First report of Corynespora cassiicola causing leaf and berry spots on Coffea canephora in Brazil
A. F. Souza A , H. Costa B , L. Zambolim C D , C. Mendes C , R. L. Freitas C , E. M. Zambolim C , W. C. Jesus Junior A and O. L. Pereira CA Departamento de Produção Vegetal, Centro de Ciências Agrárias da Universidade Federal do Espírito Santo, 29500-000, Alegre, E.S., Brazil.
B Instituto Capixaba de Pesquisa, Assistência Técnica e Extensão Rural, 29375-000, Domingos Martins, E.S., Brazil.
C Departamento de Fitopatologia, Universidade Federal de Viçosa, 36570-000, Viçosa, M.G., Brazil.
D Corresponding author. Email: zambolim@ufv.br
Australasian Plant Disease Notes 4(1) 72-74 https://doi.org/10.1071/DN09031
Submitted: 19 June 2009 Accepted: 7 July 2009 Published: 3 August 2009
Abstract
A new leaf and berry spot disease, and leaf fall caused by Corynespora cassiicola is reported on Coffea canephora in Brazil.
Brazil is the world’s second major producer of Coffea canephora cv. conilon. This species is responsible for 40% of the total coffee production of the country. It is widely cultivated in Brazil in warm areas at low altitude.
The fungus Corynespora cassiicola (Berk. & M.A. Curtis) C.T. Wei infects leaves, flowers, berries, roots and stems of several plant species of tropical and subtropical regions (Farr et al. 2009). On all known host plant species, C. cassiicola causes light-brown to dark-brown leaf spots. The genus Corynespora has never been associated with Coffea.
In November 2007, during a survey for coffee diseases in the state of Espírito Santo, in the south-east region of Brazil, leaves of C. canephora cv. conilon var. Vitória (Clone CV3) with severe spotting were collected. Samples of diseased leaves were also collected from coffee nurseries close to the coffee plantation. Leaf spots were circular to elliptical or irregular, sometimes coalescing and leading to extensive necrosis, dark brown, partially covered with fungal conidiophores and conidia.
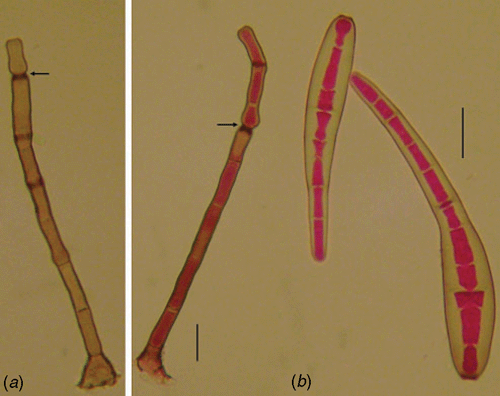
The fungus on the leaf surface of the host tissue is described as follows: conidiophores isolated, septate, dark brown, unbranched, erect, 75–225 × 7.5–10 µm. Conidia are straight to slightly curved with a rounded apex and truncate base, 55–155 × 12.5–20 µm, pale brown, with 4–17 pseudo-septa (Fig. 1). Material examined: Vic. 31208, on leaves of Coffea canephora cv. conilon, Castelo, Espírito Santo State, Brazil, coll. A. F. Souza, H. Costa and L. Zambolim, 20 Nov. 2007.
|
The fungus fits the description of Corynespora cassiicola (Ellis and Holliday 1971), a well-known fungal pathogen commonly associated with leaf spot diseases, but not previously reported causing leaf blight on C. canephora cv. conilon in Brazil.
The fungus initially causes small chlorotic spots up to 2 mm in diameter with necrotic centres on the leaves of C. canephora cv. conilon (Fig. 2). The necrotic spots become light brown and reach 7 mm in diameter, surrounded by a chlorotic halo. Spots may be solitary or coalesce and form extensive irregular lesions. Lesions on the main vein are elongated, up to 3 × 35 mm, becoming necrotic and dark. Conidia and conidiophores may be seen on the main leaf vein. Most of the leaves of infected plants are affected by the fungus in the field. Plants with high disease severity defoliate and as a consequence branches dry out, reducing berry yield.

|
On berries the initial symptoms are small necrotic lesions of 1 mm diameter (Fig. 3). Later the necrotic berry lesions expand, becoming elliptic or elongated before the berries reach maturity. Berry drop can occur prematurely from coffee plants with high disease severity. Premature leaf defoliation, under-development of the plants and death of the rooted shoots due to C. cassiicola may occur (Fig. 4). Similar symptoms on the leaves and berries were also observed on C. canephora hybrids in Ouro Preto do Oeste, State of Rondônia in the Amazon region.

|

|
The pathogenicity of the fungus was demonstrated by taking mycelial plugs (0.5 cm) from 10-day-old colonies growing on potato dextrose agar (PDA) at 26°C, and placing them on the lower side of wounded and intact healthy mature leaves of the clonal var. Vitoria (Clone CV3) of C. canephora cv. conilon, in a humid chamber. Symptoms similar to those previously observed were detected after 7–10 days on inoculated leaves. The control leaves on which PDA plugs were placed, remained healthy. Conidia of similar size and morphology to those obtained from the field were observed on the necrotic lesions after 12 days of incubation.
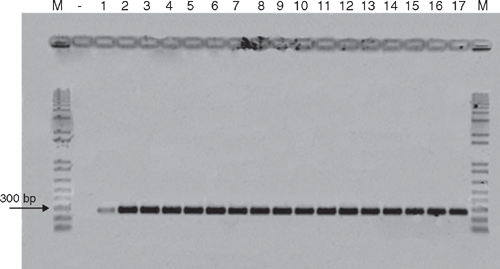
To confirm that the fungus belongs to the species C. cassiicola, genomic DNA was extracted from 12-day-old mycelia according to Raeder and Broda (1985). The ITS region of rRNA of the fungus was amplified by polymerase chain reaction (PCR) using two specific primers CCF (5′-CCC TTC GAG ATA GCA CCC-3′) and CCR (5′-ATG CCC TAA GGA ATA CCA AA-3′) (Qi et al. 2007). DNA from C. canephora cv. conilon leaves (negative control) and of 16 isolates of C. cassiicola from different hosts was compared to check their identity (Table 1). The confirmation of the presence of C. cassiicola was proved by the band correspondent to the amplified DNA fragment of ~272 bp on all the isolates tested (Fig. 5). This is the first report of C. cassiicola causing severe leaf fall on C. canephora cv. conilon plants under field conditions.
|
|
Acknowledgements
The authors thanks Professor João Batista Vida and the graduate student Ricardo Ribeiro de Oliveira of the Universidade Estadual de Maringá for the isolates of C. cassiicola from different plant species and A.F. de Souza acknowledge the Coordenação de Aperfeiçoamento de Nivel Superior – CAPES; L. Zambolim and E. M. Zambolim acknowledge the Conselho Nacional de Desenvolvimento Cientifico e Tenologico – CNPQ and Ministério da Agricultura Pecuária e Abastecimento – MAPA for providing financial support.
Qi YX,
Zhang X,
Pu JJ,
Xi YX,
Zhang HQ,
Huang SL, Zhang H
(2007) Detection of Corynespora cassiicola in Hevea rubber tree from China. Australasian Plant Disease Notes 2, 153–155.
|
CAS |

Raeder U, Broda P
(1985) Rapid preparation of DNA from filamentous fungi. Letters in Applied Microbiology 1, 17–20.
| Crossref | GoogleScholarGoogle Scholar |
CAS |



