Rock-art microbiome: influences on long term preservation of historic and culturally important engravings
Deirdre B Gleeson A C , Matthias Leopold A D , Benjamin Smith B E and John L Black B FA UWA School of Agriculture and Environment, The University of Western Australia, Crawley, WA 6009, Australia
B Centre for Rock Art Research and Management, The University of Western Australia, Crawley, WA 6009, Australia
C Email: deirdre.gleeson@uwa.edu.au
D Email: matthias.leopold@uwa.edu.au
E Email: benjamin.smith@uwa.edu.au
F Email: jblack@pnc.com.au
Microbiology Australia 39(1) 33-36 https://doi.org/10.1071/MA18009
Published: 22 February 2018
The Burrup Peninsula in north-west Western Australia is home to one of the most substantial collections of rock engravings, or petroglyphs, in the world. These petroglyphs are carved through the dark coloured patina, commonly referred to as rock varnish, into the weathering rind of the local parent rock. Rock varnish is essentially a thin layer of manganese (Mn) and iron (Fe) oxides and hydroxides with embedded clay minerals, the formation of which is relatively poorly understood. It is generally considered to be a hostile environment for microorganisms due to extreme environmental conditions including low nutrient availability, lack of water, exposure to extreme ultraviolet radiation and intense seasonal and diurnal temperature fluctuations. However, despite these environmental extremes, microorganisms have been found on and in rock varnish and have been reported as playing a significant role in the formation of rock varnish. Given this, it is likely that any change in local environmental conditions will influence the types and activities of microorganisms found in and on rock varnish and associated rock art. This article focuses on the major influences on the microbiome of culturally important rock art in the Burrup Peninsula and the implications of any environmental change on the rock art itself.
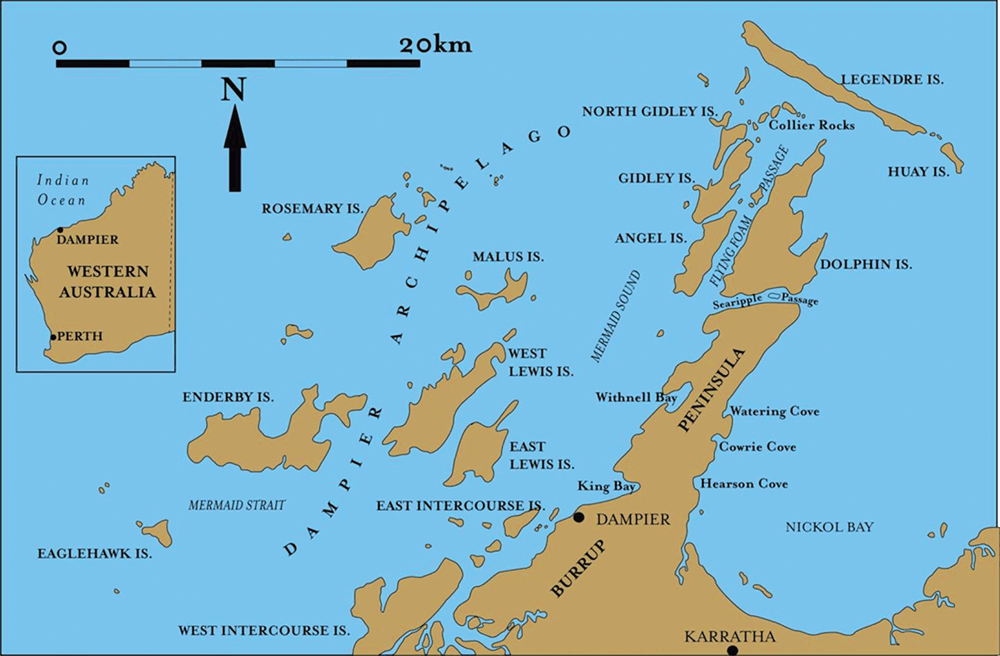
The Burrup Peninsula (see Figure 1) is estimated to contain over 1 million petroglyphs. These form one of the longest sequences of art in the world, extending back probably as much as 40 000 years. This makes the area one of the most significant rock art regions in the world1. The engravings are diverse in form and include those of many animal and bird species including extinct animals (e.g. Thylacines, the Tasmanian tiger)2. The petroglyphs (see Figure 2 as an example) on the Burrup Peninsula are carved into the rock varnish of the parent granophyre and gabbro igneous rocks. Rock varnish usually forms very slowly, at a rate of 1–10 μm per thousand years, particularly in arid desert environments where rainfall is low3,4. At its most basic rock varnish can be described as a dark coloured coating with thickness that seldom exceeds 200 μm that is composed mostly of Mn and Fe oxides that are cemented to clay minerals in a laminated structure3. The Mn and Fe present in rock varnish comes from a range of sources likely including the atmosphere, precipitation, dust and from surrounding soils5,6. Mn and Fe concentrations can vary greatly but in general Mn is enriched over Fe relative to their natural distribution with Mn oxides usually accounting for around 20% of the total oxides7.
|

|
There is some evidence suggesting that the microlaminations in rock varnish correlate with alterations in environmental conditions3. Liu et al.8 hypothesise that Fe‐rich layers are formed during dry conditions whereas darker Mn‐rich layers are formed during periods of wet3. Precipitation and solubility of Mn is generally controlled by pH and Eh conditions with low Eh and pH usually leading to dissolution of Mn whereas high Eh and pH promotes precipitation. The ratio of Mn to Fe in the varnish thus varies with climatic conditions9. It is likely that when conditions are moist this promotes the activity of bacteria and fungi which in turn produce organic acids that work to decrease local pH. Field observations by Northup and colleagues at the Black Canyon (New Mexico, US) reported a strong correlation of visibly more substantial Mn varnishing on preferential water pathways flowing on rock surfaces and cliff faces, and in ephemeral rock pothole pools10.
Rock varnish formation
The mechanism of rock varnish formation and growth is still under discussion with both biotic and abiotic processes being postulated. It has been proposed that if abiotic processes were responsible for producing rock varnish that rates would be much more rapid than empirical measurements suggest11. Evidence of a biological role has been mounting5 with significant interest being generated around likely biological mechanisms. For example the formation of β-alanine and δ-butyric acid by enzymatic carboxylation is indicative of biological activity12. There is also significant evidence that suggests that microorganisms can directly or indirectly control Mn precipitation13,14 with biomineralisation of Mn being proposed in a wide range of environments including hot springs15 and soils16. The elevated concentration of Mn that is well above that of the geological background also suggests that the formation of rock varnish is likely a biological process. This is because Mn and Fe compounds concentrated in bacteria and fungi become chemically bound in the crystalline structure and external coating of the clay minerals that cements the clays to rock surfaces17. The hypotheses that microbial communities play a role in the formation of rock varnish are also supported by the fact that a number of microbial metabolic pathways use Fe and Mn as electron donors18,19.
The role of the microbiome
Many different bacteria and fungi have been isolated and characterised from rock varnish10,20–22 but those with the ability to oxidise and precipitate Mn and Fe13,19 are of particular interest. Within this group budding bacteria, for example the genera Hyphomicrobium and Pedomicrobium, have been extensively studied as they have the ability to encrust Mn and Fe oxides within their cells23,24. These types of budding bacteria have been identified growing on rock varnishes found in warm deserts5,25 and are known to produce Mn-rich deposits on rocks present in mountain soils26, acid mine drainage19, and caves27,28. Krinsley et al.11 reported the first evidence of budding bacteria present in situ within rock varnish that were directly enhancing Mn and Fe. The authors investigated a site at Erie Barge Canal (New York) where rock varnish had completely coated the quarried sandstone over the course of approximately 100 years11. This site has rock varnish that is approximately 15 μm thick and the authors conclude that only one or two budding bacteria encrusting Mn and Fe oxides each year would be needed to generate the rock varnish11. By extrapolating from this data the authors speculate that it would take only one budding-bacterium every 400 years to explain a 20 μm thick, 10 000-year-old warm desert varnish similar to that found in the Burrup Peninsula11. We can therefore hypothesise that unique and rarely occurring environmental conditions are required to promote rock varnish formation by specific budding bacteria, for example, optimum moisture, UV and solar radiation exposure.
Industrial expansion: what does the future hold?
Recent expansion of industry in the Burrup Peninsula may potentially upset the delicate balance of environmental conditions that led to rock varnish formation. Acid rain and nitrogen deposition as a result of industrial expansion has the potential to stimulate microorganisms that may not be compatible with rock varnish formation and or which may produce organic acids that could be detrimental to the survival of the rock varnish29,30. The combined influence of acid rain and microbial organic acid production will decrease the pH of the rock art environment potentially resulting in dissolution of the Mn and Fe within the rock varnish ultimately leading to deterioration and consequent destruction of the rock art. Research is required to identify the specific organisms responsible for rock varnish formation and to assess the impact of pollution on the rock art microbiome and likely impacts on rock varnish so that we can better understand how to protect the culturally significant rock art present in the Burrup Peninsula.
Acknowledgements
The authors acknowledge and thank the Murujuga Aboriginal Corporation.
References
[1] Donaldson, M. (2011) Understanding the rocks: rock art and the geology of Murujuga (Burrup Peninsula). Rock Art Res. 28, 35–43.[2] Black, J.L. et al. (2017) Theoretical effects of industrial emissions on colour change at rock art sites on Burrup Peninsula, Western Australia. J. Archaeol. Sci. Rep. 12, 457–462.
| Theoretical effects of industrial emissions on colour change at rock art sites on Burrup Peninsula, Western Australia.Crossref | GoogleScholarGoogle Scholar |
[3] Liu, T.H. and Broecker, W.S. (2000) How fast does rock varnish grow? Geology 28, 183–186.
| How fast does rock varnish grow?Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3cXhtVemt7o%3D&md5=08e996d4e1139dba8779ad9fce2885ebCAS |
[4] Dorn, R.I. (2009) Desert Rock Coatings. In Geomorphology of Desert Environments (Parsons A.J. and Abrahams A.D. eds) pp. 153-186, Springer.
[5] Krumbein, W.E. and Jens, K. (1981) Biogenic rock varnishes of the Negev desert (Isreal) and ecological study of iron and manganese transformation by Cyanobacteria and fungi. Oecologia 50, 25–38.
| Biogenic rock varnishes of the Negev desert (Isreal) and ecological study of iron and manganese transformation by Cyanobacteria and fungi.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC1cznvVGlug%3D%3D&md5=a495f55b90f12f2a764e531b2a3e4546CAS |
[6] Thiagarajan, N. and Lee, C.T.A. (2004) Trace-element evidence for the origin of desert varnish by direct aqueous atmospheric deposition. Earth Planet. Sci. Lett. 224, 131–141.
| Trace-element evidence for the origin of desert varnish by direct aqueous atmospheric deposition.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXlvFyltLw%3D&md5=28820b470710a80582d4eb16fc163c63CAS |
[7] Potter, R.M. and Rossman, G.R. (1979) The manganese- and iron-oxide mineralogy of desert varnish. Chem. Geol. 25, 79–94.
| The manganese- and iron-oxide mineralogy of desert varnish.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaE1MXktVOntLg%3D&md5=40c40b066cba0bba80f4f194d8939d3dCAS |
[8] Jones, C.E. (1991) Characteristics and origin of rock varnish from the hyperarid coastal deserts of northern Peru. Quat. Res. 35, 116–129.
| Characteristics and origin of rock varnish from the hyperarid coastal deserts of northern Peru.Crossref | GoogleScholarGoogle Scholar |
[9] Broecker, W.S. and Liu, T. (2001) Rock varnish: recorder of desert wetness? GSA 11, 4–10.
[10] Northup, D.E. et al. (2010) Diversity of rock varnish bacterial communities from Black Canyon, New Mexico. J. Geophys. Res. Biogeosci. 115, G02007.
[11] Krinsley, D.H. et al. (2017) Mn-Fe-Enhancing Budding Bacteria in Century-Old Rock Varnish, Erie Barge Canal, New York. J. Geol. 125, 317–336.
| Mn-Fe-Enhancing Budding Bacteria in Century-Old Rock Varnish, Erie Barge Canal, New York.Crossref | GoogleScholarGoogle Scholar |
[12] Perry, R.S. et al. (2003) Amino acid analyses of desert varnish from the Sonoran and Mojave deserts. Geomicrobiol. J. 20, 427–438.
| Amino acid analyses of desert varnish from the Sonoran and Mojave deserts.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXnvVOlu7g%3D&md5=7d8877d22832ec4837b9ecbdc4056f3fCAS |
[13] Tebo, B.M. et al. (2004) Biogenic manganese oxides: Properties and mechanisms of formation. Annu. Rev. Earth Planet. Sci. 32, 287–328.
| Biogenic manganese oxides: Properties and mechanisms of formation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXkvVyisro%3D&md5=3c2d7170a1cfe30e4f8586b530795e13CAS |
[14] Nealson, K.H. (1983) The microbial manganese cycle. In Microbial Biogeochemistry (Krumbein W.E. ed) pp. 191–221, Blackwell Scientific Publishers.
[15] Mita, N. et al. (1994) A growing deposit of hydrous manganese oxide produced by microbial mediation at a hot-spring, Japan. Geochem. J. 28, 71–80.
| A growing deposit of hydrous manganese oxide produced by microbial mediation at a hot-spring, Japan.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2cXmtlCruro%3D&md5=d06edc077ca0c1967e069cba9d8169b5CAS |
[16] Stiles, C.A. et al. (2001) Pedogenic iron-manganese nodules in Vertisols: a new proxy for paleoprecipitation? Geology 29, 943–946.
| Pedogenic iron-manganese nodules in Vertisols: a new proxy for paleoprecipitation?Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXotVehtrY%3D&md5=e1088a17356834fbfae5a6a4730738cdCAS |
[17] Dorn, R.I. (2009) Rock varnish and its use to study climate change in geomorphic settings. In Geomorphology of Desert Environments (Parsons, A.J. and Abrahams, A.A. eds) pp. 657–672, Springer Science + Business Media B.V.
[18] Weber, K.A. et al. (2006) Microorganisms pumping iron: anaerobic microbial iron oxidation and reduction. Nat. Rev. Microbiol. 4, 752–764.
| Microorganisms pumping iron: anaerobic microbial iron oxidation and reduction.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XpsFOktLg%3D&md5=d43a37ada308c8a198d3cf17e4472695CAS |
[19] Tebo, B.M. et al. (2005) Geomicrobiology of manganese(II) oxidation. Trends Microbiol. 13, 421–428.
| Geomicrobiology of manganese(II) oxidation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXpsVCnsL4%3D&md5=42f66bbec6a7228ee76f961019ec0f37CAS |
[20] Gorbushina, A.A. et al. (2003) Black fungal colonies as units of survival: hyphal mycosporines synthesized by rock-dwelling microcolonial fungi. Can. J. Bot. 81, 131–138.
| 1:CAS:528:DC%2BD3sXjtlKntrw%3D&md5=4eb765dd3f98d31d01afb5d1294889b7CAS |
[21] Parchert, K.J. et al. (2012) Fungal Communities Associated with Rock Varnish in Black Canyon, New Mexico: Casual Inhabitants or Essential Partners? Geomicrobiol. J. 29, 752–766.
| Fungal Communities Associated with Rock Varnish in Black Canyon, New Mexico: Casual Inhabitants or Essential Partners?Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhtVCmu7nM&md5=9b36edb0cc628bf07f3b67d52df5092aCAS |
[22] Esposito, A. et al. (2015) Comparison of Rock Varnish Bacterial Communities with Surrounding Non-Varnished Rock Surfaces: Taxon-Specific Analysis and Morphological Description. Microb. Ecol. 70, 741–750.
| Comparison of Rock Varnish Bacterial Communities with Surrounding Non-Varnished Rock Surfaces: Taxon-Specific Analysis and Morphological Description.Crossref | GoogleScholarGoogle Scholar |
[23] Ghiorse, W.C. and Hirsch, P. (1979) Ultrastructural-study of iron and manganese deposition associated with extracellular polymers of Pedomicrobium-like budding bacteria. Arch. Microbiol. 123, 213–226.
| Ultrastructural-study of iron and manganese deposition associated with extracellular polymers of Pedomicrobium-like budding bacteria.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL3cXptVOlsQ%3D%3D&md5=bd227b94234dcc84ca01bbfd14308385CAS |
[24] Ghiorse, W.C. (1984) Biology of iron-depositing and manganese-depositing bacteria. Annu. Rev. Microbiol. 38, 515–550.
| Biology of iron-depositing and manganese-depositing bacteria.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL2cXmtVOht74%3D&md5=dc110727b7d6e5f2fa88a5f8ad2f3d6cCAS |
[25] Dorn, R.I. and Oberlander, T.M. (1981) Microbial origin of desert varnish. Science 213, 1245–1247.
| Microbial origin of desert varnish.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL3MXltlKiurg%3D&md5=3e64ecb3f5f088b099a99e289fcf3b2dCAS |
[26] Khak-mun, T. (1966) Iron- and manganese-oxidizing microorganisms in soils of South Sakhalin. Microbiology 36, 276–281.
[27] Northup, D.E. et al. (2003) Diverse microbial communities inhabiting ferromanganese deposits in Lechuguilla and Spider Caves. Environ. Microbiol. 5, 1071–1086.
| Diverse microbial communities inhabiting ferromanganese deposits in Lechuguilla and Spider Caves.Crossref | GoogleScholarGoogle Scholar |
[28] Lozano, R.P. and Rossi, C. (2012) Exceptional preservation of Mn-oxidizing microbes in cave stromatolites (El Soplao, Spain). Sediment. Geol. 255–256, 42–55.
| Exceptional preservation of Mn-oxidizing microbes in cave stromatolites (El Soplao, Spain).Crossref | GoogleScholarGoogle Scholar |
[29] MacLeod, I.D. (2005) Effects of moisture, micronutrient supplies and microbiological activity on the surface pH of rocks in the Burrup Peninsula. In: 14th Triennial Meeting (ICOM Committee for Conservation), The Hague (Verger, I. ed), pp. 385–393.
[30] Giesen, M.J. et al. (2014) Condition assessment and preservation of open-air rock art panels during environmental change. J. Cult. Herit. 15, 49–56.
| Condition assessment and preservation of open-air rock art panels during environmental change.Crossref | GoogleScholarGoogle Scholar |
Biographies
Dr Deirdre Gleeson is a microbial ecologist focussing on both natural and managed ecosystems across terrestrial and aquatic environments where she studies the microbiomes of agricultural systems, contaminated landscapes, microbioliates and seagrass and mangrove rhizospheres. She is particularly interested in microbe-mineral interactions and how these relationships relate to rock art preservation in the Burrup Peninsula.
Dr Matthias Leopold is a soil geomorphologist focusing on soils as geoarchives. He integrates knowledge from pedology and geoscience to study and understand near surface processes at various scales and environments. Matthias has broad experience in geoarchaeology and has worked with archaeologists on rock art at the Burrup Peninsula for the past three years.
Professor Benjamin Smith is Associate Dean (Research) in the Faculty of Arts, Business, Law and Education and Professor of World Rock Art at the University of Western Australia. He is President of the ICOMOS International Committee on Rock Art.
Dr John L Black AM is a Research Management Consultant specialising in the management of large programs for the animal and veterinary industries. He worked in the CSIRO Division of Animal Production for 23 years studying comparative physiology and nutrition across animal species and in the development of computer simulation models before becoming Assistant Chief of the Division. In recent years, he has been investigating, pro bono, the impacts of industrial emissions on petroglyphs on Murujuga.


