Burning poop: chemical composition and carbon dynamics of large herbivore dung burned in African savanna fires
C. Sánchez-García A , C. Santín A B * , T. Strydom C , X. L. Otero D and S. H. Doerr AA
B
C
D
Abstract
Fire and herbivores are essential to savanna ecosystems, consuming vegetation and recycling nutrients. Fire volatilises some elements and makes others readily available through ash, while herbivores redistribute nutrients via dung (excrement, faeces).
We investigate, for the first time, fire’s role in consuming dung and affecting nutrient cycling.
We examined the chemical characteristics of wild large herbivore dung (buffalo, elephant, giraffe, wildebeest, zebra) burned during African savanna fires (Kruger National Park, South Africa) and estimated carbon and nutrients losses from dung burning.
Smouldering combustion of dung led to high carbon loss to the atmosphere (C: 41% and 4.1% in unburned and burned dung) and high enrichment of nutrients (e.g. Ca, P) and metals (e.g. Cu, Fe, Zn) in the burned residue. Flaming combustion of grass resulted in lower carbon loss (C: 43% and 23% in vegetation and ash), leaving more carbon in the ash and lower relative enrichment of other nutrients and metals.
Burned dung forms nutrient hotspots with physicochemical characteristics distinct from vegetation ash.
Taking dung from wild or domestic herbivores into account in fuel inventories can improve estimations of fire-related carbon emissions and provide better understanding of fire impacts on nutrients cycling.
Keywords: Africa, biogeochemical cycles, carbon emissions, droppings, dung, grass fires, herbivores, manure, nutrient cycling, pellets, savannas, smouldering.
Background
Fire is a crucial ecological driver in savannas. The high fire frequency characteristic of these ecosystems regulates the balance between grass and trees coverage (Van Langevelde et al. 2003). Fire also acts as a key recycling agent, contributing to nutrient cycling and mobilising nutrients in various ways (Pausas and Bond 2020). Fire accelerates the mineralisation of organic matter, releasing and increasing the bioavailability of most nutrients that are part of the burned material. Although some nutrients previously stored in the organic matter can be transported over long distances as particulates and gases via smoke, other fire-affected nutrients remain on site in the resultant ash (i.e. ‘the particulate residue remaining or deposited on the ground, from the burning of wildland fuels and consisting of mineral materials and charred organic components’; Bodí et al. 2014, p. 104). Within the ash, certain elements become readily available for plants and animals. This is particularly relevant in savanna environments where soils are usually highly deficient in a range of nutrients like nitrogen (N), phosphorus (P) or calcium (Ca) (Augustine et al. 2003; Pellegrini 2016).
Along with fire, the presence of large mammalian herbivores also plays a vital role shaping the savanna ecosystems (Karp et al. 2024). These herbivores, found in high densities in many African savannas, regulate plant communities by consuming vegetation and contribute to nutrient cycling and redistribution by eating biomass and dispersing nutrients across the landscape through dung (Augustine and Frank 2001; Veldhuis et al. 2018). The concentration of nutrients in the dung can enrich soils and influence seed germination and plant growth, as well as plant community and diversity (Sitters and Olde Venterink 2021a; Guevara-Torres and Facelli 2023). After dung deposition, decomposers like dung beetles and other macrodetritivores contribute to the mineralisation and spatial distribution of dung nutrients across the landscape. Estimates suggest that within 4 days of dung deposition, approximately a quarter of the dung’s dry weight is redistributed by these organisms (Freymann et al. 2008; Veldhuis et al. 2018).
Several studies (Table 1) have assessed the chemical characteristics of herbivore dung from different regions and ecosystems (e.g. African savannas, Australian grasslands, Dutch nature reserves) but have focused their analyses mostly on carbon (C), N and P concentrations in a limited number of mammals such as elephant (e.g. Dougall 1963; Anderson and Coe 1974; Masunga et al. 2006; Stanbrook 2018; Sandhage-Hofmann et al. 2021), zebra, giraffe (e.g. Le Roux et al. 2020; Sitters and Olde Venterink 2021a), kangaroo, sheep, cattle, deer and rabbit (e.g. Holter 2016; Valdés-Correcher et al. 2019; Guevara-Torres and Facelli 2023). A small number of studies have also analysed other nutrients like Ca, Mg, Na and metals like Fe, Cu, Mn, Zn and Al (e.g. Masunga et al. 2006; Guevara-Torres and Facelli 2023). Herbivore dung is a well-known fuel source used for centuries by humans in cooking or heating (Miller 1984). Given the substantial presence of large herbivores across African savanna landscapes, these features also make dung a potentially important, but to date mostly unquantified, additional fuel component for landscape fires.
| Location | Animal | pH | C | N | P | K | Ca | Mg | Na | Fe | Cu | Mn | Zn | Al | C:N | C:P | N:P | Publication | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (%) | (mg kg−1 dung) | ||||||||||||||||||
| Nature reserves in The Netherlands and Belgium | European bison | 44.5 | 1.13 | 2130 | – | – | – | – | – | – | – | – | – | 39.8 | 224 | 5.67 | Valdés-Correcher et al. (2019) | ||
| Cow | 48 | 1.06 | 1670 | – | – | – | – | – | – | – | – | – | 45.7 | 317 | 7 | ||||
| Horse | 47.3 | 1.07 | 1290 | – | – | – | – | – | – | – | – | – | 44.2 | 376 | 8.46 | ||||
| Fallow deer | 43.2 | 1.97 | 2760 | – | – | – | – | – | – | – | – | – | 22.5 | 187 | 8.65 | ||||
| Rabbit | 43.8 | 1.66 | 1010 | – | – | – | – | – | – | – | – | – | 26.4 | 462 | 17.4 | ||||
| African savanna (Kenya) | Zebra | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 3.5 | Sitters and Olde Venterink (2021a) | ||
| Giraffe | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 6.4 | ||||
| Sub-Saharan savanna | Elephant | 39 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | Sandhage-Hofmann et al. (2021) | ||
| Aberdare NP (Kenya) | Elephant | 36.75 | 1.76 | – | – | – | – | – | – | – | – | – | – | 20.9 | – | – | Stanbrook (2018) | ||
| Tsavo NP (Kenya) | Elephant | 1.28 | – | – | – | – | – | – | – | – | – | – | – | – | – | Dougall (1963) | |||
| Tsavo NP (Kenya) | Elephant | 49.82 | 1.39 | 3900 | – | – | – | – | – | – | – | – | – | 36 | – | – | Anderson and Coe (1974) | ||
| Chobe NP (Botswana) | Elephant | 39.3 | 1.62 | 2800 | 16,600 | 10,200 | 2300 | 300 | 670 | 10.9 | 367 | 38.3 | 473.7 | 24 | – | – | Masunga et al. (2006) | ||
| Global | Cattle | 7.3 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | Holter (2016) | |
| Sheep | 8.3 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | |||
| Various ruminants | – | – | 2.51 (1.5–5.7) | – | – | – | – | – | – | – | – | – | – | 21 | – | – | |||
| Various non-ruminants | – | – | 1.88 (0.8–4.9) | – | – | – | – | – | – | – | – | – | – | – | – | – | |||
| Organic farms, wildlife parks, private stocks in Germany | Various | – | – | – | – | – | – | – | – | – | – | – | – | – | 26.8 | – | – | Frank et al. (2017) | |
| Grassland (South Australia) | Kangaroo | – | – | 1.79 | 4200 | 7000 | 16,900 | 4400 | 1500 | – | – | – | – | – | – | – | – | Guevara-Torres and Facelli (2023) | |
| Sheep | – | – | 2.98 | 9500 | 11,000 | 25,100 | 8600 | 3500 | – | – | – | – | – | – | – | – | |||
| Tropical forest in Mudamulai National Park (India) | Elephant | – | 40.8 | 1.1 | – | – | – | – | – | – | – | – | – | – | – | – | – | Chaudhary et al. (2020) | |
| Gaur | – | 38.9 | 1.6 | – | – | – | – | – | – | – | – | – | – | – | – | – | |||
| South African protected savanna | Various herbivores (grazers, browsers, mixed feeders) | – | – | 1.7–2.1 | 2800–6060 | – | – | – | – | – | – | – | – | – | – | – | 2.9–6.3 | Le Roux et al. (2020) | |
| Overall ranges (minimum and maximum values) | 36.8–49.8 | 1.06–2.9 | 1010–9500 | 7000–16,600 | 10,200–25,100 | 2300–8600 | 300–3500 | 20.9–45.7 | 187–462 | 3.5–17.4 | |||||||||
The ranges are provided in brackets (i.e. minimum and maximum) where more than one value is given for a given study and animal. NP, National Park
The intrinsic high fire recurrence in savannas results in the frequent modification of dung by burning. Observations during savanna fires suggest that dung undergoes a different burning process compared with the fine vegetation fuels that are predominant in savanna fires. Whereas fine fuels, such as grass or litter, tend to burn mostly by rapid flaming combustion, dung burns mostly via slow smouldering combustion, which often continues long after the flaming front has passed (Scholes et al. 1996; Wooster et al. 2011). The longer burning duration of smouldering combustion potentially allows a more complete volatilisation of organic compounds, and therefore, of C. Additionally, Wooster et al. (2011) noted that burning of animal dung in South African savannas could substantially alter fire emissions, with the chemical composition of the smoke plume changing compared with when only vegetation fuels were burned.
In recent decades, our understanding of the chemical composition of ash from the burning of wildland fuels (i.e. wildland fire ash) has gained traction and its role in redistributing C and other nutrients and metals across the landscape is now more widely recognised (Bodí et al. 2014; Sánchez-García et al. 2023; Girona-García et al. 2024). However, to our knowledge, the chemical characteristics of the burned dung have not been examined in detail to date. This study aims to determine the chemical composition of ash derived from burned large herbivore dung and its role in the C dynamics of African savanna fires. This should allow more accurate estimations of the overall C released during savanna fires given that they are the dominant source of global CO2 emissions from vegetation fires (~50%) owing to their high frequency and the large annual area burned (van Wees et al. 2022).
The objectives of this study are: (i) to examine the chemical characteristics of burned dung from large African savanna herbivores; and (ii) to quantify C losses from the combustion of herbivore dung and its role in the C fluxes of African savanna fires. Kruger National Park (KNP) is uniquely placed to be the focus of this study as it hosts one of the longest burning experiments on the planet, the Experimental Burn Plots (EBPs) trial, which has been running since 1954 with the aim of studying the effects of different fire regimes on South African savanna landscapes (Biggs et al. 2003). To the authors’ knowledge, dung loads have not been previously quantified in KNP. The third objective of this study is, therefore, (iii) to quantify herbivore dung loads and their contribution to the overall fuel loads during African savanna fires.
Research design and methods
Study sites
This study was performed in KNP, located in northeastern South Africa, covering an area of nearly 2 million ha. KNP is a dry savanna biome with hot semi-arid climate with humid and hot summer days. The rainy season here lasts from September to May. The EBPs trial in KNP comprise a total of 16 strings of 12–14 plots each, covering four major savanna landscapes (Fig. 1a). The EBPs are burned periodically with recurrences that vary between 1 and 6 years, and at different times of the year (late summer, autumn, late winter, following first spring rains and mid-summer; Biggs et al. 2003).
(a) Location of the Kruger National Park (KNP) in South Africa (left) and location of the Experimental Burn Plots (EBPs) in the park (right). The circles indicate the location of the four EBPs used in this study. (b) Experimental plot diagram for PB1, PB3, SB3 plots (dimensions for the MB1 plot in brackets). Solid lines represent the experimental transects (T1–T3) and dotted lines represent control transects (TA and TB). Dots along the control transects represent the location of destructive grass sampling. Asterisks along the experimental transects represent the locations of the thermocouples and the ash samples. (c) Thermocouple being installed in zebra dung (at PB1) before the experimental fire.
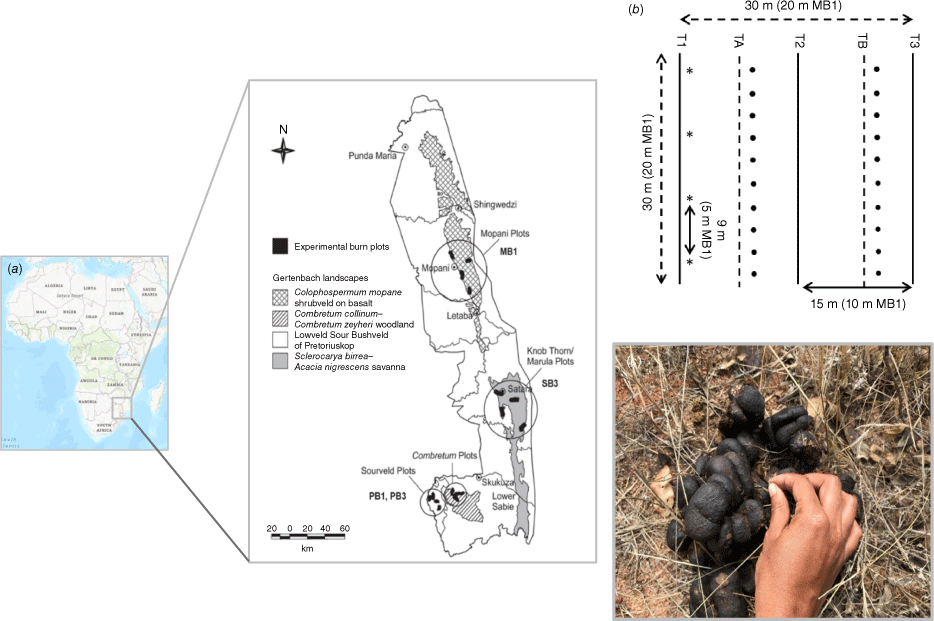
Four EBPs were selected for this study covering a range of savanna types and the two main geological substrates in KNP (sandy granitic and clayey basaltic; Table 2). Two plots were in the Pretoriuskop area (PB1, PB3), and two others in the Mopani (MB1) and Satara (SB3) areas. The selected plots represent two of the most common fire frequencies in these savannas, i.e. annually burned (PB1 and MB1), and triennially burned (PB3 and SB3). The experimental fires took place in August 2018, in the middle of the dry season, coinciding with the peak fire season in KNP.
| Plot | Area | Location (lat., long.) | Geology, soils | Dominant vegetation | Mean annual rainfall (mm) | Fire regime | ||
|---|---|---|---|---|---|---|---|---|
| Tree | Grass | |||||||
| PB1 | Pretoriuskop | 25°08′24′′S; 31°12′26′′E | Sandy granitic | Terminalia sericea, Dichrostachys cinerea | Hyperthelia dissoluta, Themeda triandra, Setaria sphacelata | 705 | Burnt annually | |
| PB3 | Pretoriuskop | 25°08′06′′S; 31°12′24′′E | Sandy granitic | Terminalia sericea, Dichrostachys cinerea | Hyperthelia dissoluta, Themeda triandra, Setaria sphacelata | 705 | Burnt triannually | |
| MB1 | Mopani | 23°33′48′′S; 31°27′24′′E | Clayey basaltic | Colophospermum mopane | Bothriochloa radicans | 451 | Burnt annually | |
| SB3 | Satara | 24°23′58″S; 31°44′12″E | Clayey basaltic | Acacia nigrescens, Sclerocarya birrea | Themeda triandra, Urochloa mosambicensis, Bothriochloa radicans | 507 | Burnt triannually | |
Experimental fire monitoring and sampling
The set-up for the experimental burns has been described in a previous publication (Sánchez-García et al. 2021) and it is therefore only summarised here. At each study site, a 30 × 30 m plot was set up within the selected EBPs before the experimental fires, except for Mopani where a 20 × 20 m plot had to be selected to ensure sufficient fuel continuity. At each plot, we selected three parallel transects (T1–T3, 30 m long, 15 m apart for PB1, PB3 and SB3, and 20 m long, 10 m apart for MB1) for fire monitoring and post-fire ash sampling. We also selected two control transects of the same length each situated parallell and 7.5 m apart from T1, T2 and T3 for PB1, PB3 and SB3, and 5 m apart for MB1 (Fig. 1b) for quantifying vegetation loads before fire (Fig. 1b). To quantify vegetation fuel loads, the grass was sampled in the two control transects using 50 × 50 cm sampling squares (10 sampling points per transect, one every 3 m; Fig. 1b). Owing to the absence of leaves and shrubs at the time of the fires, fuel loads from vegetation were restricted to the fine fuels only, specifically grass. Along each transect, we inserted K-type thermocouples and dataloggers (Lascar, Easylog) to monitor temperatures during the burns. These were installed in the soil surface (~1 cm depth) and within the grass (~50 cm above ground) at 9 m intervals in PB1, PB3 and SB3, and at 5 m intervals in MB1 (Fig. 1b, c).
Quantification and sampling of unburned (pre-fire) and burned dung were carried out, respectively, within a few hours before and after the fires. Before the fires, all dung from large herbivores (i.e. buffalo, elephant, giraffe, wildebeest and zebra) within the plots was identified, mapped and weighed. We also collected comparable unburned dung of similar freshness from adjacent areas for chemical and moisture content analyses (n = 11). For temperature monitoring, thermocouples were inserted in some in situ dung samples across the plots from different species (n = 9 for PB1 and PB3, and n = 6 for MB1 and SB3; Fig. 1c).
The fire characteristics at each plot are given in Table 3. A few hours after each fire, all dung within the respective plots was collected and weighed once it had stopped smouldering. We differentiated between burned dung and any dung that remained partially or fully unburned after the fires. On the same day as the experimental burns, we also collected the vegetation ash layer present on the soil surface using a 20 × 20 cm frame at the exact locations where thermocouples were placed in the soil (n = 12) (as described in Sánchez-García et al. 2021). All samples were air-dried to constant weight and stored in sealed containers in dry conditions in the laboratory.
| Plot | Fire date | Pre-fire dung moisture (%) | Atmospheric conditions | Tmax (°C) | Fire impacts on vegetation | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Wind speed (m s−1) | Air T (°C) | RH (%) | Soil surface | Grass | Dung | |||||
| PB1 | 19/08/18 | 23.3–36.8 | 2.3 | 26 | 41 | 40–225 | 484–744 | 32–795 | In both PB1 and PB3, the fire burned the entire experimental plot with complete combustion of fine fuels and no unburned grass left. Woody fuels were mostly unaffected and wood on the ground (down wood) and bark from standing trees and shrubs remained uncharred. | |
| PB3 | 19/08/18 | 9.9–110 | 2.3 | 31 | 30 | 40–182 | 651–918 | 22–825 | ||
| MB1 | 23/08/18 | 1.4–2.6 | 2.7 | 31 | 41 | 40–498 | 452–850 | 153–712 | In both MB1 and SB3, most (>90%) of the fine fuels on the ground were burned. In MB1, coarser woody fuels remained largely unaffected. No shrubs or trees were present in SB3. | |
| SB3 | 27/08/18 | 6.0–6.5 | 1.8 | 21 | 58 | 40–817 | 68–783 | 41–724 | ||
Dung and ash chemical analyses
The pH, electrical conductivity (EC) and total concentrations of major nutrients and metals were analysed in unburned (n = 8) and burned dung (n = 7), grass (n = 12) and vegetation ash samples (n = 12) using established methods. Briefly, pH and EC were analysed in water using a mass-to-water ratio of 1:20 after stirring for 5 min and waiting for 10 min (Buurman et al. 1996). Total C and N concentrations were determined using a Total Analyzer Leco TruSpec CHN (Leco Corp., St Joseph, MI, USA), whereas the total concentration of major nutrients and metals (Al, Ca, Co, Cr, Cu, Fe, Mg, Mn, Ni P, and Zn) was extracted in duplicate by adding 8 mL of a mixture of HNO3/HCl (3:5, v/v) in a 120 mL Teflon bomb containing 0.5 g of previously dried and ground sample and heating the mixture in an Ethos Plus microwave lab station. The efficiency of the extraction process was evaluated by analyzing certified reference material (MESS-3 and SOIL SO3), ranging from 90.1% of chromium (Cr) to 105% for lead (Pb) (Otero et al. 2016). The element concentration was measured by atomic absorption in a PerkinElmer PinAAcle 500 spectrometer. Total P was measured in the same extracts by colorimetry in a Jasco V360 spectrophotometer. All equipment used was washed thoroughly with HCl (5%) for at least 48 h, and then with ultrapure water (Milli Q).
An enrichment index (Ei), representing the change in nutrient, metal and metalloid concentration in dung and grass with fire, was calculated as follows: Ei = (concentration in burned dung or grass/concentration in unburned dung or grass) – 1; where Ei > 0 indicates that elements have undergone relative enrichment compared with their concentration in the unburned dung or grass, whereas Ei < 0 indicates that a loss of the element occurred in the burned dung or grass compared with its original concentration in the unburned dung or grass.
Data analysis
The Shapiro–Wilk test was used to test for normality in the data. The non-parametric Mann–Whitney U test was used to test for statistical differences in the chemical characteristics of unburned and burned dung, and vegetation ash (accepted at P < 0.05). The tests were performed in R (R Core Team 2014).
Results
Dung loads and burning behaviours
Before the fires, dung loads amounted, on average, to 130 kg ha−1, equivalent to 3% of the vegetation fuel loads (4225 kg ha−1) but varying across the selected EBPs, from 2% (40 kg ha−1) in SB3 to 6% (240 kg ha−1) in MB1 (Fig. 2a). Overall, the largest dung loads were observed for elephant and zebra (80 and 32 kg ha−1, respectively). However, the dung load composition varied across the plots. For instance, in PB1 and SB3, the highest dung loads were from zebra and elephant, whereas in PB3, it was zebra and buffalo. In MB1, elephant dung accounted for 72% of the total dung load (Fig. 2a).
(a) Dung loads (kg ha−1) before the experimental fires, and (b) unburned (U) and burned residue (B) dung loads after the experimental fires categorised by species in the four experimental burn plots. Note the different scale in the vertical axes.
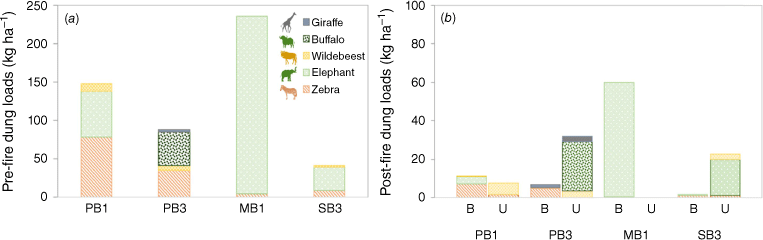
The fires led to substantial consumption of the dung (Figs 2b and 3), with a decrease of dung loads of ~85% compared with those found before the fires (mean: 20 kg ha−1) (Table 4). In most plots, some dung remained unburned (7 to 32% in PB1 and PB2, respectively; Fig. 3c), except at MB1, where nearly all the dung was consumed by the fires (Fig. 2b). Some elephant dung in MB1, however, had been used by termites and had a large soil component in it (Fig. 3d). This soil component was discounted from dung calculations, which introduces some uncertainty into the quantification of burned dung loads.
Herbivore dung after the experimental fires: (a) elephant, (b) zebra, (c) buffalo, (d) elephant used by termites with substantial mineral soil component left unburned.

| Material | Species | Loads across plots (kg ha−1) | pH | EC | C | N | P | Cu | Co | Zn | Ni | Mn | Cr | Ca | Mg | Fe | Al | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (µS cm−1) | (%) | (g kg−1) | (mg kg−1) | (g kg−1) | ||||||||||||||
| Unburnt dung | Buffalo (n = 1) | 0–43.9 | 7.4 | 2010 | 41.2 | 1.2 | 1.5 | 6.6 | 2.4 | 61.4 | 4.1 | 246.0 | 8.9 | 11.8 | 26.2 | 0.6 | 6.3 | |
| Elephant (n = 3) | 0–231.5 | 6.8–7.1 | 1558–2010 | 39.7–43.1 | 1.1–1.4 | 0.8–2.6 | 9.4–16.7 | 3.5–5.6 | 22.5–38.4 | 6.7–18.2 | 88.3–206 | 6.3–14.3 | 17.4–18.9 | 20.8–30.0 | 1.1–4.5 | 13.1–26.4 | ||
| Wildebeest (n = 2) | 0–9.1 | 7.4–7.5 | 1882–3160 | 39.6–41.1 | 1.1–1.3 | 1.4–2.9 | 6.7–8.9 | 2.1–5.03 | 53.6–60.7 | 4.3–5.6 | 158–174 | 7.7–26.2 | 10.0–10.2 | 15.4–28.5 | 1.1 | 7.2–11.5 | ||
| Zebra (n = 2) | 4.8–78.2 | 7.1–7.5 | 1115–1893 | 41.3–43.1 | 0.80–0.81 | 1.2–1.8 | 4.5–10.4 | 2.8–6.4 | 25.8–37.7 | 1.1–28.9 | 102–262 | 8.9–11.3 | 2.2–5.4 | 13.8–31.4 | 0.5–3.4 | 7.1–20.8 | ||
| Burnt dung | Buffalo (n = 1) | 0–1.8 | 9.9 | 15,580 | 5.8 | 0.5 | 12.4 | 33.2 | 11.5 | 270 | 16.7 | 1343.0 | 85.3 | 65.3 | 13.6 | 4.5 | 7.6 | |
| Elephant (n = 3) | 0–59.4 | 9.7–11.1 | 3260–15,730 | 2.6–4.4 | 0.21–0.26 | 5.6–7.6 | 41.4–81.1 | 15.0–41.6 | 135–149 | 34.2–325 | 853–1081 | 76.3–311 | 36.3–82.0 | 9.0–24.3 | 15.6–56.0 | 19.3–33.5 | ||
| Zebra (n = 3) | 0.6–7.3 | 9.8–10.9 | 2340–14,890 | 4.1–4.5 | 0.3–0.5 | 6.0–15.6 | 34.6–76.8 | 12.1–31.4 | 126–205 | 13.5–101 | 582–1729 | 84.4–237 | 23.1–35.1 | 8.7–9.9 | 5.9–54.0 | 10.2–32.1 | ||
| Overall Ei burnt dung | – | – | – | – | −0.9 | −0.7 | 4.4 | 5.1 | 4.4 | 3.1 | 7.8 | 5.1 | 12.8 | 3.0 | −0.5 | 13.7 | 0.5 | |
| Unburnt vegetation (n = 12) | – | 2559–6672 | – | – | 39.4–46.5 | 0.4–1.3 | 0.5–1.9 | 3.7–7.9 | 6.7–9.5 | 13.6–27.9 | 1.3–7.3 | 34.0–161.4 | 3.6–11.5 | 1.8–13.4 | 0.6–2.6 | 0.1–1.1 | 0.08–0.5 | |
| Vegetation ash (n = 12) | – | 384–897.4 | 9.6–10.8 | 1763–13,770 | 15.8–30.1 | 0.3–0.7 | 3.7–12.2 | 20.9–39.0 | 0.5–20.9 | 114–227 | 6.9–46.6 | 236–1687 | 39–84.6 | 15.6–48.4 | 6.4–17.9 | 4.6–8.7 | 3.4–12.7 | |
| Overall Ei vegetation ash | – | – | – | – | −0.5 | −0.4 | 5.9 | 3.9 | 0.0 | 7.8 | 4.2 | 9.7 | 6.9 | 6.6 | 6.2 | 15.5 | 33.6 | |
When n > 1, the ranges (i.e. minimum and maximum) are given. (Note: burned dung from wildebeest was not analysed.)
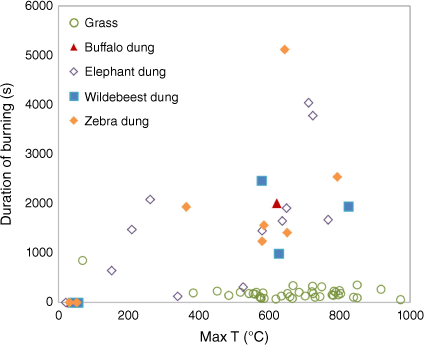
As expected, the type of combustion differed between dung and grass as indicated by the temperature profiles recorded during the fires (Fig. 4). Dung exhibited typical smouldering combustion characteristics, with lower average maximum temperatures (435°C) but longer burning durations (1780 s) compared with flaming combustion of grass (636°C and 174 s, respectively) (Fig. 4). These different fire behaviours resulted in the burned residues being also different, with ash from grass being darker in colour and ash from burned dung being lighter (Fig. 3a), which indicates the latter is the result of greater combustion completeness (Campbell et al. 2024).
Chemical characteristics of burned dung and nutrient enrichment
Among all the analysed nutrients in the burned dung, Ca, C, Fe, Al and Mg were found in the highest concentrations (mean: 47.2, 41.2, 27.3, 20.5 and 11.9 g kg−1, respectively), whereas the lowest concentrations were observed for Cr, Ni, Cu and Co (mean: 163, 92, 56 and 23 mg kg−1, respectively) (Fig. 5).
Total element content, pH and EC for the unburned (UD) and burned (DB) dung, and for unburned vegetation (UV) and vegetation ash data (VA). pH and EC data for VA were not available. Central line, bottom and top boundaries of the boxes are the median, 25th and 75th percentiles. Lines extending above and below the boxes represent maximum and minimum values. Dots are the outliers. Different letters above each boxplot (a–d) indicate significant differences between unburned and burned dung, unburned vegetation and vegetation ash, unburned dung and unburned vegetation, and burned dung and vegetation ash at P < 0.05.
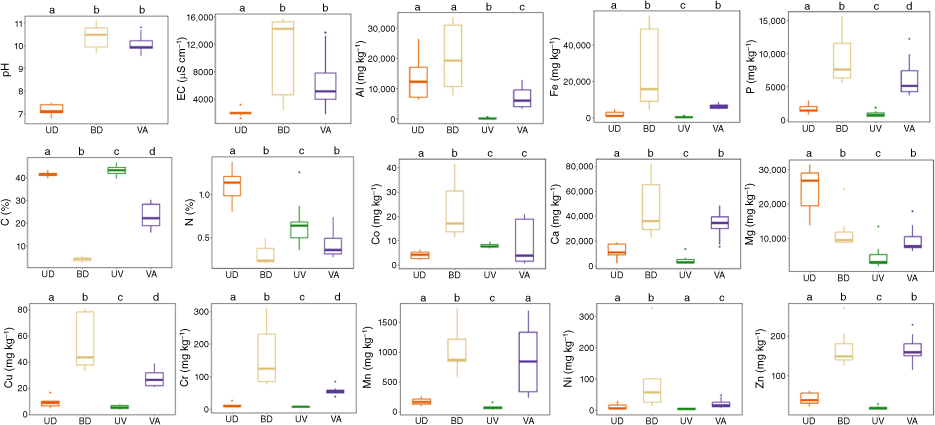
The total concentrations of all the major nutrients (C, N, Ca, Mg and P) were significantly different between unburned and burned dung (Fig. 5). C and N concentrations in the burned dung were an order of magnitude lower compared with the unburned dung (C: 41 and 4.1%, N: 1.1 and 0.3% in unburned and burned dung). Their corresponding enrichment factors were negative, at −0.9 for C and −0.7 for N. Surprisingly, the concentration of Mg also exhibited a decrease in burned dung with a mean value of 24.2 g kg−1 in unburned dung and 12.0 g kg−1 in burned dung.
In contrast, P and Ca were more than fourfold higher in burned compared with unburned dung (P: 1.7 and 9.2 g kg−1; Ca: 11.7 and 47.2 g kg−1 in unburned and burned dung, respectively) with positive enrichment factors of 4.4 and 3.0 respectively. The analysed metal concentrations (Al, Co, Cr, Cu, Fe, Mn, Ni and Zn) were all higher in burned than in unburned dung (Fig. 5), also showing positive enrichment indexes (Table 4). pH and EC increased significantly from 7.2 and 1955 µS cm−1 in unburned dung to 10.4 and 10,279 µS cm−1 in burned dung, respectively.
When compared with vegetation ash, the C content in the burned dung was an order of magnitude lower (4.1% vs 23%; Table 4). Average Mg concentration in burned dung did not differ much from vegetation ash (9.5 g kg−1); however, the enrichment indexes show Mg enrichment in vegetation ash and the opposite in burned dung (Table 4). Average Ca concentration in burned dung was similar to that observed in vegetation ash (33.7 g kg−1); however, vegetation ash showed an enrichment factor twice that of burned dung. Although the concentrations of P and metals like Cu, Co, Ni, Cr and Al were significantly higher in burned dung than in vegetation ash (which is related to the proportionally larger losses of C and N in the former during burning; Fig. 5), their enrichment indexes showed varying trends. Cu, Co, Ni and Cr showed greater enrichment in burned dung than in vegetation ash, whereas the opposite was observed for P and Al (Table 4).
The elements considered in this study did not show the same pattern when comparing unburned dung samples of the different herbivore species. Owing to the limited sample size available, meaningful statistical analyses were not feasible, so a quantitative description of these variations is provided here. In unburned dung, Cu concentration, for instance, was an order of magnitude higher in elephant dung (0.012 g kg−1) compared with other species (Table 4). Conversely, Ca was an order of magnitude lower in unburned zebra dung (3.8 g kg−1) than in the other species, whereas Fe concentration in unburned buffalo dung was an order of magnitude lower (0.6 g kg−1) than those in elephant, wildebeest and zebra dung. Elephant dung showed P and Mn concentrations an order of magnitude lower than buffalo and zebra dung (Table 4).
Carbon loads in dung and their losses during fire
Understanding how C loads differ among fuel types is key to predict C emissions associated with savanna ecosystems. In this study, C loads from vegetation fuels were two orders of magnitude higher than from herbivore dung (mean: 1842 and 53.7 kg C ha−1, respectively; Table 5). For both, grass and dung, the lowest C loads were observed in SB3 (grass: 1100, dung: 16.6 kg C ha−1) and the highest in MB1 (dung: 99.7, grass: 2942 kg C ha−1) coinciding with the lowest and highest fuel loads in these plots.
| Plot | Pre-fire C loads (kg ha−1) | Post-fire C loads (kg ha−1) | C loads released with fire (kg ha−1) | ||||
|---|---|---|---|---|---|---|---|
| Dung | Grass | Dung | Grass | Dung | Grass | ||
| PB1 | 62.1 | 1108 | 0.5 | 95.3 | 61.6 | 1013 | |
| PB3 | 36.2 | 2218 | 0.4 | 163.8 | 35.8 | 2054 | |
| MB1 | 99.7 | 2942 | 2.2 | 256.7 | 97.5 | 2686 | |
| SB3 | 16.6 | 1100 | 0.1 | 91.7 | 16.5 | 1008 | |
| Mean ± s.d. | 53.7 ± 35.9 | 1842 ± 902 | 0.8 ± 1.0 | 152 ± 77.4 | 52.9 ± 35.0 | 1690 ± 826 | |
Following the experimental fires, we observed on average a reduction of 52.9 kg C ha−1 in the total C dung loads relative to unburned dung (ranging from 16.5 to 97.5 kg C ha−1 in SB3 and MB1; Table 5), meaning that 98.5% of C was released from dung during experimental fires. This C loss of 98.5% from dung is proportionally higher than the 91.8% released into the atmosphere from vegetation (grass) burning (average: 1690 kg C ha−1).
Discussion
Effect of combustion and herbivore species on dung chemical composition
Large herbivores play an important role in maintaining the ecological balance of several ecosystems (Pringle et al. 2023). One of the most important processes in which large herbivores are involved is nutrient flow, with dung deposition being one of the main mechanisms of nutrient influx and acceleration of nutrient cycling (Sitters et al. 2017; Valdés-Correcher et al. 2019; Sitters and Olde Venterink 2021b; Barbero-Palacios et al. 2023).
Dung composition has higher concentrations of nutrients than the plant material on which herbivores feed (Pastor et al. 1993; Hobbs 1996), and a substantial fraction of the nutrients are in soluble and therefore highly bioavailable forms (Arnuti et al. 2020). In addition, the effect of the fire leads to rapid mineralisation of fuel organic materials such as dung, thus increasing the bioavailability of the nutrients present in this material (Lopez et al. 2023).
The chemical composition of the unburned dung analysed in the present study is consistent with findings reported in previous research. However, it is important to note that the composition of the excrement varies temporarily for each species, influenced by plant phenology (Verheyden et al. 2011). For instance, the average C and N contents of dung in the present study (41.3 and 1.1%, respectively) fall within the ranges reported for various herbivore species in the literature (C: 36.8–49.8%, N: 1.1–2.9%; Table 1).
A few studies have reported chemical composition for some of the species analysed here (Dougall 1963; Anderson and Coe 1974; Masunga et al. 2006; Stanbrook 2018; Sandhage-Hofmann et al. 2021). For elephant dung, for example, the concentrations of C, N and P (C: 36.8 –49.8%, N: 1.1–1.8%, P: 2.8–3.9 g kg−1; Table 1) in previous studies are in line with the values reported in this study (C: 39.7–43.1%, N: 1.1–1.4, P: 0.8–2.6 g kg−1; Table 4). Only the study by Masunga et al. (2006) reports additional element concentrations for elephant dung, with Ca, Cu, Mn and Zn of the same order of magnitude as our average concentrations, whereas Na, Fe and Al are an order of magnitude lower than ours (Tables 1 and 4).
When comparing unburned dung with burned dung, we observed that C and N concentrations are an order of magnitude lower in the latter (Fig. 5). This substantial loss of C and N during burning is expected, as these two elements are the ones starting to be volatilised at the lowest temperatures (300–500°C; Bodí et al. 2014), together with water, which becomes volatilised already at much lower temperatures. Unexpectedly, our Mg results showed a decrease in concentration in burned dung. We do not have a plausible explanation for the Mg results as the geochemical behaviour of Mg is similar to that of Ca, and it is not expected to be volatilised at temperatures lower than 1100°C (Bodí et al. 2014), which were not reached during the burning of dung (Table 3).
A key observation from the four studied savanna fires is that the C and N losses for dung during combustion are much higher than for vegetation, associated with the different burning behaviours of these two wildland fuels. Dung mostly burned by smouldering combustion whereas the fine grass vegetation burned mostly by flaming combustion. Smouldering combustion tends to result in more complete combustion and most organic matter being volatilised (Rein 2013; Fig. 3). The greater volatilisation of organic compounds also explains the higher concentration of mineral elements, like nutrients and metals, in burned dung when compared with vegetation ash. Vegetation ash produced during these fires contained a higher proportion of pyrogenic carbonaceous materials (Bird et al. 2015), and thus its composition differed substantially from the ash resulting from burned dung, with more C and N and, consequently, less enrichment of nutrients and metals. When compared with unburned dung, burned dung was also enriched in most nutrients and metals (e.g. P, Ca, Fe, Co) as shown by their positive enrichment indexes.
We also observed differences in dung combustion between species, which could be attributed to different compositions associated with digestive physiologies and feeding strategies (Demment and Van Soest 1985). For instance, wildebeest and buffalo, as grazers, eat a diet richer in fibre and cellulose, and as ruminants have more than one stomach, allowing them to digest food almost completely. In contrast, elephants, being non-ruminants and browsers, have a more varied diet than grazers, nutrient-rich and lower in fibre, producing less dense dung containing partially digested grass material, making it more flammable and easier to ignite than denser dung from species like zebra or buffalo. This is in line with our observation of elephant dung displaying more complete combustion and explains the large reduction in dung loads after fire in the MB1 plot where nearly all dung was from elephants (Fig. 2). The more complete combustion of elephant dung led to increased volatilisation of organic compounds, which resulted in the lower C, N and P concentrations than in burned dung from species like wildebeest and buffalo. The more complete combustion also resulted in the production of lighter and finer ash that was visually different from that of species such as zebra or buffalo, which maintained their shape and generated darker ash (Fig. 4). The substantially larger elephant dung loads in the northern plot from this study (MB1) than in the southern ones (PB1, PB3 and SB3) (Fig. 2a) are a result of the greater presence of elephants in relation to other species like zebra or buffalo in this area of the KNP (Abraham et al. 2021).
Burned dung as a nutrient hotspot
Our findings emphasise the role of fire as a recycling pathway for nutrients. Many elements essential for plant and microorganism growth (e.g. Ca, P, Fe, Co) are found in much higher concentrations in the burned dung than in the unburned material because these elements do not volatilise at the temperatures typically achieved during wildfires (Tuhý et al. 2021; Roshan and Biswas 2023).
After the fires, the higher pH observed in burned dung, compared both with unburned dung and vegetation ash, is likely a result of substantial concentrations of compounds that undergo alkali reactions, such as calcium oxide or potassium oxide, generated during the more complete biomass combustion (Bodí et al. 2014; Sánchez-García et al. 2023). In acid soils, the availability of nutrients for plants and micro-organisms is often limited, whereas others such as Al can become toxic (Foth and Ellis 1997). However, this higher pH in burned dung can induce an increase in the solubility and bioavailability of certain macronutrients such as P, N, Ca, Mg and K, mainly attributed to the transformation of the organic forms fixed in the biomass into soluble inorganic forms (Sertsu and Sánchez 1978; Cade-Menun et al. 2000; Roshan and Biswas 2023). This same argument also applies to trace metals. Pinedo-Gonzalez et al. (2017) found an increase in the solubility and bioavailable forms of many trace metals (both cations and anions) after fire, which is also related to the combustion of organic matter and an increase in soil pH. Something similar may occur during the combustion of faecal material. In contrast, major metals such as Fe or Mn decrease in solubility because they are transformed into poorly soluble oxides, such as haematite or magnetite in the case of Fe (Parra et al. 1996; Roshan and Biswas 2023).
The sudden pulse in nutrient bioavailability from burned dung may benefit a range of savanna plants that thrive in this post-fire environment and thus increase spatial species heterogeneity not only of plants, but also of soil fauna and microbial communities. These results show, for the first time, the role of burned dung as a nutrient and metal hotspot, which may have a localised impact on soil physical and chemical properties distinct from that of vegetation ash. This has implications for nutrient cycling and redistribution not only for savanna ecosystems, but potentially also for any ecosystems in which herbivore dung represents a notable fuel component during vegetation fires.
Some animals may make use of this resource too. There is observational evidence of big mammals such as impala, warthogs or wildebeest licking the ground in recently burned areas and they could be doing this to obtain specific minerals, such as calcium, from ash hotpots such as the ones identified in this study (Fig. 6a, b). More research is needed into this matter. Although some studies have examined the redistribution of nutrient in unburned dung by macrodetritivores like dung beetles (e.g. Holter 2016; Veldhuis et al. 2018), the redistribution of nutrients from burned dung by organisms, wind, or water is also a field that deserves further attention. In addition to burned dung giving a faster and more concentrated nutrient input than decomposing dung, chemical characteristics may also differ between the two. Fire alters the elemental concentration and form in which elements occur, so differences are expected between burned and decomposing dung. Moreover, the seasonal conditions under which these processes occur (fire: dry season vs dung decomposition: wet season) may play an important role as differing moisture levels can influence the decomposition of compounds with different recalcitrance (e.g. sugars vs lignin; Chaudhary et al. 2020). However, to our knowledge, no studies have investigated these effects.
Zebra (a) and impala (b) licking the ground of recently burned sites in the Kruger National Park (South Africa). (c) Large piece of down wood burned via smouldering combustion, also in Kruger National Park.

Finally, it is also worth noting that, although our vegetation ash resulted from grass burned via flaming combustion, during the studied fires we also observed, as anecdotal evidence, a few large pieces of down wood burned via smouldering combustion producing white ash (Fig. 6c). Although smouldering of litter and wood played a minor and unquantified role in our KNP plots, the combustion of these fuels is likely to be more significant in woodier savanna environments where these fuels are more predominant. Thus, smouldering of coarse wood could also yield nutrient hotspots that should be investigated in future studies.
Implications of dung burning for the carbon dynamics of African savannas
Our observations of dung loads suggested that herbivore dung can represent on average an additional fuel mass of 129 kg ha−1, equivalent to 3% of the vegetation fuel loads (average 4225 kg ha−1). Our estimates are consistent with those of Wooster et al. (2011) also from the KNP, where dung load estimates ranged from 100 to 500 kg ha−1. This indicates that dung can be an important but currently unaccounted for fuel component that should be included in fuel inventories, particularly in ecosystems with high densities of wild large herbivores, such as African savannas (Karp et al. 2024), or domestic grazing animals. It is, however, important to note that KNP, as a protected area, has higher densities of wild large herbivores than other African savannas, so our estimates are likely at the high end of the spectrum for these ecosystems.
In addition to wild herbivores, domesticated herbivores such as cattle are predominant in many savanna environments. We do not have data for domesticated herbivores as they are not present in Kruger, but they are widespread in other savanna environments. For example, average cattle biomass density was more than three times higher than wildlife populations (including zebra, giraffe, buffalo, gazelle) in an African savanna in northern Kenya (Hempson et al. 2017), and between 1.6 and 4.3 times higher than macropod populations in an Australian tropical savanna (Reid et al. 2020). The role of these domesticated populations should be examined in future studies investigating nutrient dynamics from burned herbivore dung. Our results show that 98.5% of the C contained in dung was volatilised into the atmosphere during fire, highlighting the notable role of dung in the C emissions of savanna fires in relation to their overall contribution to fuel loads. Although we do not know in what form C was emitted during the burns in this study, in an experiment also in KNP, Wooster et al. (2011) observed that emission ratios of CO/CO2 were up to four times higher when burning dung compared with grass. Similarly, Christian et al. (2007) reported larger amounts of CO and CH4 from burning of dung than from grass in pasture fires in Brazil. Therefore, the burning of dung should be taken into consideration from a C and nutrient dynamics point of view and when quantifying gas emissions from ecosystems where dung represents a notable fraction of the fuel loads. Nevertheless, it is important to stress that our sampling areas covered overall less than 1 ha of the approximately 2 million ha spanning the KNP, making it difficult to capture differences in animal density across the park and beyond. Future research involving a larger sampling effort is needed to quantify nutrient dynamics from burned dung from a range of herbivore species and at larger spatial scales.
Conclusions
We have studied, for the first time, the chemical composition of dung from large herbivores burning during African savanna fires and quantified its contribution to total fuel loads. Our findings improve the understanding of the role of herbivore dung and savanna fire in regulating the nutrient landscape. Burned dung was enriched in nutrients (e.g. Ca and P) compared with unburned dung and vegetation ash, except for Mg, which was unexpectedly lower in burned dung. Overall, burned dung represents a nutrient hotspot with physicochemical characteristics distinct from vegetation ash, potentially affecting localised plant and soil properties. This has implications for nutrient cycling and redistribution not only for savanna ecosystems, but potentially also for any ecosystems in which herbivore dung represents a notable fuel component during vegetation fires.
Unburned dung loads represented an additional 3% of the vegetation fuel loads with the release of C from dung being equivalent to 6% of the total C released from burning of vegetation. Our results, though limited to the study area, emphasise the significance of dung in African savanna fire dynamics and emissions, highlighting the importance of dung as a contributor to overall C released from African savanna fires, especially in areas of density of large herbivores. Additionally, owing to the different combustion processes in dung (smouldering) and the main fuel source in this ecosystem, grass (flaming), burning dung may have implications for the localised emission of more harmful gasses (CO, CH4) compared with burning grasses (CO2). Overall, these findings suggest that in ecosystems where dung makes up a substantial amount of fuel loads, such as African savannas, incorporating dung in fuel inventories and C dynamics assessments could improve the accuracy of these predictions.
Conflicts of interest
Stefan H. Doerr is one of the Editor-in-Chiefs of International Journal of Wildland Fire but was not involved in the peer review or decision-making process for this paper. The authors declare no other conflicts of interest.
Declaration of funding
During manuscript preparation C. Sánchez-García and S. H. Doerr received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement No. 101003890. Fieldwork was funded by The Leverhulme Trust (grant RPG-2014-095). Chemical analyses were supported by Natural Environment Research Council grant (NE/R011125/1) and by the Consellería de Educacion, Universidade e Formacion Profesional–Xunta de Galicia (Axudas á consolidacion e estruturacion de unidades de investigacion competitivas do SUG del Plan Galego IDT, Ambiosol Group ref. ED431C 2022/40).
Acknowledgements
We would like to thank the Scientific Services (Kruger National Park) Fire Team for applying the experimental fires that enabled this research to be conducted. Approval to conduct this study within Kruger National Park was granted as part of a registered research project (Research Code: SANTC1488) with a signed research agreement between South African National Parks and the research team. We are grateful to Maria Santiso (University of Santiago de Compostela, Spain) for performing the chemical analysis.
References
Abraham JO, Goldberg ER, Botha J, Staver AC (2021) Heterogeneity in African savanna elephant distributions and their impacts on trees in Kruger National Park, South Africa. Ecology and Evolution 11(10), 5624-5634.
| Crossref | Google Scholar | PubMed |
Anderson JM, Coe MJ (1974) Decomposition of elephant dung in an arid, tropical environment. Oecologia 14(1–2), 111-125.
| Crossref | Google Scholar | PubMed |
Arnuti F, Denardin LGdO, Nunes PAdA, Alves LA, Cecagno D, de Assis J, Schaidhauer WdS, Anghinoni I, Chabbi A, César de F. Carvalho P (2020) Sheep dung composition and phosphorus and potassium release affected by grazing intensity and pasture development stage in an integrated crop-livestock system. Agronomy 10, 1162.
| Crossref | Google Scholar |
Augustine DJ, McNaughton SJ, Frank DA (2003) Feedbacks between soil nutrients and large herbivores in a managed savanna ecosystem. Ecological Applications 13(5), 1325-1337.
| Crossref | Google Scholar |
Augustine DJ, Frank DA (2001) Effects of migratory grazers on spatial heterogeneity of soil nitrogen properties in a grassland ecosystem. Ecology 82(11), 3149-3162.
| Crossref | Google Scholar |
Barbero-Palacios L, Ferraro KM, Barrio IC, Krumins JA, Bartolomé J, Albanell E, Jarque-Bascuñana L, Lavín S, Calleja JA, Carreira JA, Serrano E (2023) Faecal nutrient deposition of domestic and wild herbivores in an Alpine grassland. Science of The Total Environment 903, 166616.
| Crossref | Google Scholar | PubMed |
Biggs R, Biggs HC, Dunne TT, Govender N, Potgieter ALF (2003) Experimental burn plot trial in the Kruger National Park: history, experimental design and suggestions for data analysis. Koedoe 46(1), 1-15.
| Crossref | Google Scholar |
Bird MI, Wynn JG, Saiz G, Wurster CM, McBeath A (2015) The pyrogenic carbon cycle. Annual Review of Earth and Planetary Sciences 43(1), 273-298.
| Crossref | Google Scholar |
Bodí MB, Martin DA, Balfour VN, Santín C, Doerr SH, Pereira P, Cerdà A, Mataix-Solera J (2014) Wildland fire ash: production, composition and eco-hydro-geomorphic effects. Earth Science Reviews 130, 8252.
| Crossref | Google Scholar |
Cade-Menun BJ, Berch SM, Preston CM, Lavkulich LM (2000) Phosphorus forms and related soil chemistry of podzolic soils on northern Vancouver Island. II. The effects of clear-cutting and burning. Canadian Journal of Forest Research 30, 1726-174.
| Crossref | Google Scholar |
Chaudhary E, Jouquet P, Rumpel C, Sukumar R (2020) Chemical parameters of decomposing dung in tropical forest as indicators of feeding behaviour of large herbivores: A step beyond classical stoichiometry. Ecological Indicators 115, 106407.
| Crossref | Google Scholar |
Campbell M, Treble PC, McDonough LK, Naeher S, Baker A, Grierson PF, Wong H, Andersen MS (2024) Combustion completeness and sample location determine wildfire ash leachate chemistry. Geochemistry, Geophysics, Geosystems 25(5), e2024GC011470.
| Crossref | Google Scholar |
Christian TJ, Yokelson RJ, Carvalho JA, Griffith DWT, Alvarado EC, Santos JC, Neto TGS, Veras CAG, Hao WM (2007) The tropical forest and fire emissions experiment: trace gases emitted by smouldering logs and dung from deforestation and pasture fires in Brazil. Journal of Geophysical Research Atmospheres 112(18), D18308.
| Crossref | Google Scholar |
Demment MW, Van Soest PJ (1985) A nutritional explanation for body-size patterns of ruminant and non-ruminant herbivores. The American Naturalist 125(5), 641-672.
| Crossref | Google Scholar |
Dougall HW (1963) On the chemical composition of elephant faeces. African Journal of Ecology 1(1), 123.
| Crossref | Google Scholar |
Foth HD, Ellis BG (1997) ‘Soil Fertility’. (CRC Press) 10.1201/9780203739341
Frank K, Brückner A, Hilpert A, Heethoff M, Blüthgen N (2017) Nutrient quality of vertebrate dung as a diet for dung beetles. Scientific Reports 7(1),.
| Crossref | Google Scholar |
Freymann BP, Buitenwerf R, Desouza O, Olff H (2008) The importance of termites (isoptera) for the recycling of herbivore dung in tropical ecosystems: a review. European Journal of Entomology 105(2), 165-173.
| Crossref | Google Scholar |
Girona-García A, Vieira D, Doerr S, Panagos P, Santín C (2024) Into the unknown: the role of post-fire soil erosion in the carbon cycle. Global Change Biology 30(6), 17354.
| Crossref | Google Scholar | PubMed |
Guevara-Torres DR, Facelli JM (2023) Choose Local: dung addition from native herbivores can produce substantial positive effects on the growth of native grasses compared to livestock dung. Journal of Soil Science and Plant Nutrition 23, 4647-4655.
| Crossref | Google Scholar |
Hempson GP, Archibald S, Bond WJ (2017) The consequences of replacing wildlife with livestock in Africa. Scientific Reports 7(1), 17196.
| Crossref | Google Scholar | PubMed |
Holter P (2016) Herbivore dung as food for dung beetles: elementary coprology for entomologists. Ecological Entomology 41(4), 367-377.
| Crossref | Google Scholar |
Hobbs NT (1996) Modification of Ecosystems by Ungulates. The Journal of Wildlife Management 60(4), 695.
| Crossref | Google Scholar |
Karp AT, Koerner SE, Hempson GP, Abraham JO, Anderson TM, Bond WJ, Burkepile DE, Fillion EN, Goheen JR, Guyton JA, Kartzinel TR, Kimuyu DM, Mohanbabu N, Palmer TM, Porensky LM, Pringle RM, Ritchie ME, Smith MD, Thompson DI, Young TP, Staver AC (2024) Grazing herbivores reduce herbaceous biomass and fire activity across African savannas. Ecology Letters 27(6), 14450.
| Crossref | Google Scholar | PubMed |
Le Roux E, Van Veenhuisen LS, Kerley GIH, Cromsigt JPGM (2020) Animal body size distribution influences the ratios of nutrients supplied to plants. Proceedings of the National Academy of Sciences of the United States of America 117(36), 22256-22263.
| Crossref | Google Scholar | PubMed |
Lopez AM, Pacheco JL, Fendorf S (2023) Metal toxin threat in wildland fires determined by geology and fire severity. Nature Communications 14, 8007.
| Crossref | Google Scholar | PubMed |
Masunga GS, Andresen Ø, Taylor JE, Dhillion SS (2006) Elephant dung decomposition and coprophilous fungi in two habitats of semi-arid Botswana. Mycological Research 110(0953), 1214-1226.
| Crossref | Google Scholar | PubMed |
Miller NF (1984) The use of dung as fuel: an ethnographic example and an archaeological application. Paléorient 71-79.
| Google Scholar |
Otero XL, Tierra W, Atiaga O, Guanoluisa D, Nunes LM, Ferreira TO, Ruales J (2016) Arsenic in rice agrosystems (water, soil and rice plants) in Guayas and Los Ríos provinces, Ecuador. Science of The Total Environment 573, 778-787.
| Crossref | Google Scholar | PubMed |
Pastor J, Dewey B, Naiman RJ, McInnes PF, Cohen Y (1993) Moose browsing and soil fertility in the boreal forests of Isle Royale National Park. Ecology 74(2), 467-480.
| Crossref | Google Scholar |
Parra JG, Rivero VC, Lopez TI (1996) Forms of Mn in soils affected by a forest fire. Science of The Total Environment 181, 231-236.
| Crossref | Google Scholar |
Pausas JG, Bond WJ (2020) On the three major recycling pathways in terrestrial ecosystems. Trends in Ecology and Evolution 35(9), 767-775.
| Crossref | Google Scholar | PubMed |
Pellegrini AFA (2016) Nutrient limitation in tropical savannas across multiple scales and mechanisms. Ecology 97(2), 313-324.
| Crossref | Google Scholar | PubMed |
Pinedo-Gonzalez P, Hellige B, West AJ, Sañudo-Wilhelmy SA (2017) Changes in the size partitioning of metals in storm runoff following wildfires: implications for the transport of bioactive trace metals. Applied Geochemistry 83, 62-71.
| Crossref | Google Scholar |
Pringle RM, Abraham JO, Anderson TM, Coverdale TC, Davies AB, Dutton CL, Gaylard A, Goheen JR, Holdo RM, Hutchinson MC, Kimuyu DM, Long RA, Subalusky AL, Veldhuis MP (2023) Impacts of large herbivores on terrestrial ecosystems. Current Biology 33, R584-R610.
| Crossref | Google Scholar | PubMed |
R Core Team (2014) R: A language and environment for statitistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org/
Reid AM, Murphy BP, Vigilante T, Bowman DMJS (2020) Distribution and abundance of large herbivores in a northern Australian tropical savanna: a multi‐scale approach. Austral Ecology 45(5), 529-547.
| Crossref | Google Scholar |
Roshan A, Biswas A (2023) Fire-induced geochemical changes in soil: implication for the element cycling. Science of The Total Environment 868, 161714.
| Crossref | Google Scholar | PubMed |
Sandhage‐Hofmann A, Linstädter A, Kindermann L, Angombe S, Amelung W (2021) Conservation with elevated elephant densities sequesters carbon in soils despite losses of woody biomass. Global Change Biology 27(19), 4601-4614.
| Crossref | Google Scholar |
Sánchez-García C, Santín C, Doerr SH, Strydom T, Urbanek E (2021) Wildland fire ash enhances short-term CO2 flux from soil in a Southern African savannah. Soil Biology and Biochemistry 160, 108334.
| Crossref | Google Scholar |
Sánchez-García C, Santín C, Neris J, Sigmund G, Otero XL, Manley J, González-Rodríguez G, Belcher CM, Cerdà A, Marcotte AL, Murphy SF, Rhoades CC, Sheridan G, Strydom T, Robichaud PR, Doerr SH (2023) Chemical characteristics of wildfire ash across the globe and their environmental and socio-economic implications. Environment International 178, 108065.
| Crossref | Google Scholar | PubMed |
Scholes RJ, Ward DE, Justice CO (1996) Emissions of trace gases and aerosol particles due to vegetation burning in southern hemisphere Africa. Journal of Geophysical Research Atmospheres 101(19), 23677-23682.
| Crossref | Google Scholar |
Sertsu SM, Sánchez PA (1978) Effects of heating on some changes in soil properties in relation to an Ethiopian land management practice. Soil Science Society of America Journal 42, 940-944.
| Crossref | Google Scholar |
Sitters J, Olde Venterink H (2021a) Herbivore dung stoichiometry drives competition between savanna trees and grasses. Journal of Ecology 109(5), 2095-2106.
| Crossref | Google Scholar |
Sitters J, Olde Venterink H (2021b) Body size–fecal nutrient patterns of mammalian herbivores. The Proceedings of the National Academy of Sciences 118(6), e2020137118.
| Crossref | Google Scholar |
Sitters J, Bakker ES, Veldhuis MP, Veen GF, Olde Venterink H, Vanni MJ (2017) The stoichiometry of nutrient release by terrestrial herbivores and its ecosystem consequences. Frontiers in Earth Science 5, 32.
| Crossref | Google Scholar |
Stanbrook RA (2018) Assessing the nutrient status of elephant dung in the Aberdare National Park, Kenya. Pachyderm 59, 86-90.
| Crossref | Google Scholar |
Tuhý M, Ettler V, Rohovec J, Matoušková Š, Mihaljevič M, Kříbek B, Mapani B (2021) Metal(loid)s remobilization and mineralogical transformations in smelter-polluted savanna soils under simulated wildfire conditions. Journal of Environmental Management 293, 112899.
| Crossref | Google Scholar | PubMed |
Valdés-Correcher E, Sitters J, Wassen M, Brion N, Olde Venterink H (2019) Herbivore dung quality affects plant community diversity. Scientific Reports 9(1), 5675.
| Crossref | Google Scholar | PubMed |
Van Langevelde F, Van De Vijver CADM, Kumar L, Van De Koppel J, De Ridder N, Van Andel J, Skidmore AK, Hearne JW, Stroosnijder L, Bond WJ, Prins HHT, Rietkerk M (2003) Effects of fire and herbivory on the stability of savanna ecosystems. Ecology 84(2), 337-350.
| Crossref | Google Scholar |
van Wees D, van der Werf GR, Randerson JT, Rogers BM, Chen Y, Veraverbeke S, Giglio L, Morton DC (2022) Global biomass burning fuel consumption and emissions at 500 m spatial resolution based on the Global Fire Emissions Database (GFED). Geoscientific Model Development 15(22), 8411-8437.
| Crossref | Google Scholar |
Veldhuis MP, Gommers MI, Olff H, Berg MP (2018) Spatial redistribution of nutrients by large herbivores and dung beetles in a savanna ecosystem. Journal of Ecology 106, 422-433.
| Crossref | Google Scholar |
Verheyden H, Aubry L, Merlet J, Petibon P, Chauveau-Duriot B, Guillon N, Duncan P (2011) Faecal nitrogen, an index of diet quality in roe deer Capreolus capreolus? Wildlife Biology 17(2), 166-175.
| Crossref | Google Scholar |
Wooster MJ, Freeborn PH, Archibald S, Oppenheimer C, Roberts GJ, Smith TEL, Govender N, Burton M, Palumbo I (2011) Field determination of biomass burning emission ratios and factors via open-path FTIR spectroscopy and fire radiative power assessment: headfire, backfire and residual smouldering combustion in African savannahs. Atmospheric Chemistry and Physics 11(22), 11591-11615.
| Crossref | Google Scholar |