been implicated as a factor in northern quoll declines prior to the year 2000, particularly in Queensland and the Northern Territory (Braithwaite and Griffiths 1994; Hill and Ward 2010; Jones et al. 2014). However, despite the subsequent introduction of environmental legislation such as the Environment Protection and Biodiversity Conservation Act 1999 (EPBC Act), designed to protect species from such direct human impacts, habitat clearing is likely continuing to threaten northern quoll populations. For example, in the period between 2000 and 2017, an estimated 1.6 million ha of potential northern quoll habitat was legally cleared (Ward et al. 2019). Northern quolls may be particularly sensitive to direct habitat loss because they require large areas (at least 220 km2) for population persistence (Brook et al. 2011): they show strong negative responses to habitat fragmentation (Rankmore 2006), and critical habitat features, such as hollows in trees and logs used for shelter, are commonly removed during land clearing and take many years to form (Woinarski and Westaway 2008).
Mining
There were few studies (n = 6) that investigated the response of northern quolls to disturbance because of mining/resource development. This is surprising, especially for the Pilbara population, where the scale of overlap between mining activity and northern quoll habitat is likely to be greatest—91% of the Pilbara bioregion is occupied by mining tenement (Environmental Protection Authority 2014). Here, rocky ridges and mesas, which are known to be important habitat for northern quolls (e.g. Moore et al. 2021), are frequently destroyed by mining companies targeting deposits of iron ore and gold (Cramer et al. 2016). Surrounding granite outcrops and tor fields are also quarried for rail formation ballast, rock armour for port infrastructure and basic raw materials for road construction. We found two studies from the Pilbara which examined the impact of mining-related habitat clearing on quolls, including a government report that found northern quolls persist at two rocky sites located in close proximity to a recently installed rail line (Dunlop et al. 2015), and an MSc thesis with similar findings at the same sites (Henderson 2015).
In addition to destroying habitat, mining activity can also impact species by introducing contaminants into the environment which can bioaccumulate, reducing the health of animals within affected areas (Nawab et al. 2015). For example, Amir Abdul Nasir et al. (2018b) found airborne manganese dust from Groote Eylandt Mining Company (a BHP Billiton subsidiary) was absorbed by northern quolls living in close proximity to mining operations. Groote Eylandt quolls accumulated manganese within their hair, testes, and brain. Quolls with higher manganese body burdens were slower at manoeuvring around corners than manganese-free quolls, which may reduce their capacity to capture prey and escape predators (Amir Abdul Nasir et al. 2018a).
Other threats
Other potential threats or sources of mortality for northern quolls are vehicle strikes (Oakwood 1997) and toxoplasmosis. However, Oakwood and Pritchard (1999) found no evidence of toxoplasmosis from 28 road-killed quolls in Kakadu National Park. Although it is clear from this review that northern quolls are exposed to multiple threats across their range, our understanding as to whether such threats to quolls are coincidentally or causally linked is poorly understood, and this can limit our capacity to manage threats effectively (Doherty et al. 2015). For example, although targeted baiting for larger predators, such as canids or foxes, may result in short-term alleviation of pressure from one predator on the northern quoll (Jolly et al. 2018a), in the long term it could potentially increase predation by mesopredators such as feral cats (Marlow et al. 2015). Additionally, total isolation from predators (i.e. in havens) can cause rapid evolutionary loss of antipredator traits that may not be easily reinstated (Jolly et al. 2018b; Jolly and Phillips 2021). Similarly, although it has been suggested that fire may increase the susceptibility of northern quolls to predation (Oakwood 1997), our understanding of how the timing, scale, or intensity of fire influences predator–prey relationships remains limited for most ecosystems and species (Geary et al. 2019a). Recognising and understanding how these threats interact may facilitate more targeted management interventions potentially leading to more desirable conservation outcomes (see Geary et al. 2019b).
Indigenous knowledge
Incorporation of Indigenous Australians and their knowledge into management actions is listed in three of eight recovery plan objectives for northern quolls (Hill and Ward 2010). A recent study found 63% of the northern quolls current range, as defined by the IUCN, occurs within the lands of Indigenous Australians (O’Bryan et al. 2020). We identified eight studies that incorporated Indigenous knowledge as part of this review, two of which included Indigenous rangers or ranger groups as co-authors (Jolly et al. 2021; Kelly et al. 2021).
The name ‘quoll’, is derived from an indigenous name for northern quolls, ‘Je-Quoll’, recorded by Joseph Banks near Cooktown in North Queensland, 1770 (Beaglehole 1963). Other names were used by Indigenous Australians to describe northern quolls across northern Australia, and many of these are recorded by (Abbott 2013). Using local Indigenous vernacular names may help acknowledge the strong connections Aboriginal people share with Australia’s fauna and flora that were honed over thousands of years of coexistence (Burbidge et al. 1988).
The most comprehensive summary of Indigenous knowledge about northern quolls is provided by Oakwood (1997). Like other quoll species (Attenbrow and Attenbrow 1987), northern quolls were consumed as food by Aboriginal people. However, there are mixed reports as to their palatability (Oakwood 1997). For example, northern quolls were considered ‘good tucker’ by the Gunwinggu people of West Arnhem Land (Goodfellow 1993). By contrast, barkuma (northern quolls) were not enjoyed as much by people of East Arnhem Land due to the buggan tumero (big smell) of the flesh (Dixon and Huxley 1985). Indigenous Australians of East Arnhem Land mentioned northern quolls as being numerous in diltji (open forest with grass) and also along beaches where cover is available, where they are said to shelter in hollow fallen logs and amongst stone.
In the Sir Edward Pellew Group of Islands of the Northern Territory, karnbulanyi (male northern quoll) or a-kaliba (female northern quoll) were also used as a food source (Bradley et al. 2006). Yanyuwa Elders from the Pellew Islands said northern quolls were once common, but younger people are less familiar with the species, and some of these island populations of quolls have been extirpated (Woinarski et al. 2011a). Further, the terms karnbulanyi and a-kaliba were later also used to describe feral cats (Bradley et al. 2006). Ziembicki et al. (2013) used Indigenous knowledge collated through a series of interviews across multiple communities in the Northern Territory to assess the extent and timing of regional mammal declines. This collation of information across communities found that range contractions were particularly pronounced for northern quolls, with the majority of decline recognised to have occurred in the 20 years prior to interviews taking place (1985–2009), and in the south of their range. Both cane toads and feral cats were implicated by observers as contributing factors in the declines, along with changing fire regimes associated with the cessation of Indigenous land management practises (Ziembicki et al. 2013).
There is significant Indigenous knowledge of the northern quoll across Australia (Abbott 2013, authors’ pers. obs.). Although some traditional knowledge has been included in a handful of studies, there is a great potential for future integration into research and management of the remaining northern quoll populations. Recently, some research groups have worked closely with Indigenous rangers and Traditional Owners to improve the conservation of northern quolls in northern Australia. Indigenous people and their knowledge are actively involved in researching and conserving northern quoll populations. One way is through Indigenous ranger programs, such as the Dhimurru, Jawoyn, Kenbi, Warddeken, and Marthakal ranger programs in the Northern Territory, the Uunguu ranger program in the Kimberley, Martu – Kanyirninpa Jukurrpa in the Great Sandy and Little Sandy Deserts and the Budadee, Murujuga, and Yindjibarndi ranger programs in the Pilbara. As part of these programs, Indigenous Australians play an important role in land management and in surveying and monitoring northern quolls, typically on lands with which they share a strong cultural connection and are part of the Indigenous estate (Jacobsen et al. 2020).
Conservation management
In 1993, despite recognition of a potential national decline in geographic range of 10–50%, the conservation status of northern quolls was recognised as ‘apparently stable’ in an action plan for the conservation of Australian marsupials and monotremes (Kennedy 1992). Four years later, a similar action plan listed northern quolls as ‘lower risk (near threatened)’ (Maxwell et al. 1996). In 2005, the northern quoll was listed as Endangered under the EPBC Act, which enacts legal responsibility for consideration in environmental impact assessments and management actions, such as the development of conservation advice and a recovery plan. Northern quolls were later listed as Critically Endangered in the Northern Territory and Endangered in Western Australia, but have remained listed as Least Concern in Queensland. In 2010, a strategic set of management priorities for the northern quoll was outlined in the ‘National Recovery Plan for the Northern Quoll’ (Hill and Ward 2010), which included eight specific objectives: (1) prevent cane toads from reaching offshore islands where northern quolls are present; (2) foster the recovery of northern quolls in populations where they coexist with cane toads; (3) halt northern quoll declines in areas where cane toads are present; (4) or absent; (5) maintain secure populations for future translocations; (6) increase knowledge of disease; (7) reduce the impacts of feral predators on northern quolls; and (8) raise public awareness of the plight of the northern quoll.
We have identified four primary management actions which have so far been applied and documented in detail that aim to achieve these objectives. These are (1) the use of islands as reserve populations through translocations, (2) cane toad control and aversion techniques, (3) feral predator control, and (4) the creation of artificial habitat.
Islands
Islands can be important tools for species conservation by harbouring species at risk from threats present on the mainland (Ringma et al. 2018). The translocation of northern quolls to three islands (Astell Island, Pobassoo Island, and Indian Island) off the coast of Northern Territory between 2003 and 2017 (Rankmore et al. 2008; Jolly and Phillips 2021; Kelly et al. 2021) has produced several positive outcomes. On Astell and Pobassoo Islands, northern quoll populations increased from a total of 64 to 5600 individuals in the 5 years that followed translocations (Rankmore et al. 2008) and populations appeared to maintain genetic diversity at least in the short term (Cardoso et al. 2009). Although the translocated populations exhibited some decline following their initial booms, both have now stabilised with high survival and recruitment rates compared to mainland populations (Griffiths et al. 2017).
In contrast to the Astell and Pobassoo Islands translocations, the Indian Island population declined sharply within a year of individuals being released and is now unlikely to be viable (Kelly et al. 2021). In addition to cane toad related mortality, the failure of the Indian Island translocation was likely contributed to by the extremely unfortunate timing of two major stochastic events (fire and cyclone) in the establishment year (Kelly et al. 2021). It is plausible that, had the timing of these events been different, the introduced quoll population on Indian Island may have taken a different trajectory (Kelly et al. 2021). The primary purpose of the Indian Island translocation, rather than establishing an insurance population, was to experimentally measure the selection on toad-smart genes and, thus, test the effectiveness of targeted gene flow (see below) (Kelly et al. 2021). This also probably influenced the fate of the population, as the experiment required quolls to be released onto an island with a resident toad population and a release cohort with appropriate population demographics (i.e. proportion of toad-smart individuals) meaning release numbers were small (n = 54).
Where quolls have been introduced to island arks for the purposes of setting up insurance populations, the success rate has been 100% (Griffiths et al. 2017). However, it is important to consider that an objective of northern quoll island introductions is to create insurance populations which can be used for future mainland reintroductions (Hill and Ward 2010). As such, it will be important for those planning future island translocations to consider the impacts of isolation on quolls in terms of both genetic diversity (Cardoso et al. 2009) and predator naivety (Jolly and Phillips 2021) in order to maximise the success of future mainland reintroductions. Translocation planners should also consider the impact northern quolls may have on prey species occupying islands that quolls are translocated to, which, in the short-term, are unlikely to be equipped with appropriate behaviours to avoid being depredated by quolls (Jolly et al. 2021). Given the right circumstances, translocations to mainland locations within the northern quoll’s historic range could also be considered. This could include properties managed in terms of both predators and fire by conservations organisation such as the Australian Wildlife Conservancy and Bush Heritage Australia, or through the Australian Government’s Indigenous Protected Areas program.
Cane toad control and aversion techniques
Although established toad populations are largely impossible to eradicate with existing tools (Tingley et al. 2017), several studies have assessed the feasibility of controlling the spread of cane toads by capitalising on their vulnerability to desiccation and blocking access to large artificial water bodies (Brook et al. 2011; Southwell et al. 2017; Tingley et al. 2013, 2017; Gregg et al. 2019). Southwell et al. (2017) suggest barriers blocking toad access to water between the Kimberley and the Pilbara could be constructed for AU$4.5 million, with such a mechanism potentially capable of stopping toad invasion from the Kimberley to the Pilbara region, even for scenarios with extremely high rainfall. Initial field trials have been successful, with toads surviving a maximum of 5 days without access to surface water under the conditions where barriers would be installed (Gregg et al. 2019). However, it is important to note that the desire of the Western Australian government and that of the pastoral industry to drought-proof the north-west pastoral industry through the increased development of alternative agriculture practises (new crops and fodder) and intensification in pastoral diversification such as central pivot irrigation (e.g. La Grange region) will limit the effectiveness of strategies based on rendering water sources inaccessible to toads. Similarly, this method of restricting toad movement will not address breaches in biosecurity procedures which enable toads to reach the Pilbara within plant nursey stock and fresh food produce from the Kimberley and Northern Territory growing regions and as hitchhikers with tourists and industrial/mining equipment.
In populations where cane toads are already established, persisting northern quolls are now ‘toad-smart’ and are less willing to depredate toads than quolls that have no previous exposure to toads (Kelly and Phillips 2017). This behaviour has been shown to be heritable, with offspring of toad-smart quolls being shown to innately avoid cane toads on their first encounter, suggesting rapid adaptive response in toad-impacted populations (Kelly and Phillips 2017, 2019). To induce similar toad-smart behaviour in toad-naïve quolls, conditioned taste aversion (CTA) trials have recently been used to alter northern quoll predatory behaviour in captivity (O’Donnell et al. 2010; Webb et al. 2015; Cremona et al. 2017b; Indigo et al. 2018; Kelly et al. 2018; Jolly et al. 2018a) and have shown promise. CTA techniques used to train quolls to avoid toads typically use cane toad flesh laced with a nausea inducing dose of thiabendazole that deters quolls from subsequently eating cane toad flesh once released. Quolls trained with non-lethal toad meals generally avoided the consumption of live and dead toads when tested in captivity (Indigo et al. 2018) and survived longer in the wild then toad-naïve quolls (O’Donnell et al. 2010; Jolly et al. 2018a).
Despite the success of CTA trials on laboratory-trained quolls, buffering wild northern quolls against cane toads using CTA techniques has proved more difficult. For example, recent unpublished research found CTA trials conducted within and adjacent to Mornington Wildlife Sanctuary in the Kimberley did not reduce toad impacts on the quoll populations (Indigo 2020). Several contributing factors are thought to be potentially responsible for the failure of these trials, including: (i) the decay of toad aversion with time since CTA exposure (Indigo et al. 2018); (ii) ineffective delivery rates of the toad sausages; and (iii) ineffective dose rates of thiabendazole within sausages (Indigo 2020). Although it has been demonstrated that intergenerational persistence of CTA trained quolls can occur in the wild following translocation (Cremona et al. 2017b), the mechanism by which toad-avoidance behaviour is transmitted across generations – via genetic or cultural means – remains unclear. Discerning the transmission mechanisms is made considerably more difficult by the fact a certain proportion of quolls, irrespective of training, have a natural tendency to avoid attacking cane toads (Kelly and Phillips 2017). The efficacy of CTA as a management strategy is largely dependent on a single factor: whether quolls can confer the learnt toad-aversion lesson between generations via trained mothers teaching their young (cultural transmission). Recently, population viability models demonstrated that the efficacy of CTA as a conservation strategy for northern quolls requires cultural transmission rates (mother training young) of >70% to prevent local extinction (Indigo et al. 2021). Unfortunately, there is currently no evidence that quolls have the ability or tendency to train their young to avoid cane toads (Indigo et al. 2021). Therefore, the transmission of behavioural aversion of cane toads in toad-averse quoll populations (e.g. Queensland) likely proceeds via genetic inheritance rather that cultural transmission.
Another cane toad mitigation strategy that could be implemented as part of future northern quoll management efforts is targeted gene flow, where quolls with heritable toad-smart genes are introduced into naïve populations to enhance their adaptive capacity (Kelly and Phillips 2016, 2019). In 2017, the first and only trial of targeted gene flow in northern quolls involved releasing 54 CTA trained northern quolls onto toad infested Indian Island. The released quolls were composed of toad-smart genotypes from Queensland, hybrid toad-smart and toad-naïve genotypes (Queensland × Northern Territory) and toad-naïve genotypes from Northern Territory. The aim of the trial was to test if selection pressure in the form of cane toads would drive toad smart genes to spread throughout the introduced quoll population with each generation. Although northern quolls failed to establish a population on Indian Island (details discussed above), genetic data collected the year after the translocation indicated selection toward toad-smarts had occurred after only a single generation (Kelly 2018; Kelly et al. 2021). The study also demonstrated the successful hybridisation of Queensland and Northern Territory northern quolls, with viable F2 hybrids and backcrosses observed, suggesting outbreeding depression (a potential barrier to the success of targeted gene flow) is not an issue for this species (Kelly et al. 2021).
Feral predator control
Dingo and wild dog control has occurred across much of mainland Australia for well over a century (Allen and Sparkes 2001). Dingo control is particularly common in pastoral areas and is mostly conducted via the deployment of meat baits containing 1080 (sodium monofluoroacetate) (Twigg et al. 2000). Sodium monofluoroacetate is a poisonous compound produced naturally by plants, mostly in the genus Gastrolobium, most of which occur in the southwest of Western Australia. Although it is typically most lethal to animals without an evolved tolerance, this depends on both the dose rate and number of baits consumed (McIlroy 1981, 1982). Using aircraft to deploy baits (aerial baiting) has dramatically increased the scale at which predator control can feasibility be implemented (Thomson 1986).
At sites where 1080 baits are deployed, dingo densities can be reduced (Thomson 1986; Twigg et al. 2000), although there are several examples of baiting programs failing to produce a decline in dingo densities (McIlroy et al. 1986; Kennedy et al. 2021). Northern quolls do eat 1080 baits (Calver et al. 1989), but the baits themselves do not appear to negatively affect quoll population sizes (King 1989). However, due to differences in their evolutionary exposure history to plants containing sodium monofluoroacetate occurring, some populations of northern quoll are likely to be more vulnerable to 1080 poisoning than others (Twigg et al. 2003). Although dingo control, if successful, may reduce dingo-related mortality in quolls, it may also have complex and unintended indirect effects (Dickman et al. 2009; Brook et al. 2012), such as mesopredator release (Crooks and Soulé 1999; Ritchie and Johnson 2009) of feral cats. However, there is conflicting evidence regarding whether feral cats are indeed released following dingo control in northern Australia (Brook et al. 2012; Kennedy et al. 2012; Leo et al. 2019; Stobo‐Wilson et al. 2020).
Feral cats can also be controlled using poison baits, albeit much less effectively (Algar et al. 2007), and with the risk of unintentionally killing dingoes (e.g Wysong et al. 2020). New baits engineered specifically for cats such as EradicatTM, 5 and HisstoryTM are now being trialled (Woinarski et al. 2019b; Johnston et al. 2013, 2020). A five-year trial is currently underway investigating the impact of a large-scale EradicatTM baiting program on northern quolls, as well as other species vulnerable to cat predation such as Rothschild’s rock wallabies (Petrogale rothschildi) and Pilbara olive pythons (Liasis olivaceus barroni) (Morris et al. 2015). Preliminary results suggest feral cat baiting does have a positive effect on northern quoll populations (Palmer 2019), without any direct impacts to the quolls themselves (Moro et al. 2019; Cowan et al. 2020a). However, confounding factors related to rainfall and fire justify the need for further research to measure benefits of feral cat baiting for northern quolls. Other technology, such as the Felixer feral cat grooming trap (Thylation), are in the process of being tested for efficacy in targeting feral cats in the presence of native fauna (Read et al. 2019).
Artificial habitat
Northern quolls, like several other CWR species in Australia, require structural complexity in the form of logs, tree hollows, termite mounds, or rocky outcrops to use as refuges (Oakwood 1997a), and reductions in habitat complexity because of fire or habitat clearing can expose these species to increased rates of predation (Oakwood 1997a). One way of mitigating these effects may be through the use of artificial refuges (Cramer et al. 2016), which provide quolls with shelter from predation and climatic exposure where natural refuges have been removed. So far, artificial refuges designed for northern quolls have been deployed in several locations across the Pilbara (Cramer et al. 2016; Cowan et al. 2020b). Each of these artificial refuges trialled were constructed in response to habitat loss as a result of mining (Cramer et al. 2016; Cowan et al. 2020b). Cowan et al. (2020b) found that although artificial refuges closely replicated the thermal conditions created by natural dens, the surrounding environment was typically less complex, potentially contributing to greater feral cat visitation and lower prey availability. It is possible that improving restoration efforts in the area surrounding refuges may increase their suitability for northern quolls, but this has yet to be tested. Overall, there remain many gaps in our knowledge of the capacity of artificial refuges to act as quoll habitat. Far more research on quoll use, survival, and breeding (among other things) within artificial refuges is required before they can be implemented as an evidence-based intervention in response to habitat loss or degradation (Cowan et al. 2021).
Future research directions
Northern quolls have been the subject of considerable research, which has improved our understanding of their threats and provided a useful basis for conservation management. However, the species remains threatened and continues to decline, with more resolute and strategic management required. Further research that addresses key knowledge gaps can contribute significantly to improving the effectiveness of conservation management for the northern quoll, and hence its overall conservation outlook. Here, we provide a non-exhaustive list of future research directions based on knowledge gaps evident from our review. If applied, each could be used to fine-tune and redirect management actions to improve conservation outcomes for northern quolls.
We acknowledge that a separate but overlapping set of research priorities have been identified for the Pilbara population (Cramer et al. 2016), including: (i) develop appropriate and standardised survey and monitoring methods; (ii) improve our understanding of habitat requirements; (iii) better understand the population dynamics of the northern quoll in the Pilbara; (iv) better understand key threats (cane toads, feral predators, mining infrastructure) and the interactions of these threats; and (v) determine the ability of the northern quoll to recolonise disturbed areas or colonise artificial habitat. We reiterate the importance of these research priorities to the conservation of Pilbara northern quolls.
1. Resolving taxonomy
Although no subspecies of northern quoll are currently recognised, several studies have found clear genetic distinctions among the four major populations based on microsatellite data, which are separated from one another by established biogeographic boundaries (Firestone et al. 2000; How et al. 2009; Woolley et al. 2015; Hohnen et al. 2016b). Determining if major populations should be treated as distinct taxonomic units is likely to be critical in informing future management interventions such as targeted gene flow and genetic rescue, where quolls from one population are translocated to another (see management section). The use of more recently available genetic techniques such as genome-wide single nucleotide polymorphisms (SNPs) analysis are likely to be important in addressing whether these genetic divergences warrant taxonomic recognition and/or whether significant evolutionary units should be assigned to populations and managed differently.
2. The status of Queensland northern quolls
The Queensland population of northern quoll previously occupied a larger area than any other northern quoll population and has since undergone larger declines than any other northern quoll population (Moore et al. 2019). Yet, we found a disproportionally small number of studies on Queensland northern quolls (although research in Queensland is ongoing). Queensland northern quolls have not been comprehensively surveyed and there are no published studies which provide estimates of abundance or density for this population (but see Woinarski et al. 2008). An explanation for this could be that northern quolls remain listed as ‘least concern’ under the Queensland Nature Conservation Act (1992), and, therefore, research funding provided by the state government for threatened species may not have been prioritised for Queensland northern quolls. We acknowledge the northern quoll research currently underway in Queensland (e.g. S. Burnett unpubl. data; G. Trewella unpubl. data), but suggest an increased research effort is valid given the scale of declines that have occurred. Further research may assist in assessing whether persisting northern quoll populations are stable, declining, or recovering, and could help prioritise conservation efforts.
3. Understand mechanisms allowing the persistence and resistance of northern quoll populations during cane toad invasions
In contrast to a trend of severe and rapid decline following the arrival of cane toads, some northern quoll populations do survive the initial wave of a toad invasion (Woinarski et al. 2008; Kelly and Phillips 2017). However, apart from a small selection of broad-scale habitat predictors (Woinarski et al. 2008), we have little knowledge of population-specific characteristics that best predict a population’s short-term likelihood of surviving a toad invasion. Understanding the initial patterns of persistence may be useful in forecasting the probability of quoll population persistence in areas yet to be invaded by cane toads (i.e. southern Kimberley, Pilbara), potentially allowing us to prioritise the management of these areas. Such lessons are likely to improve our understanding of the mechanisms and circumstances underlying a quoll populations’ persistence following cane toad invasion, but may also provide an increased mechanistic understanding of how these impacts and recoveries may play out in populations of other Australian predators that are threatened with extinction via the impacts of cane toads.
In addition to investigating factors facilitating quoll persistence through cane toad invasions, an important future research direction may be to assess if there are any signs of recovery. Have northern quolls returned to sites from which they were previously lost, and if so, what population/habitat characteristics have allowed for this return? Answering these questions are likely to provide information critical to the success of future assisted recolonisations – currently one of our most promising tools for conserving northern quolls (discussed above). This will first require the re-surveying of sites from which northern quolls have previously been confirmed to be absent following cane toad invasion (of which there are now many) to assess whether recoveries have occurred. Secondly, the physical and genetic characteristics of the recolonising quolls, along with the make-up of the recolonised habitat should be compared with sites where quolls have remained absent. Given northern quolls in Queensland have co-existed with cane toads for longer than any other northern quoll population, it is likely that recovery following cane toad-caused decline is most likely to be apparent in the Queensland population. As such, we recommend future studies addressing these questions focus on the Queensland population.
4. Quantifying the impacts of mining
Mining activity occurs across the northern quolls entire range, including important cane toad-free populations, such as Groote Eylandt and the Pilbara. Yet, few studies (see threats section) have investigated the impact of mining on northern quoll populations. As such, determining the extent to which mining activity is likely to influence the persistence of northern quoll population should be addressed in future research. This is especially true for the Pilbara populations, where overlap between the northern quolls geographic range and mining tenure is high (Cramer et al. 2016). In relation to mining activity specifically, Cramer et al. (2016) identify the impacts of linear infrastructure on northern quoll movement and the ability of the northern quoll to recolonise disturbed areas or artificial habitat as key research areas. In addition to these areas, we suggest future studies investigate secondary impacts of mining activity on northern quoll populations, such as increased predator densities surrounding mining camps driven by increased resource subsidies (e.g. food, water).
5. Population isolation and genetic rescue
A consequence of the northern quolls recent geographic decline is that many populations are now smaller and more isolated. Inbreeding and loss of genetic diversity is often unavoidable in small, isolated populations, increasing their extinction risk due to inbreeding depression (i.e. loss of fitness from low genetic diversity) and lowered adaptive potential (Frankham et al. 2017; Ralls et al. 2020). Island populations of northern quolls are particularly vulnerable to these risks, and previous work has shown they have lower genetic diversity compared to populations on the mainland (Cardoso et al. 2009). We recommend future studies expand on this work by including additional sites—both on islands as well as the mainland populations (Flanagan et al. 2018). Where isolated quoll populations are showing signs of genetic degradation, it would be wise to consider mixing populations via translocation to increase genetic diversity and adaptive potential (Aitken and Whitlock 2013; Frankham 2015; Whiteley et al. 2015).
6. Unwinding interacting threats
Northern quolls face multiple co-occurring threats across their range; however, limited research has investigated if and how these threats interact synergistically. Studies of other native mammals occurring in northern Australia demonstrate that threats may compound one another and management that addresses only individual threats in isolation of others may be ineffective (Legge et al. 2019). A better understanding northern quoll ‘threat webs’ (a group of co-occurring threats that may have additive or non-additive impacts on each other) may improve land managers’ ability to focus efforts toward ultimate threats rather than proximate threats – hopefully with improved conservation outcomes (Geary et al. 2019b). Understanding the synergistic impacts of predation, fire, and grazing on northern quolls across their existing range is likely to be important, particularly in areas where populations are already significantly degraded by toads. Currently, there is limited evidence that feral cats are driving declines in northern quolls, although the impacts of feral cats are potentially significant. Given the scale of feral cat impacts on the declining mammal fauna of northern Australia (Woinarski et al. 2019b) we suggest that the impacts of feral cats on quoll populations be investigated as a matter of priority.
An important tool in untangling these threats is the use of manipulative experiments, where at least one threat within a system is controlled (typically as part of conservation management activities), such that its relative impact on northern quoll populations can be measured in context to co-occurring threats. Although experiments of this nature are already underway (e.g. Palmer 2019), opportunities for further research are likely to exist in areas where threat management (e.g. controlled burns, predator baiting) within northern quoll habitat is planned or actively occurring already.
7. Predicting the impact of climate change
Climate change poses an extreme risk to global biodiversity (Steffen 2009), yet we found little mention of it in literature related to northern quolls. Across the northern quolls geographic range, the impacts of climate change are likely to include increased temperatures, rainfall variability, increased proportion of extreme rainfall events, and less frequent cyclones (NESP 2018). Projected changes to total annual rainfall are uncertain, although decreases in total rainfall are more likely than increases (NESP 2018). Measuring the extent to which changes in rainfall will impact resource availability and breeding success for northern quoll, among other northern Australian mammals, has obvious implications for the conservation of the species. In addition to changes in rainfall, average temperatures across Northern Australia will continue to increase and there will be more days with extreme maximum temperatures (NESP 2018). Understanding if these changes will lead to increased northern quoll mortality or reduced reproductive output as a result of thermal stress, and the implications this would have for population persistence, should be a future research priority. Identifying potential climate refugia for northern quolls (areas that they will likely persist in under various climate change scenarios) where management efforts can be concentrated should also form a future research focus.
8. Further incorporation of Indigenous knowledge
Recognition of the knowledge held by the Indigenous people of Australia – often termed ‘two-way’ or ‘right-way’ science – has improved our ecological understanding of many species (e.g. Horstman and Wightman 2001; Telfer and Garde 2006; Butler et al. 2012; Bohensky et al. 2013). There is significant Indigenous knowledge of the northern quoll across northern Australia (Abbott 2013). Some traditional knowledge has been included in a handful of studies (Dixon and Huxley 1985; Woinarski et al. 2011a; Ziembicki et al. 2013), but there is significant potential for future integration of traditional knowledge into research and on-ground management of the remaining northern quoll populations. Indigenous knowledge could potentially be important in detecting of unknown isolated populations in remote areas of the Western Desert. Given that such a large proportion of the known distribution of northern quolls is on the Indigenous Estate, future research and conservation endeavours should seek to be more inclusive of Indigenous stakeholders and aim to incorporate increased involvement of Indigenous Australians in such efforts.
9. Harnessing the heritability of toad avoidance behaviour
Individuals within quoll populations that survive the initial invasion of toads and continue to persist in sympatry with toads do so because they are innately averse to attacking toads (Kelly and Phillips 2017; Moore et al. 2019). Thus, if populations can avoid local extinction, natural selection should be rapidly acting upon heritable toad-averse traits. Harnessing the heritability of this behaviour forms the basis of targeted gene flow strategies (discussed above). Initial targeted gene flow trials using northern quolls have documented some encouraging results, however, additional research required to enhance this technique such that it can be applied on a broader scale. This may be achieved by utilising recent advancements in genetic technology to identify areas of the quolls genome that are most useful in predicting toad adverse behaviours in quolls. Future research in this area may also include attempting additional translocation trials, where captivity-bred quolls with genetic tendances to avoid toads are inserted into quoll populations predicted to be impacted by invading cane toads.
10. The role of artificial refuges
Initial trials have shown some potential for artificial refuges to provide thermally appropriate denning habitat in areas of disturbed or degraded habitat (Cowan et al. 2020b). However, this approach is not yet proven, and further research is required to determine whether artificial refuges can be a viable management tool for future northern quoll conservation. For example, evidence that northern quolls willingly use artificially constructed refuges as breeding habitat is currently lacking. Further trials, potentially incorporating differing complimentary actions (i.e. invasive predator control), are therefore required in order to address this knowledge gap. We also still have a limited understanding of the dimensions of artificially constructed den sites that maximise northern quoll use. This is particularly important in relation to natal dens. For example, existing artificial refuges range from 9 to 150 m2 in size, but offer shallower crevices compared to those northern quolls use in natural habitat (Cowan et al. 2020b). Future experiments should aim to test different sizes or arrangements of artificial refuges to determine how differences in these variables alter the thermal conditions of the refuges, and northern quoll use and survival. We also recommend that artificial refuges are trialled for northern quolls in habitats impacted by disturbances other than mining, such as fire and intense grazing, such as has already been trialled for smaller dasyurids (Bleicher and Dickman 2020).
Conflict of interests
The authors declare no conflict of interests.
Declaration of funding
H.A.M. was supported by a scholarship from the Institute of Land, Water and Society operating funds from the Faculty of Science at Charles Sturt University. L.E.V. was funded by the Australian Government’s National Environmental Science Program through the Threatened Species Recovery Hub. D.G.N. was supported by an Australian Research Council Early Career Researcher Award (DECRA).
Acknowledgements
We thank Naomi Indigo for her assistance with this review. We also thank two reviewers and the editor for useful comments on an earlier version of the manuscript.
References
Abbott, I. (2008). The spread of the cat, Felis catus, in Australia: re-examination of the current conceptual model with additional information. Conservation Science Western Australia 7, 1–17.Abbott, I. (2013). Extending the application of Aboriginal names to Australian biota:‘Dasyurus’(Marsupialia: Dasyuridae) species. Victorian Naturalist, The 130, 109.
Aitken, S. N., and Whitlock, M. C. (2013). Assisted gene flow to facilitate local adaptation to climate change. Annual review of ecology, evolution, and systematics 44, 367–388.
| Assisted gene flow to facilitate local adaptation to climate change.Crossref | GoogleScholarGoogle Scholar |
Algar, D., Angus, G., Williams, M., and Mellican, A. (2007). Influence of bait type, weather and prey abundance on bait uptake by feral cats (Felis catus) on Peron Peninsula, Western Australia. Conservation Science Western Australia 6, 109.
Allen, L., and Sparkes, E. (2001). The effect of dingo control on sheep and beef cattle in Queensland. Journal of Applied Ecology 38, 76–87.
| The effect of dingo control on sheep and beef cattle in Queensland.Crossref | GoogleScholarGoogle Scholar |
Amir Abdul Nasir, A. F., Clemente, C. J., Wynn, M. L., and Wilson, R. S. (2017). Optimal running speeds when there is a trade‐off between speed and the probability of mistakes. Functional Ecology 31, 1941–1949.
| Optimal running speeds when there is a trade‐off between speed and the probability of mistakes.Crossref | GoogleScholarGoogle Scholar |
Amir Abdul Nasir, A. F., Cameron, S. F., Niehaus, A. C., Clemente, C. J., von Hippel, F. A., and Wilson, R. S. (2018a). Manganese contamination affects the motor performance of wild northern quolls (Dasyurus hallucatus). Environmental Pollution 241, 55–62.
| Manganese contamination affects the motor performance of wild northern quolls (Dasyurus hallucatus).Crossref | GoogleScholarGoogle Scholar | 29793108PubMed |
Amir Abdul Nasir, A. F., Cameron, S. F., von Hippel, F. A., Postlethwait, J., Niehaus, A. C., Blomberg, S., and Wilson, R. S. (2018b). Manganese accumulates in the brain of northern quolls (Dasyurus hallucatus) living near an active mine. Environmental Pollution 233, 377–386.
| Manganese accumulates in the brain of northern quolls (Dasyurus hallucatus) living near an active mine.Crossref | GoogleScholarGoogle Scholar | 29096311PubMed |
Andersen, A. N., Cook, G. D., Corbett, L. K., Douglas, M. M., Eager, R. W., Russell‐Smith, J., Setterfield, S. A., Williams, R. J., and Woinarski, J. C. (2005). Fire frequency and biodiversity conservation in Australian tropical savannas: implications from the Kapalga fire experiment. Austral Ecology 30, 155–167.
| Fire frequency and biodiversity conservation in Australian tropical savannas: implications from the Kapalga fire experiment.Crossref | GoogleScholarGoogle Scholar |
Ashman, K. R., Watchorn, D. J., and Whisson, D. A. (2019). Prioritising research efforts for effective species conservation: a review of 145 years of koala research. Mammal Review 49, 189–200.
| Prioritising research efforts for effective species conservation: a review of 145 years of koala research.Crossref | GoogleScholarGoogle Scholar |
Attenbrow, V., and Attenbrow, V. (1987). ‘The Upper Mangrove Creek catchment: a study of quantitative changes in the archaeological record.’ (University of Sydney.)
Baynes, A., and McDowell, M. C. (2010). The original mammal fauna of the Pilbara biogeographic region of north-western Australia. Records of the Western Australian Museum Part 1, 285–298.
| The original mammal fauna of the Pilbara biogeographic region of north-western Australia.Crossref | GoogleScholarGoogle Scholar |
Beaglehole, J. C. (1963). ‘The Endeavour Journal of Joseph Banks, 1768-1771.’ ([Sydney]: Trustees of the Public Library of New South Wales.)
Begg, R. J. (1981). The small mammals of Little Nourlangie Rock, NT III. Ecology of Dasyurus hallucatus, the northern quoll (Marsupialia: Dasyuridae). Wildlife Research 8, 73–85.
| The small mammals of Little Nourlangie Rock, NT III. Ecology of Dasyurus hallucatus, the northern quoll (Marsupialia: Dasyuridae).Crossref | GoogleScholarGoogle Scholar |
Bleicher, S. S., and Dickman, C. R. (2020). On the landscape of fear: shelters affect foraging by dunnarts (Marsupialia, Sminthopsis spp.) in a sandridge desert environment. Journal of Mammalogy 101, 281–290.
| On the landscape of fear: shelters affect foraging by dunnarts (Marsupialia, Sminthopsis spp.) in a sandridge desert environment.Crossref | GoogleScholarGoogle Scholar |
Bohensky, E., Butler, J., and Davies, J. (2013). Integrating indigenous ecological knowledge and science in natural resource management: perspectives from Australia. Ecology and Society 18, .
| Integrating indigenous ecological knowledge and science in natural resource management: perspectives from Australia.Crossref | GoogleScholarGoogle Scholar |
BOM (2020). Climate Data Online. Bureau of Meteorology. Available at http://www.bom.gov.au/climate/data/
Bornmann, L., and Mutz, R. (2015). Growth rates of modern science: A bibliometric analysis based on the number of publications and cited references. Journal of the Association for Information Science and Technology 66, 2215–2222.
| Growth rates of modern science: A bibliometric analysis based on the number of publications and cited references.Crossref | GoogleScholarGoogle Scholar |
Bowman, D. M., Brown, G., Braby, M., Brown, J., Cook, L. G., Crisp, M., Ford, F., Haberle, S., Hughes, J., and Isagi, Y. (2010). Biogeography of the Australian monsoon tropics. Journal of Biogeography 37, 201–216.
| Biogeography of the Australian monsoon tropics.Crossref | GoogleScholarGoogle Scholar |
Bradley, J., Holmes, M., Marrngawi, D. N., Karrakayn, A. I., Wuwarlu, J. M., and Ninganga, I. (2006). Yumbulyumbulmantha ki-Awarawu: all kinds of things from country: Yanyuwa ethnobiological classification. Aboriginal and Torres Strait Islander Studies Unit Research Report Series 6, .
Braithwaite, R. W., and Griffiths, A. D. (1994). Demographic variation and range contraction in the northern Quoll, Dasyurus hallucatus (Marsupialia: Dasyuridae). Wildlife Research 21, 203–217.
| Demographic variation and range contraction in the northern Quoll, Dasyurus hallucatus (Marsupialia: Dasyuridae).Crossref | GoogleScholarGoogle Scholar |
Brook, B. W., Whitehead, P. J., and Dingle, J. K. (2011). ‘Potential cane toad short to medium term control techniques: the biological feasibility and cost of exclusion as a mitigating control strategy.’ (Department of the Environment and Heritage.)
Brook, L. A., Johnson, C. N., and Ritchie, E. G. (2012). Effects of predator control on behaviour of an apex predator and indirect consequences for mesopredator suppression. Journal of applied ecology 49, 1278–1286.
| Effects of predator control on behaviour of an apex predator and indirect consequences for mesopredator suppression.Crossref | GoogleScholarGoogle Scholar |
Burbidge, A. A., Johnson, K. A., Fuller, P. J., and Southgate, R. (1988). Aboriginal knowledge of the mammals of the central deserts of Australia. Wildlife Research 15, 9–39.
| Aboriginal knowledge of the mammals of the central deserts of Australia.Crossref | GoogleScholarGoogle Scholar |
Burnett, S. (1997). Colonizing cane toads cause population declines in native predators: reliable anecdotal information and management implications. Pacific Conservation Biology 3, 65–72.
| Colonizing cane toads cause population declines in native predators: reliable anecdotal information and management implications.Crossref | GoogleScholarGoogle Scholar |
Burnett, S., and Zwar, A. (2009). ‘Quolls (Dasyurus maculatus and D. hallucatus) in the southern Mary River catchment, south-east Queensland.’ (Wildlife Preservation Society of Queensland: Sippy Downs.)
Burnett, S., Shimizu, Y., and Middleton, J. (2013). Distribution and abundance of the Northern quoll (Dasyurus hallucatus) in far north Queensland. Report prepared by the University of the Sunshine Coast, Sunshine Coast for Ratch Australasia.
Butler, J. R., Tawake, A., Skewes, T., Tawake, L., and McGrath, V. (2012). Integrating traditional ecological knowledge and fisheries management in the Torres Strait, Australia: the catalytic role of turtles and dugong as cultural keystone species. Ecology and Society 17, 1–19.
| Integrating traditional ecological knowledge and fisheries management in the Torres Strait, Australia: the catalytic role of turtles and dugong as cultural keystone species.Crossref | GoogleScholarGoogle Scholar |
Cairns, K. M., and Wilton, A. N. (2016). New insights on the history of canids in Oceania based on mitochondrial and nuclear data. Genetica 144, 553–565.
| New insights on the history of canids in Oceania based on mitochondrial and nuclear data.Crossref | GoogleScholarGoogle Scholar | 27640201PubMed |
Calaby, J. (1973). Mammals. In ‘Alligator Rivers region environmental factfinding study: wildlife’. (CSIRO Division of Wildlife Research: Canberra.)
Calver, M., King, D., Bradley, J., Gardner, J., and Martin, G. (1989). An assessment of the potential target specificity of 1080 predator baiting in Western-Australia. Wildlife Research 16, 625–638.
| An assessment of the potential target specificity of 1080 predator baiting in Western-Australia.Crossref | GoogleScholarGoogle Scholar |
Cardoso, M. J., Eldridge, M. D., Oakwood, M., Rankmore, B., Sherwin, W. B., and Firestone, K. B. (2009). Effects of founder events on the genetic variation of translocated island populations: implications for conservation management of the northern quoll. Conservation Genetics 10, 1719–1733.
| Effects of founder events on the genetic variation of translocated island populations: implications for conservation management of the northern quoll.Crossref | GoogleScholarGoogle Scholar |
Chan, R., Dunlop, J., and Spencer, P. (2020). Highly promiscuous paternity in mainland and island populations of the endangered northern Quoll. Journal of Zoology 310, 210–220.
| Highly promiscuous paternity in mainland and island populations of the endangered northern Quoll.Crossref | GoogleScholarGoogle Scholar |
Cook, A. (2010a). Fire effect on habitat use and home-range of the northern quoll, Dasyurus hallucatus. Masters of Science thesis. University of Western Austalia, Perth, WA.
Cook, A. (2010b). ‘Habitat use and home-range of the northern quoll, Dasyurus hallucatus: effects of fire.’ (University of Western Australia.)
Cooper, C. E., and Withers, P. C. (2010). Comparative physiology of Australian quolls (Dasyurus; Marsupialia). Journal of Comparative Physiology B 180, 857–868.
| Comparative physiology of Australian quolls (Dasyurus; Marsupialia).Crossref | GoogleScholarGoogle Scholar |
Corbett, L. (2003). Terrestrial vertebrates. In ‘Fire in tropical savannas: the Kapalga experiment’. (Eds A. N. Andersen, G. D. Cook and R. J. Williams) pp. 126–152. (Springer-Verlag: New York.)
Cowan, M., Moro, D., Anderson, H., Angus, J., Garretson, S., and Morris, K. (2020a). Aerial baiting for feral cats is unlikely to affect survivorship of northern quolls in the Pilbara region of Western Australia. Wildlife Research 47, 589–598.
| Aerial baiting for feral cats is unlikely to affect survivorship of northern quolls in the Pilbara region of Western Australia.Crossref | GoogleScholarGoogle Scholar |
Cowan, M. A., Dunlop, J. A., Turner, J. M., Moore, H. A., and Nimmo, D. G. (2020b). Artificial refuges to combat habitat loss for an endangered marsupial predator: how do they measure up? Conservation Science and Practice 2, 204.
| Artificial refuges to combat habitat loss for an endangered marsupial predator: how do they measure up?Crossref | GoogleScholarGoogle Scholar |
Cowan, M. A., Callan, M. N., Watson, M. J., Watson, D. M., Doherty, T. S., Michael, D. R., Dunlop, J. A., Turner, J. M., Moore, H. A., and Watchorn, D. J. (2021). Artificial refuges for wildlife conservation: what is the state of the science? Biological Reviews , .
| Artificial refuges for wildlife conservation: what is the state of the science?Crossref | GoogleScholarGoogle Scholar | 34269510PubMed |
Cramer, V. A., Dunlop, J., Davis, R., Ellis, R., Barnett, B., Cook, A., Morris, K., and van Leeuwen, S. (2016). Research priorities for the northern quoll (Dasyurus hallucatus) in the Pilbara region of Western Australia. Australian Mammalogy 38, 135–148.
| Research priorities for the northern quoll (Dasyurus hallucatus) in the Pilbara region of Western Australia.Crossref | GoogleScholarGoogle Scholar |
Cremona, T., Crowther, M., and Webb, J. K. (2017a). High mortality and small population size prevent population recovery of a reintroduced mesopredator. Animal Conservation 20, 555–563.
| High mortality and small population size prevent population recovery of a reintroduced mesopredator.Crossref | GoogleScholarGoogle Scholar |
Cremona, T., Spencer, P., Shine, R., and Webb, J. K. (2017b). Avoiding the last supper: parentage analysis indicates multi-generational survival of re-introduced ‘toad-smart’lineage. Conservation genetics 18, 1475–1480.
| Avoiding the last supper: parentage analysis indicates multi-generational survival of re-introduced ‘toad-smart’lineage.Crossref | GoogleScholarGoogle Scholar |
Cremona, T., Baker, A. M., Cooper, S. J., Montague-Drake, R., Stobo-Wilson, A. M., and Carthew, S. M. (2020). Integrative taxonomic investigation of Petaurus breviceps (Marsupialia: Petauridae) reveals three distinct species. Zoological Journal of the Linnean Society , .
| Integrative taxonomic investigation of Petaurus breviceps (Marsupialia: Petauridae) reveals three distinct species.Crossref | GoogleScholarGoogle Scholar |
Crooks, K. R., and Soulé, M. E. (1999). Mesopredator release and avifaunal extinctions in a fragmented system. Nature 400, 563.
| Mesopredator release and avifaunal extinctions in a fragmented system.Crossref | GoogleScholarGoogle Scholar |
Dick, M., Rous, A. M., Nguyen, V. M., and Cooke, S. J. (2016). Necessary but challenging: multiple disciplinary approaches to solving conservation problems. Facets 1, 67–82.
| Necessary but challenging: multiple disciplinary approaches to solving conservation problems.Crossref | GoogleScholarGoogle Scholar |
Dickman, C., and Braithwaite, R. W. (1992). Postmating mortality of males in the dasyurid marsupials, Dasyurus and Parantechinus. Journal of Mammalogy 73, 143–147.
| Postmating mortality of males in the dasyurid marsupials, Dasyurus and Parantechinus.Crossref | GoogleScholarGoogle Scholar |
Dickman, C. R., Glen, A. S., and Letnic, M. (2009). Reintroducing the dingo: can Australia’s conservation wastelands be restored. Reintroduction of top-order predators 238, 269.
| Reintroducing the dingo: can Australia’s conservation wastelands be restored.Crossref | GoogleScholarGoogle Scholar |
Dixon, J. M., and Huxley, L. (1985). ‘Donald Thomson’s mammals and fishes of northern Australia.’ (Nelson.)
Doherty, T. S., Dickman, C. R., Nimmo, D. G., and Ritchie, E. G. (2015). Multiple threats, or multiplying the threats? Interactions between invasive predators and other ecological disturbances. Biological Conservation 190, 60–68.
| Multiple threats, or multiplying the threats? Interactions between invasive predators and other ecological disturbances.Crossref | GoogleScholarGoogle Scholar |
Dunlop, J., Johnson, B., Rayner, K., and Morris, K. (2015). Northern quoll trapping surveys at Wall Creek and Mesa 228. Report prepared for Roy Hill Pty Ltd. Department of Parks and Wildlife, Kensington.
Dunlop, J. A., Rayner, K., and Doherty, T. S. (2017). Dietary flexibility in small carnivores: a case study on the endangered northern quoll, Dasyurus hallucatus. Journal of Mammalogy 98, 858–866.
| Dietary flexibility in small carnivores: a case study on the endangered northern quoll, Dasyurus hallucatus.Crossref | GoogleScholarGoogle Scholar |
Edwards, R. D., Crisp, M. D., Cook, D. H., and Cook, L. G. (2017). Congruent biogeographical disjunctions at a continent-wide scale: quantifying and clarifying the role of biogeographic barriers in the Australian tropics. PLoS One 12, e0174812.
| Congruent biogeographical disjunctions at a continent-wide scale: quantifying and clarifying the role of biogeographic barriers in the Australian tropics.Crossref | GoogleScholarGoogle Scholar | 28376094PubMed |
Environmental Protection Authority (2014). ‘Cumulative environmental impacts of development in the Pilbara region.’ (Western Australia Government: Perth.)
Evans, M. C., and Cvitanovic, C. (2018). An introduction to achieving policy impact for early career researchers. Palgrave Communications 4, 1–12.
| An introduction to achieving policy impact for early career researchers.Crossref | GoogleScholarGoogle Scholar |
Firestone, K. B., Houlden, B. A., Sherwin, W. B., and Geffen, E. (2000). Variability and differentiation of microsatellites in the genus Dasyurus and conservation implications for the large Australian carnivorous marsupials. Conservation Genetics 1, 115–133.
| Variability and differentiation of microsatellites in the genus Dasyurus and conservation implications for the large Australian carnivorous marsupials.Crossref | GoogleScholarGoogle Scholar |
Flanagan, S. P., Forester, B. R., Latch, E. K., Aitken, S. N., and Hoban, S. (2018). Guidelines for planning genomic assessment and monitoring of locally adaptive variation to inform species conservation. Evolutionary Applications 11, 1035–1052.
| Guidelines for planning genomic assessment and monitoring of locally adaptive variation to inform species conservation.Crossref | GoogleScholarGoogle Scholar | 30026796PubMed |
Fleming, P. A., and Bateman, P. W. (2016). The good, the bad, and the ugly: which Australian terrestrial mammal species attract most research? Mammal Review 46, 241–254.
| The good, the bad, and the ugly: which Australian terrestrial mammal species attract most research?Crossref | GoogleScholarGoogle Scholar |
Frankham, R. (2015). Genetic rescue of small inbred populations: meta‐analysis reveals large and consistent benefits of gene flow. Molecular Ecology 24, 2610–2618.
| Genetic rescue of small inbred populations: meta‐analysis reveals large and consistent benefits of gene flow.Crossref | GoogleScholarGoogle Scholar | 25740414PubMed |
Frankham, R., Ballou, J. D., Ralls, K., Eldridge, M., Dudash, M. R., Fenster, C. B., Lacy, R. C., and Sunnucks, P. (2017). ‘Genetic management of fragmented animal and plant populations.’ (Oxford University Press: Oxford, UK.)
Freeland, W. J., and Martin, K. C. (1985). The rate of range expansion by Bufo marinus in Northern Australia, 1980-84. Wildlife Research 12, 555–559.
| The rate of range expansion by Bufo marinus in Northern Australia, 1980-84.Crossref | GoogleScholarGoogle Scholar |
Geary, W. L., Doherty, T. S., Nimmo, D. G., Tulloch, A. I., and Ritchie, E. G. (2019a). Predator responses to fire: a global systematic review and meta‐analysis. Journal of Animal Ecology 89, 955–971.
| Predator responses to fire: a global systematic review and meta‐analysis.Crossref | GoogleScholarGoogle Scholar |
Geary, W. L., Nimmo, D. G., Doherty, T. S., Ritchie, E. G., and Tulloch, A. I. (2019b). Threat webs: reframing the co‐occurrence and interactions of threats to biodiversity. Journal of Applied Ecology 56, 1992–1997.
| Threat webs: reframing the co‐occurrence and interactions of threats to biodiversity.Crossref | GoogleScholarGoogle Scholar |
Geyle, H. M., Woinarski, J. C., Baker, G. B., Dickman, C. R., Dutson, G., Fisher, D. O., Ford, H., Holdsworth, M., Jones, M. E., and Kutt, A. (2018). Quantifying extinction risk and forecasting the number of impending Australian bird and mammal extinctions. Pacific Conservation Biology 24, 157–167.
| Quantifying extinction risk and forecasting the number of impending Australian bird and mammal extinctions.Crossref | GoogleScholarGoogle Scholar |
Goodfellow, D. (1993) ‘Fauna of Kakadu and the Top End.’ (Wakefield Press: Kent Town, SA)
Gould, J. (1842). Characters of a new species of Perameles, and a new species of Dasyurus. Proceedings of the Zoological Society of London 10, 41–42.
Gregg, E. A., Tingley, R., and Phillips, B. L. (2019). The on‐ground feasibility of a waterless barrier to stop the spread of invasive cane toads in Western Australia. Conservation Science and Practice , e74.
| The on‐ground feasibility of a waterless barrier to stop the spread of invasive cane toads in Western Australia.Crossref | GoogleScholarGoogle Scholar |
Griffiths, A. D., and Brook, B. W. (2015). Fire impacts recruitment more than survival of small‐mammals in a tropical savanna. Ecosphere 6, 1–22.
| Fire impacts recruitment more than survival of small‐mammals in a tropical savanna.Crossref | GoogleScholarGoogle Scholar |
Griffiths, A. D., Rankmore, B., Brennan, K., and Woinarski, J. C. (2017). Demographic evaluation of translocating the threatened northern quoll to two Australian islands. Wildlife Research 44, 238–247.
| Demographic evaluation of translocating the threatened northern quoll to two Australian islands.Crossref | GoogleScholarGoogle Scholar |
Heiniger, J., Cameron, S. F., Madsen, T., Niehaus, A. C., and Wilson, R. S. (2020). Demography and spatial requirements of the endangered northern quoll on Groote Eylandt. Wildlife Research 47, 224–238.
| Demography and spatial requirements of the endangered northern quoll on Groote Eylandt.Crossref | GoogleScholarGoogle Scholar |
Henderson, M. (2015). ‘The effects of mining infrastructure on northern quoll movement and habitat.’ (Edith Cowan University: Perth.)
Hernandez Santin, L. (2017). Ecology and predator associations of the northern quoll (Dasyurus hallucatus) in the Pilbara. PhD Thesis, School of Biological Sciences, The University of Queensland, Brisbane
Hernandez-Santin, L., Goldizen, A. W., and Fisher, D. O. (2016). Introduced predators and habitat structure influence range contraction of an endangered native predator, the northern quoll. Biological Conservation 203, 160–167.
| Introduced predators and habitat structure influence range contraction of an endangered native predator, the northern quoll.Crossref | GoogleScholarGoogle Scholar |
Hernandez-Santin, L., Dunlop, J. A., Goldizen, A. W., and Fisher, D. O. (2019). Demography of the northern quoll (Dasyurus hallucatus) in the most arid part of its range. Journal of Mammalogy 100, 1191–1198.
| Demography of the northern quoll (Dasyurus hallucatus) in the most arid part of its range.Crossref | GoogleScholarGoogle Scholar |
Hernandez-Santin, L., Henderson, M., Molloy, S. W., Dunlop, J. A., and Davis, R. A. (2021). Spatial ecology of an endangered carnivore, the Pilbara northern quoll. Australian Mammalogy 43, 235–242.
| Spatial ecology of an endangered carnivore, the Pilbara northern quoll.Crossref | GoogleScholarGoogle Scholar |
Hill, B. M., and Ward, S. J. (2010). ‘National recovery plan for the northern quoll Dasyurus hallucatus.’ (Department of Natural Resources, Environment, The Arts and Sport: Darwin.)
Hohnen, R., Tuft, K., McGregor, H. W., Legge, S., Radford, I. J., and Johnson, C. N. (2016a). Occupancy of the invasive feral cat varies with habitat complexity. PLoS One 11, e0152520.
| Occupancy of the invasive feral cat varies with habitat complexity.Crossref | GoogleScholarGoogle Scholar | 27655024PubMed |
Hohnen, R., Tuft, K. D., Legge, S., Hillyer, M., Spencer, P. B., Radford, I. J., Johnson, C. N., and Burridge, C. P. (2016b). Rainfall and topography predict gene flow among populations of the declining northern quoll (Dasyurus hallucatus). Conservation Genetics 17, 1213–1228.
| Rainfall and topography predict gene flow among populations of the declining northern quoll (Dasyurus hallucatus).Crossref | GoogleScholarGoogle Scholar |
Horstman, M., and Wightman, G. (2001). Karparti ecology: recognition of Aboriginal ecological knowledge and its application to management in north‐western Australia. Ecological Management & Restoration 2, 99–109.
| Karparti ecology: recognition of Aboriginal ecological knowledge and its application to management in north‐western Australia.Crossref | GoogleScholarGoogle Scholar |
How, R., Spencer, P., and Schmitt, L. (2009). Island populations have high conservation value for northern Australia’s top marsupial predator ahead of a threatening process. Journal of Zoology 278, 206–217.
| Island populations have high conservation value for northern Australia’s top marsupial predator ahead of a threatening process.Crossref | GoogleScholarGoogle Scholar |
Hradsky, B. A., Mildwaters, C., Ritchie, E. G., Christie, F., and Di Stefano, J. (2017). Responses of invasive predators and native prey to a prescribed forest fire. Journal of Mammalogy 98, 835–847.
| Responses of invasive predators and native prey to a prescribed forest fire.Crossref | GoogleScholarGoogle Scholar |
Ibbett, M., Woinarski, J., and Oakwood, M. (2018). Declines in the mammal assemblage of a rugged sandstone environment in Kakadu National Park, Northern Territory, Australia. Australian Mammalogy 40, 181–187.
| Declines in the mammal assemblage of a rugged sandstone environment in Kakadu National Park, Northern Territory, Australia.Crossref | GoogleScholarGoogle Scholar |
Indigo, N. L. (2020). Safeguarding the northern quoll. Can we mitigate cane toad impacts through conditioned taste aversion? PhD thesis. University of Technology, Sydney.
Indigo, N., Smith, J., Webb, J. K., and Phillips, B. (2018). Not such silly sausages: evidence suggests northern quolls exhibit aversion to toads after training with toad sausages. Austral Ecology 43, 592–601.
| Not such silly sausages: evidence suggests northern quolls exhibit aversion to toads after training with toad sausages.Crossref | GoogleScholarGoogle Scholar |
Indigo, N. L., Jolly, C. J., Kelly, E., Smith, J., Webb, J. K., and Phillips, B. L. (2021). Effects of learning and adaptation on population viability. Conservation Biology , .
| Effects of learning and adaptation on population viability.Crossref | GoogleScholarGoogle Scholar | 33502048PubMed |
Jackson, S., Jackson, S. M., and Groves, C. (2015). ‘Taxonomy of Australian mammals.’ (CSIRO Publishing: Melbourne.)
Jacobsen, R., Howell, C., and Read, S. (2020). Australia’s Indigenous land and forest estate: separate reporting of Indigenous ownership, management and other special rights. Technical report 20.15. Australian Bureau of Agricultural and Resource Economics and Sciences, Department of Agriculture, Water and the Environment. Available at https://www.agriculture.gov.au/abares/forestsaustralia/publications/indigenous-estate-report
Johnston, M., O’Donoghue, M., Holdsworth, M., Robinson, S., Herrod, A., Eklom, K., Gigliotti, F., Bould, L., and Little, N. (2013). ‘Field assessment of the Curiosity® bait for managing feral cats in the Pilbara.’ (Arthur Rylah Institute for Environmental Research, Department of Sustainability and Environment: Heidelberg, Vic.)
Johnston, M., Algar, D., O’Donoghue, M., Morris, J., Buckmaster, T., and Quinn, J. (2020). Efficacy and welfare assessment of an encapsulated para-aminopropiophenone (PAPP) formulation as a bait-delivered toxicant for feral cats (Felis catus). Wildlife Research 47, 686–697.
| Efficacy and welfare assessment of an encapsulated para-aminopropiophenone (PAPP) formulation as a bait-delivered toxicant for feral cats (Felis catus).Crossref | GoogleScholarGoogle Scholar |
Jolly, C. J., and Phillips, B. L. (2021). Effects of rapid evolution due to predator‐free conservation on endangered species recovery. Conservation Biology 35, 383–385.
| Effects of rapid evolution due to predator‐free conservation on endangered species recovery.Crossref | GoogleScholarGoogle Scholar | 32378220PubMed |
Jolly, C. J., Kelly, E., Gillespie, G. R., Phillips, B., and Webb, J. K. (2018a). Out of the frying pan: reintroduction of toad‐smart northern quolls to southern Kakadu National Park. Austral Ecology 43, 139–149.
| Out of the frying pan: reintroduction of toad‐smart northern quolls to southern Kakadu National Park.Crossref | GoogleScholarGoogle Scholar |
Jolly, C. J., Webb, J. K., and Phillips, B. L. (2018b). The perils of paradise: an endangered species conserved on an island loses antipredator behaviours within 13 generations. Biology Letters 14, 20180222.
| The perils of paradise: an endangered species conserved on an island loses antipredator behaviours within 13 generations.Crossref | GoogleScholarGoogle Scholar | 29875211PubMed |
Jolly, C. J., Webb, J. K., Gillespie, G. R., and Phillips, B. L. (2020). Training fails to elicit behavioral change in a marsupial suffering evolutionary loss of antipredator behaviors. Journal of Mammalogy 101, 1108–1116.
| Training fails to elicit behavioral change in a marsupial suffering evolutionary loss of antipredator behaviors.Crossref | GoogleScholarGoogle Scholar |
Jolly, C. J., Smart, A. S., Moreen, J., Webb, J. K., Gillespie, G. R., and Phillips, B. L. (2021). Trophic cascade driven by behavioural fine-tuning as naïve prey rapidly adjust to a novel predator. Ecology 102, e03363.
| Trophic cascade driven by behavioural fine-tuning as naïve prey rapidly adjust to a novel predator.Crossref | GoogleScholarGoogle Scholar | 33830501PubMed |
Jones, M. E., Burnett, S., Claridge, A. W., Fancourt, B., Kortner, G., Morris, K., Peacock, D., Troy, S., and Woinarski, J. (2014). Australia’s surviving marsupial carnivores: threats and conservation. In ‘Carnivores of Australia: past, present and future’. (Eds A. Glen and C. Dickman) pp. 197–240. (CSIRO Publishing: Melbourne.)
Kelly, E. (2018). Targeted gene flow for conservation: northern quolls and the invasive cane toad. PhD thesis. School of BioSciences, The University of Melbourne.
Kelly, E., and Phillips, B. L. (2016). Targeted gene flow for conservation. Conservation Biology 30, 259–267.
| Targeted gene flow for conservation.Crossref | GoogleScholarGoogle Scholar | 26332195PubMed |
Kelly, E., and Phillips, B. L. (2017). Get smart: native mammal develops toad-smart behavior in response to a toxic invader. Behavioral Ecology 28, 854–858.
| Get smart: native mammal develops toad-smart behavior in response to a toxic invader.Crossref | GoogleScholarGoogle Scholar |
Kelly, E., and Phillips, B. L. (2019). Targeted gene flow and rapid adaptation in an endangered marsupial. Conservation Biology 33, 112–121.
| Targeted gene flow and rapid adaptation in an endangered marsupial.Crossref | GoogleScholarGoogle Scholar | 29896894PubMed |
Kelly, E., Phillips, B. L., and Webb, J. K. (2018). Taste overshadows less salient cues to elicit food aversion in endangered marsupial. Applied Animal Behaviour Science 209, 83–87.
| Taste overshadows less salient cues to elicit food aversion in endangered marsupial.Crossref | GoogleScholarGoogle Scholar |
Kelly, E., Jolly, C. J., Indigo, N., Smart, A., Webb, J., and Phillips, B. (2021). No outbreeding depression in a trial of targeted gene flow in an endangered Australian marsupial. Conservation Genetics 22, 23–33.
| No outbreeding depression in a trial of targeted gene flow in an endangered Australian marsupial.Crossref | GoogleScholarGoogle Scholar |
Kennedy, M. (1992) ‘Australasian marsupials and monotremes: an action plan for their conservation.’ (IUCN)
Kennedy, M., Phillips, B. L., Legge, S., Murphy, S. A., and Faulkner, R. A. (2012). Do dingoes suppress the activity of feral cats in northern Australia? Austral Ecology 37, 134–139.
| Do dingoes suppress the activity of feral cats in northern Australia?Crossref | GoogleScholarGoogle Scholar |
Kennedy, M., Kreplins, T., O’Leary, R., and Fleming, P. (2021). Responses of dingo (Canis familiaris) populations to landscape-scale baiting. Food Webs 27, e00195.
| Responses of dingo (Canis familiaris) populations to landscape-scale baiting.Crossref | GoogleScholarGoogle Scholar |
Kerle, J. A., and Burgman, M. (1984). Some aspects of the ecology of the mammal fauna of the Jabiluka Area. Northern Territory. Wildlife Research 11, 207–222.
| Some aspects of the ecology of the mammal fauna of the Jabiluka Area. Northern Territory.Crossref | GoogleScholarGoogle Scholar |
King, D. R. (1989). An assessment of the hazard posed to northern quolls (Dasyurus hallucatus) by aerial baiting with 1080 to control dingoes. Australian Wildlife Research 16, 569–574.
| An assessment of the hazard posed to northern quolls (Dasyurus hallucatus) by aerial baiting with 1080 to control dingoes.Crossref | GoogleScholarGoogle Scholar |
Kitchener, D., Keller, L., Chapman, A., McKenzie, N., Start, A., and Kenneally, K. (1981). Observations on mammals of the Mitchell Plateau area, Kimberley, Western Australia. In ‘Biological Survey of Mitchell Plateau and Admiralty Gulf, Kimberley, Western Australia.’ pp. 123–168. (Western Australian Museum: Perth.)
Knight, A. T., Cowling, R. M., Rouget, M., Balmford, A., Lombard, A. T., and Campbell, B. M. (2008). Knowing but not doing: selecting priority conservation areas and the research–implementation gap. Conservation Biology 22, 610–617.
| Knowing but not doing: selecting priority conservation areas and the research–implementation gap.Crossref | GoogleScholarGoogle Scholar | 18477033PubMed |
Leahy, L., Legge, S. M., Tuft, K., McGregor, H. W., Barmuta, L. A., Jones, M. E., and Johnson, C. N. (2016). Amplified predation after fire suppresses rodent populations in Australia’s tropical savannas. Wildlife Research 42, 705–716.
| Amplified predation after fire suppresses rodent populations in Australia’s tropical savannas.Crossref | GoogleScholarGoogle Scholar |
Legge, S., Smith, J. G., James, A., Tuft, K. D., Webb, T., and Woinarski, J. C. (2019). Interactions among threats affect conservation management outcomes: Livestock grazing removes the benefits of fire management for small mammals in Australian tropical savannas. Conservation Science and Practice 1, e52.
| Interactions among threats affect conservation management outcomes: Livestock grazing removes the benefits of fire management for small mammals in Australian tropical savannas.Crossref | GoogleScholarGoogle Scholar |
Leo, V., Reading, R. P., Gordon, C., and Letnic, M. (2019). Apex predator suppression is linked to restructuring of ecosystems via multiple ecological pathways. Oikos 128, 630–639.
| Apex predator suppression is linked to restructuring of ecosystems via multiple ecological pathways.Crossref | GoogleScholarGoogle Scholar |
Lever, C. (2001). ‘The cane toad: the history and ecology of a successful colonist.’ (Westbury Academic and Scientific Publishing: Otley, West Yorkshire, UK.)
Marlow, N. J., Thomas, N. D., Williams, A. A., Macmahon, B., Lawson, J., Hitchen, Y., Angus, J., and Berry, O. (2015). Cats (Felis catus) are more abundant and are the dominant predator of woylies (Bettongia penicillata) after sustained fox (Vulpes vulpes) control. Australian Journal of Zoology 63, 18–27.
| Cats (Felis catus) are more abundant and are the dominant predator of woylies (Bettongia penicillata) after sustained fox (Vulpes vulpes) control.Crossref | GoogleScholarGoogle Scholar |
Maxwell, S., Burbidge, A. A., and Morris, K. (1996). Spotted-tailed Quoll (SE mainland and Tas); recovery outline. In ‘The Action Plan for Australian Marsupials and Monotremes.’ pp. 85–87. Environment Australia, Canberra, ACT.
McGregor, H. W., Cliff, H. B., and Kanowski, J. (2017). Habitat preference for fire scars by feral cats in Cape York Peninsula, Australia. Wildlife Research 43, 623–633.
| Habitat preference for fire scars by feral cats in Cape York Peninsula, Australia.Crossref | GoogleScholarGoogle Scholar |
McIlroy, J. (1981). The sensitivity of Australian animals to 1080 poison. II. Marsupial and eutherian carnivores. Wildlife Research 8, 385–399.
| The sensitivity of Australian animals to 1080 poison. II. Marsupial and eutherian carnivores.Crossref | GoogleScholarGoogle Scholar |
McIlroy, J. (1982). The sensitivity of Australian animals to 1080 poison. III. Marsupial and eutherian herbivores. Wildlife Research 9, 487–503.
| The sensitivity of Australian animals to 1080 poison. III. Marsupial and eutherian herbivores.Crossref | GoogleScholarGoogle Scholar |
McIlroy, J., Cooper, R., Gifford, E., Green, B., and Newgrain, K. (1986). The effect on wild dogs, Canis familiaris, of 1080 poisoning campaigns in Kosciusko National Park, NSW. Wildlife Research 13, 535–544.
| The effect on wild dogs, Canis familiaris, of 1080 poisoning campaigns in Kosciusko National Park, NSW.Crossref | GoogleScholarGoogle Scholar |
McKenzie, N., Chapman, A., and Youngson, W. (1975). Mammals of the prince regent river reserve, north-west Kimberley, Australia. Wildlife Research Bulletin 3, 69.
Moher, D., Liberati, A., Tetzlaff, J., and Altman, D. G. (2009). Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Annals of internal medicine 151, 264–269.
| Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement.Crossref | GoogleScholarGoogle Scholar | 19622511PubMed |
Molloy, S. W., Davis, R. A., Dunlop, J. A., and van Etten, E. J. (2017). Applying surrogate species presences to correct sample bias in species distribution models: a case study using the Pilbara population of the northern Quoll. Nature Conservation 18, 25.
| Applying surrogate species presences to correct sample bias in species distribution models: a case study using the Pilbara population of the northern Quoll.Crossref | GoogleScholarGoogle Scholar |
Moore, H. A., Dunlop, J. A., Valentine, L. E., Woinarski, J. C., Ritchie, E. G., Watson, D. M., and Nimmo, D. G. (2019). Topographic ruggedness and rainfall mediate geographic range contraction of a threatened marsupial predator. Diversity and Distributions 25, 1818–1831.
| Topographic ruggedness and rainfall mediate geographic range contraction of a threatened marsupial predator.Crossref | GoogleScholarGoogle Scholar |
Moore, H. A., Michael, D. R., Ritchie, E. G., Dunlop, J. A., Valentine, L. E., Hobbs, R. J., and Nimmo, D. G. (2021). A rocky heart in a spinifex sea: occurrence of an endangered marsupial predator is multiscale dependent in naturally fragmented landscapes. Landscape Ecology 36, 1359–1376.
| A rocky heart in a spinifex sea: occurrence of an endangered marsupial predator is multiscale dependent in naturally fragmented landscapes.Crossref | GoogleScholarGoogle Scholar |
Moro, D., Dunlop, J., and Williams, M. R. (2019). Northern quoll persistence is most sensitive to survivorship of juveniles. Wildlife Research 46, 165–175.
| Northern quoll persistence is most sensitive to survivorship of juveniles.Crossref | GoogleScholarGoogle Scholar |
Morris, K., Cowan, M., Angus, J., Anderson, H., Garretson, S., Algar, D., and Williams, M. (2015). The northern quoll cat bait uptake and survivorship study, Yarraloola Land Management Area, Pilbara Region, WA: Yandicoogina JSW and Oxbow Project, Threatened Species Offset Plan. Western Australian Department of Parks and Wildlife, Perth.
Nawab, J., Khan, S., Shah, M. T., Khan, K., Huang, Q., and Ali, R. (2015). Quantification of heavy metals in mining affected soil and their bioaccumulation in native plant species. International Journal of Phytoremediation 17, 801–813.
| Quantification of heavy metals in mining affected soil and their bioaccumulation in native plant species.Crossref | GoogleScholarGoogle Scholar | 26079739PubMed |
Nelson, J. E., and Gemmell, R. T. (2003). Birth in the northern quoll, Dasyurus hallucatus (Marsupialia: Dasyuridae). Australian Journal of Zoology 51, 187–198.
| Birth in the northern quoll, Dasyurus hallucatus (Marsupialia: Dasyuridae).Crossref | GoogleScholarGoogle Scholar |
NESP (2018). Our changing climate: How will rainfall change in Northern Australia over this century? Available at https://nespclimate.com.au/wp-content/uploads/2018/06/Northern-Australia-6pp_WEB.pdf
Oakwood, M. (1997). The ecology of the northern quoll, Dasyurus hallucatus. PhD thesis thesis. Australian National University, Canberra.
Oakwood, M. (2000). Reproduction and demography of the northern quoll, Dasyurus hallucatus, in the lowland savanna of northern Australia. Australian Journal of Zoology 48, 519–539.
| Reproduction and demography of the northern quoll, Dasyurus hallucatus, in the lowland savanna of northern Australia.Crossref | GoogleScholarGoogle Scholar |
Oakwood, M. (2002). Spatial and social organization of a carnivorous marsupial Dasyurus hallucatus (Marsupialia: Dasyuridae). Journal of Zoology 257, 237–248.
| Spatial and social organization of a carnivorous marsupial Dasyurus hallucatus (Marsupialia: Dasyuridae).Crossref | GoogleScholarGoogle Scholar |
Oakwood, M. (2004a). Death after sex. Biologist 51, 5–8.
Oakwood, M. (2004b). The effect of cane toads on a marsupial carnivore, the northern quoll, Dasyurus hallucatus. Parks Australia.
Oakwood, M., and Pritchard, D. (1999). Little evidence of toxoplasmosis in a declining species, the northern quoll (Dasyurus hallucatus). Wildlife Research 26, 329–333.
| Little evidence of toxoplasmosis in a declining species, the northern quoll (Dasyurus hallucatus).Crossref | GoogleScholarGoogle Scholar |
Oakwood, M., Bradley, A. J., and Cockburn, A. (2001). Semelparity in a large marsupial. Proceedings of the Royal Society of London. Series B: Biological Sciences 268, 407–411.
| Semelparity in a large marsupial.Crossref | GoogleScholarGoogle Scholar | 11270438PubMed |
Oakwood, M., Woinarski, J., and Burnett, S. (2016). Dasyurus hallucatus. The IUCN Red List of Threatened Species. Available at https://www.iucnredlist.org/species/6295/21947321
O'Bryan, C. J., Garnett, S. T., Fa, J. E., Leiper, I., Rehbein, J. A., Fernández-Llamazares, Á., Jackson, M. V., Jonas, H. D., Brondizio, E. S., Burgess, N. D., Robinson, C. J., Zander, K. K., Molnár, Z., Venter, O., and Watson, J. E. M. (2020). The importance of indigenous peoples' lands for the conservation of terrestrial mammals. Conservation Biology 35, 1002–1008.
| The importance of indigenous peoples' lands for the conservation of terrestrial mammals.Crossref | GoogleScholarGoogle Scholar | 32852067PubMed |
O’Donnell, S., Webb, J. K., and Shine, R. (2010). Conditioned taste aversion enhances the survival of an endangered predator imperilled by a toxic invader. Journal of Applied Ecology 47, 558–565.
| Conditioned taste aversion enhances the survival of an endangered predator imperilled by a toxic invader.Crossref | GoogleScholarGoogle Scholar |
Olds, L. G., Myers, C., Reside, J., Madani, G., Dudley, A., Potter, S., Martin, R., Boona, E., Waina, T., and Taggart, D. A. (2016). Small terrestrial mammals on Doongan Station, in the Northern Kimberley bioregion, Western Australia. Australian Mammalogy 38, 164–176.
| Small terrestrial mammals on Doongan Station, in the Northern Kimberley bioregion, Western Australia.Crossref | GoogleScholarGoogle Scholar |
Ondei, S., Prior, L. D., McGregor, H. W., Reid, A. M., Johnson, C. N., Vigilante, T., Goonack, C., Williams, D., and Bowman, D. M. (2021). Small mammal diversity is higher in infrequently compared with frequently burnt rainforest–savanna mosaics in the north Kimberley, Australia. Wildlife Research 48, 218–229.
| Small mammal diversity is higher in infrequently compared with frequently burnt rainforest–savanna mosaics in the north Kimberley, Australia.Crossref | GoogleScholarGoogle Scholar |
Palmer, R. (2019). Predator control baiting and monitoring program, Yarraloola and Red Hill, Pilbara Region, Western Australia. 2016 Annual Report – Year 2. Department of Biodiversity, Conservation and Attractions, Perth.
Perry, J. J., Vanderduys, E. P., and Kutt, A. S. (2015). More famine than feast: pattern and variation in a potentially degenerating mammal fauna on Cape York Peninsula. Wildlife Research 42, 475–487.
| More famine than feast: pattern and variation in a potentially degenerating mammal fauna on Cape York Peninsula.Crossref | GoogleScholarGoogle Scholar |
Peterson, A. T. (2006). Taxonomy is important in conservation: a preliminary reassessment of Philippine species-level bird taxonomy. Bird Conservation International 16, 155–173.
| Taxonomy is important in conservation: a preliminary reassessment of Philippine species-level bird taxonomy.Crossref | GoogleScholarGoogle Scholar |
Pickett, B. D., Wallace, E. M., Ridge, P. G., and Kauwe, J. S. (2020). Lingering taxonomic challenges hinder conservation and management of global bonefishes. Fisheries 45, 347–358.
| Lingering taxonomic challenges hinder conservation and management of global bonefishes.Crossref | GoogleScholarGoogle Scholar |
Pollock, K. H. (1980). ‘Capture-recapture models: a review of current methods, assumptions and experimental design.’ (Citeseer: North Carolina, USA.)
Pollock, A. (1999). Notes on status, distribution and diet of northern quoll Dasyurus hallucatus in the Mackay-Bowen area, mideastern Queensland. Australian Zoologist 31, 388–395.
| Notes on status, distribution and diet of northern quoll Dasyurus hallucatus in the Mackay-Bowen area, mideastern Queensland.Crossref | GoogleScholarGoogle Scholar |
Potter, S., Eldridge, M. D., Taggart, D. A., and Cooper, S. J. (2012). Multiple biogeographical barriers identified across the monsoon tropics of northern Australia: Phylogeographic analysis of the brachyotis group of rock‐wallabies. Molecular Ecology 21, 2254–2269.
| Multiple biogeographical barriers identified across the monsoon tropics of northern Australia: Phylogeographic analysis of the brachyotis group of rock‐wallabies.Crossref | GoogleScholarGoogle Scholar | 22417115PubMed |
Pullin, A. S., and Knight, T. M. (2005). Assessing conservation management’s evidence base: a survey of management‐plan compilers in the United Kingdom and Australia. Conservation Biology 19, 1989–1996.
| Assessing conservation management’s evidence base: a survey of management‐plan compilers in the United Kingdom and Australia.Crossref | GoogleScholarGoogle Scholar |
Pulsford, S. A., Lindenmayer, D. B., and Driscoll, D. A. (2016). A succession of theories: purging redundancy from disturbance theory. Biological Reviews 91, 148–167.
| A succession of theories: purging redundancy from disturbance theory.Crossref | GoogleScholarGoogle Scholar | 25428521PubMed |
Radford, I. J. (2012). Threatened mammals become more predatory after small‐scale prescribed fires in a high‐rainfall rocky savanna. Austral Ecology 37, 926–935.
| Threatened mammals become more predatory after small‐scale prescribed fires in a high‐rainfall rocky savanna.Crossref | GoogleScholarGoogle Scholar |
Radford, I. J., and Andersen, A. N. (2012). Effects of fire on grass‐layer savanna macroinvertebrates as key food resources for insectivorous vertebrates in northern Australia. Austral Ecology 37, 733–742.
| Effects of fire on grass‐layer savanna macroinvertebrates as key food resources for insectivorous vertebrates in northern Australia.Crossref | GoogleScholarGoogle Scholar |
Radford, I. J., Dickman, C. R., Start, A. N., Palmer, C., Carnes, K., Everitt, C., Fairman, R., Graham, G., Partridge, T., and Thomson, A. (2014). Mammals of Australia’s tropical savannas: a conceptual model of assemblage structure and regulatory factors in the Kimberley region. PloS One 9, e92341.
| Mammals of Australia’s tropical savannas: a conceptual model of assemblage structure and regulatory factors in the Kimberley region.Crossref | GoogleScholarGoogle Scholar | 24670997PubMed |
Radford, I. J., Gibson, L. A., Corey, B., Carnes, K., and Fairman, R. (2015). Influence of fire mosaics, habitat characteristics and cattle disturbance on mammals in fire-prone savanna landscapes of the northern Kimberley. PLoS One 10, .
| Influence of fire mosaics, habitat characteristics and cattle disturbance on mammals in fire-prone savanna landscapes of the northern Kimberley.Crossref | GoogleScholarGoogle Scholar | 26121581PubMed |
Radford, I. J., Woolley, L.-A., Corey, B., Vigilante, T., Hatherley, E., Fairman, R., Carnes, K., and Start, A. N. (2020). Prescribed burning benefits threatened mammals in northern Australia. Biodiversity and Conservation 29, 2985–3007.
| Prescribed burning benefits threatened mammals in northern Australia.Crossref | GoogleScholarGoogle Scholar |
Ralls, K., Sunnucks, P., Lacy, R. C., and Frankham, R. (2020). Genetic rescue: A critique of the evidence supports maximizing genetic diversity rather than minimizing the introduction of putatively harmful genetic variation. Biological Conservation 251, 108784.
| Genetic rescue: A critique of the evidence supports maximizing genetic diversity rather than minimizing the introduction of putatively harmful genetic variation.Crossref | GoogleScholarGoogle Scholar |
Rankmore, B. R. (2006). ‘Impacts of habitat fragmentation on the vertebrate fauna of the tropical savannas of Northern Australia; with special reference to medium-sized mamals.’ (Charles Darwin University.)
Rankmore, B., Griffiths, A., Woinarski, J., Ganambarr, B., Taylor, R., Brennan, K., Firestone, K., and Cardoso, M. (2008). Island translocation of the northern quoll Dasyurus hallucatus as a conservation response to the spread of the cane toad Chaunus (Bufo) marinus in the Northern Territory, Australia. Report to the Australian Government’s Natural Heritage Trust. Department of Natural Resources, Environment and the Arts, Darwin.
Read, J. L., Bowden, T., Hodgens, P., Hess, M., McGregor, H., and Moseby, K. (2019). Target specificity of the Felixer grooming “trap”. Wildlife Society Bulletin 43, 112–120.
| Target specificity of the Felixer grooming “trap”.Crossref | GoogleScholarGoogle Scholar |
Rew-Duffy, M., Cameron, S. F., Freeman, N. J., Wheatley, R., Latimer, J. M., and Wilson, R. S. (2020). Greater agility increases probability of survival in the endangered northern quoll. Journal of Experimental Biology 223, .
| Greater agility increases probability of survival in the endangered northern quoll.Crossref | GoogleScholarGoogle Scholar |
Ringma, J., Legge, S., Woinarski, J., Radford, J., Wintle, B., and Bode, M. (2018). Australia’s mammal fauna requires a strategic and enhanced network of predator-free havens. Nature Ecology & Evolution 2, 410.
| Australia’s mammal fauna requires a strategic and enhanced network of predator-free havens.Crossref | GoogleScholarGoogle Scholar |
Ritchie, E. G., and Johnson, C. N. (2009). Predator interactions, mesopredator release and biodiversity conservation. Ecology Letters 12, 982–998.
| Predator interactions, mesopredator release and biodiversity conservation.Crossref | GoogleScholarGoogle Scholar | 19614756PubMed |
Rose, D. C., Amano, T., González-Varo, J. P., Mukherjee, N., Robertson, R. J., Simmons, B. I., Wauchope, H. S., and Sutherland, W. J. (2019). Calling for a new agenda for conservation science to create evidence-informed policy. Biological Conservation 238, 108222.
| Calling for a new agenda for conservation science to create evidence-informed policy.Crossref | GoogleScholarGoogle Scholar |
Sabath, M. D., Boughton, W. C., and Easteal, S. (1981). Expansion of the range of the introduced toad Bufo marinus in Australia from 1935 to 1974. Copeia , 676–680.
| Expansion of the range of the introduced toad Bufo marinus in Australia from 1935 to 1974.Crossref | GoogleScholarGoogle Scholar |
Salafsky, N., Boshoven, J., Burivalova, Z., Dubois, N. S., Gomez, A., Johnson, A., Lee, A., Margoluis, R., Morrison, J., and Muir, M. (2019). Defining and using evidence in conservation practice. Conservation Science and Practice 1, e27.
| Defining and using evidence in conservation practice.Crossref | GoogleScholarGoogle Scholar |
Saunders, G. R., Gentle, M. N., and Dickman, C. R. (2010). The impacts and management of foxes Vulpes vulpes in Australia. Mammal Review 40, 181–211.
| The impacts and management of foxes Vulpes vulpes in Australia.Crossref | GoogleScholarGoogle Scholar |
Schmitt, L., Bradley, A., Kemper, C., Kitchener, D., Humphreys, W., and How, R. (1989). Ecology and physiology of the northern quoll, Dasyurus hallucatus (Marsupialia, Dasyuridae), at Mitchell Plateau, Kimberley, Western Australia. Journal of Zoology 217, 539–558.
| Ecology and physiology of the northern quoll, Dasyurus hallucatus (Marsupialia, Dasyuridae), at Mitchell Plateau, Kimberley, Western Australia.Crossref | GoogleScholarGoogle Scholar |
Shine, R. (2010). The ecological impact of invasive cane toads (Bufo marinus) in Australia. The Quarterly Review of Biology 85, 253–291.
| The ecological impact of invasive cane toads (Bufo marinus) in Australia.Crossref | GoogleScholarGoogle Scholar | 20919631PubMed |
Southwell, D., Tingley, R., Bode, M., Nicholson, E., and Phillips, B. L. (2017). Cost and feasibility of a barrier to halt the spread of invasive cane toads in arid Australia: incorporating expert knowledge into model‐based decision‐making. Journal of Applied Ecology 54, 216–224.
| Cost and feasibility of a barrier to halt the spread of invasive cane toads in arid Australia: incorporating expert knowledge into model‐based decision‐making.Crossref | GoogleScholarGoogle Scholar |
Spencer, P., How, R., Hillyer, M., Cook, A., Morris, K., Stevenson, C., and Umbrello, L. (2013). Genetic analysis of northern quolls from the Pilbara region of Western Australia. Murdoch University: Perth. Final report.
Spencer, P. B., Sandover, S., Nihill, K., Wale, C. H., How, R. A., and Schmitt, L. H. (2017). Living in isolation: ecological, demographic and genetic patterns in northern Australia’s top marsupial predator on Koolan Island. Australian Mammalogy 39, 17–27.
| Living in isolation: ecological, demographic and genetic patterns in northern Australia’s top marsupial predator on Koolan Island.Crossref | GoogleScholarGoogle Scholar |
Steffen, W. (2009). ‘Australia’s biodiversity and climate change.’ (CSIRO Publishing: Melbourne.)
Stobo‐Wilson, A. M., Stokeld, D., Einoder, L. D., Davies, H. F., Fisher, A., Hill, B. M., Mahney, T., Murphy, B. P., Stevens, A., and Woinarski, J. C. (2020). Habitat structural complexity explains patterns of feral cat and dingo occurrence in monsoonal Australia. Diversity and Distributions 26, 832–842.
| Habitat structural complexity explains patterns of feral cat and dingo occurrence in monsoonal Australia.Crossref | GoogleScholarGoogle Scholar |
Telfer, W. R., and Garde, M. J. (2006). Indigenous knowledge of rock kangaroo ecology in western Arnhem Land, Australia. Human Ecology 34, 379–406.
| Indigenous knowledge of rock kangaroo ecology in western Arnhem Land, Australia.Crossref | GoogleScholarGoogle Scholar |
Thomas, O. (1926). LXVII. – The local races of Dasyurus hallucatus. Annals and Magazine of Natural History 18, 543–544.
| LXVII. – The local races of Dasyurus hallucatus.Crossref | GoogleScholarGoogle Scholar |
Thomas, H., Cameron, S. F., Campbell, H. A., Micheli-Campbell, M. A., Kirke, E. C., Wheatley, R., and Wilson, R. S. (2021). Rocky escarpment versus savanna woodlands: comparing diet and body condition as indicators of habitat quality for the endangered northern quoll (Dasyurus hallucatus). Wildlife Research , .
| Rocky escarpment versus savanna woodlands: comparing diet and body condition as indicators of habitat quality for the endangered northern quoll (Dasyurus hallucatus).Crossref | GoogleScholarGoogle Scholar |
Thomson, P. (1986). The effectiveness of aerial baiting for the control of dingoes in north-western Australia. Wildlife Research 13, 165–176.
| The effectiveness of aerial baiting for the control of dingoes in north-western Australia.Crossref | GoogleScholarGoogle Scholar |
Tingley, R., Phillips, B. L., Letnic, M., Brown, G. P., Shine, R., and Baird, S. J. (2013). Identifying optimal barriers to halt the invasion of cane toads Rhinella marina in arid Australia. Journal of Applied Ecology 50, 129–137.
| Identifying optimal barriers to halt the invasion of cane toads Rhinella marina in arid Australia.Crossref | GoogleScholarGoogle Scholar |
Tingley, R., Ward-Fear, G., Schwarzkopf, L., Greenlees, M. J., Phillips, B. L., Brown, G., Clulow, S., Webb, J., Capon, R., and Sheppard, A. (2017). New weapons in the toad toolkit: a review of methods to control and mitigate the biodiversity impacts of invasive cane toads (Rhinella marina). The Quarterly Review of Biology 92, 123–149.
| New weapons in the toad toolkit: a review of methods to control and mitigate the biodiversity impacts of invasive cane toads (Rhinella marina).Crossref | GoogleScholarGoogle Scholar | 29562120PubMed |
TSSC (2005). Northern quoll (Dasyurus hallucatus). Available at http://www.environment.gov.au/biodiversity/threatened/assessments/dasyurus-hallucatus-2005
Turpin, J. M., and Bamford, M. J. (2014). A new population of the northern quoll (Dasyurus hallucatus) on the edge of the Little Sandy Desert, Western Australia. Australian Mammalogy 37, 86–91.
Twigg, L. E., Eldridge, S. R., Edwards, G. P., Shakeshaft, B. J., dePreu, N. D., and Adams, N. (2000). The longevity and efficacy of 1080 meat baits used for dingo control in central Australia. Wildlife Research 27, 473–481.
| The longevity and efficacy of 1080 meat baits used for dingo control in central Australia.Crossref | GoogleScholarGoogle Scholar |
Twigg, L. E., Martin, G. R., Eastman, A. F., and Kirkpatrick, W. E. (2003). Sensitivity of some Australian animals to sodium fluoroacetate (1080): additional species and populations, and some ecological considerations. Australian Journal of Zoology 51, 515–531.
| Sensitivity of some Australian animals to sodium fluoroacetate (1080): additional species and populations, and some ecological considerations.Crossref | GoogleScholarGoogle Scholar |
Ujvari, B., Oakwood, M., and Madsen, T. (2013). Queensland northern quolls are not immune to cane toad toxin. Wildlife Research 40, 228–231.
| Queensland northern quolls are not immune to cane toad toxin.Crossref | GoogleScholarGoogle Scholar |
Umbrello, L. S. (2018). Evolution and diversification of dasyurid marsupials of the Australian arid zone. PhD thesis. School of Biological Sciences, University of Western Australia.
Viacava, P., Blomberg, S. P., Sansalone, G., Phillips, M. J., Guillerme, T., Cameron, S. F., Wilson, R. S., and Weisbecker, V. (2020). Skull shape of a widely distributed, endangered marsupial reveals little evidence of local adaptation between fragmented populations. Ecology and Evolution 10, 9707–9720.
| Skull shape of a widely distributed, endangered marsupial reveals little evidence of local adaptation between fragmented populations.Crossref | GoogleScholarGoogle Scholar | 33005341PubMed |
von Takach, B., Scheele, B. C., Moore, H., Murphy, B. P., and Banks, S. C. (2020). Patterns of niche contraction identify vital refuge areas for declining mammals. Diversity and Distributions 26, 1467–1482.
| Patterns of niche contraction identify vital refuge areas for declining mammals.Crossref | GoogleScholarGoogle Scholar |
Ward, M. S., Simmonds, J. S., Reside, A. E., Watson, J. E., Rhodes, J. R., Possingham, H. P., Trezise, J., Fletcher, R., File, L., and Taylor, M. (2019). Lots of loss with little scrutiny: the attrition of habitat critical for threatened species in Australia. Conservation Science and Practice 1, e117.
| Lots of loss with little scrutiny: the attrition of habitat critical for threatened species in Australia.Crossref | GoogleScholarGoogle Scholar |
Webb, J., Legge, S., Tuft, K., Cremona, T., and Austin, C. (2015). Can we mitigate cane toad impacts on northern quolls? Final report. Charles Darwin University, Darwin. Available at http://www.nespnorthern.edu.au/wp-content/uploads/2015/10/4.1.35_can_we_mitigate_cane_toad_impacts_on_northern_quolls_-_final_report.pdf
Whiteley, A. R., Fitzpatrick, S. W., Funk, W. C., and Tallmon, D. A. (2015). Genetic rescue to the rescue. Trends in Ecology & Evolution 30, 42–49.
| Genetic rescue to the rescue.Crossref | GoogleScholarGoogle Scholar |
Woinarski, J. C. (2015). Critical‐weight‐range marsupials in northern A ustralia are declining: a commentary on Fisher et al. (2014). ‘The current decline of tropical marsupials in Australia: is history repeating?’ Global Ecology and Biogeography 24, 118–122.
| Critical‐weight‐range marsupials in northern A ustralia are declining: a commentary on Fisher et al. (2014). ‘The current decline of tropical marsupials in Australia: is history repeating?’Crossref | GoogleScholarGoogle Scholar |
Woinarski, J., and Westaway, J. (2008). Hollow formation in the Eucalyptus miniata–E. tetrodonta open forests and savanna woodlands of tropical northern Australia. Final report to Land and Water Australia. Department of Natural Resources, Environment, The Arts and Sport, Darwin.
Woinarski, J., Milne, D., and Wanganeen, G. (2001). Changes in mammal populations in relatively intact landscapes of Kakadu National Park, Northern Territory, Australia. Austral Ecology 26, 360–370.
| Changes in mammal populations in relatively intact landscapes of Kakadu National Park, Northern Territory, Australia.Crossref | GoogleScholarGoogle Scholar |
Woinarski, J., Watson, M., and Gambold, N. (2002). Vertebrate monitoring and resampling in Kakadu National Park. Parks and Wildlife Commission of the Northern Territory, Palmerston, NT.
Woinarski, J., Risler, J., and Kean, L. (2004). Response of vegetation and vertebrate fauna to 23 years of fire exclusion in a tropical Eucalyptus open forest, Northern Territory, Australia. Austral Ecology 29, 156–176.
| Response of vegetation and vertebrate fauna to 23 years of fire exclusion in a tropical Eucalyptus open forest, Northern Territory, Australia.Crossref | GoogleScholarGoogle Scholar |
Woinarski, J., Rankmore, B., Fisher, A., Brennan, K., and Milne, D. (2007). The natural occurrence of northern quolls .Dasyurus hallucatus on islands of the Northern Territory: assessment of refuges from the threat posed by cane toads Bufo marinus. Report to Natural Heritage Trust, 1–40.
Woinarski, J., Oakwood, M., Winter, J., Burnett, S., Milne, D., Foster, P., Myles, H., and Holmes, B. (2008). Surviving the toads: patterns of persistence of the northern quoll Dasyurus hallucatus in Queensland. Report to The Australian Government’s Natural Heritage Trust. Available at https://denr.nt.gov.au/__data/assets/pdf_file/0003/255081/qld_quolls_finalreport.pdf
Woinarski, J., Armstrong, M., Brennan, K., Fisher, A., Griffiths, A., Hill, B., Milne, D., Palmer, C., Ward, S., and Watson, M. (2010). Monitoring indicates rapid and severe decline of native small mammals in Kakadu National Park, northern Australia. Wildlife Research 37, 116–126.
| Monitoring indicates rapid and severe decline of native small mammals in Kakadu National Park, northern Australia.Crossref | GoogleScholarGoogle Scholar |
Woinarski, J., Ward, S., Mahney, T., Bradley, J., Brennan, K., Ziembicki, M., and Fisher, A. (2011a). The mammal fauna of the Sir Edward Pellew island group, Northern Territory, Australia: refuge and death-trap. Wildlife Research 38, 307–322.
| The mammal fauna of the Sir Edward Pellew island group, Northern Territory, Australia: refuge and death-trap.Crossref | GoogleScholarGoogle Scholar |
Woinarski, J. C., Legge, S., Fitzsimons, J. A., Traill, B. J., Burbidge, A. A., Fisher, A., Firth, R. S., Gordon, I. J., Griffiths, A. D., and Johnson, C. N. (2011b). The disappearing mammal fauna of northern Australia: context, cause, and response. Conservation Letters 4, 192–201.
| The disappearing mammal fauna of northern Australia: context, cause, and response.Crossref | GoogleScholarGoogle Scholar |
Woinarski, J., Braby, M., Burbidge, A., Coates, D., Garnett, S., Fensham, R., Legge, S., McKenzie, N., Silcock, J., and Murphy, B. (2019a). Reading the black book: the number, timing, distribution and causes of listed extinctions in Australia. Biological Conservation 239, 108261.
| Reading the black book: the number, timing, distribution and causes of listed extinctions in Australia.Crossref | GoogleScholarGoogle Scholar |
Woinarski, J. C., Legge, S. M., and Dickman, C. R. (2019b). ‘Cats in Australia: companion and killer.’ (CSIRO Publishing: Melbourne.)
Woolley, P. A., Krajewski, C., and Westerman, M. (2015). Phylogenetic relationships within Dasyurus (Dasyuromorphia: Dasyuridae): quoll systematics based on molecular evidence and male characteristics. Journal of Mammalogy 96, 37–46.
| Phylogenetic relationships within Dasyurus (Dasyuromorphia: Dasyuridae): quoll systematics based on molecular evidence and male characteristics.Crossref | GoogleScholarGoogle Scholar |
Wynn, M. L., Clemente, C., Nasir, A. F. A. A., and Wilson, R. S. (2015). Running faster causes disaster: trade-offs between speed, manoeuvrability and motor control when running around corners in northern quolls (Dasyurus hallucatus). Journal of Experimental Biology 218, 433–439.
| Running faster causes disaster: trade-offs between speed, manoeuvrability and motor control when running around corners in northern quolls (Dasyurus hallucatus).Crossref | GoogleScholarGoogle Scholar |
Wysong, M. L., Iacona, G. D., Valentine, L. E., Morris, K., and Ritchie, E. G. (2020). On the right track: placement of camera traps on roads improves detection of predators and shows non-target impacts of feral cat baiting. Wildlife Research 47, 557–569.
| On the right track: placement of camera traps on roads improves detection of predators and shows non-target impacts of feral cat baiting.Crossref | GoogleScholarGoogle Scholar |
Zhang, S.-j., Wang, G.-D., Ma, P., Zhang, L.-l., Yin, T.-T., Liu, Y.-h., Otecko, N. O., Wang, M., Ma, Y.-p., and Wang, L. (2020). Genomic regions under selection in the feralization of the dingoes. Nature Communications 11, 1–10.
| Genomic regions under selection in the feralization of the dingoes.Crossref | GoogleScholarGoogle Scholar |
Ziembicki, M., Woinarski, J., and Mackey, B. (2013). Evaluating the status of species using Indigenous knowledge: novel evidence for major native mammal declines in northern Australia. Biological Conservation 157, 78–92.
| Evaluating the status of species using Indigenous knowledge: novel evidence for major native mammal declines in northern Australia.Crossref | GoogleScholarGoogle Scholar |



