HIV, hepatitis viruses and viral STIs: intertwined fates?
Tony CunninghamWestmead Millennium Institute for Medical Research
Darcy Road
PO Box 412
Westmead, NSW 2145, Australia
Tel: +61 2 9845 9001
Fax: +61 2 9845 9100
Email: tony.cunningham@sydney.edu.au
Microbiology Australia 35(2) 83-87 https://doi.org/10.1071/MA14025
Published: 5 May 2014
Although the major target cell for HIV in the body is the CD4 lymphocyte, uptake by and infection of other target cells such macrophages and dendritic cells also play a major role in pathogenesis. Macrophages are a major reservoir for HIV in most lymphoid tissue in the untreated patient and remain the major target cell in brain whether the subject is treated or not. Dendritic cells play a major role in transferring HIV efficiently to T cells particularly after viral entry through sexual transmission. Our work has focused on understanding how HIV binds to and enters these cells, trafficks through them and, during that time, alters their function to facilitate viral survival, replication and spread. Understanding of these processes is the foundation for a rational approach to the development of therapeutics (including microbicides) and vaccines.
In 1986, in response to the threat of HIV in Australia the Commonwealth Government established a national strategy for the containment of HIV, which led to the establishment of three national centres in HIV research in basic research, clinical and epidemiological research and social research. The National Centre for HIV Virology Research (NCHVR) was initially founded by Professor Ian Gust and subsequently directed by Professor John Mills. In 2003 the Feecham Review of HIV research led to the mainstreaming of basic HIV research and recommended enhanced linkages between the centres and inclusion of research into Hepatitis C in view of its rising incidence in Australia. In 2003 I was appointed Director of the Australian Centre for HIV and Hepatitis Virology Research (ACH2) which brought together HIV and Hepatitis researchers from the previous NCHVR and the Australian Centre for Hepatitis Virus Research (ACHV). A facilitated external strategic plan led to the focus of ACH2 on translational research into the development of novel vaccine and candidates, drugs and diagnostics and prognostics including molecular epidemiology for HIV and Hepatitis C. ACH2 distributes its money through short duration grants to all relevant public research centres in Australia, (mandated by the availability of funds from the Commonwealth) through a competitive peer reviewed process.
In recent years national strategies have been established separately for HIV, Hepatitis B, Hepatitis C, Sexually Transmitted Infections and Indigenous Health. Thus the remit of ACH2 now covers HIV, both Hepatitis viruses and relates these to indigenous health. To facilitate interactions between the National HIV and Hepatitis Clinical and Epidemiology Research Centre (now the Kirby Institute) and ACH2 and to support clinical trials with advanced laboratory techniques, the Immunovirology Research Network (IVRN) headed by Professor Andrew Lloyd was established within ACH2.
ACH2 has been judged to have a number of successes. It has fulfilled its initial goals through deploying diagnostic and prognostic tests into clinical practice and clinical research. It has also facilitated research to the level where it can be funded by additional development funding from agencies such as ARC (Linkage grants), NH&MRC (Development grants), Gates Foundation and private companies. Indeed ACH2 is one of the few grant funding sources outside ARC and NHMRC for virologists and immunologists, emulating alternative funding bodies for cancer and cardiovascular researchers in Australia. ACH2 also holds annual national meetings, bringing together HIV and Hepatitis basic researchers and facilitating interactions between HIV translational researchers and physicians at the annual Australian Society for HIV Medicine (ASHM) meetings. These meetings have facilitated translational research and many collaborations between HIV and Hepatitis researchers which otherwise would not have occurred e.g. in the development of HIV and Hepatitis C vaccines and immune prognostic tests.
The Centre for Virus Research at the Westmead Millennium Institute now consists of 40–50 researchers and postgraduate students, studying HIV and Herpesviruses. In particular we have focused on (i) immunology and cell biology of HIV in relation to the development of mucosal vaccines and microbicides, (ii) the immunology and neurobiology of Herpes simplex virus in relation to development of novel antiviral agents and (iii) improving the partially successful HSV vaccine candidates.
Our studies of HIV and HSV are highly complementary. Both are sexually transmitted agents which enter the anogenital mucosa where they encounter dendritic cells (DCs) and cause infection. At the epidemiologic level prior infection with HSV-2 is associated with a three-fold or greater increase in HIV acquisition. Indeed in South Africa most HIV acquisition is in the setting of concomitant sexually transmitted infections, particularly ulcerating ones such as HSV1/2 where we showed target cells of HIV activated T cells and macrophages lie in the base of the ulcer1. Recently we have been studying how HSV and HIV are taken up by and infect DCs, how this perturbs their function2–4 and how co-infection with each of the two viruses influences the other, especially as they induce opposite effects on DC biology.
HIV and macrophages
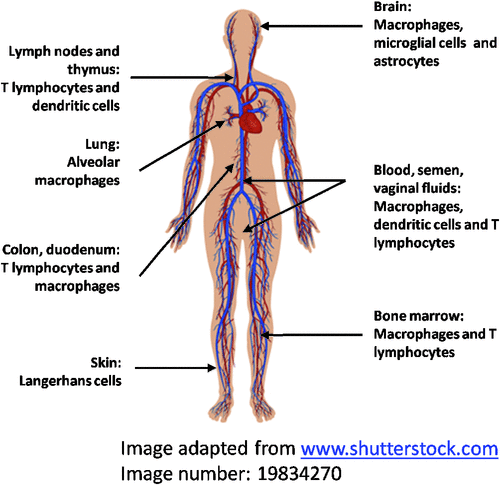
Our HIV studies commenced in 1986 elucidating the interaction of HIV with macrophages,one of the key target cells for HIV, alongside T cells and DCs. HIV replication in macrophages is at a lower level and noncytopathic compared to T cells. They are an important reservoir of HIV within the body in brain, lung, spleen, secondary lymphoid tissue, bone marrow and also in placenta5,6 (Figure 1). In the brain, microglia (specialized tissue macrophages) are the principal reservoir of HIV infection and the source of toxins which may influence neuronal function to produce HIV-associated dementia (HAD)7. HIV produced by these cells readily spreads to T cells either via direct cell to cell contact of by cell free virus.
|
Our first major discovery was that the main HIV binding (CD4) and entry (CCR5) receptors on CD14+ blood monocytes are modulated by adherence to substrate and maturation into macrophages. The maturing cells down regulate CD4 to a moderate degree but this is offset by the appearance of surface CCR5 (within several hours of adherence) which mostly determines their susceptibility to HIV infection, resolving a paradox about the HIV ‘infectability’ of monocytes and macrophages. The ability of primary isolates to infect monocytes maturing into macrophages correlated directly with the extent of CCR5 expression5,8,9. There is considerable variability in CCR5 expression in macrophages throughout the body with low levels of CCR5 being expressed in intestinal wall macrophages, apart from the rectum, leading to lower susceptibility to HIV infection10. In contrast, in placental macrophages it appears to be low CD4 expression which determines the low level infection5,6.
Macrophages from identical and non-identical twins of the Australian twin registry and primary HIV strains from patients at various stages of progressive HIV disease showed the outcome of HIV infection of these cells depends on the interaction of viral and cellular genotypes. These studies showed a marked host-cell genetic effect on the replication of primary HIV strains in macrophages and on CCR5 expression, which was independent of the CCR5delta32 genotype known to diminish or prevent HIV infection11,12.
Furthermore HIV isolates from patients with advanced disease (AIDS) showed enhanced replication within macrophages and predominant use of CCR5 for entry13. These late-emerging, viral strains require lower levels of CD4/CCR5 expression for entry, indicating stronger binding of their envelope proteins to CCR5. This was later shown to be a property of the sequence and conformation of the envelope proteins14.
A man homozygous for the CCR5Delta32 mutation was not resistant to HIV infection as expected. CCR5 surface expression was absent on T lymphocytes and macrophages. Sequencing of the full HIV genome revealed a 21 nucleotide insertion in the V1 region of gp120 probably altering the envelope protein to utilize predominantly CXCR4 for viral entry. Thus certain strains of HIV readily use CXCR4 for entry into macrophages, which is highly relevant to the pathogenesis of late-stage HIV disease15.
As the expression of chemokine receptors, CCR5 (and also CXCR4) and HIV-1 infection is under continuous regulation by a complex cytokine network produced by a variety of cells we and others have defined their important effects on HIV replication in macrophages. These effects are either inhibitory (IFN-alpha, IFN-beta, IFN-gamma, GM-CSF, IL-10, IL-13 and IL-16 and beta-chemokines), stimulatory (M-CSF, TNF-alpha, TNF-beta, IL-1, IL-6) or bifunctional, that is both inhibitory and stimulatory (IL-4). The balance of the effects of these cytokines is probably responsible for the activation of macrophages which, together with activation of T cells, is an important feature of advancing immunosuppression with HIV disease progression16,17.
Coinfection with HIV is a major factor in the resurgence of multiresistant TB in many parts of the globe and macrophages are the reservoir host cells for both pathogens. As the interactions between both pathogens in co-infected cells is poorly understood the global gene expression of human macrophages following co-infection with both pathogens was examined using cDNA microarrays. A broad range of genes was up-regulated in response to M. tuberculosis infection of primary macrophages, including those encoding pro-inflammatory chemokines and cytokines, dominating (fewer and lower) changes following HIV infection alone were fewer and lower. These studies were the foundation for further extensive studies of HIV-induced changes in gene expression in macrophages and DCs18.
Other studies of HIV
The first cases of HIV transmitted to women by artificial insemination19 provided the first definitive evidence demonstration that women could be infected vaginally through an intact mucosa.
The ‘Sydney Blood Bank Cohort’ consisted of eight subjects infected via transfusion with an attenuated strain of HIV due to deletions in the nef -LTR regions of the HIV genome, leading to an attenuated course of HIV infection. Although the initial promise of using such strains for live attenuated HIV vaccines has faded because of safety concerns, the correlates of immune protection against HIV infection have been advanced by studying this and other rare cohorts of long-term survivors20,21.
HIV infection of dendritic cells
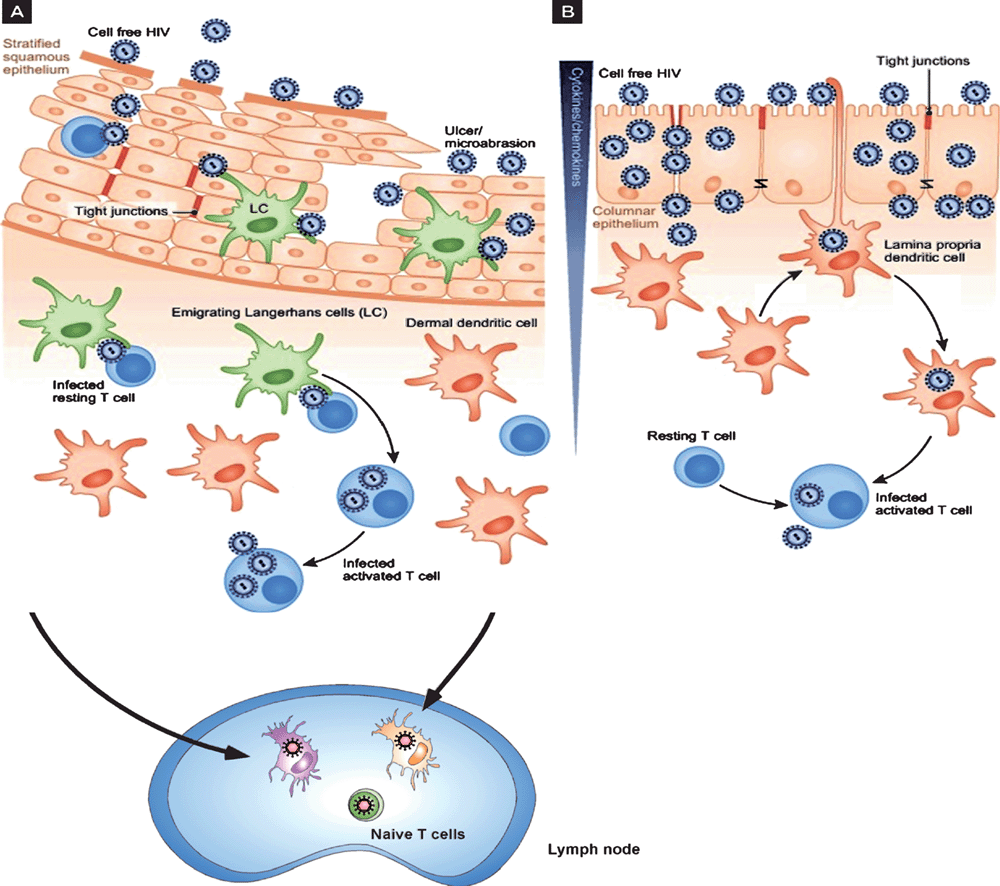
During sexual transmission, HIV from infected T cells in the semen can contact DC within the inflamed stratified squamous mucosa called Langerhan’s cells (LCs) and also encounters lamina propria DCs in rectal/cervical columnar mucosa (Figure 2). HIV interactions with these cells is different to T cells and macrophages as HIV is taken up by two different mechanisms with the prime purpose of HIV transfer to their target T cells2–4. In contrast, HSV infects both keratinocytes and LCs in the stratified squamous epidermis. Viral infection induces migration of the infected LCs to the dermis where they interact with dermal DCs prior to migration of these cells to lymph nodes to stimulate appropriate T cell responses22.
|
HIV binds strongly to C-type lectin receptors (CLRs), such as langerin on Langerhans cells from the epidermis, and DC-SIGN and mannose receptor on DCs in the dermis but most blood DCs do not express CLRs and instead bind HIV via CD4 and CCR5. We then examined the kinetics of trafficking after such CLR binding in monocyte-derived DCs as a model and demonstrated that this occurred in two temporal phases after binding to CLRs. The first consisted of uptake into ‘caves’ or virus containing compartments showing invaginations at the plasma membrane and the second via transfer from CLRs to CD4 and CCR5 and subsequent fusion with the membrane. In the former case virus was rapidly degraded probably through interactions between virus in these caves and the endolysosomal system. In the latter case virus proceeded to de novo replication albeit at a very low level23–25. Indeed it is now known that there are now a number of constitutive and inducible restriction factors which limit replication of HIV in DCs19.
This two-phase trafficking of HIV through DCs also leads to two-phase transfer of HIV to T cells23–25. This is potentially important for drugs attempting to inhibit the process by which HIV is transferred through DCs to T cells, resulting in amplification of replication in the latter.
In recent studies we have now examined HIV uptake and replication in a variety of epithelial DCs. Dr Najla Nasr and I have demonstrated that two-phase transfer occurs in Langerhans cells and both phases of uptake and transfer to T cells can be blocked by soluble langerin homologues. In our laboratory Dr Andrew Harman, in collaboration with Paul Cameron at the Burnet Institute, has also shown this two-phase transfer occurs in dermal and lamina propria DCs. (Abstract X4 2011, Keystone Symposium in HIV pathogenesis, Banff 2014.)
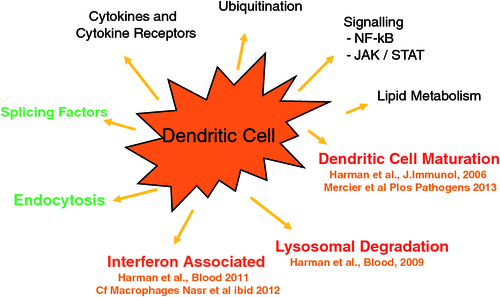
Most recently our studies have focused on how HIV subverts the cellular environment of DCs and macrophages to facilitate HIV replication and spread. HIV induces host DC gene expression in two phases corresponding to HIV trafficking in DCs26. Firstly we showed that de novo infection results in enhanced maturation of DCs and adherence to T cells to facilitate HIV transfer. Surprisingly microvesicles liberated after lytic infection of T cells contaminate the HIV inoculum and provide a major contribution to the maturation and adherence of these DCs. The full proteome of these vesicles including the HIV and cellular molecules responsible for the effect (nef and HSP90) has been defined27,28. HIV was found to inhibit lysosomal cathepsins and antigen processing26 (Figure 3).
|
Finally, HIV infection of both DCs and macrophages inhibits their ability to produce interferon, thus facilitating spread to adjacent cells (where interferon has its effect). Paradoxically HIV also induces ‘interferon-stimulated genes’ in infected DCs and macrophages to control viral replication and perhaps lysis of these cells29,30. Thus HIV induces a persistent noncytopathic infection in these cells, ideally suited to their transfer and reservoir function. Currently we are defining the exact pathways which are responsible for these effects.
A fascinating future for HIV research lies ahead as the HIV scientific community focuses on the two major aims of preventing HIV infection through vaccine/microbicide development and ‘curing’ or eliminating HIV from antiretroviral-controlled chronically infected people.
Acknowledgements
I would like to pay special tribute to the many eminent Australian microbiologists who have served on the Board of ACH2 (chaired by Dick Smallwood), Executive (especially Chris Burrell) or the Scientific Advisory Committee (chaired by John Finlay-Jones) of ACH2 and my longstanding ‘HIV’ collaborators Paul Cameron, Paul Gorry and Sharon Lewin.
References
[1] Cunningham, A.L. et al. (1985) Evolution of recurrent herpes simplex lesions. An immunohistologic study. J. Clin. Invest. 75, 226–233.| Evolution of recurrent herpes simplex lesions. An immunohistologic study.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaL2M%2FotVGrsg%3D%3D&md5=a08df63b75b7def96e2ebf7333c57504CAS | 3880773PubMed |
[2] Cunningham, A.L. et al. (2013) Initial HIV mucosal infection and dendritic cells. EMBO Mol. Med. 5, 658–660.
| Initial HIV mucosal infection and dendritic cells.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXntV2hs7c%3D&md5=7cd77757d0b5ca6b7ce1b627be640535CAS | 23653303PubMed |
[3] Cunningham, A.L. et al. (2008) Langerhans cells and viral immunity. Eur. J. Immunol. 38, 2377–2385.
| Langerhans cells and viral immunity.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXht1SnsbfP&md5=b4f64991634dee3cc0b4b054ab838093CAS | 18792031PubMed |
[4] Cunningham, A.L. et al. (2010) Manipulation of dendritic cell function by viruses. Curr. Opin. Microbiol. 13, 524–529.
| Manipulation of dendritic cell function by viruses.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXpvVaqtbo%3D&md5=2a809e72e54ada68320953644888f02cCAS | 20598938PubMed |
[5] Fear, W.R. et al. (1998) Differential tropism and chemokine receptor expression of human immunodeficiency virus type 1 in neonatal monocytes, monocyte-derived macrophages, and placental macrophages. J. Virol. 72, 1334–1344.
| 1:CAS:528:DyaK1cXlt12gtw%3D%3D&md5=19a95fe42baf75dc207f8458f3d40c24CAS | 9445034PubMed |
[6] Kesson, A.M. et al. (1993) Human immunodeficiency virus type 1 infection of human placental macrophages in vitro. J. Infect. Dis. 168, 571–579.
| Human immunodeficiency virus type 1 infection of human placental macrophages in vitro.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK3szlvFOnsA%3D%3D&md5=bbec9fb1cbb552bb3b30440d3831572bCAS | 7689088PubMed |
[7] Cunningham, A.L. et al. (1997) HIV infection of macrophages and pathogenesis of AIDS dementia complex: interaction of the host cell and viral genotype. J. Leukoc. Biol. 62, 117–125.
| 1:CAS:528:DyaK2sXksF2rsr8%3D&md5=6be2102e5dd1b2300faad2c5e8d5fc76CAS | 9226002PubMed |
[8] Kazazi, F. et al. (1989) Variations in CD4 expression by human monocytes and macrophages and their relationships to infection with the human immunodeficiency virus. J. Gen. Virol. 70, 2661–2672.
| Variations in CD4 expression by human monocytes and macrophages and their relationships to infection with the human immunodeficiency virus.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL1MXmtlSnt70%3D&md5=2072f5e7f50812b184c8d7befc5ddb36CAS | 2677236PubMed |
[9] Naif, H.M. et al. (1998) CCR5 expression correlates with susceptibility of maturing monocytes to human immunodeficiency virus type 1 infection. J. Virol. 72, 830–836.
| 1:CAS:528:DyaK1cXhvFCltQ%3D%3D&md5=80c1f63a536f020253f343df11810b32CAS | 9420295PubMed |
[10] Shen, R. et al. (2011) Stromal down-regulation of macrophage CD4/CCR5 expression and NF-κB activation mediates HIV-1 non-permissiveness in intestinal macrophages. PLoS Pathog. 7, e1002060.
| Stromal down-regulation of macrophage CD4/CCR5 expression and NF-κB activation mediates HIV-1 non-permissiveness in intestinal macrophages.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXntVajsrc%3D&md5=8687f067a126a1086ac52890c81ada06CAS | 21637819PubMed |
[11] Chang, J. et al. (1996) Twin studies demonstrate a host cell genetic effect on productive human immunodeficiency virus infection of human monocytes and macrophages in vitro. J. Virol. 70, 7792–7803.
| 1:CAS:528:DyaK28Xmt1Gksb8%3D&md5=41cd9f4c78053dd4feb2df84804962a8CAS | 8892900PubMed |
[12] Naif, H.M. et al. (1999) Definition of the stage of host cell genetic restriction of replication of human immunodeficiency virus type 1 in monocytes and monocyte-derived macrophages by using twins. J. Virol. 73, 4866–4881.
| 1:CAS:528:DyaK1MXjtFertbw%3D&md5=8817bcaa2ba428135bf72dcdac941b8cCAS | 10233948PubMed |
[13] Li, S. et al. (1999) Persistent CCR5 utilization and enhanced macrophage tropism by primary blood human immunodeficiency virus type 1 isolates from advanced stages of disease and comparison to tissue-derived isolates. J. Virol. 73, 9741–9755.
| 1:CAS:528:DyaK1MXnsV2msbw%3D&md5=74c615b353f607f0df8919f386ba07f4CAS | 10559284PubMed |
[14] Sterjovski, J. et al. (2010) An altered and more efficient mechanism of CCR5 engagement contributes to macrophage tropism of CCR5-using HIV-1 envelopes. Virology 404, 269–278.
| An altered and more efficient mechanism of CCR5 engagement contributes to macrophage tropism of CCR5-using HIV-1 envelopes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXoslaitbs%3D&md5=b1758cf9b938b187d03e79a145165327CAS | 20570309PubMed |
[15] Naif, H.M. et al. (2002) A human immunodeficiency virus type 1 isolate from an infected person homozygous for CCR5Δ32 exhibits dual tropism by infecting macrophages and MT2 cells via CXCR4. J. Virol. 76, 3114–3124.
| A human immunodeficiency virus type 1 isolate from an infected person homozygous for CCR5Δ32 exhibits dual tropism by infecting macrophages and MT2 cells via CXCR4.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38Xit1GqtbY%3D&md5=c1afb9e3a05c1c2781897198ab8f89d0CAS | 11884536PubMed |
[16] Kedzierska, K. et al. (2003) The influence of cytokines, chemokines and their receptors on HIV-1 replication in monocytes and macrophages. Rev. Med. Virol. 13, 39–56.
| The influence of cytokines, chemokines and their receptors on HIV-1 replication in monocytes and macrophages.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXht1eqtrk%3D&md5=c6c559a0f254f04d8e47720d171222cfCAS | 12516061PubMed |
[17] Kelly, M.D. et al. (1998) Dichotomous effects of β-chemokines on HIV replication in monocytes and monocyte-derived macrophages. J. Immunol. 160, 3091–3095.
| 1:CAS:528:DyaK1cXitFWntbc%3D&md5=959ad9c6b343b0ebe0ec9c84d940c4ffCAS | 9531262PubMed |
[18] Maddocks, S. et al. (2009) Gene expression in HIV-1/Mycobacterium tuberculosis co-infected macrophages is dominated by M. tuberculosis. Tuberculosis (Edinb.) 89, 285–293.
| Gene expression in HIV-1/Mycobacterium tuberculosis co-infected macrophages is dominated by M. tuberculosis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXht1ans73F&md5=57c204fbf0839d133b10c3983cdc8d09CAS | 19520608PubMed |
[19] Stewart, G.J. et al. (1985) Transmission of human T-cell lymphotropic virus type III (HTLV-III) by artificial insemination by donor. Lancet 2, 581–585.
| Transmission of human T-cell lymphotropic virus type III (HTLV-III) by artificial insemination by donor.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaL2M3psV2ktg%3D%3D&md5=6312282f8980d151dde884cd26dc14ecCAS | 2863597PubMed |
[20] Deacon, N.J. et al. (1995) Genomic structure of an attenuated quasi species of HIV-1 from a blood transfusion donor and recipients. Science 270, 988–991.
| Genomic structure of an attenuated quasi species of HIV-1 from a blood transfusion donor and recipients.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2MXptlOlurY%3D&md5=8e17ce1f5fe12326104e5a6a795a07b2CAS | 7481804PubMed |
[21] Learmont, J. et al. (1992) Long-term symptomless HIV-1 infection in recipients of blood products from a single donor. Lancet 340, 863–867.
| Long-term symptomless HIV-1 infection in recipients of blood products from a single donor.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK3s%2FitlaisA%3D%3D&md5=d12c61e6b4258ab6e43bea69b7158eb7CAS | 1357294PubMed |
[22] Puttur, F.K. et al. (2010) Herpes simplex virus infects skin γδ T cells before Langerhans cells and impedes migration of infected Langerhans cells by inducing apoptosis and blocking E-cadherin downregulation. J. Immunol. 185, 477–487.
| Herpes simplex virus infects skin γδ T cells before Langerhans cells and impedes migration of infected Langerhans cells by inducing apoptosis and blocking E-cadherin downregulation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXns1CqsLY%3D&md5=b1f076939c218fb84a679679deb9fd9fCAS | 20519652PubMed |
[23] Turville, S.G. et al. (2001) HIV gp120 receptors on human dendritic cells. Blood 98, 2482–2488.
| HIV gp120 receptors on human dendritic cells.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXnslCqs70%3D&md5=085aca2daf1be0abfa45ca5e63a5f386CAS | 11588046PubMed |
[24] Turville, S.G. et al. (2002) Diversity of receptors binding HIV on dendritic cell subsets. Nat. Immunol. 3, 975–983.
| Diversity of receptors binding HIV on dendritic cell subsets.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XnsVGrsL0%3D&md5=d7763909829829eafb2c87185dd651d8CAS | 12352970PubMed |
[25] Turville, S.G. et al. (2004) Immunodeficiency virus uptake, turnover, and 2-phase transfer in human dendritic cells. Blood 103, 2170–2179.
| Immunodeficiency virus uptake, turnover, and 2-phase transfer in human dendritic cells.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXitlequ78%3D&md5=eca87c4841486b66e8446a7ba0d96d3dCAS | 14630806PubMed |
[26] Harman, A.N. et al. (2009) HIV-1-infected dendritic cells show 2 phases of gene expression changes, with lysosomal enzyme activity decreased during the second phase. Blood 114, 85–94.
| HIV-1-infected dendritic cells show 2 phases of gene expression changes, with lysosomal enzyme activity decreased during the second phase.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhtVOkurrK&md5=89df01a13634d9ef481e4bbace563f44CAS | 19436054PubMed |
[27] Harman, A.N. et al. (2006) HIV induces maturation of monocyte-derived dendritic cells and Langerhans cells. J. Immunol. 177, 7103–7113.
| 1:CAS:528:DC%2BD28XhtFGrtrvE&md5=94b908db44f65a68eff45220e36af989CAS | 17082627PubMed |
[28] Mercier, S.K. et al. (2013) The microvesicle component of HIV-1 inocula modulates dendritic cell infection and maturation and enhances adhesion to and activation of T lymphocytes. PLoS Pathog. 9, e1003700.
| The microvesicle component of HIV-1 inocula modulates dendritic cell infection and maturation and enhances adhesion to and activation of T lymphocytes.Crossref | GoogleScholarGoogle Scholar | 24204260PubMed |
[29] Harman, A.N. et al. (2011) HIV infection of dendritic cells subverts the IFN induction pathway via IRF-1 and inhibits type 1 IFN production. Blood 118, 298–308.
| HIV infection of dendritic cells subverts the IFN induction pathway via IRF-1 and inhibits type 1 IFN production.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXpsVWmt7c%3D&md5=f93fb3b9a09424444d2f3c7baaa248d4CAS | 21411754PubMed |
[30] Nasr, N. et al. (2012) HIV-1 infection of human macrophages directly induces viperin which inhibits viral production. Blood 120, 778–788.
| HIV-1 infection of human macrophages directly induces viperin which inhibits viral production.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhtFOgurrN&md5=fb565c8f52bc46fff82bb7745ad859d4CAS | 22677126PubMed |
Biography
Professor Tony Cunningham AO is Executive Director, Westmead Millennium Institute for Medical Research and Director of the Institute’s Centre for Virus Research, Professor of Research Medicine and Sub-Dean (Research) Sydney Medical School, Westmead , the University of Sydney. Since 2003 he has been Director of the Australian Centre for HIV and Hepatitis Virology Research (ACH2), funded directly by the Australian Government. He trained in infectious diseases, clinical virology and virology research at the University of Melbourne and as a postdoctoral fellow in infectious diseases at Stanford University. His major research interests are in HIV and Herpesviruses (mainly Herpes simplex virus) biology and immunology, especially in relation to the development of vaccines and microbicides. He has published more than 280 primary refereed scientific articles and >60 invited reviews or chapters in various journals or books on these topics and has been cited more than 10,000 times. He has also played a leading international role in elucidating HIV interactions with macrophages, as reservoirs, and with dendritic cells as an initial target cell in the anogenital mucosa. His group has also made numerous key contributions to HSV immunology which has led to the development and trialling of a partially successful HSV vaccine candidate by GlaxoSmithKline. He and his colleagues also pioneered the study of anterograde transport of herpes simplex virus in neuronal axons in vitro in 1994, and have made major contributions in understanding the mechanisms since. He has participated in numerous international roundtables and often consults for global pharma on antivirals and vaccines. In 2010, Tony was made an Officer in the Order of Australia (AO) for ‘service to medicine, particularly in the field of viral research and through the development and leadership of medical and biomedical research’.


