Detection and control of off-flavour compound-producing streptomycetes on locally produced nuts using streptophages
Laura G. Dionysius A , Peter R. Brooks A and D. İpek Kurtböke A *A Centre for Bioinnovation and the School of Science, Technology and Engineering, University of the Sunshine Coast, Maroochydore DC, Qld 4558, Australia.

Laura G. Dionysius is one of the USC recent graduates with first class Honours. She also holds a BSc in the Science Program of the USC. She conducted research with Dr İpek Kurtböke over the past 2 years related to the application of various actinophages. While in Grade 11, she began studying at USC through the Head Start program and has since become the first student from her school, Peregian Beach College, to graduate with Honours and achieve first class. She is also a member of the USC Science Society Committee as the Treasurer. |

Dr Peter Brooks has a PhD in Chemistry from the University of New South Wales, awarded in 1989. He then moved to the University of Adelaide before taking up a position at La Trobe University, Bendigo. Since 2001 he has taught analytical, organic and general chemistries at the University of the Sunshine Coast. Dr Brooks has extensive research experience in analytical, environmental, and organic chemistry. His research has focused on environmental monitoring and the quantitation of bioactive compounds. |

Dr D. İpek Kurtböke is currently a senior lecturer at the University of the Sunshine Coast (USC) in Australia and one of the members of the Centre for Bioinnovation of the USC, conducting research in applied, industrial and environmental microbiology. She is an internationally reputed actinomycetologist and she has been in the field of biodiscovery since 1982 conducting research into discovery of novel and potent therapeutic compounds produced by actinomycetes in Turkey, Italy, the UK, and Australia with leading pharmaceutical companies. She has been an Executive Board member of the World Federation of Culture Collections (WFCC) since 2000, currently serving her second term as the President of the Federation. She is also one of the members of the International Committee on Taxonomy of Viruses (ICTV)’s Bacterial Viruses Subcommittee. She has editorial duties in different journals including Marine Drugs, Diversity and Frontiers Marine Science/Marine Biotechnology. |
Microbiology Australia 43(1) 36-39 https://doi.org/10.1071/MA22011
Submitted: 17 January 2022 Accepted: 17 February 2022 Published: 23 April 2022
© 2022 The Author(s) (or their employer(s)). Published by CSIRO Publishing on behalf of the ASM. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Members of the phylum Actinomycetota are the most prominent part of the soil microbiota, more specifically the species within the genus Streptomyces of this phylum. Key functions of Streptomyces species (or streptomycetes in general terms) include nutrient cycling and plant growth promotion and disease protection. However, these species can also produce volatile organic compounds, predominantly geosmin, which is responsible for musty and mildew scents that are unpleasant to humans and can negatively impact the nut crop industry as odorous nuts generally lose their market value. Bacterial viruses, called bacteriophages have been previously used successfully in agriculture and aquaculture to remove such odorous species and they may therefore be applied to the nut industry. To eliminate these compounds, the producer streptomycetes may be selectively removed from nut surfaces using streptophages. The removal of Streptomyces species from nut surfaces can then be expected to minimise geosmin production, therefore removing the unpleasant off-flavours and benefiting the nut industry.
Keywords: actinomycetes, bacteriophages, food taints, geosmin, nuts, Streptomyces, streptophage, volatile organic compounds.
Introduction
Streptomycetes as the producers of volatile organic compounds
Over a thousand microbial volatile organic compounds (VOCs)1 have been identified from streptomycetes, many of which are acids, alcohols, aldehydes, alkenes, benzenoids, esters, ketones, pyrazines, and terpenes.2 VOCs produced by Streptomyces species can benefit agriculture via the production of bioactive compounds, which can assist in bacterial and fungal growth inhibition, plant growth promotion or inhibition, and invoke resistance mechanisms.2
Geosmin (trans-1,10-dimethyl-trans-9-decalol), 2-methylisoborneol (2-MIB; 1,2,7,7-tetramethyl-exo-bicyclo-heptan-2-ol), and dimethyl disulfide are three of the major VOCs produced by Streptomyces species.3–5 Geosmin and 2-MIB are semi-volatile and terpenoid secondary metabolites.6 Streptomycetes produce these compounds via the 1-deoxy-d-xylulose 5-phosphate/2-C-methyl-d-erythritol 4-phosphate (DOXP/MEP) and melavonic (MVA) pathways.7 Likewise, dimethyl disulfide is a volatile sulfur compound produced via methionine degradation followed by methanethiol oxidation.8,9
Taste and odour compounds in environment
VOCs are also known as taste and odour compounds (T&Os) due to their detectability by humans. Humans can detect these T&Os-VOCs at concentrations of 4 ng/L, due to their olfactory sense.4,10 Furthermore, geosmin synthase genes, which enables geosmin production, are broadly distributed within the members of the genus Streptomyces.4,11 These compounds arise in food products, such as nuts and fish, via bioaccumulation from plant debris, soil, and water use.6,12,13 Further accumulation of geosmin can occur in storage silos if left unmaintained due to continued growth of streptomycetes, not only resulting in strong odours but also giving rise to organic dust toxic syndrome.14 Geosmin has an earthy flavour,15,16 yet there has been no successful technique to remove these VOCs due to the ineffectiveness and high cost of current methods.17 A recent study conducted at the University of the Sunshine Coast (USC) aimed to remove VOCs on locally-produced and openly sold nut samples using streptophages.
Bacteriophage safety in agricultural products and humans
Bacteriophages have seen a rise in use as biocontrol agents in agriculture, bioprocessing, and healthcare.18,19 Bacteriophages are regarded as safe for animals, humans, and plants, further promoting their use in the previously mentioned industries.20–22 Chibani-Chennoufi et al.23 reported that mice exposed to an oral four-phage cocktail did not experience a decline of their commensal E. coli biota. Bruttin and Brüssow24 also reported that human volunteers orally exposed to phage T4 maintained their commensal E. coli population.
Findings of an example study from the Sunshine Coast region
Streptomycetes
Eight streptomycetes were isolated from seven different locally produced and openly sold nut samples using two different isolation methods (air compaction using an air sampler25 and conventional serial dilution26) and incubation temperatures (28°C and 37°C) to maximise the chances of detection of these odorous species. Details of these isolates are given in Table 1.
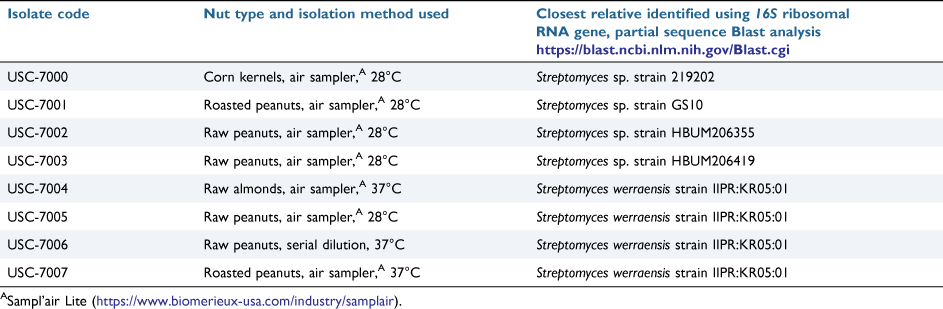
|
Streptophages
Like bacteria, bacteriophages are present in agricultural environments where the host bacteria reside.27 Usually bacteriophages are host specific, however, they also display polyvalency within the host’s taxonomic rank.22 The three major families of actinophages are Myoviridae, Siphoviridae, Podoviridae. Siphoviridae morphology is the most abundant one, particularly in soil among the actinophages.28,29 This group of phages is mostly polyvalent within the Streptomycetaceae family to which the genus Streptomyces belongs. They are commonly known as streptophages30 and morphologically consist of a long and flexible noncontractile tail. The siphoviridae heads contain portal protein at the vertices, which connects the head and tail segments, while the other vertices contain capsid proteins31 and the tails usually are 100–400 nm in length depending on the species.31
Bacteriophages target host populations via phenotype modification, predation, and lysogeny.27 Soil is the major reservoir for actinophages, and they most commonly target actinomycete genera Streptomyces, Actinoplanes and Mycobacterium.27
Application of streptophages onto nut samples and testing for the presence of the VOCs
Nine different polyvalent streptophages from USC’s Microbial Library32 were selected and used to create a composite phage suspension (Fig. 1) at a concentration of 108 pfu/mL. A composite streptomycete suspension was also created by mixing all eight streptomycete isolates at a concentration of 104 cfu/mL. This concentration was selected as it represents unacceptable contamination value determined by the NSW Food Authority in their guidelines33 for microbiological quality of ready to eat foods in Australia. Surface sterilised and UV irradiated nut samples using the methods by El-Tarabily34 and Thomas and Puthur35 were deliberately infected with this composite sample of streptomycetes. After 3 days of incubation streptophage composite sample was applied onto the streptomycete inoculated nuts with a host/phage ratio of 1:2. This ratio was selected due to past successful applications of phages onto hosts.36 VOC production was examined using Headspace-Gas chromatography mass spectrometry (HS-GC/MS) throughout the 14 days of incubation in tightly capped bottles. A mixed standard of Geosmin and 2-MIB (Sigma-Aldrich) was used to detect Geosmin, which is known to be detected at 8.83 min.

|

|
Findings indicated a sharp decrease immediately in geosmin production after the composite phage suspension application onto streptomycete infected nut samples (Fig. 2). After day 8, the geosmin levels were near zero and streptomycete cfu/mL came down to the acceptable levels by the NSW Food Authority (<102).
2-MIB was not detected on the streptomycete or streptomycete plus phage treated nut samples at any stage of this study. Yanxia et al.17 reported a strong correlation between geosmin and 2-MIB concentration, indicating that 2-MIB may be dependent on geosmin production so the sharp decrease in geosmin might be the reason of its absence.
Conclusions
The continual rise in demand of agricultural products has resulted in an increase in preferences in the use of environmentally and human health friendly methods replacing other synthetic agents. Therefore, bacteriophage treatments gained attention to minimise product losses and nutritional properties from disease causing bacteria. Like the previous successful treatments of potatoes37–39 and strawberries,40 the observed success of streptophage treatment on nuts in this study may indicate similar positive outcomes might be possible. Therefore, information generated via the studies like the one presented here can contribute toward development of effective phage biocontrol methods targeting different problems in agriculture. Such methods might subsequently reduce the economic losses of the growers due to unmarketable product including the ones possessing earthy-musty smells.
Data availability
The data that support this study will be shared upon reasonable request to the corresponding author.
Conflicts of interest
The authors declare no conflicts of interest.
Declaration of funding
This research did not receive any specific funding.
Acknowledgements
This work was enabled by use of the Central Analytical Research Facility (CARF) at the Queensland University of Technology (QUT), a Microscopy Australia linked lab.
References
[1] Whitfield, FB (1998) Microbiology of food taints. Int J Food Sci Technol 33, 31–51.| Microbiology of food taints.Crossref | GoogleScholarGoogle Scholar |
[2] Cordovez, V et al. (2015) Diversity and functions of volatile organic compounds produced by Streptomyces from a disease-suppressive soil. Front Microbiol 6, 1081.
| Diversity and functions of volatile organic compounds produced by Streptomyces from a disease-suppressive soil.Crossref | GoogleScholarGoogle Scholar | 26500626PubMed |
[3] Schrader, KK and Blevins, WT (2001) Effects of carbon source, phosphorus concentration, and several micronutrients on biomass and geosmin production by Streptomyces halstedii. J Ind Microbiol Biotechnol 26, 241–247.
| Effects of carbon source, phosphorus concentration, and several micronutrients on biomass and geosmin production by Streptomyces halstedii.Crossref | GoogleScholarGoogle Scholar | 11464274PubMed |
[4] Becher, PG et al. (2020) Developmentally regulated volatiles geosmin and 2-methylisoborneol attract a soil arthropod to Streptomyces bacteria promoting spore dispersal. Nat Microbiol 5, 821–829.
| Developmentally regulated volatiles geosmin and 2-methylisoborneol attract a soil arthropod to Streptomyces bacteria promoting spore dispersal.Crossref | GoogleScholarGoogle Scholar | 32251369PubMed |
[5] Wilkins, K (1996) Volatile metabolites from actinomycetes. Chemosphere 32, 1427–1434.
| Volatile metabolites from actinomycetes.Crossref | GoogleScholarGoogle Scholar |
[6] Jonns, JA et al. (2017) Streptophage-mediated control of off-flavour taint producing streptomycetes isolated from barramundi ponds. Synth Syst Biotechnol 2, 105–112.
| Streptophage-mediated control of off-flavour taint producing streptomycetes isolated from barramundi ponds.Crossref | GoogleScholarGoogle Scholar | 29062967PubMed |
[7] Zaitlin, B and Watson, SB (2006) Actinomycetes in relation to taste and odour in drinking water: myths, tenets and truths. Water Res 40, 1741–1753.
| Actinomycetes in relation to taste and odour in drinking water: myths, tenets and truths.Crossref | GoogleScholarGoogle Scholar | 16600325PubMed |
[8] Han, D et al. (2016) Dimethyl disulphide residue analysis and degradation kinetics determination in soil using gas chromatography–mass spectrometry. Int J Environ Anal Chem 96, 694–704.
| Dimethyl disulphide residue analysis and degradation kinetics determination in soil using gas chromatography–mass spectrometry.Crossref | GoogleScholarGoogle Scholar |
[9] Schöller, CEG et al. (2002) Volatile metabolites from actinomycetes. J Agric Food Chem 50, 2615–2621.
| Volatile metabolites from actinomycetes.Crossref | GoogleScholarGoogle Scholar |
[10] Asquith, EA et al. (2013) The role of actinobacteria in taste and odour episodes involving geosmin and 2-methylisoborneol in aquatic environments. J Water Supply: Res Technol—AQUA (Print) 62, 452–467.
| The role of actinobacteria in taste and odour episodes involving geosmin and 2-methylisoborneol in aquatic environments.Crossref | GoogleScholarGoogle Scholar |
[11] Martín-Sánchez, L et al. (2019) Phylogenomic analyses and distribution of terpene synthases among Streptomyces. Beilstein J Org Chem 15, 1181–1193.
| Phylogenomic analyses and distribution of terpene synthases among Streptomyces.Crossref | GoogleScholarGoogle Scholar | 31293665PubMed |
[12] Wood, S et al. (1983) Factors influencing geosmin production by a streptomycete and their relevance to the occurrence of earthy taints in reservoirs. Water Sci Technol 15, 191–198.
| Factors influencing geosmin production by a streptomycete and their relevance to the occurrence of earthy taints in reservoirs.Crossref | GoogleScholarGoogle Scholar |
[13] Nielsen, JL et al. (2006) Detection of activity among uncultured actinobacteria in a drinking water reservoir. FEMS Microbiol Ecol 55, 432–438.
| Detection of activity among uncultured actinobacteria in a drinking water reservoir.Crossref | GoogleScholarGoogle Scholar | 16466382PubMed |
[14] Lacey, J and Crook, B (1988) Fungal and actinomycete spores as pollutants of the workplace and occupational allergens. Ann Occup Hyg 32, 515–533.
| Fungal and actinomycete spores as pollutants of the workplace and occupational allergens.Crossref | GoogleScholarGoogle Scholar | 3067644PubMed |
[15] Lanciotti, E et al. (2003) Actinomycetes, cyanobacteria and algae causing tastes and odours in water of the River Arno used for the water supply of Florence. J Water Supply: Res Technol—AQUA 52, 489.
| Actinomycetes, cyanobacteria and algae causing tastes and odours in water of the River Arno used for the water supply of Florence.Crossref | GoogleScholarGoogle Scholar |
[16] Jørgensen, NOG et al. (2016) Relations between abundance of potential geosmin- and 2-MIB-producing organisms and concentrations of these compounds in water from three Australian reservoirs. J Water Supply: Res Technol—AQUA 65, 504.
| Relations between abundance of potential geosmin- and 2-MIB-producing organisms and concentrations of these compounds in water from three Australian reservoirs.Crossref | GoogleScholarGoogle Scholar |
[17] Zuo, Y et al. (2009) Isolation, identification and odour-producing abilities of geosmin/2-MIB in actinomycetes from sediments in Lake Lotus, China. J Water Supply: Res Technol—AQUA (Print) 58, 552–561.
| Isolation, identification and odour-producing abilities of geosmin/2-MIB in actinomycetes from sediments in Lake Lotus, China.Crossref | GoogleScholarGoogle Scholar |
[18] Aleshkin, AV et al. (2013) Bacteriophages as probiotics and decontaminating agents for food products. Asia Pac J Life Sci 7, 91–107.
[19] Moye, ZD et al. (2018) Bacteriophage applications for food production and processing. Viruses 10, 205.
| Bacteriophage applications for food production and processing.Crossref | GoogleScholarGoogle Scholar |
[20] Yamada T (2012) Bacteriophages of Ralstonia solanacearum: their diversity and utilization as biocontrol agents in agriculture. In Bacteriophages (Kurtböke DI, ed.). pp. 113–138. InTech.
[21] Jones JB et al (2021) Crop use of bacteriophages. In Bacteriophages: biology, technology, therapy (Harper DR et al, eds). pp. 839–856. Springer International Publishing.
[22] Monk, AB et al. (2010) Bacteriophage applications: where are we now? Lett Appl Microbiol 51, 363–369.
| Bacteriophage applications: where are we now?Crossref | GoogleScholarGoogle Scholar | 20796209PubMed |
[23] Chibani-Chennoufi, S et al. (2004) In vitro and in vivo bacteriolytic activities of Escherichia coli phages: implications for phage therapy. Antimicrob Agents Chemother 48, 2558–2569.
| In vitro and in vivo bacteriolytic activities of Escherichia coli phages: implications for phage therapy.Crossref | GoogleScholarGoogle Scholar | 15215109PubMed |
[24] Bruttin, A and Brüssow, H (2005) Human volunteers receiving Escherichia coli phage T4 orally: a safety test of phage therapy. Antimicrob Agents Chemother 49, 2874–2878.
| Human volunteers receiving Escherichia coli phage T4 orally: a safety test of phage therapy.Crossref | GoogleScholarGoogle Scholar | 15980363PubMed |
[25] Lacey J (1988) Actinomycetes as biodeteriogens and pollutants of the environment. In Actinomycetes in biotechnology (Goodfellow M et al, eds). pp. 359–432. Academic Press.
[26] Koch R (1883) Über die neuen Untersuchungsmethoden zum Nachweis der Mikrokosmen in Boden, Luft und Wasser (Schwalbe J, ed.). pp. 276–284. Robert Koch‐Institut.
[27] Kurtbӧke Dİ (2017) Ecology and habitat distribution of actinobacteria. In Biology and biotechnology of actinobacteria (Wink J et al, eds). pp. 123–149. Springer International Publishing.
[28] Ackermann, HW (2001) Frequency of morphological phage descriptions in the year 2000. Arch Virol 146, 843–857.
| Frequency of morphological phage descriptions in the year 2000.Crossref | GoogleScholarGoogle Scholar | 11448025PubMed |
[29] Ackermann HW (2006) Classification of bacteriophages. In The Bacteriophages, General Background of Phage Biology (Calender R, ed.). pp. 8–16. Oxford University Press.
[30] Ackermann, HW et al. (1985) New actinophage species. Intervirology 23, 121–130.
| New actinophage species.Crossref | GoogleScholarGoogle Scholar | 3988483PubMed |
[31] Sanz-Gaitero M et al (2019) Structure and function of bacteriophages. In Bacteriophages: Biology, Technology, Therapy (Harper DR et al, eds). pp. 1–73. Springer International Publishing.
[32] Kurtböke, Dİ (2006) From culture collections to biological resource centres. Microbiol Aust 27, 4–5.
[33] NSW Food Authority (2009) Guideline levels for microorganisms. In Microbiological Quality Guide for Ready-to-eat Foods (NSW Food Authority, ed.). p. 7. NSW Food Authority.
[34] El-Tarabily, KA (2008) Promotion of tomato (Lycopersicon esculentum Mill.) plant growth by rhizosphere competent 1-aminocyclopropane-1-carboxylic acid deaminase-producing streptomycete actinomycetes. Plant Soil 308, 161–174.
| Promotion of tomato (Lycopersicon esculentum Mill.) plant growth by rhizosphere competent 1-aminocyclopropane-1-carboxylic acid deaminase-producing streptomycete actinomycetes.Crossref | GoogleScholarGoogle Scholar |
[35] Thomas, DTT and Puthur, JT (2017) UV radiation priming: a means of amplifying the inherent potential for abiotic stress tolerance in crop plants. Environ Exp Bot 138, 57–66.
| UV radiation priming: a means of amplifying the inherent potential for abiotic stress tolerance in crop plants.Crossref | GoogleScholarGoogle Scholar |
[36] Abdelsattar, AS et al. (2021) How to train your phage: the recent efforts in phage training. Biologics 1, 70–88.
| How to train your phage: the recent efforts in phage training.Crossref | GoogleScholarGoogle Scholar |
[37] McKenna, F et al. (2001) Novel in vivo use of a polyvalent Streptomyces phage to disinfest Streptomyces scabies-infected seed potatoes. Plant Pathol 50, 666–675.
| Novel in vivo use of a polyvalent Streptomyces phage to disinfest Streptomyces scabies-infected seed potatoes.Crossref | GoogleScholarGoogle Scholar |
[38] Ashfield-Crook, NR et al. (2018) Assessment of the detrimental impact of polyvalent streptophages intended to be used as biological control agents on beneficial soil streptoflora. Curr Microbiol 75, 1589–1601.
| Assessment of the detrimental impact of polyvalent streptophages intended to be used as biological control agents on beneficial soil streptoflora.Crossref | GoogleScholarGoogle Scholar | 30242439PubMed |
[39] Ashfield-Crook N et al (2021) Bioactive streptomycetes from isolation to applications: a Tasmanian potato farm example. In Methods in Molecular Biology. Humana Press.
[40] Kurtböke, Dİ et al. (2016) Isolation and characterization of Enterobacteriaceae species infesting post-harvest strawberries and their biological control using bacteriophages. Appl Microbiol Biotechnol 100, 8593–8606.
| Isolation and characterization of Enterobacteriaceae species infesting post-harvest strawberries and their biological control using bacteriophages.Crossref | GoogleScholarGoogle Scholar | 27357225PubMed |


