The detection and significance of emerging insecticide resistance in mosquitoes
Nancy M Endersby-Harshman A B , Andrew R Weeks A and Ary A Hoffmann AA School of BioSciences, Bio21 Institute, 30 Flemington Road, The University of Melbourne, Vic. 3010, Australia.
B Tel: +61 3 8344 2281, Email: nancye@unimelb.edu.au
Microbiology Australia 39(2) 80-83 https://doi.org/10.1071/MA18022
Published: 6 April 2018
Mosquito-borne arboviruses are increasing in incidence around the world. Australia enjoys some protection from pests and diseases afforded by its geographic isolation coupled with strict biosecurity control at its borders. However, as the volume of global trade, travel and transport expands, risk of exotic incursions to Australia is increasing. Detection of foreign mosquitoes at airports and seaports around Australia is becoming commonplace. The Asian tiger mosquito, Aedes albopictus, which has expanded its range throughout Europe and the Americas1, has not become established in mainland Australia, but is encountered as an exotic incursion2. The yellow fever mosquito and dengue vector, Aedes. aegypti, occurs naturally in northern Queensland, but is also captured at Australia’s ports on a recurrent basis as an incursion from overseas3. Although Ae. aegypti is established in Australia, its detection as an incursion is still cause for concern. Apart from the possibility that invasive mosquitoes will carry exotic arboviruses, genetic characteristics of a foreign insect population can be very different from those observed in local mosquitoes, particularly in terms of insecticide resistance. Our recent research has shown that invading mosquitoes from overseas carry insecticide resistance alleles not found in Australia4 and our development of a global genomic database is helping us to pinpoint their source.
Disease transmission is a major human health consequence of mosquito activity and, in many cases, management of mosquito-borne diseases is addressed by interfering with the mosquito vector. Interference may take the form of physical methods such as source reduction, for example, by removing water containers around dwellings, thereby destroying the aquatic stages of the mosquito and their oviposition sites5. More recently, mosquito population replacement or suppression programs have been developed based on virus-blocking endosymbionts, particularly Wolbachia pipientis6. Variations on the sterile insect release technique using genetic modification are also being employed to reduce abundance of pest mosquitoes7. The World Health Organization (WHO) is promoting integrated vector management (IVM) to reduce the reliance on chemical insecticides for vector control8. Despite these developments, insecticide application is still the main method of mosquito control9 in tropical urban areas where the focus species are Ae. aegypti and Ae. albopictus. As vectors of dengue, Zika, chikungunya and yellow fever viruses, Ae. aegypti and Ae. albopictus are regular targets of insecticide treatment.
Pyrethroid insecticides are valuable tools for mosquito control because of their rapid action, high efficacy, low mammalian toxicity relative to some other insecticide groups and low cost10. Pyrethroids can be applied in a variety of ways such as thermal fogging of neighbourhoods11, internal domestic space and residual spraying12,13, as spatial repellents14, in lethal ovitraps15, as treatment of bed nets16 and for impregnation of clothing17. These diverse applications have led to a dependency on pyrethroids around the world, but have also resulted in their overuse18. As a consequence, multiple instances of pyrethroid resistance have developed in populations of Ae. aegypti throughout much of its range18, although some gaps in testing exist19, and there is evidence for similar developments of resistance within Ae. albopictus populations, though not to the same extent20.
Pyrethroid resistance levels and mechanisms vary by region18,20, which can help identify the significance of mosquito incursions to Australia. Resistance levels in adult mosquitoes from Central and South America are generally higher than those from Asia, Africa and the USA20. The two major pyrethroid resistance mechanisms encountered in Ae. aegypti and Ae. albopictus within these regions are detoxification by cytochrome P450 monoxygenase enzymes and modification of the pyrethroid target site which is the insect’s voltage sensitive sodium channel (Vssc gene)19,20. Combinations of different Vssc mutations also show geographic patterns20, which, together with other methods, can assist in determining the geographic origin of mosquitoes detected as incursions in Australia. Recently we discovered that Australian Ae. aegypti mosquitoes (Figure 1) show none of the common mutations in the Vssc gene that are associated with resistance to pyrethroid insecticides4. In contrast, most incursions of this species to Australia do carry pyrethroid resistance mutations which we have determined by screening Ae. aegypti with TaqMan® assays developed from DNA sequence data acquired during a study of insecticide resistance in Indonesia21.
|
Several problems are posed by the presence of resistance in exotic incursions. Firstly, insecticide resistance in mosquitoes entering the country represents a risk that the resistance alleles will become established in the Australian mosquito population where they will have an advantage on occasions when pyrethroid insecticides are applied. Secondly, there is the problem of the immediate or preventative control of mosquitoes as they arrive in the country, particularly at airports. Aircraft disinsection on international flights into Australia depends entirely on the use of pyrethroid insecticides because of their rapid knockdown of susceptible insects and low mammalian toxicity. They are the only class of insecticide recommended for disinsection by the WHO22 and are applied as a space spray (knockdown) or residual treatment. Insects that are resistant to pyrethroids generally will survive disinsection and may or may not be detected by biosecurity officers at airports, depending on the monitoring regime. Without a safe and effective disinsection method, the risk of insecticide-resistant mosquitoes becoming established in Australia is high and the risk of ‘airport cases’23 of mosquito-borne diseases becomes a possibility. It, therefore, becomes necessary to track the sources of insecticide-resistant, invasive mosquitoes in the hope that alternative pest management tactics can be employed in source locations overseas to reduce mosquito populations around airports and minimise their likelihood of dispersal by aircraft. There are alternate pathways of introduction of exotics, specifically through eggs laid on personal belongings/souvenirs. In these cases, insecticide use in aircraft and around airports will not be effective barriers to entry. If an incursion, away from an airport occurs, the insecticide resistance will hamper eradication efforts.
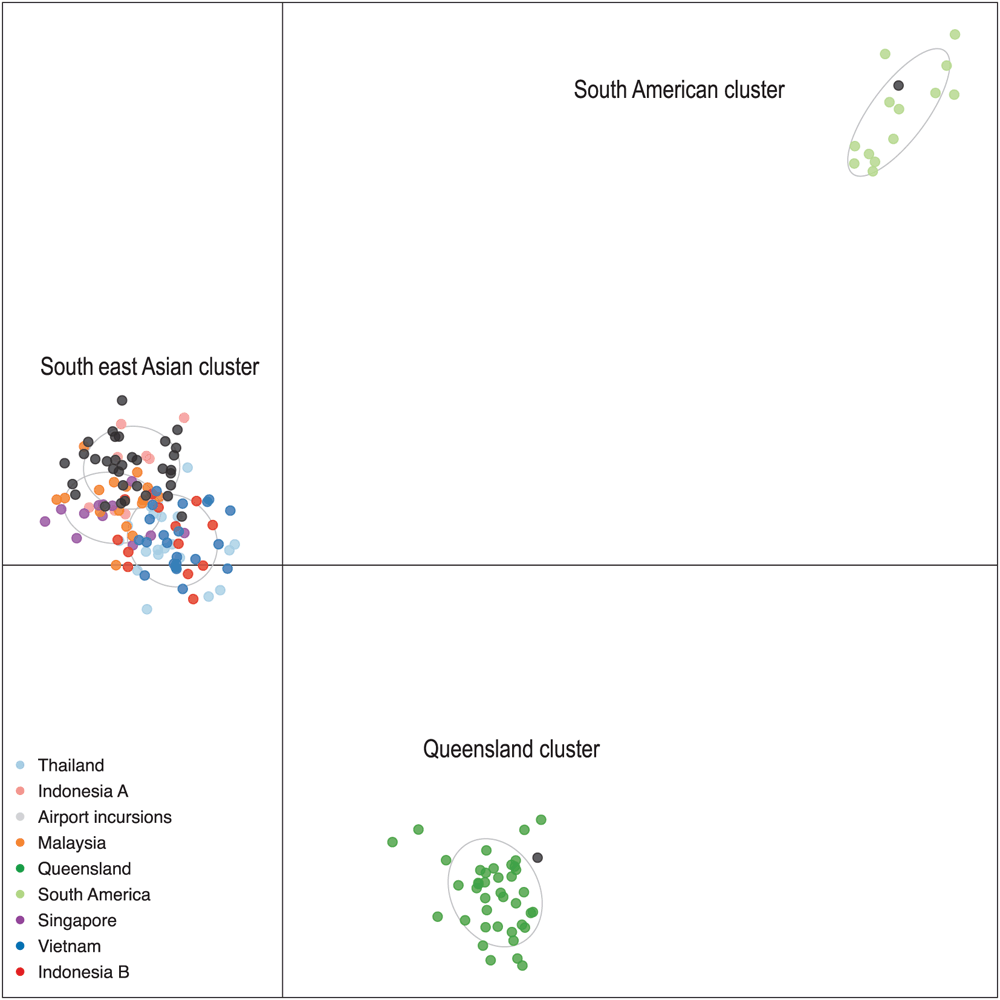
We are using genome-wide single nucleotide polymorphism (SNP) analysis to construct a comprehensive population genomic database for Ae. aegypti and Ae. albopictus from around the world. We pioneered the use of this technique for investigating broad and fine scale patterns of genetic relatedness in Aedes aegypti24 and have been building on this initial study to include mosquitoes from geographic regions relevant to risk of incursion. Genomic profiles of mosquitoes captured as exotic incursions to Australia at international airports and seaports are compared with those in the database to determine their likely origin. Using thousands of SNP loci and genotyping of the Vssc resistance loci, we established recently that most Australian incursion samples of Ae. aegypti at international airports during 2015–2016 had originated in south east Asia, with only a single incursion specimen appearing to have come from South America (Figure 2). We were also able to identify that one incursion sample originated in north Queensland, highlighting that incursions can occur through both domestic and international routes at airports.
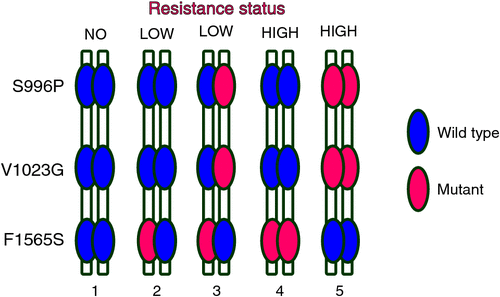
Information about place of origin and pathway to Australia enables authorities to focus on mosquito incursions from south east Asia, at least in the short term, and potentially form relevant collaborations to address the problem. Vssc mutations detected in the group of mosquito incursions from south east Asia were mostly all the same and consisted of a combined haplotype of three mutations which is known to confer resistance to Type I and Type II pyrethroids (1023G mutant homozygote, 1565F (wild type) and 996P mutant homozygote) (Figure 3) and is consistent with bioassay data showing high levels of resistance to permethrin (Type I) in Ae. aegypti from this region25. Addressing management practices in the country of origin will be the most effective way to reduce the influx of pyrethroid-resistant mosquitoes to Australia and further research should be conducted into the full resistance profile including multiple mechanisms and current efficacy of insecticide groups other than pyrethroids which may be used on other life stages of Ae. aegypti. Under some circumstances, alternative insecticides to pyrethroids can be used, for example, carbamates, that could kill pyrethroid-resistant Ae. aegypti26 and non-insecticidal methods may also be considered. Careful stewardship of pyrethroid insecticides has been practised in Queensland since the 1990s using strategic applications combined with a variety of delivery methods that include indoor residual spraying, lethal ovitraps and use of insect growth regulators as larvicides4. Under these conditions, resistance mutations in the Vssc have not been selected for in Ae. aegypti in Queensland4. Our continued research into identification of mosquito origin and monitoring of Vssc resistance mutations in incursion specimens will keep our management programs relevant, even in the event of temporal changes in mosquito distributions and levels of insecticide resistance. Continued progress should result in maintenance of insecticide susceptibility in Ae. aegypti in Australia and reduced risk of arbovirus transmission.
|
Acknowledgements
We thank the Department of Agriculture and Water Resources and Angus Sly for providing samples and funding which supported this study.
References
[1] Kotsakiozi, P. et al. (2017) Population genomics of the Asian tiger mosquito, Aedes albopictus: insights into the recent worldwide invasion. Ecol. Evol. 7, 10143–10157.| Population genomics of the Asian tiger mosquito, Aedes albopictus: insights into the recent worldwide invasion.Crossref | GoogleScholarGoogle Scholar |
[2] Muzari, M.O. et al. (2017) Holding back the tiger: successful control program protects Australia from Aedes albopictus expansion. PLoS Negl. Trop. Dis. 11, e0005286.
| Holding back the tiger: successful control program protects Australia from Aedes albopictus expansion.Crossref | GoogleScholarGoogle Scholar |
[3] Webb, C.E. et al. (2016) A guide to mosquitoes of Australia. CSIRO Publishing.
[4] Endersby-Harshman, N.M. et al. (2017) Pyrethroid susceptibility has been maintained in the dengue vector, Aedes aegypti (Diptera: Culicidae), in Queensland, Australia. J. Med. Entomol. 54, 1649–1658.
| Pyrethroid susceptibility has been maintained in the dengue vector, Aedes aegypti (Diptera: Culicidae), in Queensland, Australia.Crossref | GoogleScholarGoogle Scholar |
[5] Healy, K. et al. (2014) Integrating the public in mosquito management: active education by community peers can lead to significant reduction in peridomestic container mosquito habitats. PLoS One 9, e108504.
| Integrating the public in mosquito management: active education by community peers can lead to significant reduction in peridomestic container mosquito habitats.Crossref | GoogleScholarGoogle Scholar |
[6] Hoffmann, A.A. et al. (2011) Successful establishment of Wolbachia in Aedes populations to suppress dengue transmission. Nature 476, 454–457.
| Successful establishment of Wolbachia in Aedes populations to suppress dengue transmission.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhtVOnu7jJ&md5=516a4d7b0428a6d1ec471395aee1b648CAS |
[7] Carvalho, D.O. et al. (2015) Suppression of a field population of Aedes aegypti in Brazil by sustained release of transgenic male mosquitoes. PLoS Negl. Trop. Dis. 9, e0003864.
| Suppression of a field population of Aedes aegypti in Brazil by sustained release of transgenic male mosquitoes.Crossref | GoogleScholarGoogle Scholar |
[8] WHO/HTM/NTD/VEM/2008.2 (2008) WHO position statement on integrated vector management, World Health Organization.
[9] Manjarres-Suarez, A. and Olivero-Verbel, J. (2013) Chemical control of Aedes aegypti: a historical perspective. Rev. Costarric. Salud Pública 22, 68–75.
[10] Costa, L.G. (2015) The neurotoxicity of organochlorine and pyrethroid pesticides. Handb. Clin. Neurol. 131, 135–148.
| The neurotoxicity of organochlorine and pyrethroid pesticides.Crossref | GoogleScholarGoogle Scholar |
[11] Marcombe, S. et al. (2009) Reduced efficacy of pyrethroid space sprays for dengue control in an area of Martinique with pyrethroid resistance. Am. J. Trop. Med. Hyg. 80, 745–751.
| 1:CAS:528:DC%2BD1MXmt1Kjsrs%3D&md5=b671195e6ba70fdaa40893a30b27c1deCAS |
[12] Ritchie, S. et al. (2002) Dengue control in north Queensland, Australia: case recognition and selective indoor residual spraying WHO Dengue Bull. 26, 7–13.
[13] Vazquez-Prokopec, G.M. et al. (2017) Combining contact tracing with targeted indoor residual spraying significantly reduces dengue transmission. Sci. Adv. 3, e1602024.
| Combining contact tracing with targeted indoor residual spraying significantly reduces dengue transmission.Crossref | GoogleScholarGoogle Scholar |
[14] Bibbs, C.S. and Kaufman, P.E. (2017) Volatile pyrethroids as a potential mosquito abatement tool: a review of pyrethroid-containing spatial repellents. J. Integr. Pest Manag. 8, 21.
| Volatile pyrethroids as a potential mosquito abatement tool: a review of pyrethroid-containing spatial repellents.Crossref | GoogleScholarGoogle Scholar |
[15] Ritchie, S.A. et al. (2009) A lethal ovitrap-based mass trapping scheme for dengue control in Australia: I. Public acceptability and performance of lethal ovitraps. Med. Vet. Entomol. 23, 295–302.
| A lethal ovitrap-based mass trapping scheme for dengue control in Australia: I. Public acceptability and performance of lethal ovitraps.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD1Mjoslentw%3D%3D&md5=642d0336f817f2b4e8bf51742fa28198CAS |
[16] Lindsay, S.W. et al. (1991) Pyrethroid-treated bednet effects on mosquitoes of the Anopheles gambiae complex in The Gambia. Med. Vet. Entomol. 5, 477–483.
| Pyrethroid-treated bednet effects on mosquitoes of the Anopheles gambiae complex in The Gambia.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK387islWlsw%3D%3D&md5=9080307742b23e54d22ee1a2c8d142f8CAS |
[17] DeRaedt Banks, S. et al. (2015) Permethrin-treated clothing as protection against the dengue vector, Aedes aegypti: extent and duration of protection. PLoS Negl. Trop. Dis. 9, e0004109.
| Permethrin-treated clothing as protection against the dengue vector, Aedes aegypti: extent and duration of protection.Crossref | GoogleScholarGoogle Scholar |
[18] Du, Y. et al. (2016) Sodium channel mutations and pyrethroid resistance in Aedes aegypti. Insects 7, 60.
| Sodium channel mutations and pyrethroid resistance in Aedes aegypti.Crossref | GoogleScholarGoogle Scholar |
[19] Moyes, C.L. et al. (2017) Contemporary status of insecticide resistance in the major Aedes vectors of arboviruses infecting humans. PLoS Negl. Trop. Dis. 11, e0005625.
| Contemporary status of insecticide resistance in the major Aedes vectors of arboviruses infecting humans.Crossref | GoogleScholarGoogle Scholar |
[20] Smith, L.B. et al. (2016) Pyrethroid resistance in Aedes aegypti and Aedes albopictus: Important mosquito vectors of human diseases. Pestic. Biochem. Physiol. 133, 1–12.
| Pyrethroid resistance in Aedes aegypti and Aedes albopictus: Important mosquito vectors of human diseases.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC28XmtFCmt74%3D&md5=c26a9a63ff9641845fb98fbb891cce49CAS |
[21] Wuliandari, J.R. et al. (2015) Association between three mutations, F1565C, V1023G and S996P, in the voltage-sensitive sodium channel gene and knockdown resistance in Aedes aegypti from Yogyakarta, Indonesia. Insects 6, 658.
| Association between three mutations, F1565C, V1023G and S996P, in the voltage-sensitive sodium channel gene and knockdown resistance in Aedes aegypti from Yogyakarta, Indonesia.Crossref | GoogleScholarGoogle Scholar |
[22] WHO (2012) Guidelines for testing the efficacy of insecticide products used in aircraft. ISBN 978 92 4 150323 5.
[23] Siala, E. et al. (2015) Airport malaria: report of four cases in Tunisia. Malar. J. 14, 42.
| Airport malaria: report of four cases in Tunisia.Crossref | GoogleScholarGoogle Scholar |
[24] Rašić, G. et al. (2014) Genome-wide SNPs lead to strong signals of geographic structure and relatedness patterns in the major arbovirus vector, Aedes aegypti. BMC Genomics 15, 275.
| Genome-wide SNPs lead to strong signals of geographic structure and relatedness patterns in the major arbovirus vector, Aedes aegypti.Crossref | GoogleScholarGoogle Scholar |
[25] Hamid, P.H. et al. (2017) Knockdown resistance (kdr) of the voltage-gated sodium channel gene of Aedes aegypti population in Denpasar, Bali, Indonesia. Parasit. Vectors 10, 283.
| Knockdown resistance (kdr) of the voltage-gated sodium channel gene of Aedes aegypti population in Denpasar, Bali, Indonesia.Crossref | GoogleScholarGoogle Scholar |
[26] Vazquez-Prokopec, G.M. et al. (2017) Deltamethrin resistance in Aedes aegypti results in treatment failure in Merida, Mexico. PLoS Negl. Trop. Dis. 11, e0005656.
| Deltamethrin resistance in Aedes aegypti results in treatment failure in Merida, Mexico.Crossref | GoogleScholarGoogle Scholar |
Biographies
Nancy Endersby-Harshman is a Research Fellow working at the Bio21 Institute at the University of Melbourne. Her research interests focus on the dengue vector mosquito, Aedes aegypti, in the fields of insecticide resistance, population genetics, Wolbachia and vector ecology.
Andrew Weeks is a Senior Research Fellow working at the Bio21 Institute at the University of Melbourne. His research focuses on the population genetics of insect pests including those important for agriculture and human health.
Ary Hoffmann is a Melbourne Laureate Professor and NHMRC Principal Research Fellow at the Bio21 Institute at the University of Melbourne working in the areas of pest/disease vector control and climate change adaptation.