Zoonotic tissue parasites of Australian wildlife
David M SprattAustralian National Wildlife Collection
National Research Collections Australia, CSIRO
GPO Box 1700
Canberra, ACT 2601, Australia
Tel: +61 2 6242 1648
Email: dave.spratt@csiro.au
Microbiology Australia 37(1) 12-14 https://doi.org/10.1071/MA16005
Published: 11 February 2016
Increasing use of bushlands for recreational, commercial and scientific activities fosters movement across the urban-bushland interface. This may facilitate the transmission of parasitic diseases from wildlife to humans (zoonoses). The fashionable trend to consumption of game meats such as feral pig and crocodile, and raw fish such as sushi, sashimi and pickled herring has exacerbated the zoonotic potential of parasites of wildlife.
Transmission from wildlife to humans
Angiostrongyliasis
Angiostrongylus cantonensis is a nematode parasite of the pulmonary arteries and right ventricle of Rattus rattus and R. norvegicus in Australia1. It is the causative agent of eosinophilic meningoencephalitis, a zoonotic infection of humans. The life cycle includes an obligatory period of larval development in terrestrial or aquatic snails and slugs, and also may involve a range of paratenic or transport hosts (freshwater prawns, land crabs, planarians, frogs, lizards), which feed on gastropods. Rats become infected by ingesting intermediate or paratenic hosts. In the rat, the nematode undergoes an obligatory migration through the spinal column and brain en route to the final site in the pulmonary arteries of the lungs. Humans become infected by accidentally or deliberately eating infected gastropods or paratenic hosts, or unwashed salad greens containing these. The parasite has been reported from domestic and zoo animals, mammalian and avian wildlife and humans in Brisbane and Sydney2–4. The clinical signs of headache, vomiting, paralysis and sometimes death are induced as a consequence of the obligatory period of development of the parasite in the central nervous system. This occurs in young children who deliberately or accidentally ingest snails or slugs containing infective larvae5–7, or foolish young adults who do so for a bet8,9.
Muspiceoidosis
Haycocknema perplexum is a minute muspiceoid nematode living as adults inside individual skeletal muscle cells of humans in Australia10. Eight cases have been documented, 4 in Tasmania and 4 in north Queensland11–13 Gasser (personal communication). Eight to twelve eggs hatch inside the uterus of the female, develop to third-stage infective larvae and burst from the head region killing the adult, an efficient mechanism for auto-re-infection. Escaped larvae invade uninfected muscle cells. The occurrence of H. perplexum in intramyofibres results in eosinophilic polymyositis but no reaction within the invaded cell itself11. Progressive myopathy occurs and infection becomes life threatening. Early human diagnosis by muscle biopsy is imperative in cases of progressive myopathy associated with blood eosinophilia and elevated creatine kinase levels. Steroid treatment of patients exacerbates their infections to a life-threatening illness and may delay diagnosis by masking key diagnostic features. Treatment with albendazole, 400 mg twice daily for 8–9 weeks, is recommended12. Haycocknema perplexum is considered a zoonosis although the source of infection of humans – water, soil, plants or animals – remains unknown.
Halicephalobus gingivalis
Halicephalobus gingivalis formerly known as Micronema deletrix, is a free-living nematode of soil, manure and decaying humus known to cause opportunistic infections, primarily in horses but also in humans14. The majority of cases in horses have been fatal and usually not diagnosed before necropsy. All human cases have involved fatal meningoencephalitis including the first human case in Australia, a 74-year-old woman from Eyre Penninsula, South Australia15. In tissues, only ova, larvae and adult females are seen, reproduction in the parasitic phase presumed to be by parthenogenesis16. It is not known how H. gingivalis infects humans or horses although exposure through an oromaxillary route may explain common neurological involvement15.
Transmission from domestic animals to humans and wildlife
Toxoplasmosis
Felids, domestic cats in particular, are the only definitive host of the obligate intracellular protozoan parasite, Toxoplasma gondii17. Most species of mammals and birds are susceptible to infection and may act as intermediate hosts. Infection is usually systemic resulting in a short period of rapid multiplication in various tissues followed by the establishment of tissue cysts in the muscles and brain. These are transmitted only if ingested by predation or scavenging or if passed vertically across the placenta from mother to foetus.
The localisation of T. gondii cysts in the forebrain of rats and mice together with the immune reaction to the cysts is related to altered behaviour, in particular the attenuation of predator odour aversion and anxiety. This facilitates ingestion of the intermediate host by the cat definitive host and perpetuation of the life cycle17.
Humans become infected with T. gondii mainly by ingesting uncooked meat containing viable tissue cysts or by ingesting food or water contaminated with oocysts from the faeces of infected cats. Transplacental transmission may occur in women during their first trimester of pregnancy and this infection is then passed to the developing embryo via the placenta. This frequently results in severe brain and eye lesions in the newborn or death of the developing embryo. Research results from the past 15 years have shown that T. gondii infection is associated with several neuropsychiatric diseases and behavioural changes in humans as well animals18. Although the mechanisms are unknown a growing body of data indicates that they are complex, comprising humoral, immune, neurotransmitter, epigenetic, genetic, and structural effects19.
Echinococcosis (hydatid disease)
Australia has, on average, >80 new cases of human hydatidosis per annum caused by the larval stage of the cestode, Echinococcus granulosus20. The parasite was introduced into Australia with domestic livestock and dogs. However, a cycle in wildlife is maintained through a predator/prey interaction between dingoes, wild dogs and less importantly foxes and kangaroos and wallabies, less importantly feral pigs (Sus scrofa)21,22. The establishment of a dingo/wild dog-macropod cycle, which effectively maintains parasite transmission, acts as a spill-back reservoir of infection for sheep and cattle23. This is a major problem for control strategies focussed on human education and husbandry practices to break the domestic ‘dog-sheep’ cycle. In contrast to the situation in livestock, hydatid cysts occur primarily in the lungs rather than the liver of marsupials. Infection occurs predominantly in the eastern States but the parasite has established recently in wildlife in water catchment and forestry areas outside Perth21. Western grey kangaroos and feral pigs act as intermediate hosts. This focus of transmission may have been initiated through E. granulosus-infected domestic pig hunting dogs from the eastern states. Transmission appears to be perpetuated by dogs of local pig hunters infected through being fed the offal of locally shot kangaroos21. Hydatid disease is also an important conservation issue, especially for small endangered species and populations of Macropodidae by severely reducing effective lung volume24,25. Such reductions impact the fitness of the animals enhancing susceptibility to predation, thus ensuring perpetuation of the cycle.
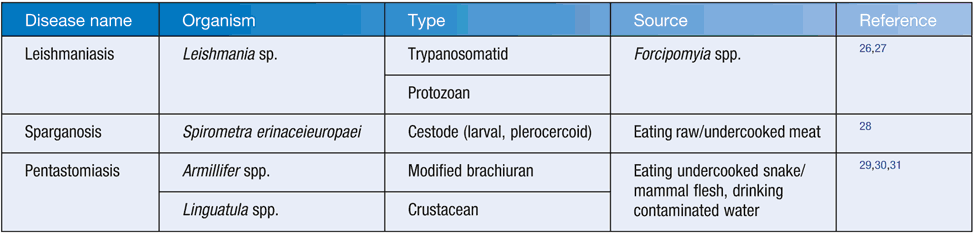
Several potential zoonotic tissue parasite infections in Australia are listed in Table 1.
|
References
[1] Mackerras, M.J. and Sandars, D.F. (1955) The life history of the rat lung-worm, Angiostrongylus cantonensis (Chen) (Nematoda: Metastrongylidae). Aust. J. Zool. 3, 1–21.| The life history of the rat lung-worm, Angiostrongylus cantonensis (Chen) (Nematoda: Metastrongylidae).Crossref | GoogleScholarGoogle Scholar |
[2] Spratt, D.M. (2005)a Australian ecosystems, capricious food chains and parasitic consequences for people. Int. J. Parasitol. 35, 717–724.
| Australian ecosystems, capricious food chains and parasitic consequences for people.Crossref | GoogleScholarGoogle Scholar | 15925595PubMed |
[3] Spratt, D.M. (2005)b Neuroangiostrongyliasis: disease in wildlife and humans. Microbiol. Aust. 26, 63–64.
[4] Ma, G. (2013) Tawny frogmouths and brushtail possums as sentinels for Angiostrongylus cantonensis, the rat lungworm. Vet. Parasitol. 192, 158–165.
| Tawny frogmouths and brushtail possums as sentinels for Angiostrongylus cantonensis, the rat lungworm.Crossref | GoogleScholarGoogle Scholar | 23218219PubMed |
[5] Prociv, P. and Tiernan, J.R. (1987) Eosinophilic meningoencephalitis with permanent sequelae. Med. J. Aust. 147, 294–295.
| 1:STN:280:DyaL2szhsVKhtQ%3D%3D&md5=ecaaae67f1259025d30a3745e8bd6cbbCAS | 3626948PubMed |
[6] Prociv, P. et al. (2000) Neuro-angiostrongyliasis: unresolved issues. Int. J. Parasitol. 30, 1295–1303.
| Neuro-angiostrongyliasis: unresolved issues.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3MzgsVSqtA%3D%3D&md5=545a40a042dcefa5a2108da8dcda7efbCAS | 11113256PubMed |
[7] Morton, N.J. et al. (2013) Severe haemorrhagic meningoencephalitis due to Angiostrongylus cantonensis among young children in Sydney, Australia. Clin. Infect. Dis. 57, 1158–1161.
| Severe haemorrhagic meningoencephalitis due to Angiostrongylus cantonensis among young children in Sydney, Australia.Crossref | GoogleScholarGoogle Scholar | 23843445PubMed |
[8] Senanayake, S.N. (2003) First case of human angiostrongyliasis acquired in Sydney. Med. J. Aust. 179, 430–431.
| 14558868PubMed |
[9] Blair, N.F. et al. (2013) Angiostrongylus meningoencephalitis: survival from minimally conscious state to rehabilitation. Med. J. Aust. 198, 440–442.
| Angiostrongylus meningoencephalitis: survival from minimally conscious state to rehabilitation.Crossref | GoogleScholarGoogle Scholar | 23641996PubMed |
[10] Spratt, D.M. et al. (1999) Haycocknema perplexum n.g., n. sp. (Nematoda: Robertdollfusidae): an intramyofibre parasite in man. Syst. Parasitol. 43, 123–131.
| Haycocknema perplexum n.g., n. sp. (Nematoda: Robertdollfusidae): an intramyofibre parasite in man.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3c%2FosFOrsA%3D%3D&md5=6a19ccd1d6de84bd1bdb83bf24fe3725CAS | 10619062PubMed |
[11] Dennett, X. et al. (1998) Polymyositis caused by a new nematode. Med. J. Aust. 168, 226–227.
| 1:STN:280:DyaK1c3gtVOhsw%3D%3D&md5=7e97862a2f0c7d0bab004085f4ee65eeCAS | 9539901PubMed |
[12] Basuroy, R. et al. (2008) Parasitic myositis in tropical Australia. Med. J. Aust. 188, 254–256.
| 18279140PubMed |
[13] McKelvie, P. et al. (2013) A further patient with parasitic myositis due to Haycocknema perplexum, a rare entity. J. Clin. Neurosci. 20, 1019–1022.
| A further patient with parasitic myositis due to Haycocknema perplexum, a rare entity.Crossref | GoogleScholarGoogle Scholar | 23664131PubMed |
[14] Anderson, R.C. et al. (1998) Halicephalobus gingivalis (Stefanski, 1954) from a fatal infection of a horse in Ontario, Canada with comments on the validity of H. deletrix and a review of the genus. Parasite 5, 255–261.
| Halicephalobus gingivalis (Stefanski, 1954) from a fatal infection of a horse in Ontario, Canada with comments on the validity of H. deletrix and a review of the genus.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK1cvksF2jsA%3D%3D&md5=9b8d01fb8aa8ebeb234564e2f1b5d45bCAS | 9772725PubMed |
[15] Lim, C.K. et al. (2015) First human case of fatal Halicephalobus gingivalis meningoencephalitis in Australia. J. Clin. Microbiol. 53, 1768–1774.
| First human case of fatal Halicephalobus gingivalis meningoencephalitis in Australia.Crossref | GoogleScholarGoogle Scholar | 25694532PubMed |
[16] Blunden, A.S. et al. (1987) Halicephalobus deletrix infection in a horse. Equine Vet. J. 19, 255–260.
| Halicephalobus deletrix infection in a horse.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaL2s3nsFOqtA%3D%3D&md5=3b34cd3a9c6356e4affe0db36220c2e2CAS | 3608968PubMed |
[17] Dubey, J.P. et al. (1998) Structures of Toxoplasma gondii tachyzoites, bradyzoites, and sporozoites and biology and development of tissue cysts. Clin. Microbiol. Rev. 11, 267–299.
| 1:STN:280:DyaK1c3isFOmsw%3D%3D&md5=3e8b4dc9820a45ea532d967e91f0718aCAS | 9564564PubMed |
[18] Evans, A.K. (2014) Patterns of Toxoplasma gondii cyst distribution in the forebrain associated with individual variation in predator odor avoidance and anxiety-related behavior in male Long-Evans rats. Brain Behav. Immun. 37, 122–133.
| Patterns of Toxoplasma gondii cyst distribution in the forebrain associated with individual variation in predator odor avoidance and anxiety-related behavior in male Long-Evans rats.Crossref | GoogleScholarGoogle Scholar | 24269877PubMed |
[19] Hinze-Selch, D. (2015) Toxoplasma gondii infection and neuropsychiatric disease: current insight. Rep. Parasitol. 4, 43–51.
| Toxoplasma gondii infection and neuropsychiatric disease: current insight.Crossref | GoogleScholarGoogle Scholar |
[20] Thompson, R.C.A. and McManus, D.P. (2002) Towards a taxonomic revision of the genus Echinococcus. Trends Parasitol. 18, 452–457.
| Towards a taxonomic revision of the genus Echinococcus.Crossref | GoogleScholarGoogle Scholar |
[21] Thompson, R.C.A. et al. (1988) Hydatid disease in urban areas of Western Australia: an unusual cycle involving western grey kangaroos (Macropus fuliginosus), feral pigs and domestic dogs. Aust. Vet. J. 65, 188–190.
| Hydatid disease in urban areas of Western Australia: an unusual cycle involving western grey kangaroos (Macropus fuliginosus), feral pigs and domestic dogs.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaL1czis1WltA%3D%3D&md5=ece31fc9fdfd6c72e26a532ca047b6dbCAS |
[22] Jenkins, D.J. and Morris, B. (2003) Echinococcus granulosus in and around the Kosciuszko National Park, south-eastern Australia. Aust. Vet. J. 81, 81–85.
| Echinococcus granulosus in and around the Kosciuszko National Park, south-eastern Australia.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD2c7ovVSlsA%3D%3D&md5=3cfd344f57159d2127c59d33b4d44c5dCAS | 15084018PubMed |
[23] Durie, P.H. and Riek, R.F. (1952) The role of the dingo and wallaby in the infestation of cattle with hydatids (Echinococcus granulosus (Batsch, 1786) Rudolphi, 1805) in Queensland. Aust. Vet. J. 28, 249–254.
| The role of the dingo and wallaby in the infestation of cattle with hydatids (Echinococcus granulosus (Batsch, 1786) Rudolphi, 1805) in Queensland.Crossref | GoogleScholarGoogle Scholar |
[24] Johnson, P.M. et al. (1998) Mortality in wild and captive rock wallabies and nailtail wallabies due to hydatid disease caused by Echinococcus granulosus. Aust. Mammal. 20, 419–423.
[25] Barnes, T.S. et al. (2008) Cystic echinococcosis in a wild population of the brush-tailed rock-wallaby (Petrogale penicillata), a threatened macropodid. Parasitology 135, 715–723.
| Cystic echinococcosis in a wild population of the brush-tailed rock-wallaby (Petrogale penicillata), a threatened macropodid.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD1czjvVarsA%3D%3D&md5=3d0b38898bb664144295103ab9558e3fCAS | 18442430PubMed |
[26] Rose, K. (2004) Cutaneous leishmaniasis in red kangaroos: isolation and characterisation of the causative organisms. Int. J. Parasitol. 34, 655–664.
| Cutaneous leishmaniasis in red kangaroos: isolation and characterisation of the causative organisms.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXjsVWgtrg%3D&md5=bdadb5d5ffc1274c31a18c8dfc63ae11CAS | 15111087PubMed |
[27] Dougall, A.M. et al. (2011) Evidence incriminating midges (Diptera: Ceratopogonidae) as potential vectors of Leishmania in Australia. Int. J. Parasitol. 41, 571–579.
| Evidence incriminating midges (Diptera: Ceratopogonidae) as potential vectors of Leishmania in Australia.Crossref | GoogleScholarGoogle Scholar | 21251914PubMed |
[28] Liu, Q. (2015) Human sparganosis, a neglected food borne zoonosis. Lancet Infect. Dis. 15, 1226–1235.
| Human sparganosis, a neglected food borne zoonosis.Crossref | GoogleScholarGoogle Scholar | 26364132PubMed |
[29] Paré, J.A. (2008) An overview of Pentastomiasis in reptiles and other vertebrates. J. Exot. Pet Med. 17, 285–294.
| An overview of Pentastomiasis in reptiles and other vertebrates.Crossref | GoogleScholarGoogle Scholar |
[30] Kelehear, C. et al. (2014) Pentastomids of wild snakes in the Australian tropics. Intl. J. Parasitol.: Para. Wldlfe. 3, 20–31.
[31] Drabick, J.J. (1987) Pentastomiasis. Rev. Infect. Dis. 9, 1087–1094.
| Pentastomiasis.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaL1c%2FpvFShtQ%3D%3D&md5=9159c40040374fb4ce3c7bc85ac6f255CAS | 3321358PubMed |
Biography
Dave Spratt is an Honorary Fellow at the Australian National Wildlife Collection, National Research Collections Australia, CSIRO, in Canberra. The major themes of Dr Spratt’s research are the study and understanding of the diseases of wildlife including disease ecology, parasite taxonomy, helminth biodiversity and zoonoses. Research topics of interest include metastrongyloid, filarioid, trichinelloid and muspiceoid nematodes, pentastomes, and small mammal succession and recolonisation of their helminth communities following wildfire.


