The geomicrobiology of mining environments
Talitha C Santini A B and Emma J Gagen A CA School of Earth and Environmental Sciences, Room 325, Steele Building, St Lucia Campus, The University of Queensland, Brisbane, Qld 4072, Australia
Email: t.santini@uq.edu.au
B School of Earth and Environmental Sciences, Room 218, Richards Building, St Lucia Campus, The University of Queensland, Brisbane, Qld 4072, Australia
C Email: e.gagen@uq.edu.au
Microbiology Australia 39(1) 37-41 https://doi.org/10.1071/MA18010
Published: 22 February 2018
As the global population increases, so does the demand for minerals and energy resources. Demand for some of the major global commodities is currently growing at rates of: copper – 1.6% p.a.1; iron ore: 1.4% p.a.2; aluminium – 5% p.a.3; rare earth elements – 7% p.a.4, driven not only by population growth in China, India, and Africa, but also by increasing urbanisation and industrialisation globally. Technological advances in renewable energy production and storage, construction materials, transport, and computing could see demand for some of these resources spike by 2600% over the next 25 years under the most extreme demand scenarios5. Coupled with declining ore grades, this demand means that the global extent of mining environments is set to increase dramatically. Land disturbance attributed to mining was estimated to be 400 000 km2 in 20076, with projected rates of increase of 10 000 km2 per year7. This will increase the worldwide extent of mining environments from around 500 000 km2 at present to 1 330 000 km2 by 2100, larger than the combined land area of New South Wales and Victoria (1 050 000 km2), making them a globally important habitat for the hardiest of microbial life. The extreme geochemical and physical conditions prevalent in mining environments present great opportunities for discovery of novel microbial species and functions, as well as exciting challenges for microbiologists to apply their understanding to solve complex remediation problems.
Major habitats in mining environments can be divided into two main groups (Figure 1): mine sites, where ore is excavated and crushed, including waste storage sites for overburden (rock and soil materials removed to access the ore body), and waste rock (sub-economic rock surrounding the higher grade ore body); and processing/refinery sites, where the ore is upgraded or purified to separate the target element or resource, including waste storage sites for by-products from either aqueous (tailings) or high temperature smelting (slags) refining techniques, and wastewaters from these processes. Not covered in this article are mining-affected environments around mining and refinery sites, which receive inputs from mine sites in the form of dust (ore, overburden, tailings, and the resource product), surface water and groundwater discharges (wastewaters), or even solid wastes (tailings, waste rock) which, in some cases, are exported by riverine or marine disposal. The severity of impacts and disturbance is far lower in mining-affected environments around the site than within the mining or refinery sites that may generate offsite impacts, and we have therefore excluded them from the primary mining environments (mines and refineries) to be discussed here.
Ore bodies are, by their definition, geochemical and mineralogical anomalies, containing target resources at elevated concentrations compared to the average in continental crusts. It should be no surprise, then, that the excavation of these ores and exposure to water and air generates unusual geochemical environments for microbial communities to inhabit and modify. Even more extreme geochemical and physical environments are created in the tailings and wastewater streams produced from ore processing and refining activities (Figure 1) as a result of the elevated temperatures and pressures used in processing and refining, and the chemical reagents added to enhance resource recovery. The processing conditions effectively sterilise tailings and wastewater streams, and the extreme geochemical and physical conditions then impose strong selection pressures on future microbial colonisers. pH values tend to be ≤4.5 or ≥8.5, due to the use of acidic or alkaline refining conditions, and/or the reaction of ore or process-generated minerals producing acidity (e.g. oxidation of sulphides; Equation 1) or alkalinity (e.g. dissolution of hydroxides or carbonates; Equation 2).
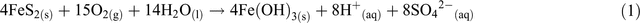

Salinity, and particularly sodicity, is usually high enough in tailings to inhibit growth of even the most salt-tolerant plant species (>4 mS cm–1) and classifies wastewaters as brackish to brine (1–35 g L–1 salt), due to the addition of various (often sodium-based) reagents during refining processes. Major biological nutrients (C, N, K, P) are present in low to negligible concentrations, because the depth at which ores are excavated during mining, and their low surface area in situ, does not allow for significant microbial colonisation and fixation of atmospheric carbon and nitrogen, and does not expose the ore to near-surface weathering processes that release K and P from minerals (commonly feldspars of the general formula (K,Na,Ca)(Al,P,Si)4O8, micas of the general formula (K,Na,Ca)(Al,Mg,Fe)2–3(Si,Al,Fe3+)4O10(OH,F)2, and apatite Ca3(PO4)2). Crushing of ore to enhance reaction kinetics during refining creates tailings materials that are prone to waterlogging, largely anaerobic, and exhibit rapid mineral weathering rates (both chemically and biologically driven) due to the large particle surface areas. The extreme pH and high mineral weathering rates release heavy metals (Pb, Hg, Cd, Co, Sn), metalloids (As, Se, Sb, B), and other elements at concentrations typically considered to be toxic for most plant and microbial life.
And yet life persists! Although generally low biomass and low diversity8–11, active microbial communities appear to be present across all mining environments. Dominant phyla tend to be those known to host lineages tolerant of one or more of the challenging environmental conditions present in mining environments, such as pH, salinity, high metals/metalloid concentrations, and lack of organic carbon. For example, acid mine drainage and sulphidic waste rock are dominated by Gammaproteobacteria, Betaproteobacteria, Actinobacteria, Nitrospira, and Firmicutes8,12, and alkaline tailings are dominated by Gammaproteobacteria, Firmicutes, Actinobacteria, and Bacteroidetes11. However, community composition diverges within mining environments at lower taxonomic levels, where the influences of site specific factors like ore type, environmental conditions, and process chemistry play a greater role11.
Cultivation and isolation of novel species from mine sites has yielded fundamental insights into processes of element cycling (e.g. arsenic13; silver14; gold15; rare earth elements16; thiocyanate17), the mechanisms and origins of pH, salt, and metal tolerances (e.g. acid and chloride tolerance18; gold19), and microbe-mineral interactions20. Some of these novel species are genetically tractable, e.g. Marinobacter subterrani from an iron mine, and are thus invaluable tools for fundamental investigations into microbial physiology and metabolism21. Others are becoming useful tools in biotechnology; for example, an Acidithiobacillus thiooxidans strain isolated from a copper mine that is now being used in industrial bioleaching22. Metal-tolerant organisms from mines also hold promise amongst the range of organisms being considered in approaches for recovery of metals, a process known as biomining, including eukaryotic microbes e.g. Euglena mutabilis and Chlorella protothecoides isolated from a copper mine23. The heavy metal tolerances of these eukaryotes also makes them useful bioindicators for metal contamination in aquatic systems. Such discoveries are facilitated in mining environments, which provide selection pressures of sufficient strength to promote the proliferation of species with these tolerances and capabilities.
At a community level, the restricted diversity present in mining environments has proved ideal for development of new bioinformatics tools, such as metagenomics from a study of microbial communities in acidic mine wastewaters24. Understanding processes of microbial community succession, and metabolic interdependencies between species is also vastly easier at low levels of microbial community diversity25 (although increasing metagenomic sequencing breadth and depth can assist for more diverse communities; cf Wrighton et al.26), and both are emerging fields of fundamental research in mining environments and environments impacted by mining and refining activities. The high concentrations of elements which are on average present at low concentrations in the Earth’s crust, and the lack of organic carbon to support alternative (higher energy yielding) metabolic pathways makes mining environments fertile ground for the discovery of novel metabolic pathways, which at present are only hypothesised by theoretical bioenergetic calculations for these reactions.
Already, insights from the geomicrobiology of mining environments have improved our understanding of the Earth’s geological past and likely future, as well as supporting advances in industrial capabilities across sectors as diverse as food processing and preservation, agriculture, mineral processing, astrobiology, pharmaceuticals, and human health. Given that this article is focussed on mining environments, we will provide a couple of examples from our research groups on application of these insights to the remediation of mining environments, for two of Australia’s largest mineral commodities, iron ore and bauxite (aluminium ore).
Accelerating iron cementation for iron ore mine site remediation
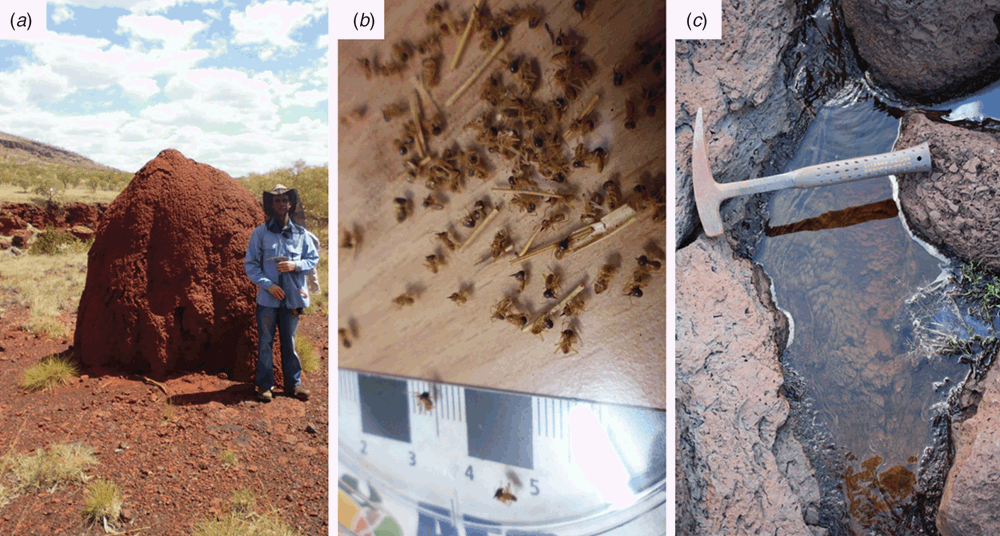
In tropical areas, iron ore that has been formed by the long-term weathering of banded iron formations (BIFs) is often capped by a hard, well-consolidated iron duricrust that hosts a unique plant ecosystem adapted to survive only in the harsh duricrust (background, Figure 2a). The iron duricrust exists as an extensive blanket covering the relatively soft iron ore below, and because it is extremely resistant to erosion, it often defines the landscape in these regions as ridges and plateaus. The duricrust itself is a ferricrete comprised of fragments of iron ore and BIF cemented together by goethite. Effective post-mining rehabilitation strategies of these iron ore areas relies on re-formation of the duricrust, which to date has not been achieved due to a lack of understanding about how the duricrust formed, and therefore how to re-establish it. Geochemical and microbial fossil evidence suggests that biological cycling of iron has contributed to the evolution of the duricrusts throughout geologic history, particularly the dissolution and reprecipitation of goethite27,28; thus, potentially, present-day biological iron cycling could be harnessed to ‘re-form’ this duricrust on a shorter time scale. Exploring the microbiomes associated with iron duricrusts has revealed that lakes, ponds and puddles perched on the duricrust are a source of both iron oxidising and iron reducing microorganisms (Figure 2c), probably working in tandem under natural conditions and actively cycling iron. The gut of termites that penetrate into the duricrust (Figure 2b) and build their nests on and in it has also proven to be a novel source of microorganisms capable of reducing some of the more crystalline iron oxides in the duricrust effectively in consortia via fermentation (unpublished data). Given the novelty of this process and its potential application biotechnologically, metagenomic approaches are being used to reconstruct the main genomes from this consortia and elucidate the mechanisms of goethite reduction. Field-scale trials using microorganisms from the iron duricrust associated ecosystems are also currently underway to test the concept of ’re-forming’ the duricrust through accelerated biological iron cycling.
Neutralising pH in alkaline alumina refining (bauxite) residue for tailings remediation
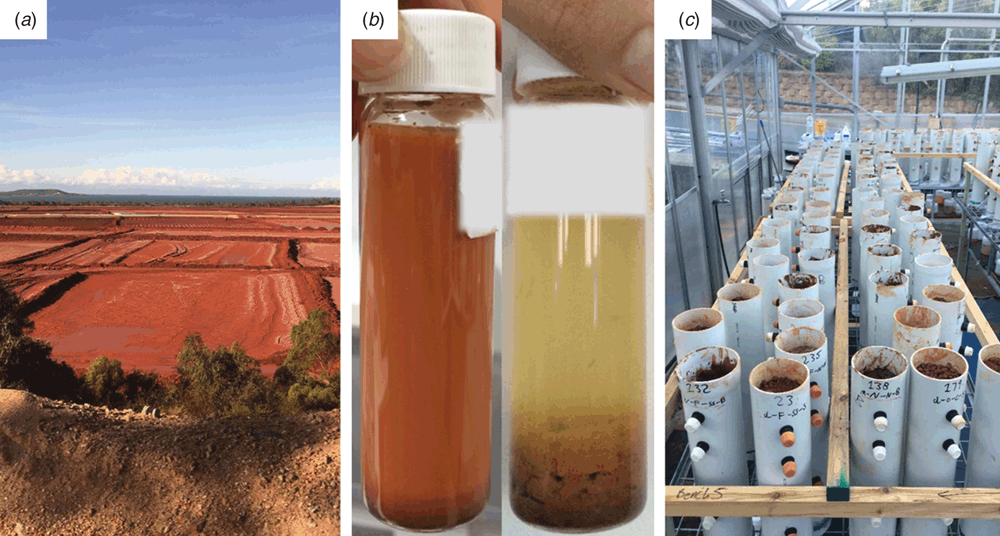
Aluminium is produced from bauxite (aluminium ore) by a two stage process, involving an alkaline hydrothermal digest (Bayer process) to release aluminium as aluminate, which is then precipitated as alumina (Al2O3), followed by an electrolysis step to recover pure aluminium metal from the alumina. The tailings produced in the first step are known as bauxite residue, and are typically discharged into tailings storage facilities (Figure 3a) at pH 11–13. One of the key goals of tailings remediation is to decrease pH to values ≤ 8.5–9. Previous work focussed on addition of chemical amendments to achieve this: carbon dioxide (atmospheric, or process-derived); weak acids; and seawater. These amendments are expensive and often most effective when completed prior to tailings discharge, making remediation of existing tailings storage areas difficult. Field work across bauxite residue storage facilities up to 40 years old suggested that microbial fermentation of organic carbon (driven by Firmicutes, a dominant phylum in bauxite residue communities) was likely playing an important, but neglected, role in neutralising pH11. Building on insights from field work characterising the structure and function of microbial communities in bauxite residues before, during, and after remediation, our research group has now developed microbially driven approaches for pH neutralisation in bauxite residue that will enable remediation of both existing and future alkaline tailings and wastewater streams29,30. These approaches have been successful at laboratory (Figure 3b) and glasshouse scale (Figure 3c), and in early 2018, will be tested in an industry-first field scale trial in Western Australia.
In summary, mining environments present unusually harsh conditions for biology with their extremes of pH, salinity, metals concentrations, and nutrient availability. However, microbial communities still thrive; and in many cases, often drive geochemical cycling under these conditions. The consequences of this can be negative (e.g. acid mine drainage, mobilisation of heavy metals) or positive (e.g. fermentation to neutralise alkaline wastes, iron cycling to stabilise and re-form surface duricrusts). With the rapidly expanding mining sector, it is important that we as microbiologists continue to strive to better understand the role of microbes in geochemical cycling in these natural and anthropogenically generated systems, and to seize opportunities to harness the novel microbial potential available to us from these unique ecosystems. This will not only expand our understanding of microbial diversity, evolution, and functional capacity, but enable us to contribute to solving some of the most urgent challenges facing the mining industry, by developing new microbially driven technologies for ore extraction, ore processing, and environmental rehabilitation. This will become even more important as the mining industry continues to explore unconventional resources such as deep seafloor and sub-seafloor deposits, and new modes of extraction such as in situ leaching.
References
[1] Northey, S. et al. (2014) Modelling future copper ore grade decline based on a detailed assessment of copper resources and mining. Resour. Conserv. Recycling 83, 190–201.| Modelling future copper ore grade decline based on a detailed assessment of copper resources and mining.Crossref | GoogleScholarGoogle Scholar |
[2] Yellishetty, M. et al. (2010) Iron ore and steel production trends and material flows in the world: is this really sustainable? Resour. Conserv. Recycling 54, 1084–1094.
| Iron ore and steel production trends and material flows in the world: is this really sustainable?Crossref | GoogleScholarGoogle Scholar |
[3] Nappi, C. (2013) The global aluminium industry 40 years from 1972. International Aluminium Institute.
[4] Humphries, M. (2012) Rare earth elements: the global supply chain. USA Congressional Research Service.
[5] Alonso, E. et al. (2012) Evaluating rare earth element availability: a case with revolutionary demand from clean technologies. Environ. Sci. Technol. 46, 3406–3414.
| Evaluating rare earth element availability: a case with revolutionary demand from clean technologies.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38Xhs1Kiurw%3D&md5=580d11845a045718ed6075ac913b36f0CAS |
[6] Hooke, R.L. et al. (2012) Land transformation by humans: a review. GSA Today 22, 4–10.
| Land transformation by humans: a review.Crossref | GoogleScholarGoogle Scholar |
[7] Hossner, L.R. and Hons, F.M. (1992) Reclamation of mine wastes. In Advances in Soil Science: Soil Restoration (Lal, R., Stewart, B.A., eds), pp. 311–350, Springer-Verlag.
[8] Baker, B.J. and Banfield, J.F. (2003) Microbial communities in acid mine drainage. FEMS Microbiol. Ecol. 44, 139–152.
| Microbial communities in acid mine drainage.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXjtFWku7c%3D&md5=d929019ff2057768ea84ecf165d945b4CAS |
[9] van der Gast, C. et al. (2003) Bacterial community structure and function in a metal-working fluid. Environ. Microbiol. 5, 453–461.
| Bacterial community structure and function in a metal-working fluid.Crossref | GoogleScholarGoogle Scholar |
[10] Mendez, M.O. et al. (2008) Characterisation of a bacterial community in an abandoned semi-arid lead-zinc mine tailing site. Appl. Environ. Microbiol. 74, 3899–3907.
| Characterisation of a bacterial community in an abandoned semi-arid lead-zinc mine tailing site.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXnvFWltr4%3D&md5=2778fe4bcaf7a2d61e0f493b62c19011CAS |
[11] Santini, T.C. et al. (2015) Microbial diversity in engineered haloalkaline environments shaped by shared geochemical drivers observed in natural analogues. Appl. Environ. Microbiol. 81, 5026–5036.
| Microbial diversity in engineered haloalkaline environments shaped by shared geochemical drivers observed in natural analogues.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXht1Ojt7jL&md5=afbc699c5e85bd657d73fb89654e52c1CAS |
[12] Johnson, D.B. (2003) Chemical and microbiological characteristics of mineral spoils and drainage waters at abandoned coal and metal mines. Water Air Soil Pollut. 3, 47–66.
| Chemical and microbiological characteristics of mineral spoils and drainage waters at abandoned coal and metal mines.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXisVKgs7o%3D&md5=36dc19acb8024123b92bce6db99fde60CAS |
[13] Krafft, T. and Macy, J.M. (1998) Purification and characterisation of the respiratory arsenate reductase of Chrysiogenes arsenatis. Eur. J. Biochem. 255, 647–653.
| Purification and characterisation of the respiratory arsenate reductase of Chrysiogenes arsenatis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXmtlyrur8%3D&md5=6039837ef21826609bc4e7ce5a01a1c9CAS |
[14] Shuster, J. et al. (2017) Biogeochemical cycling of silver in acidic weathering environments. Minerals (Basel) 7, 218.
| Biogeochemical cycling of silver in acidic weathering environments.Crossref | GoogleScholarGoogle Scholar |
[15] Reith, F. et al. (2006) Biomineralisation of gold: biofilms on bacterioform gold. Science 313, 233–236.
| Biomineralisation of gold: biofilms on bacterioform gold.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xmslansrw%3D&md5=a2b2897c43b74678f6ce48ede3e7c866CAS |
[16] Corbett, M.K. et al. (2017) Incorporation of indigenous microorganisms increases leaching rates of rare earth elements from Western Australian monazite. Sol. St. Phen. 262, 294–298.
| Incorporation of indigenous microorganisms increases leaching rates of rare earth elements from Western Australian monazite.Crossref | GoogleScholarGoogle Scholar |
[17] Watts, M.P. et al. (2017) In situ stimulation of thiocyanate biodegradation through phosphate amendment in gold mine tailings. Environ. Sci. Technol. 51, 13353–13362.
| In situ stimulation of thiocyanate biodegradation through phosphate amendment in gold mine tailings.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2sXhslSgsL3L&md5=41ce38775f1b620f04c6befa21cc69b6CAS |
[18] Zammit, C.M. et al. (2012) Bioleaching in brackish waters – effect of chloride ions on the acidophile population and proteomes of model species. Appl. Microbiol. Biotechnol. 93, 319–329.
| Bioleaching in brackish waters – effect of chloride ions on the acidophile population and proteomes of model species.Crossref | GoogleScholarGoogle Scholar |
[19] Zammit, C.M. et al. (2016) Proteomic responses to gold (III) toxicity in the bacterium Cupriavidus metallidurans CH34. Metallomics 8, 1204–1216.
| Proteomic responses to gold (III) toxicity in the bacterium Cupriavidus metallidurans CH34.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC28Xhs1Clu7rP&md5=2a378c1cf265c45dda39e00f58051513CAS |
[20] Reith, F. et al. (2010) Nanoparticle factories: biofilms hold the key to gold dispersion and nugget formation. Geology 38, 843–846.
| Nanoparticle factories: biofilms hold the key to gold dispersion and nugget formation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXksFShsr0%3D&md5=db92de1daf6a20feab0d35a52a482090CAS |
[21] Bonis, B.M. and Gralnick, J.A. (2015) Marinobacter subterrani, a genetically tractable neutrophilic Fe(II)-oxidizing strain isolated from the Soudan Iron Mine. Front. Microbiol. 6, 719.
| Marinobacter subterrani, a genetically tractable neutrophilic Fe(II)-oxidizing strain isolated from the Soudan Iron Mine.Crossref | GoogleScholarGoogle Scholar |
[22] Travisany, D. et al. (2014) A new genome of Acidithiobacillus thiooxidans provides insights into adaptation to a bioleaching environment. Res. Microbiol. 165, 743–752.
| A new genome of Acidithiobacillus thiooxidans provides insights into adaptation to a bioleaching environment.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXhsV2ltrjJ&md5=6c6f1d6bde4ff7bc6e141ffd1b068594CAS |
[23] García-García, J.D. et al. (2016) Bio-recovery of non-essential heavy metals by intra- and extracellular mechanisms in free-living microorganisms. Biotechnol. Adv. 34, 859–873.
| Bio-recovery of non-essential heavy metals by intra- and extracellular mechanisms in free-living microorganisms.Crossref | GoogleScholarGoogle Scholar |
[24] Tyson, G.W. et al. (2004) Community structure and metabolism through reconstruction of microbial genomes from the environment. Nature 428, 37–43.
| Community structure and metabolism through reconstruction of microbial genomes from the environment.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXhslCns70%3D&md5=f18dcb7c4f8a08c29dd72e38e864e1c7CAS |
[25] Anantharaman, K. et al. (2016) Thousands of microbial genomes shed light on interconnected biogeochemical processes in an aquifer system. Nat. Commun. 7, 13219.
| Thousands of microbial genomes shed light on interconnected biogeochemical processes in an aquifer system.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC28XhslGrt77L&md5=4a13e4bd159ef5401f3f24272513badfCAS |
[26] Wrighton, K.C. et al. (2014) Metabolic interdependencies between phylogenetically novel fermenters and respiratory organisms in an unconfined aquifer. ISME J. 8, 1452–1463.
| Metabolic interdependencies between phylogenetically novel fermenters and respiratory organisms in an unconfined aquifer.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXhtVKmtrnP&md5=a72e58219c7f0f90ed3dc971136a38c9CAS |
[27] Monteiro, H.S. et al. (2014) (U-Th)/He geochronology of goethite and the origin and evolution of cangas. Geochim. Cosmochim. Acta 131, 267–289.
| (U-Th)/He geochronology of goethite and the origin and evolution of cangas.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXks1KgtLs%3D&md5=38783f1790a12d14149cfed3759e373dCAS |
[28] Levett, A. et al. (2016) Evidence of biogeochemical processes in iron duricrust formation. J. S. Am. Earth Sci. 71, 131–142.
| Evidence of biogeochemical processes in iron duricrust formation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC28Xht1SltrfF&md5=b96d7143bd509893f2e07b3dc41113f0CAS |
[29] Santini, T.C. et al. (2016) pH and organic carbon dose rates control microbially driven bioremediation efficacy in alkaline bauxite residue. Environ. Sci. Technol. 50, 11164–11173.
| pH and organic carbon dose rates control microbially driven bioremediation efficacy in alkaline bauxite residue.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC28XhsFKlsbnL&md5=bd47949ccb588c9d3b381456d56b3c51CAS |
[30] Santini, T.C. and Peng, Y.G. (2017) Microbial fermentation of organic carbon substrates drives rapid pH neutralisation and element removal in bauxite residue leachate. Environ. Sci. Technol. 51, 12592–12601.
| Microbial fermentation of organic carbon substrates drives rapid pH neutralisation and element removal in bauxite residue leachate.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2sXhs1CqtLjJ&md5=7ae211e48eb18406c9210e76951a4a4cCAS |
Biographies
Talitha Santini is a Senior Lecturer in the School of Earth and Environmental Sciences at the University of Queensland, where she leads a research group in microbial ecology and biogeochemistry. A major focus of this group is understanding the links between biotic and abiotic mineral weathering and nutrient cycling processes. Much of Dr Santini’s research work is supported by industry partners, which include Alcoa, South32, BHP, Rio Tinto, Rusal and Newmont.
Emma Gagen is a Postdoctoral Research Fellow in geomicrobiology in the School of Earth and Environmental Sciences at the University of Queensland. Her primary research project focuses on the role microorganisms play in iron cycling in geologic systems and is largely supported by Vale S.A. Other projects she contributes to relate to microbial colonisation of meteorites, bacterial degradation of anhydrite, microbiology of sulphur-rich environments and formation of seafloor iron-manganese crusts. Emma’s research interests extend to all areas of environmental microbiology and she is fascinated by the role microorganisms play in geochemical processes.