Advancements in the control of genital human papillomavirus infections and related diseases: highlighting Australia’s role
Suzanne M. Garland A B C D H , Julia M. L. Brotherton E , Christopher K. Fairley F G , Dorota M. Gertig E and Marion Saville EA Department of Microbiology and Infectious Diseases, The Royal Women’s Hospital, Locked Bag 300, Parkville, Vic. 3052, Australia.
B Department of Obstetrics and Gynaecology, University of Melbourne, Parkville, Vic. 3052, Australia.
C Murdoch Childrens Research Institute, University of Melbourne, Parkville, Vic. 3052, Australia.
D Department of Microbiology, Royal Children’s Hospital, Parkville, Vic. 3052, Australia.
E Victorian Cytology Service, PO Box 161, Carlton South, Vic. 3053, Australia.
F School of Population Health, University of Melbourne, Carlton, Vic. 3053, Australia.
G Melbourne Sexual Health Centre, 580 Swanston Street, Carlton, Vic. 3053, Australia.
H Corresponding author. Email: Suzanne.garland@thewomens.org.au
Sexual Health 7(3) 227-229 https://doi.org/10.1071/SH10088
Submitted: 20 July 2010 Accepted: 26 July 2010 Published: 19 August 2010
It is timely that a special issue focusing on human papillomavirus (HPV) be published, bringing together expertise in an area where rapid gains have been made recently, particularly in Australia. In fact, the observation for linking a sexually transmitted agent to cervical cancer began hundreds of years ago, when it was realised that Catholic nuns rarely suffered from this disease.1,2 Subsequently, around 30 years ago now, and with the tools of molecular biology and sophisticated molecular epidemiological methods, oncogenic genotypes of HPV were proven to be the causative agent of cervical cancer.3 Although HPV remains uncultivatable by traditional viral culture techniques, it is the first pathogen to be recognised as the causative agent in virtually 100% of cases of any solid cancer. Recognition for this discovery resulted in Professor Harold zur Hausen being awarded the Nobel Prize in Physiology or Medicine 2008, bringing the idea that there could be effective vaccines against a cancer into the global public arena worldwide.4 More specifically, worldwide it has been shown that of the 200 HPV genotypes, types 16 and 18, are more virulent than other HPVs consistently causing around 70% of all cervical cancers, as also reported for Australia.5–7
Many Australian researchers have been instrumental in the development of this knowledge, which has resulted in successful cervical cancer vaccines. Colin Laverty with his team working at the time in the Gynaecological Histopathology Department at the King George V Hospital, Sydney, was one of the first groups to recognise and report the association with HPV and cervical dysplastic changes cytologically.8 Utilising electron microscopy, they were able to show HPV morphology in koilocytotic cells (Fig. 1).9
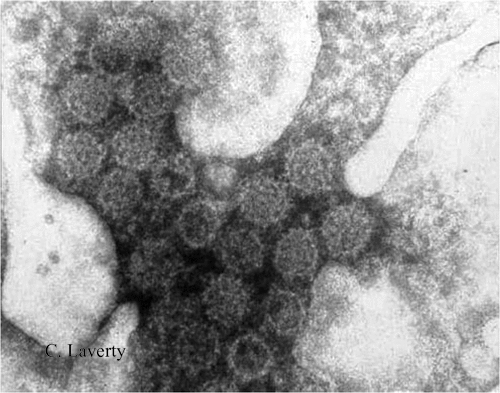
|
In the early 1990s, it was discovered that, when the HPV late protein gene (L1 gene) (which encodes for the outer viral capsid) is expressed in a vector system, that it self-assembles and produces empty viral capsids – so-called ‘virus-like particles’ (VLPs). VLPs are not infectious and have no oncogenic potential given that they do not contain any DNA (Fig. 2). These VLPs, which mimic the native infectious virions and virtually 'trick the immune system' into producing neutralising antibodies, have underpinned the successful development of the first generation of prophylactic HPV vaccines. This work was led by Zhou and Fraser in Queensland,10,11 together with others.12,13 Moreover, recognition for this work by Zhou and Frazer led to Fraser being awarded Australian of the year in 2006 (sadly occurring post-humorous for Zhou) and certainly put HPV into the public arena, particularly in Australia.
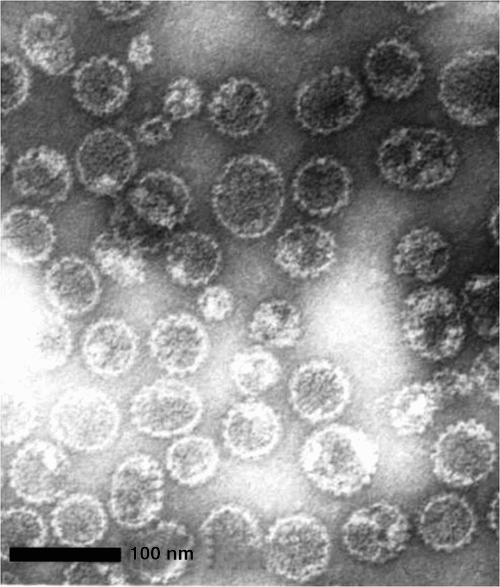
|
Currently licenced in more than a hundred countries, are a bivalent (contains VLPs of 16, 18 and marketed as Cervarix® manufactured by GlaxoSmithKline, Uxbridge, UK), as well as a quadrivalent vaccine (contains VLPs of 6, 11, 16, 18 and marketed as Gardasil® by Merck, New Jersey, USA and CSL), both of which have been shown to be efficacious against precancerous lesions (as surrogates to cancer), as well as against persistent infection with the vaccine-related HPV types in those previously naive to these genotypes.14–16 Australia has contributed to the phase 3 clinical trials of both the quadrivalent16 and the bivalent vaccine.14,17
Australia is also one of the few countries worldwide to be in a position to comprehensively measure vaccine effectiveness, given baseline HPV genotype prevalence pre-vaccination, high-quality cervical cytology registries, cancer registries and the newly developed National HPV Vaccination Program Register. The latter register will be valuable in measuring vaccination coverage, allowing for monitoring the performance of vaccine delivery programs. Linkage of the vaccination register with both cervical cytology and cancer registers will provide comprehensive information about vaccine effectiveness in the population. At the time of going to press, official release of the HPV vaccination coverage achieved in Australia’s catch up program was pending and these statistics will be published as soon as available.18 It will be particularly important to measure coverage for indigenous populations, given that the incidence and mortality rates for cervical cancer in indigenous women are around two and four times that of their non-indigenous counterparts, respectively.19 In addition, ongoing research projects in Australia will tell us about potential vaccine replacement and cross protection for HPV genotypes not currently in the first generation vaccines.20
This issue, we have asked authors to highlight their research and experiences relevant to HPV from vaccine manufacture,11 through evaluation of HPV related disease burden, screening and treatment to vaccine rollout and impact evaluation. In the Australian setting, where a comprehensive, government funded, school-based program has achieved good coverage, the disease with the shortest incubation, genital warts have already shown significant reductions in women younger than 27 years, as well as herd immunity in young heterosexual males.21–23 Hence in Australia we are already starting to witness and document a reduction in HPV disease burden as other countries begin to travel down the same path of instituting prophylactic HPV vaccines into their public health programs. In addition, with the recognition that a proportion of various other cancers are HPV related, there is the potential ultimately of a reduction in anal cancers,24 recurrent respiratory papillomatosis,25 and HPV related oropharyngeal cancers. Hence the issue of vaccination of boys is raised. In some cultures, this would take the stigma away from ‘a female considered disease’, and would also address the issue of equity in the prevention of the non-cervical HPV related disease burden.
Although both vaccines have been licenced in over a hundred countries worldwide, as yet relatively few countries have government funded public health programs, as in Australia, Canada and the UK.26 Globally, challenges include affordable prices, endorsement by governments and policy makers at all levels, as well as appropriate infrastructure for delivery to the target populations, particularly in those countries with the greatest burden of disease. However, as has been shown in demonstration projects by PATH in Vietnam, with appropriate communication and taking into account appropriate cultural issues, as well as good advocacy, high rates of coverage can be achieved.27 Some countries battle with introduction of an appropriate cervical screening program believing that this is required before a vaccination program can be considered. However, with rapid cheaper HPV DNA tests imminent, a possible future strategy could be to screen mothers with an HPV test concurrent with vaccination of their daughters.26 Moreover, as the current generation of vaccines provide protection only against the two major oncogenic HPV (16 and 18 causing 70 to 75% of cancers), well organised and integrated cervical screening programs are still required together with vaccination. A review of current cervical screening strategies will be necessary in the post-vaccination era.
Given the high uptake of vaccination in our community, it is likely that the age of commencement of screening, as well as the interval, can be safely increased.28 In the future, polyvalent vaccines with a wider range of HPV type protection or more broadly reactive products are anticipated.26
In conclusion, borrowing from the words of Elias A. Zerhouni, Director, US National Institutes of Health, ‘It is the responsibility of those of us involved in today’s biomedical research enterprise to translate the remarkable scientific innovations we are witnessing into health gains for the nation’.29 It is an imperative that we all work together for the common goal of the poorest nations worldwide gaining access to these vaccines to reduce the burden of disease from the leading cancer in women in their societies.
Conflicts of interest
SMG has received advisory board fees and grant support from CSL and GSK, and lecture fees from Merck and GlaxoSmithKline (GSK). She has received funding through her institution to conduct HPV vaccine studies for MSD and GSK. SMG is a member of the Merck Global Advisory Board as well as the Merck Scientific Advisory Committee for HPV prophylactic vaccines.
JMLB was an investigator on an investigator-driven study of HPV prevalence in Australia that received partial equal and unrestricted grant funding from CSL Ltd and (GSK). She was also an investigator on a serosurvey of HPV antibodies in Australia that received funding for the laboratory testing component from CSL Ltd.
CKF owns shares in CSL Biotherapies the manufacturer for Gardasil and has received honoraria from CSL Biotherapies.
DMG and MS have no conflicts of interest to declare.
[1] Rigoni-Stern D. Fatti statistici relativi alle malattic cancerose. Giorn Prog Patol Terap 1842; 2 507–17.

[2] Gagnon F. Contribution to the study of the etiology and prevention of cancer of the cervix of the uterus. Am J Obstet Gynecol 1950; 60 516–22.
| CAS | PubMed |

[3] Gissmann LWL, Ikenberg H, Koldovsky U, Schnurch HG, zur Hausen H. Human papillomavirus types 6 and 11 DNA sequences in genital and laryngeal papillomas and in some cervical cancers. Proc Natl Acad Sci USA 1983; 80 560–3.
| Crossref | GoogleScholarGoogle Scholar | CAS | PubMed |

[4] Zur Hausen H. Human papillomaviruses and their possible role in squamous cell carcinomas. Curr Top Microbiol Immunol 1977; 78 1–30.
| CAS | PubMed |

[5] Bosch F, Lorincz A, Munoz N, Meijer C. The causal relation between human papillomavirus and cervical cancer. J Clin Pathol 2002; 55 244–65.
| CAS | PubMed |

[6] Munoz N, Bosch FX, de Sanjose S, Herrero R, Castellsague X, Shah KV, et al. International Agency for Research on Cancer Multicenter Cervical Cancer Study Group. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med 2003; 348 518–27.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

[7] Stevens MP, Tabrizi SN, Quinn MA, Garland S. Human papillomavirus genotype prevalence in cervical biopsies from women diagnosed with cervical intraepithelial or cervical cancer in Melbourne, Australia. Int J Gynecol Cancer 2006; 16 1017–24.
| Crossref | GoogleScholarGoogle Scholar | CAS | PubMed |

[8] Laverty CR, Russell P, Hills E, Booth N. The significance of non-condylomatous wart virus infection of the cervical transformation zone: a review with discussion of two illustrative ubular strictures cases. Acta Cytol 1978; 22 195–201.
| CAS | PubMed |

[9] Hills E, Laverty CR. Electron microscopic detection of papilloma virus particles in selected koilocytotic cells in a routine cervical smear. Acta Cytol 1979; 23 53–6.
| CAS | PubMed |

[10] Kirnbauer R, Booy F, Cheng N, Lowy DR, Schiller J. Papillomavirus L1 major capsid protein self-assembles into virus-like particles that are highly immunogenic. Proc Natl Acad Sci USA 1992; 89 12180–4.
| Crossref | GoogleScholarGoogle Scholar | CAS | PubMed |

[11] Frazer IH. Cervical cancer vaccine development. Sex Health 2010; 7 230–4.
| Crossref | GoogleScholarGoogle Scholar |

[12] Zhou J, Sun XY, Stenzel DJ, Frazer IH. Expression of vaccinia recombinant HP V 16 L1 and L2 ORF proteins in epithelial cells is sufficient for assembly of HPV virion-like particles. Virology 1991; 185 251–7.
| Crossref | GoogleScholarGoogle Scholar | CAS | PubMed |

[13] Rose RC, Bonnez W, Reichman RC, Garcea RL. Expression of human papillomavirus type 11 L1 protein in insect cells: In vivo and in vitro assembly of virus-like particles. J Virol 1993; 67 1936–44.
| CAS | PubMed |

[14] Paavonen J, Naud P, Salmeron J, Wheeler CM, Chow SN, Apter DL, et al. Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women. Lancet 2009; 374 301–14.
| Crossref | GoogleScholarGoogle Scholar | CAS | PubMed |

[15] FUTURE II SG Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med 2007; 356 1915–27.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

[16] Garland SM, Hernandez AM, Wheeler CM, Perez G, Harper DM, Leodolter S, et al. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N Engl J Med 2007; 356 1928–43.
| Crossref | GoogleScholarGoogle Scholar | CAS | PubMed |

[17] Paavonen J, Jenkins D, Bosch FX, Naud P, Salmerón J, Wheeler CM, et al. Efficacy of a prophylactic adjuvanted bivalent L1 virus-like-particle vaccine against infection with human papillomavirus types 16 and 18 in young women: an interim analysis of a phase III double-blind, randomised controlled trial. Lancet 2007; 369 2161–70.
| Crossref | GoogleScholarGoogle Scholar | CAS | PubMed |

[18] Gertig DM, Brotherton JML, Saville M. Methods for measuring human papillomavirus vaccination coverage and the role of the National HPV Vaccination Program Register, Australia. Sex Health 2010;
| Crossref | GoogleScholarGoogle Scholar |

[19] Joseph T, Menzies R. Human papillomavirus vaccination and Australian Indigenous women. Sex Health 2010;
| Crossref | GoogleScholarGoogle Scholar |

[20] Brotherton JML, Kaldor JM, Garland SM. Monitoring the control of human papillomavirus (HPV) infection and related diseases in Australia: towards a national HPV surveillance strategy. Sex Health 2010; 7 310–19.
| Crossref | GoogleScholarGoogle Scholar |

[21] Cooper Robbins SC, Bernard D, McCaffery K, Skinner SR. ‘It’s a logistical nightmare!’: Recommendations for optimising human papillomavirus school-based vaccination experiences. Sex Health 2010; 7 271–8.
| Crossref | GoogleScholarGoogle Scholar |

[22] Fairley C, Hocking J, Chen M, Donovan B, Bradshaw C. Rapid decline in warts after national quadrivalent HPV vaccine program. Sex Transm Infect 2009; 85 499–502.
| Crossref | GoogleScholarGoogle Scholar | CAS | PubMed |

[23] Fairley CK, Donovan B. What can surveillance of genital warts tell us? Sex Health 2010; 7 325–7.
| Crossref | GoogleScholarGoogle Scholar |

[24] Grulich AE, Jin F, Conway EL, Stein AN, Hocking J. Cancers attributable to human papillomavirus infection. Sex Health 2010; 7 244–52.
| Crossref | GoogleScholarGoogle Scholar |

[25] Novakovic D, Cheng ATL, Cope DH, Brotherton JML. Estimating the prevalence of and treatment patterns for juvenile onset recurrent respiratory papillomatosis in Australia pre-vaccination: a pilot study. Sex Health 2010; 7 253–61.
| Crossref | GoogleScholarGoogle Scholar |

[26] Garland SM, Smith JS. Human papillomavirus vaccines: current status and future prospects. Drugs 2010; 70 1079–98.
| Crossref | GoogleScholarGoogle Scholar | PubMed |

[27] Nghi NQ, LaMontagne SD, Bingham A, Rafiq M, Mai LTP, Lien NTP, et al. Human papillomavirus vaccine introduction in Vietnam: formative research findings. Sex Health 2010; 7 262–70.
| Crossref | GoogleScholarGoogle Scholar |

[28] Canfell K. Models of cervical screening in the era of human papillomavirus vaccination. Sex Health 2010; 7 359–67.
| Crossref | GoogleScholarGoogle Scholar |

[29] Zerhouni EA. Translational and clinical science – time for a new vision. N Engl J Med 2005; 353 1621–3.
| Crossref | GoogleScholarGoogle Scholar | CAS | PubMed |



