First report of anthracnose of Acacia in Australia
H. GolzarDepartment of Agriculture and Food Western Australia, Bentley Delivery Centre, WA 6983, Australia. Email: hgolzar@agric.wa.gov.au
Australasian Plant Disease Notes 4(1) 70-71 https://doi.org/10.1071/DN09030
Submitted: 23 June 2009 Accepted: 30 June 2009 Published: 15 July 2009
Abstract
In May 2009, anthracnose symptoms were observed on Acacia seedlings, in the south-west of Western Australia. Based on morphological characteristics of the isolated fungus, disease symptoms and pathogenicity tests, Colletotrichum acutatum J.H. Simmonds was identified as the causal agent of anthracnose of Acacia. This is the first report of Anthracnose (C. acutatum) on Acacia both in Australia and worldwide.
The genus Colletotrichum contains a large number of plant pathogenic species which cause significant economic damage. It has a wide range of hosts in tropical, subtropical, and temperate regions (Bailey and Jeger 1992). Colletotrichum gloeosporioides (teleomorph Glomerella cingulata) and C. acutatum are the most common species causing anthracnose on a wide range of plant species (Mordue 1971; Dyko and Mordue 1979). In Florida, C. gloeosporioides has caused anthracnose of Acacia (Barnard and Schroeder 1984); however, there is no record of C. acutatum on Acacia internationally.
Colletotrichum acutatum causes the disease commonly known as anthracnose on a wide range of plants, including legumes, vegetables, small fruits and perennial tree crops. The disease can occur on leaves, stems and fruits of host plants. (Dyko and Mordue 1979; Peres et al. 2005; Sergeeva et al. 2008).
Acacia species are woody perennial trees belong to the family Mimosaceae commonly known as wattle in Australia. Acacia microbotrya, A. acuminata and A. saligna species are of interest for commercial utilisation in agroforestry plantings in Western Australia (Byrne and Broadhurst 2003).
The anthracnose symptoms were observed on the Acacia seedlings in the south-west of Western Australia which experiences a Mediterranean climate. Typical symptoms appeared as brown sunken circular to irregular lesions, progressing to larger necrotic areas. The fungus produced erumpent, mucilaginous, orange spore masses under high humidity conditions (Figs 1 and 2).

|

|
Pieces of infected stems and leaves were surface-sterilised by immersion in a 1.25% aqueous solution of sodium hypochlorite for 1 min, rinsed in sterile water and dried in a laminar flow cabinet. Specimens were selected randomly and either: (a) placed on potato dextrose agar (PDA), incubated at 22 ± 3°C, and after 5 days emerged fungal colonies were sub-cultured on PDA and pure cultures obtained using the ‘single spore method’; or (b) placed in trays on the moist filter paper and incubated at 25°C with a 12 : 12 h light : dark cycle.
Colletotrichum sp. was consistently isolated from the diseased specimens. Growth rate, colony morphology and morphological characteristics of the isolated fungus were determined and C. acutatum was identified as the causal agent of the anthracnose symptoms (Fig. 3).
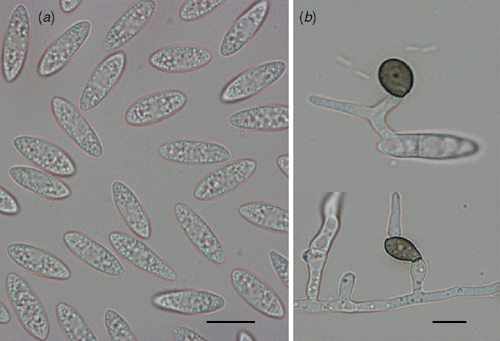
|
Pathogenicity of the fungus isolates was tested on Acacia acuminata cv. acuminata seedlings in a glasshouse experiment. Seedlings were inoculated (leaves and stems) by either wounding or non-wounding. Controls were inoculated using sterilised water (Lubbe et al. 2006). Plants were placed under mist for 48 h and then moved to the growth room chamber at 22 ± 1°C. Disease symptoms were observed 6 days post-inoculation. Koch’s postulates were fulfilled by reisolation of the C. acutatum. A culture of C. acutatum has been deposited in the WA culture collection as WAC 13265.
To our knowledge, this is the first report of anthracnose of Acacia caused by C. acutatum in Australia. Acacia is a new host for C. acutatum and it has not previously been reported worldwide.
Acknowledgements
The author would like to thank Ms Paula Mather for her technical assistance, Dr Sarah Collins for reviewing of the manuscript and Matthew Rumenos for providing Acacia samples.
Barnard EL, Schroeder RA
(1984) Anthracnose of Acacia in Florida: occurrence and fungicidal control. Proceeding of the Florida State Horticultural Society 97, 244–247.

Byrne M, Broadhurst L
(2003) Genetic diversity and the utilisation of Acacia species complexes in agroforestry in Western Australia. Australian Systematic Botany 16, 49–55.
| Crossref | GoogleScholarGoogle Scholar |

Lubbe CM,
Denman S,
Lamprecht SC, Crous PW
(2006) Pathogenicity of Colletotrichum species to Protea cultivars. Australasian Plant Pathology 35, 37–41.
| Crossref | GoogleScholarGoogle Scholar |

Peres NA,
Timmer LW,
Adaskaveg JE, Correll JC
(2005) Lifestyles of Colletotrichum acutatum. Plant Disease 89, 784–796.
| Crossref | GoogleScholarGoogle Scholar |

Sergeeva V,
Nair NG, Spooner–Hart R
(2008) Evidence of early flower infection in olives (Olea europaea) by Colletotrichum acutatum and C. gloeosporioides causing anthracnose disease. Australasian Plant Disease Notes 3, 81–82.



