Vaccine technologies used to develop COVID-19 vaccines
Paul Selleck A * and Ian Macreadie BA CSIRO Australian Centre for Disease Preparedness, Private Bag 24, Geelong, Vic. 3220, Australia.
B School of Science, RMIT University, Bundoora, Vic. 3083, Australia.

Paul Selleck has been at the Australian Animal Health Laboratory, now the Australian Centre for Disease Preparedness since, 1983. In this time he was head of the Avian Disease Diagnostic Laboratory, incorporating the National, OIE and FAO Reference Laboratory for Avian Influenza and Newcastle Disease and an OIE Reference Expert for Avian Influenza and Newcastle Disease. He was also involved in the Australian equine and swine influenza outbreaks in 2007 and 2009 respectively and has worked with Hendra, Nipah and SARS at physical containment level 4. Paul now works extensively in Asia, running training courses on biosafety and biosecurity and laboratory diagnosis. He also audits laboratories and runs training courses on quality systems and ISO laboratory accreditation. |

Ian Macreadie is an Honorary Professor of RMIT University and is Editor-in-Chief of Microbiology Australia. In the 1980s with colleagues at CSIRO he developed a viral subunit vaccine for infectious bursal disease virus, using a novel yeast expression system. He maintains a keen interest in preventatives for SARS-CoV-2. |
Microbiology Australia 43(1) 40-43 https://doi.org/10.1071/MA22013
Submitted: 1 February 2022 Accepted: 20 February 2022 Published: 14 April 2022
© 2022 The Author(s) (or their employer(s)). Published by CSIRO Publishing on behalf of the ASM. This is an open access article distributed under the Creative Commons Attribution 4.0 International License (CC BY).
Abstract
In December 2019, cases of atypical pneumonia were diagnosed in hospital patients in Wuhan, Hubei province, China. The disease, now known as COVID-19 is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). As case numbers increased and spread across the planet many companies scrambled to develop vaccines to control the infection and disease. Prior research on SARS-CoV-1, new vaccine technologies and unprecedented funding have allowed vaccines to be developed and approved in record time, without the usual pauses and bypassing any of the requirements of the vaccine approval process. This paper is a review of the current literature on some of the vaccines targeting SARS-CoV-2 and of the new technologies used to produce them.
Keywords: cell culture, COVID-19 vaccines, expression systems, recombinant virus vaccines, SARS-CoV-2, subunit vaccines, vaccines, vaccine technologies.
Introduction
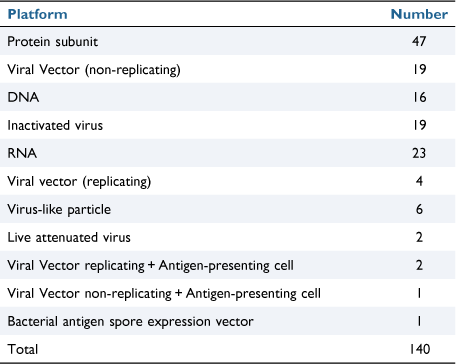
As of 29 January 2022, the WHO reported 364 191 494 confirmed cases of COVID-19, including 5 631 457 deaths globally. As of 18 January 2022, a total of 9 571 502 663 vaccine doses have been administered worldwide.1 A November 2021 study by the WHO Regional Office for Europe and European Centre for Disease Prevention and Control estimated that 470 000 lives have been saved in the 60 years and over age group by the COVID-19 vaccination campaign in 33 countries across the WHO European Region.2 Over 800 000 Americans have died so far during the U.S. COVID-19 pandemic, with more than half those deaths occurring during 2021. Without vaccines there would have been approximately 1.1 million additional COVID-19 deaths and an additional 10.3 million additional COVID-19 hospitalisations in the U.S. by November 2021.3 The WHO reports that 140 vaccines (Table 1) are at the clinical development stage and 194 vaccines at the pre-clinical development stage.4 The Australian Department of Health has approved four vaccines for use in Australia, Vaxzevria (AstraZeneca), Comirnaty (Pfizer) and Spikevax (Moderna) and Nuvaxovid (Novavax).5 The AstraZeneca vaccine is a non-replicating recombinant adenovirus, the Pfizer and Moderna vaccines are messenger RNA vaccines and the Novavax vaccine is a protein subunit vaccine.
|
Vaccine technologies
The aim of vaccination is to induce an immune response that neutralises the virus and prevents its replication. This is achieved by exposing the host to an appropriate viral protein. There are three general approaches to developing virus vaccines. Vaccines use either whole viruses, individual viral proteins or genetic material so that the vaccine directs production of the viral protein. The whole virus can be live, attenuated or killed, viral proteins can be full-length or truncated and the nucleic acid can be DNA or RNA. Protein-based vaccines can have adjuvants to enhance the host immune response and nucleic acid vaccines can have promotor sequences to enhance protein production. These also have the advantage of not requiring live virus for vaccine production or secure laboratory facilities appropriate to safely handle the agent.
Protein subunit
A protein subunit vaccine uses one or more individual viral proteins to produce an immune response against the virus. The target proteins usually contain the neutralising epitopes of the virus and are produced in either yeast, E. coli or insect cells. These vaccines contain a genetically engineered, non-infectious virus protein rather than the whole virus and have been around for many years. Many of the standard childhood vaccines are protein subunit vaccines so the technology has been thoroughly tested.6 The active ingredient of Novavax is a spike protein, SARS-CoV-2 rS (NVX-CoV2373) produced by a genetically engineered baculovirus. The baculovirus is grown in SF9 moth cells where it expresses the SARS-CoV-2 spike protein produced in moth cells. The spike protein is collected and assembled into synthetic nanoparticles. The final product consists of the nanoparticles and saponin-based Matrix-M adjuvant.7
Viral vector (non-replicating)
Viral Vector non-replicating vaccines use a genetically modified virus that has been rendered unable to replicate, usually by knocking out one or more key genes, to deliver a viral protein into the body. The gene for the viral protein of interest is inserted into the genome of the non-replicating vector virus together with a promotor. Once injected, the body’s cells transcribe and translate the viral gene into copies of the viral protein that produces an immune response against the virus.6 The AstraZeneca vaccine is a recombinant, non-replicating chimpanzee adenovirus vector (ChAdOx1) with the full-length SARS-CoV-2 spike glycoprotein gene inserted into the adenovirus genome. The AstraZeneca vaccine is derived from material cultured in an immortalised cell line, derived approximately 50 years ago from human embryonic kidney cells (HEK293). The cells contain the adenovirus genes necessary for viral replication, and the vaccine is purified to remove cellular material.8
DNA
DNA vaccines consist of a genetically engineered plasmid containing a DNA sequence coding for a viral protein of interest and promotor sequences. The DNA is transfected into cells, where the viral gene is transcribed into RNA, which is then translated into the viral protein. The body then mounts an immune response to the foreign protein. DNA vaccines are a relatively new vaccine technology. Several veterinary vaccines have been developed but before COVID-19 none had been approved for human use. Because of the COVID-19 pandemic DNA vaccine technology has developed rapidly and at least one COVID-19 DNA vaccine has been approved for use in humans and many are at the clinical development stage.6
Inactivated virus
Inactivated virus vaccines are one of the oldest technologies for producing vaccines and have been proven to be effective over almost 100 years. The virus is grown in a culture system such as cell culture, embryonated eggs or in animals. It is then inactivated using chemicals, heat or radiation to prevent the agent causing disease. Formaldehyde and β-propiolactone are now the most common inactivating chemicals used. Many human vaccines, including influenza and polio are produced this way. In the case of the influenza vaccine the virus is grown in eggs, partially purified and then killed (split) with formaldehyde to inactivate the virus. Inactivated viral vaccines often have an adjuvant added to enhance the host immune response. Inactivated virus vaccines are relatively easy to produce, can be made in reasonably large quantities, but the time of production can be quite long and can only be done in laboratories with bio-secure facilities appropriate for the agent being handled.6
RNA
RNA vaccines are a new technology. The RNA in vaccines is messenger RNA, which can be directly translated to protein by the transfected cells. As with DNA vaccines the mRNA is embedded in lipid nanoparticles. Even though mRNA vaccines are a new technology several mRNA vaccines have been approved for use to control the spread of COVID-19.6 The Pfizer mRNA vaccine contains 30 μg of BNT162b2 mRNA embedded in lipid nanoparticles encoding the SARS-CoV-2 spike protein.9 A prime dose of the Moderna mRNA vaccine contains 100 μg of mRNA embedded in SM-102 lipid nanoparticles. The booster dose contains 50 μg of mRNA.10 The RNA for both vaccines was produced using cell-free, in vitro transcription from the corresponding DNA template. The cells that take up the lipid nanoparticles are destroyed, along with the RNA.
Viral vector (replicating)
This type of vaccine is similar to viral vector (non-replicating) vaccines, however the vector virus replicates in the host. The vector virus contains one or more inserted pieces of nucleic acid coding for the SARS-CoV-2 proteins of interest that, during the replication of the vector virus, expresses SARS-CoV-2 proteins to which the host produces antibodies. While the host is protected from COVID-19, as only SARS-CoV-2 proteins are produced, rather than the whole virus, a replicating vector virus may pose a risk of infection. These vaccines can be developed rapidly once the technology has been established.6
Virus-like particles
Virus-like particles (VLPs) are protein multimer structures spontaneously assembled from viral structural proteins and containing no genetic material. VLPs have been produced by many virus families and assemble into high protein density structures, often in the identical configuration to that in the native virus. They may also contain the viral proteins responsible for cell attachment and entry resulting in efficient cell entry. VLPs have become commonly used in vaccinology as they present both B-cell and T-cell epitopes and provide a delivery system that generates both antibodies and cell-mediated immunity. It is possible to insert foreign proteins into VLPs where they can combine good safety and strong immunogenicity.6 The widely deployed hepatitis B vaccine is an example of a VLP vaccine where the 22 nm particles comprising HBV surface antigen are produced in recombinant yeast. The HPV vaccine is another example of a VLP vaccine: its development at The University of Queensland involved Ian Frazer and Jian Zhou.
Others
Vaccine delivery strategies are constantly being researched, developed and refined. Recent advances in molecular biology have made recombinant organisms, such as viruses, bacterial and other vectors, potential delivery systems for vaccines. Bacillus subtilis endospores expressing foreign antigens have been used for oral and intranasal immunisation and were shown to generate mucosal and systemic responses in a murine model.11 Antigen presenting cells have been used in presenting SARS-CoV-2 spike protein via MHC Class 1 to CD8+ cells and via MHC Class II to CD4+ cells in recombinant viral vectors.12
Conclusion
A multitude of COVID-19 vaccines have been developed or are currently undergoing pre-clinical and clinical development. Many variants of the SARS-CoV-2 virus have evolved since the virus was first identified and the evidence, and nature of the virus, suggests that evolution of new antigenic and genetic variants will continue. Therefore, the most useful vaccination strategies will be those that are easily adapted to the new variants of concern (VoC), can be produced in large volumes, have high titres and low cost. Vaccines alone will not eliminate the virus, and may even enhance its evolution, so other measures to reduce transmission, including frequent hand washing, wearing a mask, physical distancing, good ventilation and avoiding crowded places or closed settings, will continue to work against new variants by reducing the amount of viral transmission and therefore also reduce opportunities for the virus to mutate.
Data availability
Data sharing is not applicable as no new data were generated or analysed during this study.
Conflicts of interest
Paul Selleck and Ian Macreadie are on the Editorial Board of Microbiology Australia. This article has been reviewed anonymously. They declare no conflicts of interest.
Declaration of funding
This research did not receive any specific funding.
References
[1] WHO. Coronavirus (COVID-19) dashboard with vaccination data. Available at https://covid19.who.int [Accessed 29 January 2022][2] WHO/ECDC. Nearly half a million lives saved by COVID-19 vaccination in less than a year. Available at https://www.ecdc.europa.eu/en/news-events/who-ecdc-nearly-half-million-lives-saved-covid-19-vaccination [Accessed 29 January 2022]
[3] Schneider EC, et al. The U.S. COVID-19 vaccination program at one year: how many deaths and hospitalizations were averted? Commonwealth Fund; 2021.
| Crossref |
[4] WHO. COVID-19 vaccine tracker and landscape. Available at https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines [Accessed 20 January 2022]
[5] Australian Government Department of Health. Approved COVID-19 vaccines. Available at https://www.health.gov.au/initiatives-and-programs/covid-19-vaccines/approved-vaccines [Accessed 20 January 2022]
[6] WHO. The different types of COVID-19 vaccines. Available at https://www.who.int/news-room/feature-stories/detail/the-race-for-a-covid-19-vaccine-explained [Accessed 24 January 2022]
[7] Novavax. Available at https://www.health.gov.au/initiatives-and-programs/covid-19-vaccines/approved-vaccines/novavax [Accessed 24 January 2022]
[8] AstraZeneca. Available at https://www.health.gov.au/initiatives-and-programs/covid-19-vaccines/approved-vaccines/astrazeneca [Accessed 24 January 2022]
[9] Pfizer. Available at https://www.health.gov.au/initiatives-and-programs/covid-19-vaccines/approved-vaccines/pfizer [Accessed 24 January 2022]
[10] Moderna. Available at https://www.health.gov.au/initiatives-and-programs/covid-19-vaccines/approved-vaccines/moderna [Accessed 24 January 2022]
[11] Duc, LH et al.. (2003) Bacterial spores as vaccine vehicles. Infect Immun 71, 2810–2818.
| Bacterial spores as vaccine vehicles.Crossref | GoogleScholarGoogle Scholar |
[12] Rijkers, GT (2021) Antigen presentation of mRNA-based and virus-vectored SARS-CoV-2. Vaccines 9, 848.
| Antigen presentation of mRNA-based and virus-vectored SARS-CoV-2.Crossref | GoogleScholarGoogle Scholar | 34451973PubMed |


