Determining the geographic distribution and ecology of the Critically Endangered Kaputar rock skink (Egernia roomi)
Nicholas Gale A * , Jules E. Farquhar
A * , Jules E. Farquhar  B , Amelia Carlesso
B , Amelia Carlesso  A , Kylie Robert
A , Kylie Robert  A § and David G. Chapple
A § and David G. Chapple  B §
B §
A
B
Abstract
Knowledge of species’ distribution and habitat associations is fundamental for conservation planning and management, especially in the context of range-restricted taxa. The Critically Endangered Kaputar rock skink (Egernia roomi) is a high elevation species that is restricted to the Nandewar Ranges (New South Wales, Australia). The species was not formally recognised until 2019, with its distribution, ecology, and threats poorly known.
To determine the geographical distribution of the Kaputar rock skink and explore its ecology and threats.
We performed surveys throughout high elevation regions of Mount Kaputar National Park, targeting suitable habitat for the Kaputar rock skink (rock outcrops and plateaux). Species distributional modelling (SDM) was used to identify potentially suitable habitat outside of our search areas.
We detected the species at all historical record sites and at 15 new sites, increasing the species’ known area of occupancy (AOO) four-fold (from 8 km2 to 40 km2), and elevational range three-fold (from 1360–1480 m to 1147–1509 m).
The AOO for the species now exceeds the IUCN Red List threshold for Critically Endangered, but falls within the range for Endangered under Criterion B. Our SDMs indicated that all predicted suitable habitat for the species falls within the region that we surveyed in this study.
Our study provides valuable information on the geographic range of a threatened lizard species and evaluates the potential impact of large-scale fires on the persistence of the species.
Keywords: conservation status, Egernia roomi, endemic, IUCN Red List, Mount Kaputar, Nandewar bioregion, skink, species distribution models.
Introduction
It is critically important to understand the basic ecology and distribution of threatened taxa in order to effectively conserve and manage them (Mace 2004; IUCN 2022). This is particularly true as we are now in the midst of a global biodiversity crisis (IPBES 2019). Climate change is posing a threat to biota on an international level, with land use change also leading to a significant increase in biodiversity loss worldwide (Pecl et al. 2017; IPCC 2023). Moreover, recent catastrophic fire events on an unprecedented scale in eastern Australia (e.g. the summer 2019–2020 megafires) have highlighted the extent and potential severity of such threats, and the urgency needed to address their impacts (Adams et al. 2020; Nimmo et al. 2021; Legge et al. 2022a). For instance, species previously listed as Least Concern only 5–10 years ago are now being reconsidered for more severe threat categories (Ward et al. 2020; Nimmo et al. 2021; Torkkola et al. 2022). Listing species in threat categories is an important conservation process, as it assists conservation biologists in informing decisions regarding which species require management actions (Chapple et al. 2021; Cox et al. 2022). However, it is the knowledge of distribution and ecology that dictates the management actions that will have the most meaningful impact on mitigating current and potential future threats (Waldron et al. 2017; Scheele et al. 2018).
Following a 3-year drought, extensive fires began along the eastern seaboard of Australia in late 2019, burning a 97,000 km2 area (Adams et al. 2020; Ward et al. 2020). A total of 225 species of vertebrate had their ranges overlap the burn area by over 10%, with at least 76 species having over half of their known distribution burnt (Legge et al. 2022b). Species most heavily impacted by fire were habitat specialists (Legge et al. 2022b), such as those with low dispersal capabilities and restricted to rock outcrops or bogs (Dubey and Shine 2010; Senior et al. 2021; Santos et al. 2022). This is of particular concern for species from high elevations, as their specialised niches often limit them to having poor dispersal abilities and small range sizes (Dubey and Shine 2010; McCain and Colwell 2011). Such is the case for the Kaputar rock skink (Egernia roomi). The species was formally recognised as a separate species (Sadlier et al. 2019) only a month and a half prior to when fire began on Mount Kaputar in October 2019; a fire that eventually burned over 40% of the national park before it was extinguished a month later (DPIE 2021). Known only from an extremely limited area and elevational band of rock habitat (Sadlier et al. 2019), this species was immediately flagged as a conservation concern (Legge et al. 2022a).
The Kaputar rock skink had languished in taxonomic limbo for close to 35 years. It was originally described in 1985, in a controversial (Shea 1987; Wüster et al. 2021) paper by Wells and Wellington (1985). The diagnosis purported a single morphological feature (enlarged paravertebral scales) that is not unique to this species, but is shared by all congeners within the Egernia striolata species-complex (Shea and Sadlier 1999). As such, the Kaputar rock skink was not formally recognised as distinct until a recent study led by the Australian Museum confirmed the Mt Kaputar populations as a valid taxon based on combined genetic and morphological evidence (Sadlier et al. 2019). Apart from this re-description study, the species has received little research or conservation attention. Thus, we have a limited understanding of its ecology, or the true extent of its distribution throughout the Nandewar Ranges, in Mount Kaputar National Park (MKNP; New South Wales, Australia). However, due to its restricted distribution, and likely ongoing threats, the species was listed as Critically Endangered in New South Wales under the Biodiversity Conservation Act 2016 (NSW Threatened Species Scientific Committee 2021). The same Critically Endangered listing was applied federally under the Environment Protection and Biodiversity Protection Act 1999 (DCCEEW 2022). As of October 2023, the species is yet to be evaluated under the IUCN Red List of Threatened Species (IUCN 2022).
Prior to our study, the Kaputar rock skink was only known from three sites within MKNP above 1360 m in elevation, including the Mount Kaputar summit, Mt Dowe, and The Governor (Sadlier et al. 2019). Given its limited distribution, it is believed to be New South Wales’ most range-restricted vertebrate taxon (Sadlier et al. 2019). However, most of the Nandewar Range remains unsurveyed for reptiles outside of areas that have walking tracks and are easily accessible (Sadlier et al. 2019). No formal, targeted reptile survey reports of the Nandewar Ranges have been published, but there are incidental records published to online biodiversity databases such as Atlas of Living Australia (ALA) (https://www.ala.org.au/), iNaturalist (https://www.inaturalist.org) and the NSW BioNet Atlas (https://www.environment.nsw.gov.au/topics/animals-and-plants/biodiversity/nsw-bionet). Additionally, there are specimen records on the Online Zoological Collections of Australian Museums (OZCAM) (https://ozcam.org.au). Thus, it is conceivable that the Kaputar rock skink occurs more broadly throughout the Nandewar Range.
Here, we conducted comprehensive presence/absence surveys throughout MKNP to determine the distribution of the Kaputar rock skink. In addition, we performed species distribution models to estimate the potential distribution of the species and assess whether we have surveyed all high-quality areas of potential habitat for the species. During our fieldwork, we identified the potential threats to the Kaputar rock skink and used our refined knowledge of the species’ distribution to quantify its degree of distributional overlap with the 2019–2020 fires.
Materials and methods
Study species
The Kaputar rock skink (Egernia roomi) is a moderately sized (average adult snout-vent length is 105 mm), diurnal, heliothermic and saxicoline skink species, and a member of the Egernia striolata species complex (Sadlier et al. 2019). Like most of its congeners, the Kaputar rock skink is crevice obligate, and prior to this study, known only to use cracks and fissures on exposed basaltic outcrops and plateaux above 1360 m on the Nandewar Range within MKNP (Sadlier et al. 2019; Swan et al. 2022). The species is reliant on rock-on-rock microhabitat and does not construct burrows like members of the related Liopholis genus (which were once considered to be part of Egernia; Gardner et al. 2008). Complex sociality is a common trait within the Egernia genus (Chapple 2003; Riley et al. 2021), and Kaputar rock skinks have been commonly sighted occurring in pairs or small groups (NG and AC, pers. obs.). Prior to our study, the extent of occurrence (EOO) and area of occupancy (AOO) of the species (based on the three known sites) were both 8 km2 (NSW Threatened Species Scientific Committee 2021).
Study site
Located 52 km east of Narrabri in north-central NSW (Fig. 1), the Nandewar Range stands as the eroded remains of the Nandewar shield volcano, which is estimated to have formed from volcanic eruptions occurring between 21 and 17 million years ago (Abbott 1969). A westerly isolate of the Great Dividing Range, the highest elevations of the Nandewar Range (MKNP) serve as an outlying mesic upland refuge surrounded by drier slopes and plains, with the isolated, high elevation areas of the range sharing many floral and faunal communities with the disjunct New England Tableland on the Great Dividing Range to the east (Sadlier et al. 2019; Copeland and Backhouse 2022). The Kaputar Summit reaches 1509 m AHD (Australian Height Datum), while the surrounding lowland plains sit at only 230 m (Sadlier et al. 2019). MKNP (~51,400 ha) constitutes most of the remnant, native habitat within the Nandewar Bioregion, which has otherwise largely been converted into agricultural land (DPIE 2021). The park includes the declared wilderness areas of Grattai, Nandewar, and Rusden.
Mount Kaputar National Park study region, and the study species (Kaputar rock skink, Egernia roomi, top right inset). Inset map displays location of study area within the state of NSW. G, Grattai; N, Nandewar; R, Rusden designated wilderness areas. Image credit: Nicholas Gale. Aerial imagery sourced from Google Earth (https://earth.google.com, accessed February 2023).
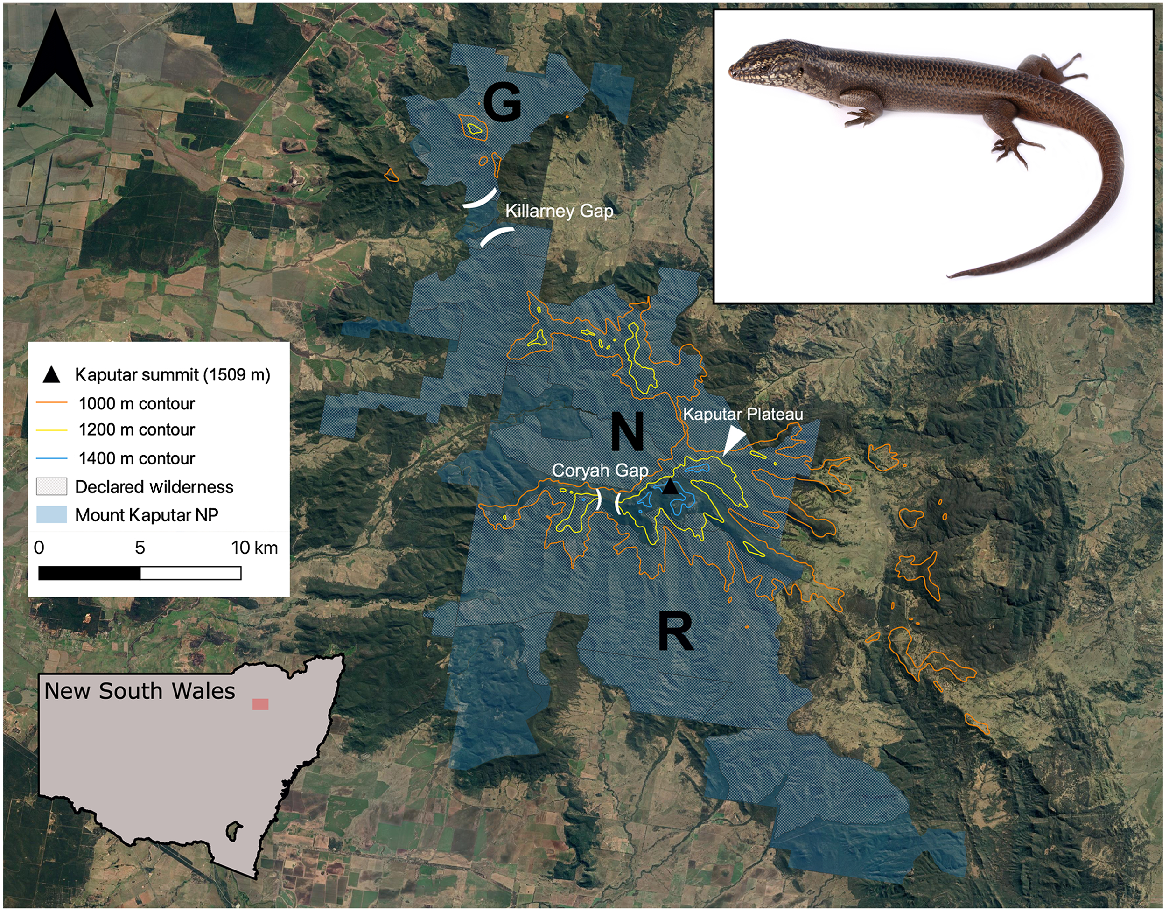
Areas over 1350 m in elevation are considered subalpine and are subjected to snowfall and prolonged frost events during winter months (DPIE 2021). Rugged rocky peaks and plateaux at this elevation are interspersed with open Snow Gum (Eucalyptus pauciflora), Ribbon Gum (Eucalyptus viminalis) and White Gum (Eucalyptus dalrylmpleana) woodland, with patches of dry heathland and Tea Tree (Leptospermum polygalifolium) thickets also present (Murphy and Shea 2015). The central section of the national park, commonly dubbed the Kaputar Plateau, encompasses the Mount Kaputar summit itself (Fig. 1), hosting the highest peaks of the range, and is the most accessible region within MKNP, possessing a partially sealed road to the summit and the majority of the maintained walking tracks within the park. While vaguely defined in literature, we define the Kaputar Plateau as the areas above the 1200 m contour east of Coryah Gap, which encompasses the summit region and associated smaller outcrops and peaks. This includes the Governor, West Kaputar Rocks, Mount Dowe, Mount Lindsay, Bundabulla Circuit/Lookout, Lindesay Rocks, and rock outcroppings east of the Horton River headwaters (Fig. 1). We consider the Bullawa Creek valley a northerly cut-off for the plateau, despite a rock-depauperate lower narrow ridge at 1000 m elevation connecting the area to the Pound Mountain area to the north (seen between sites 23 and 24 in Fig. 2).
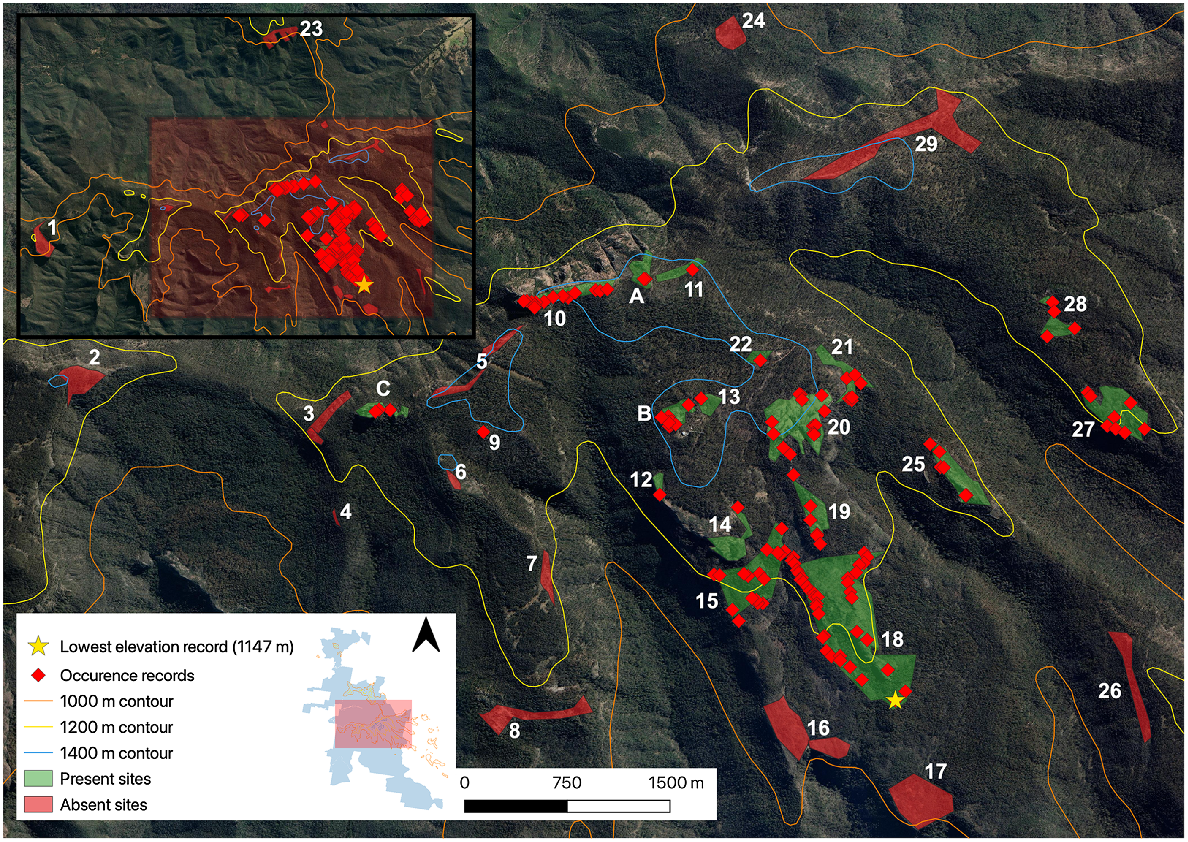
Individual occurrence records of the Kaputar rock skink detected in the central Nandewar Ranges during targeted surveys. Grattai Mountain (Site 30) is not visualised. Inset map displays survey locations within the MKNP boundary. For site information associated with site numbers, see Supplementary material. Aerial imagery sourced from Google Earth (https://earth.google.com, accessed February 2023).
Site selection and definition
Prior to our study, the Kaputar rock skink was known only from three sites: (1) the Mount Kaputar summit (Site A); (2) Mount Dowe (Site B); and (3) The Governor (Site C) (Fig. 2). Survey sites were selected using aerial satellite imagery in order to discern areas of outcropping rock within the study area (Mount Kaputar NP region >1000 m), constituting the known suitable habitat for the target species. To be certain that all accessible rock habitat was covered, all formed walking tracks (including those under a canopy) over this elevation were hiked in order to confirm rock habitat coverage. This identified one site that was hidden from aerial imagery, a westerly facing rocky cliff along Coryah Gap Fire Trail (Site 4). We considered sites as distinct if there was a greater than 50 m discontinuity in rocky habitat between them. Twelve survey sites fell within two declared wilderness areas within the park. These included the Nandewar Wilderness north of the Kaputar Plateau, and Rusden Wilderness south of the Kaputar Plateau (Fig. 1). The third wilderness area north of Killarney Gap (Grattai) was surveyed in October 2023. Sites ranged in size from <0.01 km2 (Sinclair Peak, Site 9) to 0.62 km2 (East Bundabulla Cliffs, Site 18). In total, 30 individual sites were surveyed, inclusive of Mount Lindsay and Grattai Mountain (see Supplemetary Table S3).
Visual encounter surveys
Although range restricted, the Kaputar rock skink is not cryptic, and is readily detected when basking under ideal conditions. The species can also be seen in crevices during times of inactivity, including periods of light to moderate rain and fog (NG, pers. obs.). Repeated diurnal surveys were carried out from early September 2022 to mid-January 2023, during the skink’s active season. Searches were carried out by two surveyors, and only during conditions where Kaputar rock skink detection was favourable (i.e. temperatures over 10°C, and clear of rain). Suitable habitat was scouted from afar using a combination of binoculars (Vortex Diamondback HD 10 × 42) and unaided eyes to observe basking or active lizards, and when approached, crevices were inspected using a torch to detect sheltering skinks. After a skink was detected and presence at a site was confirmed, the survey continued until all suitable habitat was exhausted, in order to maximise individual occurrence records. All sites were surveyed at least once, or twice if the Kaputar rock skink was not detected on the first visit. The iPhone application Sightings (Ug Media https://www.ugmedia.com.au) was used to record each individual sighting, taking coordinates to an accuracy of ±5 m.
Updating EOO, AOO and fine-scale habitat mapping
The minimum convex polygon surrounding all records was calculated in QGIS (ver. 3.28.3) using the attribute table field calculator to determine the EOO of the species. The IUCN recommended 2 × 2 km2 grid method was used to quantify the AOO. To achieve fine-scale habitat mapping of the Kaputar rock skink, once a site was confirmed to have the skink present, ends and beginnings of habitat were recorded in Avenza Maps (Avenza Systems; https://www.avenza.com/avenza-maps/) and modified into a polygon using QGIS to encompass discrete skink habitat; the area of these polygons (and thus habitat area) was calculated in km2. This included the three Sadlier et al. (2019) sites, which we revisited and surveyed.
Species distributional modelling (SDM)
We predicted the distribution of suitable Kaputar rock skink habitat using the Maximum Entropy species distribution modelling algorithm, MaxEnt (Phillips et al. 2006). Species distribution modelling explores the contrast between environmental conditions of occupied sites (i.e. presence locations) and potentially unoccupied sites (i.e. locations of the environmental background), to estimate habitat suitability for a species across a landscape. As a presence only algorithm, MaxEnt performs well for small sample sizes and therefore is the best choice for modelling the distribution of small range and micro-endemic species (Hernandez et al. 2006). Individual Kaputar rock skink occurrence records (118), obtained from presence/absence surveys and the visitation of historically known sites, were used to model the species’ distribution. To minimise spatial autocorrelation for the species distribution modelling, we spatially thinned the Kaputar rock skink occurrence records to 1 km using the ‘thin’ function in the R package spThin (Aiello-Lammens et al. 2015), resulting in 10 occurrence points (i.e. 10 1 km2 presence pixels) used for modelling.
Our primary aim was to model habitat suitability within a geographical area approximating the potential range of the Kaputar rock skink, to confirm all suitable habitat for the species has been surveyed. We therefore set the modelling background as the extent of all biogeographic regions (Interim Biogeographic Regionalisation for Australia (IBRA) ver. 7.0) occupied or likely to be occupied by the Kaputar rock skink. This only included the Nandewar Bioregion (NAN). Within this bioregion, the species’ currently known distribution is limited to the Kaputar subregion (NAN03). The only other subregion included in the background was the neighbouring Peel subregion (NAN04), abutting the Kaputar subregion to the east, relevant due to its possessing mountainous terrain >1000 m. The other Nandewar subregions, NAN01 (Nandewar Northern Complex) and NAN02 (Inverell Basalts) do not have any mountainous terrain above 1000 m and were therefore excluded from the modelling background.
We pre-selected a set of 27 candidate predictor variables likely affecting the species’ distribution. These variables relate to climate, topography, terrain and vegetation. We primarily included bioclimatic variables because as a high elevation ectotherm, the distribution of the Kaputar rock skink is likely influenced by temperature and precipitation regimes, as is the case for many ectothermic species (Araújo and Guisan 2006; Powney et al. 2010). As the species is known only to be associated with high elevation rock habitat (Sadlier et al. 2019; Swan et al. 2022), we included Terrain Ruggedness Index (TRI), topographic wetness, and aridity as candidate predictor variables. All environmental layers were downloaded at, or resampled to, a spatial resolution of ~1 km2 (30 arc-seconds). Variables were resampled to fit the study background region using the ‘mask’ function of the ‘raster’ package (Hijmans et al. 2013). We then extracted environmental data from the locations of the 10 thinned Kaputar rock skink occurrence points.
To investigate and reduce multicollinearity between variables, we visually inspected PCA biplots and calculated the variance inflation factor (VIF) using the ‘vifstep’ function of the usdm package for R (Naimi et al. 2014) retaining those with VIF <5. We also calculated Pearson correlation coefficients between each pair of variables and retained the variable with greater ecological relevance from each pair of highly correlated variables (P ≥0.7; (Dormann et al. 2013). The final set of variables (which were uncorrelated and biologically relevant) used for model fitting were as follows: Temperature Seasonality (Bio4), Thornthwaite Aridity Index, Normalised Difference Vegetation Index (NDVI), and TRI. Having few predictor variables is beneficial to reduce the risk of overfitting, a common issue when modelling ranges of taxa with small distributions (Anderson and Gonzalez 2011). We used the ‘ecospat.mantel.correlogram’ function in the ecospat package (Di Cola et al. 2017) to confirm that spatial autocorrelation of occurrence records was not significantly different than 0.
Models were built with regularisation multiplier options set to 1, 2 and 3 and with five different feature classes combinations (1, Linear; 2, Quadratic; 3, Linear + Quadratic; 4, Linear + Quadratic + Hinge; 5, Hinge); this resulted in 15 parameter combinations (and hence 15 candidate models). Feature classes determine the flexibility of the shape of the relationship of covariates to response. The regularisation multiplier dictates the penalty for model complexity, with lower values resulting in a model more fitted to the presences (over-fitted), and higher values in more spread out (under-fitted) model predictions (Phillips and Dudík 2008; Elith et al. 2011; Merow et al. 2013). Notably, as one of our aims was to predict new areas of potential occurrence, we did not use values of the regularisation multiplier lower than 1. Together, these settings influence model complexity by controlling the number and complexity of features in the final output. All the models were evaluated using the metrics AICc, AUCTEST, AUCDIFF, ORmtp, OM10 and Boyce Index (see Supplementary material for definitions and explanations of these metrics). We then summarised the evaluation metric scores across five models of each parameter combination. We visualised the predictions of each parameter combination’s full model, and we qualitatively evaluated their plausibility against our knowledge of the species’ ecology and of the region’s biogeography (following Petitpierre et al. 2017). We ultimately chose the parameter combination that yielded MaxEnt models with the lowest AIC score (corrected for small sample sizes; AICc; Warren and Seifert 2011), that performed well for multiple other metrics, was relatively parsimonious compared to other models (Merow et al. 2013; Coelho et al. 2019), and yielded ecologically plausible predictions.
Threats
While conducting fieldwork, we noted the presence of feral goats (Capra hircus) through (1) visual observation of animals, and (2) pellets (scats) at each site. In particular, the fouling of crevices with pellets was examined, and could be assessed during surveys whilst crevices were searched for skinks. Evidence of fire was recorded at each site, to ground truth the fire footprint. On top of this, human-induced damage was also noted, including obvious dislodgment and fracturing of large rocks and the creation of rock cairns.
Fire overlap
The minimum convex hull including all records of the species (i.e. the EOO) was overlain atop the Australian Google Earth Engine Burnt Area Map (AUS GEEBAM) (DAWE 2020) to ascertain the extent to which fire has overlapped the newly defined range of the species.
Results
Presence/absence surveys
At total of 114 occurrence observations of the Kaputar rock skink were recorded at 15 additional sites on the Nandewar Range, extending the species’ range 3.5 km east and 2.5 km south of Mount Dowe (Site B), which was previously considered the most south-easterly site for the species (Fig. 2). This brings the total number of known occurrence sites to 18, from the three previously noted sites in the recognition paper (Sadlier et al. 2019). At sites where the species was present, skinks were always found on the first survey, and time until first detection ranged from 2 to 35 min (Table 1), with the mean time until detection being 13.2 min.
| Site ID | Site name | Detection time (min) | |
|---|---|---|---|
| 9 | Sinclair Peak | 21 | |
| 10 | West Kaputar Rocks | 18 | |
| 11 | Summit East | 17 | |
| 12 | Eckford Lookout | 5 | |
| 13 | Dawson Rocks | 2 | |
| 14 | Bundabulla Lookout | 5 | |
| 15 | West Bundabulla Cliffs | 2 | |
| 18 | East Bundabulla Cliffs | 3 | |
| 19 | Mount Lindsay South | 23 | |
| 20 | Mount Lindsay | 10 | |
| 21 | Mount Lindsay lowers | 8 | |
| 22 | Barraba junction | 15 | |
| 25 | Lindesay Rocks | 4 | |
| 27 | Horton Headwaters South | 35 | |
| 28 | Horton Headwaters North | 30 |
Along with the Kaputar rock skink, eighteen other reptile species were detected during diurnal skink surveys, including the congeneric Cunningham’s Skink (Egernia cunninghami) and Tree Skink (E. striolata). This species distribution and assemblage pattern data is to be dealt with elsewhere.
A new elevational low for the species was recorded at 1147 m (Site 18, East Bundabulla Cliffs, Fig. 2), with the previously known lowest elevation for the species at The Governor recorded at 1360 m (Sadlier et al. 2019). Skinks were also observed on rocks immediately at the Summit lookout (Site A, 1509 m), with the Kaputar rock skink now known to have an elevational range of 1147–1509 m. This is a narrow band of only 362 m, although a three-fold increase from the previously noted 120 m, between 1360 and 1480 m (Sadlier et al. 2019). The species is now known from elevations that are not considered strictly subalpine (characterised by the presence of E. pauciflora woodland), but montane areas <1350 m. We did not detect the Kaputar rock skink at 14 sites (Fig. 2), including Grattai Mountain.
The species’ updated distribution is still limited to the Kaputar Plateau, as well as rock outcroppings coming off the plateau to its south (Bundabulla Cliffs east and west, Sites 15 and 18). The Kaputar rock skink’s range is bound in the east by the end of suitable rock habitat as the Nandewar Range tapers off into lowland plains, and in the west by Coryah Gap, as habitat becomes dry open sclerophyll woodland dominated by Stringybark (Eucalyptus laevopinea) and Manna Gum (E. viminalis), lacking rock habitat. Bullawa Creek and its associated deeply incised valley provides a northerly dispersal barrier, whereas the end of extensive rock on the Kaputar Plateau and the beginning of open Eucalypt woodland south of Bundabulla Cliffs constitutes the southern cut-off for the species.
We did not observe Kaputar rock skinks using trees or fallen logs, neither during surveys nor when walking between survey sites. This is despite the congeneric tree skink (E. striolata) commonly using trees and logs for shelter, including in the nearby (albeit lowland and semi-arid) Pilliga Forest (Duckett et al. 2012; Murphy and Murphy 2015). As expected, the majority of our new occurrence sites came from areas lacking walking tracks, being generally difficult to access (requiring rock scrambling or considerable hikes off formed trails in wilderness areas). New sites that were easily accessible on walking tracks included Mount Lindsay (Site 20), Eckford Lookout (Site 12) and Bundabulla Lookout (Site 14). Skink observations did not come from beside the walking track itself, but more so on the craggy cliffs bordering these aforementioned sites.
The most isolated populations, east of the Horton River (Sites 27 and 28), are noteworthy given their disjunction from the two other closest populations by what appears to be substantial dispersal barriers. They are separated from the Lindesay Rocks population (1.1 km to the west) due to the deeply incised Horton River valley. They are even further disjunct from summit populations (3.5 km to the north-west), with complete lack of habitat connectivity between these sites and Mount Capel atop the Kaputar Plateau, as the area only supports open woodland depauperate of rock habitat, now thick with vegetation regrowth (Acacia spp.) post fire. Furthermore, no E. striolata-complex skinks were detected on or around Mount Capel (Fig. 2). It is unknown whether these populations were present before the carving of the Horton River valley or later colonised from the summit population.
Lindesay Rocks (Site 25) constitutes the second-most isolated population, straddled by the Horton River in the east and Middle Creek to the west, separating the population from those at Mount Lindsay (Site 20) by 800 m (Fig. 2). While previous Kaputar rock skink records were only known from the Kaputar Plateau rim, the skink was detected on Sinclair Peak (Site 9), a miniscule, sharp rocky peak at only 1200 m2 in area, surrounded by open woodland toward the westerly extent of the plateau. No obvious habitat corridors connect the peak to The Governor, the next closest population, 600 m to the north-west (Fig. 2). The Governor remains the most westerly population of the species.
Updated EOO, AOO and fine-scale habitat mapping
The updated EOO of the species is 11.97 km2. The updated AOO is 40 km2, when calculated using the IUCN recommended 2 km × 2 km grid method. In cases where the AOO is larger than EOO, the IUCN suggests that EOO should be changed to equal the AOO for the purpose of conservation status assessment (IUCN 2022). Therefore, the EOO and AOO of the Kaputar rock skink are both 40 km2. Fine scale habitat mapping revealed 1.51 km2 of suitable rocky habitat, thus it is likely that only 1.51 km2 of habitat within the species’ AOO is ecologically suitable.
Species distributional model (SDM)
The optimal model (i.e. with the lowest ΔAICc value) performed well with an AUC of 0.997 and included a regularisation multiplier of 2 and LQ feature class. The geographical prediction of the model is in Fig. 3. Aridity and temperature seasonality (Bio4), contributed 90% and 10% to the model respectively, suggesting that aridity is the primary environmental variable that correlatively predicts the species’ distribution. NDVI and TRI, used as proxies for rock habitat, did not contribute substantially to the model.
Species distribution model (SDM) of the Kaputar rock skink, in Mount Kaputar National Park within the Kaputar Subregion. Inset map displays a close up of the Mount Coryah landform and the Kaputar Plateau, along with associated peaks and mountains important for context. Only habitat suitability grids >0.5 are visualised.
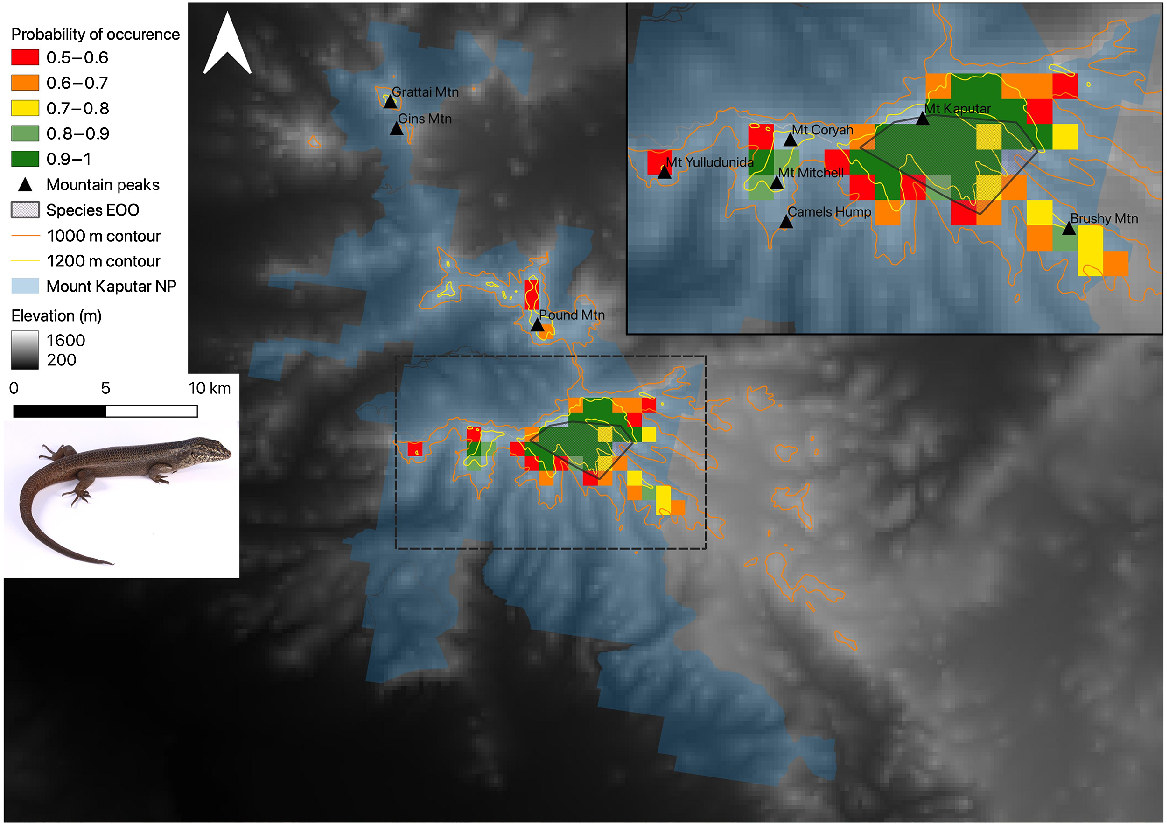
Areas of predicted habitat for the Kaputar rock skink fell exclusively in the Kaputar subregion within the modelling background, restricted above elevations of 1000 m (Fig. 3). The MaxEnt model prediction conforms closely to the ground-truthed range of the Kaputar rock skink, with the Kaputar Plateau shown to have highest probability of occurrence (0.9–1). Pound Mountain (north of Bullawa Creek), and Mount Mitchell/Mount Coryah, west of the Coryah Gap, were also predicted as having habitat suitable for the Kaputar rock skink (Fig. 3). Rock habitat to the immediate south of Pound Mountain (Site 23), which had a suitability of 0.5–0.6, was surveyed with no E. striolata-group skinks detected (Fig. 3). While Mount Mitchell was not surveyed, Mount Coryah, on the northern tip of the same landform (1.5 km north of Mount Mitchell) was surveyed with no Kaputar rock skinks detected (Fig. 2). Suitable habitat was predicted to occur around Brushy Mountain, to the south-east of the Kaputar Plateau (Fig. 3). Surveys at this area did not detect the skink. Mount Yulluduninda had only a single 1 km2 cell of 0.5–0.6 suitable habitat. No E. striolata-complex skinks were detected at this site during surveys. Modelling did not predict any suitable habitat north of Killarney Gap, with the prominent rocky mounts in the northern Grattai Wilderness including Gins Mountain (1080 m) and Grattai Mountain (1303 m) being potentially unsuitable (probability of occurrence <0.5; Fig. 3). A later survey at Grattai Mountain failed to detect the species.
Threats
At MKNP, the presence of goats was noted at almost all sites except Sinclair Peak (Site 9), by visual observations of live animals, scat, or both. However, no scats were ever found to physically contaminate crevices during skink surveys, despite being commonly found in small piles atop outcrops. Substantial human damage to rock habitat was noted at four locations with Kaputar rock skinks, all of which are easily accessible to the public and on walking tracks; these included the Summit, Mt Dowe, The Governor and Mount Lindsay.
Fire overlap
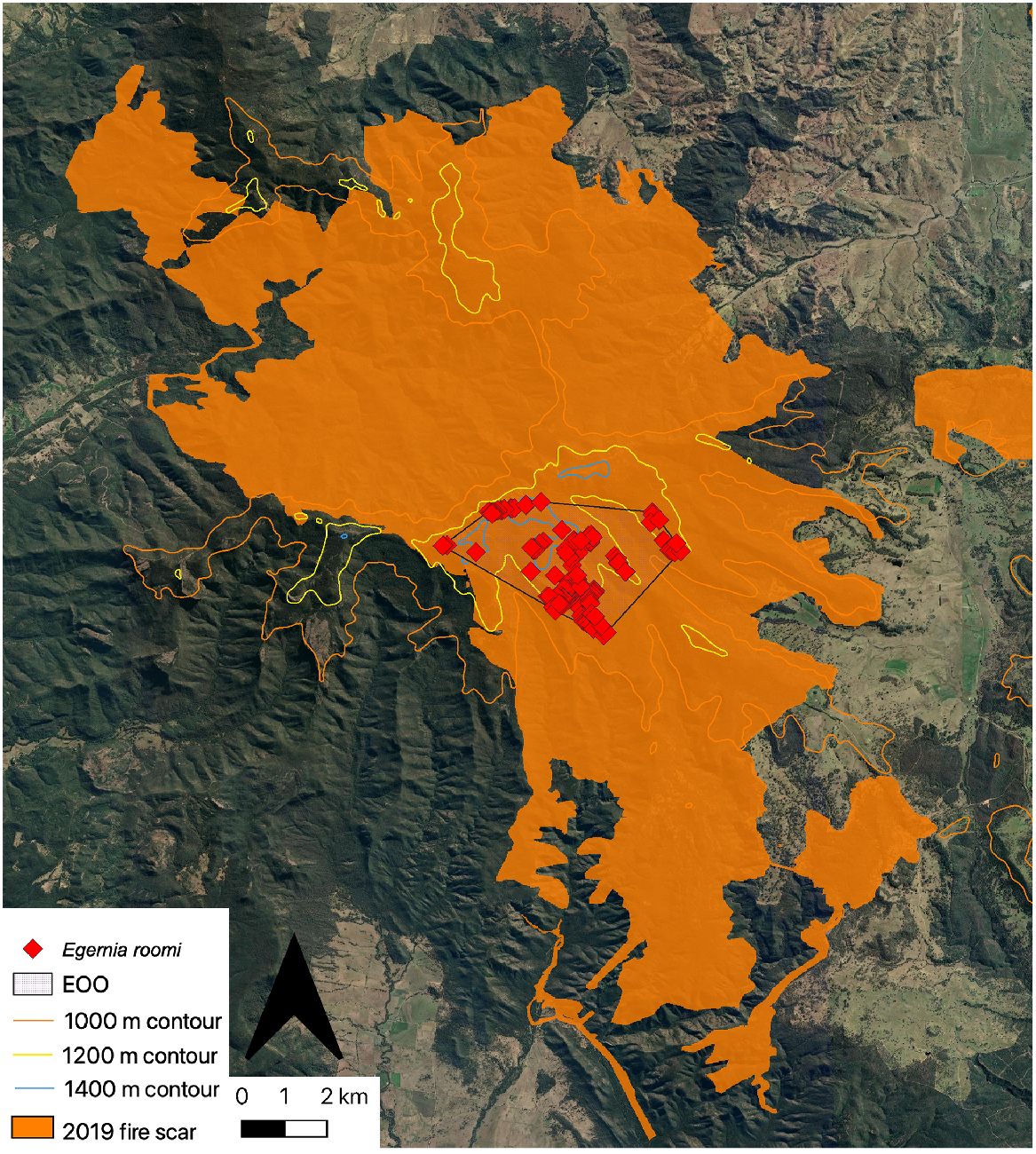
When the newly defined EOO of the Kaputar rock skink is overlain atop the Australian Google Earth Engine Burnt Area Map (AUS GEEBAM) (DAWE 2020), the entire range of the species falls within the 2019 burn scar (Fig. 4). Previous projections of the species EOO (areas above 1000 m on the Nandewar Range) led to only a 67% burn overlap estimate (Sadlier et al. 2019; Legge et al. 2022b).
Kaputar rock skink records and extent of occurence (EOO) overlapping the 2019 fire scar on the central Nandewar Range region. Aerial imagery sourced from Google Earth (https://earth.google.com, accessed February 2023).
Discussion
Distribution
Our comprehensive surveys throughout the higher elevation areas of MKNP, combined with species distribution modelling, have allowed us to determine the distributional range of the Kaputar rock skink. While we have detected the Kaputar rock skink at sites throughout a broader region of the National Park, the distribution of the species remains extremely localised and limited to small, discrete rocky habitat ‘islands’ atop the Kaputar Plateau. This mirrors the ranges of other short-range taxa endemic to the Nandewar Range, namely a community of endangered land snails (Murphy and Shea 2015; Murphy et al. 2019). Despite having the bulk of their distributions on the Kaputar Plateau and nearby high elevation peaks, these land snails have also been detected in lower elevation dry rainforest thickets (Murphy et al. 2019), with the Kaputar rock skink proving to be even more restricted than these species. Given that small distribution size is the greatest predictor of extinction risk for terrestrial vertebrates (Sekercioglu et al. 2008), the species remains a high conservation priority.
With extensive exploration and survey of the Kaputar plateau, supplemented by sampling of nearby high peaks, the distributional extent of the Kaputar rock skink is closer to being fully understood. Most accessible rock outcrops above 1000 m have been surveyed for skinks in the central region of MKNP, constituting the highest peaks of the Nandewar Range, and being the most likely habitat to harbour the Kaputar rock skink. All areas deemed potentially suitable for the species have been surveyed, making it unlikely that the species exists elsewhere. The Kaputar rock skinks’ range is now known to be smaller than the previously projected AOO (134 km2), but larger than the previously known AOO (8 km2). With our new distributional knowledge of the Kaputar rock skink, its IUCN listing can be assessed under criteria B (geographic range). With the newly calculated AOO of 40 km2, the Kaputar rock skink exceeds the threshold for Critically Endangered (<10 km2), and now only qualifies for Endangered (AOO <500 km2), under IUCN guidelines and criteria (IUCN 2022).
Threatening process
While 67% of the species’ previously modelled range (areas on the Nandewar Range above 1000 m) was burned during the 2019/2020 megafires (Legge et al. 2022a), we show that the Kaputar rock skink’s ground-truthed range now overlaps completely with that fire footprint, indicating the entirety of its distribution has been burned recently (Fig. 4). However, while completely burnt, 95% of the burn area was of low severity (Legge et al. 2022a), perhaps due to the large proportion of rock outcrops comprising the skink’s habitat. The landscape of the Nandewar Range is not naïve to fire, with six low to moderate intensity fires burning the landscape between 1937 and 2006, pre-dating the 2019/2020 megafires (DPIE 2021). While these fires each exceeded 10,000 ha individually, they did not reach the monumental 20,000 ha fires that burned ~40% of the park in 2019. Rock outcrops on the Nandewar Range appear to be mostly depauperate of vegetation and therefore of flammable material (albeit we make this observation 3 years post-fire). Rock outcrops provide a physical barrier to fire due to their inflammable nature, especially when landforms are domed or surrounded by steep cliff edges (Clarke 2002), which are common features of Kaputar rock skink habitat. While the degree of overlap between species’ ranges and burn areas can signify potential fire-impacted species, fire overlap alone does not indicate vulnerability to fire, given that differing life histories and microhabitat preferences result in diverse post-fire responses among species (Legge et al. 2022b). Although we now have a much greater understanding of the species’ range, our gauge on the species’ life history is still incomplete. The diet, reproduction and physiology of the species is essentially unknown, yet these may be important for further understanding how the species will respond to fire. We strongly recommend future research to determine these crucial aspects of the biology and ecology of the Kaputar rock skink.
Feral goats have been flagged as a major threat to the Kaputar rock skink, given their destructive nature and prevalence at Mount Kaputar (Hunter 2015; Sadlier et al. 2019). The fouling of crevices with goat faeces has been recorded in habitat of several other endangered NSW endemic reptile species, including the Barrier Range Dragon (Ctenophorus mirrityana) and the Broad-headed Snake (Hoplocephalus bungaroides). These physical barriers lead to a decrease in shelter sites for both of the species, which are reliant on this habitat (Murphy 1996; Sass and Swan 2014). Goat control is carried out via aerial shoots occurring twice yearly at MKNP, with mustering and trapping in low open country being ongoing (J. Faris, pers. comm.). Such control remains a high priority for park management (DPIE 2021). Lack of crevice degradation is perhaps indicative of current management success regarding feral goat control at MKNP. However, goat-induced ecosystem change and destruction can be difficult to detect, with the true extent of damage often unseen and therefore unknown (Russell et al. 2011).
Humans are another direct threat to the habitat of the Kaputar rock skink. While the species’ distribution is contained within a protected area, being protected under law does not prevent ongoing illegal damage from humans through the creation of rock cairns, or careless individuals searching for reptiles or invertebrates under rocks, and subsequently failing to return rocks to the same state they were found in (Michael et al. 2010; Fitzsimons and Michael 2017). This form of habitat modification is listed as a key threatening process in NSW under the Biodiversity Conservation Act, with the flow on effects of bush rock removal likely to impact not only the skink, but the outcrop community as a whole (Fitzsimons and Michael 2017). Damage of rock habitat was only noted at the Summit, Mt Dowe, The Governor, as well as Mount Lindsay, which are all on easily accessible tourist walks. Preventing the creation of rock cairns or walking tracks through skink habitat is the only tangible way to prevent further habitat degradation of this kind.
Lastly, anthropogenic climate change is perhaps one of the overall greatest threats to this species’ survival into the future. A warming future climate scenario is likely to contribute to the erosion of the skink’s physiological threshold as well as the degradation of the subalpine habitat that it lives in, with changes to vegetation likely having flow on effects to prey availability (Sekercioglu et al. 2008). Many invertebrate endemics (land snails) known from the Nandewar Range face an identical threat (Murphy and Shea 2015; Murphy et al. 2019). With similarly restricted distributions to the Kaputar rock skink, the species have nowhere to go as their habitable niche contracts, ultimately leading to their extinction.
Limitations and future direction
With our surveys being concentrated on rocky habitat, we cannot rule out the absence of the species from wooded areas, given other Egernia species readily utilise crevices in both standing trees and fallen timber (Chapple 2003; Swan et al. 2022). However, on Mount Kaputar, Eucalypt species (such as E. dalrylmpleana, E. pauciflora, and E. viminalis) from sub-alpine elevations (>1350 m) may not provide suitable shelter sites for the skink, as they possess a smooth trunk that do not crack and fissure, as opposed to Ironbark species such as Eucalyptus crebra, of which are utilised by the congeneric Tree Skink (E. striolata) in the nearby Pilliga Forest (Murphy and Murphy 2015).
Despite our surveys capturing much of the rock from the highest elevation areas of the Nandewar Range, we cannot definitively call this a full survey effort of the Nandewar Range. Rocky areas around the Pound Mountain/Doyles Peak in the Nandewar Wilderness and Gins Mountain in the Grattai Wilderness possess extensive rock that may harbour the species. Further focused surveys in wooded areas and in other high elevation rock habitat in the national park may further extend the species’ known range, as well as challenge the assumptions of the species having strictly saxicoline habits.
Conclusion
Given their 40 km2 AOO, the Kaputar rock skink meets the IUCN criteria under B2 as Endangered, being restricted to only one location (MKNP), and a continual decline in habitat extent and quality occurring due to threatening processes including fire, feral animals (goats) and damage from humans (IUCN 2022). Further research is required to fully determine the threat of fire to the species.
Although rapid conservation assessments are useful in determining which species need conservation attention (Legge et al. 2022a), many physical, behavioural and ecological traits are assumed in the process, and thus the true extent of threats are unknown. Intimate knowledge of a taxon’s ecology, life history, distribution and threats lead to the best-informed decision-making regarding management actions and conservation outcomes, allowing for the best allocation of time and funding in order to conserve a species (Scheele et al. 2018). For instance, this study has presented a rare scenario in which a species’ entire geographical range can be elucidated at a fine scale, allowing for more effective conservation measures to be implemented for its preservation.
Data availability
The occurrence data and environmental variables used in the SDM are available from the authors upon request.
Declaration of funding
This research was funded by grants from the Paddy Pallin Science Grant (Royal Zoological Society of New South Wales; to JEF) and the Australian Research Council (FT200100108; to DGC).
Acknowledgements
Catherine Bushell, Owen Lishmund, Michael Read and Wes Read assisted with fieldwork. The Read family (Michael, Jenna, Angus and Walter) generously opened their home to us during multiple fieldwork stints. Further field accommodation was provided at a reduced price by the New South Wales National Parks and Wildlife Service (NPWS). James Faris (Saving our Species), Louisa Andersen (NPWS) and Adam Fawcett (NPWS) provided insight into the Nandewar Landscape and assisted in logistical support. We thank Ross Sadlier for providing information and advice. We also thank Glenn Shea and an anonymous reviewer for their comments which improved the manuscript.
References
Abbott MJ (1969) Petrology of the Nandewar Volcano, N.S.W., Australia. Contributions to Mineralogy and Petrology 20, 115-134.
| Crossref | Google Scholar |
Adams MA, Shadmanroodposhti M, Neumann M (2020) Causes and consequences of Eastern Australia’s 2019–20 season of mega-fires: a broader perspective. Global Change Biology 26, 3756-3758.
| Crossref | Google Scholar | PubMed |
Aiello-Lammens ME, Boria RA, Radosavljevic A, Vilela B, Anderson RP (2015) spThin: an R package for spatial thinning of species occurrence records for use in ecological niche models. Ecography 38, 541-545.
| Crossref | Google Scholar |
Anderson RP, Gonzalez I, Jr. (2011) Species-specific tuning increases robustness to sampling bias in models of species distributions: an implementation with Maxent. Ecological Modelling 222, 2796-2811.
| Crossref | Google Scholar |
Araújo MB, Guisan A (2006) Five (or so) challenges for species distribution modelling. Journal of Biogeography 33, 1677-1688.
| Crossref | Google Scholar |
Chapple DG (2003) Ecology, life-history, and behavior in the Australian Scincid genus Egernia, with comments on the evolution of complex sociality in lizards. Herpetological Monographs 17, 145-180.
| Crossref | Google Scholar |
Chapple DG, Roll U, Böhm M, Aguilar R, Amey AP, Austin CC, Baling M, Barley AJ, Bates MF, Bauer AM, Blackburn DG, Bowles P, Brown RM, Chandramouli SR, Chirio L, Cogger H, Colli GR, Conradie W, Couper PJ, Cowan MA, Craig MD, Das I, Datta-Roy A, Dickman CR, Ellis RJ, Fenner AL, Ford S, Ganesh SR, Gardner MG, Geissler P, Gillespie GR, Glaw F, Greenlees MJ, Griffith OW, Grismer LL, Haines ML, Harris DJ, Hedges SB, Hitchmough RA, Hoskin CJ, Hutchinson MN, Ineich I, Janssen J, Johnston GR, Karin BR, Keogh JS, Kraus F, LeBreton M, Lymberakis P, Masroor R, McDonald PJ, Mecke S, Melville J, Melzer S, Michael DR, Miralles A, Mitchell NJ, Nelson NJ, Nguyen TQ, de Campos Nogueira C, Ota H, Pafilis P, Pauwels OSG, Perera A, Pincheira-Donoso D, Reed RN, Ribeiro-Júnior MA, Riley JL, Rocha S, Rutherford PL, Sadlier RA, Shacham B, Shea GM, Shine R, Slavenko A, Stow A, Sumner J, Tallowin OJS, Teale R, Torres-Carvajal O, Trape J-F, Uetz P, Ukuwela KDB, Valentine L, Van Dyke JU, van Winkel D, Vasconcelos R, Vences M, Wagner P, Wapstra E, While GM, Whiting MJ, Whittington CM, Wilson S, Ziegler T, Tingley R, Meiri S (2021) Conservation status of the world’s skinks (Scincidae): taxonomic and geographic patterns in extinction risk. Biological Conservation 257, 109101.
| Crossref | Google Scholar |
Clarke PJ (2002) Habitat islands in fire-prone vegetation: do landscape features influence community composition? Journal of Biogeography 29, 677-684.
| Crossref | Google Scholar |
Coelho MTP, Diniz-Filho JA, Rangel TF (2019) A parsimonious view of the parsimony principle in ecology and evolution. Ecography 42, 968-976.
| Crossref | Google Scholar |
Cox N, Young BE, Bowles P, Fernandez M, Marin J, Rapacciuolo G, Bohm M, Brooks TM, Hedges SB, Hilton-Taylor C, Hoffmann M, Jenkins RKB, Tognelli MF, Alexander GJ, Allison A, Ananjeva NB, Auliya M, Avila LJ, Chapple DG, Cisneros-Heredia DF, Cogger HG, Colli GR, de Silva A, Eisemberg CC, Els J, Fong G. A, Grant TD, Hitchmough RA, Iskandar DT, Kidera N, Martins M, Meiri S, Mitchell NJ, Molur S, Nogueira CdC, Ortiz JC, Penner J, Rhodin AGJ, Rivas GA, Rodel M-O, Roll U, Sanders KL, Santos-Barrera G, Shea GM, Spawls S, Stuart BL, Tolley KA, Trape J-F, Vidal MA, Wagner P, Wallace BP, Xie Y (2022) A global reptile assessment highlights shared conservation needs of tetrapods. Nature 605, 285-290.
| Crossref | Google Scholar | PubMed |
DAWE (2020) Australian google earth engine burnt area map. A rapid, national approach to fire severity mapping. Department of Agriculture, Water and the Environment, Commonwealth of Australia. Available at https://datasets.seed.nsw.gov.au/dataset/google-earth-engine-burnt-area-map-geebam
DCCEEW (2022) Species profile and threats database: Egernia roomi. Available at https://www.environment.gov.au/cgi-bin/sprat/public/publicspecies.pl?taxon_id=90714
Di Cola V, Broennimann O, Petitpierre B, Breiner FT, D’Amen M, Randin C, Engler R, Pottier J, Pio D, Dubuis A, Pellissier L, Mateo RG, Hordijk W, Salamin N, Guisan A (2017) ecospat: an R package to support spatial analyses and modeling of species niches and distributions. Ecography 40, 774-787.
| Crossref | Google Scholar |
Dormann CF, Elith J, Bacher S, Buchmann C, Carl G, Carré G, Marquéz JRG, Gruber B, Lafourcade B, Leitão PJ, Münkemüller T, McClean C, Osborne PE, Reineking B, Schröder B, Skidmore AK, Zurell D, Lautenbach S (2013) Collinearity: a review of methods to deal with it and a simulation study evaluating their performance. Ecography 36, 27-46.
| Crossref | Google Scholar |
DPIE (2021) Mount Kaputar National Park Plan of Management. State of NSW and Department of Planning, Industry and Environment. Available at https://www.environment.nsw.gov.au/-/media/OEH/Corporate-Site/Documents/Parks-reserves-and-protected-areas/Parks-plans-of-management/mount-kaputar-national-park-plan-of-management-210368.pdf
Dubey S, Shine R (2010) Restricted dispersal and genetic diversity in populations of an endangered montane lizard (Eulamprus leuraensis, Scincidae). Molecular Ecology 19, 886-897.
| Crossref | Google Scholar | PubMed |
Duckett PE, Morgan MH, Stow AJ (2012) Tree-dwelling populations of the skink Egernia striolata aggregate in groups of close kin. Copeia 2012, 130-134.
| Crossref | Google Scholar |
Elith J, Phillips SJ, Hastie T, Dudík M, Chee YE, Yates CJ (2011) A statistical explanation of MaxEnt for ecologists. Diversity and Distributions 17, 43-57.
| Crossref | Google Scholar |
Fitzsimons JA, Michael DR (2017) Rocky outcrops: a hard road in the conservation of critical habitats. Biological Conservation 211, 36-44.
| Crossref | Google Scholar |
Gardner MG, Hugall AF, Donnellan SC, Hutchinson MN, Foster R (2008) Molecular systematics of social skinks: phylogeny and taxonomy of the Egernia group (Reptilia: Scincidae). Zoological Journal of the Linnean Society 154, 781-794.
| Crossref | Google Scholar |
Hernandez PA, Graham CH, Master LL, Albert DL (2006) The effect of sample size and species characteristics on performance of different species distribution modeling methods. Ecography 29, 773-785.
| Crossref | Google Scholar |
Hijmans R, van Etten J, Mattiuzzi M, Sumner M, Greenberg J, Lamigueiro O, Bevan A, Racine E, Shortridge A (2013) Raster package in R. Available at https://cran.r-project.org/web/packages/raster/index.html
Hunter J (2015) Vegetation and flora of Mt Kaputar National Park. Research Gate. Available at https://doi.org/10.13140/RG.2.1.3135.3444
IPBES (2019) Summary for policymakers of the global assessment report on biodiversity and ecosystem services. Available at https://www.ipbes.net/global-assessment
IPCC (2023) Climate change 2023: synthesis report. Available at https://www.ipcc.ch/report/ar6/syr/
IUCN (2022) Guidelines for using the IUCN red list categories and criteria. Version 15. Available at https://www.iucnredlist.org/resources/redlistguidelines
Legge S, Woinarski JCZ, Scheele BC, Garnett ST, Lintermans M, Nimmo DG, Whiterod NS, Southwell DM, Ehmke G, Buchan A, Gray J, Metcalfe DJ, Page M, Rumpff L, van Leeuwen S, Williams D, Ahyong ST, Chapple DG, Cowan M, Hossain MA, Kennard M, Macdonald S, Moore H, Marsh J, McCormack RB, Michael D, Mitchell N, Newell D, Raadik TA, Tingley R, Boer M (2022a) Rapid assessment of the biodiversity impacts of the 2019–2020 Australian megafires to guide urgent management intervention and recovery and lessons for other regions. Diversity and Distributions 28, 571-591.
| Crossref | Google Scholar |
Legge S, Rumpff L, Woinarski JCZ, Whiterod NS, Ward M, Southwell DG, Scheele BC, Nimmo DG, Lintermans M, Geyle HM, Garnett ST, Hayward-Brown B, Ensbey M, Ehmke G, Ahyong ST, Blackmore CJ, Bower DS, Brizuela-Torres D, Burbidge AH, Burns PA, Butler G, Catullo R, Chapple DG, Dickman CR, Doyle KE, Ferris J, Fisher D, Gallagher R, Gillespie GR, Greenlees MJ, Hohnen R, Hoskin CJ, Hunter D, Jolly C, Kennard M, King A, Kuchinke D, Law B, Lawler I, Lawler S, Loyn R, Lunney D, Lyon J, MacHunter J, Mahony M, Mahony S, McCormack RB, Melville J, Menkhorst P, Michael D, Mitchell N, Mulder E, Newell D, Pearce L, Raadik TA, Rowley JJL, Sitters H, Spencer R, Valavi R, West M, Wilkinson DP, Zukowski S, Nolan R (2022b) The conservation impacts of ecological disturbance: time-bound estimates of population loss and recovery for fauna affected by the 2019–2020 Australian megafires. Global Ecology and Biogeography 31, 2085-2104.
| Crossref | Google Scholar |
Mace GM (2004) The role of taxonomy in species conservation. Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences 359, 711-719.
| Crossref | Google Scholar | PubMed |
McCain CM, Colwell RK (2011) Assessing the threat to montane biodiversity from discordant shifts in temperature and precipitation in a changing climate: climate change risk for montane vertebrates. Ecology Letters 14, 1236-1245.
| Crossref | Google Scholar | PubMed |
Merow C, Smith MJ, Silander JA, Jr (2013) A practical guide to MaxEnt for modeling species’ distributions: what it does, and why inputs and settings matter. Ecography 36, 1058-1069.
| Crossref | Google Scholar |
Michael DR, Lindenmayer DB, Cunningham RB (2010) Managing rock outcrops to improve biodiversity conservation in Australian agricultural landscapes. Ecological Management & Restoration 11, 43-50.
| Crossref | Google Scholar |
Murphy MJ (1996) A possible threat to the Broad-headed Snake Hoplocephalus bungaroides: degradation of habitat by the Feral Goat Capra hircus. Herpetofauna 26, 37-38.
| Google Scholar |
Murphy MJ, Murphy JK (2015) Survey of the reptiles and amphibians of Merriwindi State Conservation Area in the Pilliga forest of northern inland New South Wales. Australian Zoologist 37, 517-528.
| Crossref | Google Scholar |
Murphy MJ, Shea M (2015) Survey of the land snail fauna (Gastropoda: Pulmonata) of Mount Kaputar National Park in northern inland New South Wales, Australia, including a description of the listing of Australia’s first legally recognised endangered land snail community. Molluscan Research 35, 51-64.
| Crossref | Google Scholar |
Murphy MJ, Murphy JK, Faris JC, Mulholland MJ (2019) Marooned on an extinct volcano: the conservation status of four endemic land snails (Gastropoda: Pulmonata) at Mount Kaputar, New South Wales. Proceedings of the Linnean Society of New South Wales 141, 33-44.
| Google Scholar |
Naimi B, Hamm NAS, Groen TA, Skidmore AK, Toxopeus AG (2014) Where is positional uncertainty a problem for species distribution modelling? Ecography 37, 191-203.
| Crossref | Google Scholar |
Nimmo DG, Carthey AJR, Jolly CJ, Blumstein DT (2021) Welcome to the Pyrocene: animal survival in the age of megafire. Global Change Biology 27, 5684-5693.
| Crossref | Google Scholar | PubMed |
NSW Threatened Species Scientific Committee (2021) Final determination of the Kaputar Rock Skink Egernia roomi as critically endangered. Available at https://www.environment.nsw.gov.au/-/media/OEH/Corporate-Site/Documents/Animals-and-plants/Scientific-Committee/Determinations/2021/final-determination-kaputar-rock-skink-egernia-roomi-critically-endangered-species.pdf
Pecl GT, Araújo MB, Bell JD, Blanchard J, Bonebrake TC, Chen I-C, Clark TD, Colwell RK, Danielsen F, Evengård B, Falconi L, Ferrier S, Frusher S, Garcia RA, Griffis RB, Hobday AJ, Janion-Scheepers C, Jarzyna MA, Jennings S, Lenoir J, Linnetved HI, Martin VY, McCormack PC, McDonald J, Mitchell NJ, Mustonen T, Pandolfi JM, Pettorelli N, Popova E, Robinson SA, Scheffers BR, Shaw JD, Sorte CJB, Strugnell JM, Sunday JM, Tuanmu M-N, Vergés A, Villanueva C, Wernberg T, Wapstra E, Williams SE (2017) Biodiversity redistribution under climate change: impacts on ecosystems and human well-being. Science 355, eaai9214.
| Crossref | Google Scholar |
Petitpierre B, Broennimann O, Kueffer C, Daehler C, Guisan A (2017) Selecting predictors to maximize the transferability of species distribution models: lessons from cross-continental plant invasions. Global Ecology and Biogeography 26, 275-287.
| Crossref | Google Scholar |
Phillips SJ, Dudík M (2008) Modeling of species distributions with Maxent: new extensions and a comprehensive evaluation. Ecography 31, 161-175.
| Crossref | Google Scholar |
Phillips SJ, Anderson RP, Schapire RE (2006) Maximum entropy modeling of species geographic distributions. Ecological Modelling 190, 231-259.
| Crossref | Google Scholar |
Powney GD, Grenyer R, Orme CDL, Owens IPF, Meiri S (2010) Hot, dry and different: Australian lizard richness is unlike that of mammals, amphibians and birds. Global Ecology and Biogeography 19, 386-396.
| Crossref | Google Scholar |
Riley JL, Noble DWA, Stow AJ, Bolton PE, While GM, Dennison S, Byrne RW, Whiting MJ (2021) Socioecology of the Australian Tree Skink (Egernia striolata). Frontiers in Ecology and Evolution 9, 722455.
| Crossref | Google Scholar |
Russell BG, Letnic M, Fleming PJS (2011) Managing feral goat impacts by manipulating their access to water in the rangelands. The Rangeland Journal 33, 143-152.
| Crossref | Google Scholar |
Sadlier RA, Frankham GJ, Beatson CA, Eldridge MDB, Rowley JJL (2019) Genetic evidence in support of the recognition of the Kaputar Rock Skink, one of New South Wales’ most range-restricted vertebrate species. Records of the Australian Museum 71, 183-197.
| Crossref | Google Scholar |
Santos JL, Sitters H, Keith DA, Geary WL, Tingley R, Kelly LT (2022) A demographic framework for understanding fire-driven reptile declines in the ‘land of the lizards’. Global Ecology and Biogeography 31, 2105-2119.
| Crossref | Google Scholar |
Sass S, Swan G (2014) Factors influencing habitat occupancy of the endangered Barrier Range dragon (Ctenophorus mirrityana: Agamidae). Herpetological Review 45, 213-216.
| Google Scholar |
Scheele BC, Legge S, Armstrong DP, Copley P, Robinson N, Southwell D, Westgate MJ, Lindenmayer DB (2018) How to improve threatened species management: an Australian perspective. Journal of Environmental Management 223, 668-675.
| Crossref | Google Scholar | PubMed |
Sekercioglu CH, Schneider SH, Fay JP, Loarie SR (2008) Climate change, elevational range shifts, and bird extinctions. Conservation Biology 22, 140-150.
| Crossref | Google Scholar | PubMed |
Senior AF, Chapple DG, Atkins ZS, Clemann N, Gardner MG, While GM, Wong BBM (2021) Agonistic behavioural asymmetry in two species of montane lizard that exhibit elevational replacement. Landscape Ecology 36, 863-876.
| Crossref | Google Scholar |
Shea GM (1987) Comment on the proposed suppression for nomenclatural purposes of three works by Richard W Wells and C. Ross Wellington. The Bulletin of Zoological Nomenclature 44, 257-261.
| Crossref | Google Scholar |
Shea GM, Sadlier RA (1999) A catalogue of the non-fossil amphibian and reptile type specimens in the collection of the Australian Museum: types currently, previously and purportedly present. Technical Reports of the Australian Museum 15, 1-91.
| Crossref | Google Scholar |
Torkkola JJ, Chauvenet ALM, Hines H, Oliver PM (2022) Distributional modelling, megafires and data gaps highlight probable underestimation of climate change risk for two lizards from Australia’s montane rainforests. Austral Ecology 47, 365-379.
| Crossref | Google Scholar |
Waldron A, Miller DC, Redding D, Mooers A, Kuhn TS, Nibbelink N, Roberts JT, Tobias JA, Gittleman JL (2017) Reductions in global biodiversity loss predicted from conservation spending. Nature 551, 364-367.
| Crossref | Google Scholar | PubMed |
Ward M, Tulloch AIT, Radford JQ, Williams BA, Reside AE, Macdonald SL, Mayfield HJ, Maron M, Possingham HP, Vine SJ, O’Connor JL, Massingham EJ, Greenville AC, Woinarski JCZ, Garnett ST, Lintermans M, Scheele BC, Carwardine J, Nimmo DG, Lindenmayer DB, Kooyman RM, Simmonds JS, Sonter LJ, Watson JEM (2020) Impact of 2019–2020 mega-fires on Australian fauna habitat. Nature Ecology and Evolution 4, 1321-1326.
| Crossref | Google Scholar | PubMed |
Warren DL, Seifert SN (2011) Ecological niche modeling in Maxent: the importance of model complexity and the performance of model selection criteria. Ecological Applications 21, 335-342.
| Crossref | Google Scholar | PubMed |
Wells RW, Wellington CR (1985) A classification of the Amphibia and Reptilia of Australia. Australian Journal of Herpetology Supplementary Series 1, 1-61.
| Google Scholar |
Wüster W, Thomson SA, O’Shea M, Kaiser H (2021) Confronting taxonomic vandalism in biology: conscientious community self-organization can preserve nomenclatural stability. Biological Journal of the Linnean Society 133, 645-670.
| Crossref | Google Scholar |


