A Simple Isocratic HPLC Method for the Quantitation of 17 Cannabinoids
Peter Galettis A B C D , Michelle Williams A B C , Rebecca Gordon A B C and Jennifer H. Martin A B C
A B C D , Michelle Williams A B C , Rebecca Gordon A B C and Jennifer H. Martin A B C
A Centre for Drug Repurposing and Medicines Research, School of Medicine & Public Health, The University of Newcastle, Callaghan, NSW 2308, Australia.
B Australian Centre for Cannabinoid Clinical and Research Excellence (ACRE), The University of Newcastle, Callaghan, NSW 2308, Australia.
C Hunter Medical Research Institute, New Lambton Heights, NSW 2305, Australia.
D Corresponding author. Email: peter.galettis@newcastle.edu.au
Australian Journal of Chemistry 74(6) 453-462 https://doi.org/10.1071/CH20380
Submitted: 31 December 2020 Accepted: 12 March 2021 Published: 8 April 2021
Journal Compilation © CSIRO 2021 Open Access CC BY
Abstract
Although cannabis has been used for several thousand years, the exact composition of the cannabinoids patients are administered for different symptoms has remained largely unknown. While this absence of catalogued information may be accepted in some cultures, the use of cannabis as a human product in the registered medicines setting requires knowing its composition so that doses can be standardised between patients. This is particularly so in clinical trials that are currently under way to determine the efficacy of a product. Although the major cannabinoids of interest to prescribers are well known – tetrahydrocannabinol and cannabidiol and the corresponding acids tetrahydrocannabinolic acid and cannabidiolic acid, the cannabis plant contains many more phytocannabinoids. We have developed and validated a robust and fast (11 min) isocratic HPLC method for the analysis of 17 phytocannabinoids. The method had an analytical range of 1–150 μg mL−1 for tetrahydrocannabinolic acid and cannabidiolic acid, 0.5–75 μg mL−1 for tetrahydrocannabinol and cannabidiol, and 0.5–20 μg mL−1 for the remaining 13 cannabinoids. The method had excellent repeatability with a relative standard deviation of between 5 and 14 % and a bias of between –8.6 and 6 % for the 17 cannabinoids. The method was applied to the analysis of medicinal cannabis products, including both flos and oils with results matching the supplier’s certificate of analysis. This simple fast isocratic method with basic HPLC equipment can be easily transferred to any analytical laboratory interested in the identification and quantitation of cannabinoids.
Keywords: cannabinoids, cannabis, HPLC, quantitation, tetrahydrocannabinol, cannabidiol, medicinal, plant, oils.
Introduction
Cannabis has been used in traditional medicine for many thousands of years with records dating to the 8th century in Arabic medicine[1] with references to its use as a grain, and hallucinogenic properties far pre-dating this in Asia.[2] More recently, amidst great controversy and legal debate, some states of the USA have permitted the sale of cannabis for either medicinal or recreational purposes. In Australia, late 2014 saw the introduction of a bill supporting the legalisation of cannabis use for terminally ill patients as well as the announcement of the first clinical trials to assess the benefits and side effects of medicinal cannabis in this setting. Since this time, the Australian landscape has evolved rapidly, with clinicians assisting patients to access cannabis medicines through the Therapeutic Goods Administration’s Special Access Scheme (SAS) when standard therapies have not been helpful. Further, there are multiple clinical trials currently under way for both adults and children and a wider community awareness of medicinal cannabis.[3]
Currently, for a cannabis medicine to be available for prescription through the SAS, the Sponsor must demonstrate compliance to TGO93, the Therapeutic Goods (Standard for Medicinal Cannabis) Order 2017. This specifies that the plant or medicinal product must pass to ensure that it is free of contaminants, adulterants, and microorganisms and has the specified concentration of active ingredient(s) present. The major cannabinoids of interest to prescribers are tetrahydrocannabinol (THC) and cannabidiol (CBD) and the corresponding acids tetrahydrocannabinolic acid (THCA) and cannabidiolic acid (CBDA). However, the cannabis plant contains at least 200 phytocannabinoids[4] in addition to terpenes, fatty acids, and many other components, many of which may have chemical activity. This makes a complete characterisation difficult and limited by the availability of analytical standards and methodological factors. Existing chromatographic methods for the analysis of cannabinoids have several limitations; this includes applicability to a small number of cannabinoids[5–7] (only six to eight), complex chromatographic gradient separations[5,8] that can be difficult to replicate, expensive instrumentation costs[9] with mass spectrometry, and long run times[6] up to 25 mins, which would limit sample throughput. We aimed to develop a comprehensive method for the quantification of 17 phytocannabinoids using an isocratic separation with basic HPLC equipment that could be adopted by any analytical laboratory.
Results and Discussion
Development of HPLC Method
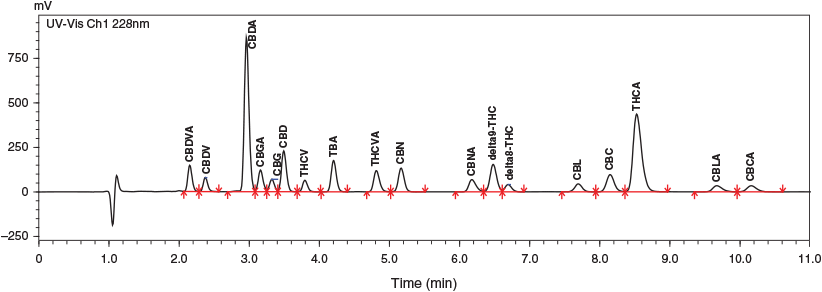
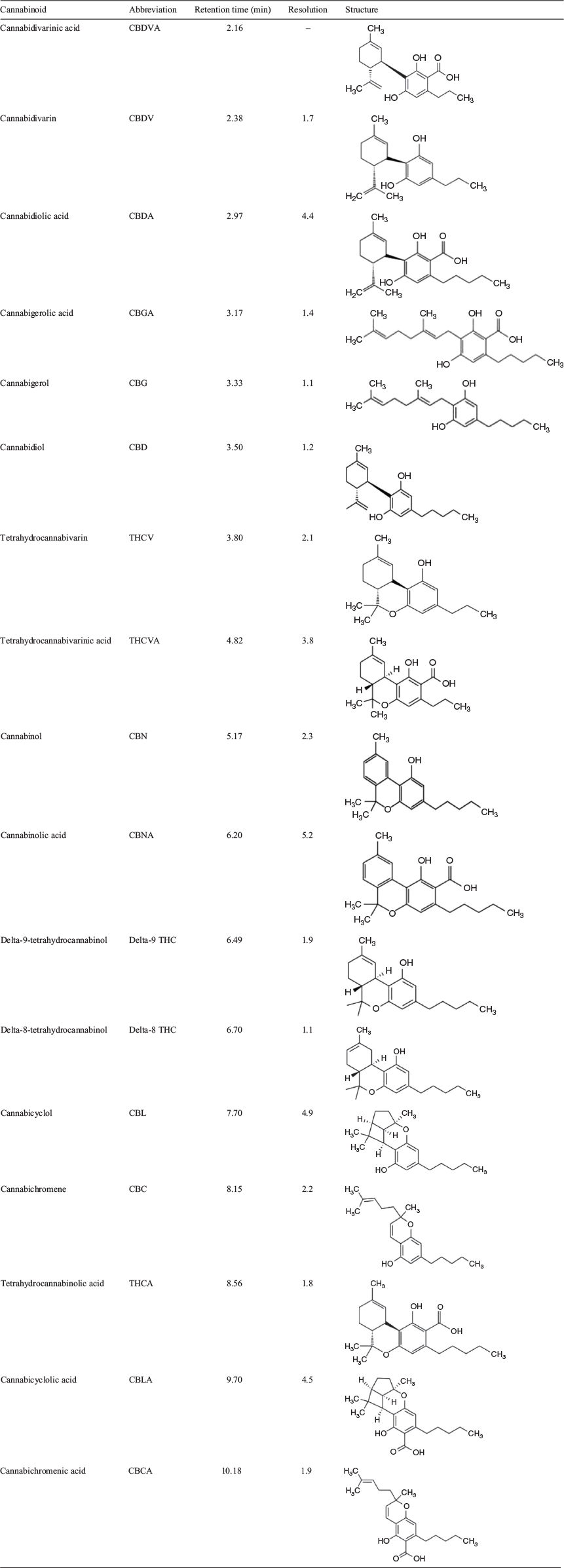
In the development of any HPLC method, several decisions need to be made in regard to the number of compounds analysed, analytical range, column for separation, mobile phase, and selection of detectors. We set out to analyse and quantitate the 17 cannabinoids listed in Table 1. Currently, UV and mass spectrometer are the predominant detectors used. Whereas mass spectrometry is typically the most sensitive and appropriate for rapid analysis, the nature and variety of the cannabinoids in plant material mean this is not necessarily the case in this setting. Of the 17 cannabinoids, cannabinol (CBN), cannabigerol (CBG), cannabinolic acid (CBNA), and cannabigerolic acid (CBGA) are the only compounds with unique molecular weights, cannabidivarin (CBDV)/tetrahydrocannabivarin (THCV) and cannabidavarinic acid (CBDVA)/tetrahydrocannabivarinic acid (THCVA) are isobaric pairs and CBDA, THCA, cannabichromenic acid (CBCA), and cannabicyclolic acid (CBLA) all share the same molecular weight. The largest group sharing the same molecular weight consists of CBD, delta-8 THC, delta-9 THC, cannabicyclol (CBL), and cannabichromene (CBC). Furthermore, all the compounds are structurally similar, with members of isobaric pairs/groups differing structurally by a minor component such as the position of a double bond or closed versus open ring structure. These structural and molecular weight similarities mean that the selective power of a mass spectrometer remains largely untapped. The concentrations of cannabinoids expected in plant material are reported and marketed as a percentage converting to grams of active drug per 100 g of plant material; these concentrations are far in excess of the nanogram to picogram capabilities of the mass spectrometer, and thus significant dilution is required to prevent detector saturation. Overall, the need for chromatographic separation because of the number of isomers in combination with the potential for extensive dilution error in mass spectrometry led to the selection of the flow-through cell and high concentration capabilities of the UV detector for the analysis of cannabinoids. A wavelength of 228 nm was chosen as this has previously[6] been shown to have some selectivity for cannabinoids.
|
Several analytical columns were tried for the separation of the 17 cannabinoids, including Phenomenex Kinetex C18, Luna C18, Luna Polar C18, Restek Raptor C18, and Raptor ARC-18. The biggest issue involved the resolution of CBG and CBD and being able to retain a run time that would be suitable in a commercial environment. The Restek Raptor ARC-18 provided the greatest resolution without significantly extending the analysis run time.
The mobile phase also had a significant effect on the separation of the cannabinoids. The number of similar cannabinoids means that the interactions of the mobile phase, column, and analyte are critical to the resolution and in turn robustness of the method. Individual aqueous mobile phases were prepared, each containing 0.1 % formic acid with variable concentrations of ammonium formate (2.5, 5, 7.5 mM). The retention time of the acidic compounds increased with higher concentrations of ammonium formate, leading to poorer separation of THCVA, CBNA, and THCA. A final concentration of 5 mM ammonium formate allowed the best chromatographic separation of all analytes (Fig. 1).
In a solution containing 17 cannabinoids, there is limited space to add an internal standard. Developing the method without one resulted in poor inter-day precision. We then tested a range of synthetic cannabinoids with little success. We found that tert-butyl anthraquinone (TBA) previously used by de Backer et al.[6] was the most promising internal standard and we further developed the method with this.
Final chromatographic separation was achieved using a Restek Raptor ARC-18 150 × 4.6 mm (2.7 µm) using an isocratic method with 5 mM ammonium formate and 0.1 % formic acid (25 %), and acetonitrile containing 0.1 % formic acid (75 %). Most compounds were baseline-separated with a resolution >1.5 (Fig. 1, Table 1). Two groups of analytes had very close retention times. These were CBDA, CBGA, CBG, and CBD in the first group, and CBNA, delta-9 THC, and delta-8 THC in the second. These two groups of compounds were considered critical in the separation because poor separation in either of these groups could lead to overestimation of concentrations present in a sample or, worse, the quantitation of one compound for another (e.g. CBG as CBD). This method provides chromatographic separation of 17 cannabinoids, which is more than previous methods that include only six to eight cannabinoids,[5–7] does not require gradient separation,[5–9] does not require the capital expense of mass spectrometry,[9] and only takes 11 min. Overall, this is a significant improvement on existing cannabinoid methods.
Validation of HPLC Method
Naturally, there is a large variation in the concentrations of cannabinoids present in plant material, with THCA and CBDA present at the highest concentrations (usually in the range of 4 to 20 %), with smaller concentrations of THC and CBD and even lower amounts of the minor cannabinoids. However, most medicinal cannabis oils have high concentrations of THC and CBD and lower concentrations of the other cannabinoids.
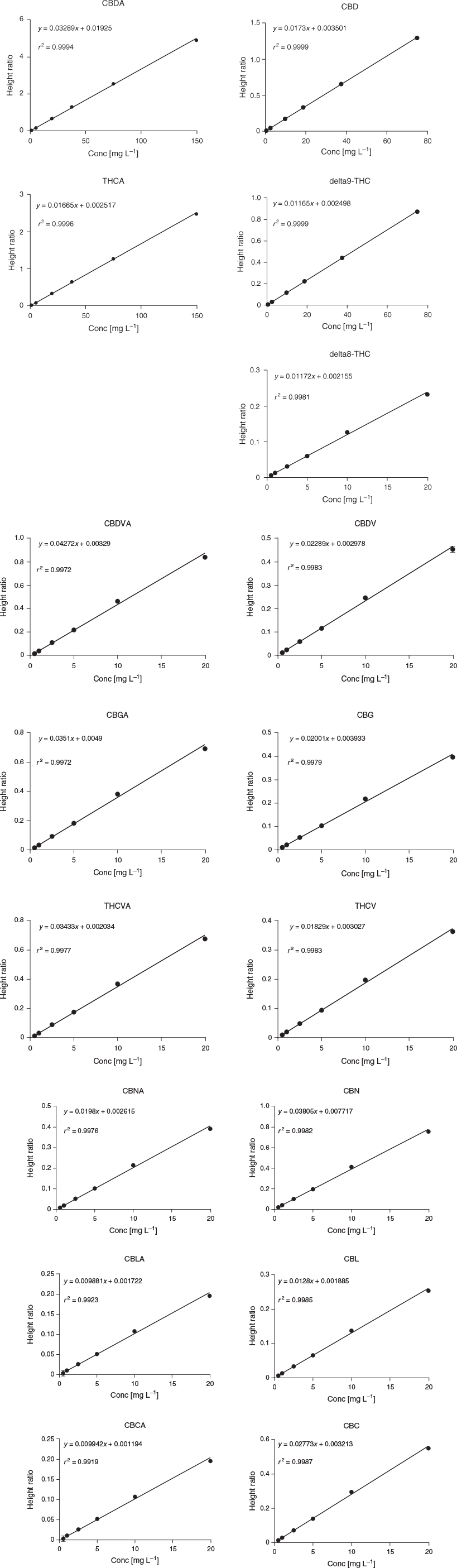
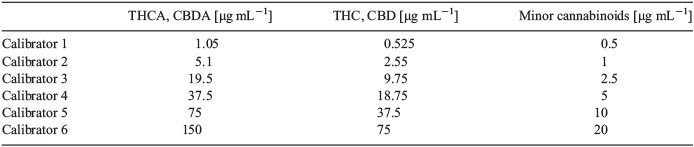
One of the limitations of this methods is that the large number of cannabinoids present also limits the maximum concentration of each compound that could be quantitated. For example, if all cannabinoids were added at the same amount, the maximum concentration of each cannabinoid that could be measured would be 58 μg mL−1. In order to account for differences in cannabinoid concentrations, we established different ranges in our calibration curves. The range for THCA and CBDA was 1–150 μg mL−1; THC and CBD had a range of 0.5–75 μg mL−1, while the remaining cannabinoids had a range of 0.5–20 μg mL−1. The cannabinoids all had linear responses over the ranges selected (Fig. 2). To determine the most appropriate calibration model, the height ratio counts from nine runs performed on 6 different days were analysed according to the procedure and code fully described by Desharnais et al.[10] A linear model with weighting of 1/x2 was selected for all compounds. The method showed no carryover after the highest calibrator for all cannabinoids apart from CBD, which had a small amount of carryover, which was less than half of the limit of quantitation.
Precision and bias (Table 2) were determined by evaluation of nine standard curves prepared on 6 different days. The relative standard deviation (RSD) across all compounds ranged from 5 to 14 % and the bias of the standards ranged from –8.6 to 5.7 %, giving acceptable precision and bias for this method based on Association of Official Analytical Collaboration (AOAC) International guidelines.[11]

|
Because of the less than ideal separation of CBDA, CBGA, CBG and CBD, and delta 9 THC and delta 8 THC, two resolution mixes were prepared and run during every run, in order to verify that suitable chromatography and quantitation were been achieved. Delta-9 and delta-8 THC were prepared at 40 and 2.5 μg mL−1 and gave values of 42 ± 0.4 (11) μg mL−1 for delta-9 THC and 2.8 ± 0.2 (11) μg mL−1 for delta-8 THC, which gives a repeatability of 1.1 and 4.5 % respectively and a bias of 4.7 and 10 % over 11 runs. The second resolution mix of CBDA (100 μg mL−1), CBGA (1 μg mL−1), CBG (1 μg mL−1), and CBD 60 μg mL−1) resulted in values of CBDA, 114 ± 1.2 (11); CBGA, 1.4 ± 0.04 (11); CBG, 1.1 ± 0.05 (11); and CBD, 58 ± 0.7 (11) μg mL−1. This is an RSD of 1.1, 2.9, 4.8 and 1.2 % and a bias of 14, 38, 5 and 15 % respectively; apart from the large positive bias for CBGA (38 %), which we suggest as being due to the concentration of CBGA overestimated at low concentrations in the presence of high concentrations of CBDA; these values are all acceptable based on AOAC international guidelines.[11]
Application of HPLC Method
In order to determine the applicability of this method, one extract and three plant medicinal cannabis products were analysed. The plant products were Bedrocan® – a cannabis flos labelled as containing 22 % THC and <1 % CBD, Bedrolite® containing <1 % THC and 9 % CBD, and Bediol® containing 6.3 % THC and 8 % CBD. These products are labelled with total THC and CBD as a percentage (g of cannabinoid/100 g of plant) as required by TGO93. Table 3 gives the breakdown of the major cannabinoids measured in each of the products. Although the total THC in each of the three products had a positive bias, apart from the Bedrolite THC, they were within the 20 % range accepted by the regulators using TGO93 guidance. There was also a positive bias in the total CBD; however, this was less than that seen with THC. The presence of minor cannabinoids was also detected. As shown in Table 3, they were generally present in low concentrations with only CBGA in Bedrocan product present at greater than 10 μg mg−1.

|
In addition to the three plant materials, the method was also applied to a medicinal cannabis oil LGP Classic 10:10, a product containing 10 mg mL−1 of both THC and CBD, with results of 10.0 ± 0.9 (8) for THC and 10.5 ± 0.9 (8) mg mL−1 for CBD. The certificate of analysis of this product stated that THC was present at 10.1 mg mL−1 and CBD at 9.4 mg mL−1. This meant that our results had a bias of –1 % for THC and 12 % for CBD.
Conclusion
Here, we present a validated method for the detection and quantitation of 17 cannabinoids in either plant material or oil extract. Although the extraction procedure is targeted for use with plants, a simplified dilution in concert with the analytical method can be applied for the detection of cannabinoids in other medicinal products such as oils. The method developed is fast, reproducible, robust, and reliable, and uses basic HPLC equipment available in most analytical laboratories. As such, it should be able to be easily transferred to other laboratories interested in the comprehensive analysis of cannabinoids.
Experimental
Reagents and Materials
Cerilliant certified reference materials of CBDVA, CBDV, CBDA, CBGA, CBG, CBD, THCV, THCVA, CBN, CBNA, delta-9 THC, delta-8 THC, CBL, CBC, THCA, CBLA, and CBCA were purchased from Novachem (Heidelberg West, Vic., Australia). Bedrocan, Bediol, and Bedrolite were also from Novachem. Formic acid and TBA were purchased from Sigma–Aldrich (Darmstadt, Germany). Water was purified with a Millipore Advantage A10 Milli-Q system. HPLC-grade acetonitrile, methanol, and ammonium formate were purchased from Bio-strategy (Campbellfield, Vic., Australia). LGP Classic 10:10 was purchased from Little Green Pharma.
HPLC Equipment
The Shimadzu HPLC system consisted of an LC20AB Pump, DGU-20A5R degasser, SIL-20AHT autosampler, CTO-20A column oven, and SPD-20A UV detector connected to a CBM-20A controller, which was controlled using LabSolutions software.
HPLC Conditions
Chromatographic separation was performed using a Restek Raptor ARC-18 150 × 4.6 mm with 2.7 µm particles. Chromatographic separation was achieved in 11 min using an isocratic method with 5 mM ammonium formate and 0.1 % formic acid (25 %) and acetonitrile containing 0.1 % formic acid (75 %). The UV detector was set to 228 nm.
Preparation of Mobile Phase
A 5 M ammonium formate stock solution was prepared by weighing 31.5 g in 100 mL water and mixing to dissolve completely. Mobile phase A was prepared by adding 1 mL each of formic acid and 5 M ammonium formate to 1 L of water. Mobile phase B was prepared by adding 1 mL of formic acid to 1 L of acetonitrile.
Preparation of Calibration Curve
Stock calibrator 1 was prepared at 300 µg mL−1 of THCA and CBDA and 150 µg mL−1 of THC and CBD. Stock calibrator 2 was prepared at 50 µg mL−1 of CBDVA, CBDV, CBGA, CBG, THCV, THCVA, CBN, CBNA, Delta-8 THC, CBL, CBC, CBCA, and CBLA. TBA was used as an internal standard at 50 µg mL−1. Stock calibrator 1 and 2 were mixed in various ratios to provide a calibration curve at the final concentrations listed in Table 4.
|
Preparation of Resolution Mixes
Resolution mix 1 was prepared by the addition of delta-9 THC and delta-8 THC to give final concentrations of 40 μg mL−1 delta-9 THC and 2.5 μg mL−1 delta-8 THC in methanol,
Resolution mix 2 was prepared by the addition of CBDA, CBGA, CBG and CBD to give final concentrations of 100 μg mL−1 CBDA, 1 μg mL−1 CBGA, 1 μg mL−1 CBG, 50 μg mL−1 CBD in methanol.
Preparation of Plant Material
All samples were stored at room temperature in the dark before extraction. Samples were extracted according to the following process: ~50 mg of plant material was weighed into a 5-mL tube, and a 7-mm stainless steel lysis ball, 0.25 mg of TBA, and 2 mL of methanol were added. Samples were then shaken for 10 min at 1500 rpm and centrifuged for 5 min at 500g and 20°C. The supernatant was transferred to a volumetric flask. A further 1.4 mL of methanol was added to the plant material, which was vortexed, spun, and transferred to the volumetric flask. This was repeated one more time and the volumetric flask made up to volume with the addition of methanol. The volumetric flask was inverted multiple times to mix and the contents were transferred to an autosampler vial for injection into the HPLC system for analysis. All samples were analysed within 72 h of extraction.
Analysis of Oils
Medicinal cannabis oils were diluted to 1 mL of methanol containing a final concentration of 50 µg mL−1 of TBA. The samples were then analysed by HPLC.
Analysis
Data are presented as mean ± s.d. (number of repeats). Relative standard deviation (RSD%) was calculated as the standard deviation divided by the mean times 100. Bias (%) was determined as the (mean – target)/target × 100.
Conflicts of Interest
The authors declare no conflicts of interest.
Declaration of Funding
This work was supported by an NSW Ministry of Health Grant and a National Health and Medical Research (NHMRC) grant GNT1135054.
Acknowledgements
We would like to thank Angus McPherson for technical assistance.
References
[1] I. Lozano, J. Cannabis Ther. 2001, 1, 63.| Crossref | GoogleScholarGoogle Scholar |
[2] M. Touw, J. Psychoactive Drugs 1981, 13, 23.
| Crossref | GoogleScholarGoogle Scholar | 7024492PubMed |
[3] Australian New Zealand Clinical Trials Registry (ANZCTR), Trial Search, in Australian New Zealand Clinical Trials Registry (ANZCTR), 2020. Available at: http://www.anzctr.org.au/TrialSearch.aspx
[4] L. O. Hanuš, S. M. Meyer, E. Muñoz, O. Taglialatela-Scafati, G. Appendino, Nat. Prod. Rep. 2016, 33, 1357.
| Crossref | GoogleScholarGoogle Scholar | 27722705PubMed |
[5] A. C. Elkins, M. A. Deseo, S. Rochfort, V. Ezernieks, G. Spangenberg, J. Chromatogr. B 2019, 1109, 76.
| Crossref | GoogleScholarGoogle Scholar |
[6] B. De Backer, B. Debrus, P. Lebrun, L. Theunis, N. Dubois, L. Decock, A. Verstraete, P. Hubert, C. Charlier, J. Chromatogr. B 2009, 877, 4115.
| Crossref | GoogleScholarGoogle Scholar |
[7] M. W. Giese, M. A. Lewis, L. Giese, K. M. Smith, J. AOAC Int. 2015, 98, 1503.
| Crossref | GoogleScholarGoogle Scholar | 26651562PubMed |
[8] E. M. Mudge, P. N. Brown, J. AOAC Int. 2020, 103, 489.
| Crossref | GoogleScholarGoogle Scholar | 31561754PubMed |
[9] L. Vaclavik, F. Benes, M. Fenclova, J. Hricko, A. Krmela, V. Svobodova, J. Hajslova, K. Mastovska, J. AOAC Int. 2019, 102, 1822.
| Crossref | GoogleScholarGoogle Scholar | 31208494PubMed |
[10] B. Desharnais, F. Camirand-Lemyre, P. Mireault, C. D. Skinner, J. Anal. Toxicol. 2017, 41, 269.
| Crossref | GoogleScholarGoogle Scholar | 28158619PubMed |
[11] Appendix F: Guidelines for Standard Method Performance Requirements, in Official Methods of Analysis of AOAC International (Ed. G. W. Latimer, Jr) 2016 (AOAC International: Rockville, MD)