From rediscovery towards recovery: a recent history of Australia’s most critically endangered marsupial, Gilbert’s potoroo (Potorous gilbertii)
J. Anthony Friend A * , Elizabeth A. Sinclair B C and Jacqueline M. Courtenay D E
B C and Jacqueline M. Courtenay D E
A
B
C
D
E
Abstract
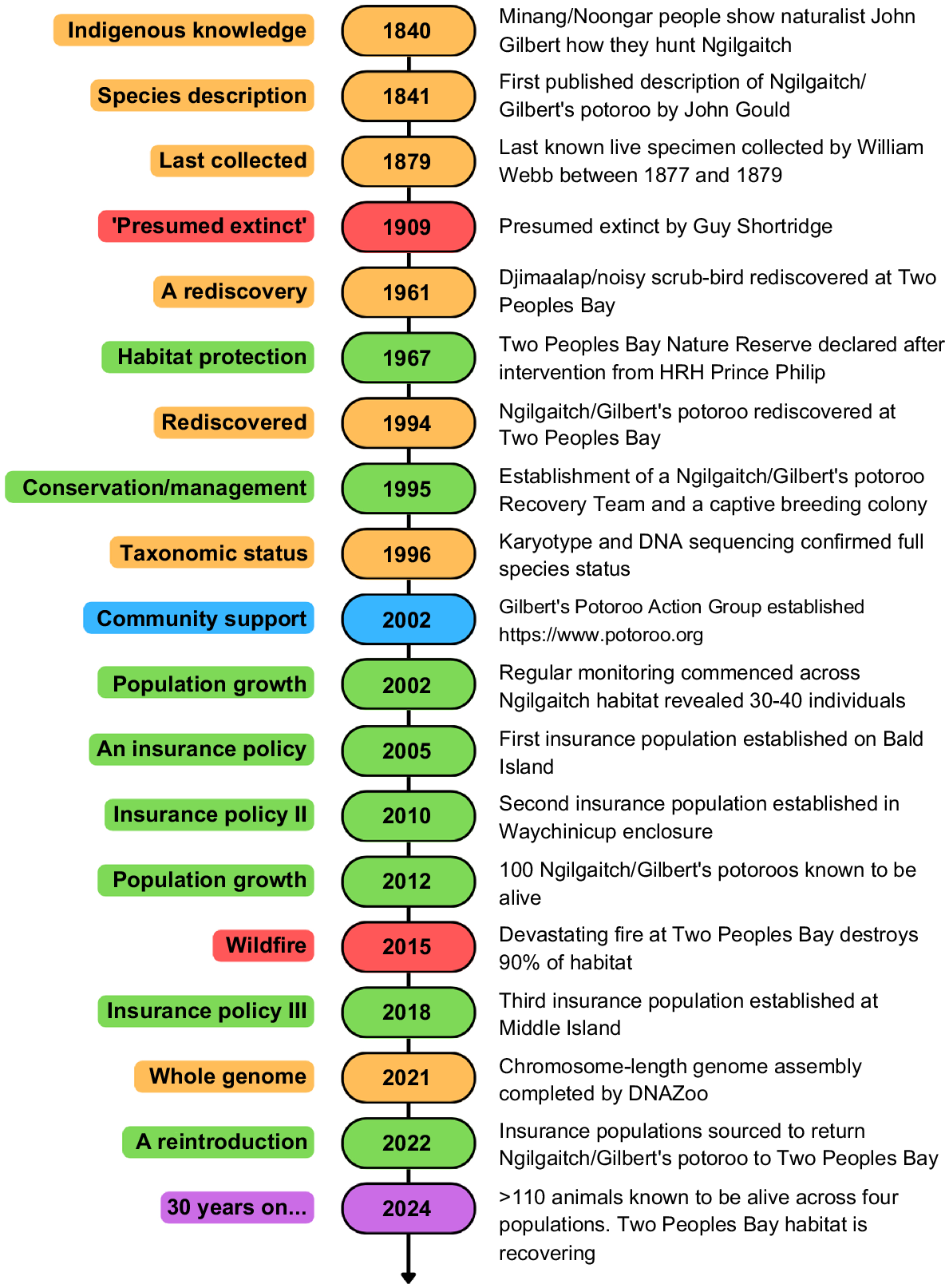
Ngilgaitch/Gilbert’s potoroo (Potorous gilbertii) was presumed extinct until its rediscovery at Two Peoples Bay Nature Reserve, Australia, in 1994.
Our paper summarises the history and rediscovery of Ngilgaitch/Gilbert’s potoroo, outlines the diverse research conducted to inform recovery, describes key management actions, and documents the fluctuating fortunes of the species between 1994 and 2024.
We summarise research and management actions to promote the species’ recovery at Two Peoples Bay Nature Reserve and nearby reserves.
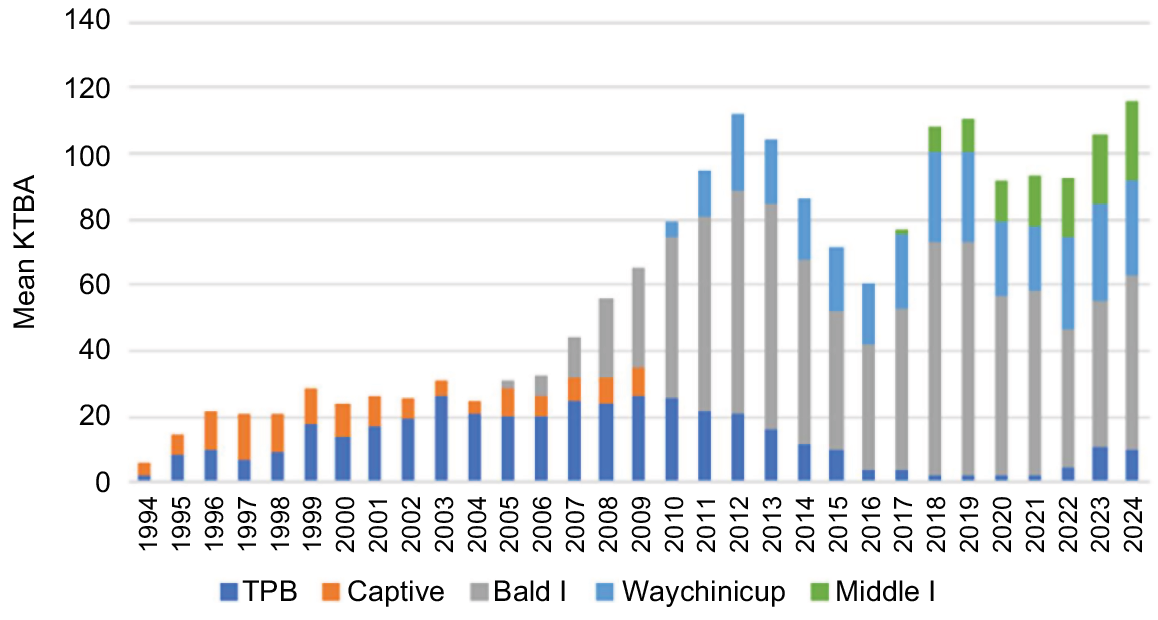
About 40 Ngilgaitch/Gilbert’s potoroo survived in heathland on the slopes of Maardjitup Gurlin/Mount Gardner, feeding almost exclusively on the fruiting bodies of hypogeal fungi. Two ‘insurance’ populations were established between 2005 and 2014 by translocation from the original population to Bald Island (810 ha) and a mainland enclosure (380 ha). These colonies proved critical to the species’ survival when a wildfire consumed most of the habitat at Two Peoples Bay in November 2015, causing the functional extinction of the population. Efforts have begun to restore this population through translocation from the insurance populations.
Establishing new potoroo populations by translocation has been the most valuable recovery technique for this species. Current and predicted climate change must be considered when choosing translocation sites. Management of all populations requires effective fire management, feral predator control, and actions derived from genetic information.
The Two Peoples Bay potoroo population was effectively lost following the 2015 fire. However, the regenerating habitat at the site remains an important resource in efforts to ensure the survival of the species.
Keywords: climate change, community advocacy, habitat management, hypogeal fungi, Ngilgaitch, population genetics and genomics, species recovery, translocation.
A brief history and taxonomy
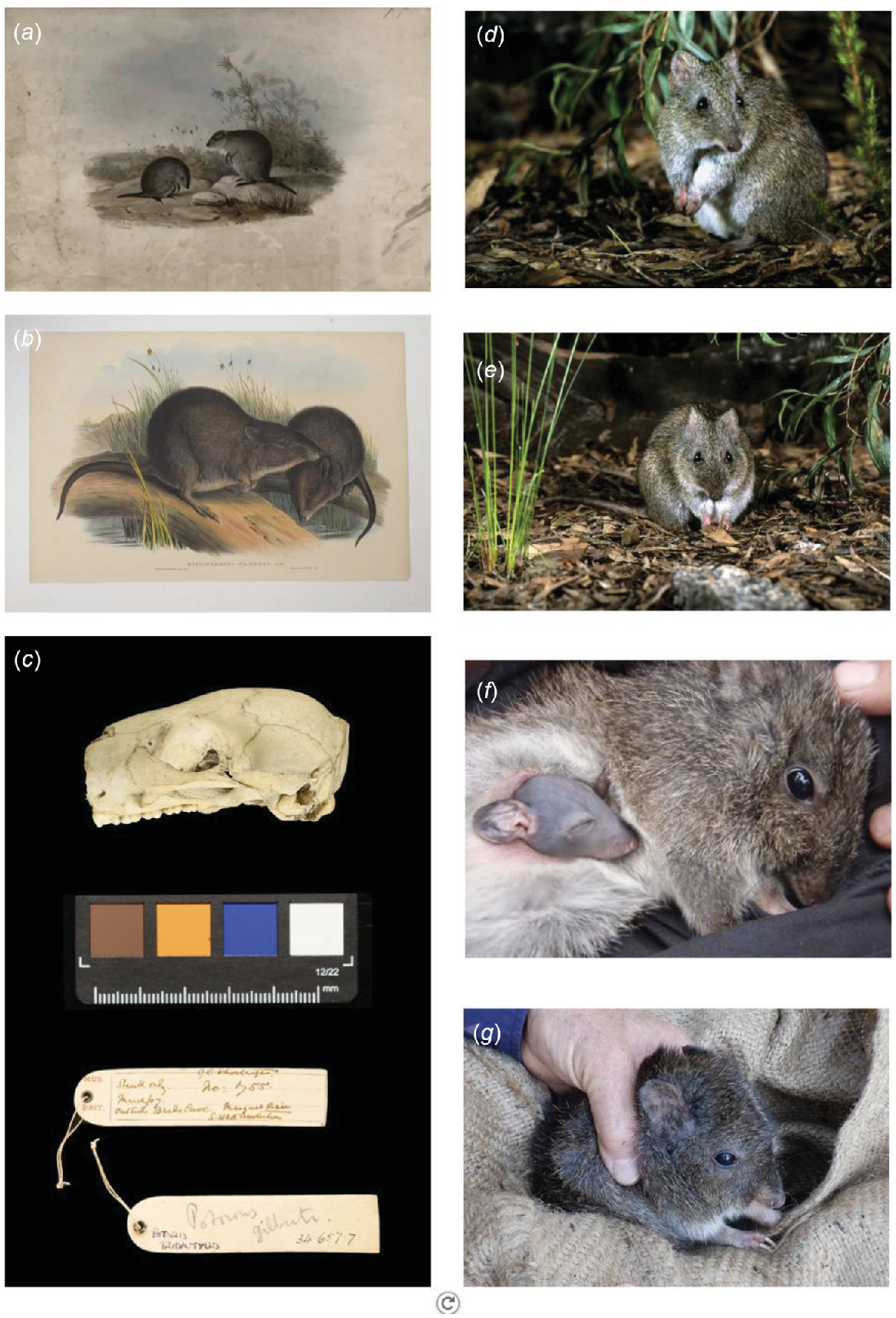
Gilbert’s potoroo (Potorous gilbertii) is one of the smallest members of the family Potoroidae, which includes potoroos, bettongs, and rat-kangaroos (Baker and Gynther 2023). Gilbert’s potoroo, known as ‘Ngilgaitch’ by Noongar Indigenous Peoples (see list of alternative names and spellings in Abbott 2001), was described by Gould (1841) as Hypsiprymnus gilbertii from specimens collected by John Gilbert (Fig. 1a, b). It was one of five species of Potorous recognised by 1888: Potorous tridactylus Kerr, 1792; P. gilbertii Gould, 1841; Potorous platyops Gould, 1844; Potorous apicalis Gould, 1851; and Potorous rufus Higgins and Petterd, 1884. Several subsequent taxonomic revisions were conducted in the absence of new Ngilgaitch/P. gilbertii samples (Ride 1970; Johnston and Sharman 1976, 1977; Johnston et al. 1984).
(a) Original drawings of Ngilgaitch/Hypsiprymnus gilbertii (now Potorous gilbertii) by H.C. Richter and (b) J. Gould and H.C. Richter 1841. (c) Shortridge skull specimen from 1907. Photograph by Amgueddfa Cymru – National Museum Wales. (d, e, f, and g) Images of adult and juvenile P. gilbertii since rediscovery in 1994. Photographs by Dick Walker/GPAG, Tony Friend, and Kathryn Greenop.
Only 10 specimens of Ngilgaitch/P. gilbertii were collected before it was ‘presumed extinct’ (Glauert 1950), held in five institutions across three countries. All were collected as live animals in the Albany-King George Sound area, on the south coast of Western Australia. Ngilgaitch/P. gilbertii is also known from over 800 fossil/subfossil specimens primarily from cave deposits along the Leeuwin-Naturaliste Ridge ranging in age from ~143,000 to ~400 years old (Glauert 1948; Cook 1963; Merrilees 1968; Baynes et al. 1975; Dortch and Wright 2010; Prideaux et al. 2010; Murray et al. 2013; Western Australian Museum palaeontology records; Fig. 2a), suggesting it has always been restricted to extreme south-western Australia. All museum specimens collected alive were from areas with long-term annual rainfall means over 800 mm (Albany/King-George Sound, 930 mm; Two Peoples Bay, 833 mm). Importantly, these totals include significant summer rainfall (December–February totals: Albany, 77.5 mm or 8% of the annual total; Two Peoples Bay, 75.7 mm or 9% of the annual total), vital for maintaining diverse year-round fungal food supply. By comparison, Perth’s long-term mean annual rainfall is 720 mm, with only 39.3 mm, or 5.4% falling in summer. The abundance of late Pleistocene–Holocene material suggests the species was once common, even up until the early years of European settlement (notes on a hunt for Ngilgaitch/P. gilbertii and Quokka/Setonix brachyurus by John Gilbert in Gould 1863, 1973):
The natives capture it by breaking down a long, narrow passage in the thicket, in which a number of them remain stationed, while others, particularly old men and women, walk through the thicket, and by beating the bushes and making a yelling noise, drive the affrighted animals before them into the cleared space, where they are immediately speared by those on the watch: in this way a tribe of natives will often kill an immense number of both species in a few hours. I have not heard of the Hypsiprymnus Gilbertii being found in any other part of the colony than King George’s Sound.
(a) Location of palaeontological records (black diamonds) concentrated along the Leeuwin-Naturaliste ridge and modern records (labelled red diamonds) for Ngilgaitch/P. gilbertii in Western Australia. Rainfall isohyets are indicated (mm). Inset: location of (a) and (b) along the south coast of Western Australia. (b) Location of Middle Island ~600 km east of Albany and (c) Maardjitup Gurlin/Mount Gardner area showing extent of the 2015 bushfire (shaded) and various locations mentioned in the text.
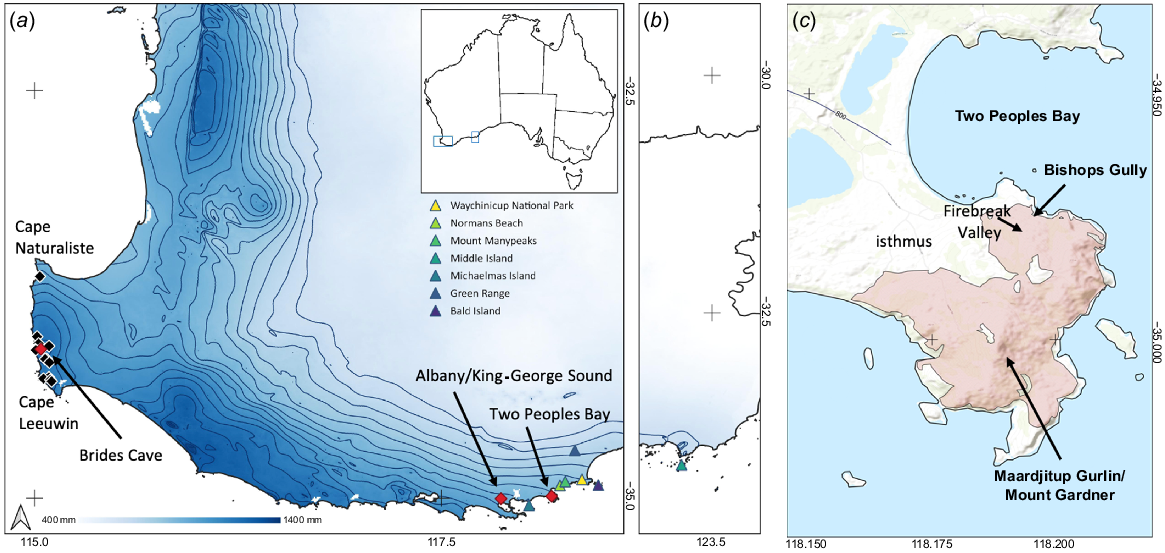
One modern specimen is known to have been collected outside the Albany area, an unsexed skull collected outside Brides Cave, near Margaret River in 1907 (Fig. 1c, part of the Shortridge collection in the National Museum in Wales, J. Gallichan, pers. comm.). Aboriginal oral history as reported to Daisy Bates between 1901 and 1902 (cited in Tilbrook 1983) suggests that the species may have persisted in the Margaret River area in sufficient numbers to be hunted at least until 1845. Abbott (2008) also reports that an ‘old timer’, V. Roberts, told him that his elder brothers had spoken of the ‘Nilgyte’ being present in coastal vegetation at Scott River east of Augusta in the 1890s, although he had not personally seen it. Ngilgaitch/P. gilbertii was ‘presumed extinct’ in Western Australia by the early 1900s (Shortridge 1909; Seebeck et al. 1989).
Rediscovery, species validation, and early records
About 120 years after the last living specimens were collected, Ngilgaitch/P. gilbertii Gilbert’s potoroo was rediscovered in Two Peoples Bay Nature Reserve, a highly important conservation area managed by the Western Australian Department of Biodiversity, Conservation and Attractions (DBCA), in December 1994 (Sinclair et al. 1996; Fig. 1d–g). Five animals were initially captured in open sandy heathland in a remote area of Maardjitup Gurlin/Mount Gardner. It was the largest Australian ‘Critical Weight Range’ mammal species rediscovered (CWR; 35–5500 g; Burbidge and McKenzie 1989) at the time. Most extinctions and declines of Australian mammals have occurred among CWR species, with this tendency strongest amongst ground-dwelling taxa (Johnson and Isaac 2009).
The newly rediscovered potoroo population met the criteria for ‘Critically Endangered’ ranking according to the IUCN criteria (IUCN 1994). However, its taxonomic status required clarification for definition of its conservation status and subsequent implementation of recovery actions. At the time of the rediscovery P. gilbertii was considered a synonym of P. tridactylus and part of the P. tridactylus tridactylus subspecies, which included all mainland populations from Queensland to the south-west of Western Australia (Johnston 1983). Early research then focused on species validation (Sinclair and Westerman 1997; Sinclair et al. 2000; Westerman et al. 2004; Frankham et al. 2012a), with full species recognition warranted and the original specific name of gilbertii Gould, 1841 reinstated. Four species of Potorous are currently recognised (P. tridactylus, P. longipes, P. gilbertii, and P. platyops), although Moorda/P. platyops is now regarded as ‘Extinct’ (Friend and Kitchener 2023). Planning and implementation of recovery actions for Ngilgaitch/P. gilbertii were subsequently given high priority by both Western Australian and Commonwealth governments, as the IUCN ranking of ‘Critically Endangered’ was confirmed. This paper summarises research, management and conservation efforts to prevent future extinction, with emphasis on the importance of the Two Peoples Bay population. A timeline of significant events is provided for context (Fig. 3).
Early field studies and the captive colony of Ngilgaitch/Gilbert’s potoroo
Early recovery actions (1994–2000) laid out in the Interim Wildlife Management Guidelines (Start and Burbidge 1995) focused on: (1) searching the south coast near Albany for additional populations; (2) establishing a captive breeding colony as insurance against natural disasters, such as wildfire and disease; and (3) conducting research into the biology and ecology of Ngilgaitch/P. gilbertii relevant to its management. Feral cats (Felis catus) and red fox (Vulpes vulpes) were both known from Two Peoples Bay. Fox control by monthly distribution of 1080 baits had been in place in the Reserve since 1988 (Department of Conservation and Land Management 1995) and continues today, complemented by feral cat control, detailed later.
Surveying new sites to understand the species distribution was a priority. Baited ‘hair tubes’ or ‘hair arches’ fitted internally with double-sided tape (Scotts and Craig 1988) have been used to collect hair samples from mammals in the wild, as a method of detecting the presence of a species at a site. This simple, less time-consuming method was used as a first approach in preference to cage trapping, as larger areas could be surveyed, and the tubes did not need to be checked every day. Ngilgaitch/P. gilbertii hair can be distinguished microscopically from other mammals known to occur along the south coast of Western Australia including Quokka (Setonix brachyurus), Quenda (Isoodon fusciventer), Mardo/yellow-footed antechinus (Antechinus flavipes), Mootit/bush rat (Rattus fuscipes), and Wawding/western ringtail possum (Pseudocheirus occidentalis) (Brunner and Coman 1974; Denmark Environment Centre Inc. 2003). Hair arch surveys were initially carried out between 1996 and 1998 to search for new populations of Ngilgaitch/P. gilbertii at Normans Beach and along the Yilberup/Mount Manypeaks ridgeline without success. Indigenous names for locations on Two Peoples Bay Nature Reserve and its surrounds are from Knapp et al. (2024).
Commencing in December 1994, a captive colony was established with four individuals (two males and two females with pouch young) captured within 2 months of the rediscovery. Subsequently, another female and its male young-at-heel were added to the colony in May 1995 and a female with a female pouch young in April 1996 (Courtenay et al. 1998). Early European collectors left few biological or ecological data relating to Ngilgaitch/P. gilbertii, so knowledge of animal husbandry, diet, and ecology were based initially on studies of the eastern Australian potoroos, particularly the long-nosed potoroo, P. tridactylus (Seebeck 1981, 1982; Scotts and Seebeck 1989; Seebeck et al. 1989; Claridge et al. 1993; Claridge and Cork 1994). By 2001, eight young potoroos had been born and raised in captivity using conventional husbandry methods (Courtenay and Friend 2004). Basic knowledge of husbandry and reproduction in Ngilgaitch/P. gilbertii was gained through this process and intensive monitoring of adults and their young (Courtenay and Friend 2004; Stead-Richardson et al. 2010). No additional births occurred after 2001, despite subsequent experimental manipulation of captive diet and ongoing attempts at breeding management. The colony dwindled through mortality due to several factors including age-related mortality and a high incidence of renal oxalosis (Forshaw et al. 2017). The breeding program ceased in 2010 with the release of the four remaining animals into a 380-ha enclosure fenced to exclude foxes and feral cats in Waychinicup National Park, described later.
Public advocacy and community support
Species extinctions typically have ecological drivers, such as habitat loss, introduced predators, inappropriate fire regimes, and climate change. However, Woinarski et al. (2017) point out that extinction events are also influenced by policy and management settings and insufficient resources. It has been shown that threatened species are more likely to survive when they have dedicated community group advocacy (Garnett et al. 2018). The Gilbert’s Potoroo Action Group (GPAG), incorporated in 2002, is based in Albany, Western Australia (Fig. 3). GPAG is a not-for-profit group with around 100 members and a smaller group of passionate community volunteers. The group’s activities fall into three broad areas: (1) fundraising to assist with research and implementation of the recovery program; (2) promoting public awareness of the critically endangered status; and (3) hands-on volunteer assistance in research activities.
GPAG has raised approximately A$1.25 million since 2002, A$1.1 M of that since the 2015 fire primarily through grant funding that was unavailable to government agencies. Substantial funds have also been generated through a range of activities (GPAG labelled wines, abseiling, head-shaving, and a truffle-themed quiz night; Fig. 4). Small, but regular, amounts are raised through donations to the ‘Containers for Change’ recycling program and donations via the website. The group endeavours to raise awareness (locally, nationally, and internationally) of the plight of Ngilgaitch/P. gilbertii through special events such as the biennial South Coast Threatened Species forum, social media, a quarterly newsletter, and a website (https://www.potoroo.org). Objective 3 in the current Recovery Plan (Friend et al. 2016), ‘to increase the awareness of, and support for the recovery of Gilbert’s potoroo’, recognises the role of GPAG as a major contributor to recovery, through education, community awareness, volunteerism, and vital fundraising. Members contribute to vegetation and fungi surveys and animal monitoring (radio-tracking and trapping). Community advocacy has an important role to play in contributing to the success of threatened species recovery in an era where alleviating the global biodiversity crisis requires an increased societal recognition of the need to be responsible for the care of country (Woinarski et al. 2024). Societal extinction is a process by which cultural knowledge, personal experience, and a species’ presence in art and storytelling is lost and can occur even while a species still survives (Jarić et al. 2022). For species such as the Ngilgaitch/P. gilbertii that are particularly at risk of societal extinction (due to their very small population, cryptic and nocturnal habits, remote location, and absence from zoos or wildlife parks), an active and ongoing community group dedicated to raising awareness and obtaining financial support is likely to be critical in helping avert its biological extinction.
Gilbert’s Potoroo Action Group (GPAG) and community volunteers in action. (a, b) Changing sand in the potoroo pens, (c) labelling the vintage at Jingalla winery, (d) Rottnest Channel swim team in 2022, (e) collaborative art fundraiser at 25th anniversary dinner, (f) abseil off the QVI building in 2019, and (g) Gilbey counting the funds.

Survey and population estimation at Two Peoples Bay Nature Reserve
Hair arch surveys for potoroos, initiated under the recovery program in 1995, continued until 2008 within Two Peoples Bay, surrounding areas, and many heathland sites mainly to the east of Albany, in the Yilberup/Mount Manypeaks area, Green Range, and other coastal areas as far east as the Jerdacuttup Lakes east of Ravensthorpe. Surveys to the west of Albany, led by project officer Douglas Tait under the auspices of the Denmark Environment Centre, concentrated on granite-dominated sites along the coast, with supplementary surveys on the Leeuwin-Naturaliste Ridge around Margaret River (Denmark Environment Centre Inc. 2003, 2004). The only new records of Ngilgaitch/P. gilbertii from hair arch surveys were obtained in 2000, near Bishops Gully on the Maardjitup Gurlin/Mount Gardner headland, 1 km from previously known potoroo locations. The overall conclusion from these surveys was that no population existed outside Two Peoples Bay Nature Reserve, and therefore that recovery of the species would be totally reliant on the original rediscovered population.
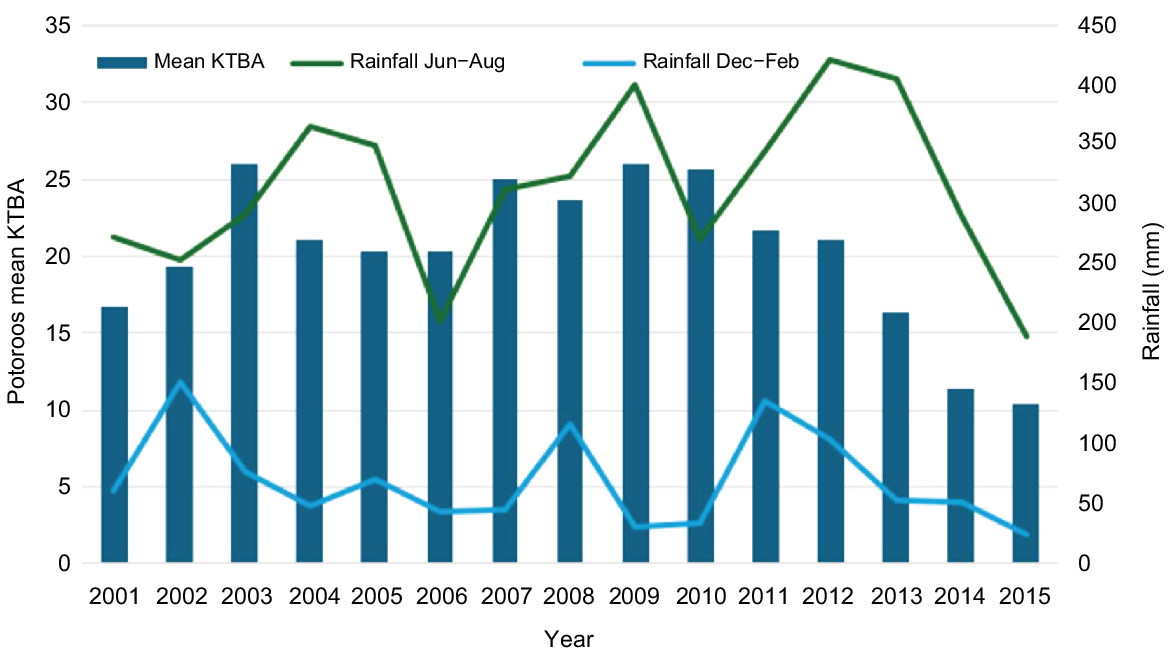
In 2001, an expanded monitoring program using trapping on nine traplines across the Maardjitup Gurlin/Mount Gardner headland was established, with the aim of assessing population size and monitoring numbers, reproduction, survival, and health parameters through time. This trapping program also provided an opportunity for postgraduate research students and other researchers, providing important knowledge to assist in the management of the species. Trapping was carried out three times per year across all traplines from 2001 until November 2015, when a large bushfire caused by lightning burned 90% of the suitable potoroo habitat that occupied approximately half of the vegetated section of the 1000-ha headland (Fig. 2c). The number of animals known to be alive (KTBA) on the traplines was estimated to represent 75% of the population in the Reserve. KTBA remained fairly constant from 2002 until 2012, surpassing 20 individuals from 2003 (Figs 5, 6). Between 2012 and 2015, a strong decrease in mean KTBA coincided with a steady decline in summer rainfall, followed by very low winter rainfall in 2014 and 2015 (Fig. 6). Summer rainfall is critical to maintain fungi that fruit through summer and provide sustenance for potoroos when sporocarps of other species are less available (Nguyen 2000, figs 5.8, 5.10). It is likely that the successive dry winters had a negative impact on the overall biomass of fungal sporocarps, further decreasing year-round food availability for potoroos. This may have reduced KTBA through higher mortality rates, particularly among younger and older individuals. The effects of the fire on this reduced population are described below.
Mean number of Ngilgaitch/P. gilbertii known to be alive (KTBA) from 1994 to 2024. Numbers are given for the wild population at Two Peoples Bay Nature Reserve, captive colony, and three translocated populations at Bald Island (2005), Waychinicup enclosure (2010) and Middle Island (2018). The 2015 fire consumed 90% of potoroo habitat on the Maardjitup Gurlin/Mount Gardner headland.
Population size and seasonal rainfall at Two Peoples Bay Nature Reserve from 2001 to 2015. The abundance of Ngilgaitch/P. gilbertii is expressed as the mean of up to three known to be alive (KTBA) counts each year on the nine regularly monitored traplines on the Maardjitup Gurlin/Mount Gardner headland. Mean seasonal rainfall is given for winter (June–August) and summer (December–February).
The presence of a small but stable and wild population of Ngilgaitch/P. gilbertii at Two Peoples Bay until at least 2012 and the existence of a captive colony allowed research into aspects of the biology, ecology, and population genetics, benefitting the recovery effort through the knowledge gained. Some of these studies are detailed in the following sections.
Diet and feeding ecology
Sporocarps of hypogeal or underground fungi form a significant part of the diet of Australian mammals, especially potoroids (Seebeck et al. 1989; Claridge et al. 1993; Claridge and Cork 1994; Nuske et al. 2017). There is a high level of endemism among truffle-like fungi in Australia (Bougher and Lebel 2001), with approximately 350 new species recently described or in the process of being described (Syme 1999; Syme et al. 2025). Among the three extant Potorous species, there is variation in both the importance of fungi and the number of fungal species within the diet, as determined from analysis of faecal samples. Plants, seeds, and invertebrates form a significant proportion of the diet in P. tridactylus (Bennett and Baxter 1989; Claridge et al. 1993). Fungal content varies between 25% and 70% seasonally, with over 50 fungal species found by Bennett and Baxter (1989) and 58 by Claridge et al. (1993). P. longipes is even more fungivorous than P. tridactylus, with fungi accounting for at least 80% (Scotts and Seebeck 1989) to over 90% (Green et al. 1999) of the dietary volume. Green et al. (1999) recorded 36 fungal species in faecal material, which also varied seasonally.
More than 90% of the diet of wild Ngilgaitch/P. gilbertii at Two Peoples Bay was made up from fungal material, with over 40 species of hypogeal fungi represented (Nguyen et al. 2005). Examples of fungal spores from Ngilgaitch/P. gilbertii scats are illustrated in Bougher and Friend (2009) and Syme et al. (2025). The small non-fungal portion of the diet consists of plants (stems, roots, and seeds) and invertebrates (Nguyen et al. 2005). The presence of seeds in the faeces of animals from Two Peoples Bay was investigated by Cochrane et al. (2005) who identified seeds from Billardiera fusiformis, Marianthus sp., Astroloma sp., and Leucopogon sp., presumably ingested when the animals ate the fleshy fruit of these species. Germination trials comparing ingested and non-ingested Billardiera seeds showed that ingestion by Quokkas/S. brachyurus and Ngilgaitch/P. gilbertii increased germination by 58% and 31%, respectively (Cochrane et al. 2005). This highlights the role of Ngilgaitch/P. gilbertii as a seed and fungal spore disperser, but also that the process improves seed germination after defaecation.
Movements, spatial organisation, and habitat preference
Knowledge of the area of habitat required by individual animals is vital information for species management and critical for management of threatened taxa. Studying home range, the area that an animal travels for its normal daily activities (Viana et al. 2018), provides a measure of the amount of habitat required for feeding, resting, caring for young, and other social activities (Winkelmann et al. 2003; Elmore et al. 2005; Le Pla et al. 2024). An early detailed study of microhabitat use of Ngilgaitch/P. gilbertii was carried out in 1996 using a modified ‘spool and line’ tracking technique (Vetten 1996; Vetten et al. 1997). Animals were fitted with a small package containing two spools of nylon thread totalling 340 m, which unravelled as the animal moved through the habitat. Six individual animals (three males, three females) were involved in the study and were spooled between one and three times each over a period of one month in August 1996, for a total of 14 spools analysed. Trapping occurred along a line of 20 traps placed approximately 10 m apart. Potoroos were caught in 10 of the 20 traps. All were released at their trap location after spools were attached, so they were familiar with surrounds to avoid ‘bolting’. Data collection involved following the spool line and measuring the distance travelled in relation to vegetation species and cover adjacent to and above the line. Diggings adjacent to the spool lines were also recorded. Vegetation and cover from each line were then compared with combined data from 16 transect lines (340 m each) representing the habitat available to Ngilgaitch/P. gilbertii within the 340-m limit of the spools, using Chi-squared contingency tables and direct comparisons. The transects were started at the two outermost trap points where animals had been captured and conducted along compass bearings at 45-degree intervals (0, 45, 90 and so on) from the starting point. This design aimed to ensure that the over-representation of the habitat adjacent to the trap lines present in the spooling data was also present in the transect data (Vetten 1996). The spool tracking showed no clear association with vegetation type or density of cover. However, the more open areas appeared to be favoured for nocturnal foraging, as most diggings were found in open and semi-open areas that tended to be in sedgeland and open heathland (Vetten 1996).
Radio-tracking groups of Ngilgaitch/P. gilbertii from fixed stations was conducted over five fortnights between 2000 and 2009 to estimate the home range size of animals in the Reserve (Friend 2001; J. A. Friend, unpubl. data). The study area in Firebreak Valley (Fig. 2c) was used for a pilot study in December 2000 and for further studies carried out in February 2002, 2004, 2006, and 2009. The valley slopes below the granite outcrops to the east and west had proved to be reliable sites to capture animals since the early days of the recovery program. The vegetation across the valley conforms with the shrub-dominated community S8 Headland Mixed Dense Low Heath described in Hopkins et al. (2024b). The dominant structural element is dense Melaleuca striata, which with M. thymoides forms a solid canopy up to 2 m above ground. The understorey is dominated by sedges including Anarthria scabra which provide cover and clumps between which Ngilgaitch/P. gilbertii often make well-hidden nests.
Trapping to secure animals for radio-tracking was carried out on three established traplines along which a total of 55 cage traps, baited with a mixture of peanut butter, rolled oats, and pistachio oil flavour were set. After an incident in 1999 in which an adult potoroo fitted with a radio-collar caught both forefeet under the neck band, collar use was abandoned. All animal tracking was conducted using movement-sensing core transmitters (Sirtrack, now Lotek NZ, Havelock North, New Zealand, http://www.lotek.com). These weighed 8–9 g and were attached temporarily to animals with elastic adhesive tape around the base of the tail. Transmitters drop off when the tape adhesive fails, usually around 4–8 weeks after deployment. Each radio-tracking station was equipped with a null-peak aerial system mounted on a 6-m rotating mast. The antennae were rotated when the beeping signal was heard, and the null-peak system provided a silent point between loud peaks when the antennae were pointing directly at the source of the signal. The direction to the animal was then read from the compass indicator. Bearings on each animal were taken every 20 min and triangulation used later to calculate the position of the animal and its precision.
The 2-week pilot study was carried out in December 2000 after fitting transmitters to six Ngilgaitch/P. gilbertii (three adult males, one sub-adult male, one juvenile male and one adult female, which was the mother of the juvenile male). The following observations were made (Friend 2001):
The animals were almost only active at night. Very few movements occurred in daylight.
Adult males moved more widely than the juvenile and sub-adult males or the female.
Individuals occupied stable home ranges during the 2 weeks of the study.
All adult males and the sub-adult male occupied discrete home ranges.
The sub-adult male had been caught first as a pouch young of female (#23) in the southern part of the study site but had clearly established a home range in the northern part.
There was extensive overlap in home ranges of the female, the juvenile male (offspring), and one of the adult males.
Two adult males appeared to make exploratory movements outside their regular home range area.
The juvenile male was still living in its mother’s home range and nested with its mother on some, but not all days.
Three tracking stations operated on four 6-h shifts in 2000 to cover the full 24-h cycle. As no diurnal activity was recorded in that study, four stations were used from 2002, providing more location accuracy in three 6-h shifts spanning the night. Animals were tracked for 2 weeks. This required up to 24 willing volunteers who were readily recruited through various forms of advertising, including through GPAG. The calculated positions for five tracked females in 2004 and for three males and three females in 2009 are mapped in Fig. 7a and b, respectively. The 2004 plots showed that females occupy discrete home ranges with little overlap. This spatial arrangement provides each female with exclusive access to the resources in the area without competition for food with other females at times of high metabolic demand when large young are being suckled. The 2009 plots confirmed that there was little overlap between males, females did not share with other females, and male home ranges overlapped with more than one female.
Locations of radio-tracked Ngilgaitch/P. gilbertii in Firebreak Valley (2-week periods). (a) February 2004, five females (F92, F69, F66, F50, F25) and (b) February 2009, two males (M94, M133) and three females (F69, F50, F128). Locations were calculated from simultaneous bearings taken from four radio-tracking stations. Sources of this material, as given in the text, are Friend 2001; J. A. Friend, unpubl. data.
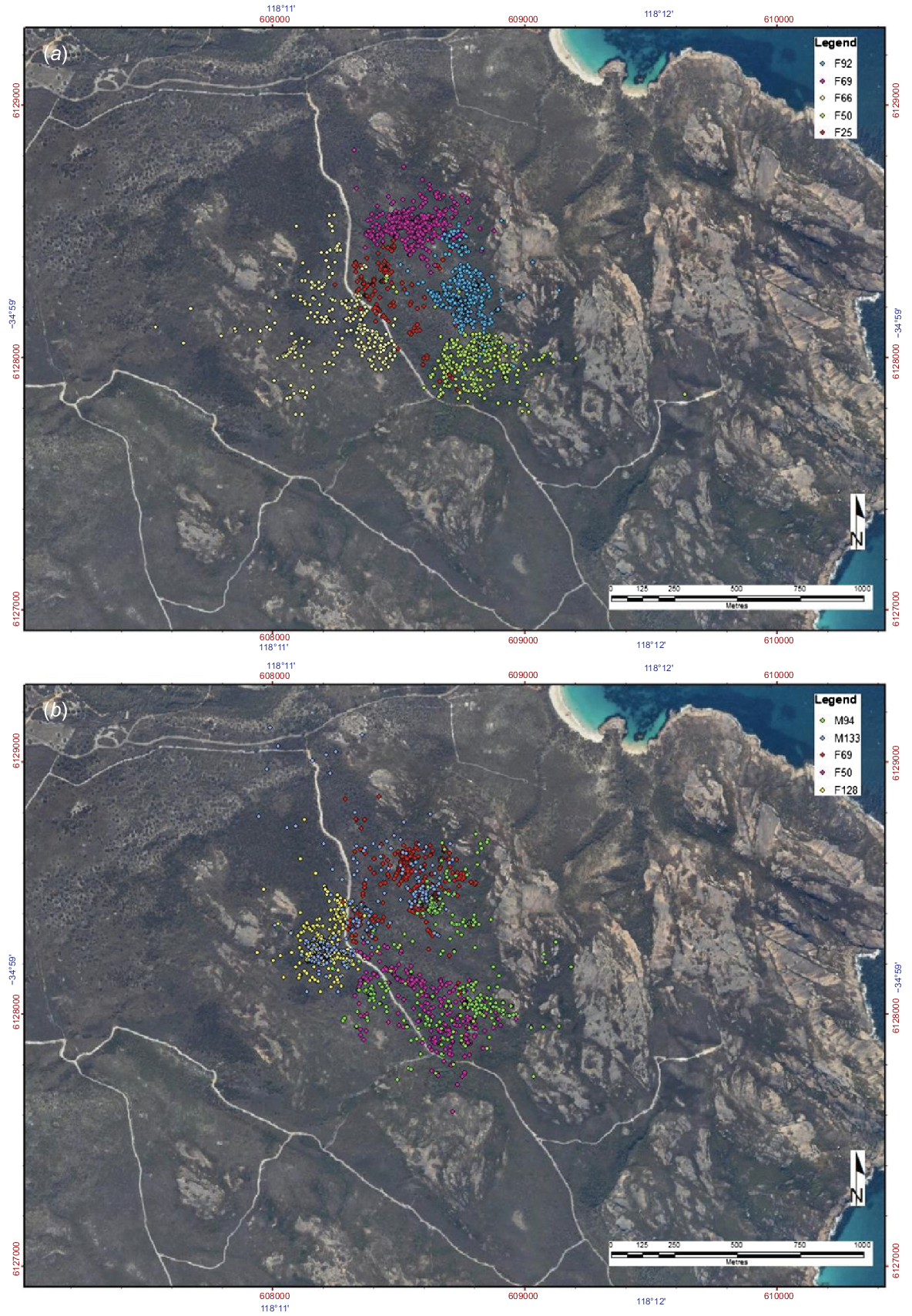
Home range size was estimated from these maps by drawing the smallest convex polygon (no inward corners) surrounding all points (Mohr and Stumpf 1966; Harris et al. 1990). Home range sizes ranged from 6.3 ha to 56.1 ha in the 4 years of study (Table 1). The mean home range size (±s.d.) was 33.7 ± 17.0 ha for males (n = 7) and 19.2 ± 7.0 ha for females (n = 14). The large difference in home range size between the sexes can be explained in terms of social behaviour, as females are sedentary, while males travel across the home ranges of several females to find individuals in oestrus. There was little movement by females up the sides of Firebreak Valley onto the exposed granite ridges, implying that they find all their feeding and nesting requirements in the dense low heath on the valley sides. Short excursions by males appear related to social, rather than feeding movements.
| Year | Potoroo (sex and number) | Home range (ha) | |
|---|---|---|---|
| 2002 | F25 | 16.7 | |
| 2002 | F50 | 9.7 | |
| 2002 | F66 | 13.5 | |
| 2002 | M24 | 26.2 | |
| 2002 | M26 | 43.8 | |
| 2002 | M65 | 6.3 | |
| 2004 | F25 | 19.8 | |
| 2004 | F50 | 23.3 | |
| 2004 | F66 | 32.3 | |
| 2004 | F69 | 17.1 | |
| 2004 | F92 | 16.9 | |
| 2006 | F50 | 12.6 | |
| 2006 | F69 | 11.6 | |
| 2006 | F92 | 32.3 | |
| 2006 | M83 | 30.7 | |
| 2006 | M94 | 23.7 | |
| 2009 | F128 | 17.1 | |
| 2009 | F50 | 23.4 | |
| 2009 | F69 | 22.2 | |
| 2009 | M133 | 48.8 | |
| 2009 | M94 | 56.1 |
Home range was estimated from radio-tracked locations using the minimum convex polygon method (Mohr and Stumpf 1966; Harris et al. 1990). All home range estimations were based on more than 170 fixes, except for M65 in 2004 (n = 51 fixes).
Comparison with published data on other potoroo species reveals strong differences in home range size among species. Green et al. (1998) found that home range areas of P. longipes at two sites in East Gippsland, Victoria, were of similar size to Ngilgaitch/P. gilbertii (Bellbird, 22.3–59.9 ha; Riley, 14.3–22.7 ha). In contrast, home ranges of P. tridactylus measured in two Victorian studies were much smaller in size. Male home ranges averaged 4.0 ha and females 1.9 ha at a site near Warrnambool in western Victoria (Long 2001), while male home ranges averaged 13.7 ha and females 6.7 ha in the Great Otway National Park (Le Pla et al. 2024). The strong difference in home range size between these potoroo species may relate to diet. Both Ngilgaitch/P. gilbertii (Nguyen et al. 2005) and the long-footed potoroo (Green et al. 1999) are highly fungi-dependent species whose diets comprise over 80% fungal sporocarps throughout the year. P. tridactylus is much more omnivorous, with a diet containing less fungal biomass, between 25% (spring–summer) and 70% (winter), supplemented with leaves, fruits, seeds, and arthropod material (Bennett and Baxter 1989).
The intensive use of the Melaleuca low dense heath by Ngilgaitch/P. gilbertii contrasts with the initial descriptions left by John Gilbert in the 1840s, who described the animal as being ‘….the constant companion of H. brachyurus [Halmaturus brachyurus is the old scientific name for the Quokka], inhabiting with them the dense thickets of spearwood and rank vegetation surrounding swamps or small running streams’ (John Gilbert 1841 published in Gould 1863, 1973). A change in preferred habitat could be due to the influence of V. vulpes, not present in south-western Australia until 80 years after Gilbert’s visit (Fairfax 2019). However, it is clear that Ngilgaitch/P. gilbertii had always favoured dense vegetation, and the structure of long-unburnt Melaleuca heath (see fire history in Orr et al. 1993) would provide some protection from fox predation. Knowledge of the spatial organisation, habitat preference, and home range size of Ngilgaitch/P. gilbertii has allowed an understanding of the requirements of the species when considering potential suitable sites for translocation.
Population genetics and mating system
Population genetics and genomics studies have been conducted using mitochondrial DNA, microsatellite DNA markers, and single nucleotide polymorphisms (SNPs) (Sinclair et al. 2002; Ottewell et al. 2023). Three maternally inherited mitochondrial DNA lineages were initially identified by Sinclair et al. (2002) from potoroos sampled between 1994 and 1996 (n = 29), of which one was common (22 out of 25 individuals). A single lineage was later detected by Ottewell et al. (2023) from potoroos sampled between 1994 and 2021 (n = 236), who suggested sequencing error may account for two of the earlier lineages or they have since declined to undetectable levels. No tissue samples with the rare lineages of Sinclair et al. (2002) were included in Ottewell et al. (2023). Effectively only a single female lineage has been breeding within the Two Peoples Bay Nature Reserve population. Evidence of a genetic bottleneck was detected also in nuclear microsatellite DNA markers; however, excess gene diversity (higher than expected heterozygosity 49.3%) and a relatively high genetic effective population size (Ne = 242–362; Sinclair et al. 2002) suggests the bottleneck was recent, most likely occurring since European colonisation. This result, implying a large historic decrease in total population, is also consistent with the ‘common’ status in collection notes by John Gilbert from the 1840s (Gould 1863, 1973).
Sample sizes were too small to assess population structure associated with fine-scale habitat use and too few markers were available in the early studies to assign paternity with confidence in wild Ngilgaitch/P. gilbertii to determine the mating system. Frankham et al. (2012b) used microsatellite DNA markers to conclude that P. tridactylus had a promiscuous/polygynous mating system, although one female had three young sired by the same male over multiple years, suggesting the mating system was not entirely promiscuous. P. longipes appears to have a monogamous mating system (Scotts and Seebeck 1989; Green and Mitchell 1998). A similar observation was made in the Ngilgaitch/P. gilbertii captive colony from 1996 to 1998, during which one female had three young sired by the same male but did not produce any young when paired with a different male known to be fertile (Courtenay 1997). Further research will be required to understand the mating system in wild Ngilgaitch/P. gilbertii.
A similar higher than expected heterozygosity was also detected in 501 SNPs, whereby Ottewell et al. (2023) suggested positive selection on heterozygotes might also be driving the maintenance of genome-wide diversity. Further research is needed to confirm these observations. Nonetheless, Ngilgaitch/P. gilbertii will be susceptible to erosion of genetic variation if the population remains small over a prolonged period of time. The new ‘insurance’ populations (see below) were shown to have lower levels of genetic diversity relative to the original population in Two Peoples Bay prior to 2015 (Ottewell et al. 2023). A draft chromosome level genome was assembled for Ngilgaitch/P. gilbertii in 2021 (https://www.dnazoo.org/assemblies/potorous_gilbertii) and will be an invaluable resource for further research into genetic issues, such as inheritance of renal oxalosis (Forshaw et al. 2017).
Predation of Ngilgaitch/Gilbert’s potoroo at Two Peoples Bay
Records of F. catus in the King George Sound area date back to 1826 (Abbott 2002). V. vulpes was introduced to Australia in the late 19th century (Rolls 1969) and was most likely present along the south coast by 1925 (Fairfax 2019). Both species remain major drivers of mammalian species decline in Australia (Woinarski et al. 2015). Heinsohn (1968) suggested that the absence of V. vulpes was a major factor in the continuing high numbers of P. tridactylus in Tasmania. In addition to foxes and feral cats, natural predators on Ngilgaitch/P. gilbertii include the Waargul/southwestern carpet python (Morelia imbricata) and potentially the masked owl (Tyto novaeholladiae) (Friend 2023). At Two Peoples Bay, regular fox baiting began in 1988 as a management strategy to protect the Djimaalap/noisy scrub-bird (Atrichornis clamosus) population. A subsequent reduction in fox numbers possibly enabled the surviving population of Ngilgaitch/P. gilbertii to increase to detectable numbers.
Predation incidents on transmittered potoroos by carpet pythons had been recorded in the early 2000s, so to gather further data on causes of mortality, transmitters were fitted to as many animals at Two Peoples Bay as resources permitted. Between 2000 and 2018, 157 transmitters were deployed on Ngilgaitch/P. gilbertii, many sequentially to the same individuals. Seven animals were taken by carpet pythons and one by a fox during that period. The fox victim and one of the carpet python victims had just been translocated from a 14-ha offsite enclosure, and it is likely that these animals were still unfamiliar with their new site and thus more vulnerable to predation than the resident animals. The other six carpet python victims were Two Peoples Bay residents before the 2015 fire, so clearly carpet pythons were a potential factor in Ngilgaitch/P. gilbertii population regulation. No predation by diurnal or nocturnal birds of prey has been confirmed to date.
Eight animals were released back into Two Peoples Bay Nature Reserve in 2022, 7 years after the fire under a reinforcement translocation. Two of these animals were predated in the weeks after their release. One potoroo was found inside a carpet python, while cat DNA was identified in swabs from the transmitter and remains of the other one. Both animals were found in the extensive area burnt in 2015, with vegetation still less dense than before the fire. Less dense cover may have been a factor in these predations, as well as the animals adjusting to the new site. Cat control by twice-yearly trapping and distribution of Eradicat baits (Algar and Burrows 2004) was already under way at Two Peoples Bay by 2015. Soon after the predation event by the cat was discovered, planned deployment of three Felixer grooming traps (Read et al. 2019) was also implemented, strengthening feral cat control on the headland. The effect of carpet python predation on Ngilgaitch/P. gilbertii population dynamics is poorly understood and the subject of ongoing research.
Parasites and other health issues
Small population size, common in threatened species, leaves species vulnerable to extinction through low levels of genetic diversity, reduced reproductive fitness and disease, and limited ability to adapt to changing environmental conditions (Frankham 2005, 2010). A highly diverse range of parasitic taxa including protists, nematodes, insects, arachnids, and crustaceans are known from Australian wildlife (Spratt and Beveridge 2018). An extensive list of known protozoan parasites has also been recorded from Australian vertebrates and invertebrates, some of which are known from populations of the eastern Australian P. tridactylus (O’Donoghue and Adlard 2000). Managing the health of captive Ngilgaitch/P. gilbertii during the early years of the recovery program, as well as the field-based program to monitor the wild population, led to a PhD study on health and disease issues previously reported in Ngilgaitch/P. gilbertii (Vaughan 2008) and another focused on haemoparasites including trypanosomes (Austen 2015). These studies led to the discovery of parasitic infections and other health issues experienced by wild and captive potoroos.
The presence of a trypanosome, along with another two haemoparasites (Clark et al. 2004), was recorded from blood samples that had been collected during routine health assessments of wild and captive animals. A new species, described as Trypanosoma copemani n.sp. by Austen et al. (2009), was present in seven wild Ngilgaitch/P. gilbertii and three S. brachuyrus captured in one trapping session within Two Peoples Bay Nature Reserve. Trypanosomes, a group of unicellular blood-borne parasitic protozoa, are generally transmitted via blood-sucking insects such as flies and ticks, with several species identified in Australian vertebrate species (Austen et al. 2009). Some trypanosomes are known to cause severe disease in animals including humans, although to date there is little information on their impact on the health of Australian native wildlife (Austen et al. 2015). Austen et al. (2009) noted there were no obvious clinical signs of disease in Ngilgaitch/P. gilbertii or S. brachyurus, and that the parasites had most likely co-evolved with their hosts. The tick, Ixodes australiensis, was identified as the vector for T. copemani, based on ticks collected from Ngilgaitch/P. gilbertii and S. brachyurus at Bald Island and Two Peoples Bay (Austen et al. 2011). Ixodes australiensis is endemic to Western Australia, but not regarded as host-specific, since it has been recorded from several small marsupials, kangaroos, dogs, cows, and humans (Roberts 1960; Austen et al. 2011).
Other parasites identified from Ngilgaitch/P. gilbertii include the early developmental stage microfilaria from a nematode (O’Donoghue and Adlard 2000) and the piroplasm Theileria gilberti n. sp. (Lee et al. 2009). Piroplasmorida are responsible for economically important diseases of domestic and wild animals, with the genus Theileria distinguished by infection of leukocytes by sporozoites, maturation of schizonts into merozoites, and subsequent infection of red blood cells to form piroplasms (Uilenberg 2006). Phylogenetic studies using piroplasm rDNA indicate marsupial Theileria species form a unique clade (Paparini et al. 2012; Mans et al. 2015; Storey-Lewis et al. 2018; Barbosa et al. 2019), although limited information is available to determine whether they are host and vector specific. Little is known about the prevalence and clinical impact of piroplasms on marsupial health (Paparini et al. 2012), although stress may have been a contributor in one case from a juvenile platypus (Ornithorhynchus anatinus) (Kessell et al. 2014).
Cryptococcosis is a yeast infection acquired from the natural environment and is commonly reported in the koala (Phascolarctos cinereus; Krockenberger et al. 2003), although cases have been reported in other marsupials (Vaughan et al. 2007). Two species have been associated with disease in potoroos, Cryptococcus gattii in P. tridactylus and Cryptococcus neoformans in Ngilgaitch/P. gilbertii (Vaughan et al. 2007).
Six Ngilgaitch/P. gilbertii in the captive colony died or were euthanised over a 12-year period, with post-mortem examinations showing severe renal oxalosis (Forshaw et al. 2017). A disorder of oxalate metabolism was subsequently confirmed and suggested the condition was similar to primary hyperoxaluria type 1 in humans. Five of the Ngilgaitch/P. gilbertii were closely related, suggesting the disease is also genetically inherited (Forshaw et al. 2017).
Wild and captive populations of Ngilgaitch/P. gilbertii had a long history of balanoposthitis (an inflammation of the penis and prepuce) with associated discharge or crusting, ulceration, and preputial discharge (Vaughan-Higgins et al. 2011). The discharge was associated with Treponema, Actinobacillus, and Pasteurella bacteria. Treponema infections in animals are not common, although the clinical correlations in Ngilgaitch/P. gilbertii were similar to those in the European rabbit (Oryctolagus cuniculus), an introduced species present in Two Peoples Bay Nature Reserve. The disease in rabbits decreases rates of conception, causes placental retention, and leads to neonatal deaths (Froberg et al. 1993; Saito et al. 2003). However, the overall impact on reproduction and health in Ngilgaitch/P. gilbertii has not been determined and infections were present in wild and captive animals. Adult females with a Treponema infection were still reproducing, although associated disease may potentially reduce fertility and fecundity (Vaughan-Higgins et al. 2011).
Development of recovery strategies
The captive breeding colony was established primarily to reduce the likelihood of extinction of Ngilgaitch/P. gilbertii through loss of the only population in the event of a large bushfire on the Maardjitup Gurlin/Mount Gardner headland. The potential of captive breeding to provide animals for translocation to new sites was also acknowledged in early recovery planning (Start and Burbidge 1995; Courtenay et al. 1998). If the existing habitat at Two Peoples Bay was at carrying capacity, then establishment of new wild colonies through translocation could increase overall numbers, providing more security for the species against catastrophe and progress overall species recovery. Translocations of threatened species to increase numbers and reduce extinction risk are now a common practice that has significantly improved the conservation status of many species worldwide (Seddon et al. 2014; Hoffmann et al. 2015).
It was clear by 2004 that the captive breeding program would neither guarantee the species’ survival if the wild population were lost, nor provide sufficient new animals for a translocation program. Alternative approaches to producing additional Ngilgaitch/P. gilbertii for translocation were examined by Friend (2004), based on the finding that the Two Peoples Bay population was at carrying capacity and more young animals were reaching independence than could be accommodated in the available habitat. The surplus young could therefore be translocated to new sites. However, removal of individuals from the tiny wild population would need to be done with great care. The impact of taking a single individual would increase with its age; removal of a breeding adult would have a much greater impact on the reproductive potential of the population than removal of a pouch young. Available methods of removal of individuals from the wild population were as follows, in order of increasing impact:
Cross-fostering of Ngilgaitch/P. gilbertii pouch young at 30–60 days (Merchant and Sharman 1966; Friend 2008; Taggart et al. 2010). This method could provide captive stock for translocation, as well as allowing the donor female to re-enter oestrus early and thus increase the production of young;
Hand rearing pouch young at 120 days. This method could also provide a captive group while reducing mortality of newly independent young at a vulnerable stage in the wild;
Removal of independent sub-adult animals; and
Removal of adult breeding animals.
Initial cross-fostering trials were carried out between 2001 and 2003. Three small Ngilgaitch/P. gilbertii pouch young were taken from the wild and placed in small incubators for transport on commercial flights to Adelaide. On arrival at the P. tridactylus facility, the young were cross-fostered to captive female P. tridactylus (Taggart et al. 2010). None of the pouch young survived to pouch exit, although they were accepted initially by the foster-mothers. A breeding colony of P. tridactylus was set up near Albany in 2006 to eliminate the 12-h pouch separation interval due to transport, so that further cross-fostering trials could be conducted. Six pouch transfers were achieved between 2006 and 2009, with two Ngilgaitch/P. gilbertii young reared successfully to pouch exit and weaning. While the successes were encouraging, a rate as low as this would not significantly increase the production of young in the wild or justify the high cost of maintaining the surrogate colony.
Hand-rearing of late-stage pouch young was trialled with two individuals, a female in 2004 and a male in 2005, both removed from their wild mothers’ pouches at around 10 days before natural pouch exit. Eunice Daubert, a volunteer wildlife carer based in Albany, took on the responsibility of caring for each of the young animals, initially feeding them with milk formula, then weaning them onto solid food. The female did well throughout the process and was eventually transferred to the Two Peoples Bay breeding colony, but the male suffered from diarrhoea and his weight fluctuated significantly before he could also join the breeding colony. The effort and stress involved were judged to be too great to persist with this method.
Translocation to Bald Island
Simultaneously in 2005, however, approval was granted for a trial translocation, involving the temporary transfer of a male and female adult Ngilgaitch/P. gilbertii to Bald Island for up to 6 weeks (Friend 2004). This 810 ha granite island, 25 km east of Two Peoples Bay, was the site of a successful introduction of the endangered Djimaalap/A. clamosus from Two Peoples Bay, commencing in 1993 (Danks 1997). The island has a natural population of Quokkas/S. brachyurus, with no snakes, foxes, or cats present. Both potoroos were fitted with mortality-sensing drop-off transmitters to allow daily status monitoring and weekly trapping to assess weight change. The two animals were flown by helicopter from Two Peoples Bay to the island on 18 February 2005 and released in Melaleuca woodland with a dense ground cover of Lepidosperma angustatum clumps. Daily status checks on the animals by radio-tracking and attempts to trap them weekly to assess their weight and condition were conducted. Radio-tracking showed that the animals found suitable nest sites every day and new diggings could be found near their locations. The animals initially separated after release, but 2 weeks later, the male found the female in a fungi-rich area, and they subsequently remained together (Friend et al. 2005).
Both animals were removed from the island after 5 weeks, as the male had dropped his transmitter, and both animals were serendipitously trapped on the same morning. Both animals had lost weight in the first 2 weeks but regained it. Identification of fungal spore-types in scats collected from the cage traps indicated that the translocated animals were able to quickly access a similar diversity of fungal species as on the mainland (Bougher et al. 2008; Bougher and Friend 2009). A second proposal for a permanent translocation for potoroos to Bald Island was approved, as the trial had shown that Ngilgaitch/P. gilbertii could sustain themselves and find nest sites. Ten animals (five males and five females) were captured at Two Peoples Bay and transferred to the island between August 2005 and December 2007. The slow rate of removal of animals was justified by the finding that the number of animals KTBA in the Two Peoples Bay population had increased over the period of removal (Fig. 5). The new island population grew quickly and in June 2010, 49 animals were recorded in one monitoring trip. The current number KTBA in 2024 is 53 potoroos, although a total of 71 had been achieved in 2012.
Translocation to Waychinicup National Park enclosure
A second insurance population was established in 2010 within a purpose-built enclosure fenced with a fox- and cat-proof floppy-top fence (Moseby and Read 2006). The fence enclosed 380 ha of woodland, sedgeland and heathland adjacent to Normans Beach in the eastern section of Waychinicup National Park, approximately 10 km from Two Peoples Bay. The location was chosen because of the presence of at least 50 ha of the heathland dominated by Melaleuca striata favoured by Ngilgaitch/P. gilbertii on Maardjitup Gurlin/Mount Gardner (S8 Headland Mixed Dense Low Heath of Hopkins et al. 2024b). The founder population comprised nine animals, three from Two Peoples Bay and six from Bald Island. The population grew more slowly than that on Bald Island, probably due to the observed predation by carpet pythons. An additional 41 animals were introduced to the enclosure from Bald Island between 2010 and 2014. The KTBA for the enclosure now varies between 25 and 30 animals that occupy granitic substrates with Melaleuca heathland and jarrah (Eucalyptus marginata) woodland.
Population effects of the 2015 fire
Following the rediscovery of Ngilgaitch/P. gilbertii in 1994, the high risk that a large fire on the Maardjitup Gurlin/Mount Gardner headland would pose to the species’ survival was quickly identified (Start and Burbidge 1995). Since 1970, management of the Two Peoples Bay Nature Reserve had focused on exclusion of fire from the Djimaalap/A. clamosus areas (Smith 1987). The successful implementation of this policy saw the scrub-birds thrive (Danks 1997), as well as allowed the single surviving population of Ngilgaitch/P. gilbertii to persist long enough for the chance rediscovery to occur. Trapping and radio-tracking of the only known population provided evidence that Ngilgaitch/P. gilbertii favoured the densest vegetation in this long-unburnt habitat (Courtenay and Friend 2004), an assumption made in initial conservation planning (Start and Burbidge 1995). The downside of fire exclusion, however, was the generation of high fuel levels on the headland, perhaps mitigated to a degree by a loss of plant biomass through an expanding Phytophthora infection (Hart et al. 2024).
Accidental fire was not absent from the headland, despite the creation of a 100-m wide, slashed low fuel zone across the isthmus (Fig. 2c). Between 2000 and 2015, there were three small fire incidents, two caused by lightning and one by a discarded cigarette, all of which occurred under light winds and were extinguished before spreading far. On 14 November 2015, however, the combined effect of two very dry winters, a severe lightning storm along the south coast, and high winds over following days resulted in the development a large fire that was difficult to contain, partly due to the steep, rocky terrain. Despite the concerted efforts of DBCA’s fire crews, the fire spread through most of the area inhabited by Ngilgaitch/P. gilbertii, with only 10% of suitable habitat remaining unburnt, on the west side of Firebreak Valley.
By chance, a trapping census had been under way at the time and nine animals had been fitted with transmitters. The signals of seven animals were located after the fire and during November and December, six of those with transmitters and another that had not been recorded since 2012 were captured and released. In December–January 2016, the seven potoroos were brought into captivity as it was believed that the remnant habitat was too small to support all the survivors. One female did not eat well, however, and was released after 5 weeks back into the unburnt patch in early March, while in June one of the males died of renal oxalosis (Forshaw et al. 2017). Meanwhile, a trial translocation of two Bald Island animals to 92 ha on Michaelmas Island off Albany in early 2016 had provided ambivalent results, when one animal did well and the other lost weight and died. Even so, translocation of four of the fire survivors to the island was supported and went ahead in July 2016. This translocation failed, however, with the loss of two females, one to starvation and the other in unexplained circumstances. The two surviving males were recaptured in late August and early September and moved via captivity out into the unburnt area in Firebreak Valley.
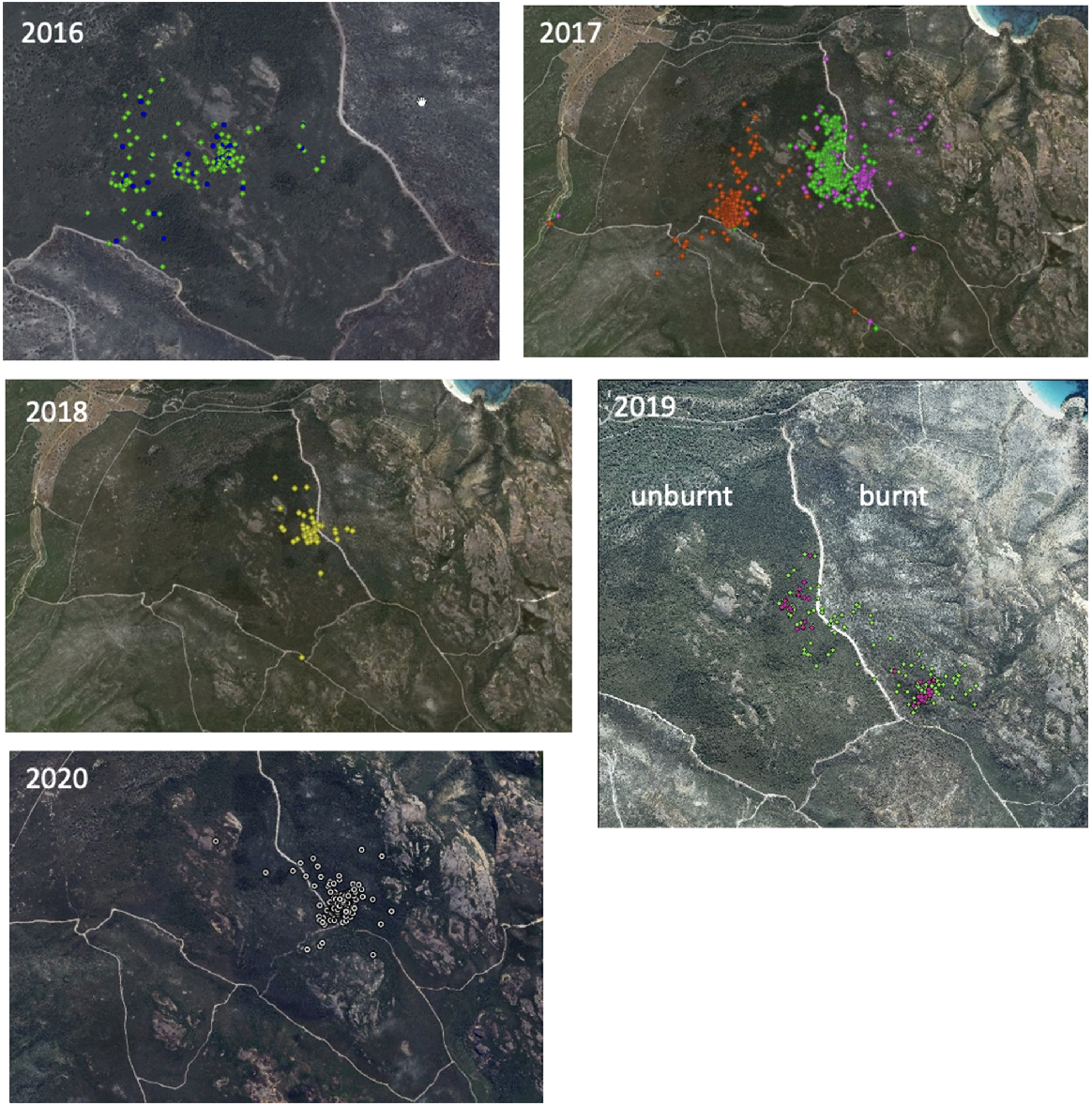
A few days earlier a new female of estimated age 2 years, clearly a survivor of the 2015 fire, had been captured in the unburnt area. An older female survivor of the fire had been released from captivity into the same area in June, so by the end of 2016 the known Ngilgaitch/P. gilbertii population at Two Peoples Bay was five individuals. These animals provided an opportunity to study the relative use of the unburnt vegetation and the burnt habitat as it recovered. Tail-mounted GPS loggers provided a detailed record of nocturnal movements and diurnal nest locations at times over the next 5 years. It was important to discover where the surviving animals were feeding and nesting, and whether they were using the burnt, but previously suitable, habitat. Ten GPS logger deployments (either ATS G10 or Lotek Pinpoint 120 units) were carried out on five animals caught in the unburnt section between 2016 and 2020. Location records during seven deployments over this period revealed progressively more use of the burnt areas, particularly on the eastern side of the firebreak separating burnt and unburnt areas (Fig. 8).
First steps towards post-fire recolonisation
By 2020, the few remaining animals were using burnt habitat extensively, but not exclusively, for both feeding at night and nesting by day. This evidence challenged long-held ideas regarding the requirement for long-unburnt habitat by Ngilgaitch/P. gilbertii (e.g. Friend et al. 2016). It also suggested that areas burnt in 2015 might be able to support animals reintroduced back to the Reserve as early as 5 years after the fire. Consequently, a 3-year program to translocate animals from Bald Island and the Waychinicup enclosure to Maardjitup Gurlin/Mount Gardner and to move smaller groups between all populations for genetic supplementation was proposed (Friend et al. 2022) and approved. In late 2021, GPAG successfully applied for a A$248,000 grant from the Australian Government’s Environment Restoration Fund, which included funding for enhanced feral and native predator management on Maardjitup Gurlin/Mount Gardner to try to reduce predation risk for translocated animals. This funding assisted DBCA to increase frequency of cat trapping, deploy three Felixer grooming traps, and attempt non-lethal control of carpet pythons through hand capture and removal. The first transfers of animals to Maardjitup Gurlin/Mount Gardner from Bald Island and Waychinicup were carried out in 2022. No potoroos that had survived the fire or young born post-fire had been recorded by trapping or camera surveillance in 2021 or 2022.
In total, eight potoroos from insurance populations were released between September and December 2022, comprising a male and a female from the Waychinicup enclosure and three males and three females from Bald Island. All were released into the unburnt area with a tail-mounted package containing a transmitter and a GPS logger, which remained on the animals for 3−6 weeks before being recovered. The most notable finding was the early movement of all animals into the areas burnt in 2015. Trapping continued in both fire ages (7 years post-fire and 50+ years post-fire), but no animals were captured in the long-unburnt vegetation where the new releases had occurred. Two of the females were taken by predators before their transmitters had fallen off: one by a feral cat 20 days after release; and one by a carpet python 30 days after release. Translocated animals were ranging over the 90% of the Reserve that had been burnt, rather than the 10% that had not, so the task of trapping the animals to monitor weight change was very difficult. Nevertheless, all but two of the six surviving potoroos had been trapped to check condition by early February 2023, providing the opportunity to assess weight change since release. All animals checked were within 5% of release weight, and although the average weight change was −3.9%, this was within the predetermined success criterion for weight loss of −5% and indicated that sufficient food was available. A further sign of the adequacy of food resources in the recovering habitat was provided when a female released in December 2022 was found bearing a pouch young in May 2023. The positive results of the first year’s work to return Ngilgaitch/P. gilbertii to Two Peoples Bay allowed the continuation of the process in 2023, with intensified cat control under way.
In response to the severe effects of the 2015 fire on the Ngilgaitch/P. gilbertii population at Two Peoples Bay, funds were sought from the Australian Government to establish a new insurance population. Funding for 1 year under the Commonwealth’s National Landcare Program was delivered via GPAG in 2017 to translocate founder animals from existing sites to 1080 ha Middle Island, 600 km east of Albany, in the Recherche Archipelago Nature Reserve (Fig. 2b). Based on the positive results of two intensively monitored trials of four potoroos in July 2017 and February 2018, a full translocation of 10 animals in July 2018 was approved and implemented. A camera array of 10 cameras across the island has revealed that in August 2024, potoroos were established on granite substrates over half of the island, now classed as a third insurance population for the species.
A future under climate change
Ngilgaitch/P. gilbertii still holds the unenviable title of the world’s rarest marsupial, despite the tripling of the original population numbers through 30 years of management, research and conservation efforts. It is likely that the exposed granite and deep gullies of the Maardjitup Gurlin/Mount Gardner area that formed a natural refuge against fire allowing the Djimaalap/A. clamosus to persist (Danks 1997) provided similar protection for small groups of Ngilgaitch/P. gilbertii. The establishment of insurance populations on Bald Island in 2005 and in Waychinicup National Park in 2010 undoubtedly prevented extinction of Ngilgaitch/P. gilbertii following the 2015 fire on Maardjitup Gurlin/Mount Gardner (Bolam et al. 2021), significantly improving its long-term conservation status. New knowledge of population dynamics, habitat preference and use, the highly specialised diet, disease challenges, and genetic diversity has contributed to a better understanding of requirements for the persistence of wild Ngilgaitch/P. gilbertii populations. Numbers in the new insurance populations have increased steadily to apparent carrying capacity, but the global population is still critically small, due largely to the currently limited availability of suitable habitat free of foxes and feral cats. Genetic and genomic information suggests the original population had already been suffering from a severe, but likely recent genetic bottleneck. On the positive side, higher-than-expected heterozygosity has been maintained, possibly through male dispersal and heterozygosity advantage.
The historical and current distributions of Ngilgaitch/P. gilbertii in the extreme south-west of Western Australia are closely linked to areas of high annual rainfall that also receive summer rainfall, required for a year-round reliance on fungi. Thus, declining rainfall and future projections for a warming environment associated with climate change in this southern-most location in Western Australia (Andrys et al. 2017; Hopkins et al. 2024a) raise deep concern for the persistence of habitat suitable for Ngilgaitch/P. gilbertii. In fact, an assessment of Australia’s most imperilled vertebrates by an expert panel (Garnett et al. 2022) suggested a 25% likelihood of extinction for this species by 2041. Understanding spatial and temporal changes in diet of wild Ngilgaitch/P. gilbertii will also be important in predicting the impact of a warming and drying climate on fungi assemblages. The establishment of isolated but managed populations along Western Australia’s south coast has enabled an increase in overall numbers and provides vital insurance against loss in a single location due to fire, disease, or introduced predators.
A range of stakeholders including Traditional Custodians, community groups, university scientists, and DBCA conservation management and science staff participated in a 2-day Structured Decision-Making workshop in June 2024 to clarify future directions for Ngilgaitch/P. gilbertii under potential management scenarios. Detailed costings of the various scenarios, with further consultations and a final report with recommendations are expected in 2025. In the meantime, a strategy of continuing to establish new populations in suitable habitat on the mainland with effective fox and cat control and possibly on additional islands must be seen as the most promising action to reduce the species’ risk of extinction. Areas with higher annual and summer rainfall should be favoured during the site selection process.
Declaration of funding
Funding has been provided by the Western Australian Department of Biodiversity, Conservation and Attractions, the Australian Commonwealth Government under various funding programs including the National Landcare Program through South Coast Natural Resource Management; Edith Cowan University, Gilbert’s Potoroo Action Group, Western Australian State Natural Resource Management Program, National Geographic Society, Earth Sanctuaries Foundation, and the BankWest Landscope Conservation Visa Card Program.
Acknowledgements
We acknowledge that Two Peoples Bay is part of Merningar Bardok Country and that Noongar Peoples are the Traditional Custodians of this Country. We thank Clemency Fisher for originally drawing our attention to the Ngilgaitch/Gilbert’s potoroo specimen at the National Museum in Wales in 2001, all members of the Gilbert’s Potoroo Recovery Team, Gilbert’s Potoroo Action Group and community volunteers. Tim Button, Stephanie Hill, Val Hack, and Susanne Schreck gave substantial and excellent technical support to the recovery program, particularly in fieldwork, data management, and captive husbandry. We are grateful for the work of Dr Denis Saunders, the Guest Editor of the Pacific Conservation Biology collection on The Natural History of Two Peoples Bay Nature Reserve, Western Australia, and two reviewers (Emeritus Professor Andrew Bennett and Dr Jeff Short) for significantly improving our manuscript.
References
Abbott I (2001) Aboriginal names of mammal species in south-west Western Australia. CALM Science 3, 433-486.
| Google Scholar |
Abbott I (2002) Origin and spread of the cat, Felis catus, on mainland Australia, with a discussion of the magnitude of its early impact on native fauna. Wildlife Research 29, 51-74.
| Crossref | Google Scholar |
Abbott I (2008) Historical perspectives of the ecology of some conspicuous vertebrate species in south-west Western Australia. Conservation Science Western Australia 6, 1-214.
| Google Scholar |
Algar D, Burrows ND (2004) Feral cat control research: Western Shield review—February 2003. Conservation Science Western Australia 5, 131-163.
| Google Scholar |
Andrys J, Kala J, Lyons TJ (2017) Regional climate projections of mean and extreme climate for the southwest of Western Australia (1970–1999 compared to 2030–2059). Climate Dynamics 48, 1723-1747.
| Crossref | Google Scholar |
Austen JM, Jeffries R, Friend JA, Ryan U, Adams PJ, Reid SA (2009) Morphological and molecular characterization of Trypanosoma copemani n. sp. (Trypanosomatidae) isolated from Gilbert’s potoroo (Potorous gilbertii) and Quokka (Setonix brachyurus). Parasitology 136, 783-792.
| Crossref | Google Scholar | PubMed |
Austen JM, Ryan UM, Friend JA, Ditcham WGF, Reid SA (2011) Vector of Trypanosoma copemani identified as Ixodes sp. Parasitology 138, 866-872.
| Crossref | Google Scholar | PubMed |
Austen JM, Reid SA, Robinson DR, Friend JA, Ditcham WGF, Irwin PJ, Ryan U (2015) Investigation of the morphological diversity of the potentially zoonotic Trypanosoma copemani in quokkas and Gilbert’s potoroos. Parasitology 142, 1443-1452.
| Crossref | Google Scholar | PubMed |
Barbosa AD, Austen J, Portas TJ, Friend JA, Ahlstrom LA, Oskam CL, Ryan UM, Irwin PJ (2019) Sequence analyses at mitochondrial and nuclear loci reveal a novel Theileria sp. and aid in the phylogenetic resolution of piroplasms from Australian marsupials and ticks. PLoS ONE 14, e0225822.
| Crossref | Google Scholar | PubMed |
Baynes A, Merrilees D, Porter JK (1975) Mammal remains from the upper levels of a late Pleistocene deposit in Devil’s Lair, Western Australia. Journal of the Royal Society of Western Australia 5, 97-117.
| Google Scholar |
Bennett AF, Baxter BJ (1989) Diet of the long-nosed potoroo, Potorous tridactylus (Marsupialia, Potoroidae), in southwestern Victoria. Wildlife Research 16, 263-271.
| Crossref | Google Scholar |
Bolam FC, Mair L, Angelico M, Brooks TM, Burgman M, Hermes C, Hoffmann M, Martin RW, McGowan PJK, Rodrigues ASL, et al. (2021) How many bird and mammal extinctions has recent conservation action prevented? Conservation Letters 14, e12762.
| Crossref | Google Scholar |
Bougher NL, Friend JA (2009) Fungi consumed by translocated Gilbert’s potoroos (Potorous gilbertii) at two sites with contrasting vegetation, south coastal Western Australia. Australian Mammalogy 31, 97-105.
| Crossref | Google Scholar |
Bougher NL, Lebel T (2001) Sequestrate (truffle-like) fungi of Australia and New Zealand. Australian Systematic Botany 14, 439-484.
| Crossref | Google Scholar |
Bougher NL, Friend JA, Bell L (2008) Fungi available to and consumed by translocated Gilbert’s Potoroos: Preliminary assessments at three translocation sites. Department of Environment and Conservation, Perth. Available at https://library.dbca.wa.gov.au/FullTextFiles/024745.pdf
Burbidge AA, McKenzie NL (1989) Patterns in the modern decline of Western Australia’s vertebrate fauna: causes and conservation implications. Biological Conservation 50, 143-198.
| Crossref | Google Scholar |
Claridge AW, Cork SJ (1994) Nutritional-value of hypogeal fungal sporocarps for the long-nosed potoroo (Potorous-tridactylus), a forest-dwelling mycophagous marsupial. Australian Journal of Zoology 42, 701-710.
| Crossref | Google Scholar |
Claridge AW, Tanton MT, Cunningham RB (1993) Hypogeal fungi in the diet of the long-nosed potoroo (Potorous tridactylus) in mixed species and regrowth Eucalypt forest stands in south-eastern Australia. Wildlife Research 20, 321-337.
| Crossref | Google Scholar |
Cochrane JA, Friend JA, Hill SJE (2005) Endozoochory and the Australian bluebell: Consumption of Billardiera fusiformis (Labill.) Payer (Pittosporaceae) seeds by three mammal species at Two Peoples Bay Nature Reserve, Western Australia. Journal of the Royal Society of Western Australia 88, 191-196.
| Google Scholar |
Cook DL (1963) The fossil vertebrate fauna of Strongs Cave, Boranup, Western Australia. Western Australian Naturalist 8, 153-162.
| Google Scholar |
Courtenay JM, Friend JA (2004) Gilbert’s Potoroo (Potorous gilbertii) Recovery Plan (2003–2008). Western Australian Wildlife Management Program No.32. Department of Conservation and Land Management. Available at https://www.dcceew.gov.au/sites/default/files/documents/p-gilbertii.pdf
Courtenay J, Start AN, Burbidge AA (1998) Gilbert’s potoroo recovery plan, 1998–2007. Department of Conservation and Land Management, Perth, WA, Australia. Available at https://library.dbca.wa.gov.au/FullTextFiles/021262.pdf
Danks A (1997) Conservation of the Noisy Scrub-bird: a review of 35 years of research and management. Pacific Conservation Biology 3, 341-349.
| Crossref | Google Scholar |
Dortch J, Wright R (2010) Identifying palaeo-environments and changes in Aboriginal subsistence from dual-patterned faunal assemblages, south-western Australia. Journal of Archaeological Science 37, 1053-1064.
| Crossref | Google Scholar |
Elmore LW, Miller DA, Vilella FJ (2005) Foraging area size and habitat use by red bats (Lasiurus borealis) in an intensively managed pine landscape in Mississippi. The American Midland Naturalist 153, 405-417.
| Crossref | Google Scholar |
Fairfax RJ (2019) Dispersal of the introduced red fox (Vulpes vulpes) across Australia. Biological Invasions 21, 1259-1268.
| Crossref | Google Scholar |
Forshaw D, Horwitz AM, Ellard K, Friend JA, Greed L, Metz M (2017) Hyperoxaluria, hyperglycoluria and renal oxalosis in Gilbert’s potoroos (Potorous gilbertii). Australian Veterinary Journal 95, 250-258.
| Crossref | Google Scholar | PubMed |
Frankham R (2005) Genetics and extinction. Biological Conservation 126, 131-140.
| Crossref | Google Scholar |
Frankham R (2010) Challenges and opportunities of genetic approaches to biological conservation. Biological Conservation 143, 1919-1927.
| Crossref | Google Scholar |
Frankham GJ, Handasyde KA, Eldridge MDB (2012a) Novel insights into the phylogenetic relationships of the endangered marsupial genus Potorous. Molecular Phylogenetics and Evolution 64, 592-602.
| Crossref | Google Scholar | PubMed |
Frankham GJ, Reed RL, Eldridge MDB, Handasyde KA (2012b) The genetic mating system of the long-nosed potoroo (Potorous tridactylus) with notes on male strategies for securing paternity. Australian Journal of Zoology 60, 225-234.
| Crossref | Google Scholar |
Friend JA (2008) Cross-fostering Gilbert’s potoroos. Landscope 23, 6-8 Available at https://library.dbca.wa.gov.au/Journals/080052/080052-23.025.pdf.
| Google Scholar |
Friend JA, Hill SJE, Button TA (2005) Bald Island getaway for Gilbert’s potoroos. Landscope 21, 48-54 Available at https://library.dbca.wa.gov.au/Journals/080052/080052-21.009.pdf.
| Google Scholar |
Friend JA, Cowen SC, Comer S (2022) Translocation proposal: Gilbert’s potoroo from Bald Island and Waychinicup enclosure to Two Peoples Bay, Waychinicup and Middle Island – population reinforcement and genetic enhancement. Department of Biodiversity, Conservation and Attractions, Perth, WA, Australia.
Froberg MK, Fitzgerald TJ, Hamilton TR, Hamilton B, Zarabi M (1993) Pathology of congenital syphilis in rabbits. Infection and Immunity 61, 4743-4749.
| Crossref | Google Scholar | PubMed |
Garnett ST, Hayward-Brown BK, Kopf RK, Woinarski JCZ, Cameron KA, Chapple DG, Copley P, Fisher A, Gillespie G, Latch P, Legge S, Lintermans M, Moorrees A, Page M, Renwick J, Birrell J, Kelly D, Geyle HM (2022) Australia’s most imperilled vertebrates. Biological Conservation 270, 109561.
| Crossref | Google Scholar |
Glauert L (1948) The cave fossils of the South-West. West Australian Naturalist 1, 100-104.
| Google Scholar |
Glauert L (1950) The development of our knowledge of the marsupials of Western Australia. Journal of the Royal Society of Western Australia 34, 115-134.
| Google Scholar |
Gould J (1863) ‘The mammals of Australia.’ (The Author: London) 10.5962/p.312839
Green K, Mitchell AT (1998) Breeding of the long-footed potoroo, Potorous longipes (Marsupialia: Potoroidae), in the wild: behaviour, births and juvenile independence. Australian Mammalogy 20, 1-7.
| Crossref | Google Scholar |
Green K, Mitchell AT, Tennant P (1998) Home range and microhabitat use by long-footed Potoroos (Potorous longipes). Wildlife Research 25, 357-372.
| Crossref | Google Scholar |
Green K, Tory MK, Mitchell AT, Tennant P, May TW (1999) The diet of the long-footed potoroo (Potorous longipes). Australian Journal of Ecology 24, 151-156.
| Crossref | Google Scholar |
Hart RP, Freebury G, Barrett S (2024) Phytophthora cinnamomi: extent and impact in Two Peoples Bay Nature Reserve, Western Australia (1983–2024). Pacific Conservation Biology 30, PCB24028.
| Crossref | Google Scholar |
Heinsohn GE (1968) Habitat requirements and reproductive potential of the macropod marsupial Potorous tridactylus in Tasmania. Mammalia 32, 30-43.
| Crossref | Google Scholar |
Hoffmann M, Duckworth JW, Holmes K, Mallon DP, Rodrigues ASL, Stuart SN (2015) The difference conservation makes to extinction risk of the world’s ungulates. Conservation Biology 29, 1303-1313.
| Crossref | Google Scholar | PubMed |
Hopkins AJM, Smith GT, Saunders DA (2024a) Introduction to the special issue of The Natural History of Two Peoples Bay Nature Reserve, Western Australia. Pacific Conservation Biology 30, PC24023.
| Crossref | Google Scholar |
Hopkins AJM, Williams AAE, Harvey JM, Hopper SD (2024b) A new vegetation classification for Western Australia’s Two Peoples Bay Nature Reserve and its significance for fire management. Pacific Conservation Biology 30, PC24036.
| Crossref | Google Scholar |
Jarić I, Roll U, Bonaiuto M, Brook BW, Courchamp F, Firth JA, Gaston KJ, Heger T, Jeschke JM, Ladle RJ, Meinard Y, Roberts DL, Sherren K, Soga M, Soriano-Redondo A, Veríssimo D, Correia RA (2022) Societal extinction of species. Trends in Ecology & Evolution 37, 411-419.
| Crossref | Google Scholar | PubMed |
Johnson CN, Isaac JL (2009) Body mass and extinction risk in Australian marsupials: the ‘Critical Weight Range’ revisited. Austral Ecology 34, 35-40.
| Crossref | Google Scholar |
Johnston PG, Sharman GB (1976) Studies on populations on Potorous Desmarest (Marsupialia) I. Morphological variation. Australian Journal of Zoology 24, 573-588.
| Crossref | Google Scholar |
Johnston PG, Sharman GB (1977) Studies on populations of potorous Desmarest (Marsupialia) II. Electrophoretic, chromosomal and breeding studies. Australian Journal of Zoology 25, 733-747.
| Crossref | Google Scholar |
Johnston PG, Davey RJ, Seebeck JH (1984) Chromosome homologies in Potorous tridactylus and P. longipes (Marsupialia: Macropodidae) based on G-banding patterns. Australian Journal of Zoology 32, 319-324.
| Crossref | Google Scholar |
Kessell AE, Boulton JG, Dutton GJ, Woodgate R, Shamsi S, Peters A, Connolly JH (2014) Haemolytic anaemia associated with Theileria sp. in an orphaned platypus. Australian Veterinary Journal 92, 443-449.
| Crossref | Google Scholar | PubMed |
Knapp L, Cummings D, Cummings S, Fielder PL, Hopper SD (2024) A Merningar Bardok family’s Noongar oral history of Two Peoples Bay Nature Reserve and surrounds. Pacific Conservation Biology 30, PC24018.
| Crossref | Google Scholar |
Krockenberger MB, Canfield PJ, Malik R (2003) Cryptococcus neoformans var. gattii in the koala (Phascolarctos cinereus): a review of 43 cases of cryptococcosis. Medical Mycology 41, 225-234.
| Crossref | Google Scholar | PubMed |
Lee JY, Ryan UM, Jeffries R, McInnes LM, Forshaw D, Friend JA, Irwin PJ (2009) Theileria gilberti n. sp. (Apicomplexa: Theileriidae) in the Gilbert’s Potoroo (Potorous gilbertii). Journal of Eukaryote Microbiology 56, 290-295.
| Crossref | Google Scholar | PubMed |
Le Pla M, Hradsky BA, Di Stefano J, Farley-Lehmer TC, Birnbaum EK, Pascoe JH (2024) Movement and ranging behaviour of long-nosed potoroos (Potorous tridactylus) in south-west Victoria, Australia. Wildlife Research 51, WR23013.
| Crossref | Google Scholar |
Long KI (2001) Spatio-temporal interactions among male and female long-nosed potoroos, Potorous tridactylus (Marsupialia: Macropodoidea): mating system implications. Australian Journal of Zoology 49, 17-26.
| Crossref | Google Scholar |
Mans BJ, Pienaar R, Latif AA (2015) A review of Theileria diagnostics and epidemiology. International Journal for Parasitology: Parasites and Wildlife 4, 104-118.
| Crossref | Google Scholar | PubMed |
Merchant JC, Sharman GB (1966) Observations on the attachment of marsupial pouch young to the teats and on the rearing of pouch young by foster-mothers of the same or different species. Australian Journal of Zoology 14, 593-609.
| Crossref | Google Scholar |
Merrilees D (1968) Man the destroyer: late Quaternary changes in the Australian marsupial fauna. Journal of the Royal Society of Western Australia 51, 1-24.
| Google Scholar |
Mohr CO, Stumpf WA (1966) Comparison of methods for calculating areas of animal activity. The Journal of Wildlife Management 30, 293-304.
| Crossref | Google Scholar |
Moseby KE, Read JL (2006) The efficacy of feral cat, fox and rabbit exclusion fence designs for threatened species protection. Biological Conservation 127, 429-437.
| Crossref | Google Scholar |
Murray DC, Haile J, Dortch J, White NE, Haouchar D, Bellgard MI, Allcock RJ, Prideaux GJ, Bunce M (2013) Scrapheap challenge: a novel bulk-bone metabarcoding method to investigate ancient DNA in faunal assemblages. Scientific Reports 3, 3371.
| Crossref | Google Scholar | PubMed |
Nguyen VP, Needham AD, Friend JA (2005) A quantitative dietary study of the ‘Critically Endangered’ Gilbert’s Potoroo (Potorous gilbertii). Australian Mammalogy 27, 1-6.
| Crossref | Google Scholar |
Nuske SJ, Vernes K, May TW, Claridge AW, Congdon BC, Krockenberger A, Abell SE (2017) Redundancy among mammalian fungal dispersers and the importance of declining specialists. Fungal Ecology 27, 1-13.
| Crossref | Google Scholar |
O’Donoghue PJ, Adlard RD (2000) Catalogue of protozoan parasites recorded in Australia. Memoirs of the Queensland Museum 45, 1-163.
| Google Scholar |
Paparini A, Ryan UM, Warren K, McInnes LM, de Tores P, Irwin PJ (2012) Identification of novel Babesia and Theileria genotypes in the endangered marsupials, the woylie (Bettongia penicillata ogilbyi) and boodie (Bettongia lesueur). Experimental Parasitology 131, 25-30.
| Crossref | Google Scholar | PubMed |
Prideaux GJ, Gully GA, Couzens AMC, Ayliffe LK, Jankowski NR, Jacobs Z, Roberts RG, Hellstrom JC, Gagan MK, Hatcher LM (2010) Timing and dynamics of Late Pleistocene mammal extinctions in southwestern Australia. Proceedings of the National Academy of Sciences of the United States of America 107, 22157-22162.
| Crossref | Google Scholar |
Read JL, Bowden T, Hodgens P, Hess M, McGregor H, Moseby K (2019) Target specificity of the felixer grooming “trap”. Wildlife Society Bulletin 43, 112-120.
| Crossref | Google Scholar |
Roberts FHS (1960) A systematic study of the Australian species of the genus Ixodes (Acarina: Ixodidae). Australian Journal of Zoology 8, 392-486.
| Crossref | Google Scholar |
Saito K, Tagawa M, Hasegawa A (2003) Rabbit syphilis diagnosed clinically in household rabbits. The Journal of Veterinary Medical Science 65, 637-639.
| Crossref | Google Scholar | PubMed |
Scotts DJ, Craig SA (1988) Improved hair-sampling tube for the detection of rare mammals. Australian Wildlife Research 15, 469-472.
| Crossref | Google Scholar |
Seddon PJ, Griffiths CJ, Soorae PS, Armstrong DP (2014) Reversing defaunation: restoring species in a changing world. Science 345, 406-412.
| Crossref | Google Scholar | PubMed |
Seebeck JH (1981) Potorous tridactylus (Kerr) (Marsupialia: Macropodidae): its distribution, status and habitat preferences in Victoria. Australian Wildlife Research 8, 285-306.
| Crossref | Google Scholar |
Shortridge GC (1909) An account of the geographical distribution of the marsupials and monotremes of south-west Australia, having special reference to the specimens collected during the Balston expedition of 1904–1907. Proceedings of the Zoological Society of London 79, 803-848.
| Crossref | Google Scholar |
Sinclair EA, Westerman M (1997) Phylogenetic relationships within the genus Potorous (Marsupialia: Macropodidae) based on allozyme electrophoresis and sequence analysis of the cytochrome b gene. Journal of Mammalian Evolution 4, 147-161.
| Crossref | Google Scholar |
Sinclair EA, Danks A, Wayne AF (1996) Rediscovery of Gilbert’s potoroo, Potorous tridactylus in Western Australia. Australian Mammalogy 19, 69-72.
| Crossref | Google Scholar |
Sinclair EA, Murch AR, Di Renzo M, Palermo M (2000) Chromosome morphology in Gilbert’s Potoroo, Potorous gilbertii (Marsupialia: Potoroidae). Australian Journal of Zoology 48, 281-287.
| Crossref | Google Scholar |
Sinclair EA, Costello B, Courtenay JM, Crandall KA (2002) Detecting a genetic bottleneck in Gilbert’s Potoroo (Potorous gilbertii) (Marsupialia: Potoroidae), inferred from microsatellite and mitochondrial DNA sequence data. Conservation Genetics 3, 191-196.
| Crossref | Google Scholar |
Smith GT (1987) The changing environment for birds in the south-west of Western Australia; some management implications. In ‘Nature conservation: the role of remnants of native vegetation’. (Eds DA Saunders, GW Arnold, AA Burbidge, AJM Hopkins) pp. 269–277. (Surrey Beatty & Sons: Chipping Norton, NSW, Australia)
Spratt DM, Beveridge I (2018) Wildlife parasitology in Australia: past, present and future. Australian Journal of Zoology 66, 286-305.
| Crossref | Google Scholar |
Stead-Richardson E, Bradshaw D, Friend T, Fletcher T (2010) Monitoring reproduction in the critically endangered marsupial, Gilbert’s potoroo (Potorous gilbertii): preliminary analysis of faecal oestradiol-17β, cortisol and progestagens. General and Comparative Endocrinology 165, 155-162.
| Crossref | Google Scholar | PubMed |
Storey-Lewis B, Mitrovic A, McParland B (2018) Molecular detection and characterisation of Babesia and Theileria in Australian hard ticks. Ticks and Tick-borne Diseases 9, 471-478.
| Crossref | Google Scholar | PubMed |
Syme K, Lebel T, Hilton RN (2025) Macrofungi of Two Peoples Bay Nature Reserve, Western Australia. Pacific Conservation Biology 31, PC24091.
| Crossref | Google Scholar |
Taggart DA, Schultz DJ, Fletcher TP, Friend JA, Smith IG, Breed WG, Temple-Smith PD (2010) Cross-fostering and short-term pouch young isolation in macropod marsupials: implications for conservation and species management. In ‘Macropods: the biology of Kangaroos, Wallabies and Rat-kangaroos’. (Eds G Coulson, M Eldridge) pp. 263–278. (CSIRO Publishing: Collingwood, Vic, Australia)
Tilbrook L (1983) ‘Nyungar tradition: glimpses of Aborigines of south-western Australia, 1829–1914.’ (University of Western Australia Press: Nedlands, WA, Australia) Available at https://aiatsis.gov.au/sites/default/files/catalogue_resources/m0022954.pdf
Uilenberg G (2006) Babesia—A historical overview. Veterinary Parasitology 138, 3-10.
| Crossref | Google Scholar | PubMed |
Vaughan RJ, Vitali SD, Eden PA, Payne KL, Warren KS, Forshaw D, Friend JA, Horwitz AM, Main C, Krockenberger MB, Malik R (2007) Cryptococcosis in Gilbert’s and long-nosed potoroo. Journal of Zoo and Wildlife Medicine 38, 567-573.
| Crossref | Google Scholar |
Vaughan-Higgins R, Buller N, Friend JA, Robertson I, Monaghan CL, Fenwick S, Warren K (2011) Balanoposthitis, dyspareunia, and treponema in the Critically Endangered Gilbert’s Potoroo (Potorous gilbertii). Journal of Wildlife Diseases 47, 1019-1025.
| Crossref | Google Scholar | PubMed |
Vetten S, Courtenay J, Needham A (1997) Microhabitat use by Gilbert’s Potoroo (Potorous tridactylus gilbertii Gould) in relation to vegetation associations and ground cover. Newsletter of the Australian Mammal Society 2, 22.
| Google Scholar |
Viana DS, Granados JE, Fandos P, Pérez JM, Cano-Manuel FJ, Burón D, Fandos G, Párraga Aguado MA, Figuerola J, Soriguer RC (2018) Linking seasonal home range size with habitat selection and movement in a mountain ungulate. Movement Ecology 6, 1.
| Crossref | Google Scholar | PubMed |
Westerman M, Loke S, Springer MS (2004) Molecular phylogenetic relationships of two extinct potoroid marsupials, Potorous platyops and Caloprymnus campestris (Potoroinae: Marsupialia). Molecular Phylogenetics and Evolution 31, 476-485.
| Crossref | Google Scholar | PubMed |
Winkelmann JR, Bonaccorso FJ, Goedeke EE, Laura J, Ballock LJ (2003) Home range and territoriality in the Least Blossom Bat, Macroglossus minimus, in Papua New Guinea. Journal of Mammalogy 84, 561-570.
| Crossref | Google Scholar |
Woinarski JCZ, Burbidge AA, Harrison PL (2015) Ongoing unraveling of a continental fauna: Decline and extinction of Australian mammals since European settlement. Proceedings of the National Academy of Sciences of the United States of America 112, 4531-4540.
| Crossref | Google Scholar |
Woinarski JCZ, Garnett ST, Legge SM, Lindenmayer DB (2017) The contribution of policy, law, management, research, and advocacy failings to the recent extinctions of three Australian vertebrate species. Conservation Biology 31, 13-23.
| Crossref | Google Scholar | PubMed |
Woinarski JCZ, Garnett ST, Legge SM (2024) No more extinctions: recovering Australia’s biodiversity. Annual Review of Animal Biosciences 13,.
| Crossref | Google Scholar |