Seasonal truffle consumption by long-nosed bandicoots (Perameles nasuta) in a mixed rainforest–open forest community in north-eastern New South Wales
Karl VernesEcosystem Management, University of New England, Armidale, NSW 2351, Australia. Email: kvernes@une.edu.au
Australian Mammalogy 36(1) 113-115 https://doi.org/10.1071/AM13040
Submitted: 14 November 2013 Accepted: 11 December 2013 Published: 4 March 2014
Abstract
Scats of long-nosed bandicoots (Perameles nasuta) from north-eastern New South Wales were examined for seasonal occurrence of fungi. Fungus was detected in bandicoot diets in all seasons, but samples from autumn and winter were more likely to contain fungi, and more taxa were consumed in these seasons, compared with spring and summer. Individual scat samples also contained more spore types in autumn and winter than in spring and summer. My results support other work in temperate south-eastern Australia that indicate an autumn and winter peak in fungal availability, and a stronger focus on fungal consumption by mammals at this time of year.
Introduction
Mycophagous (‘fungus eating’) mammals are an important component of healthy forest ecosystems. These mammals excavate and consume below-ground mycorrhizal fungi, thereby providing a spore-dispersal mechanism for fungi that are vital symbionts to forest trees (Johnson 1996). Despite mycophagy by mammals receiving increasing attention in recent decades (Maser et al. 2008), much remains to be understood about the relationship between mammals, fungi and trees in Australian forests, including a basic understanding of which mammals consume and disperse fungal spores (Vernes 2007).
The long-nosed bandicoot (Perameles nasuta) is a common medium-sized mammal of wet forest communities in eastern Australia from southern Victoria to north-eastern Queensland (Dickman and Stodart 2008). Although P. nasuta is known to include fungi in its omnivorous diet, few studies have focussed specifically on the fungal diet of this species. Claridge (1993) revealed the mycophagous dietary habits of P. nasuta by showing that at two wet forest sites in south-eastern Australia, P. nasuta consumed at least 25 different fungal taxa, including many hypogeous truffle-forming types. From just two opportunistically collected scat samples, Reddell et al. (1997) also showed P. nasuta to have consumed a range of truffle-forming fungi in north-eastern Queensland. Focusing only on arbuscular fungi (Phylum Glomeromycota), McGee and Baczocha (1994) detected a further three fungal species in the diet of P. nasuta at Sydney Harbour National Park. Most recently, using a combination of stable-isotope analysis of blood and microscopic analysis of scats, Thums et al. (2005) showed that P. nasuta on the New South Wales central coast was mycophagous, and that more fungi occurred in the winter diet than the summer diet; however, dietary diversity was low, with only two fungal taxa identified.
This paper documents the seasonal changes in fungal consumption by P. nasuta in wet forest at Gibraltar Range and Washpool National Parks in north-eastern New south Wales. This is the first description of the fungal diet of P. nasuta in north-eastern New South Wales, and only one of a limited number that examines fungal consumption by the species anywhere in its range.
Methods
Fresh scat samples deposited by P. nasuta were collected from forest tracks at Gibraltar Range National Park (29°31′S, 152°18′E) and adjacent Washpool National Park (29°28′S, 152°20′E) on the Great Escarpment in north-eastern New South Wales at an elevation of ~1000 m (for a detailed description of the site, see Vernes et al. 2006). All samples came from areas that contained a mix of wet sclerophyll forest and warm temperate rainforest. Although P. nasuta was frequently seen during spotlighting surveys of the area, and had been trapped there on a few occasions (see Vernes et al. 2006), my inability to catch P. nasuta in traps as part of a larger study of mammal–fungal community dynamics constrained me to collecting scats from the forest floor in order to describe its fungal diet. Scats of P. nasuta have a smaller diameter (pers. obs.) than those of the northern brown bandicoot (Isoodon macrourus), which also occurs in the broader region (Vernes et al. 2006). However, I. macrourus has not been recorded from the wetter forest localities we worked in at Gibraltar Range and Washpool; rather, it has only been recorded much further to the north of our collection sites (see Atlas of Living Australia, http://www.ala.org.au). We never saw (by spotlight) or captured I. macrourus in the study area, but we did see P. nasuta regularly. For these reasons, I am confident my samples are from P. nasuta, not I. macrourus.
Scats were briefly softened in 5% KOH, macerated, and then rinsed through a 125-µm mesh. A few drops of the filtrate was then mixed on a glass slide with a few drops of Meltzer’s Reagent; when dry, the preparation was mounted in glycerine jelly and sealed with a glass coverslip. Slides were scanned at 400× magnification; if necessary, spores were observed at 1000× under oil immersion to confirm identification. Photographs of representative spore morphotypes were taken using an Olympus CX microscope with digital capture capability. Samples were also examined with a scanning electronic microscope (JEOL JSM-5600 SEM operating at 10 kV and 8–48-mm working distance) to obtain representative photographs of as many morphotypes as possible. Using available guides, published papers, and assistance from expert mycologists, identification based on morphological characters (size, shape, ornamentation, wall thickness and symmetry) was made to genus where possible, although some spores could be identified only to family. For a few spore types no identification could be reliably assigned.
Results
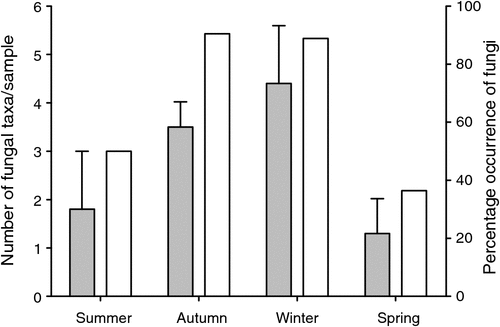
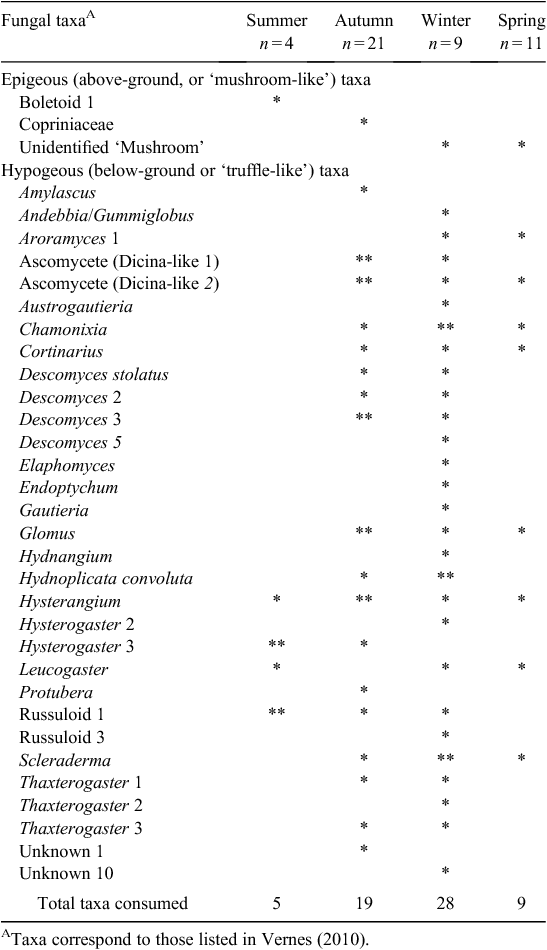
A total of 45 samples from P. nasuta was analysed for the occurrence of fungal spores; 33 of these contained fungus. P. nasuta consumed fungi in all seasons, but peaks in the proportion of scats containing fungi occurred in autumn and winter (Fig. 1). The number of spore types per sample changed significantly with season (F3,41 = 2.97, P = 0.043), and this also peaked in autumn and winter. At least 34 types of fungi, most of them hypogeous spore-forming taxa, were recorded in the diet (Table 1). About a third of these fungi (11 taxa) were recorded only in winter diets, which had considerably higher taxon richness (28 taxa), compared with autumn (19 taxa), spring (9 taxa) and summer (5 taxa) diets.
|
|
Discussion
These data (although limited in terms of sample sizes and uneven numbers of samples between seasons) show that P. nasuta consumes many species of fungi over the course of a year, including many hypogeous truffle-forming mycorrhizal taxa. Although P. nasuta is omnivorous, eating a wide variety of non-fungal foods, including plant material and invertebrates (O’Hara et al. 2012), my results support the assertion by Claridge (1993) that P. nasuta also consumes much fungus, and is an important spore disperser of hypogeous mycorrhizal fungi in eastern Australian forests.
These data also indicate that the fungal diet of P. nasuta in the study area shifted significantly over the course of a year, from low consumption of just a few species of fungus in spring and summer, to high consumption of many species in autumn and winter. Thums et al. (2005) determined that P. nasuta consumed more fungus in winter at their New South Wales central coast study site; my work supports this observation, but also adds support to a more general model of fungal consumption and dispersal by mammals in eastern Australia having a strong autumn and winter bias. In north-eastern New South Wales, several small mycophagous macropods consume fungus most often in autumn and winter, and the number of unique spore types per sample also peak at these times (Vernes 2010). This seasonal trend is also apparent in the tropics, where Vernes et al. (2001) showed that more fungal taxa were consumed by northern bettongs (Bettongia tropica) during the winter dry season than at other times of the year. Other researchers elsewhere in eastern Australia, working on a range of mycophagous mammals, have shown fungal consumption to be typically greatest in autumn and winter (Claridge et al. 1993a; Johnson 1994). These dietary observations reflect fruiting patterns in eastern Australia, with peaks in truffle production occurring during autumn and/or winter both in temperate regions (Claridge et al. 1993b, 2000; Danks et al. 2013) and in the tropics (Vernes et al. 2004; Abell et al. 2006).
At my Gibraltar Range study site in north-eastern New South Wales, previous work has revealed a diverse mycophagous mammal community that includes several forest rodents (bush rat, Rattus fuscipes; swamp rat, R. lutreolus; New Holland mouse, Pseudomys novaehollandiae; fawn footed melomys, Melomys cervinipes), a small dasyurid (brown antechinus, Antechinus stuartii), a range of medium-sized macropodids (red-necked pademelon, Thylogale thetis; parma wallaby, Macropus parma; swamp wallaby, Wallabia bicolor), the long-nosed potoroo (Potorous tridactylus) and two possums (bobuck, Trichosurus caninus; eastern pygmy possum, Cercartetus nanus) (Vernes and Dunn 2009; Vernes 2010; Vernes, unpubl. data). The current work confirms P. nasuta as an additional truffle spore-disperser at this site, and suggests that it is one of the more strongly mycophagous mammals in this community. Together with previous work, this paper highlights the complex interrelationships between mammals and fungi that are operating in Australian temperate forests.
Acknowledgements
I thank Stuart Green (UNE) for field assistance, and the NSW NPWS for accommodating us at Gibraltar Range National Park. Mike Castellano (USDA), James Trappe (Oregon State University), and Teresa Lebel (Royal Botanic Gardens, Melbourne) assisted with spore identification, for which I am most grateful, Pam Brook prepared the samples for analysis and Jim Ehrman (Digital Microscope Facility, Mount Allison University) operated the SEM. I also thank an anonymous reviewer and journal editor Ross Goldingay for valuable suggestions that improved the manuscript. This research was supported under the Australian Research Council’s Discovery Projects funding scheme (DP0557022).
References
Abell, S. E., Gadek, P. A., Pearce, C. A., and Congdon, B. C. (2006). Seasonal resource availability and use by an endangered tropical mycophagous marsupial. Biological Conservation 132, 533–540.| Seasonal resource availability and use by an endangered tropical mycophagous marsupial.Crossref | GoogleScholarGoogle Scholar |
Claridge, A. W. (1993). Fungal diet of the long-nosed bandicoot (Perameles nasuta) in south-eastern Australia. The Victorian Naturalist 110, 86–91.
Claridge, A. W., Tanton, M. T., and Cunningham, R. B. (1993a). Hypogeal fungi in the diet of the long-nosed potoroo (Potorous tridactylus) in mixed-species and regrowth eucalypt forest stands in south-eastern Australia. Wildlife Research 20, 321–338.
| Hypogeal fungi in the diet of the long-nosed potoroo (Potorous tridactylus) in mixed-species and regrowth eucalypt forest stands in south-eastern Australia.Crossref | GoogleScholarGoogle Scholar |
Claridge, A. W., Robinson, A. P., Tanton, M. T., and Cunningham, R. B. (1993b). Seasonal production of hypogeal fungal sporocarps in a mixed-species eucalypt forest stand in south-eastern Australia. Australian Journal of Botany 41, 145–167.
| Seasonal production of hypogeal fungal sporocarps in a mixed-species eucalypt forest stand in south-eastern Australia.Crossref | GoogleScholarGoogle Scholar |
Claridge, A. W., Barry, S. C., Cork, S. J., and Trappe, J. M. (2000). Diversity and habitat relationships of hypogeous fungi. II. Factors influencing the occurrence and number of taxa. Biodiversity and Conservation 9, 175–199.
| Diversity and habitat relationships of hypogeous fungi. II. Factors influencing the occurrence and number of taxa.Crossref | GoogleScholarGoogle Scholar |
Danks, M., Lebel, T., Vernes, K., and Andrew, N. (2013). Truffle-like fungi sporocarps in a eucalypt-dominated landscape: patterns in diversity and community structure. Fungal Diversity 58, 143–157.
| Truffle-like fungi sporocarps in a eucalypt-dominated landscape: patterns in diversity and community structure.Crossref | GoogleScholarGoogle Scholar |
Dickman, C. R., and Stodart, E. (2008). Long-nosed bandicoot, Perameles nasuta. In ‘The Mammals of Australia’. 3rd edn. (Eds S. Van Dyck and R. Strahan.) pp. 189–190. (Reed New Holland: Sydney.)
Johnson, C. N. (1994). Nutritional ecology of a mycophagous marsupial in relation to production of hypogeous fungi. Ecology 75, 2015–2021.
| Nutritional ecology of a mycophagous marsupial in relation to production of hypogeous fungi.Crossref | GoogleScholarGoogle Scholar |
Johnson, C. N. (1996). Interactions between mammals and ectomycorrhizal fungi. Trends in Ecology & Evolution 11, 503–507.
| Interactions between mammals and ectomycorrhizal fungi.Crossref | GoogleScholarGoogle Scholar |
Maser, C., Claridge, A. W., and Trappe, J. M. (2008). ‘Trees, Truffles, and Beasts: How Forests Function.’ (Rutgers University Press: New Brunswick, NJ.)
McGee, P. A., and Baczocha, N. (1994). Sporocarpic Endogonales and Glomales in the scats of Rattus and Perameles. Mycological Research 98, 246–249.
| Sporocarpic Endogonales and Glomales in the scats of Rattus and Perameles.Crossref | GoogleScholarGoogle Scholar |
O’Hara, P. J., Murray, P. J., and Klieve, A. V. (2012). Review of the nutrition of Australian peramelid marsupials. Australian Mammalogy 34, 133–144.
| Review of the nutrition of Australian peramelid marsupials.Crossref | GoogleScholarGoogle Scholar |
Reddell, P., Spain, A. V., and Hopkins, M. (1997). Dispersal of spores of mycorrhizal fungi in scats of native mammals in tropical forests of northeastern Australia. Biotropica 29, 184–192.
| Dispersal of spores of mycorrhizal fungi in scats of native mammals in tropical forests of northeastern Australia.Crossref | GoogleScholarGoogle Scholar |
Thums, M., Klaassen, M., and Hume, I. D. (2005). Seasonal changes in the diet of the long-nosed bandicoot (Perameles nasuta) assessed by analysis of faecal scats and of stable isotopes in blood. Australian Journal of Zoology 53, 87–93.
| Seasonal changes in the diet of the long-nosed bandicoot (Perameles nasuta) assessed by analysis of faecal scats and of stable isotopes in blood.Crossref | GoogleScholarGoogle Scholar |
Vernes, K. (2007). Are diverse mammal communities important for maintaining plant–fungal associations and ecosystem health? Australasian Plant Conservation 15, 16–18.
Vernes, K. (2010). Mycophagy in a community of macropodoid species. In ‘Macropods: the Biology of Kangaroos, Wallabies and Rat-kangaroos’. (Eds G. M. Coulson, and M. D. B. Eldridge.) pp. 155–169. (CSIRO Publishing: Melbourne.)
Vernes, K., and Dunn, L. (2009). Mammal mycophagy and fungal spore dispersal across a steep environmental gradient in eastern Australia. Austral Ecology 34, 69–76.
| Mammal mycophagy and fungal spore dispersal across a steep environmental gradient in eastern Australia.Crossref | GoogleScholarGoogle Scholar |
Vernes, K., Castellano, M., and Johnson, C. N. (2001). Effects of season and fire on the diversity of hypogeous fungi consumed by a tropical mycophagous marsupial. Journal of Animal Ecology 70, 945–954.
| Effects of season and fire on the diversity of hypogeous fungi consumed by a tropical mycophagous marsupial.Crossref | GoogleScholarGoogle Scholar |
Vernes, K., Johnson, C. N., and Castellano, M. A. (2004). Fire-related changes in hypogeous sporocarp biomass at foraging points used by a tropical mycophagous marsupial, the northern bettong (Bettongia tropica). Mycological Research 108, 1438–1446.
| Fire-related changes in hypogeous sporocarp biomass at foraging points used by a tropical mycophagous marsupial, the northern bettong (Bettongia tropica).Crossref | GoogleScholarGoogle Scholar | 15757180PubMed |
Vernes, K., Green, S., Howes, A., and Dunn, L. (2006). Species richness and habitat associations of non-flying mammals in Gibraltar Range National Park. Proceedings of the Linnean Society of New South Wales 127, 93–105.


