Immune reconstitution disorders in HIV patients receiving antiretroviral therapy
Martyn A FrenchSchool of Pathology and Laboratory Medicine, University of Western Australia, Perth, WA, Australia
Department of Clinical Immunology, Royal Perth Hospital and PathWest Laboratory Medicine, Perth, WA, Australia
Email: martyn.french@uwa.edu.au
Microbiology Australia 35(2) 97-98 https://doi.org/10.1071/MA14030
Published: 16 April 2014
Suppression of HIV infection by antiretroviral therapy (ART) resolves much of the immune dysfunction caused by HIV replication. However, reconstitution of the immune system during ART may, paradoxically, cause immunological disease that presents clinically as an immune reconstitution disorder. These disorders include autoimmune disease, mainly Graves’ disease, and immune-mediated inflammatory disease, such as sarcoidosis. However, by far the most common type of immune reconstitution disorder results from restoring immune responses against opportunistic pathogens.
Restoration of immune responses against opportunistic pathogens causes immunopathology in up to 40% of HIV patients with severe CD4+ T cell deficiency, during the first 3 months of ART. The immune response may be directed against dead micro-organisms, in patients whose opportunistic infection has been treated, or against a subclinical active infection that is unmasked by the immune response. Disease events may be indistinguishable from typical disease presentations, such as first presentations of tuberculosis (TB) or dermatomal zoster, and flares of hepatitis associated with hepatitis B virus and hepatitis C virus infection, that occur after commencing ART. In contrast, an immune reconstitution inflammatory syndrome (IRIS) may occur. These syndromes are associated with infection by a wide variety of opportunistic pathogens and are characterised by inflammatory responses that are exaggerated and/or atypical and often cause significant morbidity and sometimes mortality.
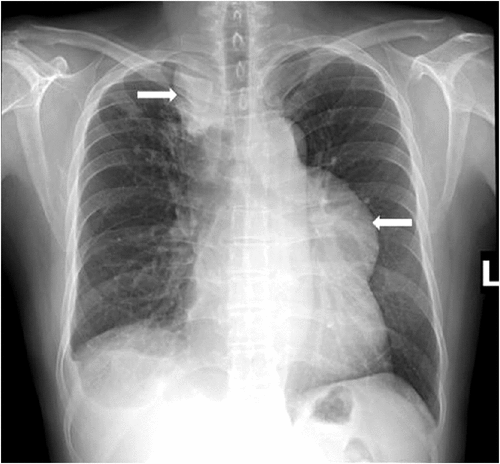
Globally, the most significant types of IRIS in HIV patients are associated with Mycobacterium tuberculosis and Cryptococcus sp. infection. Tuberculosis-associated IRIS (TB-IRIS) and cryptococcosis-associated IRIS (C-IRIS) affect about 25% of HIV patients with severe immunodeficiency (CD4+ T cell count <50/μL) when ART is commenced (Figure 1). Mortality from these conditions is highest when the central nervous system is involved, usually in patients with meningitis. Both TB-IRIS and C-IRIS, as well as IRIS associated with other pathogens, place a substantial burden on antiretroviral treatment programmes in resource-poor countries. Therefore, understanding the immunology of the various types of IRIS will not only provide valuable information about host-pathogen interactions but also assist in developing strategies for the prevention, diagnosis and treatment of these conditions.
|
Following the first description of an IRIS in HIV patients during the 1990s1, I have been rewarded by collaborations with researchers from Australia (Patricia Price, Dino Tan, Christina Chang, Julian Elliott, Sharon Lewin, Suzanne Crowe, David Cooper), Cambodia (Mean Chi Vun, Saphonn Vonthanak), India (Ramachandran Vignesh, Nagalingeswaran Kumarasamy), Malaysia (Hong Yien Tan, Adeeba Kamarazulaman), South Africa (Thumbi Ndung’u, Yunus Moosa) and the USA (Irini Sereti) to undertake research studies on the immunopathogenesis of immune reconstitution disorders.
Early studies in Australian patients highlighted the role of pro-inflammatory cytokines, particularly interleukin (IL)-6, in an IRIS associated with several pathogens and are now being confirmed 10 years later in studies conducted in patients and experimental animals with Mycobacterium avium IRIS2. It has also been shown that dysregulation of chemokine production also contributes to inflammation. For example, impaired production of CCL2 before ART and persistently increased production of CXCL10 during ART are observed in the blood of HIV patients with treated TB who develop TB-IRIS3, whereas HIV patients with treated cryptococcal meningitis who develop C-IRIS exhibit higher ratios of CCL2/CXCL10 and CCL3/CXCL10 in CSF before ART is commenced4. It is unclear whether infection by HIV, the opportunistic pathogen, or both, affect chemokine responses but it is likely that dysregulated chemokine production affects the function and/or trafficking of T cells, NK cells, monocytes or neutrophils at sites of infection by opportunistic pathogens before and after ART is commenced.
Given that recovery of CD4+ T cell numbers is the most notable effect of ART on the immune system of HIV patients, the various types of IRIS are often seen as the result of increased pathogen-specific CD4+ T cell responses. However, the role that T cells play in the immunopathogenesis of the various types of IRIS remains unclear. While CD4+ T cells reactive with M. tuberculosis antigens increase in HIV-TB patients after ART is commenced, TB-IRIS is not clearly associated with a larger number of these cells. For example, we have shown that type 1 helper T cell (Th1) responses, which are characterised by production of IFN-γ and interferon-induced chemokines, such as CXCL9 and CXCL10, are higher in TB-IRIS patients but may just reflect expansion of higher pre-ART Th1 responses5. Also, in HIV patients with C-IRIS, we could not demonstrate higher blood T cell responses to cryptococcal mannoprotein (CMP) in C-IRIS patients using whole blood assays, although whole blood T cell IFN-γ responses to CMP were lower before ART in patients who subsequently developed C-IRIS6. In contrast, Th1 responses to M. tuberculosis antigens are more prominent in HIV patients with subclinical TB that is unmasked by ART7. Studies by Australian colleagues have shown that an IRIS associated with non-tuberculous mycobacterial infection is associated with increased numbers of dysfunctional regulatory T cells8.
The inflammatory response to pathogens in the various types of IRIS is not only determined by the immune responses restored by ART but also by the pathogen load. The clearest demonstration of this was provided by studies in HIV patients with treated cryptococcal meningitis, which demonstrated that positive CSF cultures and a higher CSF quantitative cryptococcal count before commencing ART were strong predictors of C-IRIS9. While there are several possible causes of a higher cryptococcal antigen load prior to ART, including inadequate anti-fungal therapy, our data suggest that a greater degree of immunodeficiency, as manifested by lower blood CD4+ T cell counts and whole blood IFN-γ responses to CMP prior to starting ART, is a contributory factor6,9.
Taken together, the findings of studies co-ordinated from Australia and overseas indicate that an IRIS in HIV patients commencing ART is triggered by a high pathogen load and associated with innate immune responses, and in some cases T cell responses, against pathogens. Defining critical inflammatory mediators2 will lead to more effective therapy for this troublesome complication of ART.
References
[1] French, M.A. (2012) Immune reconstitution inflammatory syndrome: immune restoration disease 20 years on. Med. J. Aust. 196, 318–321.| Immune reconstitution inflammatory syndrome: immune restoration disease 20 years on.Crossref | GoogleScholarGoogle Scholar | 22432669PubMed |
[2] Barber, D.L. et al. (2014) Role of IL-6 in Mycobacterium avium-associated immune reconstitution inflammatory syndrome. J. Immunol. , .
| Role of IL-6 in Mycobacterium avium-associated immune reconstitution inflammatory syndrome.Crossref | GoogleScholarGoogle Scholar | 24591367PubMed |
[3] Oliver, B.G. et al. (2010) Mediators of innate and adaptive immune responses differentially affect immune restoration disease associated with Mycobacterium tuberculosis in HIV patients beginning ART. J. Infect. Dis. 202, 1728–1737.
| Mediators of innate and adaptive immune responses differentially affect immune restoration disease associated with Mycobacterium tuberculosis in HIV patients beginning ART.Crossref | GoogleScholarGoogle Scholar | 20977362PubMed |
[4] Chang, C. et al. (2013) Chemokine levels and chemokine receptor expression in blood and the CSF of HIV-infected patients with cryptococcal meningitis and C-IRIS. J. Infect. Dis. 208, 1604–1612.
| Chemokine levels and chemokine receptor expression in blood and the CSF of HIV-infected patients with cryptococcal meningitis and C-IRIS.Crossref | GoogleScholarGoogle Scholar | 23908492PubMed |
[5] Vignesh, R. et al. (2013) TB-IRIS after initiation of antiretroviral therapy is associated with expansion of preexistent Th1 responses against Mycobacterium tuberculosis antigens. J. Acquir. Immune Defic. Syndr. 64, 241–248.
| TB-IRIS after initiation of antiretroviral therapy is associated with expansion of preexistent Th1 responses against Mycobacterium tuberculosis antigens.Crossref | GoogleScholarGoogle Scholar | 23774879PubMed |
[6] Chang, C.C. et al. (2013) Cryptococcosis-IRIS is associated with lower Cryptococcus-specific IFN-gamma responses before antiretroviral therapy but not higher T-cell responses during therapy. J. Infect. Dis. 208, 898–906.
| Cryptococcosis-IRIS is associated with lower Cryptococcus-specific IFN-gamma responses before antiretroviral therapy but not higher T-cell responses during therapy.Crossref | GoogleScholarGoogle Scholar | 23766525PubMed |
[7] Oliver, B.G. et al. (2012) Tuberculosis after commencing antiretroviral therapy for HIV infection is associated with elevated CXCL9 and CXCL10 responses to Mycobacterium tuberculosis antigens. J. Acquir. Immune Defic. Syndr. 61, 287–292.
| Tuberculosis after commencing antiretroviral therapy for HIV infection is associated with elevated CXCL9 and CXCL10 responses to Mycobacterium tuberculosis antigens.Crossref | GoogleScholarGoogle Scholar | 22732467PubMed |
[8] Seddiki, N. et al. (2009) Proliferation of weakly suppressive regulatory CD4+ T cells is associated with over-active CD4+ T-cell responses in HIV-positive patients with mycobacterial immune restoration disease. Eur. J. Immunol. 39, 391–403.
| Proliferation of weakly suppressive regulatory CD4+ T cells is associated with over-active CD4+ T-cell responses in HIV-positive patients with mycobacterial immune restoration disease.Crossref | GoogleScholarGoogle Scholar | 19180462PubMed |
[9] Chang, C.C. et al. (2013) Clinical and mycological predictors of Cryptococcosis-associated immune reconstitution inflammatory syndrome. AIDS 27, 2089–2099.
| Clinical and mycological predictors of Cryptococcosis-associated immune reconstitution inflammatory syndrome.Crossref | GoogleScholarGoogle Scholar | 23525034PubMed |
Biography
Martyn French is a Winthrop Professor in Clinical Immunology in the School of Pathology and Laboratory Medicine of the University of Western Australia. He also practices as a Clinical Immunologist and Immunopathologist at Royal Perth Hospital and PathWest Laboratory Medicine. He has conducted research on various aspects of HIV immunology, and contributed to national and international clinical trials of therapies for HIV patients, during almost 30 years.


