First report of Coniothyrium minitans, a mycoparasite of Sclerotinia sclerotiorum, in Iran
M. R. OjaghianDepartment of Plant Protection, Agriculture Faculty, Bu-Ali Sina University, Hamadan, Iran. Email: smro59@gmail.com
Australasian Plant Disease Notes 4(1) 75-77 https://doi.org/10.1071/DN09032
Submitted: 6 July 2009 Accepted: 16 July 2009 Published: 3 August 2009
Abstract
To study possible biocontrol agents against Sclerotinia sclerotiorum on potato plants in Hamadan, Iran, a sampling of sclerotia was conducted in potato fields, and after culturing one of these sclerotia on potato dextrose agar, a coelomycetous fungus was observed. After fulfilment of Koch’s postulates and assessment of morphological characteristics, this fungus was identified as Coniothyrium minitans. This is reported for the first time in Iran.
Sclerotinia sclerotiorum is an ascomycetous, homothallic and cosmopolitan fungus (Atallah and Johnson 2004), which attacks over 400 species of broad- leaved agricultural and horticultural crops (Boland and Hall 1994), and its resting bodies (sclerotia) can survive for more than 5 years in soil (Gerlagh et al. 1999). This fungus has recently caused stem rot on potato plants (Solanum tuberosum) in a large number of fields in Hamadan, a province in the west of Iran (Ojaghian 2009).
To assess possible biological control agents against this pathogen, a sampling of sclerotia was conducted in the main fields of diseased potato in August and September 2007. During culturing some sclerotia were placed onto handmade potato dextrose agar (PDA, infusion of 200 g of potato, 20 g of dextrose, and 15 g of agar for 1 L of medium). The isolate SS4–4 produced a slow-growing and non-aerial mycelium and after 3 weeks, pycnidia developed on the culture medium, mainly around the sclerotium. This abnormal sclerotium had been obtained from the soil of a potato field in Bahar, Hamadan. In order to fulfil Koch’s postulates, a spore suspension of this coelomycetous fungus was prepared by adding 10 mL of sterile distilled water to a 30-day culture grown on PDA, and rubbing the surface of the colony using a sterilised glass spatula. The suspension was filtered through four layers of cheesecloth for removal of mycelial fragments, and the conidial concentration (109 conidia/mL) was determined using a haemocytometer and a compound microscope (Yang et al. 2007). Some surface-sterilised sclerotia were placed on autoclaved sand in a Petri dish, and sprayed with the conidial suspension. To provide adequate humidity for fungal growth, 8 mL of sterile distilled water was added weekly to the Petri dish. This experiment was conducted over five replicates and after 40–45 days, the pycnidia were observed on 70% of sclerotia. After assessment of morphological characteristics, this sclerotial mycoparasite was identified as Coniothyrium minitans and is reported in Iran for the first time.
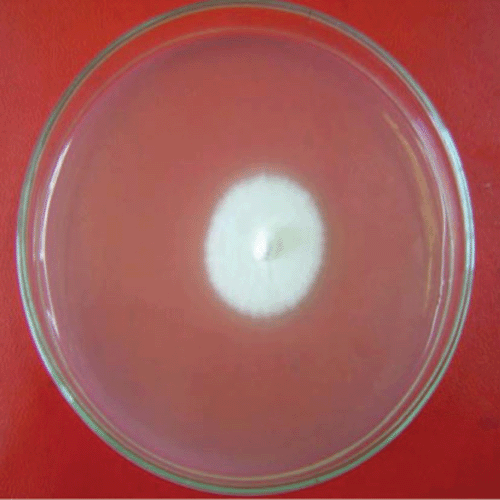
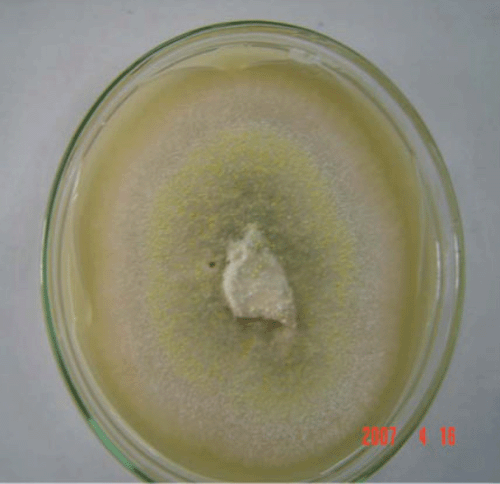
Under laboratory condition (25 ± 2°C and natural–fluorescent light) and with 10 replicates on PDA, the colony averaged 3.4 cm in diameter after 10 days. The fungal colony was initially white and without aerial mycelia (Fig. 1) and after 2 weeks, the colour of the colony changed in the centre to grey (Fig. 2).
|
|
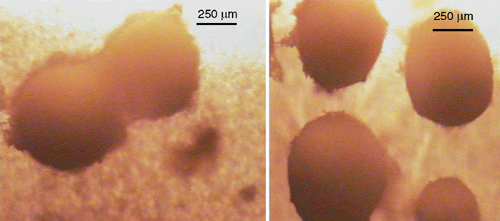
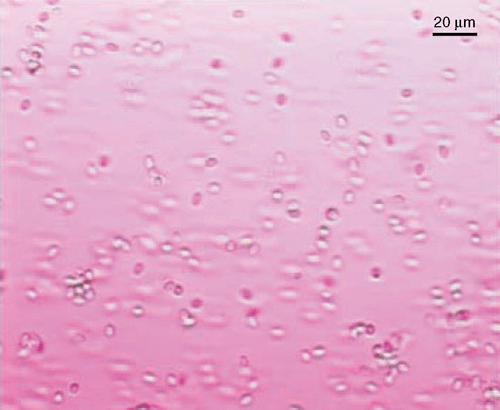
Coniothyrium minitans hyphae are 3–6 µm in diameter, smooth, simple with numerous septa in larger hyphae, and darken and become roughened with age (Phillips 1985; Whipps and Gerlagh 1992). Individual or dual pycnidia are 150–700 µm in diameter, ostiolate, with an even or rough surface, brown to black, subglobose at maturity, usually superficial on sclerotia and sometimes immersed, and become very dark with age (Fig. 3). Pycnidiospores are dark brown in mass, ovoid to ellipsoid or shortly cylindrical or nearly globose (Fig. 4), smooth to roughened and 4–7 × 2.5–4 µm (Punithalingam 1982).
|
|
Coniothyrium minitans was first described for biological control of Sclerotinia sclerotiorum in California (Campbell 1947) and has been usually isolated from sclerotia in soil from more than 30 countries on all continents except Antarctica (Sandys-Winsch et al. 1993). This fungus is an ecologically fastidious mycoparasite (Whipps et al. 2008) on sclerotia of many ascomycetous fungi such as Sclerotinia sclerotiorum, S. minor, S. trifoliorum, Botrytis spp. and some strains of Sclerotium cepivorum, but it is not able to parasitise the sclerotia of Ciborinia camellia (Van Toor et al. 2005) and basidiomycetous sclerotia (Whipps and Gerlagh 1992). There are several phenotypes of this fungus that differ in colony morphology and other biological characteristics, but the most important feature is the ability to parasitise sclerotia of S. sclerotiorum (Whipps et al. 2008).
Coniothyrium minitans has displayed a good ability to infect and degrade sclerotia in soil and has a potential to control S. sclerotiorum by decreasing carpogenic germination and viability of sclerotia (Whipps and Budge 1990; Jones and Whipps 2002). This fungus was systematically classified in the order of Pleosporales and was reclassified as Paraconiothyrium minitans, based on anamorphic characteristics, maximum parsimony analysis of ITS and SSU nrDNA sequences (Verkley et al. 2004) but due to common usage, Coniothyrium minitans continues to be used in current articles. Because of variable biocontrol efficacy of isolates on different sclerotium-forming pathogens (Gerlagh et al. 1996), it is necessary to search for more efficient isolates.
Atallah ZK, Johnson DA
(2004) Development of Sclerotinia stem rot in potato fields in south-central Washington. Plant Disease 88, 419–423.
| Crossref | GoogleScholarGoogle Scholar |

Boland GJ, Hall R
(1994) Index of plant hosts of Sclerotinia sclerotiorum. Canadian Journal of Plant Pathology 16, 93–100.

Campbell WA
(1947) A new species of Coniothyrium parasitic on sclerotia. Mycologia 39, 190–195.
| Crossref | GoogleScholarGoogle Scholar |

Gerlagh M,
Goossen-van de Geijn HM,
Fokkema NJ, Vereijken PFG
(1999) Long-term biosanitation by application of Coniothyrium minitans on Sclerotinia sclerotiorum-infected crops. Phytopathology 89, 141–147.
| Crossref | GoogleScholarGoogle Scholar |
CAS |
PubMed |

Gerlagh M,
Whipps JM,
Budge SP, Goossen-van de Geijn HM
(1996) Efficiency of isolates of Coniothyrium minitans as mycoparasites of Sclerotinia sclerotiorum, Sclerotium cepivorum and Botrytis cinerea on tomato stem pieces. European Journal of Plant Pathology 102, 787–793.
| Crossref | GoogleScholarGoogle Scholar |

Jones EE, Whipps JM
(2002) Effect of inoculum rates and sources of Coniothyrium minitans on control of Sclerotinia sclerotiorum disease in glasshouse lettuce. European Journal of Plant Pathology 108, 527–538.
| Crossref | GoogleScholarGoogle Scholar |

Ojaghian MR
(2009) First report of Sclerotinia sclerotiorum on potato plants in Iran. Australasian Plant Disease Notes 4, 39–41.

Phillips AJL
(1985)
Coniothyrium minitans on sclerotia of Sclerotinia sclerotiorum in South Africa. Phytophylactica 17, 217–219.

Sandys-Winsch C,
Whipps JM,
Gerlagh M, Kruse M
(1993) World distribution of the sclerotial mycoparasite Coniothyrium minitans. Mycological Research 97, 1175–1178.
| Crossref | GoogleScholarGoogle Scholar |

Van Toor RF,
Jaspers MV, Stewart A
(2005) Effect of soil microorganisms on viability of sclerotia of Ciborinia camelliae, the causal agent of camellia flower blight. New Zealand Journal of Crop and Horticultural Science 33, 149–160.

Verkley GJM,
da Silva M,
Wicklow DT, Crous PW
(2004)
Paraconiothyrium, a new genus to accommodate the mycoparasite Coniothyrium minitans, anamorphs of Paraphaeosphaeria, and four new species. Studies in Mycology 50, 323–335.

Whipps JM, Budge SP
(1990) Screening for sclerotial mycoparasites of Sclerotinia sclerotiorum. Mycological Research 94, 607–612.
| Crossref | GoogleScholarGoogle Scholar |

Whipps JM, Gerlagh M
(1992) Biology of Coniothyrium minitans and its potential for use in disease biocontrol. Mycological Research 96, 897–907.
| Crossref | GoogleScholarGoogle Scholar |

Whipps JM,
Sreenivasaprasad S,
Muthumeenakshi S,
Rogers CW, Challen MP
(2008) Use of Coniothyrium minitans as a biocontrol agent and some molecular aspects of sclerotial mycoparasitism. European Journal of Plant Pathology 121, 323–330.
| Crossref | GoogleScholarGoogle Scholar |

Yang L,
Miao HJ,
Li GQ,
Yin LM, Huang HC
(2007) Survival of the mycoparasite Coniothyrium minitans on flower petals of oilseed rape under field conditions in central China. Biological Control 40, 179–186.
| Crossref | GoogleScholarGoogle Scholar |



