Integrative taxonomy of the cycad-associated weevils of the Tranes group, with a revision of Tranes Schoenherr, a key to all taxa and an assessment of host specificity in the group (Coleoptera: Curculionidae: Molytinae)
Yun Hsiao A B C * and Rolf G. Oberprieler B *
A B C * and Rolf G. Oberprieler B *
A
B
C
Handling Editor: Bruno Medeiros
Abstract
Australia is a main centre of diversity for extant cycads (Cycadophyta), harbouring 4 genera and 85 named species and subspecies. Three cycad genera, Bowenia, Lepidozamia and Macrozamia, serve as hosts for four weevil genera of the Tranes group, Tranes Schoenherr, Miltotranes Zimmerman, Demyrsus Pascoe and Siraton Hustache. Several morphologically based taxonomic studies have been undertaken on some of these genera recently, but their classification, diversity and species delineations have not been evaluated using an integrative taxonomic approach. In the present study, we combine morphological characters and mitochondrial DNA data to assess the taxonomic status of taxa in this group. Different methods of molecular species delimitation, especially distance-based ones, generally provide strong support for taxon concepts derived from morphological characteristics, demonstrating that these are well able to delineate natural species and assess taxonomic diversity in this group of weevils. Exceptions are that molecular analyses indicate Siraton internatus (Pascoe) to be more closely related to Demyrsus than to S. roei (Boheman), rendering Siraton a paraphyletic taxon, and a genetically distinct but morphologically cryptic species of Miltotranes to occur south of Cairns. A key to all genera and species of the Tranes group is presented. The genus Tranes and its four previously named species are redescribed and six species are newly described, T. chadwicki sp. nov., T. forsteri sp. nov., T. kgariensis sp. nov., T. occidentalis sp. nov., T. terryae sp. nov. and T. tinctipennis sp. nov., and a lectotype is designated for the name Tranes insignipes Lea, 1929. The salient characters and distribution ranges of all Tranes species are illustrated, and their host specificities are assessed.
ZooBank: urn:lsid:zoobank.org:pub:45DE986E-A8B3-4247-B056-DF3126D4B31D
Keywords: Australia, cryptic species, cycad weevils, identification key, mitochondrial DNA, morphology, new species, taxonomy, species delineation.
Introduction
Cycads are ancient palm-like gymnosperms, representing one of the oldest groups of living plants due to their mid-Permian origin (Nagalingum et al. 2011). With four genera and nearly 90 recognised species and subspecies (M. Calonje, D. W. Stevenson and R. Osborne, The World List of Cycads, see http://www.cycadlist.org), Australia is one of the main centres of cycad diversity worldwide. Even though cycad tissues are imbued with potent defensive toxins, several insect groups have evolved intimate trophic interactions with cycads (Schneider et al. 2002; Salzman et al. 2018). In Australia, the foremost of these cycadophagous insects are four weevil genera of the Tranes group, two of which (Demyrsus Pascoe and Siraton Hustache) bore in their trunks and cataphylls and the other two (Miltotranes Zimmerman and Tranes Schoenherr) develop in their microstrobili (male cones) and serve as their main or sole pollinators. All play important ecological roles in terms of pollination and nutrient recycling, thus in cycad reproduction, survival and conservation, but also economic ones in the horticulture industry, both positive as pollinators and negative as destructive cycad ‘pests’ (e.g. Jones 2002).
The taxonomic history of the Tranes group is intricate. The genus Tranes was originally described for only two Australian species, T. vigorsii Boheman, 1843 and T. sparsus Boheman, 1843 (Schoenherr 1843), whereas another similar Australian species described in the same publication was placed in a different genus, Iphipus Schoenherr, 1835 (now restricted to South America), as I. roei Boheman, 1843. Pascoe (1870) subsequently transferred this species to Tranes and shortly afterwards described yet another similar Australian species as Platyphaeus lyterioides, placed in Baridinae (Pascoe 1875). Marshall (1939) transferred also P. lyterioides to Tranes and synonymised the names Platyphaeus with Tranes and P. lyterioides with T. sparsus, but the latter synonymy was erroneous (based on specimens misidentified by Arthur Lea) and rejected by later authors (May 1994; Zimmerman 1994; Oberprieler 1995a). Another seven species were described in Tranes by Pascoe (1870, 1874) and Lea (1898, 1929), but only one of them (T. insignipes Lea, 1929) is now still retained in the genus.
Tranes was originally described in the subfamily Erirhininae (Schoenherr 1843) but later placed in a subfamily Amalactinae together with the genera Amalactus Schoenherr, 1835, Aorus Schoenherr, 1835 and Iphipus (Lacordaire 1863; Schenkling and Marshall 1936), which are associated with monocotyledonous plants. Kuschel (1987) downgraded Amalactinae to a tribe of Molytinae and excluded Tranes and Iphipus from it, though without assigning these two genera to any other molytine tribe. Zimmerman (1994) later again transferred Tranes from Amalactinae to Molytinae and divided it into five genera, Howeotranes Zimmerman, 1994, Melanotranes Zimmerman, 1994, Miltotranes Zimmerman, 1994, Paratranes Zimmerman, 1994 and Tranes, and he also added another Australian cycad-associated genus, Demyrsus Pascoe, 1872, to the Tranes group (Fig. 1). This concept was adopted by subsequent authors (Oberprieler 1995a, 2004; Alonso-Zarazaga and Lyal 1999; Pullen et al. 2014). Oberprieler and Caldara (2012) discovered that Siraton devillei Hustache, 1934, described from Italy but unrecognised there afterwards, is conspecific with the Australian Melanotranes internatus (Pascoe, 1870), resulting in the synonymy of the names Melanotranes with Siraton and devillei with internatus and the conclusion that the species had been introduced to Europe in cycad trunks, as had been Demyrsus meleoides Pascoe, 1872 later (Covassi 1974). Three additional species were described in the Tranes group recently, one each in Demyrsus, Miltotranes and Paratranes (Hsiao and Oberprieler 2020b, 2021a, 2022), bringing to the total number to 14 species in six genera in Australia. A single species from outside of Australia also appears to belong in the Tranes group, occurring in New Caledonia and described as Hylobius pipitzi Pascoe, 1888 but representing an undescribed genus (Ch. Lyal, pers. comm.), and Hsiao et al. (2023) recently found that also the South-east Asian genus Lyterius Schoenherr, 1844 is closely related to this group.
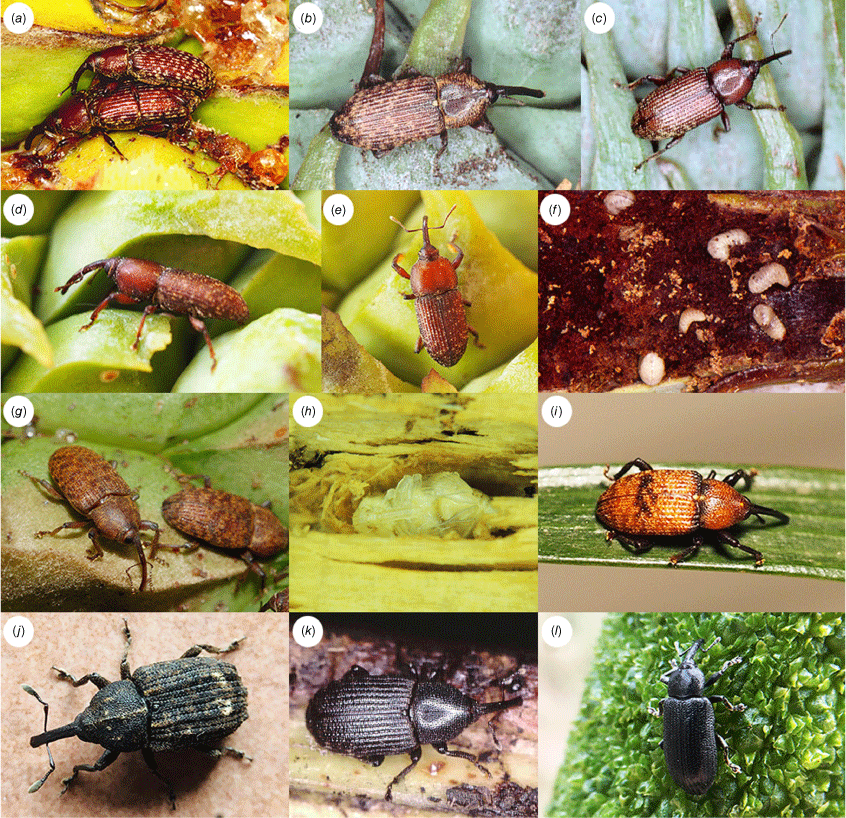
The diversity of the Tranes group in Australia. (a) T. vigorsii; (b) T. sparsus; (c) T. occidentalis; (d, e) T. lyterioides, adults; (f) T. lyterioides, larvae; (g) T. terryae, adults; (h) T. terryae, pupa; (i) Miltotranes prosternalis; (j) Demyrsus meleoides; (k) Siraton internatus; (l) Paratranes zimmermani. Photographs courtesy of (a) Kirke M. Fisher and (g) Nicholas Fisher.
The systematic placement of the Tranes group remains uncertain and somewhat controversial. Zimmerman (1994) did not assign the group to any molytine tribe, and it was consequently treated as incertae sedis in Molytinae (or Curculioninae: Molytini) by Oberprieler (1995a, 2004), Alonso-Zarazaga and Lyal (1999), Oberprieler and Caldara (2012), Lyal (2014) and Pullen et al. (2014). Following the discovery by Shin et al. (2018) of a phylogenetic relationship between Tranes and the New-Guinean Araucaria-associated genus Vanapa Pouillaude, 1915, classified in the tribe Orthorhinini of Molytinae, Anderson et al. (2018) included the Tranes group in this tribe. In the same year, Legalov (2018) erected a separate tribe for the group, but without providing any proper definition or diagnostic characters for such a tribe, listing only a few superficial differences from Amalactini (long known to be an unrelated New-World group). Our recent phylogenetic analysis, using mitogenomic data generated by low-coverage whole-genome sequencing (lcWGS) method and a reasonably comprehensive taxon sampling of Molytinae and the Tranes group (Hsiao et al. 2023), indicated the group to be more closely related to the tribes Molytini, Lixini and Pissodini than to Orthorhinini. A similar placement was found by the nuclear-gene phylogenomic analysis of Haran et al. (2023), showing Tranes nested in a clade comprising the tribes Cryptoplini, Cryptorhynchini, Lixini, Mesoptiliini, Molytini and Thecesternini (but Orthorhinini not sampled). Hsiao et al. (2023) also recovered a close relationship of the Tranes group with the South-east Asian genus Lyterius, previously misplaced in the tribe Madarini of Baridinae (Alonso-Zarazaga and Lyal 1999; Prena et al. 2023). An older tribal (family-group) name is founded on Lyterius, Lyteriides Lacordaire, 1866, although this was based on a misidentification of the genus (applied to an unrelated New-World taxon) and therefore treated as an invalid name by Alonso-Zarazaga and Lyal (1999). However, cases of misidentified type genera that threaten nomenclatural stability or universality have to be submitted to the Commission of the ICZN for a ruling (Art. 65.2.1), which has not been done for Lyteriides (Bouchard et al. 2011), so that this name remains available for a tribal entity including the true Lyterius and the Tranes group. Until this matter is resolved, we continue to refer to the Tranes group by this name. The concept and composition of Lyterius were recently revised by Prena et al. (2023).
The natural history of the Tranes group is varied. Lyterius (with five described species) is associated with Pandanus (Pandanaceae) (Prena et al. 2023), but the life histories of all species appear unknown; the larvae may develop in the flowers or fruits. The two species of Paratranes are exclusively associated with grasstrees (Xanthorrhoea, Asphodelaceae), the adults feeding on the flower stalks and also found in the green caudex of regenerating plants (Hsiao and Oberprieler 2021a) and the larvae apparently developing in the same plant organs. The single species of Howeotranes, H. insularis (Pascoe, 1874), which is restricted to Lord Howe Island, appears to be associated with the mountain palm Hedyscepe canterburyana (and perhaps also with the smaller Lepidorrhachis mooreana). It was once considered extinct (NSW Department of Environment and Climate Change 2007), but a few specimens have recently been found on Mount Gower on Hedyscepe flowers at night (C. Reid, pers. comm. 2024; Friends of Lord Howe Island 2024). The remaining 4 Australian genera, with 11 named species, are all truly associated with cycads of the genera Bowenia, Lepidozamia and Macrozamia and are here referred to as the cycad-associated Tranes group. The larvae of the two species of Demyrsus and the two of Siraton bore in trunks and caudices of moribund or dying plants of Lepidozamia and Macrozamia (Oberprieler and Caldara 2012; Hsiao and Oberprieler 2020b, 2021b), and these taxa are hence referred to as the cycad-trunk-boring Tranes group (Hsiao et al. 2023). By contrast, the seven named species of Miltotranes and Tranes are exclusively associated with cycad cones, the larvae developing in the sporophylls and rachis of male cones and pollen-dusted adults flying between cones, in at least some species visiting also receptive female cones and pollinating their ovules (hence called the cycad-pollinating Tranes group; Hsiao et al. 2023). Miltotranes is associated with Bowenia and comprises three species, M. subopacus (Lea, 1929) in eastern central Queensland associated with B. serrulata, M. prosternalis (Lea, 1929) in northern Queensland associated with B. spectabilis and M. wilsoni Hsiao & Oberprieler, 2022 in Far North Queensland associated with the isolated Bowenia population in the McIlwraith Range currently also treated as B. spectabilis (Hsiao and Oberprieler 2022). Tranes includes four named and several undescribed species (Forster et al. 1994; Zimmerman 1994; Oberprieler 1995a, 2004; Terry 2001; Hall et al. 2004; Terry et al. 2005; Pullen et al. 2014), which form four main lineages (Hsiao et al. 2023): the T. vigorsii lineage associated with Macrozamia in western Australia, the T. sparsus lineage associated with Macrozamia in western and eastern Australia, the T. insignipes lineage associated with Lepidozamia hopei in northern Queensland and the T. lyterioides lineage associated with Macrozamia and Lepidozamia peroffskyana in eastern Australia.
The taxonomy of the smaller genera of the Tranes group has recently been revised, of Demyrsus and Siraton by Hsiao and Oberprieler (2020b), of Paratranes by Hsiao and Oberprieler (2021a) and of Miltotranes by Hsiao and Oberprieler (2022), and this paper revises that of the largest genus, Tranes. It presents an integrative approach of combining morphological characters and molecular data to derive robust delimitations of species and species groups in the genus, redescribes the previously named species and describes six new ones, accompanied by illustrations of their diagnostic external and genital structures and their distribution, and it provides an identification key to all the genera and species of the Tranes group in Australia and an assessment of their host specificities.
Material and methods
Specimen repositories
The specimens examined in this study are deposited in the following collections:
ANIC, Australian National Insect Collection, CSIRO, Canberra, ACT, Australia.
DPIRD, Department of Primary Industries and Regional Development, Perth, WA, Australia.
MVMA, Museums Victoria, Melbourne, Vic., Australia.
NHMUK, Natural History Museum, London, UK.
QDPI, Queensland Department of Primary Industries, Brisbane, Qld, Australia.
QMBA, Queensland Museum, Brisbane, Qld, Australia.
SAMA, South Australian Museum, Adelaide, SA, Australia.
SMNH, Swedish Museum of Natural History (Naturhistoriska Riksmuseet), Stockholm, Sweden.
WAMPA, Western Australia Museum, Perth, WA, Australia.
Taxon sampling, sequencing, bioinformatic workflow and phylogenetic analyses
All the mitogenomic data used in the present study are derived from our project on the molecular phylogeny of Australian cycad-associated weevils using lcWGS data (NCBI BioProject: PRJNA862328) and downloaded from NCBI. The details of DNA extraction, library building and sequencing, bioinformatic workflow of data processing and sequence assembly and phylogenetic analyses are described by Hsiao et al. (2023, appendix S3). In brief, 85 specimens were sampled for this study, representing Demyrsus (4), Siraton (7), Miltotranes (7), Tranes (50), other genera of the Tranes group (10) and outgroups (7) (see Supplementary Table S1 for sample list). The taxon sampling includes all morphospecies that we were able to recognise except for Demyrsus digmon Hsiao & Oberprieler, from whose few old specimens we were unable to extract DNA. GenBank accession numbers for new and previously published data used in the analyses are provided in Supplementary Table S1. All mitochondrial protein-coding genes were aligned with MAFFT plugin (ver. 3.710, see https://www.geneious.com/plugins/mafft; Katoh and Standley 2013) implemented in Geneious Prime (ver. 2022.1.1, see https://www.geneious.com; Kearse et al. 2012) software. The concatenated nucleotide alignment of 10,191 bp (Supplementary File S1) was used to estimate phylogenetic relationships using maximum-likelihood inference in IQ-TREE (ver. 2.1.3, see http://www.iqtree.org/; Minh et al. 2020 Nguyen et al. 2015). The dataset was partitioned by gene (13 blocks) and codon position. The best-fitting partitioning scheme and substitution models were determined by ModelFinder (see http://www.iqtree.org/ModelFinder/; Lanfear et al. 2014; Kalyaanamoorthy et al. 2017). Statistical node support was estimated for each tree using Ultrafast Bootstrap (UFBoot) (Hoang et al. 2018) with 10,000 replicates and Shimodaira–Hasegawa approximate likelihood ratio test with 10,000 replicates (SH-aLRT) (Shimodaira and Hasegawa 1999). The resulting tree was visualised and edited using iTOL (see https://itol.embl.de/; Letunic and Bork 2021).
All samples referred to in this study were sourced ethically.
Molecular species delimitation
To delineate species boundaries and evaluate species diversity among the Australian cycad-associated weevils, we applied two distance-based methods, ABGD (Puillandre et al. 2012) and ASAP (Puillandre et al. 2021), and four tree-based approaches, bPTP and mlPTP (Zhang et al. 2013), mPTP (Kapli et al. 2017) and GMYC (Pons et al. 2006). As Automatic Barcode Gap Discovery (ABGD) and Assemble-Species-by-Automatic-Partitioning (ASAP) analyses aim to sort sequences into hypothetical species based on a ‘barcode’ gap, only COX1 gene sequences were used for the distance-based methods. The outgroups were excluded in order to improve the delimitation results, and specimens from which no COX1 sequences could be obtained were removed from the analyses as well. The final alignment of 70 taxa was uploaded to the ABGD webserver (see https://bioinfo.mnhn.fr/abi/public/abgd/abgdweb.html). Kimura (K80) distance was used for analysis with TS/TV = 2.0, P = 0.001–0.1 and X (relative gap width) = 0.05. The prior maximal distance was set as P = 0.02 following Jin et al. (2020). For the ASAP analysis, the same COX1 alignment was uploaded to the ASAP webserver (see https://bioinfo.mnhn.fr/abi/public/asap/). Kimura (K80) distance with TS/TV = 2.0 was used for analysis.
For the Poisson Tree Process (PTP) modelling analyses, the NT tree resulting from the previous IQ-TREE analysis was applied as input. The PTP analyses with Bayesian implementation (bPTP) and maximum-likelihood implementation (mlPTP) were performed using the PTP webserver (see https://species.h-its.org), and outgroups were excluded for the same reason. We used MCMC generation of 0.5 million and burn-in of 0.1, and trace was visualised in Tracer (ver. 1.7.2, see https://github.com/beast-dev/tracer/releases/tag/v1.7.2; Rambaut et al. 2018) to check for convergence. Multirate PTP analysis (mPTP) was performed using the webserver (see https://mptp.h-its.org/#/tree).
The General Mixed Yule Coalescent (GMYC) approach was applied based on the ultrametric tree reconstructed in BEAST 2 (ver. 2.6.6, see https://www.beast2.org/; Bouckaert et al. 2019) using the concatenated NT alignment. The GTR model and an uncorrelated relaxed-clock model (Drummond et al. 2006) with log-normal distribution were applied and MCMC generation set to 100 million. The first 25% of generations was discarded, a posterior-probability limit set to 0.5 and the log-likelihood trace visualised in Tracer to check for convergence. The ultrametric tree thus produced was then used to delimit species using GMYC utilising R and splits (ver. 1.0, T. Ezard, T. Fijisawa and T. Barraclough, see https://rdrr.io/rforge/splits/man/splits-package.html).
Morphological study, specimen preparation, photography and measurements
Morphological terms are used as in Oberprieler et al. (2014), and the methods of specimen preparation and measurements are those of Hsiao and Oberprieler (2020b). Photographs were mainly taken using a Leica DFC500 camera mounted on a Leica M205C stereomicroscope, except for photographs of the dorsal habitus of males of Tranes lyterioides and T. vigorsii, which were taken using a Dun Inc. BK Lab Plus system. Images taken at different focus planes were aligned and stacked using the software program Leica Application Suite (LAS; ver. 4.9, see https://www.leica-microsystems.com/products/microscope-software/p/leica-application-suite/) or Helicon Focus (see https://www.heliconsoft.com/heliconsoft-products/helicon-focus/; only for dorsal habitus of T. lyterioides and T. vigorsii) and edited using the software program Photoshop CS6 (Adobe Inc., see https://www.adobe.com/tw/products/photoshop.html). Some ultramorphological characteristics were illustrated by low-vacuum scanning-electron microscopy (SEM) using a Hitachi TM3030 Plus tabletop scanning electron microscope (5-kV acceleration voltage). Only specimens collected during the same collecting event as the holotype are designated as paratypes for new species. Label data of type specimens are cited verbatim, with a double slash (//) denoting data on different labels and a single slash (/) those on different lines on the same label. Label data of other specimens are standardised and sorted in alphabetical order of the localities to facilitate retrieval of distributional information. The full collection details of these specimens as given on their labels are provided in the ‘Full details of non-type specimens’ section in the Supplementary material. Abbreviation: ex., specimen(s) of unidentified sex.
Distribution maps
Locality data from specimen labels and the online database of the Atlas of Living Australia (see http://www.ala.org.au) were converted into standard GPS format (decimal degree) using Google Maps. These data were imported into GPS Visualizer (see www.gpsvisualizer.com) using the ‘JPEG map’ option. The map was created using the ‘OpenStreetMap (Mundialis)’ background. The final map was edited using QGIS (ver. 3.36.3, see https://www.qgis.org/) and Photoshop CS6.
Results
Morphological studies
Examination and evaluation of standard morphological characters as used in modern weevil taxonomy resulted in the identification and delineation of 10 species of Tranes, four described previously and six newly described here, one each in the T. sparsus and T. insignipes lineages and four in the T. lyterioides lineage. Together with the seven species recognised in the recent taxonomic revisions of the genera Demyrsus, Siraton and Miltotranes (Hsiao and Oberprieler 2020b, 2022), the cycad-associated weevil fauna in Australia now comprises 17 species and the entire Tranes group in Australia 20 species (adding the two species of Paratranes and one of Howeotranes).
Phylogenetic analysis
As shown in our phylogenetic analysis of the Australian cycad weevils (Hsiao et al. 2023, Fig. 2), the concepts of all 17 morphospecies of this group are phylogenetically supported, except for the populations of Miltotranes south of Cairns being identified as a morphologically indistinct but genetically divergent lineage from the M. prosternalis populations north of Cairns (and Demyrsus digmon not included) (Fig. 2).
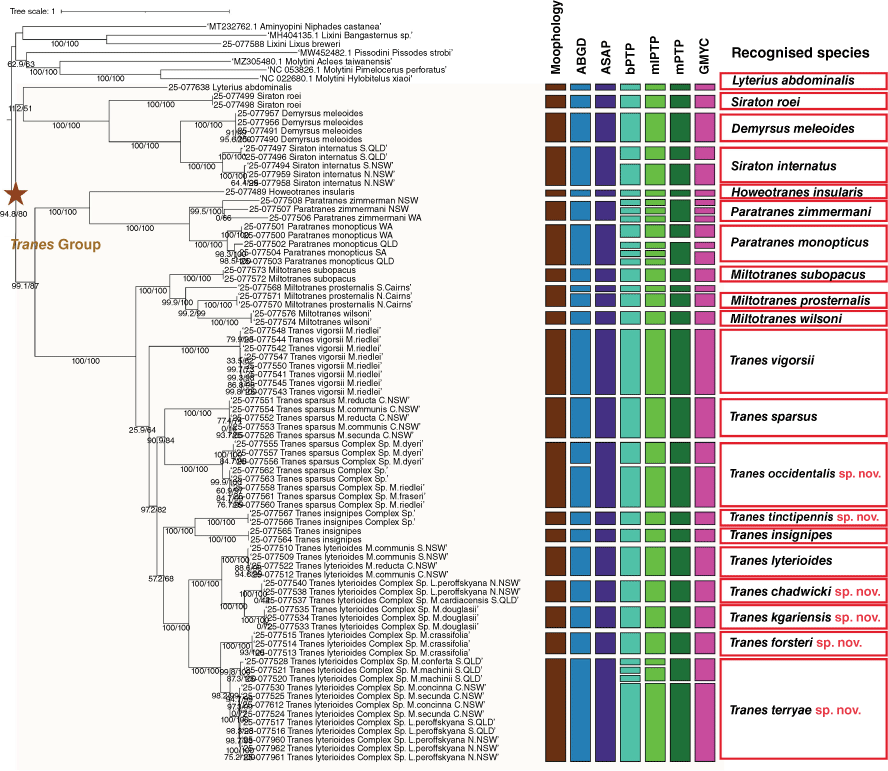
Maximum likelihood tree of Tranes group based on the nucleotide dataset of 13 mitochondrial protein-coding genes. Statistical support values presented as Shimodaira–Hasegawa approximate likelihood ratio test values (SH-aRLT)/Ultrafast Bootstrap (UFBoot). Species delimitation based on the morphology, Automatic Barcode Gap Discovery (ABGD), Assemble Species by Automatic Partitioning (ASAP), Poisson tree process modeling analyses with the Bayesian implementation (bPTP) and maximum likelihood implementation (mlPTP), multirate PTP analysis (mPTP) and General Mixed Yule-Coalescent (GMYC) are shown by bars next to species names.
Molecular species delimitation
Two distance-based methods (ABGD and ASAP) and four tree-based methods (bPTP, mlPTP, mPTP, GMYC) were used to estimate the number of species partitions (genetic or hypothetical species) contained in our genetic dataset and to assign individuals to these (Fig. 2). The ABGD analysis of 70 COX1 sequences identified 22 hypothetical species in the Tranes group (prior maximal distance (P) set as 0.02), including the single species of Lyterius incorporated in the taxon set. The ASAP analysis produced a best species partition (asap-score of 1.50) with 21 hypothetical species, largely congruent with the ABGD results except for lumping the two undescribed species of the T. sparsus lineage into a single one. By contrast, the bPTP analysis of the maximum-likelihood phylogenetic tree based on nucleotide data estimated 31 hypothetical species (the seven outgroups excluded), with support values ranging from 0.5 to 1.0 but 26 species having a support value >0.9, the larger number being due to much finer splitting of Paratranes in particular but also of the Tranes lyterioides complex. The result of the mlPTP analysis was very similar to that of the bPTP method, identifying 30 hypothetical species, with support values ranging from 0.5 to 1.0 and also 26 species having a support value >0.9. The mPTP analysis did not split Paratranes and the Tranes lyterioides complex as excessively and estimated 25 hypothetical species, but the GMYC analysis did so again in Paratranes and recovered 28 hypothetical species. The results produced by the two distance-based methods were largely congruent and also consistent with the morphological results, whereas the four tree-based methods were prone to split lineages along geographical lines (populations) into distinct species.
Taxonomic assessment
In order to avoid oversplitting species (into geographical but morphologically unrecognisable entities), only taxonomic units supported by both morphological characteristics and DNA evidence are considered to constitute natural species. Thus, 16 species of cycad-associated weevils of the Tranes group are recognised, including six unnamed species of Tranes, which are described as new species below. Together with Demyrsus digmon (not included in the analysis due to unavailability of genetic material) and the morphologically cryptic species of Miltotranes identified by the phylogenetic tree structure and all molecular delimitation methods, at least 18 species are currently identifiable among the Australian cycad weevils of the Tranes group. The genetic divergence revealed by the tree-based species delimitation methods in Siraton internatus, Tranes occidentalis and T. terryae indicates some geographical structure and possible isolation of populations that may also warrant species recognition, but denser and wider sampling of the taxa in question is necessary to explore this.
Taxonomy
Tranes group, incertae sedis
After Zimmerman (1994) and Hsiao and Oberprieler (2020b, 2022).
| 1 | Metanepisternal sutures lined with sclerolepidia (Fig. 3)...2 Metanepisternal sutures without sclerolepidia...5 |
| 2 | Dorsum of body and legs densely covered with brownish to whitish coarse setae (Hsiao and Oberprieler 2020b, fig. 3); antennal clubs as long as or longer than funicles (Hsiao and Oberprieler 2020b, fig. 7a, b); surface of pronotum punctorugulose (Hsiao and Oberprieler 2020b, fig. 8a, b)...3 (Demyrsus) Dorsum of body and legs lacking setae, at most sparsely and finely pubescent (Hsiao and Oberprieler 2020b, fig. 5); antennal clubs distinctly shorter than funicles (Hsiao and Oberprieler 2020b, fig. 7c, d); surface of pronotum sparsely punctate (Hsiao and Oberprieler 2020b, fig. 10a, b)...4 (Siraton) |
| 3 | Metaventrite and abdominal ventrites 1 and 2 of male medially indistinctly sparsely hirsute (Hsiao and Oberprieler 2020b, fig. 9a); ventrite 5 of male nearly glabrous, with ~10 short setae apically (Hsiao and Oberprieler 2020b, fig. 9c); penis with sides apically extended only into simple, broadly rounded lobes (Hsiao and Oberprieler 2020b, fig. 11d); distribution: coastal central New South Wales and southern Queensland...D. meleoides Metaventrite and abdominal ventrites 1 and 2 of male medially distinctly densely hirsute, especially on ventrites 1 and 2 (Hsiao and Oberprieler 2020b, fig. 9b); ventrite 5 of male distinctly hirsute, with ~20 longer setae apically (Hsiao and Oberprieler 2020b, fig. 9d); penis with sides apically in addition to broadly rounded lobes extended into a short, stout, anteriadly directed process proximally of the lobes (Hsiao and Oberprieler 2020b, fig. 11j); distribution: coastal northern Queensland...D. digmon |
| 4 | Pronotum densely and coarsely punctate, semilustrous (Hsiao and Oberprieler 2020b, fig. 10a); femora ventrally with small subapical tooth (Hsiao and Oberprieler 2020b, fig. 10e); rostrum in female as long as pronotum (Hsiao and Oberprieler 2020b, fig. 6b); distribution: eastern New South Wales and coastal Queensland...S. internatus Pronotum sparsely and finely punctate, lustrous (Hsiao and Oberprieler 2020b, fig. 10b); femora without ventral tooth (Hsiao and Oberprieler 2020b, fig. 10f); rostrum in female usually longer than pronotum (Hsiao and Oberprieler 2020b, fig. 6d); distribution: south-western Western Australia...S. roei |
| 5 | Body and legs completely black; procoxae contiguous (Hsiao and Oberprieler 2021a, fig. 1b)...6 Body and legs orange to dark brown; procoxae separate...8 |
| 6 | Large (length ≥11 mm); femora not sulcate beneath; elytra densely covered with coarse yellowish setae in apical third; distribution: Lord Howe Island...Howeotranes (H. insularis) Small to middle-sized (length ≤10 mm); femora distinctly sulcate beneath (Hsiao and Oberprieler 2021a, fig. 6c, d); elytral derm nearly nude, sparsely covered with very short setae (Hsiao and Oberprieler 2021a, fig. 1); distribution: mainland Australia...7 (Paratranes) |
| 7 | Pronotum narrow, sides weakly arcuate (Hsiao and Oberprieler 2021a, fig. 3a, b); femora ventrally with subapical tooth (Hsiao and Oberprieler 2021a, fig. 6c); protibial uncus distinct (Hsiao and Oberprieler 2021a, fig. 6e); tarsal claws thin (Hsiao and Oberprieler 2021a, fig. 6g); distribution: south-western Western Australia, eastern South Australia, coastal central New South Wales and coastal Queensland...P. monopticus Pronotum wide, sides strongly arcuate (Hsiao and Oberprieler 2021a, fig. 3c, d); femora ventrally without subapical tooth (Hsiao and Oberprieler 2021a, fig. 6d); protibial uncus minute (Hsiao and Oberprieler 2021a, fig. 6f); tarsal claws thick (Hsiao and Oberprieler 2021a, fig. 6h); distribution: south-western Western Australia and coastal central New South Wales...P. zimmermani |
| 8 | Forehead narrower than width of rostrum at base (Hsiao and Oberprieler 2022, fig. 3a); pronotum completely punctorugulose (Hsiao and Oberprieler 2022, fig. 4a–c); meso- and metatibiae with distal setal comb restricted to apical margin (Hsiao and Oberprieler 2022, fig. 4d–f)...9 (Miltotranes) Forehead as wide as width of rostrum at base; pronotum mostly flat, only punctorugulose laterally; meso- and metatibiae with distal setal comb extending to apical quarter of tibia (Fig. 4–26)...11 (Tranes) |
| 9 | Body uniformly dark brown (Hsiao and Oberprieler 2022, fig. 1c, d); antennae inserted in middle of rostrum in male, slightly behind middle in female (Hsiao and Oberprieler 2022, fig. 1c, d, 2c, d); abdominal ventrite 5 in female distinctly depressed posteriorly (Hsiao and Oberprieler 2022, fig. 7b); distribution: Byfield district of eastern Central Queensland...M. subopacus Body orange to dark red with large black macula in middle of elytra (Hsiao and Oberprieler 2022, fig. 1a, b, e, f); antennae inserted slightly before middle in male, in middle in female (Hsiao and Oberprieler 2022, fig. 1a, b, e, f, 2a, b, e, f); abdominal ventrite 5 in female flat, without depression (Hsiao and Oberprieler 2022, fig. 7a, c); distribution: northern Queensland...10 |
| 10 | Pronotum with a pair of triangular black marks at posterior margin and black elytral macula irregular, broken and mosaic-like (Hsiao and Oberprieler 2022, fig. 1a, b); pronotal and elytral setae densely distributed, clustered in parts to somewhat obscure derm (Hsiao and Oberprieler 2022, fig. 1a, b, 4a); pronotum ~0.8–0.9× as broad as elytra at humeri (Hsiao and Oberprieler 2022, fig. 1a, b); protibiae ~6.0× as long as wide (Hsiao and Oberprieler 2022, fig. 6a); distribution: Far North Queensland around Cairns...M. prosternalis Pronotum unicolourous, without black marks, and black elytral macula entire (Hsiao and Oberprieler 2022, fig. 1e, f); pronotal and elytral setae sparsely distributed (Hsiao and Oberprieler 2022, fig. 1e, f, 4c); pronotum ~0.7–0.8× as broad as elytra at humeri (Hsiao and Oberprieler 2022, fig. 1e, f); protibiae ~7.5× as long as wide (Hsiao and Oberprieler 2022, fig. 6c); distribution: McIlwraith Range of Far North Queensland...M. wilsoni |
| 11 | Pronotum with a pair of black marks at base medially, elytra with black anterior margin and a pair of irregular black marks medially...12 (T. insignipes lineage) Pronotum and elytra unicolourous...13 |
| 12 | Rostrum ventrally distinctly curved (Fig. 9a, b); antennae inserted slightly behind middle of rostrum in both sexes; pronotal collar with teeth (Fig. 13a); pro- and mesotibiae without tooth (Fig. 17a); distribution: Far North Queensland, mainly south of Cairns...T. insignipes Rostrum nearly straight (Fig. 9c, d); antennae inserted slightly before middle of rostrum in male, in middle in female; pronotal collar without teeth (Fig. 13b); pro- and mesotibiae with well-developed tooth medially (Fig. 17b); distribution: Far North Queensland, mainly north of Cairns...T. tinctipennis |
| 13 | Large (length ≥11 mm, usually 13–14 mm); rostrum of male and female subequal in length (Fig. 9i, j); distribution: south-western Western Australia...T. vigorsii (T. vigorsii lineage) Small to medium-sized (length <11 mm, ~3.5–10.5 mm); rostrum longer in female (Fig. 9e–h, 10)...14 |
| 14 | Medium-sized (length ~6.0–10.5 mm); body elongate–oval (Fig. 7e, h); prosternum of male flat, without prominent protuberance (Fig. 14c, d); rostrum of female ~1.3–1.5× as long as pronotum (Fig. 9f, h)...15 (T. sparsus lineage) Small (length ~3.5–8.5 mm); body broadly oval (Fig. 8); prosternum of male convex, with strongly prominent protuberance (Fig. 14f–j); rostrum of female ~1.5–1.7× as long as pronotum (Fig. 10b, d, f, h, j)...16 (T. lyterioides lineage) |
| 15 | Antennae inserted slightly before middle of rostrum in male, in middle in female (Fig. 9e, f); prosternum of male without tubercles (Fig. 14c); protibiae of male without tibial brush; abdominal ventrite 5 of female with large depression posteriorly (Fig. 19f); distribution: eastern central New South Wales...T. sparsus Antennae inserted in middle of rostrum in male, slightly behind middle in female (Fig. 9g, h); prosternum of male with small tubercles (Fig. 14d); protibiae of male with well-developed tibial brush; abdominal ventrite 5 of female with apunctate rounded patch posteriorly (Fig. 19h); distribution: south-western Western Australia...T. occidentalis |
| 16 | Body dark red and legs dark red to black (Fig. 8e, f); body weakly convex (Fig. 10e, f); antennae inserted before middle of rostrum in male (Fig. 10e); abdominal ventrites 1 and 2 deeply depressed (Fig. 18h); distribution: coastal south-east Queensland...T. forsteri Body and legs completely reddish brown (Fig. 8a–d, g–j); body nearly flat (Fig. 10a–d, g–j); antennae inserted in middle or behind middle of rostrum in male (Fig. 10a, c, g, i); abdominal ventrites 1 and 2 slightly depressed (Fig. 18f, g, i, j)...17 |
| 17 | Body and legs densely covered with setae (Fig. 8i, j); pronotum of male distinctly narrower than elytra; prosternal protuberance of male without ridged anterior face (Fig. 14j); distribution: north-eastern New South Wales...T. terryae Body and legs sparsely to moderately covered with setae (Fig. 8a–d, g, h); pronotum of male nearly as wide as elytra; prosternal protuberance of male with ridged anterior face (Fig. 14f, g, i)...18 |
| 18 | Antennae inserted behind middle of rostrum in male (Fig. 10g); prosternal protuberance of male with strongly and sharply ridged anterior face (Fig. 14i); median pit on mesoventrite shallowly depressed (Fig. 16i); distribution: K’gari (Fraser Island) in south-east Queensland...T. kgariensis Antennae inserted in middle of rostrum in male (Fig. 10a, c); prosternal protuberance of male with moderately to weakly ridged anterior face (Fig. 14f, g); median pit on mesoventrite deeply depressed (Fig. 16f, g); distribution: New South Wales and southern Queensland...19 |
| 19 | Prosternal protuberance of male with weakly ridged anterior face (Fig. 14g); prosternellum distinctly shorter than wide (Fig. 14g); elytra distinctly narrowing apicad in apical third (Fig. 8c, d); penis ~0.4 mm long, subtruncate apically (Fig. 25c); distribution: coastal south-east Queensland...T. chadwicki Prosternal protuberance of male with moderately ridged anterior face (Fig. 14f); prosternellum nearly as long as wide (Fig. 14f); elytra distinctly narrowing apicad in apical quarter (Fig. 8a, b); penis ~0.5 mm long, rounded apically (Fig. 25a); distribution: coastal New South Wales...T. lyterioides |
Genus Tranes Schoenherr, 1843
Tranes Schoenherr, 1843, p. 129. – Lacordaire 1863, p. 508 (key, taxonomy). Pascoe 1870, p. 199 (taxonomy), 1874, p. 387 (taxonomy). Gemminger 1871, p. 2451 (catalogue). Westwood 1886, p. 128 (infestation of Siraton internatus; as Tranes). Olliff 1889, p. 91 (faunistic record of Howeotranes insularis; as Tranes). Froggatt 1896, p. 77 (ecology of Paratranes; as Tranes). Lea 1898, p. 592 (taxonomy), 1929, pp. 537, 539 (key, taxonomy). Walker 1906, p. 23 (ecology of Siraton internatus and T. sparsus). Schenkling and Marshall 1936, p. 1 (catalogue). Marshall 1939, p. 582 (synonymy). Mulder 1964, p. 12 (ecology of Paratranes monopticus; as Tranes). Covassi 1974, p. 215 (morphological comparison between Demyrsus and Siraton; as Tranes). Glass 1980, p. 7 (infestation of Siraton internatus; as Tranes). Hawkeswood 1985, p. 164 (ecology of Paratranes monopticus; as Tranes). Kuschel 1987, p. 13 (classification). Ornduff 1989, p. 243 (ecology), 1990, p. 96 (ecology of Siraton internatus; as Tranes), 1991, p. 10 (ecology), 1993, pp. 122, 123 (ecology of T. vigorsii and Siraton internatus; as Tranes). Kennedy 1991, p. 22 (ecology), 1992, p. 20 (infestation of Siraton internatus; as Tranes), 2011, p. 14 (infestation of Siraton internatus; as Tranes). Crowson 1992 (ecology). Chadwick 1993, p. 78 (ecology), 1998, p. 15 (ecology), 1999, p. 15 (ecology). Zimmerman 1992, p. 580 (colour photographs), 1994, pp. 694, 696 (key, classification). Connell and Ladd 1993, p. 98 (pollination biology). Jones 1993, p. 59 (ecology), 2002, p. 51 (ecology). May 1993, p. 12 (conservatism of larval traits), 1994, pp. 618, 625 (key, larva). Wilson 1993, p. 14 (ecology of Miltotranes subopacus; as Tranes), 2002, p. 440 (ecology). Forster et al. 1994, p. 218 (ecology). Oberprieler 1995a, pp. 305, 329 (classification, catalogue, ecology), 1995b, p. 338 (ecology), 1997, p. 25 (ecology), 2004, pp. 174, 183 (classification, ecology). Forster 1996, p. 13 (ecology). Wilson and Rowles 1997, p. 14 (ecology). Marvaldi and Morrone 1998, p. 104 (classification). Alonso-Zarazaga and Lyal 1999, p. 210 (catalogue). Hill and Osborne 2001, p. 4 (ecology). Terry 2001, p. 1293 (pollination biology). Hall et al. 2004, p. 334 (pollination biology). Terry et al. 2004, p. 234, 2005, p. 931, 2012, pp. 356, 363 (pollination biology). Lyal et al. 2006, p. 236 (morphological comparison). Downie et al. 2008, p. 114 (pollination biology). Oberprieler and Caldara 2012, p. 57 (classification, ecology). Wallenius et al. 2012, p. 397 (pollination biology). Lyal 2014, p. 560 (classification, ecology). Pullen et al. 2014, p. 289 (catalogue). Wallenius 2014, p. 26 (pollination biology). Anderson et al. 2018, p. 2 (classification). Legalov 2018, p. 345 (key, catalogue, classification). Toon et al. 2020, p. 1044 (ecology). Hsiao and Oberprieler 2020a, p. 372 (ecology), 2020b, p. 677 (classification), 2021a, pp. 122, 128 (morphological comparison, ecology), 2022, p. 3 (morphological comparison), 2024, pp. 18, 19 (taxonomy of Zimmiodes Hsiao & Oberprieler; morphological comparison, ecology). Suinyuy and Johnson 2021, p. 234 (ecology). Hsiao et al. 2023, [pp. 1–11] (biology, composition, phylogenetic relationships). Haran et al. 2023, [pp. 1–11] (phylogenetic relationships). Prena et al. 2023, p. 27 (taxonomy of Lyterius; phylogenetic relationships).
Type species, by original designation: Tranes vigorsii Boheman, 1843.
Platyphaeus Pascoe, 1875, p. 66. – Hustache 1938, p. 158 (catalogue). Marshall 1939, p. 582 (synonymy). Alonso-Zarazaga and Lyal 1999, p. 210 (catalogue). Pullen et al. 2014, p. 289 (catalogue).
Type species, by monotypy: Platyphaeus lyterioides Pascoe, 1875.
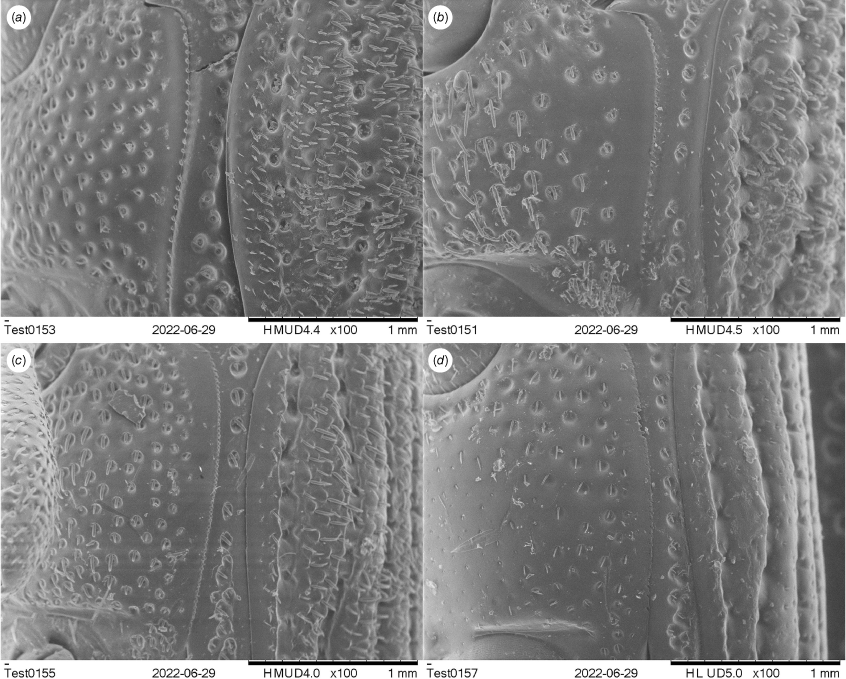
Body broadly oval to elongate–oval, length ~3.5–15.0 mm in both sexes, width ~0.4–0.5× length (Fig. 7, 8). Body and legs dark red to reddish brown, semilustrous, covered with yellowish, coarse, sublanceolate and subsquamiform setae, setae condensed in clusters in some parts (Fig. 7–10). Rostrum moderately long, usually longer in females, slightly to strongly downcurved (Fig. 9, 10). Eyes dorsally well separated, with forehead as wide as rostrum at base (Fig. 4a); ventrally narrowly separated, interocular distance variable, narrower to wider than distance between procoxae. Antennae inserted slightly before to behind middle of rostrum in males, in middle to behind middle in females; funicles distinctly 7-segmented, segments 1 and 2 longer than remaining segments, 2 longer than apical width of scape and shorter than segments 3 + 4; clubs stout and short, distinctly shorter than funicles (Fig. 11). Pronotum roundly trapezoidal, as wide as to narrower than elytra at humeri, sides weakly to strongly arcuate; surface sparsely and distinctly punctate, weakly to strongly punctorugulose laterally, with elongate longitudinal apunctate region medially (Fig. 12). Prothorax anteriorly strongly constricted into short collar, margin of collar laterally not extended into ocular lobes, with sparse small teeth in males of most species (Fig. 13); prosternum sparsely and finely punctate, densely covered with small tubercles in males of most species, forming pair of prominent protuberances or with large, elevated plate in males of some species (Fig. 14); procoxal cavities narrowly to widely separate; prosternellum elongate to broad, widened posteriorly; intermesocoxal process trapezoidal, sparsely to densely setose (Fig. 15); mesoventrite with median pit (Fig. 16), metanepisterna without sclerolepidia. Elytra oval, jointly ~0.6–0.7× as broad as long, sides narrowing apicad; surface nearly flat, without well-developed tubercles. Femora with ventral tooth; protibiae with well-developed inner tibial brush in males of most species (Fig. 4b); meso- and metatibiae with distal setal combs continued around apex and extending to apical fourth of tibia (Fig. 4c). Terminalia: tergite VII of males transverse, moderately emarginate medially (Fig. 4d), of females subtrapezoidal, posterior margin subtruncate with posterior angles obtuse (Fig. 4e) or strongly truncate with posterior angles angular (Fig. 4f); tergite VIII of males subtrapezoidal to subquadrate, with posterior margin subtruncate to rounded (Fig. 5a, b), of females subtrapezoidal or subtriangular, distinctly narrowing apicad laterally, with posterior margin subtruncate to rounded (Fig. 5c, d); sternite VIII of males narrowly roundly subtrapezoidal or angular at posterior margin, largely sclerotised but medially membranous, of females with sclerotised parts of apical lobes slender, linear, laterally slightly curved or abruptly angled (Fig. 21); spiculum gastrale asymmetrical, widely concave apically or pointed, not concave apically; tegmen with complete oval ring, manubrium longer to shorter than parameroid lobes (Fig. 22); penis thick to elongate (~1.4–2.7× as long as wide), distinctly narrowing apicad in apical third to fifth; endophallus apically with basal membranous sleeve of asperities, apically without or with anchor-shaped or crescentic sclerite, medially without or with a complex sclerite composed of omega (Ω) shaped and dentate sclerites (Fig. 23–25); ovipositor short, moderately longer than wide, gonocoxites basally broad, narrowing apicad, apically bluntly rounded; gonostyli cylindrical, setose apically; bursa copulatrix without bands of spicules; spermatheca thick, right-angled, gland small, swollen, narrowing apicad or elongate, rounded apically (Fig. 26).
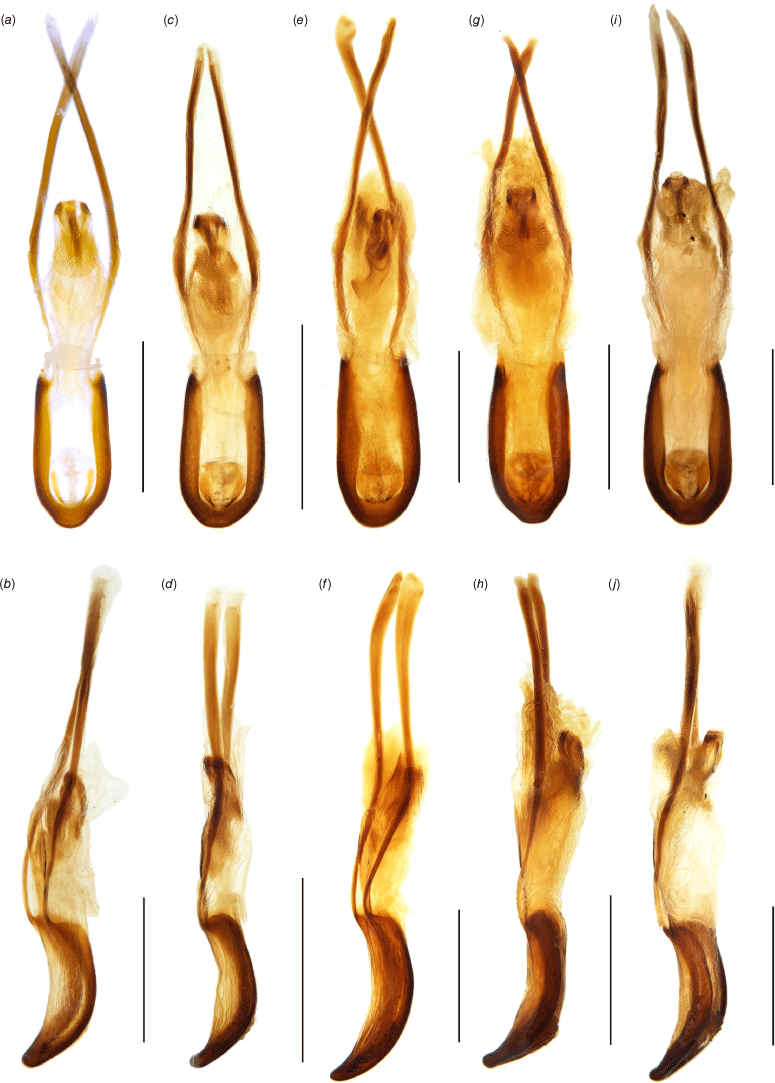
Diagnostic external characters and terminalia of Tranes. (a) T. vigorsii, head and base of rostrum, dorsal view; (b) T. vigorsii, left protibia of male, dorsal view; (c) T. vigorsii, left mesotibia of male, dorsal view; (d) T. vigorsii, tergite VII of male, dorsal view; (e) T. vigorsii, tergite VII of female, dorsal view; (f) T. insignipes, tergite VII of female, dorsal view. Scale bars: (a–c) 1.0 mm; (d–f) 0.5 mm.
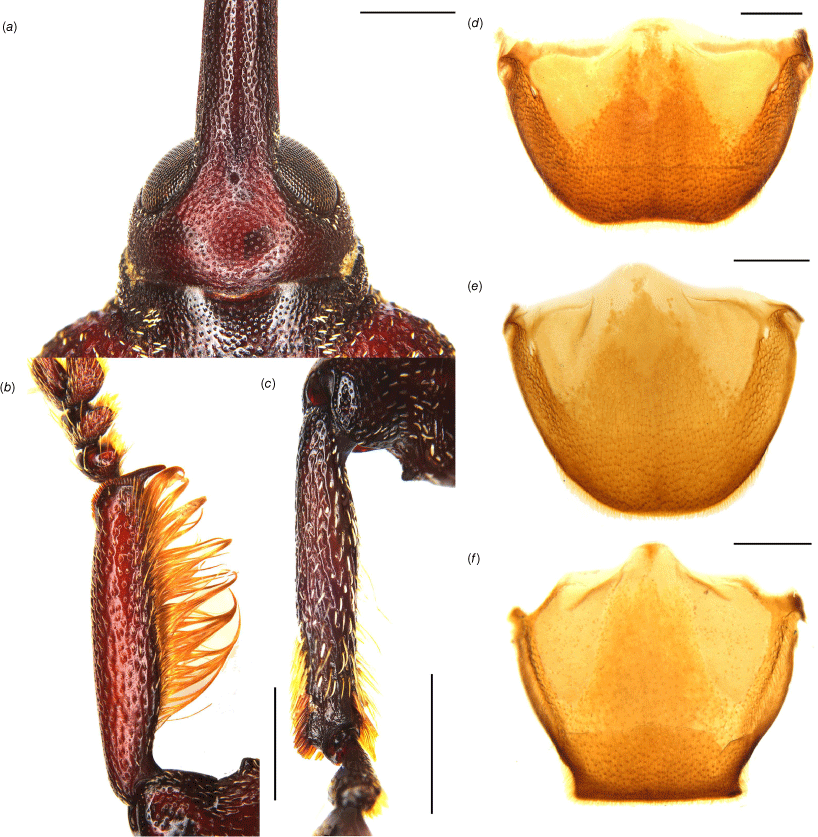
Diagnostic terminalia of Tranes. (a) T. vigorsii, tergite VIII of male, dorsal view; (b) T. insignipes, tergite VIII of male, dorsal view; (c) T. vigorsii, tergite VIII of female, dorsal view; (d) T. sparsus, tergite VIII of female, dorsal view. Scale bars: 0.5 mm.
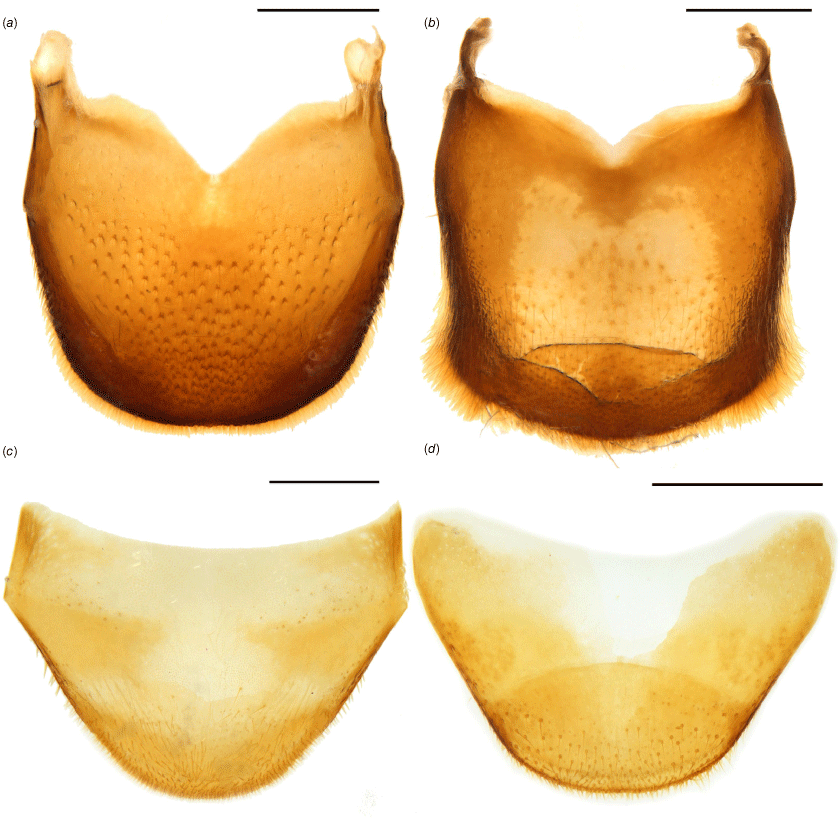
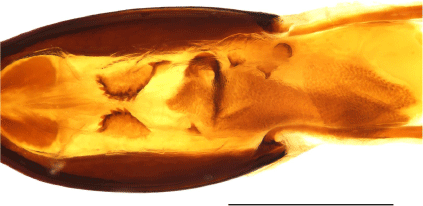
Name-bearing types of Tranes. (a) T. insignipes; (b) T. sparsus; (c) Platyphaeus lyterioides.
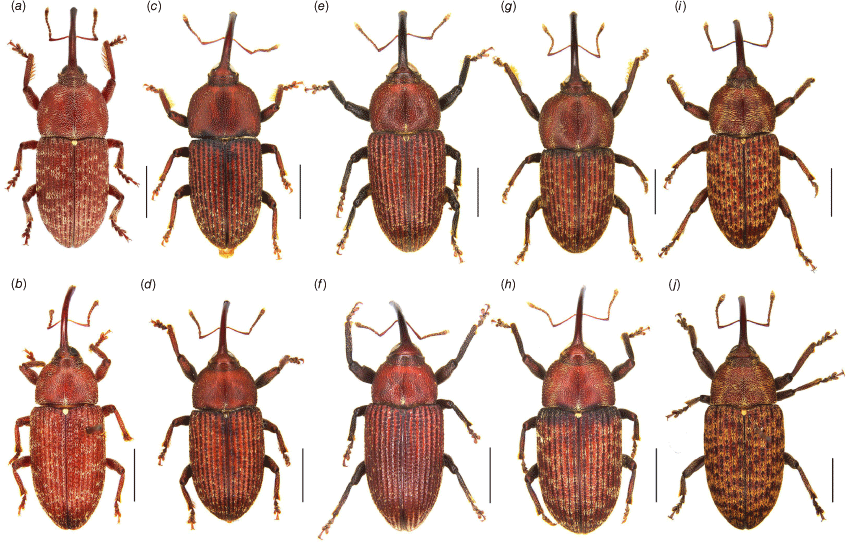
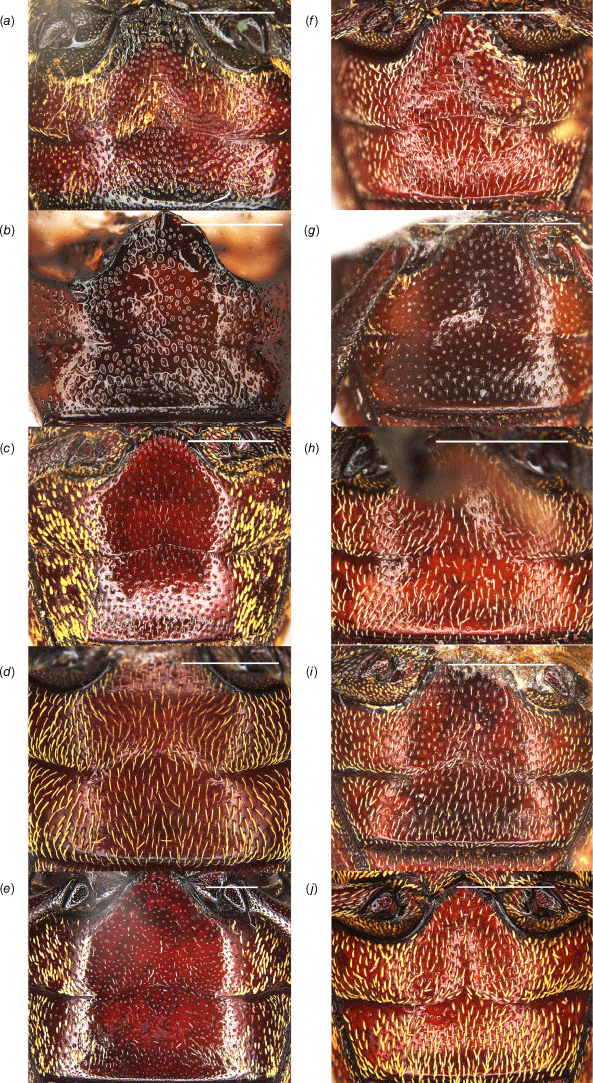
Habitus of Tranes adults, dorsal view. (a) T. insignipes, male; (b) T. insignipes, female; (c) T. tinctipennis, male, holotype; (d) T. tinctipennis, female; (e) T. sparsus, male; (f) T. sparsus, female; (g) T. occidentalis, male, holotype; (h) T. occidentalis, female; (i) T. vigorsii, male; (j) T. vigorsii, female. Scale bars: 5.0 mm.
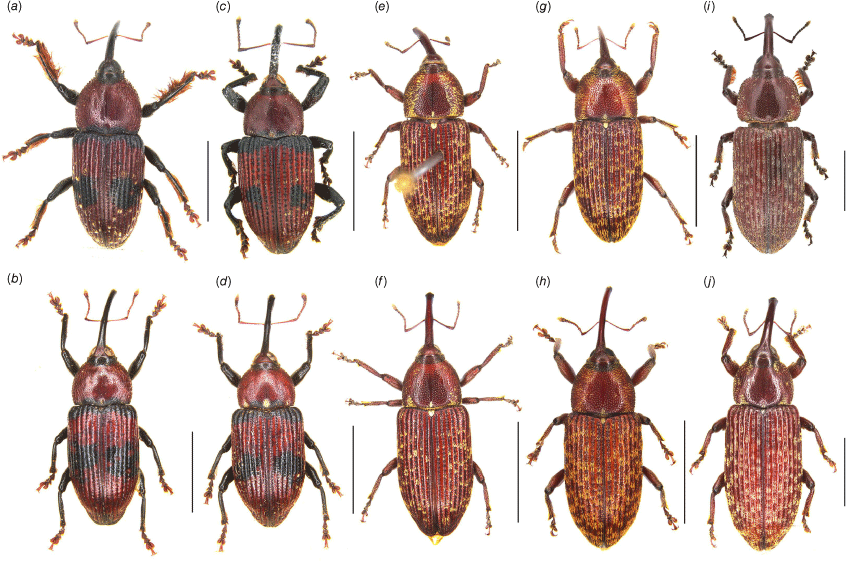
Habitus of Tranes adults, dorsal view. (a) T. lyterioides, male; (b) T. lyterioides, female; (c) T. chadwicki, male, holotype; (d) T. chadwicki, female; (e) T. forsteri, male, holotype; (f) T. forsteri, female; (g) T. kgariensis, male, holotype; (h) T. kgariensis, female; (i) T. terryae, male, holotype; (j) T. terryae, female. Scale bars: 2.0 mm.
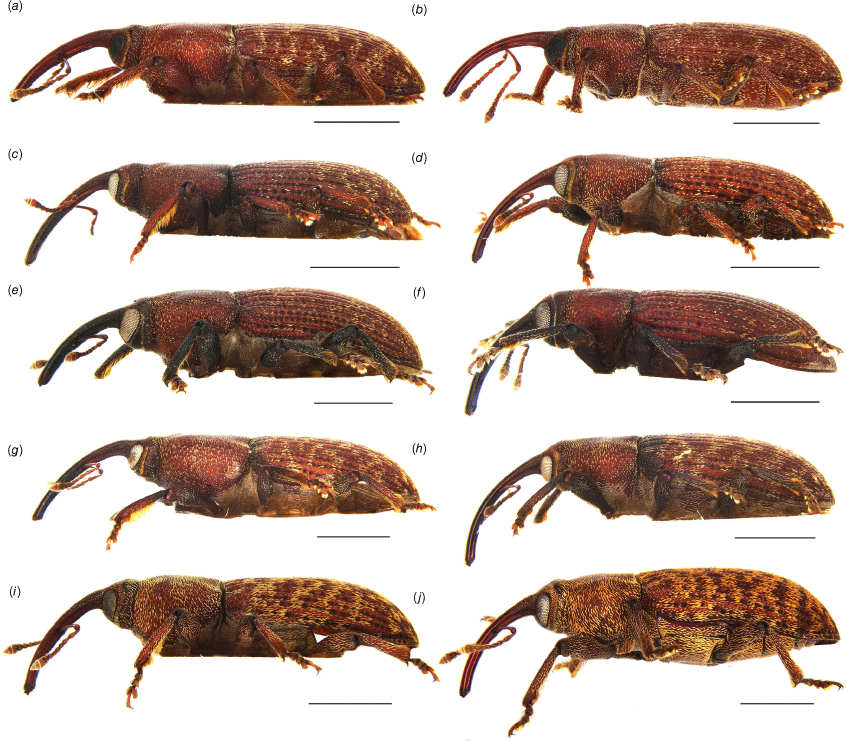
Habitus of Tranes adults, lateral view. (a) T. insignipes, male; (b) T. insignipes, female; (c) T. tinctipennis, male, holotype; (d) T. tinctipennis, female; (e) T. sparsus, male; (f) T. sparsus, female; (g) T. occidentalis, male, holotype; (h) T. occidentalis, female; (i) T. vigorsii, male; (j) T. vigorsii, female. Scale bars: 5.0 mm.
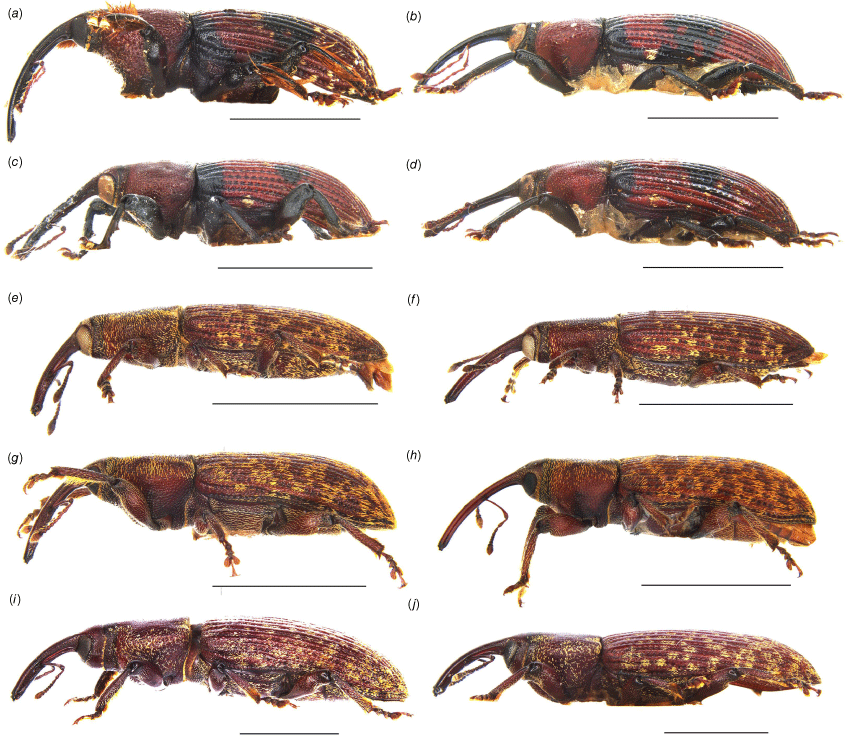
Habitus of Tranes adults, lateral view. (a) T. lyterioides, male; (b) T. lyterioides, female; (c) T. chadwicki, male, holotype; (d) T. chadwicki, female; (e) T. forsteri, male, holotype; (f) T. forsteri, female; (g) T. kgariensis, male, holotype; (h) T. kgariensis, female; (i) T. terryae, male, holotype; (j) T. terryae, female. Scale bars: 2.0 mm.
Left antenna of Tranes weevils. (a) T. insignipes; (b) T. tinctipennis; (c) T. sparsus; (d) T. occidentalis; (e) T. vigorsii; (f) T. lyterioides; (g) T. chadwicki; (h) T. forsteri; (i) T. kgariensis; (j) T. terryae. Scale bars: 0.5 mm.

Pronotum of Tranes species, males. (a) T. insignipes; (b) T. tinctipennis; (c) T. sparsus; (d) T. occidentalis; (e) T. vigorsii; (f) T. lyterioides; (g) T. chadwicki; (h) T. forsteri; (i) T. kgariensis; (j) T. terryae. Scale bars: 1.0 mm.
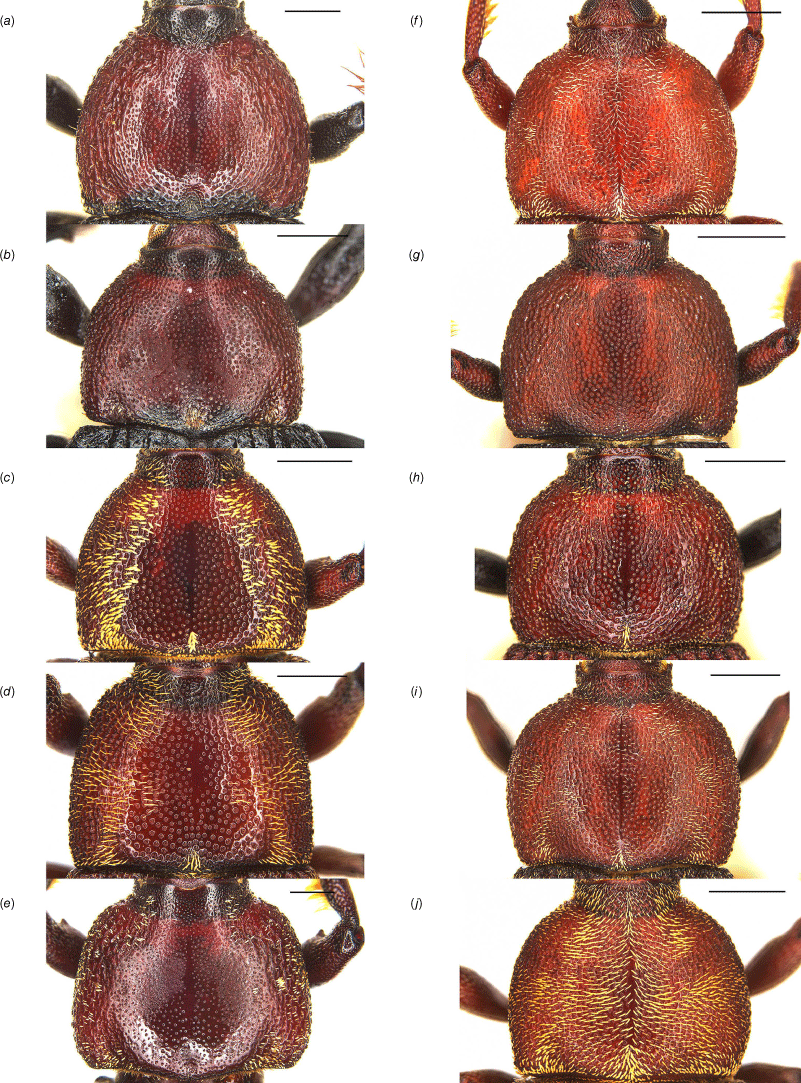
Pronotal collar of Tranes species, males. (a) T. insignipes; (b) T. tinctipennis; (c) T. sparsus; (d) T. occidentalis; (e) T. vigorsii; (f) T. lyterioides; (g) T. chadwicki; (h) T. forsteri; (i) T. kgariensis; (j) T. terryae. Scale bars: 0.5 mm.
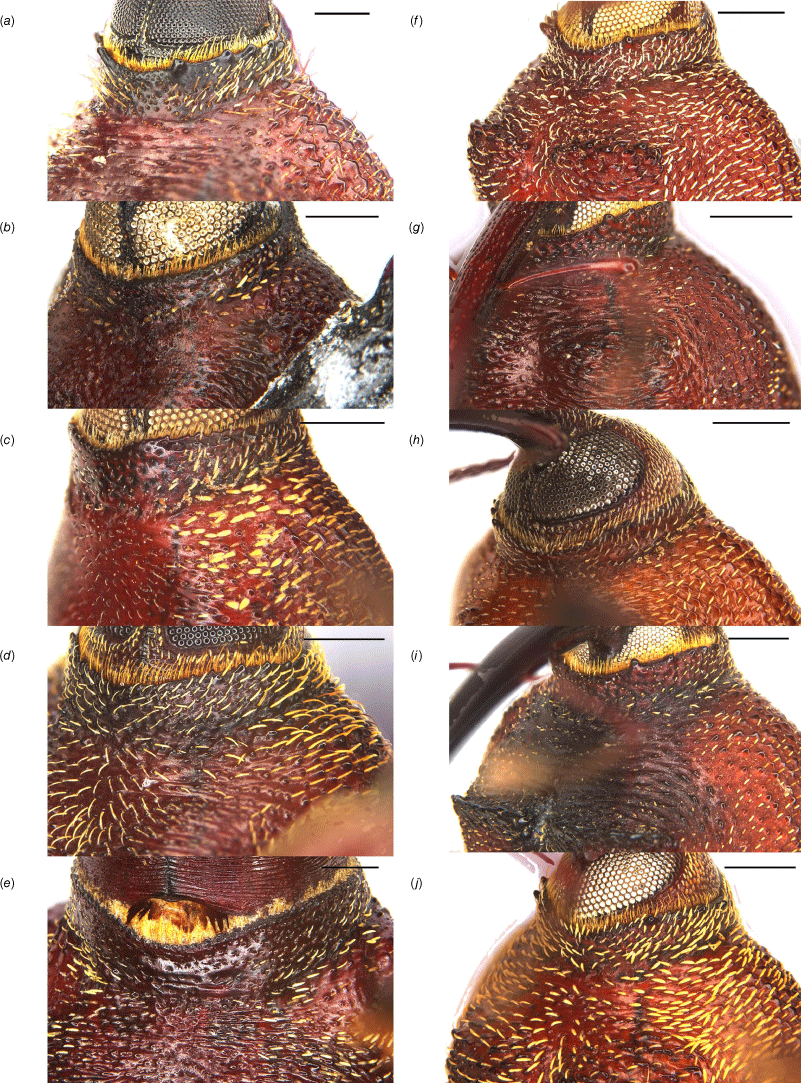
Prosternum of Tranes species, males. (a) T. insignipes; (b) T. tinctipennis; (c) T. sparsus; (d) T. occidentalis; (e) T. vigorsii; (f) T. lyterioides; (g) T. chadwicki; (h) T. forsteri; (i) T. kgariensis; (j) T. terryae. Scale bars: 0.5 mm.
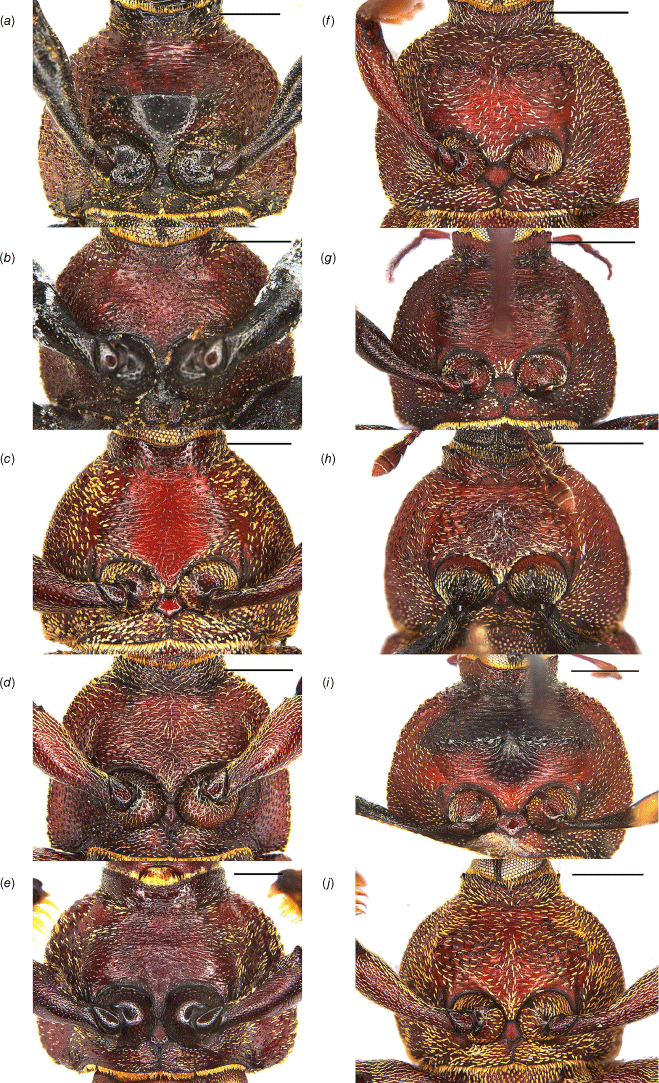
Intermesocoxal process of Tranes species, males. (a) T. insignipes; (b) T. tinctipennis; (c) T. sparsus; (d) T. occidentalis; (e) T. vigorsii; (f) T. lyterioides; (g) T. chadwicki; (h) T. forsteri; (i) T. kgariensis; (j) T. terryae. Scale bars: 0.5 mm.
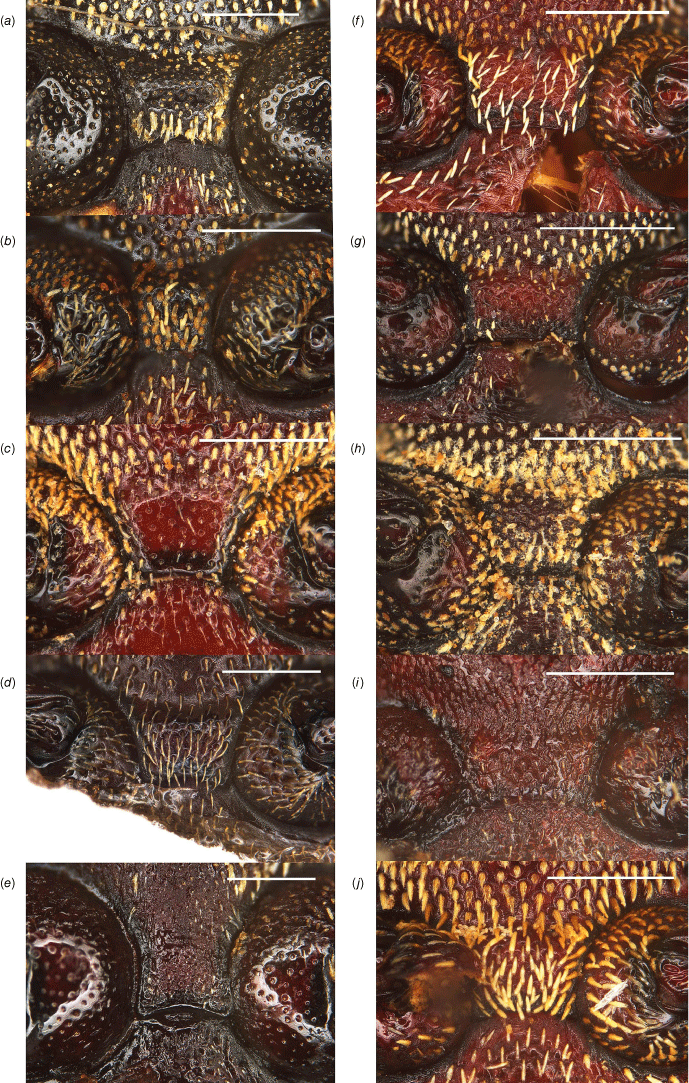
Median pit on mesoventrite of Tranes species, males. (a) T. insignipes; (b) T. tinctipennis; (c) T. sparsus; (d) T. occidentalis; (e) T. vigorsii; (f) T. lyterioides; (g) T. chadwicki; (h) T. forsteri; (i) T. kgariensis; (j) T. terryae. Scale bars: 0.5 mm.
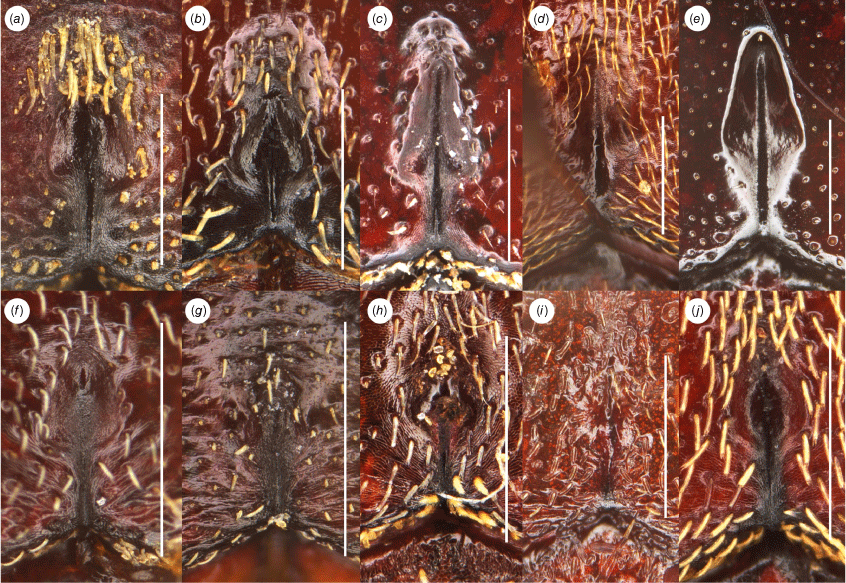
Left protibia of male of Tranes insignipes lineage, dorsal view. (a) T. insignipes; (b) T. tinctipennis. Scale bars: 1.0 mm.

Abdominal ventrite 1 and 2 of Tranes species, males. (a) T. insignipes; (b) T. tinctipennis; (c) T. sparsus; (d) T. occidentalis; (e) T. vigorsii; (f) T. lyterioides; (g) T. chadwicki; (h) T. forsteri; (i) T. kgariensis; (j) T. terryae. Scale bars: 1.0 mm.
Abdominal ventrite 5 of Tranes species. (a) T. insignipes, male; (b) T. insignipes, female; (c) T. tinctipennis, male; (d) T. tinctipennis, female; (e) T. sparsus, male; (f) T. sparsus, female; (g) T. occidentalis, male; (h) T. occidentalis, female; (i) T. vigorsii, male; (j) T. vigorsii, female. Scale bars: 0.5 mm.

Abdominal ventrite 5 of Tranes species. (a) T. lyterioides, male; (b) T. lyterioides, female; (c) T. chadwicki, male; (d) T. chadwicki, female; (e) T. forsteri, male; (f) T. forsteri, female; (g) T. kgariensis, male; (h) T. kgariensis, female; (i) T. terryae, male; (j) T. terryae, female. Scale bars: 0.5 mm.

Abdominal sternite VIII of Tranes species. (a) T. insignipes, male; (b) T. insignipes, female; (c) T. tinctipennis, male; (d) T. tinctipennis, female; (e) T. sparsus, male; (f) T. sparsus, female; (g) T. occidentalis, male; (h) T. occidentalis, female; (i) T. vigorsii, male; (j) T. vigorsii, female; (k) T. lyterioides, male; (l) T. lyterioides, female; (m) T. chadwicki, male; (n) T. chadwicki, female; (o) T. forsteri, male; (p) T. forsteri, female; (q) T. kgariensis, male; (r) T. kgariensis, female; (s) T. terryae, male; (t) T. terryae, female. Scale bars: 0.5 mm.
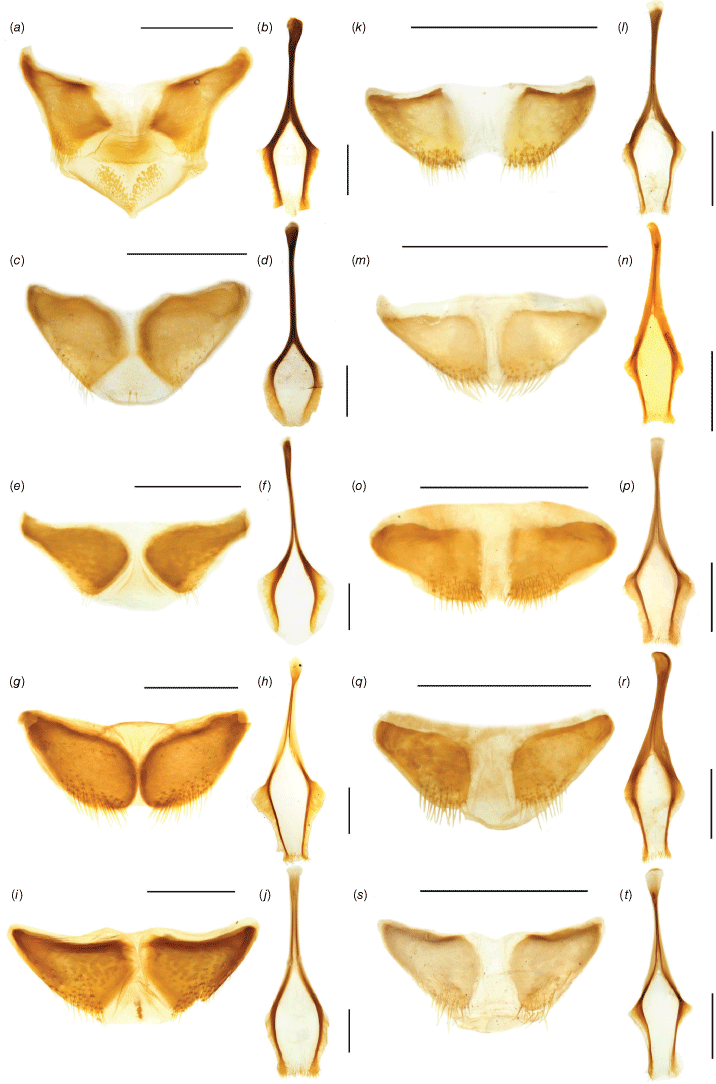
Spiculum gastrale and tegmen ring of Tranes species, males. (a) T. insignipes, spiculum gastrale; (b) T. insignipes, tegmen ring; (c) T. tinctipennis, spiculum gastrale; (d) T. tinctipennis, tegmen ring; (e) T. sparsus, spiculum gastrale; (f) T. sparsus, tegmen ring; (g) T. occidentalis, spiculum gastrale; (h) T. occidentalis, tegmen ring; (i) T. vigorsii, spiculum gastrale; (j) T. vigorsii, tegmen ring; (k) T. lyterioides, spiculum gastrale; (l) T. lyterioides, tegmen ring; (m) T. chadwicki, spiculum gastrale; (n) T. chadwicki, tegmen ring; (o) T. forsteri, spiculum gastrale; (p) T. forsteri, tegmen ring; (q) T. kgariensis, spiculum gastrale; (r) T. kgariensis, tegmen ring; (s) T. terryae, spiculum gastrale; (t) T. terryae, tegmen ring. Scale bars: 0.5 mm.
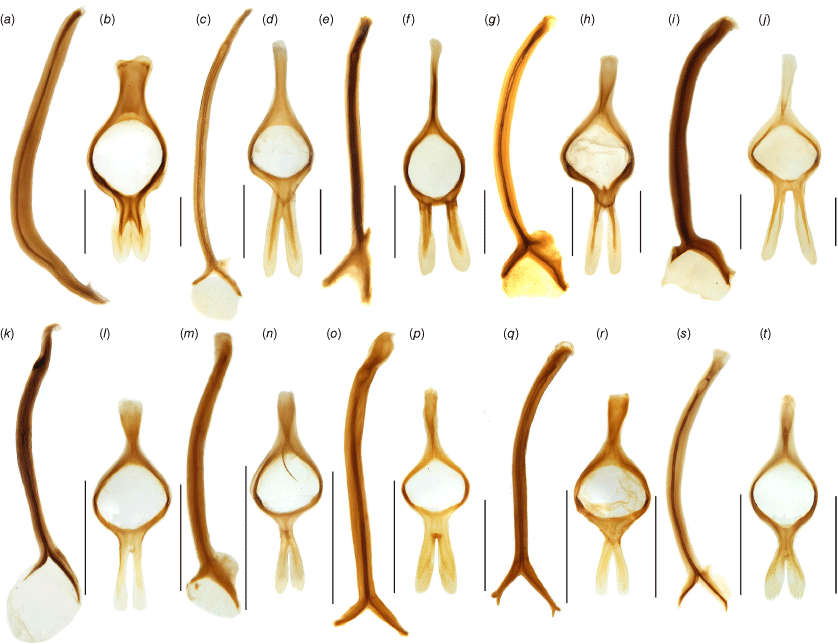
Penis of Tranes species. (a) T. insignipes, dorsal view; (b) T. insignipes, lateral view; (c) T. tinctipennis, dorsal view; (d) T. tinctipennis, lateral view; (e) T. sparsus, dorsal view; (f) T. sparsus, lateral view; (g) T. occidentalis, dorsal view; (h) T. occidentalis, lateral view; (i) T. vigorsii, dorsal view; (j) T. vigorsii, lateral view. Scale bars: 0.5 mm.

Penis of Tranes species. (a) T. lyterioides, dorsal view; (b) T. lyterioides, lateral view; (c) T. chadwicki, dorsal view; (d) T. chadwicki, lateral view; (e) T. forsteri, dorsal view; (f) T. forsteri, lateral view; (g) T. kgariensis, dorsal view; (h) T. kgariensis, lateral view; (i) T. terryae, dorsal view; (j) T. terryae, lateral view. Scale bars: 0.5 mm.
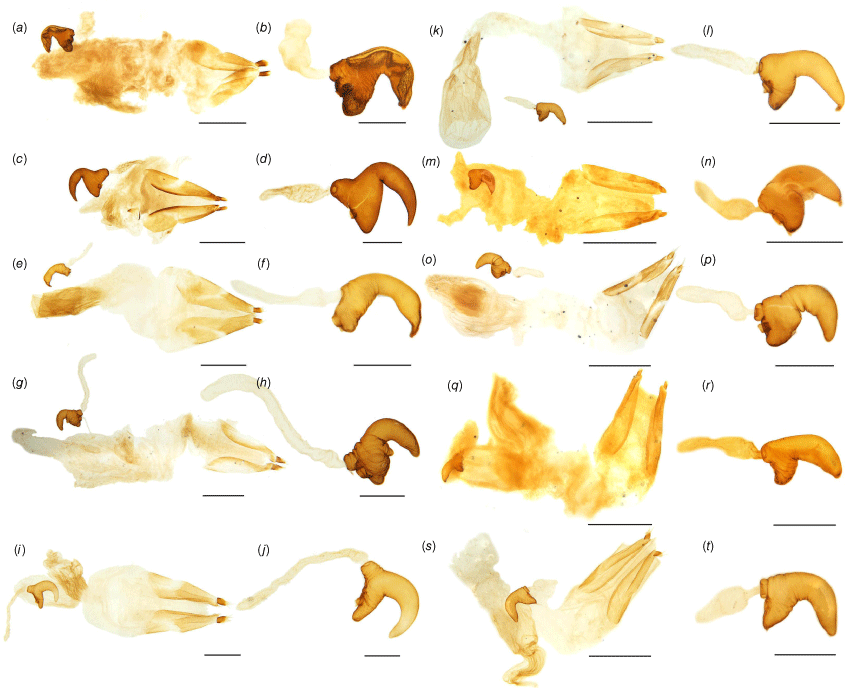
Female genital structures of Tranes species. (a) T. insignipes, genitalia; (b) T. insignipes, spermatheca with gland; (c) T. tinctipennis, genitalia; (d) T. tinctipennis, spermatheca with gland; (e) T. sparsus, genitalia; (f) T. sparsus, spermatheca with gland; (g) T. occidentalis, genitalia; (h) T. occidentalis, spermatheca with gland; (i) T. vigorsii, genitalia; (j) T. vigorsii, spermatheca with gland; (k) T. lyterioides, genitalia; (l) T. lyterioides, spermatheca with gland; (m) T. chadwicki, genitalia; (n) T. chadwicki, spermatheca with gland; (o) T. forsteri, genitalia; (p) T. forsteri, spermatheca with gland; (q) T. kgariensis, genitalia; (r) T. kgariensis, spermatheca with gland; (s) T. terryae, genitalia; (t) T. terryae, spermatheca with gland. Scale bars: (a, c, e, g, i, k, m, o, q, s) 0.5 mm; (b, d, f, h, j, l, n, p, r, t) 0.2 mm.
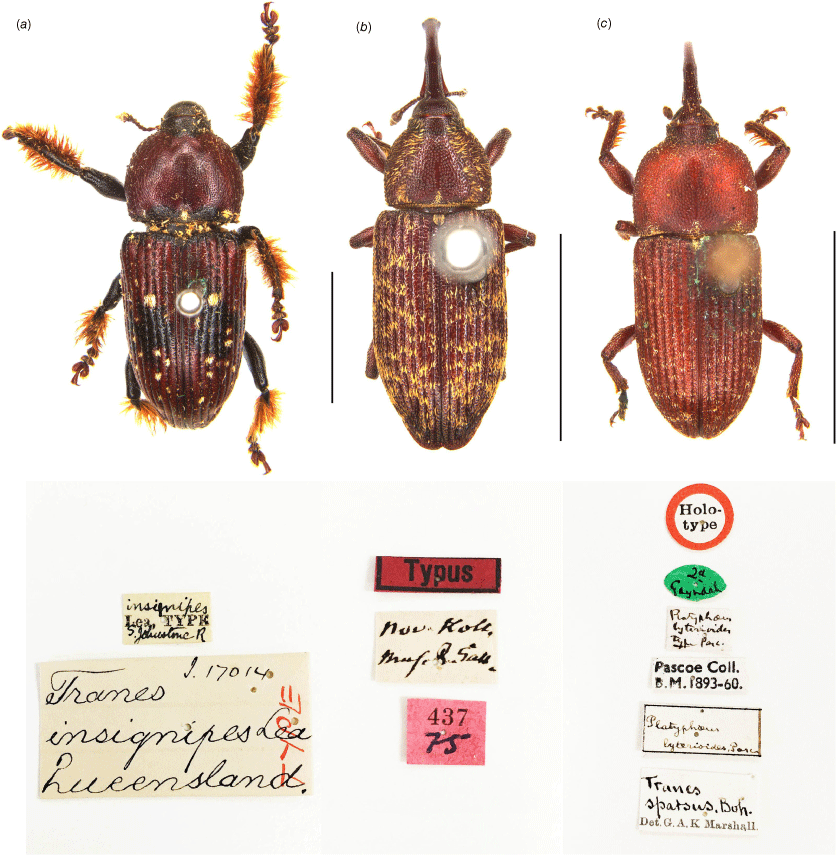
Tranes insignipes Lea, 1929
(Fig. 4f, 5b, 6a, 7a, b, 9a, b, 11a, 12a, 13a, 14a, 15a, 16a, 17a, 18a, 19a, b, 21a, b, 22a, b, 23a, b, 26a, b, 27a.)
Tranes insignipes Lea, 1929, p. 537. – Lea 1929, p. 539 (key). Schenkling and Marshall 1936, p. 1 (catalogue). Zimmerman 1992, p. 580, pl. 594, fig. 5, 6, 1994, p. 696 (classification). Oberprieler 1995a, p. 329 (catalogue), 1997, p. 25 (ecology). Wilson and Rowles 1997, p. 14 (ecology, host plant). Jones 2002, p. 53 (ecology). Hall et al. 2004, p. 340 (ecology). Pullen et al. 2014, p. 289 (catalogue). Toon et al. 2020, p. 1044 (ecology). Hsiao et al. 2023, [pp. 2, 6, 8] (biology, phylogenetic relationships).
Type locality: South Johnstone River, Queensland, Australia.
Lectotype (Fig. 6a), ♂. ‘insignipes / Lea, TYPE / S. Johnstone R // Tranes I. 17014 / insignipes Lea / Queensland. / TYPE // LECTOTYPE / Tranes insignipes / Lea, 1929 / des. Hsiao & Oberprieler, 2025’ (SAMA).
Paralectotypes. 1♂, ‘S. Johnstone R. / Queensland / H W. Brown // insignipes / Lea, Co-type // Specimen / figured / ECZ // Tranes 21288 / insignipes Lea / Queensland / Cotype // PARALECTOTYPE / Tranes insignipes / Lea, 1929 / des. Hsiao & Oberprieler, 2025’ (SAMA), 1♀, ‘S. Johnstone R. / Queensland / H. W. Brown // insignipes / Lea, Co-type / Type♀ // Specimen / figured / ECZ // PARALECTOTYPE / Tranes insignipes / Lea, 1929 / des. Hsiao & Oberprieler, 2025’ (SAMA), 1♂, ‘S. Johnstone R. / Queensland / H. W. Brown // 4453 // insignipes / Lea, Co-type // Tranes / insignipes Lea / Queensland. / Cotype // PARALECTOTYPE / Tranes insignipes / Lea, 1929 / des. Hsiao & Oberprieler, 2025’ (QMBA).
Queensland: The Boulders (1♀, QDPI); Garradunga, edge of Seymour Range, 17.28°S, 146.00°E (1♀, SAMA); Garradunga, 2 km E, NNE of Innisfail, 17.28°S, 146.01°E (1♀, ANIC); Innisfail (1♂, ANIC); Polly Ck, Seymour Rg, near Garradunga (1♀, ANIC); Polly Ck, Garradunga (3♂, 6♀, ANIC); Polly Ck, Garradunga, 17.458°S, 146.020°E (1♀, ANIC); Polly Ck, Garradunga, 17.459°S, 146.021°E (2♀, ANIC, ANIC Database Number 25 077564, Number 25 077565); S. Johnstone River (3♂, ANIC; 1♂, QDPI, INSECOLL 0-056367; 1♂, MVMA, COL-109452); Stone Ck, Garradunga (1♀, ANIC); Stone Ck, 17°28′S, 146°01′E (2♀, QMBA); Moresby (4♂, 1♀, ANIC); Mt Lewis, via Julatten (3♂, 5♀, ANIC); Mt Lewis (2♀, ANIC); Mt Lewis Rd (1♀, ANIC); Mt Lewis (2♀, ANIC); Turpentine Road near Thornton Beach, near Cape Tribulation, 16.11°S, 145.25°E (4♀, ANIC); (no data) (1♂, 1♀, ANIC; 2♂, QDPI, INSECOLL 0-056368–0-056369).
See differential diagnosis of Tranes insignipes lineage in Table 1.
| T. insignipes | T. tinctipennis | ||
|---|---|---|---|
| Rostrum | Distinctly ventrally curved (Fig. 9a, b ) | Nearly straight (Fig. 9c, d ) | |
| Antennae | Inserted slightly behind middle of rostrum in both male and female (Fig. 9a, b ) | Inserted slightly before middle of rostrum in male and in middle in female (Fig. 9c, d ) | |
| Pronotal collar | With teeth (Fig. 13a ) | Without tooth (Fig. 13b ) | |
| Pronotum of male | Nearly as wide as elytra in male (Fig. 7a ) | Distinctly narrower than pronotum (Fig. 7c ) | |
| Lateral margins of pronotum | Rounded in posterior two-thirds (Fig. 12a ) | Subparallel-sided in posterior two-thirds (Fig. 12b ) | |
| Surface on pronotum | Distinctly punctorugulose laterally (Fig. 12a ) | Slightly punctorugulose laterally (Fig. 12b ) | |
| Prosternum of male | Densely covered with small tubercles, with a large, semicircular, elevated plate in large-sized male (>10.0 mm) (Fig. 14a ) | Without such tubercle and elevated plate (Fig. 14b ) | |
| Pro- and mesotibiae | Without tooth (Fig. 17a ) | With a well-developed tooth medially (Fig. 17b ) | |
| Abdominal ventrite 1 and 2 | Flat, slightly depressed (Fig. 18a ) | Distinctly depressed (Fig. 18b ) | |
| Abdominal ventrite 5 | With a rounded depression (Fig. 19a, b ) | With a large, rounded depression and two small irregular depressions (Fig. 19c, d ) | |
| Tibiae and abdominal ventrite 5 of male | Densely covered with long brown bristles (Fig. 18a , 19a ) | Without such long bristles (Fig. 18c , 19c ) | |
| Abdominal sternite VIII of male | With well-developed angular apical margin, with a V-shaped patch and both lateral angles distinctly punctate and covered with setae (Fig. 21a ) | Apical margin rounded to truncate, with a triangular patch finely punctate and covered with very fine setae (Fig. 21c ) | |
| Abdominal sternite VIII of female | Abruptly angled laterally (Fig. 21b ) | Linear, curved laterally (Fig. 21d ) | |
| Spiculum gastrale | Without concave apex (Fig. 22a ) | Widely concave apically (Fig. 22c ) | |
| Penis | Moderately narrowing apicad in apical third, acutely pointed apically (Fig. 23a ) | Strongly narrowing apicad in apical fourth, obtusely pointed apically (Fig. 23c ) | |
| Endophallus | Without apical anchor-shaped sclerite (Fig. 23a ) | With anchor-shaped sclerite apically (Fig. 23c ) |
Body broadly oval (Fig. 7a, b), length 7.6–10.8 mm in both sexes, width ~0.4× of length, weakly convex in lateral view (Fig. 9a, b).
Head black, with vertex orange to dark red, antennae reddish brown; thorax orange to dark red, pronotum black along posterior margin, with a pair of black marks at base medially, prosternal elevated process in male black, elytra orange to dark red, with black anterior margin and a pair of irregular black marks medially; abdomen with ventrites 1 and 2 orange to dark red, posterior margin of ventrite 2 and ventrites 3–5 black; coxae, trochanters, femora and tibiae black, tarsi reddish brown; body and legs semilustrous, body and legs covered with coarse, sublanceolate and subsquamiform, yellowish setae, clustered in some parts to somewhat obscure derm, especially on pronotum, posterior margin of metaventrite, scutellar shield, elytra and abdominal ventrites 1 and 2 in male.
Rostrum: moderately long, longer in female (~1.2× as long as pronotum in male, 1.5× in female), robust, ventrally curved, dorsoventrally flattened, slightly broadened apically in dorsal view, coarsely punctate dorsally, punctures slightly smaller in distal half, proximal half with paired dorsomedian and dorsolateral carinae, the latter lower than the former. Eyes: subcircular in outline, slightly convex but not protruding. Antennae: inserted slightly behind middle of rostrum in both male and female (Fig. 9a, b); scapes not reaching eye; funicles with segment 1 longest, ~1.8×, 3.1×, 3.7×, 4.4×, 4.4× and 3.7× as long as segments 2–7 respectively; clubs stout, ~1.5× as long as wide, densely and finely pubescent in apical two segments (Fig. 11a).
Pronotum: Roundly trapezoidal, apex ~0.5× as narrow as base (Fig. 12a), broader in male (nearly as wide as elytra in male, distinctly narrower than elytra in female); anterior margin subtruncate, slightly emarginate medially, with 5 small teeth on pronotal collar laterally (Fig. 13a), posterior margin protruding medially, forming obtuse median lobe, lateral margins mostly rounded but distinctly narrowed anteriorly; disc weakly and evenly convex; surface distinctly punctate, distinctly punctorugulose laterally, with median longitudinal apunctate ridge, punctures separate on disc but confluent and vague laterally. Prosternum: surface sparsely and finely punctate, densely covered with small tubercles, with a large, semicircular, elevated plate in large males (length >10.0 mm) (Fig. 14a); prosternellum elongate, widened posteriorly (Fig. 14a). Mesoventrite: intermesocoxal process trapezoidal, faintly declivous (Fig. 15a); median pit rounded to elongate (Fig. 16a). Scutellar shield: roundly subpentagonal. Elytra: ~1.8–2.3× as long as pronotum, jointly ~0.6× as broad as long, broader than base of pronotum; humeri broadly rounded, slightly protruding; surface uneven, deeply and coarsely punctate in rows, forming distinct striae, interstriae convex. Legs: femora with small ventral subapical tooth; tibiae with premucro smaller than uncus; tibiae stronger, densely covered with well-developed long brown bristles in male (Fig. 17a); meso- and metatibiae with distal setal combs continuing around apex and extending to apical fourth of tibia; tarsi with claws simple, free, divergent.
Abdomen: Ventrites 1 and 2 flat, slightly depressed (Fig. 18a); ventrite 5 flat, with large, rounded depression posteriorly, depression in male densely and finely punctate, covered with long brown bristles (Fig. 19a, b). Terminalia. Male: sternite VIII pentagonal, sclerotised, apical margin angular, with a V-shaped patch and both lateral angles distinctly punctate and densely covered with short setae, basal margin strongly sclerotised (Fig. 21a); spiculum gastrale not concave apically, base lightly sclerotised (Fig. 22a); tegmen with complete ring, manubrium nearly as long as parameroid lobes (Fig. 22b); penis elongate (~2.3–2.5× as long as wide), subparallel-sided, gradually narrowing apicad in apical third, acutely pointed apically, abruptly strongly narrowing apicad slightly before the apex (Fig. 23a, b); endophallus apically with membranous sleeve of asperities. Female: sternite VIII abruptly angled laterally (Fig. 21b); gonocoxites thick, short, apically bluntly rounded (Fig. 26a); gonostyli short, conical, bluntly rounded and setose apically; bursa copulatrix without bands of spicules; spermatheca thick, right-angled, gland small, swollen, rounded apically (Fig. 26b).
Lea (1929) did not specify the derivation of the name insignipes, but it is evident that he named the species for the conspicuously setose forelegs of the male.
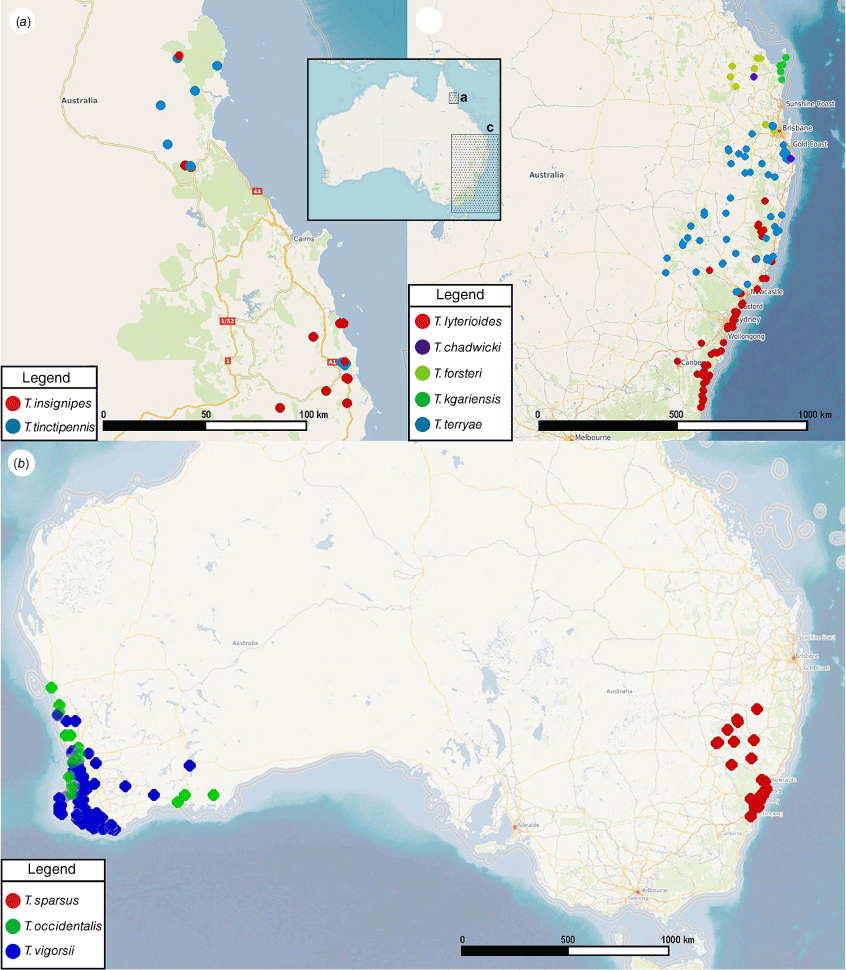
This species occurs in the coastal regions of northern Queensland (Fig. 27a), mainly recorded from the Innisfail region around the type locality but with isolated records also from Mount Lewis and the Daintree area, where it is sympatric with T. tinctipennis. Given that its host plant, Lepidozamia hopei, is widely distributed between these areas and also occurs slightly further south, T. insignipes may occur throughout the range of its host plant and also be more broadly sympatric with T. tinctipennis.
This species is exclusively associated with Lepidozamia hopei, the adults ovipositing in the sporophylls of dehiscing male cones and the larvae developing in the sporophylls (Wilson and Rowles 1997) and probably the rachis as well. Even though no experimental study of its involvement in the pollination of L. hopei has been conducted, the large amount of pollen found on its tibial brushes indicates that it is likely to be a major, if not the sole, pollinating agent of L. hopei (Wilson and Rowles 1997). Adults appear to be active mainly at night, as indicated by the label data of specimens attracted to light. Adults have been collected together with those of the similar T. tinctipennis at the same time and place and may co-inhabit male cones of L. hopei with those of the latter species.
Lea (1929) described Tranes insignipes based on specimens from the South Johnstone River in Far North Queensland. The description states ‘Types in South Australian Museum; cotype in Queensland Museum’ but does not give an exact number of the specimens. We examined a male labelled ‘Type’ and a pair labelled ‘co-type’ in Lea’s collection (SAMA) and another male labelled ‘co-type’ in QMBA. Although Lea labelled one male in his collection as ‘TYPE’ (Fig. 6a), he did not designate this as the primary (name-bearing) type in his description, and therefore both it and the three ‘co-type’ specimens are syntypes of equal nomenclatural status. In order to fix the species name to a single, name-bearing type, we here designate the male syntype labelled ‘TYPE’ (Fig. 6a), which is well prepared and agrees well with Lea’s description, as the lectotype of Tranes insignipes and the ‘co-type’ specimens as paralectotypes and have labelled them accordingly.
Tranes tinctipennis Hsiao & Oberprieler, sp. nov.
(Fig. 7c, d, 9c, d, 11b, 12b, 13b, 14b, 15b, 16b, 17b, 18b, 19c, d, 21c, d, 22c, d, 23c, d, 26c, d, 27a.)
ZooBank: urn:lsid:zoobank.org:act:342D4C81-C11A-4D51-8748-32734499549B
Type locality: Turpentine Road (16°06′36″S, 145°15′00″E) near Thornton Beach, near Cape Tribulation, Queensland, Australia.
Holotype, ♂. ‘16.11°S, 145.25°E / Turpentine Road, nr. / Thornton Beach, nr. / Cape Tribulation, Qld. / 10–11 December 1994 / J. Balderson & S. Lamond // HOLOTYPE / Tranes tinctipennis / Hsiao & Oberprieler, 2025’ (ANIC).
Paratypes (all labelled ‘PARATYPE / Tranes tinctipennis / Hsiao & Oberprieler, 2025’). 10♀, same as holotype (ANIC).
Queensland: Cooktown (1♀, NHMUK); Cape Tribulation, Noah Ck (1♂, NHMUK); Daintree (2♂, 6♀, NHMUK); Garradunga (2♀, ANIC, ANIC Database Number 25 077567); 17.459°S, 146.021°E, Polly Ck, Garradunga (1♀, ANIC, ANIC Database Number 25 077566); Mt Lewis (1♂, 1♀, NHMUK); Mt Lewis Rd (1♀, ANIC); Mt Lewis Rd, 30 road km from Rex Range Rd, ~16.32°S, 145.17°E (1♀, ANIC); Stone Ck, 17°28′S, 146°01′E (2♀, QMBA).
See Table 1.
Body broadly oval (Fig. 7c, d), length 6.7–9.4 mm in both sexes, width ~0.4× of length, weakly convex in lateral view (Fig. 9c, d).
Head black with vertex dark red, antennae reddish brown; thorax dark red, pronotum with a pair of black marks at base medially, elytra dark red, with black anterior margin and a pair of irregular black marks medially; abdomen dark red, femora and tibiae black, tarsi reddish brown; body and legs semilustrous, body and legs covered with coarse, sublanceolate and subsquamiform, yellowish setae, clustered in some parts to somewhat obscure derm, especially on posterior margin of pronotum, posterior margin of metaventrite, scutellar shield and elytra.
Rostrum: moderately long, longer in female (~1.5× as long as pronotum in male, 1.6× in female), robust, nearly straight, ventrally slightly curved apically, dorsoventrally flattened, slightly broadened apically in dorsal view, coarsely punctate dorsally, punctures slightly smaller in distal half, proximal half with paired dorsomedian and dorsolateral carinae, the latter lower than the former. Eyes: subcircular in outline, slightly convex but not protruding. Antennae: inserted slightly before middle of rostrum in male (Fig. 9c), in middle in female (Fig. 9d); scapes not reaching eye; funicles with segment 1 longest, ~2.1×, 3.1×, 2.8×, 3.1×, 2.6× and 2.5× as long as segments 2–7 respectively; clubs stout, ~1.5× as long as wide, densely and finely pubescent in apical two segments (Fig. 11b).
Pronotum: roundly trapezoidal, apex ~0.5× as narrow as base (Fig. 12b), distinctly narrower than elytra in both sexes; anterior margin subtruncate, slightly emarginate medially, without tooth on pronotal collar (Fig. 13b), posterior margin protruding medially, forming obtuse median lobe, lateral margins mostly rounded but distinctly narrowed anteriorly; disc weakly and evenly convex; surface distinctly punctate, slightly punctorugulose laterally, with median longitudinal apunctate ridge, punctures separate on disc but confluent and vague laterally. Prosternum: surface sparsely and finely punctate (Fig. 14b); prosternellum elongate, widened posteriorly (Fig. 14b). Mesoventrite: intermesocoxal process trapezoidal, faintly declivous (Fig. 15b); median pit rounded to elongate (Fig. 16b). Scutellar shield: roundly subpentagonal. Elytra: ~2.1–2.4× as long as pronotum, jointly ~0.7× as broad as long, broader than base of pronotum; humeri broadly rounded, slightly protruding; surface uneven, deeply and coarsely punctate in rows, forming distinct striae, interstriae convex. Legs: femora with small ventral subapical tooth; tibiae with premucro smaller than uncus; pro- and mesotibiae with a tooth medially (Fig. 17b); meso- and metatibiae with distal setal combs continuing around apex and extending to apical fourth of tibia; tarsi with claws simple, free, divergent.
Abdomen: ventrites 1 and 2 distinctly depressed (Fig. 18b); ventrite 5 flat, with a large median rounded depression posteriorly and small irregular depressions at both sides anteriorly, densely punctate in male, median depression in male densely covered with short brown bristles (Fig. 19c, d). Terminalia. Male: sternite VIII subtrapezoidal, sclerotised, apical margin subtruncate, with a triangular patch finely punctate and densely covered with very fine setae, basal margin strongly sclerotised (Fig. 21c); spiculum gastrale widely concave apically, base lightly sclerotised (Fig. 22c); tegmen with complete ring, manubrium nearly as long as parameroid lobes (Fig. 22d); penis elongate (~2.3–2.6× as long as wide), subparallel-sided, slightly widened medially, strongly narrowing apicad in apical fourth, obtusely pointed apically (Fig. 23c, d); endophallus apically with basal membranous sleeve of asperities and apical anchor-shaped sclerite. Female: sternite VIII curved laterally (Fig. 21d); gonocoxites thick, short, apically bluntly rounded (Fig. 26c); gonostyli short, conical, bluntly rounded and setose apically; bursa copulatrix without bands of spicules; spermatheca thick, right-angled, gland small, swollen, narrowing apicad, rounded apically (Fig. 26d).
The specific epithet is a Latin adjective meaning ‘painted-winged’, in reference to the contrasting pattern of deep red and black colours of the elytra that give them a painted or coloured-in appearance.
This species occurs in the coastal regions of Far North Queensland (Fig. 27a), in largely the same area as T. insignipes but most records being from the Mount Lewis and Daintree areas north of Cairns and only a few from the southern region around Innisfail. The collecting data indicate that it is at least partly sympatric with T. insignipes and even active at the same localities and time of year, perhaps co-inhabiting the male cones of the same plants.
Although no specific information is available on the biology and ecology of T. tinctipennis, it is presumably also associated with Lepidozamia hopei, with the larvae developing in the male cones and the adults likely also playing a role in its pollination. Its interactions with T. insignipes are unknown, but both species may occur together in the male cones of L. hopei.
This species corresponds to the ‘Tranes insignipes lineage sp.’ in the molecular phylogenetic analysis of Australian cycad-associated weevils (Hsiao et al. 2023).
Tranes sparsus Boheman, 1843
(Fig. 1b, 5d, 6b, 7e, f, 9e, f, 11c, 12c, 13c, 14c, 15c, 16c, 18c, 19e, f, 21e, f, 22e, f, 23e, f, 26e, f, 27b.)
Tranes sparsus Boheman in Schoenherr, 1843, p. 131. – Lacordaire (1863), p. 508 (morphological comparison). Gemminger 1871, p. 2451 (catalogue). Lea 1898, p. 593 (taxonomy of Tranes sensu lato), 1929, p. 539 (key, morphological comparison). Walker 1906, p. 23 (ecology). Schenkling and Marshall 1936, p. 1 (catalogue). Marshall 1939, p. 582 (synonymy, an error, see Remarks). Chadwick 1993, p. 78 (ecology), 1998, p. 15 (ecology). Forster et al. 1994, p. 219 (ecology). May 1994, pp. 619, 627 (key, larva). Zimmerman 1994, p. 696 (classification). Oberprieler 1995a, pp. 306, 329 (classification, catalogue, ecology), 1995b, p. 338 (ecology). Alonso-Zarazaga and Lyal 1999, p. 210 (synonymy, an error, see Remarks). Terry 2001, p. 1295 (ecology). Jones 2002, p. 53 (ecology). Pullen et al. 2014, p. 289 (catalogue). Toon et al. 2020, p. 1044 (ecology). Hsiao et al. 2023, [pp. 2, 6, 8] (biology, phylogenetic relationships).
Type locality: Nova Hollandia (Australia).
Holotype, Tranes sparsus (Fig. 6b), ♀. ‘Typus // Nov. Holl. / Mus. R. Gall. // 437 / 75 // HOLOTYPE / Tranes sparsus / Boheman in Schoenherr, / 1843 / Hsiao & Oberprieler, 2025’ (SMNH).
New South Wales: Avalon (18♂, 14♀, ANIC); Berkshire Park (5♂, 4♀, ANIC); Cromer (2♂, 7♀, ANIC); Engadine (6♂, 2♀, ANIC, ANIC Database Number 25 077613–25 077614); Engadine, near Sydney, 34.04°S, 151.01°E (1♂, ANIC); Kurnell (1♀, ANIC); Menai (1♂, ANIC); Mullaley (1♂, ANIC); Munmorah State Conservation Area (12♂, 29♀, ANIC); Noraville (127♂, 157♀, 2 ex., ANIC, ANIC Database Number 25 077553–25 077554; 4♂, 4♀, WMA); Noraville, 33°16′S, 151°34′E (21♂, 10♀, ANIC); Tudibaring (1♂, ANIC); Wahroonga (1♂, ANIC); Cessnock, 32°50′S, 151°21′E (16♂, 18♀, ANIC, ANIC Database Number 25 077551–25 077552); Goonoowigall Wildnerness near Inverell, 29°42′S, 151°07′E (1♂, 2♀, ANIC); 9 km W of Coonabarabran, 149.11°E, 31.17°S (1♂, 1♀, ANIC); Mt Kaputar N. P., 150.10°E, 30.17°S (6♂, 2♀, ANIC); Coryah Gap, Mt Kaputar, 30°16′S, 150°07′E (17♂, 12♀, ANIC; 2♂, 2♀, QDPI); X-Line road, Pilliga East State Forest 266, 30°38′S, 149°35′E (9♂, 4♀, ANIC; 3♂, QDPI); Flagstaff Hill, Tamworth, 31°05′11″S, 150°57′41″E (10♂, 15♀, ANIC; 2♂, 2♀, QDPI); W of Salisbury Trig., Wingen Maid N. R. (1♀, ANIC); Talbragar fossil site, ~25 km north-east of Gulgong, 32°10′S, 149°48′E (1♀, ANIC, ANIC Database Number 25 077526); Mt Sugarloaf, Newcastle (1♂, NHMUK).
See differential diagnosis of Tranes sparsus and T. vigorsii lineages in Table 2.
| T. sparsus | T. occidentalis | T. vigorsii | ||
|---|---|---|---|---|
| Body size (length) | Median, <11.0 mm | Large, ≥11.0 mm, usually 13.0–14.0 mm | ||
| Length of rostrum | Distinctly longer in female (Fig. 9e–h ) | Subequal in male and female (Fig. 9i, j ) | ||
| Width of rostrum | Subequal in male and female (Fig. 7e, f ) | Distinctly narrower in female (Fig. 7g, h ) | Subequal in male and female (Fig. 7i, j ) | |
| Antennae | Inserted slightly before middle of rostrum in male and in middle in female (Fig. 9e, f ) | Inserted in middle of rostrum in male and slightly behind middle in female (Fig. 9g, h ) | Inserted slightly before middle of rostrum in male and in middle in female (Fig. 9i, j ) | |
| Pronotal collar | Without tooth (Fig. 13c ) | With teeth (Fig. 13d ) | Without tooth (Fig. 13e ) | |
| Prosternum of male | Without tubercle (Fig. 14c ) | Covered with small tubercles (Fig. 14d, e ) | ||
| Prosternellum | Broad (Fig. 14c ) | Elongate (Fig. 14d, e ) | ||
| Protibiae of male | Without tibial brush | With well-developed tibial brush | ||
| Abdominal ventrite 5 of male | Flat, sparsely pubescent (Fig. 19e ) | Convex medially, densely pubescent (Fig. 19g ) | Convex medially, with a large, rounded patch posteriorly, densely pubescent while sparsely and finely pubescent and punctate on rounded patch (Fig. 19i ) | |
| Abdominal ventrite 5 of female | With a large depression posteriorly (Fig. 19f ) | With an apunctate rounded patch posteriorly (Fig. 19h, j) | ||
| Penis | Elongate, strongly narrowing apicad in apical fifth, rounded apically, apex with a longitudinal dark brown stripe (Fig. 23e ) | Thick, strongly narrowing apicad in apical third, rounded apically (Fig. 23g ) | Thick, moderately narrowing apicad in apical fourth, truncate apically (Fig. 23i ) | |
| Endophallus | With crescent sclerite apically (Fig. 23e ) | With a complex sclerite medially, composed of a omega (Ω) shaped sclerite and a small sclerite with two inward denticles basally and a pair of sclerites with several inward denticles distally (Fig. 23g , 24) | With anchor-shaped sclerite apically (Fig. 23i ) | |
| Gland of female genitalia | Short, nearly as long as spermatheca (Fig. 26f ) | Long, longer than spermatheca (Fig. 26h, j ) | ||
Body elongate–oval (Fig. 7e, f), length 7.3–10.4 mm in both sexes, width ~0.4× of length, nearly flat in lateral view (Fig. 9e, f).
Body and legs completely reddish brown; body and legs semilustrous; body and legs covered with coarse, sublanceolate and subsquamiform, yellowish setae, clustered in some parts to somewhat obscure derm, especially on pronotum, lateral and posterior margins of prosternum, lateral margins of meso- and metaventrites and abdominal ventrites 1–4, scutellar shield and elytra.
Rostrum: moderately long, longer in female (~1.1× as long as pronotum in male, 1.3–1.4× in female), robust, ventrally curved, dorsoventrally flattened, slightly broadened apically in dorsal view, coarsely punctate dorsally, punctures slightly smaller in distal half, proximal half with paired dorsomedian and dorsolateral carinae, the latter lower than the former. Eyes: subcircular in outline, slightly convex but not protruding. Antennae: inserted slightly before middle of rostrum in male (Fig. 9e), in middle in female (Fig. 9f); scapes not reaching eye; funicles with segment 1 longest, ~1.4×, 1.9×, 2.2×, 2.2×, 2.6× and 2.2× as long as segments 2 to 7 respectively; clubs stout, ~2.0× as long as wide, densely and finely pubescent (Fig. 11c).
Pronotum: roundly trapezoidal, apex ~0.5× as narrow as base (Fig. 12c), distinctly narrower than elytra in both sexes; anterior margin subtruncate, slightly emarginate medially, without tooth on pronotal collar (Fig. 13c), posterior margin protruding medially, forming obtuse median lobe, lateral margins mostly rounded but distinctly narrowed anteriorly; disc nearly flat; surface distinctly punctate, slightly punctorugulose laterally, with median longitudinal apunctate ridge, punctures separate on disc but confluent and vague laterally. Prosternum: surface sparsely and finely punctate, without tubercle and prominent protuberance (Fig. 14c); prosternellum broad, widened posteriorly (Fig. 14c). Mesoventrite: intermesocoxal process trapezoidal, strongly declivous (Fig. 15c); median pit groove-like (Fig. 16c). Scutellar shield: roundly subpentagonal. Elytra: longer in female, ~2.1–2.2× as long as pronotum in male, 2.4–2.5× in female, jointly ~0.6× as broad as long, broader than base of pronotum; humeri broadly rounded, slightly protruding; surface nearly flat, deeply and coarsely punctate in rows, forming distinct striae, interstriae convex. Legs: femora with small ventral subapical tooth; tibiae with premucro smaller than uncus; protibiae without tibial brush; meso- and metatibiae with distal setal combs continuing around apex and extending to apical fourth of tibia; tarsi with claws free, divergent.
Abdomen: ventrites 1 and 2 flat, slightly depressed (Fig. 18c); ventrite 5 flat, of female with a large depression posteriorly (Fig. 19e, f). Terminalia. Male: sternite VIII subtrapezoidal, sclerotised, apical margin subtruncate, finely punctate and sparsely covered with short setae, basal margin strongly sclerotised (Fig. 21e); spiculum gastrale widely concave apically, base lightly sclerotised (Fig. 22e); tegmen with complete ring, manubrium longer than parameroid lobes (Fig. 22f); penis elongate (~2.7× as long as wide), subparallel-sided, slightly widened apicad, strongly narrowing apicad in apical fifth, rounded apically, apex with a longitudinal dark brown stripe (Fig. 23e, f); endophallus apically with basal membranous sleeve of asperities and apical crescent sclerite. Female: sternite VIII abruptly angled laterally (Fig. 21f); gonocoxites thick, short, apically bluntly rounded (Fig. 26e); gonostyli short, conical, bluntly rounded and setose apically; bursa copulatrix without bands of spicules; spermatheca thick, right-angled, gland small, elongate, rounded apically (Fig. 26f).
This species occurs on the Northern (New England) Tablelands as well as in the central coastal regions of New South Wales (Fig. 27b), but it is unclear whether the latter distribution is autochthonous or due to human introduction. On the Tablelands T. sparsus is sympatric with T. terryae and at the central coast with T. lyterioides, which co-inhabit the male cones of the same Macrozamia species as T. sparsus does (Chadwick 1993; label data).
This species has been recorded from male cones of Macrozamia communis, M. concinna, M. glaucophylla, M. humilis, M. reducta, M. secunda and M. stenomera. Walker (1906) also reported it from the crown of M. spiralis (as the ‘burrawong’) at Woy Woy, but as this species was then confused with M. communis (Jones 1993, 2002) and as also T. sparsus and T. lyterioides were confused at the time (see below), this record most probably refers to T. lyterioides occurring on M. communis (see also Material Examined under that species). As no adults of T. sparsus have been found to visit female cones (Chadwick 1993) and as the male cones of all its host species are also inhabited by adults of T. lyterioides and T. terryae, which are demonstrated pollinators of their hosts, it appears that T. sparsus may not be involved in the pollination of any of its hosts. Chadwick (1993) observed a few adults resting under the bark of Eucalyptus trees to overwinter.
Only one specimen labelled as a type specimen of T. sparsus was obtained from the Schoenherr collection in SMNH for examination. Even though Boheman in Schoenherr (1843) did not specify the number of specimens in the original description, the single locality suggests that the type series includes only one specimen. The label data of this type specimen are congruent with the information about its origin and repository given in the original description (‘Nova Hollandia. E Mus. Reg. Gall.’), thus indicating that this specimen is the holotype of the name Tranes sparsus (Fig. 6b).
Marshall (1939) synonymised the older name Tranes sparsus with the younger Platyphaeus lyterioides Pascoe, 1875 (and labelled the holotype of P. lyterioides as T. sparsus; see below), but this was based on females of T. lyterioides in the NHMUK misidentified as T. sparsus by Arthur Lea (E. C. Zimmerman, pers. comm., 1975). Zimmerman (1994) consequently treated T. lyterioides as a valid species again, followed by Chadwick (1993, 1998), Forster et al. (1994), May (1994), Oberprieler (1995a, 1995b), Terry (2001), Jones (2002) and Pullen et al. (2014) but not by Alonso-Zarazaga and Lyal (1999) (who seemingly overlooked Zimmerman’s treatment). Our examination of the holotypes of both names confirm that they are different species (see below under T. lyterioides).
Tranes occidentalis Hsiao & Oberprieler, sp. nov.
(Fig. 1c, 7g, h, 9g, h, 11d, 12d, 13d, 14d, 15d, 16d, 18d, 19g, h, 21g, h, 22g, h, 23g, h, 24, 26g, h, 27b.)
ZooBank: urn:lsid:zoobank.org:act:1AE299EC-ECCA-4A2A-AF9C-3EE416A2FCFE
Type locality: Yanchep National Park, 53.7 km N Perth (31°33′04.1″S, 115°41′13.5″E), 37 m, Western Australia, Australia.
Holotype, ♂. ‘24.X.2012 / AU: WA: 31°33′04.1″S / 115°41′13.5″E / Yanchep NP, 53.7 km / N Perth, 37 m / H. Escalona & S. / Pinzon leg. // Host: Macrozamia / riedlei, in male cones // HOLOTYPE / Tranes occidentalis / Hsiao & Oberprieler, 2025’ (ANIC).
Paratypes (all labelled ‘PARATYPE / Tranes occidentalis / Hsiao & Oberprieler, 2025’). 17♂, 34♀, 1 larva, same data as holotype (ANIC).
Western Australia: Avon Valley (3♂, 3♀, WMA); Blue Rock, ~4.5 km E of Jarrahdale (1♀, WMA); Boothendarra, 38.5 km E Jurien Bay, 30°13′24.3″S, 115°24′02.2″E (1♂, 4♀, ANIC); Bullsbrook (1♂, ANIC; 3♂, 3♀, MVMA, COL-109486–109491); Bunbury (1♂, WMA); Chittering (1♀, WMA); Collie S. F., 10 km W Collie, 32°22′05.1″S, 116°05′25.7″E (1♂, ANIC, ANIC Database Number 25 077558); Edwards Rd 21.1 km NW Collie, 33°16′39.8″S, 115°56′54.4″E (1♂, ANIC); Eneabba (1♂, 1♀, ANIC; 2♂, 3♀, DPIRD, Agriculture (Dept) Western Australia 64012–64016); 20 km N Eneabba (1♀, ANIC); Fremantle (3♀, ANIC); Geraldton (1♂, ANIC); Guildford (1♂, WMA); Icy Creek, Lane Pool Reserve (1♀, ANIC); Icy Creek, Lane Pool Reserve (1♀, ANIC); King George Sound (1♂, ANIC); Lake Grace (6♀, QMBA); Leeuwin–Naturaliste N.P., Conto Campgr. to Lake Cave Trail (2♀, ANIC, ANIC Database Number 25 077562–25 077563); Mandurah (1♂, 1♀, ANIC); Melville, 32.03°S, 115.48°E (1♂, ANIC); Melville Park (1♀, DPIRD, Agriculture (Dept) Western Australia 64027); Midland Junction (1♀, MVMA, COL-109461); Mundaring Weir Catchment Area (1♀, WAMPA); Nilgen, 143 km N Perth, Indian Ocean Drive, 30°51′41.3″S, 115°21′46.9″E (1♂, ANIC, ANIC Database Number 25 077561); Perth (2♂, QMBA); Salter Point (1♂, WMA); Swan River (1♂, 1♀, SAMA; 1♂, DPIRD, Agriculture (Dept) Western Australia 64034; 1♂, MVMA, COL-109367); Stokes N. P., 78 km W Esperance, 33°49.003′S, 121°8.958′E (1♂, ANIC, ANIC Database Number 25 077555); Thomas River, 101 km E of Esperance, 33.51°S, 121.53°E (1♀, ANIC, ANIC Database Number 25 077557); Thomas River, 63 miles [~101.4 km] E of Esperance, 33.51°S, 121.53°E (2♀, ANIC, ANIC Database Number 25 077556); Thomas River, 23 km NW by W of Mt Arid, 33.51°S, 123.00°E (5♂, 2♀, ANIC); Thompson’s Lake, Jandakot (3♀, ANIC); Wandi (3♂, 4♀, DPIRD, Agriculture (Dept) Western Australia 112575–112481); Walyunga N. P. (2♀, ANIC); Waste Transfer Station, Matherson Rd, Childlow, 31°52′31.91″S, 116°15′8.9″E (1♀, DPIRD, EHB lure, #P0030); Wembley Park [Perth] (1♀, SAMA); Woodridge, Guildford (1♀, ANIC); Yalgorup N. P., 123 km S Perth, Ellis Rd, 32°55′45.5″S, 115°42′33.5″E (9♂, 5♀, ANIC).
See Table 2.
Body elongate–oval (Fig. 7g, h), length 5.8–10.2 mm in both sexes, width ~0.4× of length, nearly flat in lateral view (Fig. 9g, h).
Body and legs completely reddish brown; body and legs semilustrous; body and legs covered with coarse, sublanceolate and subsquamiform, yellowish setae, clustered in some parts to somewhat obscure derm, especially on pronotum, lateral margins of meso- and metaventrites and abdominal ventrites 1–4, posterior margin of abdominal ventrite 5 in male, scutellar shield and elytra.
Rostrum: moderately long, longer in female (~1.1–1.3× as long as pronotum in male, 1.5× in female), robust, ventrally curved, dorsoventrally flattened, slightly broadened apically in dorsal view, coarsely punctate dorsally, punctures slightly smaller in distal half, proximal half with paired dorsomedian and dorsolateral carinae, the latter lower than the former. Eyes: subcircular in outline, slightly convex but not protruding. Antennae: inserted in middle of rostrum in male (Fig. 9g), slightly behind middle in female (Fig. 9h); scapes not reaching eye; funicles with segment 1 longest, ~1.5×, 2.0×, 2.3×, 2.3×, 2.3× and 2.0× as long as segments 2–7 respectively; clubs stout, ~1.3× as long as wide, densely and finely pubescent (Fig. 11d).
Pronotum: roundly trapezoidal, apex ~0.5–0.6× as narrow as base (Fig. 12d), distinctly narrower than elytra in both sexes; anterior margin subtruncate, slightly emarginate medially, with 3–4 small teeth on pronotal collar laterally in male (Fig. 13d), posterior margin protruding medially, forming obtuse median lobe, lateral margins mostly rounded but distinctly narrowed anteriorly; disc nearly flat; surface distinctly punctate, slightly punctorugulose laterally, with median longitudinal apunctate ridge, punctures separate on disc but confluent and vague laterally. Prosternum: surface sparsely and finely punctate, of male densely covered with small tubercles, without prominent protuberance (Fig. 14d); prosternellum elongate, widened posteriorly (Fig. 14d). Mesoventrite: intermesocoxal process trapezoidal, strongly declivous (Fig. 15d); median pit groove-like (Fig. 16d). Scutellar shield: roundly subpentagonal. Elytra: length subequal in both male and female, ~2.1–2.6× as long as pronotum in male, 2.5–2.6× in female, jointly ~0.6× as broad as long, broader than base of pronotum; humeri broadly rounded, slightly protruding; surface nearly flat, deeply and coarsely punctate in rows, forming distinct striae, interstriae convex. Legs: femora with small ventral subapical tooth; tibiae with premucro smaller than uncus; protibiae with well-developed tibial brush in male; meso- and metatibiae with distal setal combs continuing around apex and extending to apical fourth of tibia; tarsi with claws free, divergent.
Abdomen: ventrites 1 and 2 flat, slightly depressed (Fig. 18d); ventrite 5 convex medially, densely pubescent, of male with denser pubescence (Fig. 19g), of female with an apunctate rounded patch posteriorly, pubescence on rounded patch extremely sparse and short (Fig. 19h). Terminalia. Male: sternite VIII subtrapezoidal, sclerotised, apical margin rounded, finely punctate and densely covered with short setae, basal margin strongly sclerotised (Fig. 21g); spiculum gastrale widely concave apically, base lightly sclerotised (Fig. 22g); tegmen with complete ring, manubrium shorter than parameroid lobes (Fig. 22h); penis thick (~1.9–2.0× as long as wide), subparallel-sided, slightly narrowing apicad, strongly narrowing apicad in apical third, rounded apically (Fig. 23g, h); endophallus apically with basal membranous sleeve of asperities, medially with a complex sclerite composed of an omega (Ω) shaped sclerite and a small sclerite with two inward denticles basally and a pair of sclerites with several inward denticles distally (Fig. 24). Female: sternite VIII abruptly angled laterally (Fig. 21h); gonocoxites thick, short, apically bluntly rounded (Fig. 26g); gonostyli short, conical, bluntly rounded and setose apically; bursa copulatrix without bands of spicules; spermatheca thick, right-angled, gland elongate, longer than spermatheca, rounded apically (Fig. 26h).
The specific epithet is an adjective derived from the Latin noun occidens, meaning ‘west’, and refers to the distribution of the species in Western Australia.
This species is restricted to the coastal regions of south-western Western Australia, where it occurs partly sympatrically with T. vigorsii (Fig. 27b).
The hosts recorded for T. occidentalis comprise all three named species of Macrozamia in Western Australia, i.e. M. dyeri, M. fraseri and M. riedlei, the last of which it shares with T. vigorsii. It appears to be exclusively associated with the male cones of these cycads, but, as with T. vigorsii and also with T. sparsus in eastern Australia, no involvement in the pollination of its hosts has been recorded. The adults spend the day inside the cones or the crown and fly in the evening and night, when they may be attracted to lights.
This species corresponds to the ‘Tranes sparsus lineage sp.’ in the molecular phylogenetic analysis of Australian cycad-associated weevils (Hsiao et al. 2023).
Tranes vigorsii Boheman, 1843
(Fig. 1a, 4a–e, 5a, c, 7i, j, 9i, j, 11e, 12e, 13e, 14e, 15e, 16e, 18e, 19i, j, 21i, j, 22i, j, 23i, j, 26i, j, 27b.)
Tranes vigorsii Boheman in Schoenherr (1843), p. 130. – Lacordaire 1863, p. 508 (morphological comparison). Gemminger 1871, p. 2451 (catalogue). Lea 1898, p. 593 (taxonomy of Tranes s. lat.; as vigorsi), 1929, pp. 538, 539 (key, morphological comparison; as vigorsi). Schenkling and Marshall 1936, p. 1 (catalogue; as vigorsi). Ornduff 1991, p. 10, 1993, p. 123 (ecology; as vigorsi). Connell and Ladd 1993, p. 98 (pollination biology). Forster et al. 1994, p. 219 (ecology; as vigorsi). Zimmerman 1994, pp. 695, 696 (key, classification). Oberprieler 1995a, pp. 307, 329 (classification, catalogue, ecology), 1995b, p. 338 (ecology). Alonso-Zarazaga and Lyal 1999, p. 210 (catalogue). Jones 2002, p. 53 (ecology). Pullen et al. 2014, p. 289 (catalogue). Toon et al. 2020, p. 1037 (ecology), p. 1044 (ecology; as vigorsi). Hsiao et al. 2023, [pp. 2, 6] (biology, phylogenetic relationships).
Type locality: Swan River, Nova Hollandia (Australia).
Holotype, ♂. ‘Swan River, N. Holl. Hope’ (SMNH), examined by E. C. Zimmerman in 1975 and compared with specimens so labelled in the ANIC.
Western Australia: W. Australia (1♂, ANIC); Albany (1♂, QMBA; 1♂, DPIRD, Agriculture (Dept) Western Australia 64030); Augusta (1♀, WAMPA); Augusta, 13.4 km NW, Caves Rd, 23 m, 34°15′19.4″S, 115°03′32.1″E (7♂, 5♀, ANIC); Augusta, Allnutt Terrace, 34°19′19.9″S, 115°09′36.5″E (7♂, 7♀, ANIC); Badgerup (1♀, ANIC); Banganup, Wattleup (2♂, WAMPA); Boothendarra, 38.5 km E Jurien Bay, 159 m, 30°13′24.3″S, 115°24′02.2″E (1♂, 2♀, ANIC, ANIC Database Number 25 077549–25 077550); Booyana, Norseman district (1♂, ANIC); Boranup Hut, 4 miles [~6.4 km] W of 191 mile peg on Bussetton Highway (1♂, WAMPA); Boranup Karri Forest, 67 m, 34°05′59.6″S, 115°03′12.6″E (1 ex., ANIC); Bridgetown (1♂, DPIRD, Agriculture (Dept) Western Australia 64021); Bridgetown, 13 km SW, Mokerdillup Rd, 296 m, 34°03′11″S, 116°05′19.8″E (1♂, 3♀, ANIC); Bunbury (2♂, ANIC); Bunbury, 45 km N, 33.00°S, 115.54°E (1♂, ANIC); Bunbury, 55 km N, 33.00°S, 115.54°E (1♀, ANIC); Calgardup (2♂, 6♀, WAMPA); Cave Rd, W side, 0.5 km N of its junction with Boranup Rd (N junction) btwn Auguata & Margaret River (2♂, 5♀, ANIC); Cockleshell Gully (1♀, DPIRD, Agriculture (Dept) Western Australia 64020); Collie S. F., 10 km W Collie, 218 m, 32°22′05.1″S, 116°05′25.7″E (2♂, 1♀, ANIC); Crawley (1♂, ANIC); Deepdene (1♀, ANIC); Deepdene, Karridale (1♂, ANIC); Denmark (3♂, 9♀, WAMPA); Denmark, 34°58′S, 117°21′E (1♂, 1♀, WAMPA); Denmark, 11.7 km SW, Lights Rd, 31 m, 35°00′41.8″S, 117°16′40.2″E (1♂, 3♀, ANIC); Donnybrook (1♀, DPIRD, Agriculture (Dept) Western Australia 64032); Donnybrook, 32.6 km SE, near Grimwade Rd, 33°45′36.3″S, 116°01′06.2″E (5♂, 8♀, ANIC); Drakesbrook (1♂, MVMA, COL-109484); Dwellingup, 32.43°S, 116.04°E (1♀, ANIC); Frankland River rest area (1♂, ANIC); Frankland River, 70 km E Manjimup, Unicup Rd, 34°22′03.5″S, 116°46′17.4″E (4♂, 5♀, ANIC); Gnangara Pine Plantation, Possum Rd (near dried swamp), 31°46′22.34″S, 115°55′6.11″E (1♀, DPIRD, EHB lure, #P0014); Gooseberry Hill N.P., SE Perth (1♀, ANIC); Gosnells (1♂, 2♀, ANIC); Granite Peaks S. F., 30 km NW Walpole, Beardmore Rd, 34°49′00.3″S, 116°36′09.1″E (1♂, 1♀, ANIC); Icy Creek, Lane Pool Reserve (3♂, 2♀, ANIC); Irwin Inlet (1♂, WAMPA); Jandakot, Thompson’s Lake (1♂, ANIC); King George Sound (4♂, 4♀, ANIC; 1♂, SAMA); Lake Grace (2♂, 4♀, QMBA); Lake Logue N, ~14 km W Eneabba (1♂, WAMPA); Lake Muir, 60 km SE Manjimup (3♂, 3♀, ANIC); Lake Muir (33♂, 28♀, ANIC); Lane Poole Reserve, 15 km Dwellingup (1♂, 1♀, ANIC); Leeuwin-Naturaliste N.P., Bonarup Forest Caves Rd at Giants Cave (3♂, 3♀, ANIC); Leeuwin-Naturaliste N.P., Conto Campgr. to Lake Cave Trail (6♂, 5♀, ANIC); Leeuwin-Naturaliste N. P., Yallingup Caves Rd, 33°38′38.1″S, 115°02′22″E (4♂, 8♀, ANIC); Leeuwin-Naturaliste N. P., Yallingup Caves Rd, 33°38′35.2″S, 115°02′09.1″E (6♂, 2♀, ANIC); Lowden (1♀, DPIRD, Agriculture (Dept) Western Australia 64035); Ludlow (1♂, DPIRD, Agriculture (Dept) Western Australia 64018); Manjimup, 13.8 km NW, Donnelly Rd, 34°10′17.3″S, 116°03′02″E (4♂, 3♀, ANIC); Margaret River, 2 miles [~3.2 km] SE, 34.57S, 115.04E (1♂, ANIC); Margaret River E, Neilson Rd, 78 m, 33°57′14.6″S, 115°06′20.7″E (1♀, ANIC); Margaret River, 6 km SW 88 m, 33°59′36′S, 115°02′52.9″E (2♂, 3♀, ANIC, ANIC Database Number 25 077545–25 077546); Margaret River, 10.2 km SW, Redgate Rd, 43 m, 34°01′34.5″S, 115°01′41.8″E (2♂, ANIC); Margaret River, 16 km SW, Caves Road, 34.05°S, 115.04°E (14♂, 6♀, ANIC); Melville Park (2♂, 2♀, DPIRD, Agriculture (Dept) Western Australia 64023–64026); Mount Barker (1♀, DPIRD, Agriculture (Dept) Western Australia 64022); Mount Cooke (1♂, DPIRD, Agriculture (Dept) Western Australia 64019); Muir Hwy, 50 km E of Manjimup, 8.9 km E Radburn Rd, N. side Hwy, Stoate S. F. (3♂, 4♀, ANIC); Mumballup S. F., 17 km SW Collie, Mungalup Rd, 33°27′11.1″S, 116°02′54.0″E (1♂, 4♀, ANIC); Mundaring (1♀, DPIRD, Agriculture (Dept) Western Australia 64028); Mundaring Weir Catchment Area (1♂, WAMPA); Nornalup (1♀, DPIRD, Agriculture (Dept) Western Australia 64031); Norseman (1♀, MVMA, COL-109501); Peel Estate (1♂, 1♀, ANIC; 1♂, MVMA, COL-109497); Pemberton (1♂, DPIRD, Agriculture (Dept) Western Australia 64017); Pemberton, 8 miles [~12.9 km] S.W. (1♂, 1♀, ANIC); Perillup Rd, 45 km NW Mount Baker, 34°30′45.4″S, 117°14′47.3″E (1♂, ANIC); Perth (2♂, MVMA, COL-109495–109496; 1♂, QMBA); Perth, Kings Park (2♂, 1♀, ANIC); Ravensthorpe (1♀, ANIC); Scarborough (6♂, 6♀, WAMPA); Serpentine N. P., 11.4 km E Serpentine, Scarp Rd, 32°22′10.8″S, 116°02′59.4″E (4♂, 3♀, ANIC, ANIC Database Number 25 077541–25 077542); South West Highway, 3 mile peg (7♂, 9♀, DPIRD, Agriculture (Dept) Western Australia 63996–64011); Star Swamp, North Beach (1♀, WAMPA); Swan River (5♂, 1♀, ANIC; 3♂, 3♀, MVMA, COL-109379, 109492–109494, 109499–109500; 1♂, 1♀, SAMA); Tingledale, 25 km NE Walpole, Settlers Boundary Rd, 34°56′33.1″S, 116°54′18.1″E (19♂, 19♀, ANIC); Walpole Coalmine Beach Camping area (1♀, WAMPA); Walpole-Nornalup N. P., 6.8 km E Walpole, Hilltop Rd, 34°58′56.5″S, 116°47′25.5″E (22♂, 12♀, ANIC, ANIC Database Number 25 077547–25 077548); Walyunga N. P. (4♂, ANIC); Wandi (5♂, 15♀, DPIRD, Agriculture (Dept) Western Australia 112555–112574); Wanneroo (1♀, WAMPA); Watheroo N. P. (8♂, 2♀, ANIC); Wellington S. F., 14.5 km E Roelands, Edwards Rd, 33°16′41.1′S, 115°56′59.2″E (9♂, 5♀, ANIC); Wellington S. F., 21.1 km NW Collie, Edwards Rd, 33°16′39.8″S, 115°56′54.4″E (6♂, 3♀, ANIC, ANIC Database Number 25 077543–25 077544); Wembley (2♀, WAMPA); Wembley Park (1♂, 1♀, ANIC); West Pinjar (1♀, ANIC); Wilga (11♂, 11♀, ANIC); Wilga, 4 km E (3♂, 7♀, ANIC); Wilga, 33.42°S, 116.14°E (1♂, ANIC); Williams (1♀, SAMA); Wilsons Inlet (3 ex., QDPI, INSECOLL 0-056370–0-056372; 1♂, 3♀, QMBA, UQIC Reg. #77825–77828); Woodridge, Guildford (1♂, ANIC); Yalgorup N. P., 123 km S Perth, Ellis Rd, 32°55′45.5″S, 115°42′33.5″E (4♂, 1♀, ANIC); Yallingup, 1.5 km S, 33.39°S, 115.02°E (1♂, WAMPA); Yanchep (1♂, WAMPA); Yanchep N. P., 53.7 km N Perth, 37 m, 31°33′04.1″S, 115°41′13.5″E (2♀, ANIC).
See Table 2.
Body elongate–oval (Fig. 7i, j), length 11.2–14.9 mm in both sexes, width ~0.4× of length, nearly flat in lateral view (Fig. 9i, j).
Body and legs completely reddish brown; body and legs semilustrous; body and legs covered with coarse, sublanceolate and subsquamiform, yellowish setae, clustered in some parts to somewhat obscure derm, especially on prothorax, lateral margins of meso- and metaventrites and abdominal ventrites 1–4, abdominal ventrite 5, scutellar shield and elytra.
Rostrum: moderately long, subequal in both sexes (~1.1–1.2× as long as pronotum), robust, ventrally curved, dorsoventrally flattened, slightly broadened apically in dorsal view, coarsely punctate dorsally, punctures slightly smaller in distal half, proximal half with paired dorsomedian and dorsolateral carinae, the latter lower than the former. Eyes: subcircular in outline, slightly convex but not protruding. Antennae: inserted slightly before middle of rostrum in male (Fig. 9i), in middle in female (Fig. 9j); scapes not reaching eye; funicles with segment 1 longest, ~1.7×, 2.8×, 2.8×, 2.8×, 2.8× and 2.2× as long as segments 2–7 respectively; clubs stout, ~1.4–1.6× as long as wide, densely and finely pubescent (Fig. 11e).
Pronotum: roundly trapezoidal, apex ~0.5–0.6× as narrow as base (Fig. 12e), distinctly narrower than elytra in both sexes; anterior margin subtruncate, slightly emarginate medially, without teeth on pronotal collar (Fig. 13e), posterior margin protruding medially, forming obtuse median lobe, lateral margins mostly rounded but distinctly narrowed anteriorly; disc nearly flat; surface distinctly punctate, slightly punctorugulose laterally, with median longitudinal apunctate ridge, punctures separate on disc but confluent and vague laterally. Prosternum: surface sparsely and finely punctate, of male densely covered with small tubercles, without prominent protuberances (Fig. 14e); prosternellum elongate, widened posteriorly (Fig. 14e). Mesoventrite: intermesocoxal process trapezoidal, strongly declivous (Fig. 15e); median pit groove-like (Fig. 16e). Scutellar shield: roundly subpentagonal. Elytra: longer in female, ~2.5–2.6× as long as pronotum in male, 2.8× in female, jointly ~0.6× as broad as long, broader than base of pronotum; humeri broadly rounded, slightly protruding; surface nearly flat, deeply and coarsely punctate in rows, forming distinct striae, interstriae convex. Legs: femora with small ventral subapical tooth; tibiae with premucro smaller than uncus; protibiae with well-developed tibial brush in male; meso- and metatibiae with distal setal combs continuing around apex and extending to apical fourth of tibia; tarsi with claws free, divergent.
Abdomen: ventrites 1 and 2 flat, slightly depressed (Fig. 18e); ventrite 5 convex medially, with a large, rounded patch posteriorly, pubescence on rounded patch extremely sparse and short, of male with rounded patch sparsely and finely punctate (Fig. 19i), of female with rounded patch more well developed and apunctate (Fig. 19j). Terminalia. Male: sternite VIII subtrapezoidal, sclerotised, apical margin subtruncate, finely punctate and sparsely covered with short setae, basal margin strongly sclerotised (Fig. 21i); spiculum gastrale widely concave apically, base lightly sclerotised (Fig. 22i); tegmen with complete ring, manubrium shorter than parameroid lobes (Fig. 22j); penis thick (~1.8–2.0× as long as wide), subparallel-sided, slightly widened apicad, moderately narrowing apicad in apical fourth, truncate apically (Fig. 23i, j); endophallus apically with basal membranous sleeve of asperities and apical anchor-shaped sclerite. Female: sternite VIII abruptly angled laterally (Fig. 21j); gonocoxites thick, short, apically bluntly rounded (Fig. 26i); gonostyli short, conical, bluntly rounded and setose apically; bursa copulatrix without bands of spicules; spermatheca thick, right-angled, gland elongate, longer than spermatheca, rounded apically (Fig. 26j).
Boheman in Schoenherr (1843) did not specify the derivation of the species name but wrote that it had been proposed by Frederick William Hope (1797–1862), from whom he had received the type specimen for description. Hope had evidently proposed the name for Nicholas Aylward Vigors (1785–1840), an Irish zoologist and politician who was a founding member of the Zoological Society of London in 1826 and (together with Hope) of the Royal Entomological Society of London in 1833 (Long 2009). The type may have been collected by John Septimus Roe (1797–1878), the first Surveyor-General of the Swan River Colony (later Perth) in Western Australia (Pullen et al. 2014).
This species occurs in the coastal regions of south-western Western Australia (Fig. 27b).
The only host plant of T. vigorsii appears to be Macrozamia riedlei, as recorded by several authors (e.g. Ornduff 1991, 1993; Connell and Ladd 1993; Oberprieler 1995b). The larvae of T. vigorsii have been reported tunnelling in the sporophylls and rachis of both male and female cones and also in the sarcotesta of some seeds (Connell and Ladd 1993). This contrasts with other Tranes species, whose larvae have almost never been found in female cones, and that only very few larvae of T. lyterioides were able to develop in female cones of M. communis in our laboratory rearings. Connell and Ladd (1993) concluded from their preliminary pollination study of M. riedlei that pollen is transferred by both insects and wind, as the experiment showed no significant difference in seed set between exclusion of either vector, but the mesh insect exclusion bags were not designed to exclude the small thrips that inhabit the cones (Cycadothrips emmaliami Mound & Marullo) and are surmised to be the pollinating agents of this cycad species (Mound and Marullo 1998). Owing to its large size, T. vigorsii is most unlikely to be directly involved in the pollination of M. riedlei. The adult weevils are largely nocturnal and attracted to light.
Tranes lyterioides (Pascoe, 1875)
(Fig. 1d–f, 6c, 8a, b, 10a, b, 11f, 12f, 13f, 14f, 15f, 16f, 18f, 20a, b, 21k, l, 22k, l, 25a, b, 26k, l, 27c.)
Platyphaeus lyterioides Pascoe, 1875, p. 66. – Hustache 1938, p. 158 (catalogue). Marshall 1939, p. 582 (synonymy, an error, see Remarks). Alonso-Zarazaga and Lyal 1999, p. 210 (synonymy, an error, see Remarks). Pullen et al. 2014, p. 289 (catalogue).
Tranes lyterioides (Pascoe, 1875) – Marshall 1939, p. 582. Ornduff 1989, p. 243 (misidentification), 1990, p. 98 (misidentification). Kennedy 1991, p. 22 (misidentification), 1992, p. 21 (ecology). Chadwick 1993, p. 78 (ecology), 1998, p. 15 (ecology), 1999, p. 15 (ecology). Jones 1993, p. 59 (ecology), 2002, p. 53 (ecology). Forster et al. 1994, p. 219 (ecology). May 1994, pp. 619, 625 (key, larva). Zimmerman 1994, p. 696 (classification). Oberprieler 1995a, pp. 306, 329 (classification, catalogue, ecology), 1995b, p. 338 (ecology), 1997, p. 25 (misidentification). Terry 2001, p. 1294 (pollination biology). Hall et al. 2004, p. 340 (ecology). Terry et al. 2004, p. 242 (pollination biology), 2005, p. 932 (pollination biology). Wallenius et al. 2012, p. 398 (pollination biology). Pullen et al. 2014, p. 289 (catalogue). Wallenius 2014, p. 28 (pollination biology). Toon et al. 2020, p. 1044 (ecology). Hsiao and Oberprieler 2021a, p. 122 (morphological comparison), 2022, p. 4 (morphological comparison). Hsiao et al. 2023, [pp. 2, 5, 6, 8] (biology, co-evolution, phylogenetic relationships).
Type locality: Gayndah, Queensland, Australia (evidently in error; see Remarks).
Holotype (Fig. 6c), ♂. ‘Holo- / type // Qd. / Gayndah // Platyphaeus / lyterioides / Type Pac. // Pascoe Coll. / B.M. 1893-60. // Platyphaeus / lyterioides. Pascoe // Tranes / sparsus. Boh. / Det. G.A.K. Marshall. // HOLOTYPE / Platyphaeus lyterioides / Pascoe, 1875 / des. Hsiao & Oberprieler, 2025’ (NHMUK).
New South Wales: Avalon (13♂, 12♀, ANIC); Balgowlah (15♂, 25♀, ANIC); Bawley Point, 35.30°S, 150.24°E (7♂, 10♀, ANIC); Bawley Point (1♀, ANIC); Bermagui, 7 miles [~11.3 km] S (1♂, ANIC); Bermagui, 36°25′45″S, 150°04′35″E (38♂, 10♀, ANIC); Big Hill, 16.5 miles [~26.6 km] from Wollomombi turn off (6♂, 6♀, ANIC); Big Hill (13♂, 21♀, ANIC); Big Hill, 25 km from Wollomombi on Armidale–Kempsey road, Styx River S. F., 30°38′S, 152°11′E (14♂, 32♀, ANIC; 4♀, QDPI); Bodalla Forest Park, 8 km N of Narooma, 36.10°S, 150.07°E (13♂, 24♀, 41 larvae, ANIC); Bouddi (2♂, ANIC); Broulee (62♂, 83♀, 6 larvae, 1 pupa, ANIC, ANIC Database Number 25 077509–25 077510); Broulee, 35.51°S, 150.11°E (12♂, 18♀, 23 larvae, ANIC); Bungwahl, 6 km E, 32°23′05″S, 152°30′15″E (2♂, 7♀, ANIC Database Number 25 077509–25 077510, ANIC); Burri Heights, 1 km N Tomakin, 35.49°S, 150.12°E (26♂, 27♀, ANIC); Carrai S. F. (2♂, 8♀, ANIC); Cessnock, 32°50′S, 151°21′E (14♂, 20♀, ANIC, ANIC Database Number 25 077525); Clyde River (1♀, ANIC); Clyde Mt (35♂, 28♀, 27 larvae, ANIC); Clyde Mt E slope, Lyons Rd, 0.5 km N of Kings Highway (1♀, ANIC); Clyde Mt, West Distributor Rd (7♂, 5♀, ANIC); Cromer (1♂, ANIC); Crowdy Bay N.P., southern section (1♀, ANIC); Currowan S. F. (6♀, ANIC); Currowan S. F., near Nelligen (2♂, 2♀, ANIC); Currowan S. F., 35°37′S, 150°02′E (7♂, 5♀, ANIC); Currowan S. F., Black Flat Rd (8♂, 9♀, ANIC); Depot Beach, 10 miles [~16.1 km] NE of Batemans Bay (1♀, ANIC); Depot Beach, 16 km NE of Batemans Bay (8♂, 9♀, ANIC); Durras (1♀, ANIC); Engadine, Sydney (9♂, 6♀, 4 larvae, ANIC); Fingal Bay (3♂, 8♀, ANIC); Gerroa (1♀, ANIC); Illawarra (1♂, QMBA, UQIC Reg. #77829); Kurnell (10♂, 23♀, ANIC); Longreach (2♂, ANIC); Merriwa, 20 miles [∼32.2 km] (3♂, 4♀, ANIC); Monga S. F., 35°34′31″S, 149°50′01″E (1♂, 3♀, ANIC); Moon Bay (107♂, 111♀, ANIC); Moruya (4♂, ANIC); Mulbring, 10 km (1♂, ANIC); Mt Dromedary (1♀, ANIC); Mt Sugarloaf (14♂, 33♀, ANIC); Munmorah S. R. A. (Munmorah State Conservation Area, Elizabeth Bay Drive, Lake Munmorah) (16♂, 21♀, ANIC); Myall Lake, N of Mungo Brush (1♂, ANIC); Nelson Beach, 36°41′S, 149°59′E (20♀, ANIC); Noraville (121♂, 168♀, ANIC; 6♂, 2♀, WMA); Nowra (6♂, 5♀, ANIC); Pee Dee (2♂, 1♀, ANIC); Potoroo Rd (10♂, 1♀, ANIC); Potoroo Road, 5 km west, Dingo S. F. (2♂, 1♀, ANIC); Potoroo Road, Wadsworth Trail, Dingo S. F. (5♂, 1♀, ANIC); Seven Mile Beach (1067♂, 1324♀, ANIC); South Coast (12♂, 18♀, ANIC); Styx River (1♀, ANIC); Sydney (1♂, NHMUK; 3♂, 4♀, SAMA); Sydney, Royal Botanic Gardens (5♂, 12♀, ANIC, ANIC Database Number 25 077511–25 077512); Tomakin, 35°49′S, 150°12′E (3♀, 4 larvae, 5 pupae, ANIC); Tomakin (7♂, 16♀, ANIC); Willi Willi East (11♂, 4♀, ANIC); Woy Woy (2♀, MVMA, COL-109476); Yalwal (4♂, ANIC). Australian Capital Territory: 35.16°S, 149.06°E, Black Mountain SE slope (1♂, ANIC).
See differential diagnosis of Tranes lyterioides lineage in Table 3.
| T. lyterioides | T. chadwicki | T. kgariensis | T. forsteri | T. terryae | ||
|---|---|---|---|---|---|---|
| Coloration | Body and legs completely reddish brown (Fig. 8a, d, g, h ) | Body dark red and legs dark red to black (Fig. 8e, f ) | Body and legs completely reddish brown (Fig. 8i, j ) | |||
| Body | Nearly flat (Fig. 10a, d, g, h ) | Weakly convex (Fig. 10e, f ) | Nearly flat (Fig. 10i, j ) | |||
| Vestiture | Sparsely to moderately covered with setae (Fig. 8a–h ) | Densely covered with setae (Fig. 8i, j ) | ||||
| Antennae of male | Inserted in middle of rostrum (Fig. 10a, c ) | Inserted behind middle of rostrum (Fig. 10g ) | Inserted before middle of rostrum (Fig. 10e ) | Inserted behind middle of rostrum (Fig. 10i ) | ||
| Pronotum of male | Nearly as wide as elytra (Fig. 8a, c, g ) | Distinctly narrower than elytra (Fig. 8e, i ) | ||||
| Prosternal protuberance of male | With moderately ridged anterior face (Fig. 14f ) | With weakly ridged anterior face (Fig. 14g ) | With strongly and sharply ridged anterior face (Fig. 14i ) | Without ridged anterior face (Fig. 14h, j ) | ||
| Prosternellum | Broad (Fig. 14f, g, i ) | Elongate (Fig. 14h, j ) | ||||
| Median pit on mesoventrite | Deeply depressed (Fig. 16f, g ) | Shallowly depressed (Fig. 16i ) | Deeply depressed (Fig. 16h, j ) | |||
| Abdominal ventrites 1 and 2 | Slightly depressed (Fig. 18f, g, i ) | Distinctly depressed (Fig. 18h ) | Slightly depressed (Fig. 18j ) | |||
| Elytra | Distinctly narrowing apicad in apical fourth (Fig. 8a, b ) | Distinctly narrowing apicad in apical third (Fig. 8c–j ) | ||||
| Length of penis (mm) | ~0.5 | ~0.4 | ~0.5 | ~0.7 | ~0.6 | |
| Lateral margins of penis | Subparallel-sided, strongly narrowing apicad in apical fourth, rounded apically (Fig. 25a ) | Subparallel-sided, strongly narrowing apicad in apical fourth, subtruncate apically (Fig. 25c ) | Subparallel-sided, strongly narrowing apicad in apical third, subtruncate apically (Fig. 25g ) | Slightly arcuate, strongly narrowing apicad in apical third, rounded apically (Fig. 25e ) | Subparallel-sided, strongly narrowing apicad in apical fourth, subtruncate apically (Fig. 25i ) | |
Body broadly oval (Fig. 8a, b), length 5.5–8.2 mm in both sexes, width ~0.4–0.5× of length, nearly flat in lateral view (Fig. 10a, b).
Body and legs completely reddish brown; body and legs semilustrous; body and legs covered with coarse, sublanceolate and subsquamiform, yellowish setae, clustered in some parts to somewhat obscure derm, especially on pronotum, scutellar shield and elytra.
Rostrum: moderately long, longer in female (~1.1–1.2× as long as pronotum in male, 1.6–1.7× in female), robust, ventrally curved, dorsoventrally flattened, slightly broadened apically in dorsal view, coarsely punctate dorsally, punctures slightly smaller in distal half, proximal half with paired dorsomedian and dorsolateral carinae, the latter lower than the former. Eyes: subcircular in outline, slightly convex but not protruding. Antennae: inserted in middle of rostrum in male (Fig. 10a), slightly behind middle in female (Fig. 10b); scapes not reaching eye; funicles with segment 1 longest, ~1.6×, 2.0×, 3.2×, 3.2×, 2.7× and 2.7× as long as segments 2–7 respectively; clubs stout, ~1.5× as long as wide, densely and finely pubescent (Fig. 11f).
Pronotum: roundly trapezoidal, apex ~0.5× as narrow as base (Fig. 12f), broader in male (nearly as wide as elytra in male, distinctly narrower than elytra in female); anterior margin subtruncate, slightly emarginate medially, with 3–4 small teeth on pronotal collar laterally in male (Fig. 13f), posterior margin protruding medially, forming obtuse median lobe, lateral margins mostly rounded but distinctly narrowed anteriorly; disc nearly flat; surface distinctly punctate, slightly punctorugulose laterally, with median longitudinal apunctate ridge, punctures separate on disc but confluent and vague laterally. Prosternum: surface sparsely and finely punctate, of male densely covered with small tubercles, forming paired prominent protuberance, with moderately ridged anterior face (Fig. 14f); prosternellum broad, widened posteriorly (Fig. 14f). Mesoventrite: intermesocoxal process trapezoidal, strongly declivous (Fig. 15f); median pit rounded to elongate (Fig. 16f). Scutellar shield: roundly subpentagonal. Elytra: longer in female, ~1.7–2.0× as long as pronotum in male, 2.4–2.6× in female, jointly ~0.6–0.7× as broad as long, broader than base of pronotum; humeri broadly rounded, slightly protruding; surface nearly flat, deeply and coarsely punctate in rows, forming distinct striae, interstriae convex. Legs: femora with small ventral subapical tooth; tibiae with premucro smaller than uncus; protibiae with well-developed tibial brush in male; meso- and metatibiae with distal setal combs continued around apex and extending to apical fourth of tibia; tarsi with claws free, divergent.
Abdomen: ventrites 1 and 2 slightly depressed (Fig. 18f); ventrite 5 flat, with a large depression posteriorly (Fig. 20a, b). Terminalia. Male: sternite VIII subtrapezoidal, sclerotised, apical margin subtruncate, distinctly punctate and densely covered with short setae, basal margin strongly sclerotised (Fig. 21k); spiculum gastrale widely concave apically, base lightly sclerotised (Fig. 22k); tegmen with complete ring, manubrium shorter than parameroid lobes (Fig. 22l); penis thick (~1.9–2.0× as long as wide), subparallel-sided, slightly widened apicad, strongly narrowing apicad in apical fourth, rounded apically (Fig. 25a, b); endophallus apically with basal membranous sleeve of asperities and apical anchor-shaped sclerite. Female: sternite VIII abruptly angled laterally (Fig. 21l); gonocoxites thick, short, apically bluntly rounded (Fig. 26k); gonostyli short, conical, bluntly rounded and setose apically; bursa copulatrix without bands of spicules; spermatheca thick, right-angled, gland small, swollen, narrowing apicad, rounded apically (Fig. 26l).
Pascoe (1875) did not provide a derivation for the name lyterioides, but he evidently named the species for its similarity to the genus Lyterius Schoenherr, which was usually classified in the subfamily Baridinae (e.g. Lacordaire 1866; Alonso-Zarazaga and Lyal 1999) but is misplaced there (Prena et al. 2023) and in fact quite closely related to the Tranes group (Hsiao et al. 2023).
This species occurs in the coastal regions of New South Wales between Tathra in the south and almost Coffs Harbour in the north, but it appears to be most common along the coast south of Newcastle (Fig. 27c). Its range is largely congruent with that of its host plant species, Macrozamia communis, M. reducta and M. montana, and in the northern half of its range (north of Newcastle) it is sympatric with T. terryae.
The hosts recorded for Tranes lyterioides are Macrozamia communis, M. montana and M. reducta. Prior to their description as distinct species (Hill 1998), M. montana and M. reducta were included in the concept of M. communis, so that records of T. lyteriodes from M. communis at localities around Cessnock (Mount Sugarloaf, Mulbring, Merriwa) and along the Kempsey–Armidale road (Big Hill, Pee Dee, Willi Willi) before 1998 refer to M. reducta and M. montana respectively. At Cessnock, T. lyterioides occurs on M. reducta together with T. terryae, but further north it does not seem to share its hosts with the latter species. The interactions between the two species on M. reducta and their roles in its pollination are unknown. Tranes lyterioides has been shown to be the main pollinator of M. communis, and its pollination mutualism with M. communis has been well studied over the past few decades (Chadwick 1993; Terry 2001; Wallenius et al. 2012; Wallenius 2014). In brief, adults of T. lyterioides aggregate on immature male cones at the onset of the annual coning period and, when the sporophylls separate, enter the cones to feed on pollen, mate and oviposit in the sporophylls. During the dehiscence (pollen-shedding) phase of the male cones, pollen-laden weevils leave the cones at sunset when these become thermogenic and emit high concentrations of volatile organic compounds. During such mass exodus events from male cones, some weevils also enter receptive female cones in the vicinity and, when they crawl down along the rachis, deposit their pollen on the micropyles of the developing seeds and fertilise their ovules (Chadwick 1993; Terry 2001; Wallenius et al. 2012; Wallenius 2014). The larvae first develop in the tissues of the male sporophylls but later tunnel into the rachis and peduncle of the cones, where they eventually pupate. Newly enclosed adults may overwinter in the old cones or shelter in the crown and among leaf bases until the next coning period. It is likely that T. lyterioides is also the main or sole pollinator of M. montana and M. reducta, casting doubt on their status as species distinct from M. communis.
Pascoe (1875) did not specify the number of specimens he had before him for the original description, but the single measurement and locality indicate that he based the description on only one specimen. There is also only a single specimen in Pascoe’s collection in the NHMUK, labelled as the holotype (Fig. 6c). Our examination of this specimen confirmed its conspecificity with the Tranes species associated with M. communis, M. montana and M. reducta in New South Wales, having the same sparse vestiture, flat pronotum, equal width of pronotum and elytra, well-developed teeth on the pronotal collar, moderately ridged anterior face of the prosternal protuberance, broad prosternal process and prosternellum, flat intermesocoxal process and elytra 1.9× as long as the pronotum. Not conforming with this conspecificity is that the holotype is labelled as having been collected at Gayndah in south-eastern Queensland, a locality that falls in the range of a different species, T. chadwicki, forcing the conclusion that the locality stated on the label of the holotype and given in the description of T. lyterioides must be erroneous.
Pascoe (1875) originally placed T. lyterioides in a monotypic genus Platyphaeus, whose name was synonymised with Tranes by Marshall (1939), who then also treated the name T. lyterioides as a junior synonym of T. sparsus, evidently based on females of T. lyterioides in the NHMUK misidentified as T. sparsus by Arthur Lea (E. C. Zimmerman, pers. comm., 1975). Zimmerman (1994) consequently rejected the species synonymy, followed by Chadwick (1993, 1998), Forster et al. 1994, May (1994), Oberprieler (1995a, 1995b), Terry (2001), Jones (2002) and Pullen et al. (2014). Our comparison of the holotypes of T. lyterioides and T. sparsus and our molecular phylogenetic analysis of the Tranes group (Hsiao et al. 2023; Fig. 2) confirm that these two species are different but that they do belong to the same genus.
Tranes chadwicki Hsiao & Oberprieler, sp. nov.
(Fig. 8c, d, 10c, d, 11g, 12g, 13g, 14g, 15g, 16g, 18g, 20c, d, 21m, n, 22m, n, 25c, d, 26m, n, 27c.)
ZooBank: urn:lsid:zoobank.org:act:81F05280-C7CE-4EDC-8FDC-315E914D574D
Type locality: 25°39′05″S, 152°01′18″E, Mt Walsh National Park, Palm Valley road near Cardiac Hill, Queensland, Australia.
Holotype, ♂. ‘29.XI.2002 / AU: 25°39′05″S, 152°01′18″E, Mt. / Walsh National Park, Palm Valley road / nr. Cardiac Hill, QLD / P.I. Forster leg. // Host: Macrozamia cardiacensis / (Voucher: P.I. Forster PIF26495), in / male cones dehiscing pollen // HOLOTYPE / Tranes chadwicki / Hsiao & Oberprieler, 2025’ (ANIC).
Paratypes (all labelled ‘PARATYPE / Tranes chadwicki / Hsiao & Oberprieler, 2025’). Same data as holotype (10♂, 16♀, ANIC, ANIC Database Numbe 25 077536–25 077537; 1♂, 2♀, QDPI).
See Table 3.
Body broadly oval (Fig. 8c, d), length 3.6–6.5 mm in both sexes, width ~0.4–0.5× of length, nearly flat in lateral view (Fig. 10c, d).
Body and legs completely reddish brown; body and legs semilustrous; body and legs covered with coarse, sublanceolate and subsquamiform, yellowish setae, clustered in some parts to somewhat obscure derm, especially on pronotum, scutellar shield and elytra.
Rostrum: moderately long, longer in female (~1.2–1.5× as long as pronotum in male, 1.5–1.7× in female), robust, ventrally curved, dorsoventrally flattened, slightly broadened apically in dorsal view, coarsely punctate dorsally, punctures slightly smaller in distal half, proximal half with paired dorsomedian and dorsolateral carinae, the latter lower than the former. Eyes: subcircular in outline, slightly convex but not protruding. Antennae: inserted in middle of rostrum in male (Fig. 10c), slightly behind middle in female (Fig. 10d); scapes not reaching eye; funicles with segment 1 longest, ~1.5×, 2.6×, 2.6×, 2.8×, 2.6× and 2.3× as long as segments 2–7 respectively; clubs stout, ~1.6–1.8× as long as wide, densely and finely pubescent (Fig. 11g).
Pronotum: roundly trapezoidal, apex ~0.5× as narrow as base (Fig. 12g), broader in male (nearly as wide as elytra in male, distinctly narrower than elytra in female); anterior margin subtruncate, slightly emarginate medially, with 3–4 small teeth on pronotal collar laterally in male (Fig. 13g), posterior margin protruding medially, forming obtuse median lobe, lateral margins mostly rounded but distinctly narrowed anteriorly; disc nearly flat; surface distinctly punctate, slightly punctorugulose laterally, with median longitudinal apunctate ridge, punctures separate on disc but confluent and vague laterally. Prosternum: surface sparsely and finely punctate, of male densely covered with small tubercles, forming paired prominent protuberance, with weakly ridged anterior face (Fig. 14g); prosternellum broad, widened posteriorly (Fig. 14g). Mesoventrite: intermesocoxal process trapezoidal, weakly declivous (Fig. 15g); median pit groove-like (Fig. 16g). Scutellar shield: roundly subpentagonal. Elytra: longer in female, ~1.7–2.1× as long as pronotum in male, 2.2–2.4× in female, jointly ~0.6–0.7× as broad as long, broader than base of pronotum; humeri broadly rounded, slightly protruding; surface nearly flat, deeply and coarsely punctate in rows, forming distinct striae, interstriae convex. Legs: femora with small ventral subapical tooth; tibiae with premucro smaller than uncus; protibiae with well-developed tibial brush in male; meso- and metatibiae with distal setal combs continuing around apex and extending to apical fourth of tibia; tarsi with claws free, divergent.
Abdomen: ventrites 1 and 2 slightly depressed (Fig. 18g); ventrite 5 flat, with a large depression posteriorly (Fig. 20c, d). Terminalia. Male: sternite VIII subtrapezoidal, sclerotised, apical margin subtruncate, distinctly punctate and densely covered with short setae, basal margin strongly sclerotised (Fig. 21m); spiculum gastrale widely concave apically, base lightly sclerotised (Fig. 22m); tegmen with complete ring, manubrium shorter than parameroid lobes (Fig. 22n); penis thick (~1.9× as long as wide), subparallel-sided, slightly widened apicad, strongly narrowing apicad in apical fourth, subtruncate apically (Fig. 25c, d); endophallus apically with basal membranous sleeve of asperities and apical anchor-shaped sclerite. Female: sternite VIII abruptly angled laterally (Fig. 21n); gonocoxites thick, short, apically bluntly rounded (Fig. 26m); gonostyli short, conical, bluntly rounded and setose apically; bursa copulatrix without bands of spicules; spermatheca thick, right-angled, gland small, swollen, narrowing apicad, rounded apically (Fig. 26n).
The species is named for Clarence Earl (Clarry) Chadwick (1909–2004), entomologist at the N.S.W. Department of Agriculture in Sydney and Orange and, after his retirement in 1974, Research Associate at the Australian Museum in Sydney until 1988 (Owen 2005). In his retirement years Clarry devoted a lot of time and energy to the observation and collection of insects associated with Macrozamia communis, which he donated to the ANIC in 1999. The numerous records of Tranes weevils incorporated into this revision are a testament to the significant contribution he made to the knowledge of the natural history and distribution of these weevils (see also Chadwick 1993, 1998, 1999).
As known to date, this species occurs only in a small area near Biggenden in south-eastern Queensland (Fig. 27c). The isolated record from Mooball National Park, ~350 km further south in northern New South Wales (near Murwillumbah), is most likely due to a labelling error and refers to T. terryae instead, as all other specimens from this locality and from nearby ones (Murwillumbah, Lamington N. P., Springbrook) are T. terryae (see there). Tranes chadwicki appears to have a restricted range that is likely congruent with that of its host species, Macrozamia cardiacensis.
This species appears to be associated only with M. cardiacensis. The isolated record from Lepidozamia peroffskyana in Mooball National Park is probably based on a labelling error; the specimens agree with T. chadwicki on morphological characters and molecular data (Fig. 2), but the locality and host data are probably erroneous, applying to T. terryae instead. Tranes chadwicki is likely the main or sole pollinator of M. cardiacensis, but its life cycle and involvement in the pollination of its host remain to be elucidated.
This species corresponds to the ‘Tranes lyterioides lineage sp. B’ in the molecular phylogenetic analysis of Australian cycad-associated weevils (Hsiao et al. 2023).
Tranes forsteri Hsiao & Oberprieler, sp. nov.
(Fig. 8e, f, 10e, f, 11h, 12h, 13h, 14h, 15h, 16h, 18h, 20e, f, 21o, p, 22o, p, 25e, f, 26o, p, 27c.)
ZooBank: urn:lsid:zoobank.org:act:702AE941-F194-4723-95E9-DBA571FA2AA0
Type locality: Utopia, 14 km SSE of Biggenden, Rock Pools road (25°38′43″S, 152°05′30″E), Queensland, Australia.
Holotype, ♂. ‘5. I. 1992 / AU: 25°38′43″S, 152°05′30″E, Utopia, / 14 km SSE of Biggenden, Rock Pools / road, QLD / P.I. Forster leg. 929318 // Host: Macrozamia / parcifolia (P.I. / Forster PIF 93188), in male cones / dehiscing pollen // HOLOTYPE / Tranes forsteri / Hsiao & Oberprieler, 2025’ (ANIC).
Paratypes (all labelled ‘PARATYPE / Tranes forsteri / Hsiao & Oberprieler, 2025’). Same as holotype (1♂, 3♀, ANIC; 1♂, 1♀, QDPI).
Queensland: Auburn S. F., 25°38′18″S, 151°07′27″E (12♂, 11♀, QDPI, ANIC Database Number 25 077513–25 077515); Barrett’s road near Isis River, Vacant Crown Land, 25°13′48″S, 152°26′07″E (9♂, 3♀, ANIC; 2♂, 2♀, QDPI); Beeron Holding, 25.29°S, 151.20°E (6♂, 5♀, ANIC); Beeron Holding, 4 km W of ‘Toondahra’ Homestead, 25°59′S, 151°20′E (6♂, 4♀, ANIC; 3♀, QDPI); Biggenden, 14 km SSE, 25.38°S, 152.05°E (6♂, 5♀, 9 larvae, ANIC); Brisbane Forest Park, Brisbane, 27°26′19″S, 152°49′31″E (1♂, 1♀, 7 larvae, ANIC); State Forest 840, 20 km SSW of Bundaberg, 25°02′52″S, 152°17′47″E (15♂, 7♀, 9 larvae, ANIC; 2♂, 2♀, QDPI); State Forest 840, S of Bundaberg, 25.03°S, 152.17°E (10♂, 12♀, 15 larvae, ANIC); Utopia, 14 km SSE of Biggenden, Rock Pools road, 25°38′43″S, 152°05′30″E (8 larvae, ANIC).
See Table 3.
Body broadly oval (Fig. 8e, f), length 4.9–7.1 mm in both sexes, width ~0.4–0.5× of length, weakly convex in lateral view (Fig. 10e, f).
Body completely dark red, legs dark red to black; body and legs semilustrous; body and legs covered with coarse, sublanceolate and subsquamiform, yellowish setae, clustered in some parts to somewhat obscure derm, especially on pronotum, scutellar shield and elytra.
Rostrum: moderately long, longer in female (~1.2–1.3× as long as pronotum in male, 1.6–1.7× in female), robust, ventrally curved, dorsoventrally flattened, slightly broadened apically in dorsal view, coarsely punctate dorsally, punctures slightly smaller in distal half, proximal half with paired dorsomedian and dorsolateral carinae, the latter lower than the former. Eyes: subcircular in outline, slightly convex but not protruding. Antennae: inserted slightly before middle of rostrum in male (Fig. 10e), slightly behind middle in female (Fig. 10f); scapes not reaching eye; funicles with segment 1 longest, ~1.6×, 2.2×, 2.6×, 2.6×, 2.6× and 2.6× as long as segments 2–7 respectively; clubs stout, ~1.5× as long as wide, densely and finely pubescent (Fig. 11h).
Pronotum: roundly trapezoidal, apex ~0.6× as narrow as base (Fig. 12h), distinctly narrower than elytra in both male and female; anterior margin subtruncate, slightly emarginate medially, with 3–5 small teeth on pronotal collar laterally in male (Fig. 13h), posterior margin protruding medially, forming obtuse median lobe, lateral margins mostly rounded but distinctly narrowed anteriorly; disc weakly convex; surface distinctly punctate, slightly punctorugulose laterally, with median longitudinal apunctate ridge, punctures separate on disc but confluent and vague laterally. Prosternum: surface sparsely and finely punctate, of male densely covered with small tubercles, forming paired prominent protuberance, evenly convex, without ridged anterior face (Fig. 14h); prosternellum elongate, widened posteriorly (Fig. 14h). Mesoventrite: intermesocoxal process trapezoidal, strongly declivous (Fig. 15h); median pit rounded to elongate (Fig. 16h). Scutellar shield: roundly subpentagonal. Elytra: longer in female, ~1.9–2.3× as long as pronotum in male, 2.4–2.5× in female, jointly ~0.6–0.7× as broad as long, broader than base of pronotum; humeri broadly rounded, slightly protruding; surface weakly convex, deeply and coarsely punctate in rows, forming distinct striae, interstriae convex. Legs: femora with small ventral subapical tooth; tibiae with premucro smaller than uncus; protibiae with well-developed tibial brush in male; meso- and metatibiae with distal setal combs continuing around apex and extending to apical fourth of tibia; tarsi with claws free, divergent.
Abdomen: ventrites 1 and 2 distinctly depressed (Fig. 18h); ventrite 5 flat, with a large depression posteriorly (Fig. 20e, f). Terminalia. Male: sternite VIII subtrapezoidal, sclerotised, apical margin subtruncate, distinctly punctate and densely covered with short setae, basal margin strongly sclerotised (Fig. 21o); spiculum gastrale widely concave apically, base lightly sclerotised (Fig. 22o); tegmen with complete ring, manubrium shorter than parameroid lobes (Fig. 22p); penis thick (~2.0–2.1× as long as wide), lateral margins slightly arcuate, slightly widened apicad, strongly narrowing apicad in apical third, rounded apically (Fig. 25e, f); endophallus apically with basal membranous sleeve of asperities and apical anchor-shaped sclerite. Female: sternite VIII abruptly angled laterally (Fig. 21p); gonocoxites thick, short, apically bluntly rounded (Fig. 26o); gonostyli short, conical, bluntly rounded and setose apically; bursa copulatrix without bands of spicules; spermatheca thick, right-angled, gland small, elongate, narrowing apicad, rounded apically (Fig. 26p).
The species is named for Paul Forster, botanist at the Queensland Herbarium, who made many contributions to the biology and conservation of Australian cycads and collected Tranes weevils from many localities and Macrozamia species and made them available for this research.
This species appears to be restricted to the Bundaberg–Mundubbera regions of south-eastern Queensland (Fig. 27c), where it seems to be parapatric with T. chadwicki. The two records from Brisbane appear to reflect an error or an introduction (see below).
The species has been recorded from several closely related Macrozamia species, M. crassifolia, M. lomandroides, M. parcifolia and M. pauli-guilielmi, which all appear to be genuine hosts of the species. The isolated record from M. lucida in Brisbane Forest Park refers to two adults and seven larvae found in a female cone with maturing seeds, rather than to a series of specimens having been found in male cones, and it is thus likely erroneous. If this record can be confirmed, the species may have been introduced to this region and not be widespread and abundant there, but without such possible confirmation this host and distributional record cannot be taken to be valid.
This species was first recorded by Forster et al. (1994) as ‘Tranes sp. 2’ from M. lomandroides, M. sp. aff. pauli-guilielmi No. 1 (M. crassifolia) and M. sp. aff. pauli-guilielmi No. 2 (M. parcifolia) but mixed with T. kgariensis (from M. douglasii) and T. terryae (from M. sp. aff. plurinervia, i.e. M. machinii). It was similarly confounded by Toon et al. (2020). It corresponds to the ‘Tranes lyterioides lineage sp. C’ in the molecular phylogenetic analysis of Australian cycad-associated weevils (Hsiao et al. 2023).
Tranes kgariensis Hsiao & Oberprieler, sp. nov.
(Fig. 8g, h, 10g, h, 11i, 12i, 13i, 14i, 15i, 16i, 18i, 20g, h, 21q, r, 22q, r, 25g, h, 26q, r, 27c.)
ZooBank: urn:lsid:zoobank.org:act:FBE92597-0323-4BBE-A047-8E67B2C9715A
Type locality: Great Sandy National Park (24°59′38″S, 153°16′16″E), K’gari (Fraser Island), Queensland, Australia.
Holotype, ♂. ‘2. XI. 2002 / AU: 24°59′38″S, 153°16′16″E, Great / Sandy National Park, Fraser Island, / Warhumba Swamp road, 4 km SW of / Orchid Beach, QLD / P.I. Forster leg. // Host: Macrozamia douglasii (Voucher: / P.I. Forster PIF29003), in male cones / dehiscing pollen // HOLOTYPE / Tranes kgariensis / Hsiao & Oberprieler, 2025’ (ANIC).
Paratypes (all labelled ‘PARATYPE/Tranes kgariensis/Hsiao & Oberprieler, 2025’). 14♂, 14♀, same data as holotype (ANIC).
Queensland: Central Station, Fraser Is. (1♀, QMBA); Central Station, Forestry Camp near Eurong, Fraser Is., 25.28°S, 153.04°E (2♂, ANIC, ANIC Database Number 25 077533); Central Station, Fraser Is. (2♂, NHMUK); Fraser Is., Coomboo Lake, 25°14′S, 153°10′E (1♂, 1♀, QMBA, ANIC Database Number 25 077534); Great Sandy National Park, Fraser Is., Warhumba Swamp road, 4 km SW of Orchid Beach (1♂, 3♀, QDPI); Poona Lake, ~1 km N, Cooloola N. P., 25°57′16.6″S, 153°06′16.6″E (1♀, ANIC, ANIC Database Number 25 077535).
See Table 3.
Body broadly oval (Fig. 8g, h), length 5.1–8.1 mm in both sexes, width ~0.4–0.5× of length, nearly flat in lateral view (Fig. 10g, h).
Body and legs completely reddish brown; body and legs semilustrous; body and legs covered with coarse, sublanceolate and subsquamiform, yellowish setae, clustered in some parts to somewhat obscure derm, especially on pronotum, scutellar shield and elytra.
Rostrum: moderately long, longer in female (~1.3× as long as pronotum in male, 1.6× in female), robust, ventrally curved, dorsoventrally flattened, slightly broadened apically in dorsal view, coarsely punctate dorsally, punctures slightly smaller in distal half, proximal half with paired dorsomedian and dorsolateral carinae, the latter lower than the former. Eyes: subcircular in outline, slightly convex but not protruding. Antennae: inserted slightly behind middle of rostrum in both male and female (Fig. 10g, h); scapes not reaching eye; funicles with segment 1 longest, ~1.8×, 2.5×, 3.3×, 3.3×, 3.3× and 2.9× as long as segments 2–7 respectively; clubs stout, ~1.6–1.7× as long as wide, densely and finely pubescent (Fig. 11i).
Pronotum: roundly trapezoidal, apex ~0.5× as narrow as base (Fig. 12i), broader in male (nearly as wide as elytra in male, distinctly narrower than elytra in female); anterior margin subtruncate, slightly emarginate medially, with 3–4 small teeth on pronotal collar laterally in male (Fig. 13i), posterior margin protruding medially, forming obtuse median lobe, lateral margins mostly rounded but distinctly narrowed anteriorly; disc nearly flat; surface distinctly punctate, slightly punctorugulose laterally, with median longitudinal apunctate ridge, punctures separate on disc but confluent and vague laterally. Prosternum: surface sparsely and finely punctate, of male densely covered with small tubercles, forming paired prominent protuberance, with strongly and sharply ridged anterior face (Fig. 14i); prosternellum broad, widened posteriorly (Fig. 14i). Mesoventrite: intermesocoxal process trapezoidal, weakly declivous (Fig. 15i); median pit groove-like (Fig. 16i). Scutellar shield: roundly subpentagonal. Elytra: longer in female, ~1.6–1.7× as long as pronotum in male, 2.1–2.2× in female, jointly ~0.7× as broad as long, broader than base of pronotum; humeri broadly rounded, slightly protruding; surface nearly flat, deeply and coarsely punctate in rows, forming distinct striae, interstriae convex. Legs: femora with small ventral subapical tooth; tibiae with premucro smaller than uncus; protibiae with well-developed tibial brush in male; meso- and metatibiae with distal setal combs continuing around apex and extending to apical fourth of tibia; tarsi with claws free, divergent.
Abdomen: ventrites 1 and 2 slightly depressed (Fig. 18i); ventrite 5 flat, with a large depression posteriorly (Fig. 20g, h). Terminalia. Male: sternite VIII subtrapezoidal, sclerotised, apical margin subtruncate, distinctly punctate and densely covered with short setae, basal margin strongly sclerotised (Fig. 21q); spiculum gastrale widely concave apically, base lightly sclerotised (Fig. 22q); tegmen with complete ring, manubrium nearly as long as parameroid lobes (Fig. 22r); penis thick (~1.9–2.0× as long as wide), subparallel-sided, strongly narrowing apicad in apical third, subtruncate apically (Fig. 25g, h); endophallus apically with basal membranous sleeve of asperities and apical anchor-shaped sclerite. Female: sternite VIII abruptly angled laterally (Fig. 21r); gonocoxites thick, short, apically bluntly rounded (Fig. 26q); gonostyli short, conical, bluntly rounded and setose apically; bursa copulatrix without bands of spicules; spermatheca thick, right-angled, gland small, elongate, narrowing apicad, rounded apically (Fig. 26r).
The specific epithet is a Latin adjective derived from the name K’gari (Fraser Island), which denotes a large sand island situated along the south-eastern Queensland coast in the Wide Bay–Burnett region.
This species occurs mainly on K’gari (Fraser Island) but has also been taken near Poona Lake in the Great Sandy National Park on the mainland south of the island (Fig. 27c) and recorded from the Tuan State Forest (Forster et al. 1994), adjacent to the south-western side of K’gari, where its host species also grows.
The only host plant recorded for Tranes kgariensis is Macrozamia douglasii, of which it likely is the sole pollinator. Forster et al. (1994) briefly described the habits of the species. As in other Tranes species, the adults congregate in large numbers on the male cones, and the feeding activity of the larvae causes the disintegration of cones 2 weeks after their dehiscence. Dispersal of adults from dehiscing male cones apparently also takes place in the evening, as the weevils are attracted to lights at night.
This species was first recorded by Forster et al. (1994), from M. douglasii in Tuan State Forest, but treated as the same species (‘Tranes sp. 2’) as those associated with M. crassifolia, M. lomandroides and M. parcifolia (T. forsteri) and M. machinii (T. terryae). It was similarly confused by Toon et al. (2020). It corresponds to the ‘Tranes lyterioides lineage sp. A’ in the molecular phylogenetic analysis of Australian cycad-associated weevils (Hsiao et al. 2023).
Tranes terryae Hsiao & Oberprieler, sp. nov.
(Fig. 1g, h, 8i, j, 10i, j, 11j, 12j, 13j, 14j, 15j, 16j, 18j, 20i, j, 21s, t, 22s, t, 25i, j, 26s, t, 27c.)
ZooBank: urn:lsid:zoobank.org:act:DC8017B3-8E8E-4FC6-9567-84A32BDB3C8E
Type locality: Springbrook National Park, Queensland, Australia.
Holotype, ♂. ‘6-7.I.2017 / AU: QLD: Springbrook / N.P., Ankida / D.D. McKenna leg. / DDM2017-027 // HOLOTYPE / Tranes terryae / Hsiao & Oberprieler, 2025’ (ANIC).
Paratypes (all labelled ‘PARATYPE / Tranes terryae / Hsiao & Oberprieler, 2025’). 13♂, 6♀, same as holotype (ANIC, ANIC Database Number 25 077516–25 077517).
New South Wales: Barrington Tops S. F., Moppy Lockout, 31.54°S, 151.33°E (1♀, ANIC); Broken Head N. R., 8 km S Byron Bay, 28.43°S, 153.37°E (5♂, 8♀, ANIC); Bruxner Park Flora Pres., Sealy Lookout, N of Coffs Harbour (18♂, 20♀, ANIC); Cessnock, 32°50′S, 151°21′E (3♂, 5♀, ANIC); Clarence Town (1♂, SAMA); Coonabarabran (2♀, ANIC, ANIC Database Number 25 077531–25 077532); Dandry road NNE of Coonabarabran, 31°08′S, 149°20′E (2♂, 5♀, ANIC; 4♀, QDPI); Dandry road NNE of Coonabarabran, 31°13′S, 149°17′E (2♂, 8♀, ANIC; 1♂, 1♀, QDPI); Doonarang N. P., rainforest track, 31.6567°S, 152.7755°E (8♂, 46♀, ANIC, ANIC Database Number 25 077960–25 077962); Dorrigo (3♀, ANIC); Dorrigo N. P. (1♀, NHMUK); Flagstaff Hill, Tamworth, 31°05′11″S, 150°57′41″E (6♂, 9♀, ANIC; 1♂, 1♀, QDPI); Gilgandra (1♀, ANIC); Goonoowigall Wildnerness near Inverell, 29°42′S, 151°07′E (4♂, 3♀, ANIC; 3♂, 1♀, QDPI); Grassy Head near Macksville (6♂, 6♀, ANIC); Grassy Head, N of Kempsey, 31.05°S, 152.50°E (3♂, 5♀, ANIC); Lansdowne (1♂, ANIC); Lansdowne S. F., N of Taree (1♂, ANIC); Lansdowne S. F., Starrs Creek (3♂, 5♀, ANIC); Lorien Wildlife Refuge, 3 km N Lansdowne/Taree (1♂, 1♀, ANIC); Mooball (1♂, 1♀, ANIC); Mooball S. F. near Murwillumbah (3♂, 3♀, ANIC); Mt Hillston, 14 km SW from Bundarra, 30°12′S, 150°57′E (5♂, 6♀, ANIC, 1 ♂, 1 ♀, QDPI); Mt Kaputar N. P., 150.10E 30.17S (1♂, ANIC); Mt Kaputar, Coryah Gap, 30°16′S, 150°07′E (18♂, 15♀, ANIC; 2♂, 2♀, QDPI); Mt Warning (1♂, ANIC); Mullaley (1♂, ANIC); Murwilllumbah, 28.20°S, 153.24°E (1♂, 2♀, ANIC); Neath, South Maitland Railway (1♂, ANIC); North Brother near Laurieton (3♂, 3♀, ANIC); Pilliga East State Forest 266, X-Line road, 30°38′S, 149°35′E (5♂, 3♀, ANIC; 1♂, QDPI); Potoroo road, Dingo S. F., Wadsworth Trail (1♀, ANIC); South Arm via Bowraville (1♂, 1♀, ANIC); Springbrook N. P., Ankida (13♂, 35♀, ANIC); State Forest 708, Branch Creek near Tabulam, 28°55′S, 152°35′E (5♂, 15♀, ANIC; 2♂, 2♀, QDPI); State Forest 708, Branch Creek near Tabulam, 28.54°S, 152.35°E (4♂, 3♀, ANIC); State Forest 794, Royal Camp, 5 km along Pebble Ridge road, 29°00′S, 152°50′E (2♂, 2♀, ANIC; 2♂, QDPI); Talbragar fossil site, ~25 km NE Gulgong, 32°10′S, 149°48′E (3♂, 5♀, 7 larvae, 5 pupae, ANIC, ANIC Database Number 25 077524–25 077525); Wingen Maid N. R, ‘Sans Tache’, 31°54′26″S, 150°49′11″E (33♂, 31♀, ANIC, ANIC Database Number 25 077529–25 077530); Wingen Maid N. R., W of Salisbury Trig. (5♂, 8♀, ANIC, ANIC Database Number 25 077611–25 077612). Queensland: Bracker S. F., eastern boundary, 28°33′32″S, 151°08′32″E (1♂, 3♀, ANIC; 2♂, 1♀, QDPI); D’Aguilar Range, State Forest 809, 4.8 km by road NW of The Summit, 27°17′S, 152°43′E (1♂, 1♀, ANIC; 1♂, QDPI); Dalmorton S. F., 29.50°S, 152.27°E (1♀, ANIC); Dinosaur’s Playground, State Forest 809, 5 km N of Mt Glorious, 27°17′03″S, 152°45′46″E (22♂, 17♀, ANIC; 1♂, 3♀, QDPI); Eungai, near Macksville, 30°51′S, 152°54′E (3♂, 1♀, ANIC); Jimna House, 7 km SWbyS Tallebudgera, 28.12°S, 153.23°E (1♂, 1♀, ANIC); Lamington N. P. (1♀, QDPI, INSECOLL 0-056375); Mt D’Aguilar (2♂, 2♀, QDPI, INSECOLL 0-056376); Mt Gammie N (7♂, 10♀, 1 larva, ANIC, ANIC Database Number 25 077527–25 077528); Mt Gannon summit, via West Burleigh (7♂, 4♀, QMBA); Mt Glorious (1♀, ANIC; 1♂, 1♀, QMBA); Mt Glorious, 27.20°S, 152.46°E (12♂, 12♀, ANIC); Rocky Glen vicinity (1♂, WMA); State Forest 444, Durikai, 28°19′S, 151°42′E (1♂, 1♀, ANIC; 1♂, QDPI); Sundown N. P., near Red Rock Gorge road to Severn River, 28°52′S, 151°42′E (7♂, 13♀, ANIC; 1♂, 3♀, QDPI); Tamborine Mountain (4♂, 8♀, ANIC; 4♂, 2♀, MVMA, COL-109477, 143351–143354; 5♂, 2♀, QDPI, INSECOLL 0-056373–0-056374; 2♂, 3♀, QMBA); Toowoomba (4♂, 3♀, ANIC); Wondul Range, N of Inglewood, 28.06°S, 151.03°E (13♂, 10♀, ANIC); Wondul Range, N of Inglewood (5♂, 1♀, ANIC, ANIC Database Number 25 077520–25 077521); Wondul Range near Inglewood, Stock Route, 28°13′S, 151°27′E (61♂, 106♀, 28 larvae, ANIC, ANIC Database Number 25 077518–25 077519; 4♂, 8♀, QMBA, UQIC Reg. #77593–77604); Wondul Range, Portion 82, ~30 km N of Inglewood, 28°07′S, 151°04′E (9♂, 8♀, ANIC; 3♂, 3♀, QDPI); Wyberba, Portion 90, 28°50′S, 151°54′E (19♂, 34♀, ANIC; 2♂, 2♀, QDPI).
See Table 3.
Body broadly oval (Fig. 8i, j), length 5.7–8.6 mm in both sexes, width ~0.4–0.5× of length, nearly flat in lateral view (Fig. 10i, j).
Body and legs completely reddish brown; body and legs semilustrous; body and legs covered with coarse, sublanceolate and subsquamiform, yellowish setae, clustered in some parts to somewhat obscure derm, especially on prothorax, meso- and metaventrites, scutellar shield, elytra and abdominal ventrites 1–4.
Rostrum: moderately long, longer in female (~1.2–1.4× as long as pronotum in male, 1.5–1.7× in female), robust, ventrally curved, dorsoventrally flattened, slightly broadened apically in dorsal view, coarsely punctate dorsally, punctures slightly smaller in distal half, proximal half with paired dorsomedian and dorsolateral carinae, the latter lower than the former. Eyes: subcircular in outline, slightly convex but not protruding. Antennae: inserted slightly behind middle of rostrum in both male and female (Fig. 10i, j); scapes not reaching eye; funicles with segment 1 longest, ~1.5×, 2.1×, 2.1×, 2.5×, 2.1× and 2.1× as long as segments 2–7 respectively; clubs stout, ~1.6× as long as wide, densely and finely pubescent (Fig. 11j).
Pronotum: roundly trapezoidal, apex ~0.5× as narrow as base (Fig. 12j), distinctly narrower than elytra in both sexes; anterior margin subtruncate, slightly emarginate medially, with 3–4 small teeth on pronotal collar laterally in male (Fig. 13j), posterior margin protruding medially, forming obtuse median lobe, lateral margins mostly rounded but distinctly narrowed anteriorly; disc nearly flat; surface distinctly punctate, slightly punctorugulose laterally, with median longitudinal apunctate ridge, punctures separate on disc but confluent and vague laterally. Prosternum: surface sparsely and finely punctate, of male densely covered with small tubercles, forming paired prominent protuberance, evenly convex, without ridged anterior face (Fig. 14j); prosternellum elongate, widened posteriorly (Fig. 14j). Mesoventrite: intermesocoxal process trapezoidal, weakly declivous (Fig. 15j); median pit rounded to elongate (Fig. 16j). Scutellar shield: roundly subpentagonal. Elytra: longer in female, ~1.8–2.1× as long as pronotum in male, 2.3–2.4× in female, jointly ~0.7× as broad as long, broader than base of pronotum; humeri broadly rounded, slightly protruding; surface nearly flat, deeply and coarsely punctate in rows, forming distinct striae, interstriae convex. Legs: femora with small ventral subapical tooth; tibiae with premucro smaller than uncus; protibiae with well-developed tibial brush in male; meso- and metatibiae with distal setal combs continuing around apex and extending to apical fourth of tibia; tarsi with claws free, divergent.
Abdomen: ventrites 1 and 2 slightly depressed (Fig. 18j); ventrite 5 flat, with a large depression posteriorly (Fig. 20i, j). Terminalia. Male: sternite VIII subtrapezoidal, sclerotised, apical margin subtruncate, distinctly punctate and densely covered with short setae, basal margin strongly sclerotised (Fig. 21s); spiculum gastrale widely concave apically, base lightly sclerotised (Fig. 22s); tegmen with complete ring, manubrium nearly as long as or shorter than parameroid lobes (Fig. 22t); penis thick (~1.9–2.3× as long as wide), subparallel-sided, slightly widened apicad, strongly narrowing apicad in apical fourth, subtruncate apically (Fig. 25i, j); endophallus apically with basal membranous sleeve of asperities and apical anchor-shaped sclerite. Female: sternite VIII abruptly angled laterally (Fig. 21t); gonocoxites thick, short, apically bluntly rounded (Fig. 26s); gonostyli short, conical, bluntly rounded and setose apically; bursa copulatrix without bands of spicules; spermatheca thick, right-angled, gland small, swollen, narrowing apicad, rounded apically (Fig. 26t).
The species is named for Irene Terry, University of Utah, in recognition of the major and significant contributions she made to the understanding of the mutualisms existing between cycads and their pollinators, especially those between Macrozamia cycads and Tranes weevils.
This species occurs over a large area in eastern Australia, from around Cessnock in New South Wales in the south to Brisbane in southern Queensland in the north. In the southern part of its range it is sympatric with T. lyterioides along the coast (Fig. 27c).
Coinciding with its fairly large distribution range, T. terryae also has a considerably wide range of hosts, spanning Lepidozamia peroffskyana and 14 species of Macrozamia: M. concinna, M. conferta, M. diplomera, M. fawcettii, M. glaucophylla, M. humilis, M. lucida, M. machinii, M. occidua, M. polymorpha, M. reducta, M. secunda, M. stenomera and M. viridis. Whether it breeds regularly in the male cones of all these species remains to be determined, however. Its life history and role in the pollination of L. peroffskyana and M. machinii have been studied by Hall et al. (2004) and Terry et al. (2004, 2005), revealing a pattern highly congruent with the pollination mutualism existing between T. lyterioides and M. communis. The number of weevils congregating on ripe male cones can be much larger, however, Hall et al. (2004) reporting more than 700 weevils from a single male cone of L. peroffskyana, compared to ~200 of T. lyterioides recorded per cone of M. communis (Chadwick 1993), but this difference is probably mainly due to the larger size of the L. peroffskyana cones.
Tranes terryae has been reported several times in the literature, earlier treated as T. lyterioides (Ornduff 1989; Kennedy 1991; Chadwick 1993; Oberprieler 1997) but later as one or several undescribed species (Forster et al. 1994; Hall et al. 2004; Terry et al. 2004, 2005; Toon et al. 2020). In the molecular phylogenetic analysis of Australian cycad-associated weevils (Hsiao et al. 2023) it corresponds to the species referred to as ‘Tranes lyterioides lineage sp. D’.
Discussion
Review of the taxonomy of the Tranes group based on molecular and morphological analysis
The phylogenetic analysis based on mitogenomic data revealed that the Tranes group includes not only Australian weevils associated with cycads and grasstrees but also a South-east Asian genus that appears to be associated with Pandanus (Hsiao et al. 2023; Prena et al. 2023), and another, as yet undescribed genus in New Caledonia evidently belongs to this group as well. Legalov (2018) formalised the Tranes group as a distinct tribe and attempted to define it, but due to the high degree of morphological heterogeneity in the group it resulted in a very poor definition and distinction from other relevant molytine groups. For instance, in Legalov’s (2018) key to the tribes of Molytinae, couplet 36 distinguishes the Tranes group and similar tribes from other molytines by the protibiae having a carina with a longitudinal comb of setae, but this character only applies to Howeotranes, Miltotranes and Tranes, not to any of the other genera. Similarly, couplet 37 differentiates the Tranes group from other similar molytine tribes by ventrally subcontiguous eyes and densely setose protibiae, yet the eyes of Demyrsus are ventrally distinctly separate and densely setose protibiae occur only in males of Miltotranes and some Tranes species. Furthermore, Lyterius, an apparent close relative of the Tranes group (Hsiao et al. 2023), has a flattened body with a smooth surface, ventrally widely separated eyes and a distinct discrimen on the metaventrite (Prena et al. 2023), thus increasing the morphological variability of the Tranes group as now understood. Moreover, the concepts and compositions of apparently closely related molytine groups, such as Cryptorhynchini, Mesoptiliini, Molytini, Lixini and Pissodini (Hsiao et al. 2023; Haran et al. 2023) and also Orthorhinini and similar groups (Shin et al. 2018; Anderson et al. 2018), are equally unclear, and a much more comprehensive phylogenetic analysis of Molytinae and related taxa is required before the classificatory status of the Tranes group can be properly assessed.
Further phylogenetic study is also required for the Tranes group itself due to the nature of mitogenomic data (e.g. maternal inheritance and lack of recombination), which may affect the pattern observed in the phylogenetic analysis or overemphasise geographical structure in species delineation. Our molecular analysis (Hsiao et al. 2023) indicates that Siraton may not be a monophyletic genus, S. internatus being more closely related to Demyrsus meleoides than to S. roei. This suggests that S. roei may be more suitably treated as a different genus or that Siraton be subsumed into Demyrsus. However, morphological support (or not) for such a relationship remains to be carefully assessed. Denser sampling of specimens is also required to assess in more depth the differences between Queensland and New South Wales populations of S. internatus as revealed by our tree-based species delineation methods, both morphological and genetic (by use of more genetic markers, e.g. nuclear genes) (see Hsiao and Oberprieler 2020b). Also, the second species of Demyrsus, D. digmon Hsiao & Oberprieler, which we could not include in our analysis due to inability to extract viable DNA from the only available (old) specimens, needs to be incorporated in a more comprehensive analysis of the trunk-boring members of the Tranes group.
A similar situation of intraspecific genetic variation is evident in the grasstree-associated genus Paratranes. Three of our tree-based species delineation methods (bPTP, mlPTP, GMYC) suggested the three specimens of P. zimmermani included in the analysis to represent distinct species, though without any geographical pattern (the specimen from Western Australia intermixed with the two from New South Wales) and thus likely due to missing data resulting from incompletely sequenced mitogenomes, whereas the other delineation methods (morphology, ABGD, ASAP, mPTP) interpreted all three specimens as being conspecific. Likewise, in P. monopticus the morphological and the distance-based methods of species delimitation (ABGD, ASAP) identified a single species occurring in both western and eastern Australia, whereas the tree-based methods (bPTP, mlPTP, mPTP, GMYC) segregated the western and eastern populations into separate lineages (with bPTP, mlPTP and GMYC even dividing the latter into multiple ones, though without clear geographical pattern). Although the morphological study of Hsiao and Oberprieler (2021a) detected subtle differences in the male genitalia between the western and eastern populations (the apical margin of the endophallic sclerite medially slightly emarginate v. straight or slightly lobed), the absence of more significant differences, the results of the ABGD and ASAP delimitation methods and the very shallow genetic divergence between the western and eastern populations (less than in Siraton internatus) support the recognition of P. monopticus as a single species. Nevertheless, comparable analyses of larger samples of specimens and localities would be needed to corroborate this result.
In the cycad-pollinating Tranes group, the differentiation of Miltotranes from Tranes (Zimmerman 1994) is well supported by the genetic data, as is that of the former genus into three species, M. subopacus, M. prosternalis and M. wilsoni (Hsiao and Oberprieler 2022), which are recovered by all methods of molecular species delimitation. However, all molecular methods of analyses also revealed a genetic differentiation between the populations of M. prosternalis occurring north and south of Cairns, which are morphologically almost identical except for slight differences in the rhombic sclerite of the endophallus (Hsiao and Oberprieler 2022). Even though the molecular data appear to support a species-level differentiation of the M. prosternalis populations north and south of Cairns, this is based on only few specimens (two from Daintree in the north, one from Garradunga in the south), and the clustering of the northern population with M. wilsoni, which is morphologically well distinct from M. prosternalis, is implausible from a morphological perspective. A more comprehensive analysis, based on larger samples of specimens and localities and also on more genetic markers, is required to properly resolve the taxonomy of the M. prosternalis complex.
Tranes, the largest genus of the Tranes group, comprises four major lineages, the T. vigorsii, T. sparsus, T. insignipes and T. lyterioides groups. The T. vigorsii lineage, both on morphological evidence and on all our molecular species delimitation methods, which included multiple samples from five localities across the distributional range of the lineage, comprises a single species, the large (~15-mm body length) T. vigorsii, which is restricted to south-western Western Australia and also the type species of Tranes.
In the T. sparsus lineage, which comprises very similar but smaller (~10-mm body length) weevils in both western and eastern Australia, our molecular analyses support the morphological data in delineating one species in the east, T. sparsus, and another in the west, here described as T. occidentalis. The latter is divided into two geographical populations (Fig. 2), a western one associated with Macrozamia fraseri and M. riedlei and an eastern one with M. dyeri. Five of our methods of molecular species delimitation (ABGD, bPTP, mlPTP, mPTP, GMYC) segregated these two populations as different species, but the absence of significant and consistent morphological differences, the short tree length and the best species partition suggested by ASAP, which is an improved version of ABGD, indicate that they represent a single species (T. occidentalis). It seems likely that these populations were in the recent past, or may still be, geographically connected, as T. vigorsii is over the same area, but more intensive sampling and further analysis is needed to investigate this case.
The T. insignipes lineage, long considered to comprise a single species, in fact contains two similar but morphologically as well as genetically well distinct species, T. insignipes and T. tinctipennis, the latter newly described herein. Although these two species mostly occur in different areas, T. insignipes in the Innisfail area south of Cairns and T. tinctipennis in the Daintree–Cooktown area north of Cairns, they are in fact sympatric (assuming the label data to be correct) and may both be more continuously distributed throughout the range of their common host plant, Lepidozamia hopei.
The T. lyterioides lineage includes the smaller (≤10-mm body length) species of the genus and is also the most diverse one. It comprises two major clades, one containing three distinct species congruently delineated by morphological characters and all molecular species delimitation methods, namely T. chadwicki, T. kgariensis and T. lyterioides, which are mostly distributed along the coast or just inland from it, and the other containing two distinct species, T. forsteri and T. terryae, based on morphological and the distance-based molecular species delimitation analyses. The tree-based methods, however, segregated the specimens of T. terryae from Inglewood and surrounding area from those from the remaining region of the distributional range, and the bPTP and mlPTP methods even divided the specimens from the Inglewood area into different species (though with a low support value of <0.9). Given also the short tree lengths of these clusters, we interpret T. terryae as being a single species with a wide range extending well inland and associated with at least 14 species of Macrozamia as well as with Lepidozamia peroffskyana.
Assessment of host specificity
Detailed taxonomic study determining how many species a higher insect taxon such as a genus comprises is important in addressing questions about how host-specific the taxon may be. However, the most critical factor in determining host specificity is the species concept (and species delineation method) applied to both the insects and the hosts, and incongruences in their species concepts can lead to vastly different assessments of host specificity. The Australia cycad flora is regarded as comprising 41 named species of Macrozamia, 2 of Lepidozamia, 2 of Bowenia and 34 of Cycas (The World List of Cycads, see http://www.cycadlist.org). However, the taxonomic validity of many Australian cycad species is questionable, as cycad taxonomy operates with a ‘morphogeographic’ species concept (Walters et al. 2004) that tends to treat ecotypes with slight morphological differences in adaptation to different habitats as distinct species. The application of such a species concept is highly likely to inflate the number of actual species, which then leads to incorrect assumptions of the specificity of their insect associates, especially when the taxonomy of the latter is also poorly known (Oberprieler 1995b).
Our integrative taxonomic study of the Australian cycad-associated weevils, combining morphological and different molecular species delimitation approaches, resulted in the recognition of only 17 species of Australian cycad-associated species, and among them are 4 trunk-boring ones known for their low level of host specificity. The other 13 species, which breed exclusively in the male cones of their hosts and in at least some cases pollinate them, are expected to be considerably more host-specific. From the large number of specimens and locality and host records of these species incorporated into our study, it is possible to make an assessment of their host specificity and any incongruences between weevil and cycad host species. In Miltotranes, M. subopacus is exclusively associated with Bowenia serrulata, M. prosternalis with B. spectabilis and M. wilsoni with a geographically isolated northern population of Bowenia currently treated as B. spectabilis (Hsiao and Oberprieler 2022), thus exhibiting an extremely high level of host specificity. In Tranes, the two species of the T. insignipes group, T. insignipes and T. tinctipennis, are also highly host-specific, both associated only with Lepidozamia hopei, and sympatrically as well, although the exact nature of their association with this cycad species requires further investigation. In Western Australia, the large T. vigorsii is apparently exclusively associated with Macrozamia riedlei, whereas the smaller T. occidentalis occurs on this as well as on the other two species recognised in the region, M. dyeri and M. fraseri. The latter two are, however, closely related to M. riedlei (Hill and Osborne 2001) and were in the past included in the concept of this species (Hill 1993; Jones 1993, 2002), so that T. occidentalis would have been regarded as highly host-specific until c. 1993 but not so now that M. dyeri and M. fraseri are treated as distinct species. A similar situation exists in the T. lyterioides group in eastern Australia. Tranes kgariensis is specific to M. douglasii and T. chadwicki apparently to M. cardiacensis, and T. lyterioides would have been considered specific to M. communis up to 1998, when M. montana and M. reducta were separated as distinct species from M. communis (Hill 1998). Likewise, of the four recorded hosts of T. forsteri, M. crassifolia, M. parcifolia and M. pauli-guilielmi belong in the M. pauli-guilielmi complex of the Section Parazamia (Jones and Forster 1994; Hill and Osborne 2001), with M. lomandroides deemed to be a more isolated species in Parazamia (Hill and Osborne 2001), so that T. forsteri would have also been considered to be host-specific prior to the early 1990s, when the concept of M. pauli-guilielmi was a wider one (Hill 1993). Only two Tranes species have a demonstrably wide range of hosts, T. sparsus (recorded from seven species) and T. terryae (from 14 Macrozamia species and Lepidozamia peroffskyana), whereby it remains to be ascertained whether all these records reflect true hosts, whether some host taxa may also be complexes of closely related entities (potentially ‘morphogeographic’ forms of single species) and whether some may reflect recent introductions (of T. terryae from Macrozamia onto L. peroffskyana and of both species from the section Parazamia onto Macrozamia, i.e. onto M. communis/M. reducta along the New South Wales north coast). It thus appears that there is a fairly high degree of host specificity in Miltotranes as well as in Tranes but that, in the latter, the taxonomy and species concepts of Macrozamia need to be placed on a more secure footing (supported by molecular-genetic delimitation) before the pattern of host specificity can be elucidated more resolutely.
Integrative taxonomy and species diversity of Australian cycad-associated weevils of the Tranes group
In recent years, following the increasing availability of DNA sequencing technologies, many exploratory methods of species delimitation have been developed for proposing de novo species partitions. These methods can be classified into two main categories. The first category requires no input tree and estimates the number of species using pairwise genetic distances, whereas the methods in the second category infer the number of species from an input phylogenetic tree. Even though the results of these different methods are generally congruent with one another and also agree with other independent data, such as morphological characters, direct comparison indicates that sometimes some or even most of them fail to infer species limits correctly and only one or a few manage to do so (Puillandre et al. 2021). Furthermore, it is evident that the tree-based methods tend to oversplit species entities (Fujisawa and Barraclough 2013; Kekkonen and Hebert 2014), whereas distance-based methods may be prone to lump them (Pentinsaari et al. 2017). Different methods of species delineation should therefore be applied jointly and compared with other independent data (e.g. from different genetic markers, morphology, ecology) to achieve an objective taxonomic assessment (Ducasse et al. 2020).
The present study introduces an integrative approach combining traditional morphological comparison, DNA-based species delineation and ecological data such as distribution records to evaluate the species diversity of the Australian cycad weevils of the Tranes group. Anatomical comparison, which has the benefit of assessing large numbers of specimens from all available localities, can readily determine morphological boundaries and variability in different taxonomic units, whereas molecular species delimitation can be considered as a valuable tool analysing a different, independent dataset (i.e. DNA sequences) to support or refute morphological concepts and delineations. The molecular species delimitation and phylogenetic analysis employed in this study identified altogether at least 20 distinct species and geographical entities of Australian cycad weevils of the Tranes group. In combination with the assessment of morphological characters, relying on the usually highly species-diagnostic characters of the male genitalia, 17 of these entities can be recognised as natural species (including Demyrsus digmon, from which we failed to extract DNA but which possesses distinctive morphological characteristics). As in previous studies of weevils (Hsiao and Oberprieler 2024; Ren et al. 2024), our molecular analyses showed that tree-based methods are prone to oversplitting and interpreting geographical populations as distinct species. The PTP methods with Bayesian and maximum-likelihood implementation (bPTP and mlPTP) sometimes inferred unexpected and implausible species partitions by segregating specimens from the same collecting event into different species, which can, however, be overcome by the mPTP method. In contrast to the tree-based methods, the distance-based methods strongly supported our morphology-based identification, especially the ASAP method, which produced perfectly congruent results.
Conclusions
The present study applies an integrative approach by combining analysis of morphological characteristics and mitochondrial DNA data to estimate the species diversity and evaluate the taxonomic status of the Australian cycad-associated weevils of the Tranes group. Altogether, the molecular analyses identified 20 distinct species and geographical populations of Australian cycad-associated weevils of the Tranes group, including a morphologically cryptic species of Miltotranes. In combination with the morphological analysis, we recognise 17 of them as valid natural species, including Demyrsus digmon, which was unavailable for molecular analysis, and six hitherto undescribed species of Tranes, which are described as T. chadwicki sp. nov., T. forsteri sp. nov., T. kgariensis sp. nov., T. occidentalis sp. nov., T. terryae sp. nov. and T. tinctipennis sp. nov.
Data availability
The data underlying this article containing the complete set of unedited photographs are available in Zenodo at https://zenodo.org (see doi:10.5281/zenodo.13983601). This paper forms part of the PhD thesis of Yun Hsiao (2023).
Acknowledgements
We express our cordial thanks to Hermes E. Escalona (ANIC), Max Barclay (NHMUK), Susan Wright (QMBA) and Justin S. Bartlett (QDPI) for their assistance in borrowing specimens, and to Hermes E. Escalona and Debbie Jennings (ANIC) for taking partial photographs of Tranes. We are also grateful to the photographers Kirke M. Fisher and Nicholas Fisher, who allowed us to use their photographs of the Tranes group. The authors are also indebted to the editor and anonymous reviewers for their suggestions in improving the manuscript.
References
Anderson RS, Oberprieler RG, Setliff GP (2018) A review of the Araucaria-associated weevils of the tribe Orthorhinini (Coleoptera: Curculionidae: Molytinae), with description of new species of Ilacuris Pascoe, 1865 and Notopissodes Zimmerman & Oberprieler, 2014 and a new genus, Kuschelorhinus Anderson & Setliff. Diversity 10, 54.
| Crossref | Google Scholar |
Bouchard P, Bousquet Y, Davies AE, Alonso-Zarazaga MA, Lawrence JF, Lyal CHC, Newton AF, Reid CAM, Schmitt M, Slipiński SA, Smith ABT (2011) Family-group names in Coleoptera (Insecta). ZooKeys 88, 1-972.
| Crossref | Google Scholar | PubMed |
Bouckaert R, Vaughan TG, Barido-Sottani J, Duchêne S, Fourment M, Gavryushkina A, Heled J, Jones G, Kühnert D, De Maio N, Matschiner M, Mendes FK, Müller NF, Ogilvie HA, du Plessis L, Popinga A, Rambaut A, Rasmussen D, Siveroni I, Suchard MA, Wu CH, Xie D, Zhang C, Stadler T, Drummond AJ (2019) BEAST 2.5: an advanced software platform for Bayesian evolutionary analysis. PLOS Computational Biology 15(4), e1006650.
| Crossref | Google Scholar | PubMed |
Chadwick CE (1993) The roles of Tranes lyterioides and T. sparsus Boh (col., Curculionidae) in the pollination of Macrozamia communis (Zamiaceae). In ‘The Biology, Structure and Systematics of the Cycadales. Proceedings of Cycad 90, the Second International Conference on Cycad Biology’, 22–28 July 1990, Townsville, Qld, Australia. (Eds DW Stevenson, KJ Norstog) pp. 77–88. (Palm & Cycad Societies of Australia: Brisbane, Qld, Australia)
Chadwick CE (1998) Some insects and other invertebrates associated with the Australian cycad Macrozamia communis L. Johnson (Zamiaceae). Encephalartos 54, 10-18.
| Google Scholar |
Chadwick CE (1999) Invertebrates associated with Macrozamia communis (Zamiaceae) – again. Encephalartos 59, 14-16.
| Google Scholar |
Connell SW, Ladd PG (1993) Pollination biology of Macrozamia riedlei — the role of insects. In ‘The Biology, Structure and Systematics of the Cycadales. Proceedings of Cycad 90, the Second International Conference on Cycad Biology’, 22–28 July 1990, Townsville, Qld, Australia. (Eds DW Stevenson, KJ Norstog) pp. 96–102. (Palm & Cycad Societies of Australia: Brisbane, Qld, Australia)
Covassi M (1974) Il Demyrsus meleoides Pascoe: un potenziale nemico delle cicadee ornamentali introdotto in Italia (Coleoptera, Curculionidae). Redia 55, 211-217 [In Italian].
| Google Scholar |
Downie DA, Donaldson JS, Oberprieler RG (2008) Molecular systematics and evolution in an African cycad–weevil interaction: Amorphocerini (Coleoptera: Curculionidae: Molytinae) weevils on Encephalartos. Molecular Phylogenetics and Evolution 47(1), 102-116.
| Crossref | Google Scholar | PubMed |
Drummond AJ, Ho SY, Phillips MJ, Rambaut A (2006) Relaxed phylogenetics and dating with confidence. PLoS Biology 4(5), e88.
| Crossref | Google Scholar | PubMed |
Ducasse J, Ung V, Lecointre G, Miralles A (2020) LIMES: a tool for comparing species partition. Bioinformatics 36(7), 2282-2283.
| Crossref | Google Scholar | PubMed |
Forster PI (1996) Lepidozamia hopei (Zamiaceae), the world’s tallest cycad. Encephalartos 48, 12-15.
| Google Scholar |
Forster PI, Machin PJ, Mound LA, Wilson GW (1994) Insects associated with reproductive structures of cycads in Queensland and northeast New South Wales, Australia. Biotropica 26, 217-222.
| Crossref | Google Scholar |
Friends of Lord Howe Island (2024) Extinct beetle “rediscovered”. Available at https://friendslhi.com.au/news-item/extinct-beetle-rediscovered/
Froggatt WW (1896) The entomology of the grass-trees (Xanthorrhoea). Proceedings of the Linnean Society of New South Wales 21, 74-87.
| Google Scholar |
Fujisawa T, Barraclough TG (2013) Delimiting species using single-locus data and the Generalized Mixed Yule Coalescent approach: a revised method and evaluation on simulated data sets. Systematic Biology 62(5), 707-724.
| Crossref | Google Scholar | PubMed |
Glass C (1980) Tranes beetle now a pest in the U.S. The Cycad Newsletter 3, 7.
| Google Scholar |
Hall JA, Walter GH, Bergstrom DM, Machin P (2004) Pollination ecology of the Australian cycad Lepidozamia peroffskyana (Zamiaceae). Australian Journal of Botany 52(3), 333-343.
| Crossref | Google Scholar |
Haran J, Li X, Allio R, Shin S, Benoit L, Oberprieler RG, Farrell BD, Brown SDJ, Leschen RAB, Kergoat GJ, McKenna DD (2023) Phylogenomics illuminates the phylogeny of flower weevils (Curculioninae) and reveals ten independent origins of brood-site pollination mutualism in true weevils. Proceedings of the Royal Society of London – B. Biological Sciences 290, 20230889.
| Crossref | Google Scholar | PubMed |
Hawkeswood TJ (1985) Notes on some beetles (Coleoptera) associated with Xanthorrhoea johnsonii (Xanthorrhoeaceae) in the Brisbane area, south-east Queensland. Victorian Naturalist 102, 162-166.
| Google Scholar |
Hill KD (1993) History of the taxonomy of Australian cycads. In ‘The Biology, Structure and Systematics of the Cycadales. Proceedings of Cycad 90, the Second International Conference on Cycad Biology’, 22–28 July 1990, Townsville, Qld, Australia. (Eds DW Stevenson, KJ Norstog) pp. 250–259. (Palm & Cycad Societies of Australia: Brisbane, Qld, Australia)
Hoang DT, Chernomor O, von Haeseler A, Minh BQ, Vinh LS (2018) UFBoot2: improving the ultrafast bootstrap approximation. Molecular Biology and Evolution 35(2), 518-522.
| Crossref | Google Scholar | PubMed |
Hsiao Y (2023) Systematics and evolutionary biology of the weevils associated with cycads in Australia. PhD thesis, The Australian National University, Canberra, ACT, Australia. 10.25911/X743-QN30
Hsiao Y, Oberprieler RG (2020a) Bionomics and rearing of Miltotranes prosternalis (Lea, 1929) (Coleoptera: Curculionidae), a mutualistic cycad pollinator in Australia. Entomological Science 23(4), 369-373.
| Crossref | Google Scholar |
Hsiao Y, Oberprieler RG (2020b) A review of the trunk-boring cycad weevils in Australia, with description of a second species of Demyrsus Pascoe, 1872 (Coleoptera: Curculionidae). Austral Entomology 59(4), 677-700.
| Crossref | Google Scholar |
Hsiao Y, Oberprieler RG (2021a) A review of Paratranes Zimmerman, 1994, Xanthorrhoea-associated weevils of the Tranes group (Coleoptera, Curculionidae, Molytinae), with description of a new species. European Journal of Taxonomy 767, 117-141.
| Crossref | Google Scholar |
Hsiao Y, Oberprieler RG (2021b) Bionomics and new host plant records of the Australian trunk-boring cycad weevils (Coleoptera: Curculionidae). The Coleopterists Bulletin 75(3), 695-699.
| Crossref | Google Scholar |
Hsiao Y, Oberprieler RG (2022) Taxonomic revision of the genus Miltotranes Zimmerman, 1994 (Coleoptera: Curculionidae: Molytinae), the Bowenia-pollinating cycad weevils in Australia, with description of a new species and implications for the systematics of Bowenia. Insects 13(5), 456.
| Crossref | Google Scholar | PubMed |
Hsiao Y, Oberprieler RG (2024) An integrative taxonomic and phylogenetic approach reveals a new genus of Australasian Cycas-pollinating weevils (Coleoptera: Curculionidae: Cossoninae). Zoological Journal of the Linnean Society 202, zlad190.
| Crossref | Google Scholar |
Hsiao Y, Oberprieler RG, Zwick A, Zhou Y-L, Ślipiński A (2023) Museomics unveil systematics, diversity and evolution of Australian cycad-pollinating weevils. Proceedings of the Royal Society of London – B. Biological Sciences 290, 20231385.
| Crossref | Google Scholar | PubMed |
Jin M, Zwick A, Ślipiński A, de Keyzer R, Pang H (2020) Museomics reveals extensive cryptic diversity of Australian prionine longhorn beetles with implications for their classification and conservation. Systematic Entomology 45(4), 745-770.
| Crossref | Google Scholar |
Jones DJ, Forster PI (1994) Seven new species of Macrozamia section Parazamia (Miq.) Miq. (Zamiaceae section Parazamia) from Queensland. Austrobaileya 4(2), 269-288.
| Google Scholar |
Kalyaanamoorthy S, Minh BQ, Wong TKF, von Haeseler A, Jermiin LS (2017) ModelFinder: fast model selection for accurate phylogenetic estimates. Nature Methods 14, 587-589.
| Crossref | Google Scholar | PubMed |
Kapli P, Lutteropp S, Zhang J, Kobert K, Pavlidis P, Stamatakis A, Flouri T (2017) Multi-rate Poisson tree processes for single-locus species delimitation under maximum likelihood and Markov chain Monte Carlo. Bioinformatics 33(11), 1630-1638.
| Crossref | Google Scholar | PubMed |
Katoh K, Standley DM (2013) MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Molecular Biology and Evolution 30(4), 772-780.
| Crossref | Google Scholar | PubMed |
Kearse M, Moir R, Wilson A, Stones-Havas S, Cheung M, Sturrock S, Buxton S, Cooper A, Markowitz S, Duran C, Thierer T, Ashton B, Meintjes P, Drummond A (2012) Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 28(12), 1647-1649.
| Crossref | Google Scholar | PubMed |
Kekkonen M, Hebert PDN (2014) DNA barcode-based delineation of putative species: efficient start for taxonomic workflows. Molecular Ecology Resources 14(4), 706-715.
| Crossref | Google Scholar | PubMed |
Kennedy P (1991) Cycad–insect relationships. Encephalartos 27, 22-25.
| Google Scholar |
Kennedy P (1992) Cycad–insect relationships. – Destruction. Encephalartos 29, 20-22.
| Google Scholar |
Kennedy P (2011) Trunk-boring weevils – Melanotranes internatus in New South Wales. Encephalartos 105, 14-20.
| Google Scholar |
Kuschel G (1987) The subfamily Molytinae (Coleoptera: Curculionidae): general notes and description of new taxa from New Zealand and Chile. New Zealand Entomologist 9(1), 11-29.
| Crossref | Google Scholar |
Lanfear R, Calcott B, Kainer D, Mayer C, Stamatakis A (2014) Selecting optimal partitioning schemes for phylogenomic datasets. BMC Evolutionary Biology 14, 82.
| Crossref | Google Scholar | PubMed |
Lea AM (1898) Descriptions of new species of Australian Coleoptera. Part V. Proceedings of the Linnean Society of New South Wales 23(4), 521-645.
| Google Scholar |
Lea AM (1929) Descriptions of new species of Australian Coleoptera. XX. Proceedings of the Linnean Society of New South Wales 54(5), 519-549.
| Google Scholar |
Legalov AA (2018) Annotated key to weevils of the world. Part 2. Subfamily Molytinae (Coleoptera, Curculionidae). Ukrainian Journal of Ecology 8, 340-350.
| Google Scholar |
Letunic I, Bork P (2021) Interactive Tree Of Life (iTOL) v5: an online tool for phylogenetic tree display and annotation. Nucleic Acids Research 49(1), W293-W296.
| Crossref | Google Scholar | PubMed |
Long P (2009) Vigors, Nicholas Aylward. In ‘Dictionary of Irish Biography’. (Royal Irish Academy: Dublin, Ireland) 10.3318/dib.008818.v1
Lyal CHC, Douglas DA, Hine SJ (2006) Morphology and systematic significance of sclerolepidia in the weevils (Coleoptera: Curculionoidea). Systematics and Biodiversity 4(2), 203-241.
| Crossref | Google Scholar |
Marshall GAK (1939) LXVI. — New tropical African Curculionidae (Col.). Annals and Magazine of Natural History 11(3), 561-583.
| Crossref | Google Scholar |
Marvaldi AE, Morrone JJ (1998) Immature stages of Rhyparonotus altarensis (Olliff) (Coleoptera: Curculionidae: Molytinae) with comments on larval characters in Anchonini and Molytinae. Journal of the New York Entomological Society 106(2–3), 95-104.
| Google Scholar |
May BM (1993) Larvae of Curculionoidea (Insecta: Coleoptera): a systematic overview. Fauna of New Zealand 28, 1-223.
| Google Scholar |
Minh BQ, Schmidt HA, Chernomor O, Schrempf D, Woodhams MD, von Haeseler A, Lanfear R (2020) IQ-TREE 2: new models and efficient methods for phylogenetic inference in the genomic era. Molecular Biology and Evolution 37(5), 1530-1534.
| Crossref | Google Scholar |
Mound LA, Marullo R (1998) Biology and identification of Aeolothripidae (Thysanoptera) in Australia. Invertebrate Taxonomy 12(6), 929-950.
| Crossref | Google Scholar |
Mulder RH (1964) Insects associated with ‘Xanthorrhoea sp.’. Journal of the Entomological Society of Australia 1, 12.
| Google Scholar |
Nagalingum NS, Marshall CR, Quental TB, Rai HS, Little DP, Mathews S (2011) Recent synchronous radiation of a living fossil. Science 334(6057), 796-799.
| Crossref | Google Scholar |
Nguyen LT, Schmidt HA, von Haeseler A, Minh BQ (2015) IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Molecular Biology and Evolution 32, 268-274.
| Crossref | Google Scholar | PubMed |
NSW Department of Environment and Climate Change (2007) Appendices. Lord Howe Island Biodiversity Management Plan. (NSW DECC: Sydney, NSW, Australia) Available at https://www.dcceew.gov.au/sites/default/files/documents/lord-howe-island-appendices.pdf
Oberprieler RG (1995a) The weevils (Coleoptera: Curculionoidea) associated with cycads. 1. Classification, relationships and biology. In ‘Proceedings of the Third International Conference on Cycad Biology’, 5–9 July 1993, Pretoria, South Africa. (Ed. P Vorster) pp. 295–334. (The Cycad Society of South Africa: Stellenbosch, South Africa)
Oberprieler RG (1995b) The weevils (Coleoptera: Curculionoidea) associated with cycads. 2. Host specificity and implications for cycad taxonomy. In ‘Proceedings of the Third International Conference on Cycad Biology’, 5–9 July 1993, Pretoria, South Africa. (Ed. P Vorster) pp. 335–365. (The Cycad Society of South Africa: Stellenbosch, South Africa)
Oberprieler RG (1997) Cycad weevils: comments on articles by P.I. Forster and E. Rouwenhorst. Encephalartos 49, 25-26.
| Google Scholar |
Oberprieler RG, Caldara R (2012) Siraton devillei Hustache (Coleoptera: Curculionidae), the mysterious weevil from the Isle of Elba: exiled no longer. Zootaxa 3573(1), 55-58.
| Crossref | Google Scholar |
Oberprieler RG, Anderson RS, Marvaldi AE (2014) 3 Curculionoidea Latreille, 1820: Introduction, Phylogeny. In ‘Handbook of Zoology. Arthropoda: Insecta. Coleoptera, Beetles. Volume 3: Morphology and Systematics (Phytophaga)’. (Eds RAB Leschen, RG Beutel) pp. 285–300. (Walter de Gruyter) 10.1515/9783110274462.285
Olliff AS (1889) The insect fauna of Lord Howe Island. Australian Museum Memoir 2(4), 77-98.
| Crossref | Google Scholar |
Ornduff R (1989) Size distribution and coning behaviour of the Australian cycad Lepidozamia peroffskyana. Australian Journal of Ecology 14(2), 241-245.
| Crossref | Google Scholar |
Ornduff R (1990) Geographic variation in reproductive behavior and size structure of the Australian cycad Macrozamia communis (Zamiaceae). American Journal of Botany 77(1), 92-99.
| Crossref | Google Scholar |
Ornduff R (1991) Coning phenology of the cycad Macrozamia riedlei (Zamiaceae) over a five-year interval. Bulletin of the Torrey Botanical Club 118(1), 6-11.
| Crossref | Google Scholar |
Ornduff R (1993) Features of the reproductive biology of Macrozamia species and Cycas media in Australia. In ‘The Biology, Structure and Systematics of the Cycadales. Proceedings of Cycad 90, the Second International Conference on Cycad Biology’, 22–28 July 1990, Townsville, Qld, Australia. (Eds DW Stevenson, KJ Norstog) pp. 121–124. (Palm & Cycad Societies of Australia: Brisbane, Qld, Australia)
Owen G (2005) OBITUARY: Clarence Earl CHADWICK, BSc (Syd.). May 1909–18 November 2004. General and Applied Entomology: The Journal of the Entomological Society of New South Wales 34, 1-6 Available at https://www.entsocnsw.org.au/images/stories/media/34%20chadwick%20obituary.pdf.
| Google Scholar |
Pascoe FP (1870) Descriptions of some genera and species of Australian Curculionidae. Transactions of the Entomological Society of London 18(2), 181-209.
| Crossref | Google Scholar |
Pascoe FP (1874) LV.—Additions to the Australian Curculionidæ. Part VI. The Annals and Magazine of Natural History; Zoology, Botany, and Geology 13(77), 383-389.
| Crossref | Google Scholar |
Pascoe FP (1875) IV.—Additions to the Australian Curculionidæ. Part VIII. Annals and Magazine of Natural History 16(91), 55-67.
| Crossref | Google Scholar |
Pentinsaari M, Vos R, Mutanen M (2017) Algorithmic single-locus species delimitation: effects of sampling effort, variation and nonmonophyly in four methods and 1870 species of beetles. Molecular Ecology Resources 17(3), 393-404.
| Crossref | Google Scholar | PubMed |
Pons J, Barraclough TG, Gomez-Zurita J, Cardoso A, Duran DP, Hazell S, Kamoun S, Sumlin WD, Vogler AP (2006) Sequence-based species delimitation for the DNA taxonomy of undescribed insects. Systematic Biology 55(4), 595-609.
| Crossref | Google Scholar | PubMed |
Prena J, Hsiao Y, Oberprieler RG (2023) New combinations and synonymies in the weevil genus Lyterius Schönherr (Coleoptera, Curculionidae), with a conspectus of historical works on Daldorff’s Sumatran beetles. Zootaxa 5380(1), 26-36.
| Crossref | Google Scholar | PubMed |
Puillandre N, Lambert A, Brouillet S, Achaz G (2012) ABGD, Automatic Barcode Gap Discovery for primary species delimitation. Molecular Ecology 21(8), 1864-1877.
| Crossref | Google Scholar | PubMed |
Puillandre N, Brouillet S, Achaz G (2021) ASAP: assemble species by automatic partitioning. Molecular Ecology Resources 21(2), 609-620.
| Crossref | Google Scholar | PubMed |
Pullen KR, Jennings D, Oberprieler RG (2014) Annotated catalogue of Australian weevils (Coleoptera: Curculionoidea). Zootaxa 3896(1), 1-481.
| Crossref | Google Scholar | PubMed |
Rambaut A, Drummond AJ, Xie D, Baele G, Suchard MA (2018) Posterior summarization in Bayesian phylogenetics using Tracer 1.7. Systematic Biology 67, 901-904.
| Crossref | Google Scholar | PubMed |
Ren J, Ren L, Zhang R (2024) Delimiting species, revealing cryptic diversity, and population divergence in Qinghai–Tibet Plateau weevils through DNA barcoding. Ecology and Evolution 14(7), e11592.
| Crossref | Google Scholar | PubMed |
Salzman S, Whitaker M, Pierce NE (2018) Cycad-feeding insects share a core gut microbiome. Biological Journal of the Linnean Society 123(4), 728-738.
| Crossref | Google Scholar |
Schneider D, Wink M, Sporer F, Lounibos P (2002) Cycads: their evolution, toxins, herbivores and insect pollinators. Naturwissenschaften 89(7), 281-294.
| Crossref | Google Scholar | PubMed |
Schoenherr CJ (1843) ‘Genera et species curculionidum, cum synonymia hujus familiae. Species novae aut hactenus minus cognitae, descriptionibus a Dom. L. Gyllenhal, C. H. Boheman, O. J. Fahraeus et entomologis aliis illustratae. Tomus Septimus. – Pars Secunda. Supplementum continens.’ (Roret: Paris, France; and Fred. Fleischer: Lipsia [Leipzig, German Confederation]) [In Latin]
Shimodaira H, Hasegawa M (1999) Multiple comparisons of log-likelihoods with applications to phylogenetic inference. Molecular Biology and Evolution 16(8), 1114.
| Crossref | Google Scholar |
Shin S, Clarke DJ, Lemmon AR, Moriarty Lemmon E, Aitken AL, Haddad S, Farrell BD, Marvaldi AE, Oberprieler RG, McKenna DD (2018) Phylogenomic data yield new and robust insights into the phylogeny and evolution of weevils. Molecular Biology and Evolution 35(4), 823-836.
| Crossref | Google Scholar | PubMed |
Suinyuy TN, Johnson SD (2021) Evidence for pollination ecotypes in the African cycad Encephalartos ghellinckii (Zamiaceae). Botanical Journal of the Linnean Society 195(2), 233-248.
| Crossref | Google Scholar |
Terry I (2001) Thrips and weevils as dual, specialist pollinators of the Australian cycad Macrozamia communis (Zamiaceae). International Journal of Plant Sciences 162(6), 1293-1305.
| Crossref | Google Scholar |
Terry I, Moore CJ, Walter GH, Forster PI, Roemer RB, Donaldson JD, Machin PJ (2004) Association of cone thermogenesis and volatiles with pollinator specificity in Macrozamia cycads. Plant Systematics and Evolution 243(3–4), 233-247.
| Crossref | Google Scholar |
Terry LI, Walter GH, Donaldson JS, Snow E, Forster PI, Machin PJ (2005) Pollination of Australian Macrozamia cycads (Zamiaceae): effectiveness and behavior of specialist vectors in a dependent mutualism. American Journal of Botany 92(6), 931-940.
| Crossref | Google Scholar | PubMed |
Terry I, Tang W, Taylor A, Donaldson J, Singh R, Vovides A, Cibrián Jaramillo A (2012) An overview of cycad pollination studies. Memoirs of the New York Botanical Garden 106, 352-394.
| Crossref | Google Scholar |
Toon A, Terry LI, Tang W, Walter GH, Cook LG (2020) Insect pollination of cycads. Austral Ecology 45(8), 1033-1058.
| Crossref | Google Scholar |
Walker JJ (1906) Antipodean field notes III. A sketch of the entomology of Sydney. N.S.W. Entomologists Monthly Magazine 17, 22-27, 50–55.
| Google Scholar |
Wallenius TC (2014) Chemical Ecology and Pollination Biology of the Australian Cycad Macrozamia communis. PhD thesis, The Australian National University, Canberra, ACT, Australia. 10.25911/5d515556812bf
Wallenius TC, Peakall R, Wanjura WJ, Chyb S, Oberprieler RG (2012) Chapter 25. Volatile emissions, thermogenesis and dehiscence patterns of Macrozamia communis (Zamiaceae): implications for cycad pollination research. In ‘Proceedings of Cycad 2008: The 8th International Congress on Cycad Biology’, 13–15 January 2008, Panama City, Panama. (Eds DW Stevenson, R Osborne, AST Blake) pp. 395–418. (The New York Botanical Garden Press: New York, NY, USA) 10.21135/893275150.025
Walters T, Osborne R, Decker D (2004) We hold these truths… In ‘Cycad Classification: Concepts and Recommendations’. (Eds T Walters, R Osborne) pp. 1–11. (CAB International: Wallingford, CT, USA) 10.1079/9780851997414.0001
Westwood JO (1886) Observations upon species of Curculionidae injurious to Cycadeae, especially to plants of the genus Zamia. Annales de la Société Entomologique de Belgique 30, 125-130.
| Google Scholar |
Wilson GM (1993) Initial observations of the reproductive behaviour and an insect pollination agent of Bowenia serrulata (W. Bull) Chamberlain. Encephalartos 36, 13-18.
| Google Scholar |
Wilson GM (2002) Insect pollination in the cycad genus Bowenia Hook. ex Hook. f. (Stangeriaceae). Biotropica 34(3), 438-441.
| Crossref | Google Scholar |
Wilson GM, Rowles PC (1997) Notes on the biology of Lepidozamia hopei Regel (Zamiaceae). Encephalartos 52, 12-17.
| Google Scholar |
Zhang J, Kapli P, Pavlidis P, Stamatakis A (2013) A general species delimitation method with applications to phylogenetic placements. Bioinformatics 29, 2869-2876.
| Crossref | Google Scholar | PubMed |
Zimmerman EC (1992) ‘Australian Weevils (Coleoptera: Curculionoidea). Vol. VI.’ (CSIRO: Melbourne, Vic., Australia) 10.1071/9780643104945
Zimmerman EC (1994) ‘Australian Weevils (Coleoptera: Curculionoidea). Vol. 1. Orthoceri. Anthribidae to Attelabidae. The Primitive Weevils.’ (CSIRO: Melbourne, Vic., Australia) 10.1071/9780643104907