Feasibility of accelerometry in a self-directed upper limb activity program of a subacute setting with stroke survivors
Tamara Tse A B * , Yvonne Y. K. Mak-Yuen A B C , Wesley Young D and Susan Darzins
A B * , Yvonne Y. K. Mak-Yuen A B C , Wesley Young D and Susan Darzins  D
D
A
B
C
D
Abstract
Wearable devices, such as accelerometers, offer novel approaches to measuring post-stroke upper limb activity. Limited studies have explored feasibility of accelerometry. Guided by the Bowen Feasibility Framework, this feasibility study aimed to examine the practicality, acceptability, and limited efficacy of accelerometry in a self-directed upper limb program with stroke survivors using a pre-post study of sequentially eligible inpatients.
Key metrics were: practicality (60% of participants had 10 hours of wear per day for 3 or more days), acceptability (adherence to recommended wear-time), and limited efficacy (correlation between Wolf Motor Function Test (WMFT) and upper limb use from accelerometry data).
Twelve stroke survivors were recruited over 7 months, mean age 73 years (range 39–94 years). Eight participants (67%) met the practicality and acceptability criteria. A moderate positive correlation existed between WMFT and upper limb use at admission (rs = 0.33, P = 0.42) and at discharge (rs = 0.42, P = 0.34).
Wearable devices were feasible and acceptable for most stroke survivors, however, one-third found the devices uncomfortable, and this should be factored into sample size calculations of future studies.
Keywords: accelerometry, activity trackers, arm use, physical activity, rehabilitation, wearable devices, wearable electronic devices, upper extremity.
Introduction
Stroke is a leading cause of disability in Australia (Australian Institute of Health and Welfare 2018); it affects stroke survivors’ abilities to carry out everyday activities unassisted (Deloitte Access Economics 2020) and causes significant economic costs for post-stroke care (Feigin et al. 2022). Stroke survivors commonly experience impacts on body function and structures, activities, and participation in daily life. Up to 80% experience motor or sensory impairments of their affected arm (Carey and Matyas 2011) and 50% give up at least one meaningful activity as a result of impairments after their stroke (Tse et al. 2022).
Australian and New Zealand Clinical Guidelines for Stroke Management (Stroke Foundation n.d.) recommend a minimum of 2 h of daily active therapy for stroke survivors with upper limb weakness, and for stroke survivors to continue with self-directed active task practice outside scheduled therapy sessions. Clinicians have often relied on self-report measures such as diaries to determine stroke survivors’ engagement in self-directed task practice, which are subject to reporting biases. Wearable devices, such as accelerometers, capture and quantify movements and are showing promise as an emerging objective measure of upper limb activity in stroke survivors (Doman et al. 2016). Although accelerometry has been criticised for the inability to distinguish between maladaptive movement and functional movement (Maceira-Elvira et al. 2019), a recent meta-analysis provided preliminary evidence in support of accelerometry. This study demonstrated strong correlations with functional assessments of upper limb, ability to compare symmetry of movement of both upper limbs during bilateral tasks, and the ability to quantify upper limb use during everyday life activities. Most of the studies included in the meta-analysis were conducted in clinics. There is minimal evidence indicating whether accelerometry is feasible to use in a sub-acute hospital setting with people who have experienced a stroke with upper limb impairments.
The Bowen Feasibility Framework (Bowen et al. 2009) is one of the most widely used frameworks to determine whether an approach is appropriate for further testing (Klaic et al. 2022). There are eight areas of focus: acceptability, domain, implementation, practicality, adaption, integration, expansion, and limited efficacy. This study applied the Bowen Feasibility Framework to explore acceptability, practicality, and limited efficacy. A self-directed upper limb program for improving upper limb function after stroke that incorporated accelerometry to complement the measurement of upper limb activity was developed. Therefore, the aim of this study was to test the feasibility of using accelerometry in a self-directed upper limb program conducted in an inpatient rehabilitation unit with stroke survivors. Specific objectives were to explore (1) whether the accelerometers were practical to wear during the day and for extended periods of time while in hospital, (2) whether they were acceptable from the perspective of the stroke survivors, and (3) whether data obtained from the accelerometers were comparable to a reference standard measure of upper limb function.
Materials and methods
Study design
This study utilised data from a small single-group pre-post pilot study testing a self-directed upper limb program for improving upper limb function after stroke. Bowen’s Feasibility Framework was used to guide the selection of questions and methods to identify if accelerometry is practical and acceptable to use in an inpatient setting and whether the data obtained yielded similar outcomes as reference standard measures of upper limb function, known as limited efficacy (Bowen et al. 2009). Ethical approval was sought and approved by the St Vincent’s Hospital Melbourne Human Research Ethics Committee (HREC) (203/19, project ID number 57974) and the Australian Catholic University HREC (2019/376R).
Setting and participants
Stroke survivors with upper limb impairment admitted to the 23-bed sub-acute inpatient rehabilitation unit at St Vincent’s Hospital Melbourne were invited to participate in the pilot study. Inclusion criteria were (1) prescribed use of the self-directed upper limb program by the treating therapists, and (2) ability to provide informed consent, including use of interpreter if communication barriers and ability to understand commands and sustain attention, as assessed by the Montreal Cognitive Assessment (MoCA).
To describe the participant group, routinely gathered patient information, including age, sex, pre-stroke hand dominance, post-stroke affected arm, stroke type, time since stroke, stroke severity (National Institute of Health Stroke Scale; Brott et al. 1989), length of stay, and living situation, were gathered from participants’ medical files.
Procedure and intervention program
Eligible patients were screened by the treating occupational therapists between January and September 2020, during the COVID-19 pandemic. Those meeting the criteria were invited to participate in the study via a third-party therapist or researcher. All participants provided written informed consent and had the option to withdraw at any time.
The self-directed upper limb program is based on repetitive task practice and consists of a behavioural agreement, the upper limb activity kit, and homework sheets (Marino et al. 2021). The upper limb activity kit contained an individualised program with fine and gross motor activities, and functional activities from the Graded Repetitive Arm Supplementary Program (GRASP) (Harris et al. 2009), revised weekly by the treating therapist. The behavioural agreement, signed by the participant and therapist, outlined the daily self-directed activity program and weekly reviews with program adjustments.
The upper limb activity kits consisted of nine sets of items designed to progress participants’ fine and gross motor skills with an occupational focus on selected tasks (Marino et al. 2021). Four of the kits were designed for fine motor skills and are graded from easiest to hardest via colours: yellow, red, green, and blue. Two of the kits were designed for gross motor skills: yellow/red for the easier level, green/blue for the harder level. The remaining three kits included the three levels of the GRASP (Harris et al. 2009). The therapist selected the activities and the number of repetitions on homework sheets for participants to complete outside direct therapy sessions.
Measurements
ActiGraph accelerometers (ActiGraph wGT3X-BT, https://theactigraph.com/wearable-devices) are wearable sensors manufactured in North America that measure movement in three dimensions and have been shown to measure upper limb activity levels (Noorkõiv et al. 2014). Participants wore an ActiGraph GT9X Link accelerometer on each upper limb just above the wrist, throughout their stay. The accelerometers captured data at 30 Hz and the research team used the Acti-Life software to convert the data into activity use in 1-s epochs. The accelerometer data from the accelerometers followed the method for quantifying upper limb performance using accelerometers developed by Lang et al. (2017) to determine the average upper limb activity use in hours per day for each upper limb. Epochs with a non-zero vector magnitude were counted during the recording period (06:00–19:59 hours) when patients were more likely to be awake and moving. The epochs were converted to hours for each arm for the duration of the participant’s stay.
Participants’ upper limb function was measured at baseline and at discharge by the treating therapist. The graded Wolf Motor Function Test (WMFT) was used, consisting of 17 items; items 1–6 are timed functional tasks, items 7–8 measure strength (weight lifted and grip), and items 9–17 measure quality of functional movement (Wolf et al. 2005). The maximum score is 75, and lower scores are indicative of lower functioning. The Minimally Clinically Important Difference of the WMFT is 1.0 points for the dominant side affected and 1.2 points for the non-dominant side affected (Lang et al. 2008). Instrument validation research supports use of the WMFT with stroke patients (Wolf et al. 2001).
Practicality is the extent an idea, program, process, or measure can be carried out with intended participants (Bowen et al. 2009). Practicality was met if 60% of participants that consented to the study wore the accelerometer on both arms for 10 h of wear per day and for at least 3 days during their hospital admission. These criteria were based on previous studies, including a feasibility study using accelerometry on the wrist (Clark et al. 2021), where 60% of participants had 3 or more days of valid wear (10 or more hours of wear per day), and two studies investigating accelerometry with stroke survivors (Uswatte et al. 2005; Noorkõiv et al. 2014). One found most participants wore the accelerometers for approximately 83% of waking hours (approximately 10 h) (Uswatte et al. 2005). The other, a systematic review, found the most frequent duration was 3 days (Noorkõiv et al. 2014).
Acceptability is the extent a new idea, program, process, or measure is judged as suitable, satisfying, or attractive (Bowen et al. 2009). Acceptability in this study was concerned with the wearability of the accelerometer and was determined by the proportion of eligible participants consenting to the study that wore the accelerometers for the duration of their inpatient stay.
Limited efficacy is whether a new idea, program, process, or measure shows promise of being successful with the intended population (Bowen et al. 2009). Efficacy of using accelerometers to measure upper limb activity was determined by comparing upper limb use from accelerometry data on the first and last day against the WMFT assessed at admission and discharge. In addition, graphical methods were used to display observable changes in magnitude and direction of upper limb activity use, with accelerometry data.
Analysis
Participant characteristics were measured and reported descriptively using measures of central tendency and variance. Descriptive and frequency statistics were used to calculate the number and percentage of participants that wore the accelerometers for 10 h of wear per day for at least 3 days during their hospital admission (practicality), and the number and percentage of participants consenting to the study that wore the accelerometers for the duration of their inpatient stay (acceptability). Among those that completed the feasibility study, data were collected from the accelerometers as described above. Accelerometer data were generated on the average hours of upper limb activity per day for each arm for the duration of the participants’ stay and analysed using Microsoft Excel, excluding nocturnal movements (19:59–06:00 hours). Upper limb activity were charted over the participants hospital admission using simple line graphs with a gradient line of best fit to describe the pattern of upper limb activity measured by accelerometry. Missing activity data were removed from the graphs.
Comparison between accelerometry data and performance on the WFMT at admission and discharge was undertaken using Spearman’s correlations. Spearman’s correlation coefficient (rs) of 0.10–0.29 indicate a small effect size, scores of 0.20–0.49 indicate a moderate effect size and scores equal to or above 0.50 indicate a large effect size (Cohen 2013). Analysis was performed using SPSS 28 (IBM Corp., ver. 28.0, Armonk, NY, USA).
Results
Twelve stroke survivors with a mean age of 73 years (s.d. 15.6, range 39–94) were recruited. All were right hand dominant and had experienced varying types of stroke (three haemorrhagic, seven ischemic, and one bilateral stroke affecting both upper limbs). Eight of the 12 had left-sided weakness. All participants were ≤37 days post-stroke on admission and spent between 22 and 71 days (mean = 45.43, s.d. = 17.29) on the sub-acute ward (Table 1).
| Participant characteristics | ||
|---|---|---|
| Age (years) | ||
| Range | 39–94 | |
| Mean (s.d.) | 73.5 (15.6) | |
| Gender, number of females (%) | 5 (42) | |
| Length of stay (days) | ||
| Range | 22–71 | |
| Mean (s.d.) | 45 (16.6) | |
| Stroke type (n% ischaemic) | 7 (58) | |
| Affected side (n% left) | 8 (67) | |
| FIM (admission), mean (s.d.) | 62 (17.8) | |
| FIM (discharge), mean (s.d.) | 100 (22.2) | |
| WMFT (admission), 0–75 | ||
| Mean (s.d.) | 48.5 (17.9) | |
| Median (IQR) | 49.5 (31.3, 63.5) | |
| WMFT (discharge), 0–75 | ||
| mean (s.d.) | 65.9 (8.6) | |
| median (IQR) | 69.0 (63.0, 72.0) | |
| Upper limb activity (admission), h | ||
| Mean (s.d.) | 2.3 (1.5) | |
| Median (IQR) | 1.8 (1.0, 4.0) | |
| Upper limb activity (discharge), h | ||
| Mean (s.d.) | 3.2 (1.9) | |
| Median (IQR) | 3.0 (1.4, 4.9) | |
s.d., standard deviation; FIM, functional independence measure; WMFT, Wolf Motor Function Test; IQR, interquartile range.
Practicality
The criterion of practicality was met. Eight participants (67%) were able to comply with the wearing regime and wore the accelerometers for ≥10 h wear per day and ≥3 days during their hospital admission.
Acceptability
Eight of the 12 (67%) wore the accelerometers throughout their hospital admission, with three unable to tolerate them due to reported discomfort and interference with everyday activities such as dressing, and one felt uneasy with electronic devices.
Limited efficacy
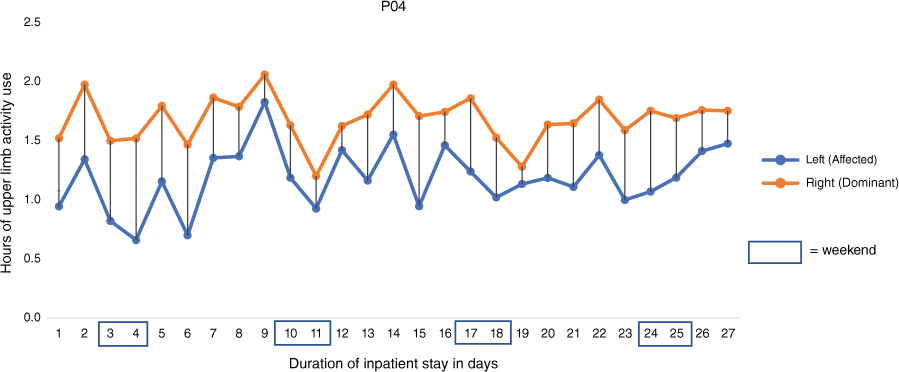
COVID-19 isolation prevented one participant’s WMFT assessment at discharge. Among the seven assessed, six improved their WMFT scores from admission to discharge (change range: 2–27), indicating clinically meaningful improvements in upper limb function (Lin et al. 2009). The Spearman’s correlation coefficient between upper limb activity and WMFT at admission was moderate (rs = 0.43, P = 0.34) and large at discharge (rs = 0.71, P = 0.11), see Table 2. Upper limb activity measured between 06:00 and 19:59 hours of a representative participant is shown in Fig. 1 (see Supplementary File S1 for all participants). The changes in magnitude and direction of upper limb activity use over the duration of the participants hospital stay highlights an upward trend in six of eight participants, and days of lower upper limb activity use during weekends.
| Patient ID | WMFT_(admission), 0–75 | WMFT_(discharge), 0–75 | Change in WMFT (admission to discharge) | Upper limb activity (admission), h | Upper limb activity (discharge), h | Change in upper limb activity (admission to discharge), h | |
|---|---|---|---|---|---|---|---|
| P02 | 47 | 66 | 19 | 1.94 | 2.65 | 0.71 | |
| P03 | 44 | 69 | 5 | 1.75 | 1.32 | −0.43 | |
| P04 | 65 | 70 | 5 | 0.94 | 1.48 | 0.54 | |
| P05 | 21 | 48 | 27 | 0.88 | 1.34 | 0.46 | |
| P08 | 73 | 73 | 0 | 4.8 | 6.23 | 1.43 | |
| P09 | 59 | 72 | 12 | 1.22 | 3.62 | 2.4 | |
| P11 | 52 | – | – | 4.43 | 3.44 | −0.99 | |
| P12 | 27 | 63 | 36 | 2.6 | 5.3 | 2.7 | |
| Total, mean (s.d.) | 48.5 (17.9) | 65.9 (8.6) | 14.9 (13.1) | 2.3 (1.5) | 3.2 (1.9) | 0.9 (1.3) | |
| Total, median (IQR) | 49.5 (31.3, 63.5) | 69.0 (63.0, 72.0) | 12 (5, 23) | 1.8 (1.0, 4.0) | 3.0 (1.4, 4.9) | 0.6 (0.2, 1.7) |
WMFT, Wolf Motor Function Test; s.d., standard deviation; IQR, interquartile range.
Discussion
The findings of this study highlight the feasibility of using accelerometers to monitor upper limb activity in a clinical setting. The majority of participants wore the accelerometers for at least 10 h per day for 3 days or more during their hospital stay, which suggests the potential value of this technology in measuring upper limb activity in an inpatient setting. The ability to capture participants’ upper limb activity outside therapy time and during weekends was another valuable feature of accelerometry that can be used to enhance knowledge about use of the upper limb beyond direct therapy time (Martino Cinnera et al. 2024). Although most studies that have used accelerometry have tended to use the data to capture duration of movement and feedback on movement (Wang et al. 2017; Rast and Labruyère 2020), there is growing evidence to support the use of accelerometry beyond these features to include quality and quantity of movement between affected and unaffected limbs (Martino Cinnera et al. 2024).
Although not powered to analyse intervention effects, analysis indicated a correlation between data captured with the accelerometers and functional assessment of the upper limb. These findings suggest accelerometry may be a useful outcome measure of upper limb recovery, which has also been supported by a recent systematic review and meta-analysis (Martino Cinnera et al. 2024). Martino Cinnera et al. (2024) pooled data from 213 stroke survivors across 10 studies to investigate correlations between kinematic data from wearable devices and clinical assessments. The findings demonstrate similar correlations (r = 0.69) to those of this current study (rs = 0.71), supporting the use of accelerometry as a measure of upper limb use in everyday life activities.
There are potential challenges of using accelerometry. Approximately one-third of participants did not adhere to the recommended wear time while in hospital. This was primarily attributed to discomfort caused by the accelerometers and concerns about how they affected participants engagement in daily activities. A similar dropout rate (29%) was reported in a recent feasibility study using wrist-worn accelerometers with stroke survivors in Denmark (Langerak et al. 2023). The reasons for dropping out were also similar, including the burden of wearing the devices during rehabilitation programs and discomfort from allergic reactions to nickel. These findings serve as important considerations for researchers when recruiting participants for studies involving wearable devices, as it suggests that up to one-third of participants may withdraw from the study due to limited acceptability.
The cost to purchase the six devices and the software was just over US$3000, and is another potential challenge of using accelerometry in clinical settings. In this current study, a software program developed by the manufacturers of the accelerometer was needed to convert the data into a usable format. The software was able to provide a visual summary that could be used as feedback to patients. These costs may be a potential barrier to wide use across inpatient settings.
Study limitations
This study had limitations, including a small sample size, implementation in a single setting, and pandemic challenges affecting ability to use the WMFT. Additional limitations may include limited diversity of participants and the short-term nature of the observations rendering the study period as too limited to observe any long-term trends in patient recovery.
Implications of findings
Using accelerometry to measure upper limb use was feasible and acceptable for 67% of eligible participants, however, a third found the wearable devices uncomfortable and withdrew from the study. These findings should be factored into future sample size calculations for studies involving wearable devices. Efficacy of using accelerometry shows promise. Further work is needed to better understand the relationship between clinical measures and upper limb accelerometry data in stroke rehabilitation.
Data availability
Data that support this study will be shared upon reasonable request to the corresponding author.
Declaration of funding
This project was supported by St Vincent’s Research Endowment Fund [grant number 90185, 2020], the Arthur A Thomas Trust [grant number AATHOM002, 2019], and the Eirene Lucas Foundation [no grant number, 2019].
Ethics standard
The authors asserts that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
Acknowledgements
Thanks is extended to the patients, family, and staff at St George’s Rehabilitation ward, Kew, Victoria, Australia, who participated in, or supported, this project.
References
Bowen DJ, Kreuter M, Spring B, Cofta-Woerpel L, Linnan L, Weiner D, Bakken S, Kaplan CP, Squiers L, Fabrizio C, Fernandez M (2009) How we design feasibility studies. American Journal of Preventative Medicine 36(5), 452-457.
| Crossref | Google Scholar | PubMed |
Brott T, Adams HP, Jr, Olinger CP, Marler JR, Barsan WG, Biller J, Spilker J, Holleran R, Eberle R, Hertzberg V (1989) Measurements of acute cerebral infarction: a clinical examination scale. Stroke 20(7), 864-870.
| Crossref | Google Scholar | PubMed |
Carey LM, Matyas TA (2011) Frequency of discriminative sensory loss in the hand after stroke in a rehabilitation setting. Journal of Rehabilitation Medicine 43(3), 257-263.
| Crossref | Google Scholar | PubMed |
Clark ELM, Gulley LD, Hilkin AM, Rockette-Wagner B, Leach HJ, Lucas-Thompson RG, Tanofsky-Kraff M, Nadeau KJ, Scott SM, Sheeder JL, Shomaker LB (2021) Feasibility and Acceptability of Accelerometer Measurement of Physical Activity in Pregnant Adolescents. International Journal of Environmental Research and Public Health 18(5), 2216.
| Crossref | Google Scholar | PubMed |
Deloitte Access Economics (2020) The economic impact of stroke in Australia, 2020. Available at https://www.deloitte.com/au/en/services/economics/perspectives/economic-impact-stroke-australia.html
Doman CA, Waddell KJ, Bailey RR, Moore JL, Lang CE (2016) Changes in Upper-Extremity Functional Capacity and Daily Performance During Outpatient Occupational Therapy for People With Stroke. American Journal of Occupational Therapy 70(3), 7003290040p1-7003290040p11.
| Crossref | Google Scholar | PubMed |
Feigin VL, Brainin M, Norrving B, Martins S, Sacco RL, Hacke W, Fisher M, Pandian J, Lindsay P (2022) World Stroke Organization (WSO): Global Stroke Fact Sheet 2022. International Journal of Stroke 17(1), 18-29.
| Crossref | Google Scholar | PubMed |
Harris JE, Eng JJ, Miller WC, Dawson AS (2009) A self-administered Graded Repetitive Arm Supplementary Program (GRASP) improves arm function during inpatient stroke rehabilitation: a multi-site randomized controlled trial. Stroke 40(6), 2123-2128.
| Crossref | Google Scholar | PubMed |
Klaic M, Kapp S, Hudson P, Chapman W, Denehy L, Story D, Francis JJ (2022) Implementability of healthcare interventions: an overview of reviews and development of a conceptual framework. Implementation Science 17(1), 10.
| Crossref | Google Scholar | PubMed |
Lang CE, Edwards DF, Birkenmeier RL, Dromerick AW (2008) Estimating minimal clinically important differences of upper-extremity measures early after stroke. Archives of Physical Medicine and Rehabilitation 89(9), 1693-1700.
| Crossref | Google Scholar | PubMed |
Lang CE, Waddell KJ, Klaesner JW, Bland MD (2017) A Method for Quantifying Upper Limb Performance in Daily Life Using Accelerometers. Journal of Visualized Experiments 21(122), 55673.
| Crossref | Google Scholar | PubMed |
Langerak AJ, Regterschot GRH, Evers M, Van Beijnum BF, Meskers CGM, Selles RW, Ribbers GM, Bussmann JBJ (2023) A Sensor-Based Feedback Device Stimulating Daily Life Upper Extremity Activity in Stroke Patients: A Feasibility Study. Sensors 23(13), 5868.
| Crossref | Google Scholar | PubMed |
Lin KC, Hsieh YW, Wu CY, Chen CL, Jang Y, Liu JS (2009) Minimal detectable change and clinically important difference of the Wolf Motor Function Test in stroke patients. Neurorehabilitation and Neural Repair 23(5), 429-434.
| Crossref | Google Scholar | PubMed |
Maceira-Elvira P, Popa T, Schmid AC, Hummel FC (2019) Wearable technology in stroke rehabilitation: towards improved diagnosis and treatment of upper-limb motor impairment. Journal of NeuroEngineering and Rehabilitation 16(1), 142.
| Crossref | Google Scholar | PubMed |
Marino R, Mak-Yuen Y, Roberts E, Young W, Darzins S, Fotheringham V, Stevens N, Tse T (2021) Oral Presentations – Thursday 24 June 2021. Australian Occupational Therapy Journal 68(S1), 7-91.
| Crossref | Google Scholar | PubMed |
Martino Cinnera A, Picerno P, Bisirri A, Koch G, Morone G, Vannozzi G (2024) Upper limb assessment with inertial measurement units according to the international classification of functioning in stroke: a systematic review and correlation meta-analysis. Topics in Stroke Rehabilitation 31(1), 66-85.
| Crossref | Google Scholar | PubMed |
Noorkõiv M, Rodgers H, Price CI (2014) Accelerometer measurement of upper extremity movement after stroke: a systematic review of clinical studies. Journal of NeuroEngineering and Rehabilitation 11, 144.
| Crossref | Google Scholar | PubMed |
Rast FM, Labruyère R (2020) Systematic review on the application of wearable inertial sensors to quantify everyday life motor activity in people with mobility impairments. Journal of Neuroengineering and Rehabilitation 17(1), 148.
| Crossref | Google Scholar | PubMed |
Stroke Foundation (n.d.) Clinical Guidelines for Stroke Management. Available at https://informme.org.au/guidelines/living-clinical-guidelines-for-stroke-management
Tse T, Lentin P, Douglas J, Carey LM (2022) Understanding activity participation 3-months after stroke: a mixed methodology study. Disability and Rehabilitation 44(12), 2868-2878.
| Crossref | Google Scholar | PubMed |
Uswatte G, Foo WL, Olmstead H, Lopez K, Holand A, Simms LB (2005) Ambulatory monitoring of arm movement using accelerometry: an objective measure of upper-extremity rehabilitation in persons with chronic stroke. Archives of Physical Medicine and Rehabilitation 86(7), 1498-1501.
| Crossref | Google Scholar | PubMed |
Wang Q, Markopoulos P, Yu B, Chen W, Timmermans A (2017) Interactive wearable systems for upper body rehabilitation: a systematic review. Journal of NeuroEngineering and Rehabilitation 14(1), 20.
| Crossref | Google Scholar | PubMed |
Wolf SL, Catlin PA, Ellis M, Archer AL, Morgan B, Piacentino A (2001) Assess-ing Wolf motor function test as outcome measure for research in patients after stroke. Stroke 32(7), 1635-1639.
| Crossref | Google Scholar | PubMed |
Wolf SL, Thompson PA, Morris DM, Rose DK, Winstein CJ, Taub E, Giuliani C, Pearson SL (2005) The EXCITE trial: attributes of the Wolf Motor Function Test in patients with subacute stroke. Neurorehabilitation and Neural Repair 19(3), 194-205.
| Crossref | Google Scholar | PubMed |