Global emergence of Rickettsia felis infections: the hidden threat in pets and their fleas
Lucas G. Huggins A * and Vito Colella AA

Dr Lucas Huggins is a postdoctoral research fellow specialising in molecular techniques at The University of Melbourne Veterinary School. His current research focus is on the development of pan-pathogen molecular tools for humans and animals of veterinary importance that can aid us in understanding parasite diversity and epidemiology, particularly in some of the most neglected regions of the world. |

Dr Vito Colella is a senior research fellow and senior lecturer in the Department of Veterinary Biosciences at The University of Melbourne. His current research focuses on the development of novel diagnostics for neglected tropical diseases and intervention strategies to mitigate the impact of zoonotic parasites on animal and human populations in the Asia-Pacific region. |
Abstract
The globally emerging pathogen Rickettsia felis uses the cat flea as a biological vector and can cause serious infections in humans. Rickettsia felis can be transmitted to numerous mammalian hosts, but to date, only dogs have been demonstrated as an amplifier host that is infectious to naïve cat fleas. When infected, canines typically do not show clinical signs making them ideal pathogen reservoirs through which fleas can silently transmit R. felis from pets to co-habiting humans. Once infected, humans develop the multi-systemic disease flea-borne spotted fever with symptoms ranging from mild fevers, rashes and muscle aches through to severe disease. Given the close associations between fleas, pets and humans, it is not surprising that serosurveys of R. felis in people have found high levels of exposure, particularly in at-risk demographics, such as veterinarians. Nonetheless, although sensitive diagnostic tools for the detection of R. felis exist, a deep understanding of R. felis epidemiology and transmission remains lacking. A greater research focus must be placed on using such diagnostic tools to resolve outstanding questions surrounding R. felis pathophysiology and biology, including the role of companion animals in pathogen transmission.
Keywords: canine, companion animals, Ctenocephalides felis, domestic dog, flea-borne spotted fever, metabarcoding, rickettsiosis, zoonosis.
From its initial detection and characterisation in the 20th Century through to its first isolation in 2001, our understanding of the importance and impact of the emergent zoonotic bacterium Rickettsia felis has, over the last two decades, risen dramatically.1–3 Taxonomically positioned within the order Rickettsiales, which includes the important animal pathogen genera Anaplasma and Ehrlichia, R. felis shares its genus with a plethora of highly pathogenic species, including Rickettsia rickettsii, the causative agent of Rocky Mountain spotted fever and Rickettsia prowazekii, which causes epidemic typhus.1,4,5 To date, the cosmopolitan cat flea Ctenocephalides felis felis is the only confirmed biological vector of Rickettsia felis and its arthropod reservoir – however, a few other arthropods have been found to carry R. felis DNA (e.g. booklice).1,6 In humans, infection with R. felis produces the multi-systemic disease flea-borne spotted fever (FBSF) that generates pyrexia, maculopapular rash, headache, myalgia and the formation of a dry dark scab (eschar) at the site of flea bite.4,7 Nonetheless, the underlying pathophysiology of R. felis remains poorly understood, particularly when compared to better studied species such as R. rickettsii, which causes disease by increasing vascular permeability to generate oedema.8 Although FBSF caused by R. felis has typically been characterised as a mild disease, more recent reports have implicated this pathogen in fatal cases of meningoencephalitis as well as a serious case of encephalitis in a pregnant woman.9,10
Rickettsia felis belongs to the spotted fever group of Rickettsia, with phylogenetic analyses indicating the species is most closely related to sister species such as Rickettsia australis, Candidatus Rickettsia asemboensis and Rickettsia hoogstraalii, among others.1,11 Interestingly, substantial genetic diversity across key taxonomic barcoding genes from some R. felis isolates has underscored the possibility that R. felis represents a species complex that, to date, remains unresolved.1
The distribution of R. felis is global, with the pathogen’s epidemiology and transmission intimately tied to that of its vectors and vertebrate hosts.4 Despite their name, C. f. felis fleas feed on a wide variety of vertebrate hosts and are frequently found as the dominant ectoparasite infesting dogs and cats (e.g. in Asia).12,13 Moreover, C. f. felis infected with R. felis have been shown to maintain this pathogen for up to 12 generations by transovarial and transstadial transmission (Fig. 1), meaning that this pathogen is recurrently transmitted from mother to offspring and between flea life cycle stages.14 The ability of fleas to persist in domestic settings means that fleas can continuously reinfest our pets from an environmental reservoir, thereby further increasing the risks posed by R. felis.15 Adult fleas are typically the only part of the life cycle observed during infestations; however, they only represent a small proportion (~1–5%) of the local flea population, with the remainder being egg, larval or highly resilient pupal stages that may make flea control more challenging.15
Current understanding of the life cycle and transmission routes of Rickettsia felis. Rickettsia felis can be transmitted vertically to eggs and other life cycle stages of the cat flea Ctenocephalides felis felis, by transovarial and transstadial transmission for up to 12 generations. Horizontal transmission occurs through flea biting and transmission of R. felis to competent mammalian hosts as well as by a potential flea co-feeding route. Canines develop rickettsaemia without outward clinical signs, working to silently amplify R. felis and lead to onward transmission. If humans are bitten by a R. felis infected flea, then the multisystemic disease flea-borne spotted fever can develop. It is unknown if; (i) other mammalian species are competent R. felis reservoirs; and (ii) if arthropod vectors, apart from fleas, are capable of naturally transmitting R. felis.
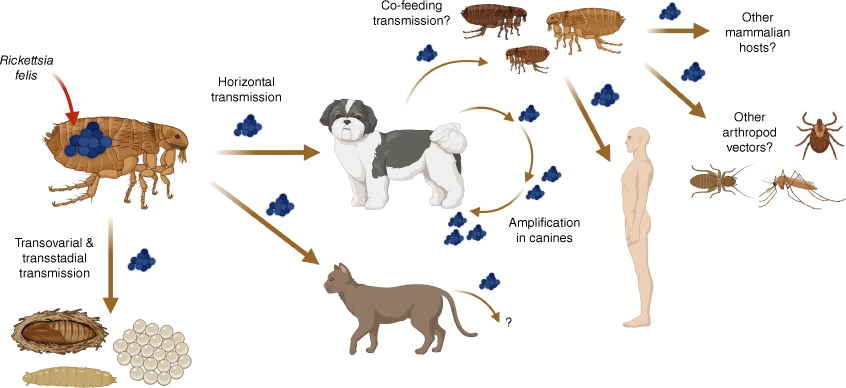
Horizontal transmission (e.g. through vector co-feeding on a host Fig. 1), is also essential for ecological maintenance of R. felis within a population and, although there is substantial evidence that a wide spectrum of mammals can become infected, a mammalian reservoir remained elusive until 2020.4,7,16 Critically, at this time, experimental infections of the domestic dog with R. felis were shown to be infectious to C. f. felis fleas and vice versa. Furthermore, after 3–6 days post infection, canine rickettsaemia was observed and found to persist for up to 100 days, demonstrating the role domestic dogs play as an infectious source of R. felis to fleas (Fig. 1).16 Importantly, dogs experimentally infected with R. felis showed an absence of, or only mild, clinical signs highlighting a potential tolerance of the host towards the pathogen that further demonstrates the role of dogs as a reservoir species for R. felis.16
Epidemiological surveys of canines have increasingly highlighted their role in the maintenance of R. felis, while also emphasising the risk dogs play in transmission to humans.16,17 When investigated within the Asia-Pacific region, high levels of both canine exposure, i.e. serologically positive, and active infection, i.e. polymerase chain reaction (PCR) positive, to R. felis have frequently been demonstrated, particularly in countries such as Australia where there has been more investigation.17–19 For example, in one study conducted by Hii et al. over half of the canines tested in Queensland and the Northern Territory were found seropositive to R. felis, indicative of exposure to this pathogen.19
Studies exploring R. felis seroprevalence in humans have also found that levels of exposure to this pathogen can be high, particularly in certain demographics.20,21 For instance, serological studies investigating exposure to this pathogen in ‘high-risk’ cohorts identified a seroprevalence to R. felis in 29% of patients with a suspected case of rickettsiosis and 16% in veterinarians.20,21 The latter finding underscores an occupational risk for veterinary professionals due to their greater contact with infected animals.21
Given the role mammals and fleas play in R. felis maintenance, it is not surprising that there have been well-documented cases of transmission caused by close contact between humans and companion animals.20–22 In 2009, five members of a family living in Melbourne, Australia, became sick with a rickettsial disease concordant with FBSF, after receiving kittens infested with fleas from a nearby farm.22 The patients were serologically positive for typhus group Rickettsia, whereas fleas collected from the cat family group were found positive for R. felis DNA by PCR.22 These findings are further supported by research that has explicitly looked at risk factors associated with R. felis infection. For example, one study reported that high-risk occupations that entail close contact with domestic animals increase the odds ratio of human infection by almost six times.23 Given these data, simple measures to reduce flea-burden in companion animals such as the use of ectoparasiticides, regular grooming and pet owner education will likely act to significantly reduce the risk of FBSF in humans.19 Thankfully, when human R. felis infections do occur, treatment can be simple, with a course of the antibiotic doxycycline established as being highly effective.24
Clinical diagnosis of R. felis in humans is challenging because of the overlap of symptoms of infection with many other rickettsial and non-rickettsial diseases.1,4,25 This makes the accurate diagnosis of R. felis infections with serological and molecular tools essential.1,25 Immunofluorescent antibody assays are still largely considered the reference method for detection of most rickettsial infections and can be highly sensitive (Fig. 2).26 Nonetheless, although specific serological tests for R. felis exposure in humans exist, cross reactivity to other Rickettsia, such as Rickettsia typhi has been demonstrated, meaning that definitive diagnosis with such methods may typically require further testing.3,4,26,27 Moreover, such methods do not provide any information on current infection status, with antibodies to R. felis remaining detectable up to 4 years post-exposure.28
Diagnostic techniques and sample types used for the detection of Rickettsia felis and other Rickettsia species.
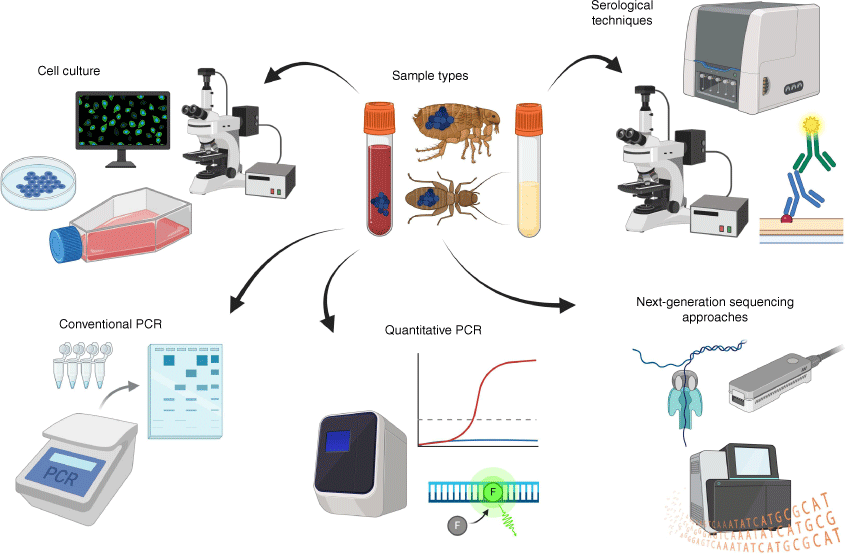
Considering this, molecular diagnostics for the detection of R. felis in humans offer many advantages over serology due to their greater specificity and ability to detect active infections.1,26 Numerous conventional PCR (cPCR) and quantitative PCR (qPCR) assays have been developed for the specific detection of R. felis and have been effectively utilised for the identification of this pathogen from vectors, humans and other vertebrate hosts (Fig. 2).4,26 These assays target genes that are informative for diagnosing Rickettsia infections at the species-level, such as the citrate synthase (gltA) or outer membrane protein B (ompB) genes.4,26 However, despite the strengths of such approaches they are hampered by challenges associated with intermittent rickettsaemia, meaning that bloodstream-circulating DNA may rapidly wane, precluding PCR-based detection.4,25 More recently, advanced molecular techniques such as next-generation sequencing (NGS)-based approaches have come to the fore, due to their ability to explore questions that are difficult to address using classical molecular methods (Fig. 2). With NGS, conserved genes found across all bacteria or, more specifically all Rickettsia, can be simultaneously targeted and sequenced in a technique termed ‘metabarcoding’ to provide both sensitive and specific detection.17,29 Crucially, unlike cPCR, these metabarcoding approaches are not limited to the detection of just one or a few species at a time, meaning that they can easily characterise bacterial coinfections as well as Rickettsia that are potentially novel.17,29 Metabarcoding has already been used for the accurate detection of rickettsial species, such as R. felis, and shows great promise as a tool for helping us in identifying novel vectors as well as understanding the epidemiology, and transmission dynamics of this important pathogen.29,30
Rickettsia felis is a complex, globally distributed and emergent zoonotic pathogen of significant importance for human health. However, many elements of its biology and epidemiology are still poorly understood and are at the centre of a large body of ongoing research. Key questions remain to be answered, including:
What is the precise mechanism by which humans become infected by R. felis and what is the role of companion animals in this pathogen’s transmission?
Do arthropods apart from fleas play a significant role as vectors?
Do non-canine reservoir hosts exist?
What are the pathophysiological changes associated with R. felis infection in both humans and animals?
What is the extent of genetic diversity within R. felis and does this organism represent a species complex?
Continuing research efforts across the 21st Century will undoubtedly work to further unpick the threat posed to us by R. felis and our pets. Improved R. felis detection methods and a better understanding of this pathogen’s biology will provide us with critical insights for more effective control strategies, ultimately safeguarding both human and animal health.
Data availability
Data sharing is not applicable as no new data were generated or analysed during this study.
References
1 Angelakis E et al. (2016) Rickettsia felis: the complex journey of an emergent human pathogen. Trends Parasitol 32, 554-564.
| Crossref | Google Scholar | PubMed |
2 Parola P (2011) Rickettsia felis: from a rare disease in the USA to a common cause of fever in sub-Saharan Africa. Clin Microbiol Infect 17, 996-1000.
| Crossref | Google Scholar | PubMed |
3 Adams JR et al. (1990) Infection of colonized cat fleas, Ctenocephalides felis (Bouché), with a rickettsia-like microorganism. Am J Trop Med Hyg 43, 400-409.
| Crossref | Google Scholar | PubMed |
4 Reif KE, Macaluso KR (2009) Ecology of Rickettsia felis: a review. J Med Entomol 46, 723-736.
| Crossref | Google Scholar | PubMed |
5 Bechah Y et al. (2008) Epidemic typhus. Lancet Infect Dis 8, 417-426.
| Crossref | Google Scholar | PubMed |
6 Abdad MY et al. (2011) Rickettsia felis, an emerging flea-transmitted human pathogen. Emerg Health Threats J 4, 7168.
| Crossref | Google Scholar | PubMed |
7 Brown LD, Macaluso KR (2016) Rickettsia felis, an emerging flea-borne rickettsiosis. Curr Trop Med Rep 3, 27.
| Crossref | Google Scholar | PubMed |
8 Walker DH et al. (2003) Pathogenic mechanisms of diseases caused by Rickettsia. Ann N Y Acad Sci 990, 1-11.
| Crossref | Google Scholar | PubMed |
9 Mawuntu AHP et al. (2020) Rickettsia felis identified in two fatal cases of acute meningoencephalitis. PLoS Negl Trop Dis 14, e0007893.
| Crossref | Google Scholar | PubMed |
10 Qiu J et al. (2024) A case of Rickettsia felis infection-induced encephalitis in a pregnant woman. World J Emerg Med 15, 150.
| Crossref | Google Scholar |
11 Pérez-Osorio CE et al. (2008) Rickettsia felis as emergent global threat for humans. Emerg Infect Dis 14, 1019.
| Crossref | Google Scholar | PubMed |
12 van der Mescht L et al. (2021) New taxonomic and evolutionary insights relevant to the cat flea, Ctenocephalides felis: a geographic perspective. Mol Phylogenet Evol 155, 106990.
| Crossref | Google Scholar | PubMed |
13 Colella V et al. (2020) Zoonotic vectorborne pathogens and ectoparasites of dogs and cats in Eastern and Southeast Asia. Emerg Infect Dis 26, 1221-1233.
| Crossref | Google Scholar | PubMed |
14 Wedincamp J, Jr, Foil LD (2002) Vertical transmission of Rickettsia felis in the cat flea (Ctenocephalides felis Bouché). J Vector Ecol 27, 96-101.
| Google Scholar | PubMed |
15 Halos L et al. (2014) Flea control failure? Myths and realities. Trends Parasitol 30, 228-233.
| Crossref | Google Scholar | PubMed |
16 Ng-Nguyen D et al. (2020) Domestic dogs are mammalian reservoirs for the emerging zoonosis flea-borne spotted fever, caused by Rickettsia felis. Sci Rep 10, 4151.
| Crossref | Google Scholar | PubMed |
17 Huggins LG et al. (2023) Advanced approaches for the diagnosis and chemoprevention of canine vector-borne pathogens and parasites – implications for the Asia-Pacific region and beyond. Adv Parasitol 120, 1-85.
| Crossref | Google Scholar | PubMed |
18 Inpankaew T et al. (2016) Canine vector-borne pathogens in semi-domesticated dogs residing in northern Cambodia. Parasit Vectors 9, 253.
| Crossref | Google Scholar | PubMed |
19 Hii SF et al. (2013) Seroprevalence and risk factors for Rickettsia felis exposure in dogs from southeast Queensland and the Northern Territory, Australia. Parasit Vectors 6, 159.
| Crossref | Google Scholar | PubMed |
20 Teoh YT et al. (2016) Evidence of exposure to Rickettsia felis in Australian patients. One Health 2, 95-98.
| Crossref | Google Scholar | PubMed |
21 Teoh YT et al. (2017) Serological evidence of exposure to Rickettsia felis and Rickettsia typhi in Australian veterinarians. Parasit Vectors 10, 129.
| Crossref | Google Scholar | PubMed |
22 Williams M et al. (2011) First probable Australian cases of human infection with Rickettsia felis (cat-flea typhus). Med J Aust 194, 41-43.
| Crossref | Google Scholar | PubMed |
23 Bernabeu-Wittel M et al. (2006) Seroepidemiological study of Rickettsia felis, Rickettsia typhi, and Rickettsia conorii infection among the population of southern Spain. Eur J Clin Microbiol Infect Dis 25, 375-381.
| Crossref | Google Scholar | PubMed |
24 Botelho-Nevers E et al. (2012) Treatment of Rickettsia spp. infections: a review. Expert Rev Anti Infect Ther 10, 1425-1437.
| Crossref | Google Scholar |
25 Portillo A et al. (2017) Guidelines for the detection of Rickettsia spp. Vector Borne Zoonotic Dis 17, 23-32.
| Crossref | Google Scholar | PubMed |
26 Hun L, Troyo A (2012) An update on the detection and treatment of Rickettsia felis. Res Rep Trop Med 3, 47-55.
| Crossref | Google Scholar | PubMed |
27 Angelakis E et al. (2012) Comparison of real-time quantitative PCR and culture for the diagnosis of emerging rickettsioses. PLoS Negl Trop Dis 6, e1540.
| Crossref | Google Scholar | PubMed |
28 Lim MY et al. (2012) Rickettsia felis infections, New Zealand. Emerg Infect Dis 18, 167.
| Crossref | Google Scholar | PubMed |
29 Huggins LG et al. (2022) Nanopore sequencing using the full length 16S rRNA gene for the detection of blood-borne bacteria in dogs reveals a novel species of haemotropic mycoplasma. Microbiol Spectr 10, e03088-22.
| Crossref | Google Scholar |
30 Greay TL et al. (2021) Illuminating the bacterial microbiome of Australian ticks with 16S and Rickettsia-specific next-generation sequencing. Curr Res Parasitol Vector Borne Dis 1, 100037.
| Crossref | Google Scholar | PubMed |
 Dr Lucas Huggins is a postdoctoral research fellow specialising in molecular techniques at The University of Melbourne Veterinary School. His current research focus is on the development of pan-pathogen molecular tools for humans and animals of veterinary importance that can aid us in understanding parasite diversity and epidemiology, particularly in some of the most neglected regions of the world. |
 Dr Vito Colella is a senior research fellow and senior lecturer in the Department of Veterinary Biosciences at The University of Melbourne. His current research focuses on the development of novel diagnostics for neglected tropical diseases and intervention strategies to mitigate the impact of zoonotic parasites on animal and human populations in the Asia-Pacific region. |


