Current WHO protocols for mass drug administration in helminth control
Richard S Bradbury A and Patricia M Graves BA School of Medical and Applied Sciences
Central Queensland University
Rockhampton, Qld, Australia
Email: rbradbury@cqu.edu.au
B College of Public Health
Medical and Veterinary Sciences
Division of Tropical Health and Medicine
James Cook University
Cairns Qld, Australia
Email: patricia.graves@jcu.edu.au
Microbiology Australia 37(1) 10-12 https://doi.org/10.1071/MA16004
Published: 12 February 2016
Soil transmitted helminths (STH), comprising Ascaris, Trichuris, Strongyloides and the hookworms remain a significant cause of morbidity amongst people in many parts of the world, including Australia. Other important helminth infections include lymphatic filariasis (LF), schistosomiasis and onchocerciasis. Preventive chemotherapy (mass drug administration [MDA]) campaigns are frequently conducted for these helminth infections in endemic areas, but the target population groups, duration of campaigns, cointerventions (e.g. vector control) criteria for inclusion, drugs used and doses of drugs differ.
The benefits of deworming individuals, especially children, who are infected with soil-transmitted helminths and schistosomiasis, include reduction in anaemia and improved growth. Rarer, but more severe presentations, such as intestinal obstruction with A. lumbricoides and rectal prolapse due to T. trichiura infection, will also be reduced1. Treatment for filariasis and onchcocerciasis in childhood will prevent the development of later severe consequences of these diseases including lymphoedema, hydrocoele, elephantiasis and blindness.
In situations of moderate to high endemicity, it has been considered more efficient and cost-effective to treat the entire eligible population in particular age groups or communities for these diseases, rather than first testing individuals to determine who is infected. The goals of such MDA are morbidity control in some cases and interruption of transmission through vectors in others. For these reasons, MDA for STH is usually undertaken for school age children, while for schistosomiasis, MDA is performed either in children or in eligible people of all ages, depending on the endemicity level. For the insect borne helminths, onchocerciasis and LF, the goal is transmission interruption and the entire community is eligible for MDA.
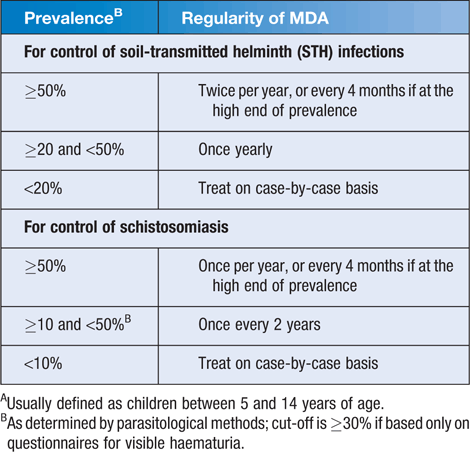
The frequency of MDA for STH in school-age children is dependent on the prevalence of infections in a given population (Table 1). Current WHO protocols for STH control recommend MDA with a single oral dose of albendazole (400 mg), mebendazole (500 mg) or levamisole (80 mg)1. Mebendazole is more effective than albendazole for T. trichiura, whilst albendazole is slightly more effective against hookworm than mebendazole. The two drugs have equally high efficacy when used against Ascaris lumbricoides1. Despite these differences, in practice when administered biennially over a number of years, either drug used on its own is effective for overall STH control1.
|
Onchocerciasis control and/or elimination requires annual or biannual treatment with ivermectin for many years (up to 20 in some cases), while lymphatic filariasis uses annual administration with albendazole and either ivermectin or diethylcarbamazine (DEC) with at least 65% population coverage for at least five years. Thus ivermectin and/or albendazole administration may occur in some communities annually or biannually as part of onchocerciasis or LF elimination programs. Where this occurs, STH control programs should be harmonised to ensure that there is a 6 month delay between albendazole administrations1. In communities with endemic schistosomiasis, the addition of praziquantel (40 mg/kg) is recommended (Table 1). Reductions in the frequency of MDA may be considered after 5–6 years of consistent >75% population coverage, after testing of the residual prevalence of helminths in that population. Such a decision is based on several factors, specific details of which may be found in the World Health Organization guide for managers of control programmes1.
Reliance on albendazole and mebendazole in WHO recommendations for MDA will result in a lower impact on the clearance of Strongyloides stercoralis, for which ivermectin and thiabendazole are more effective drugs2. Thiabendazole was discontinued in Australia in 2003 and due to the lower rate of side effects, ivermectin has been recommended by some as the treatment of choice2. Due to the auto-infective cycle of this helminth, some authors have recommended re-treatment at one and two months to ensure elimination3. Only one randomised trial has been performed thus far, in which treatment twice at 2 weeks apart was found to have no greater benefit than a single dose4. Both the authors of this paper and others2 recommend further studies into the optimal dose schedules for ivermectin in the control of Strongyloidiasis within a larger cohort of participants.
Recently, there has been discussion about the outcomes and optimal age range for MDA programs to control STH and more evidence on the impacts is required5–7; this controversy is outside the scope of this review. A novel concept of elimination of STH by MDA ‘one village at a time’ rather than by wide-scale MDA has been proposed for remote areas with low populations, such as many remote Australian Aboriginal communities. This approach is currently being trialled in a remote area of the Solomon Islands8. It advocates allowing individual communities to act as autonomous units and to employ control options specifically tailored to the geographic, cultural, economic, aetiological and environmental factors influencing STH transmission in their own community8 (Figure 1).

|
Deworming of school-age children has been a mainstay of helminth control for many years. Discussion continues on the optimal method of MDA for this purpose. In some cases, such as where high rates of strongyloidiasis or onchocerciasis are present, the addition of other drugs may be warranted. Further consideration of new therapies, combination therapies, the reconsideration of use of the use of ‘old’ anti-helminthic therapies have all been postulated as mechanisms by which to improve absolute cure rates in MDA programs and to reduce the possible development of antihelminthic resistance9. Ways to improve MDA participation, as well as paediatric formulations of praziquantel for schistosomiasis prevention in preschool children may also be added to this list. The current WHO protocols for MDA provide an important baseline guide to those undertaking MDA for helminth control in endemic areas.
References
[1] World Health Organization (2011) Helminth control in school-age children: a guide for managers of control programmes. 2nd edn. Geneva: World Health Organization.[2] Bisoffi, Z. et al. (2011) Randomized clinical trial on ivermectin versus thiabendazole for the treatment of strongyloidiasis. PLoS Negl. Trop. Dis. 5, e1254.
| Randomized clinical trial on ivermectin versus thiabendazole for the treatment of strongyloidiasis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhtVGqsLrN&md5=3e5ac7643521e9c62bfd937f1bc5ba85CAS | 21814588PubMed |
[3] Adams, M. et al. (2003) Strongyloidiasis: an issue in Aboriginal communities. Rural Remote Health 3, 152.
| 1:STN:280:DC%2BD2M3kt1SmsA%3D%3D&md5=a788a19b1ec1aab8b1f64cadedb5d9a2CAS | 15877491PubMed |
[4] Suputtamongkol, Y. et al. (2011) Efficacy and safety of single and double doses of ivermectin versus 7-day high dose albendazole for chronic strongyloidiasis. PLoS Negl. Trop. Dis. 5, e1044.
| Efficacy and safety of single and double doses of ivermectin versus 7-day high dose albendazole for chronic strongyloidiasis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXmtFKhu70%3D&md5=62dbc34dcee4722316414477a7845060CAS | 21572981PubMed |
[5] Anderson, R.M. et al. (2015) Should the goal for the treatment of soil transmitted Helminth (STH) infections be changed from morbidity control in children to community-wide transmission elimination? PLoS Negl. Trop. Dis. 9, e0003897.
| Should the goal for the treatment of soil transmitted Helminth (STH) infections be changed from morbidity control in children to community-wide transmission elimination?Crossref | GoogleScholarGoogle Scholar | 26291538PubMed |
[6] Taylor-Robinson, D.C. et al. (2015) Deworming drugs for soil‐transmitted intestinal worms in children: effects on nutritional indicators, haemoglobin and school performance. Cochrane Database Syst. Rev. 7, CD000371.
| Deworming drugs for soil‐transmitted intestinal worms in children: effects on nutritional indicators, haemoglobin and school performance.Crossref | GoogleScholarGoogle Scholar | 26202783PubMed |
[7] Hicks, J.H. et al. (2015) The case for mass treatment of intestinal helminths in endemic areas PLoS Negl. Trop. Dis. 9, e0004214.
| The case for mass treatment of intestinal helminths in endemic areasCrossref | GoogleScholarGoogle Scholar | 26492528PubMed |
[8] Harrington, H. et al. (2015) Prevalence of soil transmitted helminths in remote villages in East Kwaio, Solomon Islands. Western Pac. Surveill. Response J. 6, 1–8.
| Prevalence of soil transmitted helminths in remote villages in East Kwaio, Solomon Islands.Crossref | GoogleScholarGoogle Scholar |
[9] Savioli, L. (2014) Preventive anthelmintic chemotherapy – expanding the armamentarium. N. Engl. J. Med. 370, 665–666.
| Preventive anthelmintic chemotherapy – expanding the armamentarium.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXis1eqtL4%3D&md5=c1c63b897c0d180184d5c9cd6b00217cCAS | 24521114PubMed |
Biographies
Dr Richard Bradbury is an Australian Parasitologist with an interest in all fields of parasitology. He was recently appointed as the Team Lead in the Parasite Diagnostics and Biology Laboratory of the Centers for Disease Control and Prevention in Atlanta, USA. He is writing this work in both his personal capacity and in his capacity as an adjunct academic at Central Queensland University.
Dr Patricia Graves is a vector-borne disease epidemiologist with research, field, laboratory and consulting experience in the control and elimination of malaria and filariasis. She is Director of the JCU/WHO Collaborating Centre for Control of LF, STH and other NTDs in the Division of Tropical Health and Medicine, James Cook University.


