Science behind management of Shark Bay and Florida Bay, two P-limited subtropical systems with different climatology and human pressures
Gary A. Kendrick A G , James W. Fourqurean B , Matthew W. Fraser A , Michael R. Heithaus C , Gary Jackson D , Kim Friedman A E and David Hallac FA Oceans Institute and School of Plant Biology, University of Western Australia, Perth, 35 Stirling Highway, Crawley, WA 6009, Australia.
B Department of Biological Sciences and Southeast Environmental Research Center, Florida International University, 3000 NE 151st Street, North Miami, FL 33181, USA.
C Department of Biological Sciences, School of the Environment, Arts and Society, Florida International University, 3000 NE 151st Street, North Miami, FL 33181, USA.
D Western Australian Fisheries and Marine Research Laboratories, Department of Fisheries, PO Box 20, North Beach, WA 6920, Australia.
E DEC Marine Science Program, Department of Environment and Conservation, 17 Dick Perry Avenue, Kensington, WA 6151, Australia.
F Yellowstone Center for Resources, PO Box 168, Yellowstone National Park, WY 82190, USA.
G Corresponding author. Email: gary.kendrick@uwa.edu.au
Marine and Freshwater Research 63(11) 941-951 https://doi.org/10.1071/MF12280
Submitted: 30 September 2012 Accepted: 2 October 2012 Published: 26 November 2012
Abstract
This special issue on ‘Science for the management of subtropical embayments: examples from Shark Bay and Florida Bay’ is a valuable compilation of individual research outcomes from Florida Bay and Shark Bay from the past decade and addresses gaps in our scientific knowledge base in Shark Bay especially. Yet the compilation also demonstrates excellent research that is poorly integrated, and driven by interests and issues that do not necessarily lead to a more integrated stewardship of the marine natural values of either Shark Bay or Florida Bay. Here we describe the status of our current knowledge, introduce the valuable extension of the current knowledge through the papers in this issue and then suggest some future directions. For management, there is a need for a multidisciplinary international science program that focusses research on the ecological resilience of Shark Bay and Florida Bay, the effect of interactions between physical environmental drivers and biological control through behavioural and trophic interactions, and all under increased anthropogenic stressors. Shark Bay offers a ‘pristine template’ for this scale of study.
Introduction
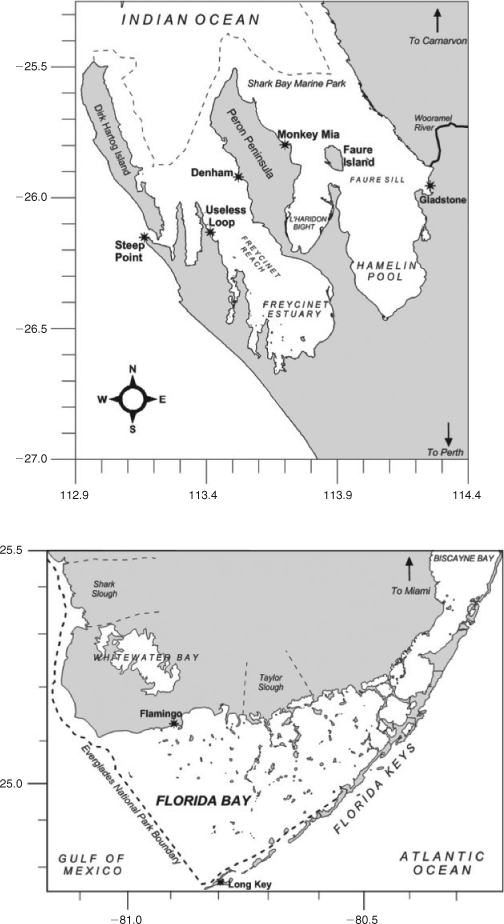
Stewardship of natural areas is increasingly important as the global population increases. This stewardship of critical ecosystems will only be effective when management plans are underpinned by science at a system-scale level. This special issue updates our contemporary knowledge of two subtropical embayments, Shark Bay and Florida Bay (Fig. 1), two UN World Heritage Sites juxtaposed with large terrestrial National Parks. The research papers in this special issue summarise science and management programs in Shark Bay (20+) and Florida Bay (6), updating our understanding of these subtropical bays with an emphasis on comparing the environmental drivers, threats to biology and strategies for sustainable management. There has been a significant amount of research in Florida Bay, driven by large-scale changes to river inputs (resulting in changing nutrient loadings) and rapid population growth in the Miami and southern Florida region (summarised in 15 research papers in Estuaries 1999, and updated in Kruczinsky and Fletcher 2012). Here we compare that with Shark Bay, with the goal of influencing future directions of research and management in both systems. In particular, we emphasise the critical importance of updating our knowledge on the ecosystem drivers, species interactions and management of resources in the marine environments of the Shark Bay World Heritage Area.
|
Shark Bay and Florida Bay are both semi-enclosed evaporative basins, which are common in subtropical regions. They are characterised by greater evaporation than rainfall and freshwater inputs from terrestrial watersheds. Both bays are open to exchange with the coastal ocean and have hypersaline regions, at least seasonally. Oceanic sources of seawater dominate water and nutrient inputs, and terrestrial run-off and river discharge add relatively small amounts of water and nutrients. In addition to these similarities, there are also major differences between the bays, primarily in climatology and anthropogenic pressures. The watershed of Florida Bay receives much more precipitation than Shark Bay (see Hydrology/climatology). While hypersalinity in Shark Bay appears to be a permanent feature of a relatively pristine environment, hypersalinity in Florida Bay depends on inter- and intra-annual variations in rainfall and has been exacerbated by the reduction in freshwater flows associated with large-scale flood control developed by the US Army Corps of Engineers in the 1950s and 1960s. Shark Bay is a pristine system where human influence is relatively small: the residential population is less than 1000, and; there are between 100 000 and 200 000 tourists per annum. Southern Florida is, and has been for over 200 years, heavily influenced by a large and growing human population and industrial and urban development. The population of Southern Florida is ~5 000 000. This special issue will address a range of physical, ecological and social values of these unique systems, underlining the need for conservation of subtropical embayments in general.
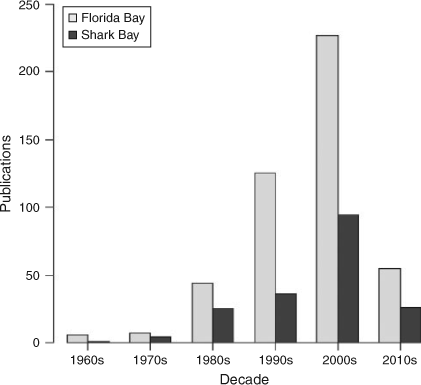
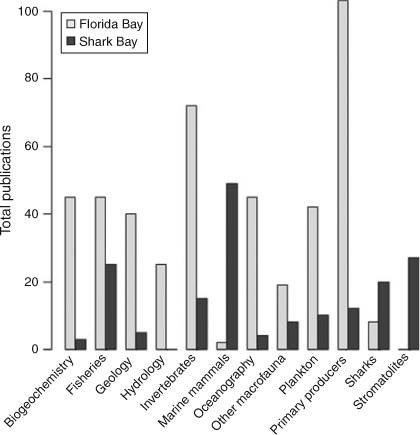
A Web of Science (http://wokinfo.com/; Thomson Reuters, New York, USA) search for the terms ‘Shark Bay’ and ‘Florida Bay’ from 1960 to present indicated exponential increases in the total number of research publications published per decade in both systems (Fig. 2) with greater numbers from Florida Bay in every decade. For example, 36 papers were published studying Shark Bay between 1990 and 1999, while 125 papers were published examining Florida Bay over the same period. Increased research focus in Florida Bay has been driven by major environmental issues observed in the 1980s and 1990s, summarised in a special issue in Estuaries with an emphasis on the threats to Florida Bay (Fourqurean and Robblee 1999). The large difference in numbers of publications reflects the remoteness of Shark Bay versus the proximity of Florida Bay to a major population centre and historical watershed restoration throughout south Florida. For Florida Bay, there has been a greater focus on publications on the topics of biogeochemistry, geology, hydrology, oceanography, plankton, benthic primary producers (mainly seagrass) and invertebrates (Fig. 3). This has been consistent from the 1960s to the present. In Shark Bay, there has been greater emphasis on bony fishes, sharks, marine mammals and stromatolites. Both marine mammals (dugongs and dolphins) and stromatolites in Shark Bay have been a focus of decades of international research, and are among the key natural values of the Shark Bay Marine Park and World Heritage Site. In addition, the top-down influence of sharks on Shark Bay food webs has been a focus of concentrated research over the last 15 years, led by the Florida International University’s Shark Bay Ecosystem Research Project. The only areas of research with similar numbers of publications between the two bays were fisheries science and studies on macrofauna (mostly fish biology) since 2000. This reflects the commercial imperatives for sustainable fishing in both regions as populations and human pressures increase.
|
|
We compare the physical environments between Shark Bay and Florida Bay (geology, geomorphology, hydrology, climate, oceanography), then summarise the biology and anthropogenic threats for each bay. In doing so, we display the key knowledge gaps for each system, and how papers in this special issue address some of these gaps. We conclude with a summary of future directions for these sub-tropical bays.
Comparison between Shark Bay and Florida Bay
Shark Bay and Florida Bay are relatively recent (<10 000-year old) subtropical marine embayments at similar latitudes that are developed on carbonate-rich sediments, demonstrate hypersalinity and are phosphorus (P)-limited for algal and seagrass growth. The degree of hypersalinity in both systems differs substantially and reflects the wetter climate of Florida. They are also dominated by seagrasses, although temperate species dominate Shark Bay and tropical species Florida Bay. They differ in the levels of anthropogenic pressures, freshwater management and resource extraction.
Geology/geomorphology/sedimentology
Shark Bay is a shallow marine embayment, with a mean depth of <10 m (Logan and Cebulski 1970). Shark Bay was formed by a marine transgression into a coastal environment primarily composed of Pleistocene sediments approx. 7000–8000 years ago (Logan and Cebulski 1970). This flooding created a series of inlets and broad gulfs. The water mass is cut off from the Indian Ocean by a barrier ridge and islands composed of eolianite dunes. Water circulation has further been restricted in Shark Bay by a series of ridges and sills dominated by calcareous sediments. The largest of these is the Faure Sill, which runs from the mainland to the eastern coast of Peron Peninsula (Fig. 1).
Shark Bay has a permanent inter-annual salinity gradient that has been used to divide the bay into different zones: an oceanic zone (salinity 35–40‰); a metahaline zone (salinity 40–56‰); and a hypersaline zone (salinity 56–70‰) (Logan and Cebulski 1970). Oceanic and metahaline zones occupy the middle–northern sections of Shark Bay, while the hypersaline zone is restricted to Hamelin Pool. Sediments in Shark Bay are mainly calcareous sediments that are derived by in situ biogenic production (Logan and Cebulski 1970). Organisms that contribute to the production of these calcareous sediments include coralline algae, molluscs, forams, echinoids, serpulids and bryozoans (Logan and Cebulski 1970). For example, epiphytic coralline red algae growing on seagrass leaves have increased sediment depths by 0.5 mm year–1 (Walker and Woelkerling 1988). In contrast to the majority of Shark Bay, sediments at the Wooramel Delta are fine and anoxic with a high content of terrigenous muds transported into the sea by the infrequently flowing Wooramel River (mean time between flooding reported as 8 years).
Florida Bay is separated from the Atlantic Ocean by a chain of Pleistocene limestone islands. Florida Bay can be further compartmentalised into semi-isolated basins, split by a series of mud banks dominated by calcareous sediments (Fourqurean and Robblee 1999). Similar to Shark Bay, these sediments are biogenic in origin, with coralline algae, epiphytes, forams, molluscs and corals all contributing to sediment production (Bosence 1989a, 1989b, 1995). The flooding of Florida Bay occurred fairly recently; the south-western section of the bay flooded 4500 years ago, while the eastern parts of the bay only flooded as recently as 1500 years ago (Fourqurean and Robblee 1999). The mud banks in Florida Bay are similar to the ridges and sills in Shark Bay in that they restrict water circulation. Florida Bay has been zoned based on sediment dynamics: an inner and outer destructional zone (where banks are being eroded quicker than they are accumulating); a western constructional zone (where sediment supply is high enough for accretion); and a central migrational zone (where erosion and deposition are quasi-equal so that sediment balance is in an equilibrium) (Wanless and Tagett 1989).
Hydrology/climatology
There are significant differences in climatology between Florida Bay and Shark Bay. The climate of Shark Bay is extremely hot and dry, with annual potential evaporation (2000 mm) exceeding precipitation (200 mm) by an order of magnitude (Logan and Cebulski 1970; Burling et al. 1999). Rainfall is highest in winter and lowest in summer in Shark Bay, while evaporation is highest in summer and lowest in winter. However, evaporation rates are higher than precipitation rates across the entire year (Smith and Atkinson 1983). In comparison, Florida Bay receives ~1200 mm in rain every year, with 75% falling in the wet season. Annual evaporation is 830–1290 mm lower in Florida Bay than in Shark Bay, and annual precipitation is 980 mm higher (Nuttle et al. 2000). Rainfall in Florida Bay is concentrated in the summer and autumn months (July–October). This highlights a major difference between the two bays: Shark Bay has a permanent and larger net evaporation of water compared with the lower and seasonal freshwater deficit in Florida Bay.
Direct river run-off into Florida Bay is primarily through the Taylor Slough. Although freshwater run-off can be an important source of nutrient inputs for the enclosed mangrove-lined embayments along the coastal margin, freshwater run-off into Florida Bay accounts for only 3% of P and 12% of N inputs (Rudnick et al. 1999). Water and nutrients delivered in freshwater run-off are minor compared with atmospheric deposition and exchange with the Gulf of Mexico (Sutula et al. 2001). In addition, groundwater may also be a significant source of N and P; providing as much N and P as surface inputs from Taylor Slough (Corbett et al. 1999), especially along the northern mangrove-lined shore of Florida Bay (Price et al. 2006). Only one river flows into Shark Bay, the Wooramel River, into the eastern gulf. However, periods of flow are restricted to episodic flooding events, after cyclonic events in the summer or winter storms (Smith and Atkinson 1983; Nott 2011). The fluxes of fresh water, nutrients and organic matter into Shark Bay from episodic flooding of the Wooramel River are not known, and could potentially influence adjacent benthic communities. The importance of groundwater inputs of nutrients into Shark Bay is also poorly known.
Oceanography
Hypersalinity is a persistent feature in Shark Bay, but a seasonal and inter-annual feature in Florida Bay. In Shark Bay, a combination of high evaporation rates and restricted circulation with oceanic water results in a permanent gradient of increasing salinity southwards into the Bay, and is particularly pronounced in the eastern embayment, where salinities in southern Hamelin Pool can reach over 65‰ (Walker 1985). In comparison, hypersalinity in Florida Bay is temporally variable, present as a result of cyclic drought conditions in south Florida (Fourqurean and Robblee 1999) and probably aggravated by reduced freshwater inflows as a result of anthropogenic alterations (Marshall et al. 2009). Salinities in central Florida Bay can reach as high as 72‰ during drought conditions (Tabb et al. 1962).
The shallow, compartmentalised nature of both embayments results in local tidal anomalies. Tides vary considerably across Florida Bay, and are strongly influenced by a combination of bottom friction and restricted movement due to submerged banks (Wang et al. 1994). For example, tides are diurnal with a mean amplitude of 0.3 m at the western extent of Florida Bay, but are semi-diurnal with a mean amplitude of 0.17 m at the Long Key area (Wang et al. 1994). Tidal energy is attenuated by the shallow banks in Florida Bay, leading to no lunar tide over central and north-eastern sections of the Bay. There is some tidal exchange between the Atlantic Ocean and the bay, despite the restrictions in circulation. Similar patterns have been observed in Shark Bay. For example, tides on eastern Hopeless Reach are mainly semi-diurnal, while tides are diurnal at western Freycinet Reach (Burling et al. 2003). Again, bottom friction and restriction due to submerged banks play a significant role in influencing tides across Shark Bay (Burling et al. 2003). Significant tidal attenuation and lag occur over the Faure Sill region, which result in strong tidal currents of up to 100 cm s–1 (Burling et al. 2003).
Carbon, nitrogen and phosphorus
Both Shark Bay and Florida Bay have been considered as examples of systems where low P concentrations limit primary production (Smith 1984; Atkinson 1987; Powell et al. 1989; Fourqurean and Zieman 1992; Fourqurean et al. 1993). As a result of the sorption of dissolved inorganic phosphorus (DIP) onto carbonate particles (de Kanel and Morse 1978) and high rates of primary production, DIP concentrations in both bays are exceptionally low, often at or below the detection level of 20 nM (Atkinson 1987; Fourqurean et al. 1993). Dissolved inorganic nitrogen (DIN) tends to be higher in Florida Bay (median value 3.3 μM) than Shark Bay (0.2–0.6 μM) (Atkinson 1987; Fourqurean et al. 1993). Nutrient inputs into both embayments are dominated by exchange with oceanic water. Florida Bay has been subdivided into zones based on planktonic nutrient concentrations; with the western zone having a N : P ratio near the Redfield ratio, the central Bay having a higher N : P ratio, and the eastern zone with very high N : P ratios due to high DIN concentrations (Boyer et al. 1997).
Seagrasses
Seagrasses are the defining benthic communities covering a large area of the subtidal in both Shark and Florida Bays (Walker et al. 1988; Fourqurean et al. 2001). Shark Bay has a diverse seagrass assemblage of 12 tropical and temperate species (Walker et al. 1988). The occurrence and dominance of temperate seagrass species in Shark Bay is impressive: 3676 km2 of shallow banks and sills are covered with Amphibolis antarctica and 208 km2 of deeper channels and edges of sills with Posidonia australis (Walker et al. 1988). Florida Bay is more tropically influenced with 7 species occurring and is dominated by Thalassia testudinium (Fourqurean et al. 2001).
Salinity has an effect on the distribution of seagrasses and seagrass-associated biota in Shark Bay and Florida Bay. In Shark Bay, the increasing salinities have resulted in strong zonation of benthic biota (Logan and Cebulski 1970). The seagrasses A. antarctica and P. australis dominate benthic communities where mean salinity is less than ~55‰ (Walker et al. 1988), but they are absent at higher salinities, replaced by stromatolites and other microbial communities. There are also decreases in diversity in other seagrass-dependent biota with increased salinity. For example, both epiphytic and benthic seaweeds decline in species richness and abundance with increased salinity (Kendrick et al. 1988, 1990). The distribution of seagrass species throughout Florida Bay is driven by salinity (Zieman et al. 1989). Recent modelling efforts have projected that seagrass species distribution throughout Florida Bay would change with increasing freshwater flow associated with restoration of upstream wetlands (Herbert et al. 2011). Similarly, hypersalinity has been hypothesised as a contributor to seagrass die-off in Florida Bay, when combined with sulfide intrusion and hypoxia (Koch et al. 2007), and restructuring of seagrass communities (Herbert et al. 2011).
The other major driver of seagrass distribution in both Shark and Florida Bay is nutrient availability (Fourqurean et al. 1995). Indeed, changes to N and P loads entering these systems are the greatest threat to both species dominance and distribution. In Florida Bay, N : P ratios of seagrass leaves are generally higher in nearshore areas, indicating P-limited seagrass beds were growing nearshore and N-limited seagrass beds were growing offshore (Fourqurean and Zieman 2002). Fourqurean et al. (2003) demonstrated the spatial impacts of increased P and N additions to Florida Bay with increased run-off and riverine inputs would shift seagrass dominance from the slower growing Thalassia testudinium to weedy species like Ruppia maritima. These changes would have flow-on effects for habitat utilisation and secondary production. In Shark Bay, it has been assumed that the major source of P for benthic communities in the eastern embayment comes from Indian Ocean water in the north (Smith and Atkinson 1983; Atkinson 1987). As such, N : P of seagrasses increases from north to south in Shark Bay, indicative of N-limited growth near the mouth of Shark Bay, P-limited growth in the most isolated landward reaches, and light-limited growth in the middle regions (Burkholder et al. in press).
Marine mammals and sea turtles
Marine mammals are prominent components of the Shark Bay and Florida Bay ecosystems, with both bays supporting substantial populations of bottlenose dolphins (Tursiops cf. aduncus and T. truncatus, respectively). The dolphin population of Shark Bay has been the subject of long-term studies of social structure and behaviour since the 1980s (Connor et al. 1992; Smolker et al. 1992; Mann et al. 2000; Heithaus and Dill 2002). In Florida Bay, dolphin studies have focussed on habitat affinities (Torres et al. 2008). In both locations, dolphins exhibit foraging specialisations that allow them to consume a diversity of teleost prey (Lewis and Schroeder 2003; Torres and Read 2009; Sargeant et al. 2007). Dolphins could be important upper-trophic-level predators in both bays because of their high metabolic rates and relatively high population densities. However, the impacts of dolphin predation on prey population dynamics remain to be investigated. There is no evidence that population size of dolphins has been impacted by humans in either bay, but there have been declines in the abundance of individuals within areas of high boat use in Shark Bay (Bejder et al. 2006). Data on these shifts were used to develop current management practices for dolphin ecotourism in the bay.
Sirenian (manatees and dugongs) and green turtle populations in Shark Bay appear to have remained at or near historical levels (Preen et al. 1997), but have declined dramatically in Florida Bay (Jackson et al. 2001). Given the large dugong and sea turtle population in Shark Bay, it represents an important location for understanding the ecological roles of sirenians and sea turtles under natural population densities (Heithaus et al. 2008). Early dugong research in Shark Bay focussed on dugong behaviour, and habitat suitability (Anderson 1982, 2009; Marsh et al. 1994; Preen et al. 1997). Recent studies have focussed on population size, distribution and connectivity along the Western Australian coast (Gales et al. 2004; Holley et al. 2006) as well as habitat selection and foraging behaviour (Wirsing et al. 2007). Florida Bay has already experienced the consequences of near extinction of manatees and sea turtles. For example, Jackson et al. (2001) suggested that seagrass die-offs in Florida Bay may have been exacerbated by the loss of large herbivores, primarily green turtles, although seagrass canopy heights were greater because of relaxed grazing pressure.
Fish
There are ~250 fish species in the marine waters of Everglades National Park, which includes Florida Bay (Loftus 2000). Florida Bay provides important habitat for economically important species including snook (Centropomus undecimalis), tarpon (Megalops atlanticus), spotted seatrout (Cynoscion nebulosus), red drum (Sciaenops ocellatus), spiny lobster (Panulirus argus) and pink shrimp (Penaeus duorarum). Florida Bay is also a nursery ground for spiny lobsters, which spend several years in the Bay, and then migrate to coral reef habitat along the Florida Keys (Davis and Dodrill 1989). Gray snapper (Lutjanus griseus), spotted seatrout, red drum, sheepshead (Archosagrus probatocephalus), and black drum (Pogonias cromis) make up the majority of recreational fishing harvest (Tilmant 1989). Similar to seagrasses, distribution and diversity of fishes in Florida Bay may be influenced by salinity gradients (Ley et al. 1999; Thayer et al. 1999).
Fish diversity in Shark Bay is exceptionally high with the region located near the northern limit of an ecological transition zone between temperate and tropical marine fish faunas on the west coast of Australia (Hutchins 1990). Hutchins (1990) recorded 323 species in South Passage where the fish communities are dominated by tropical species while Kangas et al. (2007) recorded 241 mostly tropical species in the deeper waters to the north of Cape Peron. Other studies have shown that species diversity and the dominance of tropical taxa both decrease as one moves southwards into the inner gulfs (Lenanton 1977; Travers and Potter 2002).
Elasmobranch fish diversity is high in both Florida Bay and Shark Bay. Within Florida Bay there are ~11 species of sharks and five species of rays, with an additional 11 elasmobranch species found in marine waters outside or adjacent to the bay (Loftus 2000; Torres et al. 2006; Wiley and Simpfendorfer 2007). The diversity of elasmobranchs in Shark Bay is remarkably high (White and Potter 2004; Vaudo and Heithaus 2009). In total, 28 species of sharks and rays have been recorded from the Eastern Gulf of Shark Bay, and at least 13 other elasmobranch species have been recorded from Shark Bay (Vaudo and Heithaus 2009). The abundance of large sharks – primarily tiger sharks – is much higher in Shark Bay than Florida Bay (Heithaus 2001; Wiley and Simpfendorfer 2007), likely reflecting different histories of exploitation (Heithaus et al. 2007). The abundance of sharks within Florida Bay varies among regions, with more found in broad areas that appear to have the highest abundance of potential teleost prey and the greatest connection to open marine waters (Torres et al. 2006; Wiley and Simpfendorfer 2007). Few studies have addressed the potential ecological role of sharks and rays in Florida Bay. In contrast, Shark Bay has served as a model ecosystem for elucidating the ecological role of top predators in intact coastal ecosystems.
Anthropogenic pressures and their management
Anthropogenic pressures vary significantly between Shark Bay and Florida Bay. In Shark Bay anthropogenic pressures are so small that it may serve as a pristine template to assess the effects of interacting human pressures on subtropical embayments worldwide. Denham, the largest township in Shark Bay, is situated on the western coast of Peron Peninsula, and supports a residential population of roughly 1000 people. Shark Bay is a major tourism destination particularly during the cooler months (April–September) when large numbers of seasonal visitors significantly increase the local population. Much of this tourism is recreational fishing related (Shaw 2000) and is concentrated in Denham and Monkey Mia, a small tourism development on the eastern coast of the Peron Peninsula where dolphin-tourism draws tens of thousands of people to the bay. In addition, commercial fishing has a long history in Shark Bay (Shaw 2000). The only industrial development in the Shark Bay area is at Useless Loop, containing Shark Bay Salt Joint Venture (SBSJV), a salt works that has also loading facilities for large ships in Shark Bay. From a development point of view, Shark Bay can be considered pristine, with little human development surrounding the Bay beyond sheep rearing. Interestingly, the increases observed in research publications in Shark Bay in the 1980s and 1990s (Fig. 2) reflect the opening up of the region to tourism (the road to Denham was bituminised in 1986), a large UWA-funded research program in the 1980s (UWA Shark Bay Research Program), publication of long-term shark and dolphin research and increased levels of awareness of the region resulting in the World Heritage Listing (1991) and declaration of Shark Bay Marine Park (1997) in the 1990s.
In comparison, development adjacent to Florida Bay is more intense. The watershed of Florida Bay is the Everglades, and is extremely important in determining abiotic conditions throughout the Bay (Herbert et al. 2011). However, the Everglades have a history of engineered land-use and water-use changes to accommodate a growing population in South Florida. Such changes include the Florida Overseas Railway (1907–1911) and a series of canals, levees and water-control structures used to control hydrology around the Everglades (Herbert et al. 2011). Although these structures increased the amount of arable and habitable land in the Everglades, they also decreased freshwater heads and inflows from the Everglades, altering the historical salinity climates within Florida Bay (Herbert et al. 2011). These changes in salinity have caused shifts in benthic communities within the bay. For example, Thalassia testudinum has replaced Halodule wrightii as the dominant seagrass species in much of north and north-eastern Florida Bay due to increased salinities favouring the former species (Zieman et al. 1989; Fourqurean et al. 2003). Impacts of salinity changes have also occurred for higher-trophic-level species, such as wading birds (Lorenz et al. 2009).
Summary of contributing papers in this Special Issue
This special issue represents a significant addition to the body of literature on subtropical estuaries and to our scientific knowledge of Shark Bay and Florida Bay ecosystems. The papers demonstrate general similarities but subtle differences in the hydrology, biochemistry and ecosystem dynamics between the two systems. The physical environment has a significant effect on the chemistry and biology of these systems. There are important feedback mechanisms from the dominant biota that are similar in both embayments, including the production of significant organic and inorganic carbon, which play a role in the formation of sediment banks and in sequestration of carbon and nutrients. The role of top predators on trophic webs and the importance of mega-grazers in controlling primary production are also investigated using diverse observational, experimental, chemical and behavioural tools. Shark Bay is unique in the size of populations of mega-grazers (e.g. dugongs, green turtles) and top predators (e.g. tiger sharks) and their overall role in system productivity and recycling is large and rarely studied using such a range of methods. Management of fish stock and other human activities also has been addressed in a series of stock assessments, impacts of fishing and surveys for fisheries in Shark Bay. These will form the scientific basis for future management of the system. The shift from commercial to recreational-only fishing in Florida Bay is in stark contrast to the diverse and sustainable commercial and recreational fisheries of Shark Bay.
The sources and residence times of water within both systems and its influence on the seasonal (Florida Bay) or permanent (Shark Bay) salinity gradients defines the physical environment and therefore the drivers of species distribution and abundance in both systems. Price et al. (2012) compare the sources and exchanges among water bodies within Florida Bay and Shark Bay, and describe for the first time the isotopic signature of riverine waters entering Shark Bay via the Wooramel River and the extensive groundwater that underlies much of the central Shark Bay region.
The sedimentary fate of carbon and nutrients in both Shark Bay and Florida Bay is addressed by Fourqurean et al. (2012). The shallow banks and shoals in both systems are major sinks and stores of organic carbon, rivalling terrestrial forests in their storage capacity. Organic carbon within sediments varied between ~150 and 250 Mg Corg ha–1. These very high values place both bays among those coastal ecosystems that have the highest carbon storage, globally. While down-core profiles show that nutrient availability and productivity have increased through time in Florida Bay, similar profiles from Shark Bay suggest that nutrient availability and productivity have decreased since the Faure Sill was deposited in Shark Bay.
To predict the influence of climate change on both the Shark Bay and Florida Bay systems, more detailed bathymetry and tide data are required. Interestingly, in this issue data are presented that demonstrate astronomical tides do not play a major part (accounting for 15% of total water variation effects) on the depth distribution of different cyanobacterial mats including stromatolites in Hamelin Pool, Shark Bay (Burne and Johnson 2012).
The nature of nutrient limitation for the seagrasses in Florida Bay has been studied intensively but less so in Shark Bay. Fraser et al. (2012) address nutrient limitation in the eastern embayment of Shark Bay and demonstrate that P content of seagrass leaves does not decrease southward across the Faure Sill in salinities between 42‰ and 55‰. Fraser et al. (2012) corroborate the larger regional picture from Burkholder et al. (in press), and show little indication of P limitation of seagrasses across the Faure Sill based on N : P ratios of seagrass leaves, suggesting P sources were not limiting seagrass growth during their study. It is unknown whether this was driven by an unseasonal flooding of the Wooramel River, remineralisation of P, or inputs from alternate P sources.
Factors other than nutrients are important for changes in seagrass productivity across the salinity gradient. High salinities can reduce oxygen concentrations and speciation of sulfur in sediments than can adversely impact seagrasses. Cambridge et al. (2012) investigate the levels of sulfides in sediments and inside the tissues of seagrasses in the highly reduced sediments of Shark Bay. Sulfide intrusion into seagrasses in Florida Bay resulted in large-scale death of seagrasses and sulfides were partly to blame (Koch et al. 2007). Cambridge et al. (2012) found seagrasses in Shark Bay less impacted although tissues were equally intruded and propose that the different seagrass response was driven by greater anoxia in sediments from Florida.
Similarly, mega-grazers can reduce overall standing stock and net primary production in seagrasses. The nutritional quality of leaves in the temperate seagrass species Posidonia australis and Amphibolis antarctica is low, indicating they are not a preferred food for mega-grazers and implying their abundance may be also due in part to their lack of palatability to grazers (Burkholder et al. 2012). This hypothesis requires further testing.
Local impacts to seagrasses have been documented as a result of recreational boating in Florida Bay and managers seek to mitigate impacts through education and zoning (Hallac et al. 2012). Clearly, changes in local and regional land practices are a threat to these coastal ecosystems and restoration is one of the approaches to address impacts. Statton et al. (2012) assessed seagrass restoration efforts focussing on Shark Bay and Florida Bay and note very poor long-term success in these programs. Clearly, losses become even more critical if restoration is not a viable alternative.
The possibility of top-down impacts that cascade throughout the Shark Bay food web are explored in a series of papers. Heithaus et al. (2012) review 15 years of research investigating the possibility that tiger sharks structure Shark Bay communities primarily through non-consumptive (e.g. behavioural) effects on their prey. Burkholder et al. (2012) extend these studies by investigating the gazing preferences of both meso- and mega-grazers while Wirsing and Heithaus (2012) show that dugongs modify the duration of their behaviours in response to threats from tiger sharks. Other contributions explore the drivers of movements of rays (Vaudo and Heithaus 2012) and provide some of the first data on movements and residency patterns of adult male loggerhead turtles (Olson et al. 2012). Interestingly, fatty acid and isotope studies paint a complex food web, with detrital sources of carbon more prevalent for fish and invertebrate grazers, ctenophore and macroalgae more important for green turtles, and loggerhead and green turtles potentially being an important component of tiger shark diet (Belicka et al. 2012). The predominance of detrital sources of carbon are also seen in the predominance of dissolved organic carbon derived from seagrass detritus dominating the lower bays and reaches of Shark Bay (Cawley et al. 2012).
Fishing represents one of the most direct human influences on marine ecosystems, in particular through the removal of fish and invertebrates. In Florida Bay, commercial fisheries ceased in the late 1980s and the system is now managed for recreational fishing only. In contrast, Shark Bay continues to support important commercial fisheries as well as being a highly popular recreational fishing destination. Commercial fishing dates back to the early 1900s for snapper (line fishing), the 1940s for whiting (beach seine netting) and the early 1960s for prawn trawling. Fisheries management in Shark Bay has historically been focussed on the sustainability of the target species and has used a range of measures including extensive temporal and spatial closures to protect habitats, particularly those critical to early life-history stages of target species. More recently, fisheries in Shark Bay and Western Australia more broadly have moved towards a more holistic management approach in line with community environmental concerns, which have required a greater understanding of the broader ecological impacts of fishing.
All major commercial fisheries in Shark Bay have had dedicated research programs since the 1960s, and more recently these have had an added focus on secondary fishing impacts. The fisheries papers in this special issue build on previous research and increase understanding of the effect of fisheries on the Shark Bay ecosystem. Chandrapavan et al. (2012) examined the performance of square mesh panels fitted to standard 100 mm scallop trawls in minimising discards of small scallops and by-catch species. Geostatistical modelling has been used to provide a useful tool for understanding patterns in the abundance and spatial distribution of scallop recruitment in Shark Bay (Mueller et al. 2012). The development of a dedicated trap fishery for blue swimmer crabs from 1998 onwards has seen the Shark Bay fishery become the largest commercial blue swimmer crab fishery in Australia (Harris et al. 2012). The finfish stocks in Shark Bay have also made it an important destination for recreational boat-based fishers since the 1960s at least, with local snapper stocks one of the main attractions (Jackson and Moran 2012). Research undertaken since the late 1990s on the depletion of snapper (Jackson and Moran 2012; Norriss et al. 2012) has been successfully used within an adaptive management approach to recover these important snapper stocks. Wise et al. (2012) demonstrate the value of long-term data sets for the management of recreational fishing in popular locations within areas of high conservation value such as Shark Bay.
Future directions
The most vital set of questions requiring immediate study relate to the ecological resilience of Shark Bay and Florida Bay and the combined interactions between physical environmental drivers, biological control through behavioural and trophic interactions, and the increased anthropogenic demands. These systems show resilience to perturbations, both short-term and sustained, but past events in Florida Bay have had major ramifications on the seagrasses and associated biota across large regions driven by increases in salinity, declines in freshwater run-off, decline in P availability, de-oxygenation and loss of fish and mega-grazers. Clearly, we need to understand the influence of these multiple interacting stressors on both the pristine natural environment of Shark Bay and the anthropogenically altered Florida Bay ecosystems.
The large spatial scale of Shark Bay and Florida Bay, and their level of complexity and heterogeneity, require a greater understanding of within-system heterogeneity to aid in management decisions and actions. The papers in this special issue are a good start to our understanding, but lead to a series of interesting questions about hydrological inputs, nutrient gradients, drivers of primary production, trophic connectivity and sustainability of commercial fisheries. The compartmentalised nature of these ecosystems can be seen in the compartmentalisation of distributions of snapper and dolphin populations. Within-system heterogeneity is also well illustrated in differing levels of P limitation in seagrasses down a limiting P and salinity gradient across the Faure Sill, and the importance of event-driven (cyclones) point-source freshwater inputs in supplying P to seagrasses for growth.
Finally, Shark Bay should be viewed as a semi-pristine ecosystem and a ‘pristine template’ to restoration efforts in Florida Bay and other subtropical embayments. Yet presently the system as a whole is poorly studied (even compared with the rest of the Western Australian coastline), despite it having been granted World Heritage status over 20 years ago. Interestingly, studies of the megafauna in Shark Bay are much advanced compared with those of Florida Bay, but studies in Florida Bay are much further ahead in other research areas. We need to coordinate research across national boundaries to address the potential trajectories of natural ecosystems under increased anthropogenic perturbations. To do this we highly recommend a more coordinated multi-institutional and multi-discipline approach to our somewhat piecemeal research in both Shark Bay and Florida Bay. Enhancing studies in both locations could provide important general insights into the dynamics of coastal ecosystems, including anthropogenic effects, and could help build a blueprint for more effective management or, in the cases of degraded ecosystems, restoration.
Acknowledgements
The idea of a special issue focussing on Shark Bay and Florida Bay came out of a UWA Institute for Advanced Studies workshop titled ‘Comparative Ecosystems Studies – Shark Bay and Southern Florida’ organised by Fourqurean and Kendrick and held in March 2011. JWF received support in the form of a Gledden Senior Visiting Fellowship from the Institute for Advanced Studies at the University of Western Australia. Support for the Special issue came from: the Western Australian Marine Science Institution through NHT II – Caring for our Country funding (OC11-00643); the Florida Coastal Everglades Long-Term Ecological Research program under National Science Foundation Grant Nos. DBI-0620409 and DEB-9910514; the Shark Bay Ecosystem Research Project under the direction of Michael Heithaus, funded by NSF Grant Nos. OCE-0526065 and OCE-0745606; The Western Australian Departments of Fisheries and Environment and Conservation; and through a grant from the University of Western Australia to GAK and JWF.
References
Anderson, P. K. (1982). Studies of dugongs at Shark Bay, Western Australia. II. Surface and subsurface observations. Australian Wildlife Research 9, 85–99.| Studies of dugongs at Shark Bay, Western Australia. II. Surface and subsurface observations.Crossref | GoogleScholarGoogle Scholar |
Anderson, P. K. (2009). Shark Bay dugongs (Dugong dugon) in summer. II Foragers in a Haladule-dominated community. Mammalia 62, 409–426.
Atkinson, M. J. (1987). Low phosphorus sediments in a hypersaline marine bay. Estuarine, Coastal and Shelf Science 24, 335–347.
| Low phosphorus sediments in a hypersaline marine bay.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL2sXksVGrs7g%3D&md5=dd50bd1e4d5e7dea82658d7f3f3074baCAS |
Bejder, L., Samuels, A., Whitehead, H., Gales, N., Mann, J., Connor, R., Heithaus, M., Watson-Capps, J., Flaherty, C., and Kruetzen, M. (2006). Decline in relative abundance of bottlenose dolphins exposed to long-term disturbance. Conservation Biology 20, 1791–1798.
| Decline in relative abundance of bottlenose dolphins exposed to long-term disturbance.Crossref | GoogleScholarGoogle Scholar |
Belicka, L. L., Burkholder, D., Fourqurean, J. W., Heithaus, M. R., Macko, S. A., and Jaffe, R. (2012). Stable isotope and fatty acid biomarkers of seagrass, epiphytic, and algal organic matter to consumers in a pristine seagrass ecosystem. Marine and Freshwater Research 63, 1085–1097.
| Stable isotope and fatty acid biomarkers of seagrass, epiphytic, and algal organic matter to consumers in a pristine seagrass ecosystem.Crossref | GoogleScholarGoogle Scholar |
Bosence, D. (1989a). Biogenic carbonate production in Florida Bay. Bulletin of Marine Science 44, 419–433.
Bosence, D. (1989b). Surface sublittoral sediments of Florida Bay. Bulletin of Marine Science 44, 434–453.
Bosence, D. (1995). Anatomy of a recent biodetrital mud-mound, Florida Bay, USA. Special Publication of the International Association of Sedimentologists 23, 475–493.
Boyer, J. N., Fourqurean, J. W., and Jones, R. D. (1997). Spatial characterization of water quality in Florida Bay and Whitewater Bay by multivariate analyses: zones of similar influence. Estuaries 20, 743–758.
| Spatial characterization of water quality in Florida Bay and Whitewater Bay by multivariate analyses: zones of similar influence.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXjtVSmuw%3D%3D&md5=ea6f63e6ec0e1be81d70b64b0f834a26CAS |
Burkholder, D. A., Heithaus, M. R., and Fourqurean, J. W. (2012). Feeding preferences of herbivores in a relatively pristine subtropical seagrass ecosystem. Marine and Freshwater Research 63, 1051–1058.
| Feeding preferences of herbivores in a relatively pristine subtropical seagrass ecosystem.Crossref | GoogleScholarGoogle Scholar |
Burkholder, D., Fourqurean, J. W., and Heithaus, M. R. (). Spatial pattern in seagrass stoichiometry indicates both N-limited and P-limited regions of an iconic P-limited subtropical bay. Marine Ecology Progress Series , .
Burling, M. C., Ivey, G. N., and Pattiaratchi, C. B. (1999). Connectively driven exchange in a shallow coastal embayment. Continental Shelf Research 19, 1599–1616.
| Connectively driven exchange in a shallow coastal embayment.Crossref | GoogleScholarGoogle Scholar |
Burling, M. C., Pattiaratchi, C. B., and Ivey, G. N. (2003). The tidal regime of Shark Bay, Western Australia. Estuarine, Coastal and Shelf Science 57, 725–735.
| The tidal regime of Shark Bay, Western Australia.Crossref | GoogleScholarGoogle Scholar |
Burne, R., and Johnson, K. (2012). Sea-level variation and the zonation of microbialites in Hamelin Pool, Shark Bay. Marine and Freshwater Research 63, 994–1004.
| Sea-level variation and the zonation of microbialites in Hamelin Pool, Shark Bay.Crossref | GoogleScholarGoogle Scholar |
Cambridge, M. L., Fraser, M. W., Holmer, M., Kuo, J., and Kendrick, G. A. (2012). Hydrogen sulfide intrusion in seagrasses from Shark Bay, Western Australia. Marine and Freshwater Research 63, 1027–1038.
| Hydrogen sulfide intrusion in seagrasses from Shark Bay, Western Australia.Crossref | GoogleScholarGoogle Scholar |
Cawley, K. M., Ding, Y., Fourqurean, J. W., and Jaffe, R. (2012). Characterising the sources and fate of dissolved organic matter in Shark Bay, Australia: a preliminary study using optical properties and stable carbon isotopes. Marine and Freshwater Research 63, 1098–1107.
| Characterising the sources and fate of dissolved organic matter in Shark Bay, Australia: a preliminary study using optical properties and stable carbon isotopes.Crossref | GoogleScholarGoogle Scholar |
Chandrapavan, A., Kangas, M. I., and Sporer, E. (2012). Performance of square-mesh codends in reducing discards and by-catch in the Shark Bay scallop fishery. Marine and Freshwater Research 63, 1142–1151.
| Performance of square-mesh codends in reducing discards and by-catch in the Shark Bay scallop fishery.Crossref | GoogleScholarGoogle Scholar |
Connor, R. C., Smolker, R. A., and Richards, A. F. (1992). Two levels of alliance formation among male bottlenose dolphins Tursiops sp. Proceedings of the National Academy of Sciences of the United States of America 89, 987–990.
| Two levels of alliance formation among male bottlenose dolphins Tursiops sp.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3MrmtFejtQ%3D%3D&md5=513508e84e4d5a42067a7c594fea35a8CAS |
Corbett, D. R., Chanton, J., Burnett, W., Dillon, K., Rutkowski, C., and Fourqurean, J. W. (1999). Patterns of groundwater discharge into Florida Bay. Limnology and Oceanography 44, 1045–1055.
| Patterns of groundwater discharge into Florida Bay.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXktFOiu7Y%3D&md5=993b954517dddefafccab90e67ba3d7eCAS |
Davis, G. E., and Dodrill, J. W. (1989). Recreational fishery and population dynamics of spiny lobsters, (Panulius Argus) in Florida Bay, Everglades. Bulletin of Marine Science 44, 78–88.
de Kanel, J., and Morse, J. W. (1978). The chemistry of orthophosphate uptake from seawater onto calcite and aragonite. Geochimica et Cosmochimica Acta 42, 1335–1340.
| The chemistry of orthophosphate uptake from seawater onto calcite and aragonite.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaE1MXhtFSnu7g%3D&md5=499c2241f6160e228d1d7b376dc1e1c5CAS |
Fourqurean, J. W., and Robblee, M. B. (1999). Florida Bay: a history of recent ecological changes. Estuaries 22, 345–357.
| Florida Bay: a history of recent ecological changes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXmsVOmsbY%3D&md5=345965c3752d2e381cc7a34bf1850d5dCAS |
Fourqurean, J. W., and Zieman, J. C. (1992). Phosphorus limitation of primary production in Florida Bay: evidence from C : N : P ratios of the dominant seagrass Thalassia testudinum. Limnology and Oceanography 37, 162–171.
| Phosphorus limitation of primary production in Florida Bay: evidence from C : N : P ratios of the dominant seagrass Thalassia testudinum.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK38Xktleitbs%3D&md5=5bee7e8b99f1608ecb2b3c8104e86969CAS |
Fourqurean, J. W., and Zieman, J. C. (2002). Nutrient content of the seagrass Thalassia testudinum reveals regional patterns of relative availability of nitrogen and phosphorus in the Florida Keys USA. Biogeochemistry 61, 229–245.
| Nutrient content of the seagrass Thalassia testudinum reveals regional patterns of relative availability of nitrogen and phosphorus in the Florida Keys USA.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XntVKjurY%3D&md5=1d7518f5d2ae5464f051214fdec7aefeCAS |
Fourqurean, J. W., Jones, R. D., and Zieman, J. C. (1993). Processes influencing water column nutrient characteristics and phosphorus limitation of phytoplankton biomass in Florida Bay, FL, USA: inferences from spatial distributions. Estuarine, Coastal and Shelf Science 36, 295–314.
| Processes influencing water column nutrient characteristics and phosphorus limitation of phytoplankton biomass in Florida Bay, FL, USA: inferences from spatial distributions.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3sXlsVKjsb0%3D&md5=b37dbcb38d3f6ba9726736af93ab236eCAS |
Fourqurean, J. W., Powell, G. V. N., Kenworthy, W. J., and Zieman, J. C. (1995). The effects of long-term manipulation of nutrient supply on competition between the seagrasses Thalassia testudinum and Halodule wrightii in Florida Bay. Oikos 72, 349–358.
| The effects of long-term manipulation of nutrient supply on competition between the seagrasses Thalassia testudinum and Halodule wrightii in Florida Bay.Crossref | GoogleScholarGoogle Scholar |
Fourqurean, J. W., Willsie, A., Rose, C. D., and Rutten, L. M. (2001). Spatial and temporal pattern in seagrass community composition and productivity in south Florida. Marine Biology 138, 341–354.
| Spatial and temporal pattern in seagrass community composition and productivity in south Florida.Crossref | GoogleScholarGoogle Scholar |
Fourqurean, J. W., Boyer, J. N., Durako, M. J., Hefty, L. N., and Peterson, B. J. (2003). Forecasting responses of seagrass distributions to changing water quality using monitoring data. Ecological Applications 13, 474–489.
| Forecasting responses of seagrass distributions to changing water quality using monitoring data.Crossref | GoogleScholarGoogle Scholar |
Fourqurean, J. W., Kendrick, G. A., Collins, L. S., Chambers, R. M., and Vanderklift, M. A. (2012). Carbon, nitrogen and phosphorus storage in subtropical seagrass meadows: examples from Florida Bay and Shark Bay. Marine and Freshwater Research 63, 967–983.
| Carbon, nitrogen and phosphorus storage in subtropical seagrass meadows: examples from Florida Bay and Shark Bay.Crossref | GoogleScholarGoogle Scholar |
Fraser, M. W., Kendrick, G. A., Grierson, P. F., Fourqurean, J. W., Vanderklift, M. A., and Walker, D. I. (2012). Nutrient status of seagrasses cannot be inferred from system-scale distribution of phosphorus in Shark Bay, Western Australia. Marine and Freshwater Research 63, 1015–1026.
| Nutrient status of seagrasses cannot be inferred from system-scale distribution of phosphorus in Shark Bay, Western Australia.Crossref | GoogleScholarGoogle Scholar |
Gales, N., McCauley, R. D., Lanyon, J., and Holley, D. (2004). Change in abundance of dugongs in Shark Bay, Ningaloo and Exmouth Gulf, Western Australia: evidence for large-scale migration. Wildlife Research 31, 283–290.
| Change in abundance of dugongs in Shark Bay, Ningaloo and Exmouth Gulf, Western Australia: evidence for large-scale migration.Crossref | GoogleScholarGoogle Scholar |
Hallac, D., Sadle, J., Pearlstine, L., Herling, F., and Shinde, D. (2012). Boating impacts in Florida Bay, Everglades National Park, Florida USA: links with physical and visitor-use factors and implications for management. Marine and Freshwater Research 63, 1117–1128.
| Boating impacts in Florida Bay, Everglades National Park, Florida USA: links with physical and visitor-use factors and implications for management.Crossref | GoogleScholarGoogle Scholar |
Harris, D., Johnston, D., Sporer, E., Kangas, M., Felipe, N., and Caputi, N. (2012). Biology and management of a multi-sector blue swimmer crab fishery in a subtropical embayment – Shark Bay, Western Australia. Marine and Freshwater Research 63, 1165–1179.
| Biology and management of a multi-sector blue swimmer crab fishery in a subtropical embayment – Shark Bay, Western Australia.Crossref | GoogleScholarGoogle Scholar |
Heithaus, M. R. (2001). The biology of tiger sharks, Galeocerdo cuvier, in Shark Bay, Western Australia: sex ratio, size distribution, diet, and seasonal changes in catch rates. Environmental Biology of Fishes 61, 25–36.
| The biology of tiger sharks, Galeocerdo cuvier, in Shark Bay, Western Australia: sex ratio, size distribution, diet, and seasonal changes in catch rates.Crossref | GoogleScholarGoogle Scholar |
Heithaus, M. R., and Dill, L. M. (2002). Food availability and tiger shark predation risk influence bottlenose dolphin habitat use. Ecology 83, 480–491.
| Food availability and tiger shark predation risk influence bottlenose dolphin habitat use.Crossref | GoogleScholarGoogle Scholar |
Heithaus, M. R., Wirsing, A. J., Dill, L. M., and Heithaus, L. I. (2007). Long-term movements of tiger sharks satellite-tagged in Shark Bay, Western Australia. Marine Biology 151, 1455–1461.
| Long-term movements of tiger sharks satellite-tagged in Shark Bay, Western Australia.Crossref | GoogleScholarGoogle Scholar |
Heithaus, M. R., Frid, A., Wirsing, A. J., and Worm, B. (2008). Predicting ecological consequences of marine top predator declines. Trends in Ecology & Evolution 23, 202–210.
| Predicting ecological consequences of marine top predator declines.Crossref | GoogleScholarGoogle Scholar |
Heithaus, M. R., Wirsing, A. J., and Dill, L. M. (2012). The ecological importance of intact top-predator populations: a synthesis of 15 years of research in a seagrass ecosystem. Marine and Freshwater Research 63, 1039–1050.
| The ecological importance of intact top-predator populations: a synthesis of 15 years of research in a seagrass ecosystem.Crossref | GoogleScholarGoogle Scholar |
Herbert, D. A., Perry, W. B., Cosby, B. J., and Fourqurean, J. W. (2011). Projected reorganization of Florida Bay seagrass communities in response to the increased freshwater inflow of Everglades restoration. Estuaries and Coasts 34, 973–992.
| Projected reorganization of Florida Bay seagrass communities in response to the increased freshwater inflow of Everglades restoration.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXpsFartrk%3D&md5=5b35d95254c41084a23435b735a18468CAS |
Holley, D. K., Lawler, I. R., and Gales, N. J. (2006). Summer survey of dugong distribution and abundance in Shark Bay reveals additional key habitat area. Wildlife Research 33, 243–250.
| Summer survey of dugong distribution and abundance in Shark Bay reveals additional key habitat area.Crossref | GoogleScholarGoogle Scholar |
Hutchins, B. (1990). ‘Fish Survey of South Passage, Shark Bay, Western Australia.’ (Western Australian Museum: Perth.)
Jackson, G., and Moran, M. (2012). Recovery of inner Shark Bay snapper (Pagrus auratus) stocks: relevant research and adaptive recreational fisheries management in a World Heritage context. Marine and Freshwater Research 63, 1180–1190.
| Recovery of inner Shark Bay snapper (Pagrus auratus) stocks: relevant research and adaptive recreational fisheries management in a World Heritage context.Crossref | GoogleScholarGoogle Scholar |
Jackson, J. B. C., Kirby, M. X., Berger, W. H., Bjorndal, K. A., Botsford, L. W., Bourque, B. J., Bradbury, R. H., Cooke, R., Erlandson, J., Estes, J. A., Hughes, T. P., Kidwell, S., Lange, C. B., Lenihan, H. S., Pandolfi, J. M., Peterson, C. H., Steneck, R. S., Tegner, M. J., and Warner, R. R. (2001). Historical overfishing and the recent collapse of coastal ecosystems. Science 293, 629–637.
| Historical overfishing and the recent collapse of coastal ecosystems.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXls1Khu7o%3D&md5=98ccd86049f9fdc8323e27948a1366d1CAS |
Kangas, M. I., Morrison, S., Unsworth, P., Lai, E., Wright, I., and Thomson, A. (2007). Development of biodiversity and habitat monitoring systems for key trawl fisheries in Western Australia. Department of Fisheries, Government of Western Australia, Perth.
Kendrick, G. A., Walker, D. I., and McComb, A. J. (1988). Changes in distribution of macro-algal epiphytes on stems of the seagrass Amphibolis antarctica along a salinity gradient in Shark Bay, Western Australia. Phycologia 27, 201–208.
| Changes in distribution of macro-algal epiphytes on stems of the seagrass Amphibolis antarctica along a salinity gradient in Shark Bay, Western Australia.Crossref | GoogleScholarGoogle Scholar |
Kendrick, G. A., Huisman, J. M., and Walker, D. I. (1990). Benthic macroalgae of Shark Bay, Western Australia. Botanica Marina 33, 47–54.
| Benthic macroalgae of Shark Bay, Western Australia.Crossref | GoogleScholarGoogle Scholar |
Koch, M. S., Schopmeyer, S. A., Holmer, M., Madden, C. J., and Khyn-Hansen, C. (2007). Thalassia testudinum response to the interactive stressors hypersalinity, sulfide and hypoxia. Aquatic Botany 87, 104–110.
| Thalassia testudinum response to the interactive stressors hypersalinity, sulfide and hypoxia.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXntF2rtb4%3D&md5=997f32279c1fdf56ce67f1bcd8770026CAS |
Kruczinsky, W. L., and Fletcher, P. J. (2012). ‘Tropical Connections: South Florida’s Marine Environment.’ (IAN Press: Cambridge, MD.)
Lenanton, R. C. J. (1977). Fishes from hypersaline waters of the stromatolite zone of Shark Bay, Western Australia. Copeia , 387–390.
| Fishes from hypersaline waters of the stromatolite zone of Shark Bay, Western Australia.Crossref | GoogleScholarGoogle Scholar |
Lewis, J. S., and Schroeder, W. W. (2003). Mud plume feeding, a unique foraging behaviour of the bottlenose dolphin in the Florida Keys. Gulf of Mexico Science 21, 92–97.
Ley, J. A., McIvor, C. C., and Montague, C. L. (1999). Fishes in mangrove prop-root habitats of northeastern Florida Bay: distinct assemblages across an estuarine gradient. Estuarine, Coastal and Shelf Science 48, 701–723.
| Fishes in mangrove prop-root habitats of northeastern Florida Bay: distinct assemblages across an estuarine gradient.Crossref | GoogleScholarGoogle Scholar |
Loftus, W. F. (2000). Inventory of fishes of Everglades National Park. Florida Scientist 63, 27–47.
Logan, B. W., and Cebulski, D. E. (1970). Sedimentary environments of Shark Bay, Western Australia. In ‘Carbonate Sedimentation and Environments, Shark Bay, Western Australia’. (Ed. B. W. Logan.) pp. 1–38. (The American Association of Petroleum Geologists: Tulsa, OK.)
Lorenz, J. J., Langan-Mulrooney, B., Frezza, P. E., Harvey, R. G., and Mazzotti, F. J. (2009). Roseate spoonbill reproduction as an indicator for restoration of the Everglades and the Everglades estuaries. Ecological Indicators 9, S96–S107.
| Roseate spoonbill reproduction as an indicator for restoration of the Everglades and the Everglades estuaries.Crossref | GoogleScholarGoogle Scholar |
Mann, J., Connor, R. C., Barre, L. M., and Heithaus, M. R. (2000). Female reproductive success in bottlenose dolphins (Tursiops sp.): life history, habitat, provisioning, and group-size effects. Behavioral Ecology 11, 210–219.
| Female reproductive success in bottlenose dolphins (Tursiops sp.): life history, habitat, provisioning, and group-size effects.Crossref | GoogleScholarGoogle Scholar |
Marsh, H., Prince, R. I. T., Saalfeld, W. K., and Shepherd, R. (1994). The distribution and abundance of the dugong in Shark Bay, Western Australia. Wildlife Research 21, 149–161.
| The distribution and abundance of the dugong in Shark Bay, Western Australia.Crossref | GoogleScholarGoogle Scholar |
Marshall, F. E., Wingard, G. L., and Pitts, P. (2009). A simulation of historic hydrology and salinity in Everglades National Park: coupling paleoecologic assemblage data with regression models. Estuaries and Coasts 32, 37–53.
| A simulation of historic hydrology and salinity in Everglades National Park: coupling paleoecologic assemblage data with regression models.Crossref | GoogleScholarGoogle Scholar |
Mueller, U., Kangas, M., Sporer, E., and Caputi, N. (2012). Variability in the spatial and temporal distribution of the saucer scallop Amusium balloti, in Shark Bay – management implications. Marine and Freshwater Research 63, 1152–1164.
| Variability in the spatial and temporal distribution of the saucer scallop Amusium balloti, in Shark Bay – management implications.Crossref | GoogleScholarGoogle Scholar |
Norriss, J., Moran, M., and Jackson, G. (2012). Tagging studies reveal restricted movement of snapper (Pagrus auratus) within Shark Bay, supporting fine-scale fisheries management. Marine and Freshwater Research 63, 1191–1199.
| Tagging studies reveal restricted movement of snapper (Pagrus auratus) within Shark Bay, supporting fine-scale fisheries management.Crossref | GoogleScholarGoogle Scholar |
Nott, J. (2011). A 6000 year tropical cyclone record from Western Australia. Quaternary Science Reviews 30, 713–722.
| A 6000 year tropical cyclone record from Western Australia.Crossref | GoogleScholarGoogle Scholar |
Nuttle, W. K., Fourqurean, J. W., Cosby, B. J., Zieman, J. C., and Robblee, M. B. (2000). Influence of net freshwater supply on salinity in Florida Bay. Water Resources Research 36, 1805–1822.
| Influence of net freshwater supply on salinity in Florida Bay.Crossref | GoogleScholarGoogle Scholar |
Olson, E. L., Salomon, A. K., Wirsing, A. J., and Heithaus, M. R. (2012). Large-scale movement patterns of male loggerhead sea turtles (Caretta caretta) in Shark Bay, Australia. Marine and Freshwater Research 63, 1108–1116.
| Large-scale movement patterns of male loggerhead sea turtles (Caretta caretta) in Shark Bay, Australia.Crossref | GoogleScholarGoogle Scholar |
Powell, G. V. N., Kenworthy, W. J., and Fourqurean, J. W. (1989). Experimental evidence for nutrient limitation of seagrass limitation of seagrass growth in a tropical estuary with restricted circulation. Bulletin of Marine Science 44, 324–340.
Preen, A. R., Marsh, H., Lawler, I. R., Prince, R. I. T., and Shepherd, R. (1997). Distribution and abundance of dugongs, turtles, dolphins and other megafauna in Shark Bay, Ningaloo Reef and Exmouth Gulf, Western Australia. Wildlife Research 24, 185–208.
| Distribution and abundance of dugongs, turtles, dolphins and other megafauna in Shark Bay, Ningaloo Reef and Exmouth Gulf, Western Australia.Crossref | GoogleScholarGoogle Scholar |
Price, R. M., Swart, P. K., and Fourqurean, J. W. (2006). Coastal groundwater discharge – an additional source of phosphorus for the oligotrophic wetlands of the Everglades. Hydrobiologia 569, 23–36.
| Coastal groundwater discharge – an additional source of phosphorus for the oligotrophic wetlands of the Everglades.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XnsFyjtbw%3D&md5=23ee208287b568f53fede5e9a230642dCAS |
Price, R. M., Skrzypek, G., Grierson, P. F., Swart, P. K., and Fourqurean, J. W. (2012). The use of stable isotopes of oxygen and hydrogen to identify water sources in two hypersaline estuaries with different hydrologic regimes. Marine and Freshwater Research 63, 952–966.
| The use of stable isotopes of oxygen and hydrogen to identify water sources in two hypersaline estuaries with different hydrologic regimes.Crossref | GoogleScholarGoogle Scholar |
Rudnick, D. T., Chen, Z., Childers, D. L., Boyer, J. N., and Fontaine, T. D. (1999). Phosphorus and nitrogen inputs into Florida Bay: the importance of the Everglades watershed. Estuaries 22, 398–416.
| Phosphorus and nitrogen inputs into Florida Bay: the importance of the Everglades watershed.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1MXmsVOmtr4%3D&md5=674667a389529e48a4a5f1a143d43565CAS |
Sargeant, B. L., Wirsing, A. J., Heithaus, M. R., and Mann, J. (2007). Can environmental heterogeneity explain individual foraging variation in wild dolphins (Tursiops sp.)? Behavioral Ecology and Sociobiology 61, 679–688.
Shaw, J. (2000). Fisheries environmental management review: Gascoyne region. Fisheries Western Australia, Perth.
Smith, S. V. (1984). Phosphorus limitation of net production in a confined aquatic ecosystem. Nature 307, 626–627.
| Phosphorus limitation of net production in a confined aquatic ecosystem.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL2cXhtVykur0%3D&md5=8ff3fd50b697f4fc38f346518807fa6eCAS |
Smith, S., and Atkinson, M. (1983). Mass balance of carbon and phosphorus in Shark Bay, Western Australia. Limnology and Oceanography 28, 625–639.
| Mass balance of carbon and phosphorus in Shark Bay, Western Australia.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL3sXltFSgsLY%3D&md5=28825cacfaab30d66e36e58af857e441CAS |
Smolker, R. A., Richards, A. F., Connor, R. C., and Pepper, J. W. (1992). Sex differences in patterns of association among Indian Ocean bottlenose dolphins. Behaviour 123, 38–69.
| Sex differences in patterns of association among Indian Ocean bottlenose dolphins.Crossref | GoogleScholarGoogle Scholar |
Statton, J., Dixon, K. W., Hovey, R. K., and Kendrick, G. A. (2012). A comparative assessment of approaches and outcomes for seagrass revegetation in Shark Bay and Florida Bay. Marine and Freshwater Research 63, 984–993.
| A comparative assessment of approaches and outcomes for seagrass revegetation in Shark Bay and Florida Bay.Crossref | GoogleScholarGoogle Scholar |
Sutula, M., Day, J. W., Cable, J., and Rudnick, D. T. (2001). Hydrological and nutrient budgets of freshwater and estuarine wetlands of Taylor Slough in Southern Everglades, Florida (U.S.A.). Biogeochemistry 56, 287–310.
| Hydrological and nutrient budgets of freshwater and estuarine wetlands of Taylor Slough in Southern Everglades, Florida (U.S.A.).Crossref | GoogleScholarGoogle Scholar |
Tabb, D. C., Dubrow, D. L., and Manning, R. B. (1962). The ecology of northern Florida Bay and adjacent estuaries. State of Florida Board of Conservation, Miami, FL.
Thayer, G. W., Powell, A. B., and Hoss, D. E. (1999). Composition of larval, juvenile, and small adult fishes relative to changes in environmental conditions in Florida Bay. Estuaries 22, 518–533.
| Composition of larval, juvenile, and small adult fishes relative to changes in environmental conditions in Florida Bay.Crossref | GoogleScholarGoogle Scholar |
Tilmant, J. T. (1989). A history and an overview of recent trends in the fisheries of Florida Bay. Bulletin of Marine Science 44, 3–22.
Torres, L. G., and Read, A. J. (2009). Where to catch a fish? The influence of foraging tactics on the ecology of bottlenose dolphins (Tursiops truncatus) in Florida Bay, Florida. Marine Mammal Science 25, 797–815.
| Where to catch a fish? The influence of foraging tactics on the ecology of bottlenose dolphins (Tursiops truncatus) in Florida Bay, Florida.Crossref | GoogleScholarGoogle Scholar |
Torres, L. G., Heithaus, M. R., and Delius, B. (2006). Influence of teleost abundance on the distribution and abundance of sharks in Florida Bay, USA. Hydrobiologia 569, 449–455.
| Influence of teleost abundance on the distribution and abundance of sharks in Florida Bay, USA.Crossref | GoogleScholarGoogle Scholar |
Torres, L. G., Read, A. J., and Halpin, P. (2008). Fine-scale habitat modeling of a top marine predator: do prey data improve predictive capacity? Ecological Applications 18, 1702–1717.
| Fine-scale habitat modeling of a top marine predator: do prey data improve predictive capacity?Crossref | GoogleScholarGoogle Scholar |
Travers, M. J., and Potter, I. C. (2002). Factors influencing the characteristics of fish assemblages in a large subtropical marine embayment. Journal of Fish Biology 61, 764–784.
| Factors influencing the characteristics of fish assemblages in a large subtropical marine embayment.Crossref | GoogleScholarGoogle Scholar |
Vaudo, J. J., and Heithaus, M. R. (2009). Spatiotemporal variability in a sandflat elasmobranch fauna in Shark Bay, Australia. Marine Biology 156, 2579–2590.
| Spatiotemporal variability in a sandflat elasmobranch fauna in Shark Bay, Australia.Crossref | GoogleScholarGoogle Scholar |
Vaudo, J. J., and Heithaus, M. R. (2012). Diel and seasonal variation in the use of a nearshore sandflat by a ray community in a near pristine system. Marine and Freshwater Research 63, 1077–1084.
| Diel and seasonal variation in the use of a nearshore sandflat by a ray community in a near pristine system.Crossref | GoogleScholarGoogle Scholar |
Walker, D. I. (1985). Correlations between salinity and growth of the seagrass Amphibolis antarctica (labill.) Sonder & Aschers., in Shark Bay, Western Australia, using a new method for measuring production rate. Aquatic Botany 23, 13–26.
| Correlations between salinity and growth of the seagrass Amphibolis antarctica (labill.) Sonder & Aschers., in Shark Bay, Western Australia, using a new method for measuring production rate.Crossref | GoogleScholarGoogle Scholar |
Walker, D. I., and Woelkerling, W. J. (1988). Quantitative study of sediment contribution by epiphytic coralline red algae in seagrass meadows in Shark Bay, Western Australia. Marine Ecology Progress Series 43, 71–77.
| Quantitative study of sediment contribution by epiphytic coralline red algae in seagrass meadows in Shark Bay, Western Australia.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL1cXit1Knsb0%3D&md5=e0026d6ff987f4e0f65cd2a19c5804e9CAS |
Walker, D. I., Kendrick, G. A., and McComb, A. J. (1988). The distribution of seagrass species in Shark Bay, Western Australia, with notes on their ecology. Aquatic Botany 30, 305–317.
| The distribution of seagrass species in Shark Bay, Western Australia, with notes on their ecology.Crossref | GoogleScholarGoogle Scholar |
Wang, J. D., van de Kreeke, J., Krishnan, N., and Smith, D. (1994). Wind and tide response in Florida Bay. Bulletin of Marine Science 54, 579–601.
Wanless, H. R., and Tagett, M. G. (1989). Origin, growth and evolution of carbonate mudbanks in Florida Bay. Bulletin of Marine Science 44, 454–489.
White, W. T., and Potter, I. C. (2004). Habitat partitioning among four elasmobranch species in nearshore, shallow waters of a subtropical embayment in Western Australia. Marine Biology 145, 1023–1032.
| Habitat partitioning among four elasmobranch species in nearshore, shallow waters of a subtropical embayment in Western Australia.Crossref | GoogleScholarGoogle Scholar |
Wiley, T. R., and Simpfendorfer, C. A. (2007). The ecology of elasmobranchs occurring in the Everglades National Park, Florida: implications for conservation and management. Bulletin of Marine Science 80, 171–189.
Wirsing, A. J., and Heithaus, M. R. (2012). Behavioural transition probabilities in dugongs change with habitat and predator presence: implications for sirenian conservation. Marine and Freshwater Research 63, 1069–1076.
| Behavioural transition probabilities in dugongs change with habitat and predator presence: implications for sirenian conservation.Crossref | GoogleScholarGoogle Scholar |
Wirsing, A. J., Heithaus, M. R., and Dill, L. M. (2007). Living on the edge: dugongs prefer to forage in microhabitats that allow escape from rather than avoidance of predators. Animal Behaviour 74, 93–101.
| Living on the edge: dugongs prefer to forage in microhabitats that allow escape from rather than avoidance of predators.Crossref | GoogleScholarGoogle Scholar |
Wise, B. S., Telfer, C. F., Lai, E. K. M., Hall, N. G., and Jackson, G. (2012). Long-term monitoring of boat-based recreational fishing in Shark Bay, Western Australia: providing scientific advice for sustainable management in a World Heritage Area. Marine and Freshwater Research 63, 1129–1141.
| Long-term monitoring of boat-based recreational fishing in Shark Bay, Western Australia: providing scientific advice for sustainable management in a World Heritage Area.Crossref | GoogleScholarGoogle Scholar |


