Impact of Phytophthora dieback on a key heathland species Xanthorrhoea australis (Asphodelaceae) (austral grasstree) and floristic composition in the eastern Otways, Victoria
B. A. Wilson A * , S. P. Casey A , M. J. Garkaklis B , C. Learmonth A and T. Wevill A
A * , S. P. Casey A , M. J. Garkaklis B , C. Learmonth A and T. Wevill A
A
B
Abstract
The plant pathogen Phytophthora cinnamomi causes severe declines in susceptible vegetation, including loss of plant species, vegetation structure and fauna abundance. Grasstrees (Xanthorrhoea spp.) are keystone species that provide optimal habitat for vertebrates and invertebrates and are highly susceptible to the pathogen. Although effects in the Otway Ranges have been assessed at specific sites, there is less knowledge across the landscape on the extent of loss of Xanthorrhoea australis (austral grasstree).
The aims were thus to assess impacts at three Heathy Woodland sites and to determine the magnitude of loss of X. australis and susceptible species losses.
Floristic composition, species cover or abundance, and basal area of X. australis were recorded in quadrats within treatments (uninfested, infested, post-infested vegetation). Analyses included floristics (PRIMER v7), significant effects (ANOSIM), species contribution to similarity/dissimilarity (SIMPER). Species richness and susceptible species cover were analysed using two-way crossed ANOVAs to detect the influence of site, treatment, and interactions.
Species composition of uninfested vegetation was significantly different to infested and post-infested vegetation, with susceptible species more abundant in uninfested areas. Post-infested vegetation had the lowest percentage cover of susceptible species. The mean percentage cover of X. australis in uninfested vegetation (43%) was 10-fold greater than in infested areas (4.3%) and extremely low in post-infested vegetation (0.9%).
Susceptible species were subject to density declines and extirpation, and the loss of X. australis resulted in major structural vegetation changes.
These results have severe implications for heathy woodland communities and reliant fauna. Limiting the spread of P. cinnamomi and protecting grasstrees is critical for their security.
Keywords: austral grasstree, dieback, disease, Great Otway National Park, heathlands, keystone species, pathogen, Phytophthora cinnamomi, vegetation floristics, Xanthorrhoea australis.
Introduction
Many forest and woodland communities across the world have been affected by invasive plant pathogens resulting in significant changes to vegetation productivity, plant diversity, and ecosystem function (Castello et al. 1995; Burgess et al. 2017). The plant pathogen Phytophthora cinnamomi poses a major threat to both native and agricultural systems and is recognised as one of the world’s most significant invasive alien species (Lowe et al. 2000). The epidemic of P. cinnamomi ‘dieback’ is a major concern in areas such as Australia, Europe, USA, New Zealand and South Africa (Hansen 2008; Burgess et al. 2017).
Phytophthora cinnamomi is a soil-borne oomycete initiating root and collar rot, which results in the restriction of transpiration, nutrient uptake, and death of susceptible plant species (Dawson and Weste 1984; Marks and Smith 1991; Aberton et al. 2001). Disease infestation begins at the root tip of a host plant (Dawson and Weste 1984; Marks and Smith 1991). Under favourable conditions microscopic mycelial threads penetrate the dermal layer of host tissue, whereas under less favourable conditions they produce chlamydospores, which can survive for years within soil and dead plant material. Chlamydospores are the primary structure leading to spread of the pathogen through movement of infested soil and plant material (Cahill et al. 2008).
The symptoms of ‘phytophthora dieback’ vary widely between hosts but can include leaf chlorosis, root and collar lesions and retarded growth of plants (Podger et al. 1965; Podger 1968; Marks and Smith 1991; Podger and Vear 1998; Cahill et al. 2008). Three factors, the presence of the pathogen, a susceptible plant host and favourable environmental conditions for infection, known as the disease triangle, determine whether reproduction and spread of the pathogen is successful (Cahill et al. 2008). The inclusion of time then reveals that the longer an optimal environment persists, the more likely the disease will occur and further spread (Cahill et al. 2008). However, although zoospore infection is favoured by seasonal rain, symptom development is exacerbated by seasonal water stress, thus weather patterns can significantly affect disease development and vegetation changes.
The lethal epidemic of ‘phytophthora dieback’ can impact plant species richness and abundance, consequently altering the structure of sclerophyllous vegetation throughout Australia (Weste 1974; Weste et al. 2002). In the majority of cases this directly causes significant decline in plant species, loss of susceptible flora, degradation of fauna habitat, fauna declines and ecosystem deterioration (Wilson et al. 1994, 2009; Garkaklis et al. 2004; Wilson et al. 2020).
Phytophthora cinnamomi was first identified in Australia in 1930 and research in the jarrah (Eucalyptus marginata) forests of Western Australia in 1965 described it causing the epidemic disease ‘phytophthora dieback’ (Podger et al. 1965; Podger 1968; Marks and Smith 1991; Podger and Vear 1998). The pathogen is now widespread in Australia and reported in all states and territories (O’Gara et al. 2005). Within natural systems, the disease is listed as a ‘key threatening process’ to the Australian environment under the Environment Protection and Biodiversity Conservation Act 1999 (Cth) (EPBC) (Environment Australia 2001). Over 1000 native plant taxa have been listed as susceptible (McDougall 2005) and the pathogen threatens the existence of ~10% of all species listed as nationally threatened under the EPBC Act (1999) (Commonwealth of Australia 2018b). Plants from the families Dilleniaceae, Epacridaceae, Fabaceae, Proteaceae, Myrtaceae and Asphodelaceae are most susceptible to infection, conversely species from the Asteraceae and Poaceae families are less at risk (Cahill et al. 2008). These highly susceptible families are often found in heathland and woodland communities, where the underlying geology facilitates the rapid movement of P. cinnamomi through nutrient-poor tertiary soils, which are prone to waterlogging (Wilson 1990; Wilson et al. 1990, 1997). Species including many grasses can be infected but are asymptomatic hosts (Cahill et al. 2008).
Iconic grasstrees including Xanthorrhoea australis, (austral grasstree), X. preissii (balga) and X. semiplana are highly susceptible to infection by P. cinnamomi (Aberton et al. 2001; Weste et al. 1999; Lamont et al. 2004). These perennial monocotyledonous species are long lived and known for their arborescent trunk, inflorescences on upright spikes (1.5 m−2.5 m) and thin linear leaves grown in a terminal crown (Bedford 1986; Borsboom 2005; Lamont et al. 2004). They may grow over 3 metres tall, and their susceptibility is due to the species possessing a large surface area of fleshy adventitious roots within the soil (Weste et al. 1999; Aberton et al. 2001). Infection begins when the root epidermis is rapidly colonised by zoospores. Hyphae then move both intra- and intercellularly within the cortex, xylem and phloem vessels, resulting in decline of water uptake (Cahill and Weste 1983; Cahill et al. 1985, 1989). Visual disease symptoms observed in the field are similar to that of drought stress, including yellowing of leaves, browning and total crown and trunk collapse (Aberton et al. 2001). In most cases, plants will die rapidly between 6 and 12 months after first exhibiting disease symptoms.
There is evidence that grasstrees are used by many vertebrate and invertebrate species (Borsboom 2005). Grasstrees provide optimal habitat for some small native mammal species, supplying dense skirts as refuge from weather and predation and protection for nesting sites (Garkaklis et al. 2004; Marchesan and Carthew 2004; Laidlaw and Wilson 2006; Swinburn et al. 2007; Armistead 2008). Species such as the southern bush rat (Rattus fuscipes) and Kangaroo Island dunnart (Sminthopsis aitkeni) use grasstrees for shelter (Marchesan and Carthew 2004; Frazer and Petit 2007) and species such as the mardo (Antechinus flavipes), southern brown bandicoot (Isoodon obesulus) and northern bettong (Bettongia tropica) use grasstrees for nest sites (Paull 1993; Vernes and Pope 2001; Swinburn et al 2007; Armistead 2008).
A National Threat Abatement Plan for the disease caused by P. cinnamomi has been developed with major priorities to promote the recovery of threatened species and ecological communities under threat and to limit the spread of the pathogen into areas where it may lead to further species or communities becoming threatened (Environment Australia 2001; Commonwealth of Australia 2018b). There are a range of methods to minimise the spread of the pathogen to uninfested sites, by restricting access, implementing hygiene procedures when entering and exiting uninfested sites; and to alleviate the impact at infested sites, by application of the biodegradable fungicide phosphite, which effectively reduces or prevents infestation (Shearer and Tippett 1989; Hardy et al. 2001; Cahill et al. 2008; Barrett and Rathbone 2018; Commonwealth of Australia 2018a; Havlin and Schlegel 2021). The strategic application of phosphite has been shown to reduce the rate of autonomous spread of the pathogen, enhance the survival of susceptible species and ameliorate impacts on plant community structure (Barrett and Rathbone 2018; Commonwealth of Australia 2018a).
Diseased vegetation infected with P. cinnamomi was first observed in the Otway Ranges of southern Victoria in 1972 (Land Conservation Council 1985; Wilson 1990; Wilson et al. 1990, 1997). Dieback disease symptoms have been observed in heathy open forest, heathy woodland, Bald Hills heathland and riparian open forest (Wark et al. 1987; Laidlow and Wilson 1989; Wilson et al. 1990, 2000, 2003; Annett 2008). An assessment of the relationship between site factors and the distribution of P. cinnamomi found that the pathogen was widespread, occurring at 76% of sites, and that presence was negatively associated with elevation and positively associated with a sun index (Wilson et al. 2000, 2003). A detailed examination of the floristic and structural changes in heathland communities was undertaken at two sites within the eastern Otways from 1988 to 1995 (Laidlaw and Wilson 2003). The pathogen significantly affected not only the floristic diversity, but also vegetation structure in the area. Diseased vegetation, when compared with non-diseased areas, had less cover of the austral grasstree Xanthorrhoea australis and shrub species, and a greater cover of sedges, grasses and open ground. Structural differences observed included a decline in cover in diseased vegetation, between 0- and 0.6-m height strata. Studies on the impacts of P. cinnamomi on small mammals found capture frequency was lower in post-disease areas and captures of individual species (Antechinus agilis (agile Antechinus), Rattus fuscipes, (bush rat), Rattus lutreolus (swamp rat) and Sminthopsis leucopus (white-footed dunnart)) were greatest in non-diseased vegetation and were less frequent in areas of diseased vegetation. Radio-tracking studies found that X. australis provided important nesting sites for three species; Antechinus agilis, Cercartetus nanus (eastern pygmy possum) and Sminthopsis leucopus (Garkaklis et al. 2004; Laidlaw and Wilson 2006).
Long-term studies (26 years) of disease progression and impacts on vegetation floristics and structure in a heathy woodland site found that disease progressed dramatically between 1989 and 2005, and by 2015 only 0.08% of the site was non-diseased (Wilson et al. 2020). There were significant declines in plant species richness and numbers of susceptible species such as X. australis and increases in percentage cover of resistant sedges and grasses overall, and in cover of Leptospermum continentale (prickly tea-tree) in post-disease areas.
Much of the long-term work was conducted at few sites thus the objective here was to assess impacts at three sites more widely distributed across Heathy Woodlands. Specifically the aim was to determine susceptible species losses post-infestation across the landscape; and assess any differences, thus contributing to preceding work conducted at few sites.
A major focus in this paper was on the extent of loss of the keystone species X. australis. Although previous research revealed the significant role of X. australis within the landscape in providing refuge for many small mammal species there had been no estimation of the extent of loss. Our basal area data provides a baseline to monitor future loss and thus this work increases understanding of the integral relationship between grasstrees and overall ecosystem health and function.
Materials and methods
Study region
The study was conducted in the Anglesea Heathlands, an area of more than 7000 hectares within the Great Otway National Park, Victoria, Australia (Fig. 1). The underlying geology is associated with nutrient-poor soils, which include aeolian or outwash sands and tertiary sand/clay (Land Conservation Council 1985). The region has a mean annual rainfall of 620.4 mm and mean temperature of 18.4°C (Bureau of Meteorology 2021). The heathlands are a biodiversity hotspot comprising over 700 plant species, equivalent to one quarter of Victoria’s total floral diversity (Carr 2017; Wilson et al. 2020). All study sites were located within Ecological Vegetation Class (EVC) 48, Heathy Woodland (Victorian Government Department of Sustainability and Environment 2004). Heathy woodlands are typified by low woodland tree species, predominantly Eucalyptus obliqua (messmate stringybark) with a canopy to 10 m in height and average 15% cover. The understorey supports a diverse array of sclerophyllous shrubs and graminoids, as well as many endemic species, and 31% of Victoria’s native orchid species. (Forster and McDonald 2009; Carr 2017).
Map of the eastern Otways showing location of the three study sites 1 (Edwards Creek Track), 2 (Hurst Rd 1), 3 (Hurst Rd 2) within the Anglesea Heathy Woodlands. Map inset: location within Victoria.

The three sites selected for this study (Fig. 1) represent locations previously unsampled where there was a known infestation front, as well as uninfested and post-infested vegetation were:
Site 1 (Edwards Creek Track); approximately 25 metres north of Bald Hills Rd (38°22′37.6″S 144°06′41.8″E). The site occurs on a northern facing slope, providing favourable conditions for X. australis growth, and a warm and wet environment for the potential spread of P. cinnamomi. Phytophthora cinnamomi infestation appears closely linked with the nearby management vehicle track and illegal mountain biking trails.
Site 2 (Hurst Rd 1) retains a high density of X. australis. It is located 50 metres north-west of Hurst Rd (38°23′04.6″S 144°13′35.4″E) on a north-west facing slope, with P. cinnamomi infestation closely linked with the nearby management vehicle track and mountain biking trails, developed by management within the Great Otway National Park.
Site 3 (Hurst Rd 2) is situated 200 metres east of Hurst Rd (38°23′19.7″S 144°13′31.8″E), on a southern facing slope.
Earlier work in the area provided extensive information on the assessment of Phytophthora and dieback bike trails and development of Standard Operations for management of the pathogen (Wilson and Garkaklis 2019a, 2019b). The mechanisms for spread of the pathogen identified along the tracks included erosion, compaction and ponding following rain that spread the inoculum. Pathogen sampling found that areas visually assessed as Phytophthora dieback free did not have P. cinnamomi present. Samples from areas assessed as likely to be infested with the pathogen returned positive results. Importantly, P. cinnamomi identification did occur from directly on the track itself.
Study design
At each of the three sites, three treatment classes were identified. (1) Uninfested – sites free from any symptoms of Phytophthora ‘dieback’ disease, (2) Infested – sites which exhibited active ‘dieback’ symptoms, chlorotic and dead plants, (3) Post-infested – sites where there were no symptoms of active ‘dieback’, an absence of living indicator species such as X. australis and Isopogon ceratophyllus (horny cone-bush), and dead stumps of X. australis present (indicating the prior presence of X. australis on these sites). Stratified random sampling was implemented to independently sample within each treatment class and provide an accurate representation of vegetation floristics at each site. To align with strict hygiene protocols, all vegetation surveys were conducted within uninfested treatments prior to moving into infested and post-infested areas.
Pathogen sampling
Soil baiting was undertaken utilising methodologies described previously (Burgess et al. 2021). Three samples were taken per site from different plants displaying disease symptoms, if no disease symptoms were present samples were obtained from any plant within the quadrat, to the depth of 10 cm and 0.5 metres away from the base of the sampled plant. These samples, comprising mostly of fine roots, were mixed in distilled water in sterile cups until combined into a slurry. Three 3-week-old Eucalyptus sieberi dicotyledons were added before covering with foil and incubating at 25°C for 5–7 days. The Eucalyptus sieberi dicotyledons were then surface sterilised and plated onto agar selective for Phytophthora before resealing and incubating at 25°C for 5 days. Samples were confirmed for P. cinnamomi presence using microscopy.
Vegetation sampling
To determine floristic composition and species cover or abundance, 10 m × 2 m quadrats were established within all treatment classes and replicated at all three sites. Due to the nature of Phytophthora spread, small quadrats (10 m × 2 m) were used so as to ensure each replicate quadrat fell into a single treatment category (no quadrats had a mix of active disease with non-diseased status, nor of active disease with post-diseased status). All vascular plant species present were recorded and identified to species level and percent cover recorded. At uninfested sites four 10 m × 10 m quadrats were also established and species presence recorded, to validate whether the 10 m × 2 m quadrats were sufficient to detect representative species diversity (total sample size = 105 quadrats).
To determine X. australis density and basal area in uninfested treatments, one 50 m × 50 m quadrat was established at each site. These 50 m × 50 m quadrats enabled extrapolation of basal area to basal area per hectare and aligned with forestry guidelines for the assessment of tree species (Cunningham et al. 2013). Basal area of X. australis individuals was measured around the trunk of plants, using a diameter tape 10 cm above ground level (DBA). Immature X. australis individuals lacking a trunk were unable to be measured but presence was still recorded. Xanthorrhoea australis basal area was also recorded for all 10 m × 2 m quadrats of uninfested, infested and post-infested status.
Statistical analyses
Analyses of floristic composition/cover data was completed using PRIMER v7 (Clark and Gorley 2015) and RStudio (R Core Team 2021). Two dimensional (2D) non-metric multidimensional scaling (nMDS) plots were produced with 100 restarts using PRIMER v7, providing a visual representation of dissimilarity in species cover between sites and treatments. To test for significant effects of site and treatment class on vascular plant species cover, analysis of similarities (ANOSIM) tests with 999 permutations were also completed. Where ANOSIM detected significant effects, Bray–Curtis similarity percentages (SIMPER) analyses were conducted to identify those species contributing to percentage cover similarity/dissimilarity, between both site and treatment variables.
Species richness and susceptible species cover were analysed using two-way crossed ANOVAs in RStudio (R Core Team 2021), to detect the influence of site, treatment, and interactions between site and treatment. Statistical assumptions including normal distribution and equal variances were checked and transformations performed where necessary. Data was presented in box and whisker plots using the package ggplot2 (Wickham 2016) and Tukey’s post hoc pairwise comparisons used the emmeans package (Lenth 2022).
To determine whether basal area of Xanthorrhoea australis differed between uninfested, infested and post-infested treatments, basal area data was analysed using a two-way crossed ANOVA in RStudio (R Core Team 2021). All statistical assumptions were checked and posthoc pairwise comparisons tested using the emmeans package (Lenth 2022).
Results
Pathogen sampling
All samples baited for the presence of P. cinnamomi in infested and post-infested vegetation at the study sites Edwards Creek and Hurst Road except two were confirmed for P. cinnamomi presence using microscopy (Table 1).
| Date | Sample no. | Longitude | Latitude | P. cinnamomi presence (+/−) | |
|---|---|---|---|---|---|
| 25/3/22 | ECI1 | 144.06815 | –38.22382 | + | |
| 25/3/22 | ECI2 | 144.06817 | –38.22378 | + | |
| 25/3/22 | ECI3 | 144.06825 | –38.22359 | + | |
| 25/3/22 | ECI4 | 144.06823 | –38.22365 | + | |
| 25/3/22 | ECI5 | 144.06828 | –38.22378 | + | |
| 25/3/22 | ECI6 | 144.06828 | –38.22371 | + | |
| 25/3/22 | ECPI1 | 144.06824 | –38.22396 | − | |
| 25/3/22 | ECPI2 | 144.06819 | –38.22403 | − | |
| 25/3/22 | ECPI3 | 144.06801 | –38.22417 | + | |
| 29/3/22 | ECPI4 | 144.06801 | –38.22428 | + | |
| 29/3/22 | ECPI5 | 144.06796 | –38.22400 | + | |
| 29/3/22 | ECPI6 | 144.06823 | –38.22415 | + | |
| 27/3/22 | HRI1 | 144.13657 | –38.23044 | + | |
| 27/3/22 | HRI2 | 144.13655 | –38.23052 | + | |
| 27/3/22 | HRI3 | 144.13597 | –38.23060 | + | |
| 27/3/22 | HRI4 | 144.13589 | –38.23065 | + | |
| 27/3/22 | HRI5 | 144.13598 | –38.23080 | + | |
| 27/3/22 | HRI6 | 144.13597 | –38.23074 | + | |
| 11/9/23 | ECP1 | 144.06761 | –38.22547 | + | |
| 11/9/23 | ECP2 | 144.06764 | –38.22542 | + | |
| 11/9/23 | ECP3 | 144.06792 | –38.22534 | + | |
| 11/9/23 | ECP4 | 144.06795 | –38.22529 | + | |
| 11/9/23 | ECP5 | 144.06798 | –38.22494 | + | |
| 11/9/23 | ECP6 | 144.06801 | –38.22488 | + | |
| 11/9/23 | ECP7 | 144.06828 | –38.22522 | + | |
| 2/10/23 | ECP8 | 144.06831 | –38.22528 | + | |
| 2/10/23 | ECP9 | 144.06822 | –38.22492 | + | |
| 2/10/23 | ECP10 | 144.06822 | –38.22486 | + | |
| 2/10/23 | ECP11 | 144.06803 | –38.22472 | + | |
| 2/10/23 | ECP12 | 144.06796 | –38.22468 | + |
Effect of Phytophthora cinnamomi on floristic composition
A total of 97 individual vascular plant species were recorded at our sites, with 24 of these susceptible to infection by P. cinnamomi. Sixty-six species were identified within the uninfested treatments, 76 within the infested and 72 at post-infested. Species richness ranged from 8–34 species per quadrat. nMDS of species cover showed clear clustering by treatment class, with greater separation of uninfested plots from infested or post-infested plots (Fig. 2). Plots at Edwards Creek Track tended to be more segregated from those at Hurst Rd sites (irrespective of treatment class). Two-factor ANOSIM determined that when pooling treatments, species cover was significantly higher at Edwards Creek than Hurst Road sites, significantly higher in uninfested treatments and lowest at post-infested treatments (pooling sites) (R = 0.284, P = <0.01) and treatments (R = 0.367, P = <0.01). Pairwise comparisons detected significant differences among all treatment classes and sites (Table 2).
2D non-metric multidimensional scaling (nMDS) of species cover among site and treatment classes, within the Anglesea Heathy Woodlands (stress = 0.12). Site 1 (Edwards Creek Track) triangles, Site 2 (Hurst Rd 1), squares, Site 3 (Hurst Rd 2) circles.

| R | P-value | |||
|---|---|---|---|---|
| Treatment groups | ||||
| Global test | 0.367 | <0.01 | ||
| Pairwise tests | Uninfested, Infested | 0.419 | <0.01 | |
| Uninfested, Post-infested | 0.623 | <0.01 | ||
| Infested, Post-infested | 0.084 | 0.027 | ||
| Site groups | ||||
| Global test | 0.284 | <0.01 | ||
| Pairwise tests | Site 1, Site 2 | 0.417 | <0.01 | |
| Site 1, Site 3 | 0.365 | <0.01 | ||
| Site 2, Site 3 | 0.091 | 0.031 |
SIMPER analysis of species cover detected high dissimilarities between treatments (uninfested to post-infested 76.46%, uninfested to infested 68.51%, infested to post-infested 59.28%). When comparing uninfested to both infested and post-infested, three species susceptible to infection by P. cinnamomi contributed more than half of that dissimilarity, (Xanthorrhoea australis, Eucalyptus obliqua, Leptospermum myrsinoides). These were less abundant at infested sites when compared to uninfested and less abundant at post-infested sites, when compared to uninfested sites. The species with the largest contribution to that dissimilarity was X. australis (26% at uninfested compared to both infested and post-infested sites). Hurst Rd 1 presented the highest average cover at 21.03% (±0.60), closely followed by Hurst Rd 2 at 20.67% (±0.62). When comparing infested to post-infested sites, two sedges were more abundant at post-infested sites (Gahnia radula and Hypolaena fastigiata)
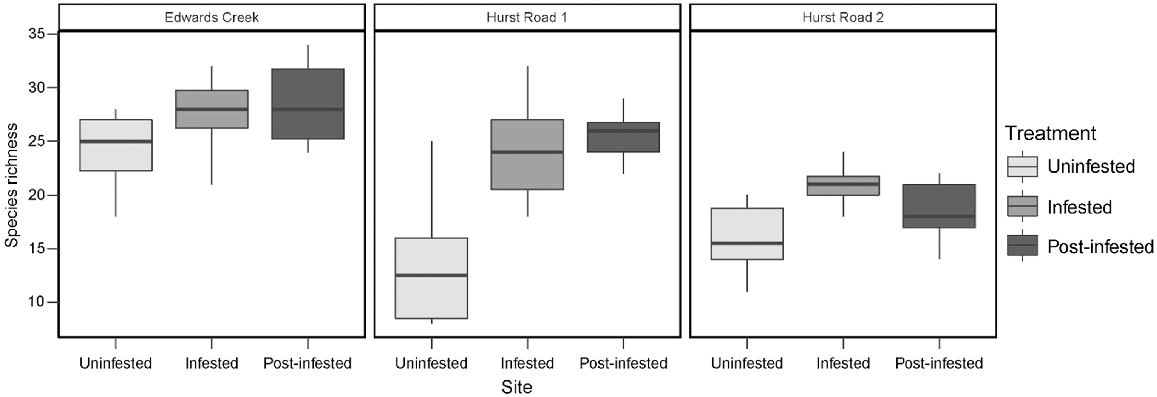
Two-factor crossed ANOVA of species richness detected a significant difference between sites (d.f. = 2, F = 44.6, P < 0.001) and treatments (d.f. = 2, F = 31.6, P < 0.001), and a site × treatment interaction (d.f. = 4, F = 6.3, P < 0.001). Uninfested species richness was significantly lower when compared to infested and post-infested treatments (Fig. 3). Post hoc Tukey’s tests indicated that species richness in the uninfested treatments at both Hurst Rd sites was significantly lower than infested and post-infested treatments at Hurst Rd sites, and than all treatments at Edwards Creek Track. The post- infested treatment at Hurst Rd 2 was also significantly lower than all treatments at Edwards Creek Track. Lastly, Hurst Rd 2 infested was significantly lower than Edwards Creek Track post-infested (Table 3).
| ECU | ECI | ECP | H1U | H1I | H1P | H2U | H2I | H2P | ||
|---|---|---|---|---|---|---|---|---|---|---|
| ECU | *** | *** | ||||||||
| ECI | *** | *** | ** | *** | ||||||
| ECP | *** | *** | *** | |||||||
| H1U | ||||||||||
| H1I | *** | *** | * | |||||||
| H1P | *** | *** | *** | |||||||
| H2U | ||||||||||
| H2I | *** | *** | * | |||||||
| H2P | ** | * |
The matrix diagonal represents the site × treatment interaction compared against itself.
EC, Edwards Creek Track; H1, Hurst Road 1; H2, Hurst Road 2; U, uninfested; I, infested; P, post-infested.
*P < 0.05, **P < 0.01, ***P < 0.001.
A total of 24 species susceptible to infection by P. cinnamomi were recorded within the study (Table 4). Edwards Creek Track had the highest mean susceptible species richness at 11.1 per quadrat, Hurst Rd 1 was slightly lower at 8.13 and Hurst Rd 2 the lowest at 6.97 (pooling treatments). The mean number of susceptible species recorded in infested treatments was 9.6 per quadrat, in comparison to 8.4 within post-infested, and 8.2 in uninfested treatments (pooling sites).
| Species | Family | Susceptibility rating | |
|---|---|---|---|
| Acrotriche serrulata | Ericaceae | S | |
| Allocasuarina misera | Casuarinaceae | S | |
| Amperea xiphoclada | Euphorbiaceae | S, FR | |
| Argentipallium obtusifolium | Asteraceae | S | |
| Banksia marginata | Proteaceae | S | |
| Chamaescilla corymbosa | Asphodeliaceae | S, FR | |
| Daviesia brevifolia | Fabaceae | S | |
| Dillwynia glaberrima | Fabaceae | S, HS | |
| Dillwynia sericea | Fabaceae | S, HS | |
| Epacris impressa | Ericaceae | S, LS | |
| Eucalyptus obliqua | Myrtaceae | S, LS | |
| Eucalyptus willisii | Myrtaceae | S | |
| Goodenia lanata | Goodeniaceae | S | |
| Hakea ulicina | Proteaceae | S | |
| Hibbertia fasciculata var. prostrata | Dillenaceae | S | |
| Hibbertia riparia | Dillenaceae | S | |
| Isopogon ceratophyllus | Proteaceae | S | |
| Leptospermum continentale | Myrtaceae | S | |
| Leptospermum myrsinoides | Myrtaceae | S | |
| Leucopogon virgatus | Ericaceae | S | |
| Platylobium obtusangulum | Fabaceae | S | |
| Tetratheca ciliata | Elaeocarpaceae | S | |
| Xanthorrhoea australis | Asphodeliaceae | S, HS | |
| Xanthorrhoea minor subsp. lutea | Asphodeliaceae | S, HS |
HS, highly susceptible; S, susceptible; LS, low susceptibility; FR, field resistant (or tolerant) (McDougall 2005).
Two-factor crossed ANOVA of susceptible species cover indicated no significant difference between sites (d.f. = 2, F = 1.8, P = 0.18), but a significant difference between treatments (d.f. = 2, F = 39.4, P < 0.001). No site × treatment interaction was detected (d.f. = 2, F = 1.1, P = 0.36). Post-infested treatments had the lowest percentage cover of susceptible species (Fig. 4). Pairwise comparisons between treatments and sites detected that susceptible species cover in the uninfested treatments was significantly higher than both infested and post-infested treatments at all sites (Table 5). The only exception was Edwards Creek Track, where the infested treatment was not significantly different to either of the Hurst Rd uninfested sites. Edwards Creek Track infested was also significantly higher than Hurst Rd 2 post-infested (Table 5).
Susceptible species percentage cover between site and treatment classes in Anglesea Heathy Woodlands.
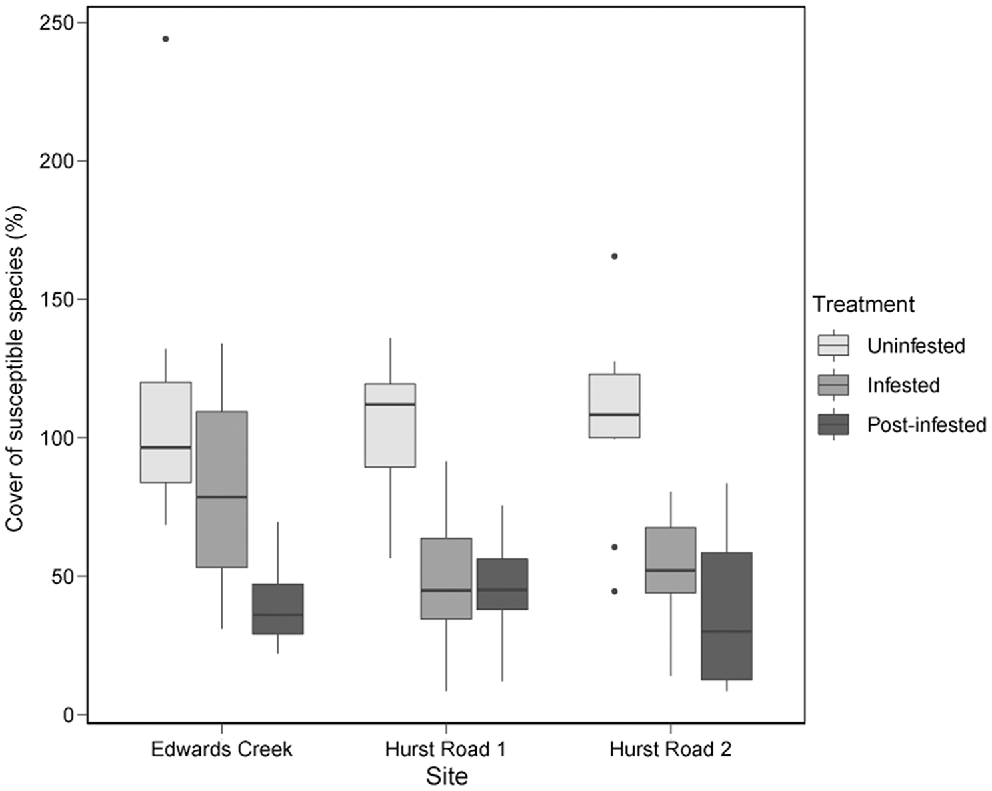
| ECU | ECI | ECP | H1U | H1I | H1P | H2U | H2I | H2P | ||
|---|---|---|---|---|---|---|---|---|---|---|
| ECU | ||||||||||
| ECI | * | |||||||||
| ECP | *** | *** | *** | |||||||
| H1U | ||||||||||
| H1I | *** | ** | ** | |||||||
| H1P | *** | *** | *** | |||||||
| H2U | ||||||||||
| H2I | *** | ** | ** | |||||||
| H2P | *** | *** | *** |
The matrix diagonal represents the site × treatment interaction compared against itself.
EC, Edwards Creek Track; H1, Hurst Road 1; H2, Hurst Road 2; U, uninfested; I, infested; P, post-infested.
*P < 0.05, **P < 0.01, ***P < 0.001.
Xanthorrhoea australis density
At Edwards Creek Track 562 X. australis/X. minor individuals were recorded within the 50 m × 50 m uninfested (control) quadrat, whereas 990 were recorded at Hurst Rd 1 and 964 at Hurst Rd 2. A 10-fold greater mean percentage cover of X. australis was recorded within the uninfested treatment class (43% ± 1.07), in comparison to the infested treatment (4.3% ± 0.70). Mean percentage cover was lowest within post-infested treatments (0.9% ± 0.11), with many quadrats recording zero cover.
All X. australis individuals were mapped and P. cinnamomi infestation status recorded in-field using the Fulcrum data management platform. Overall, large infestation fronts leading to dieback were most evident at Edwards Creek Track. When pooling basal area by site, Edwards Creek Track had a mean basal area of 0.91 m2 per hectare, whereas Hurst Rd 1 and Hurst Rd 2 were almost four times the basal area at 3.52 m2 per hectare and 3.47 m2 per hectare, respectively (Fig. 5). When pooling data by treatment, healthy uninfested communities of X. australis recorded a mean basal area of 4.31 m2 per hectare. Infested vegetation had almost one third lower mean basal area with 2.8 m2 per hectare, and post-infested vegetation had only one fifth the basal area of uninfested sites with 0.81 m2 per hectare. Analysis of basal area by size-class confirmed a lack of recent recruitment with only eight individuals <10 cm DBA recorded in our 10 m × 2 m quadrats, across all sites (Fig. 6). At both Hurst Road sites most individuals were between 10–40 cm DBA (Fig. 6a and b), most likely representing a pulse of recruitment following the Ash Wednesday fire of 1983, whereas at Edwards Creek track there were no individuals between 20 and 90 cm DBA (Fig. 6c).
Mean basal area per hectare of X. australis between treatments over all three sites within the Anglesea Heathy Woodlands. Error bars represent standard deviation (±) from the mean.

Mean number of X. australis individuals in 10 m × 2 m quadrats, in 10 cm basal area size classes, at three sites within the Anglesea Heathy Woodlands (a) Hurst Rd 1, (b) Hurst Rd 2, (c) Edwards Creek Track.

Analysis of variance of X. australis basal area in 10 m × 2 m quadrats detected a significant difference between sites (d.f. = 2, F = 3.36, P < 0.05) and treatment (d.f. = 2, F = 9.76, P < 0.01), with a site × treatment interaction (d.f. = 4, F = 3.1, P < 0.05). Post hoc Tukey’s tests determined that the uninfested treatment at both Hurst Rd sites was significantly higher than uninfested and post-infested at Edwards Creek Track and compared to post-infested at Hurst Rd 1. Hurst Rd 1 uninfested was also significantly higher than Hurst Rd 2 post-infested. Other significant pairwise comparisons were detected (Table 6).
| ECU | ECI | ECP | H1U | H1I | H1P | H2U | H2I | H2P | ||
|---|---|---|---|---|---|---|---|---|---|---|
| ECU | *** | *** | ||||||||
| ECI | *** | |||||||||
| ECP | *** | *** | *** | |||||||
| H1U | ||||||||||
| H1I | * | |||||||||
| H1P | *** | *** | *** | |||||||
| H2U | ||||||||||
| H2I | *** | |||||||||
| H2P | ** | * |
The matrix diagonal represents the site × treatment interaction compared against itself.
EC, Edwards Creek Track; H1, Hurst Road 1; H2, Hurst Road 2; U, uninfested; I, infested; P, post-infested.
Discussion
Research on the detrimental impacts of the plant pathogen Phytophthora cinnamomi has been conducted in the Great Otway National Park since the 1970s (Wilson 1990; Wilson et al. 1990, 1997, 2020; Laidlaw and Wilson 2003). One focus has been the disease impact on the keystone species Xanthorrhoea australis (Aberton et al. 2001; Daniel et al. 2005; Laidlaw and Wilson 2006; Cahill et al. 2008) but most of our knowledge on floristic change in the Anglesea Heathy Woodlands comes from relatively few sites.
This research assessed impacts of P. cinnamomi at three different sites across the Heathy Woodlands. A range of susceptible species subject to density declines and extirpation have been identified, and species composition of uninfested vegetation was significantly different to infested and post-infested vegetation with three susceptible species (X. australis, E. obliqua, B. marginata) being more abundant in uninfested areas. Post-infested vegetation had the lowest percentage cover of susceptible species. The mean percentage cover of the keystone species X. australis in uninfested vegetation was 10-fold greater than infested, with post-infested vegetation extremely low. There was also a significant difference in mean basal area per hectare: uninfested (4.31 m2), infested (2.8 m2) and post-infested (0.81 m2).
This research will be used to inform targeted phosphite application by land managers to enhance the management of Phytophthora cinnamomi and improve Australian biodiversity conservation.
Vegetation floristics
Site and Phytophthora cinnamomi infestation status were shown to influence floristic composition within the Anglesea Heathy Woodlands. At Site 1 (Edwards Creek Track) P. cinnamomi infestation was clearly linear with the track boundary progressing downslope through the landscape, characteristic as zoospores become extremely motile with increasing soil moisture/porosity and a decreasing gradient (Wilson et al. 2000, 2020; Laidlaw and Wilson 2003). Signs of previous vehicle entry were found surrounding the infestation treatment, potentially linking the spread of P. cinnamomi to fire management vehicles (Lewis and Colquhoun 2000). Species contributing most to within site similarity included four susceptible tree/shrub species and four sedge/rush species (Supplementary Table S1). Previous studies in the Grampians (Victoria), Kinglake (Victoria) and eastern Otways have detected a similar shift to sedge, rush and grass life forms following P. cinnamomi invasion (Weste et al. 2002; Barrett and Rathbone 2018; Wilson et al. 2020). Recreational activity including motorbike/mountain-bike riding was a regular occurrence at Edwards Creek Track and likely explains the increasing shift towards sedge dominance at post-infested sites.
Access to Site 2 (Hurst Rd 1) was from a management vehicle track adjacent to Hurst Rd. Infestation was most likely linked to both the management vehicle track and recently implemented mountain bike trails through the landscape (Wilson and Garkaklis 2019b; Wilson et al. 2020). A major contributor to increasing P. cinnamomi at Hurst Rd 1 is the north-western aspect of the site, as warmer temperatures may increase favourable conditions for disease spread (Wilson et al. 2000; Laidlaw and Wilson 2003). Within site compositional similarity was largely due to the dominance of X. australis, E. obliqua and Spyridium parvifolium, with only one sedge species, G. radula, dominating (Table S2). Disease impact is yet to reach the severity indicated at Edwards Creek Track.
The third site (Hurst Rd 2) was located on a southern facing slope. The large infestation fronts recorded at this site correlate with the lowest species richness of all sites monitored within the study. Three sedge/rush species contributed to within site similarity (Table S1). The site has not burnt since 1983 and consequently, understorey species may not have received appropriate fire-stimulated germination cues. Lower species richness may reflect vegetation at a late successional stage (Wills and Read 2002; Freestone et al. 2015) and an interaction between the effects of burn regime and disease status.
The absence of susceptible species at infested and post-infested treatments resulted in greater within-treatment similarity than for uninfested treatments, and in greater dissimilarity between uninfested compared to both infested and post-infested treatments. These results highlight that susceptible species are being lost as Phytophthora spreads and that they are not recruiting post-infestation.
In 1997, a 24-year study in the Grampians, Victoria, recorded regeneration of 30 susceptible species within previously infested areas, due to a potential decline in pathogen potential (Weste et al. 2002). This was associated with lower mean rainfall, which likely decreased zoospore production, and prescribed burning, which may have initiated obligate seeding and resprouting (Weste et al. 2002). In the current study, rainfall in 2020–2021 was ~100 mm above the average of 620.4 mm for the region, potentially contributing to a lack of susceptible species recruitment under enhanced conditions for disease spread (Bureau of Meteorology 2021). It is recommended that sites be monitored for disease spread as increased yearly rainfall during La Niña weather patterns, combined with a warming climate, will lead to increased disease spread without effective management solutions.
The species with the single greatest contribution to differences between uninfested and either infested or post-infested treatments was Xanthorrhoea australis, clearly showing that subsequent to P. cinnamomi infestation X. australis density is not returning to pre-infestation densities at post-infested sites.
Species richness was lowest in uninfested treatments and highest in post-infested treatments. These results do not align with other studies, as research in the Grampians, Victoria (Weste et al. 2002), kwongan heath, Western Australia (Barrett and Rathbone 2018) and banksia woodlands, Western Australia (Shearer and Dillon 1995, 1996), all demonstrate that species richness is negatively correlated with P. cinnamomi infestation status. Lower species richness at Hurst Road uninfested sites was associated with very high cover by X. australis, and a lack of fire at these sites since 1983 (compared to Edwards Creek track, which had a planned burn in 2012). Species richness in heathy systems declines with time since fire (Wills and Read 2002; Freestone et al. 2015). Hence, a lack of space for recruitment and a lack of recruitment triggers explain the patterns in richness observed in our study.
Furthermore, although species richness was lower within the uninfested treatment class, percentage cover of susceptible species such as X. australis, E. obliqua, B. marginata, and L. myrsinoides was highest. There was a greater percentage cover within post-infested sites of sedges/lilies/grasses (SLGs), that are not impacted by P. cinnamomi infestation (Wilson et al. 2020). Examples of SLGs identified within the study include Gahnia radula, Isolepis marginata, Lepidosperma filiforme, Lepidosperma semiteres and Lomandra filiformis. Previous studies in the eastern Otways demonstrated that SLGs contributed ~10% of all plant species in 1989 and by 2015 this had increased to ~20%, as a direct result of disease spread (Dawson et al. 1985; Weste and Kennedy 1997; Wilson et al. 2020). Our study confirms the shift to SLG dominated understoreys as a result of disease spread.
The effect of disease on Xanthorrhoea australis density
Site and Phytophthora cinnamomi infestation status was shown to significantly influence X. australis density within the Anglesea Heathy Woodlands. Uninfested treatments within the study recorded a mean X. australis basal area five times higher than post-infested treatments and post-infested treatments are not showing signs of X. australis regeneration. The size-class distribution of data strongly indicates a pulse of recruitment following the fires of 1983 and that this cohort has persisted better at uninfested than infested sites. Research in the Brisbane Ranges (Dawson et al. 1985; Weste 2003), NSW (McDougall and Summerell 2003) and Wilson’s Promontory (Bluett et al. 2003), has indicated regeneration of X. australis from the seed bank is possible with appropriate fire cues under decreased rainfall and lower temperatures. At the time of this study, La Niña conditions resulted in higher rainfall. Even if appropriate fire cues occurred, it is likely that most X. australis seedlings would succumb to reinfestation and die, given the more optimal conditions for disease spread (Bluett et al. 2003; McDougall and Summerell 2003; Wilson et al. 2020).
When comparing X. australis basal area by site the Hurst Rd sites recorded almost four times the density of Edwards Creek Track. Recreational activity such as mountain bike/motor-bike riding is considered to be a major contributor to density declines at Edwards Creek Track, and the recent implementation of mountain biking trails at Hurst Rd 1 is predicted to result in similar declines of X. australis density.
Two large patches of uninfested X. australis individuals were found at Hurst Rd 2 yet ~100 metres north, a vast infestation front was recorded. The disease was moving south-west downslope, with hundreds of X. australis individuals displaying chlorotic symptoms. It is highly recommended that application of the biodegradable fungicide phosphite (Havlin and Schlegel 2021) be implemented by land managers to prevent further spread of the pathogen, protect uninfested grasstrees and provide support for resistance to the pathogen to lightly infested grasstrees.
Implications for biodiversity and management
Loss of grasstrees results in major structural change of vegetation within many landscapes (Spencer et al. 2005; Frazer and Petit 2007; Swinburn et al. 2007; Wilson et al. 2020). Species such as X. australis represent the ‘old-growth’ mature and waning stands in heathy woodland vegetation (Aberton et al. 2001; Cheal 2010; Wilson et al. 2020). Previous studies have identified the significance of grasstree stumps and canopies for nesting, refuge and shelter for a range of native mammal species including the agile antechinus (Antechinus agilis), eastern pygmy possum (Cercartetus nanus), white-footed dunnart (Sminthopsis leucopus), bush rat (Rattus fuscipes), southern brown bandicoot (Isoodon obesulus obesulus), mardo (Antechinus flavipes leucogaster), ash-grey mouse (Pseudomys albocinereus) and the New Holland mouse (Pseudomys novaehollandiae) (Paull 1993; Laidlaw et al. 1996; Laidlaw and Wilson 2003, 2006; Spencer et al. 2005; Frazer and Petit 2007; Swinburn et al. 2007; Annett 2008; Lazenby et al. 2007; Haby et al. 2013; Smith et al. 2018). Protecting grasstrees and limiting the spread of P. cinnamomi is critical not only for the protection of floral diversity but also for fauna species and the habitat they depend on.
Currently, few techniques have been identified for the containment and/or eradication of Phytophthora cinnamomi from sites. However, treatments including host removal, herbicide application, fungicide application, soil fumigation and physical root barriers have been demonstrated to eliminate the pathogen to depths of 2 m from up to 18 months to 8 years after treatment, some in forest sites and National Parks (Dunne et al. 2011; Dunstan et al 2010, 2020; Crone et al 2014). A fallow approach together with exposure to high summer soil temperatures in a mine site has also been found to eradicate the pathogen within 12 months on haul roads and result in declines in recovery from bunds and stockpiles but not as rapidly or to the same degree (Dunstan et al 2010, 2020). Although these studies demonstrate the potential to eradicate this pathogen, in many cases the costs and risks would be considered prohibitive.
As a result, management options are often limited to reducing the potential for disease to spread into uninfested regions through hygiene procedures, and to reduce the impacts of current infestation through application of phosphite (Shearer and Tippett 1989; Hardy et al. 2001; Commonwealth of Australia 2018a).
Previous research has shown that X. australis seedlings treated with potassium phosphonate (phosphite), display more intense cellular responses to challenge pathogens (Daniel et al. 2005). Post-inoculation, the phloem-translocated phosphite ion limits intracellular hyphal growth of P. cinnamomi. The chemical initiates retraction of the cell membrane from the cell wall and the accumulation of electron dense substances around the cell wall of infected cells (Daniel et al. 2005). Successful phosphite treatment within the eastern Otways has been previously undertaken (Aberton et al. 1999; Laidlaw and Wilson 2003; Wilson et al. 2020). Recent targeted applications by hand and aerially across the Otways landscape by researchers and land managers promises to control the disease progress and protect the highly significant grass trees X. australis (Garkaklis et al. 2022; Garkaklis and Wilson 2023; Wilson and Garkaklis 2023a, 2023b).
Conclusion
This study has assessed significant infestations fronts within the Anglesea Heathy Woodlands. Floristic surveys indicated a range of susceptible species subject to density declines and extirpation, as a direct result of P. cinnamomi infestation. Research was focused on the density of the key indicator species Xanthorrhoea australis. Previous research revealed its significant role within the landscape in providing refuge for many small mammal species within the Anglesea Heathy Woodlands and an integral relationship between the species and overall ecosystem health and function. Edwards Creek Track has recorded a shift to vegetation dominated by SLGs, correlating with increasing recreational activities facilitating disease spread. Newly created mountain bike trails at Hurst Rd sites will spread the disease through the landscape and a similar shift in vegetation life forms is predicted without appropriate management.
It is now critical that land managers deploy strategic phosphite application at the recorded infestation fronts, to reduce disease extension and impact on species assemblages. Following phosphite treatment, increased signage and wash down stations would assist land managers in controlling the disease front at Hurst Rd sites. This study has shown that susceptible species at post-infested sites are not recovering following P. cinnamomi infestation and therefore may require further aided restoration.
Data availability
The data that support this study are available in the article and accompanying online supplementary material.
Declaration of funding
This project was supported by Corangamite Catchment Management Authority, through funding from the Australian Government’s Environmental Restoration Fund.
Acknowledgements
The authors thank colleagues who assisted in fieldwork and data analyses and for their guidance and knowledge; in particular, Mitchell Johnston, Emma Sumner, Jeronimo Ramirez and Assoc. Prof. Susanna Venn. Thanks to Hamish Martin (Department of Environment, Land, Water and Planning) for consultation with site planning. The research was conducted under Department of Environment, Land, Water and Planning Permit no.: 10009996.
References
Aberton MJ, Wilson BA, Cahill DM (1999) The use of potassium phosphonate to control Phytophthora cinnamomi in native vegetation at Anglesea, Victoria. Australasian Plant Pathology 28, 225-234.
| Crossref | Google Scholar |
Aberton MJ, Wilson BA, Hill J, Cahill DM (2001) Development of disease caused by Phytophthora cinnamomi in mature Xanthorrhoea australis. Australian Journal of Botany 49, 209-219.
| Crossref | Google Scholar |
Barrett S, Rathbone D (2018) Long-term phosphite application maintains species assemblages, richness and structure of plant communities invaded by Phytophthora cinnamomi. Austral Ecology 43, 360-374.
| Crossref | Google Scholar |
Bluett V, Weste G, Cahill D (2003) Distribution of disease caused by Phytophthora cinnamomi in Wilsons Promontory National Park and potential for further impact. Australasian Plant Pathology 32, 479-491.
| Crossref | Google Scholar |
Borsboom AC (2005) ‘Xanthorrhoea: a review of current knowledge with a focus on X. johnsonii and X. latifolia, two Queensland protected plants-in-trade.’ pp. 1–88. (Environmental Protection Agency: Qld, Australia). doi:10.13140/RG.2.1.1598.7043
Bureau of Meteorology (2021) Climate statistics for Australian locations. Available at http://www.bom.gov.au/climate/data/?ref=ftr
Burgess TI, Scott JK, McDougall KL, Stukely MJ, Crane C, Dunstan WA, Brigg F, Andjic V, White D, Rudman T, Arentz F, Ota N, Hardy GESJ (2017) Current and projected global distribution of Phytophthora cinnamomi, one of the world’s worst plant pathogens. Global Change Biology 23, 1661-1674.
| Crossref | Google Scholar | PubMed |
Burgess TI, López-Villamor A, Paap T, Williams B, Belhaj R, Crone M, Dunstan W, Howard K, Hardy GESJ (2021) Towards a best practice methodology for the detection of Phytophthora species in soils. Plant Pathology 70(3), 604-614.
| Crossref | Google Scholar |
Cahill D, Weste G (1983) Changes in respiration of seedling roots inoculated with Phytophthora cinnamomi. Journal of Phytopathology 106, 51-62.
| Crossref | Google Scholar |
Cahill D, Weste G, Grant B (1985) Leakage from seedling roots inoculated with Phytophthora cinnamomi. Journal of Phytopathology 114, 348-364.
| Crossref | Google Scholar |
Cahill D, Legge N, Grant B, Weste G (1989) Cellular and histological changes induced by Phytophthora cinnamomi in a group of plant species ranging from fully susceptible to fully resistant. Phytopathology 79, 417-424.
| Crossref | Google Scholar |
Cahill DM, Rookes JE, Wilson BA, Gibson L, McDougall KL (2008) Phytophthora cinnamomi and Australia’s biodiversity: impacts, predictions and progress towards control. Australian Journal of Botany 56, 279-310.
| Crossref | Google Scholar |
Castello JD, Leopold DJ, Smallidge PJ (1995) Pathogens, patterns, and processes in forest ecosystems. BioScience 45, 16-24.
| Crossref | Google Scholar |
Clark KR, Gorley RN (2015) PRIMER v7: User Manual/Tutorial Plymouth Routines in Multivariate Ecological Research. Available at https://www.primer-e.com/download/
Commonwealth of Australia (2018a) Threat abatement plan for disease in natural ecosystems caused by Phytophthora cinnamomi. (Department of Agriculture, Water and the Environment) Available at http://environment.gov.au/biodiversity/threatened/publications/threat-abatement-plan-disease-natural-ecosystems-caused-phytophthora- cinnamomi-2018 [Verified 6 June 2021]
Commonwealth of Australia (2018b) Background: threat abatement plan for disease in natural ecosystems caused by Phytophthora cinnamomi. (Department of Agriculture, Water and the Environment) Available at http://environment.gov.au/biodiversity/threatened/publications/threat-abatement-plan-disease-natural-ecosystems-caused-phytophthora- cinnamomi-2018 [Verified 6 June 2021]
Crone M, McComb JA, O’Brien PA, Hardy GEST (2014) Host removal as a potential control method for Phytophthora cinnamomi on severely impacted black gravel sites in the jarrah forest. Pathology 44, 154-159.
| Crossref | Google Scholar |
Daniel R, Wilson BA, Cahill DM (2005) Potassium phosphonate alters the defence response of Xanthorrhoea australis following infection by Phytophthora cinnamomi. Australasian Plant Pathology 34, 541-548.
| Crossref | Google Scholar |
Dawson P, Weste G (1984) Impact of root infection by Phytophthora cinnamomi on the water relations of two Eucalyptus species that differ in susceptibility. Phytopathology 74, 486-490.
| Crossref | Google Scholar |
Dawson P, Weste G, Ashton D (1985) Regeneration of vegetation in the Brisbane Ranges after fire and infestation by Phytophthora cinnamomi. Australian Journal of Botany 33, 15-26.
| Crossref | Google Scholar |
Dunne CP, Crane CE, Biddulph D, Lee M, Young G, Massenbauer T, Barrett S, Comer S, Freebury GJC, Utber DJ, Grant MJ, Shearer BL (2011) A review of the catchment approach techniques used to manage Phytophthora cinnamomi infestation of native plant communities of the Fitzgerald River National Park on the south coast of Western Australia. New Zealand Journal of Forestry Science 41, S121-S132.
| Google Scholar |
Dunstan WA, Rudman T, Shearer BL, Moore NA, Paap T, Calver MC, Dell B, Hardy GESJ (2010) Containment and spot eradication of a highly destructive, invasive pathogen (Phytophthora cinnamomi) in natural ecosystems. Biological Invasions 12, 913-925.
| Crossref | Google Scholar |
Dunstan WA, Howard K, Grigg A, Shaw C, Burgess TI, Hardy GESJ (2020) Towards eradication of Phytophthora cinnamomi using a fallow approach in a mediterranean climate. Forests 11, 1101.
| Crossref | Google Scholar |
Frazer DS, Petit S (2007) Use of Xanthorrhoea semiplana (grass-trees) for refuge by Rattus fuscipes (southern bush rat). Wildlife Research 34, 379-386.
| Crossref | Google Scholar |
Freestone M, Wills TJ, Read J (2015) Post-fire succession during the long-term absence of fire in coastal heathland and a test of the chronosequence survey method. Australian Journal of Botany 63, 572-580.
| Crossref | Google Scholar |
Garkaklis MJ, Calver MC, Wilson BA, Hardy GESJ (2004) Habitat alteration caused by an introduced plant disease, Phytophthora cinnamomi: a potential threat to the conservation of Australian forest fauna. In ‘Conservation of Australia’s Forest Fauna’. (Ed. D Lunney) pp. 899–913. (Royal Zoological Society of New South Wales) doi:10.7882/fs.2004.899
Garkaklis MJ, Learmonth C, Wilson BA (2022) Wild Otways Initiative – Project 3: Protecting Plant and Animal biodiversity in the Otway Ranges, Bells Beach (Ironbark Basin) and Great Ocean Road hinterland from Phytophthora dieback - Trial Phosphite spray and phytotoxicity survey at Eumeralla and Peregrine Track, Eastern Otways. Unpublished Report to the Corangamite Catchment Management Authority, Victoria.
Haby NA, Conran JG, Carthew SM (2013) Microhabitat and vegetation structure preference: an example using southern brown bandicoots (Isoodon obesulus obesulus). Journal of Mammalogy 94, 801-812.
| Crossref | Google Scholar |
Hansen EM (2008) Alien forest pathogens: Pytophthora species are changing world forests. Boreal Environment Research 13, 33-41.
| Google Scholar |
Hardy GESJ, Barrett S, Shearer BL (2001) The future of phosphite as a fungicide to control the soilborne plant pathogen Phytophthora cinnamomi in natural ecosystems. Australasian Plant Pathology 30, 133-139.
| Crossref | Google Scholar |
Havlin JL, Schlegel AJ (2021) Review of phosphite as a plant nutrient and fungicide. Soil Systems 5, 52.
| Crossref | Google Scholar |
Laidlaw WS, Wilson BA (2003) Floristic and structural characteristics of a coastal heathland exhibiting symptoms of Phytophthora cinnamomi infestation in the eastern Otway Ranges, Victoria. Australian Journal of Botany 51, 283-293.
| Crossref | Google Scholar |
Laidlow WS, Wilson BA (1989) Distribution and habitat preferences of small mammals in the eastern section of the Angahook-Lorne State Park. Victorian Naturalist 106, 224-236.
| Google Scholar |
Laidlaw WS, Wilson BA (2006) Habitat utilisation by small mammals in a coastal heathland exhibiting symptoms of Phytophthora cinnamomi infestation. Wildlife Research 33, 639-649.
| Crossref | Google Scholar |
Laidlaw WS, Hutchings S, Newell GR (1996) Home range and movement patterns of Sminthopsis leucopus (Marsupialia: Dasyuridae) in coastal dry heathland, Anglesea, Victoria. Australian Mammalogy 19, 1-9.
| Crossref | Google Scholar |
Lamont BB, Wittkuhn R, Korczynskyj D (2004) Ecology and ecophysiology of grasstrees. Australian Journal of Botany 52(5), 561-582.
| Crossref | Google Scholar |
Lazenby BT, Pye T, Richardson A, Bryant SA (2007) Towards a habitat model for the New Holland mouse Pseudomys novaehollandiae in Tasmania – population vegetation associations and an investigation into individual habitat use. Australian Mammalogy 29, 137-148.
| Crossref | Google Scholar |
Lenth RV (2022) emmeans: Estimated Marginal Means, aka Least-Squares Means. Available at https://github.com/rvlenth/emmeans
Marchesan D, Carthew SM (2004) Autecology of the yellow-footed antechinus (Antechinus flavipes) in a fragmented landscape in southern Australia. Wildlife Research 31, 273-282.
| Crossref | Google Scholar |
McDougall KL (2005) The responses of native Australian plant species to Phytophthora cinnamomi. Appendix 4. In ‘Management of Phytophthora cinnamomi for Biodiversity Conservation in Australia: Part 2. National Best Practice’. (Eds E O’Gara, K Howard, B Wilson, GESJ Hardy) pp. 1–52. (Department of the Environment and Heritage: Canberra, ACT, Australia)
McDougall KL, Summerell BA (2003) The impact of Phytophthora cinnamomi on the flora and vegetation of New South Wales – a re-appraisal. In ‘Phytophthora in Forests and Natural Ecosystems, 2nd International IUFRO Working Party Meeting’, October 2003, Albany, WA, Australia. (Eds JA McComb, GS Hardy, I Tommerup) pp. 49–56. (Murdoch University: Perth, WA, Australia)
Podger FD, Doepel RF, Zentmyer GA (1965) Association of Phytophthora cinnamomi with a disease of Eucalyptus marginata forest in Western Australia. Plant Disease Reporter 49, 943-947.
| Google Scholar |
R Core Team (2021) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Available at http://R-project.org [Verified 22 December 2021]
Shearer BL, Dillon M (1995) Susceptibility of plant species in Eucalyptus marginata forest to infection by Phytophthora cinnamomi. Australian Journal of Botany 43, 113-134.
| Crossref | Google Scholar |
Shearer BL, Dillon M (1996) Susceptibility of plant species in Banksia woodland on the Swan Coastal Plain, Western Australia, to infection by Phytophthora cinnamomi. Australian Journal of Botany 44, 433-445.
| Google Scholar |
Smith KJ, Fleming PA, Kreplins TL, Wilson BA (2018) Population monitoring and habitat utilisation of the ash-grey mouse (Pseudomys albocinereus) in Western Australia. Australian Mammalogy 41(2), 170-178.
| Crossref | Google Scholar |
Spencer R-J, Cavanough VC, Baxter GS, Kennedy MS (2005) Adult free zones in small mammal populations: response of Australian native rodents to reduced cover. Austral Ecology 30, 868-876.
| Crossref | Google Scholar |
Swinburn ML, Fleming PA, Craig MD, Grigg AH, Garkaklis MJ, Hobbs RJ, Hardy GESJ (2007) The importance of grasstrees (Xanthorrhoea preissii) as habitat for mardo (Antechinus flavipes leucogaster) during post-fire recovery. Wildlife Research 34, 640-651.
| Crossref | Google Scholar |
Vernes K, Pope LC (2001) Fecundity, pouch young survivorship and breeding season of the northern bettong (Bettongia tropica) in the wild. Australian Mammalogy 23(2), 95-100.
| Crossref | Google Scholar |
Victorian Government Department of Sustainability and Environment (2004) Otway Plain bioregion Ecological Vegetation Class bioregion benchmark. Available at https://www.environment.vic.gov.au/biodiversity/bioregions-and-evc-benchmarks
Wark MC, White MD, Robertson DJ, Marriott PF (1987) Regeneration of heath and heath woodland in the north-eastern Otway Ranges, Australia, following the wildfire of February 1983. Proceedings of the Royal Society of Victoria 99, 51-88.
| Google Scholar |
Weste G (1974) Phytophthora cinnamomi – the cause of severe disease in certain native communities in Victoria. Australian Journal of Botany 22, 1-8.
| Crossref | Google Scholar |
Weste G (2003) The dieback cycle in Victorian forests: a 30-year study of changes caused by Phytophthora cinnamomi in Victorian open forests, woodlands and heathlands. Australasian Plant Pathology 32, 247-256.
| Crossref | Google Scholar |
Weste G, Kennedy J (1997) Regeneration of susceptible native species following a decline of Phytophthora cinnamomi over a period of 20 years on defined plots in the Grampians, Western Victoria. Australian Journal of Botany 45, 167-190.
| Crossref | Google Scholar |
Weste G, Walchhuetter T, Walshe T (1999) Regeneration of Xanthorrhoea australis following epidemic disease due to Phytophthora cinnamomi in the Brisbane Ranges, Victoria. Australasian Plant Pathology 28, 162-169.
| Crossref | Google Scholar |
Weste G, Brown K, Kennedy J, Walshe T (2002) Phytophthora cinnamomi infestation – a 24-year study of vegetation change in forests and woodlands of the Grampians, Western Victoria. Australian Journal of Botany 50, 247-274.
| Crossref | Google Scholar |
Wills TJ, Read J (2002) Effects of heat and smoke on germination of soil-stored seed in south-eastern Australian sand heathland. Australian Journal of Botany 50, 197-206.
| Crossref | Google Scholar |
Wilson BA, Garkaklis MJ (2023a) Wild Otways Initiative – Project 3: Protecting Plant and Animal biodiversity in the Otway Ranges, Bells Beach (Ironbark Basin) and Great Ocean Road hinterland from Phytophthora dieback – Threat Abatement Plan (TAP) for Phytophthora dieback in the Otway Ranges, Victoria. Unpublished TAP to the Corangamite Catchment Management Authority, Victoria.
Wilson BA, Garkaklis MJ (2023b) Two-year Report – Protecting plant and animal diversity in the Otway Ranges, Bells Beach (Ironbark Basin) and Great Ocean Road hinterland from Phytophthora cinnamomi dieback; Phosphite treatment of high-risk Phytophthora infestation points. Unpublished Report to the Corangamite Catchment Management Authority, Victoria.
Wilson BA, Robertson D, Moloney DJ, Newell GR, Laidlaw WS (1990) Factors affecting small mammal distribution and abundance in the eastern Otway Ranges, Victoria. In ‘Australian ecosystems: 200 years of utilization, degradation and reconstruction. Proceedings of the Ecological Society of Australia. Vol. 16’. (Eds DA Saunders, AJM Hopkins, RA How) pp. 379–396. (Surrey Beatty: Sydney, NSW, Australia)
Wilson BA, Newell G, Laidlaw WS, Friend GR (1994) Impact of plant diseases on faunal communities. Journal of the Royal Society of Western Australia 77, 139-143.
| Google Scholar |
Wilson BA, Aberton J, Cahill DM (2000) Relationships between site factors and distribution of Phytophthora cinnamomi in the Eastern Otway Ranges, Victoria. Australian Journal of Botany 48, 247-260.
| Crossref | Google Scholar |
Wilson BA, Lewis A, Aberton J (2003) Spatial model for predicting the presence of cinnamon fungus (Phytophthora cinnamomi) in sclerophyll vegetation communities in southeastern Australia. Austral Ecology 28, 108-115.
| Crossref | Google Scholar |
Wilson BA, Kinloch J, Sonneman T, Swinburn M (2009) Distribution and impacts of Phytophthora cinnamomi. In ‘Biodiversity Values and Threatening Processes of the Gnangara Groundwater System’. (Eds BA Wilson, LE Valentine) A report to the Gnangara Sustainability Strategy and the Department of Environment and Conservation pp. 1–56. (Department of Environment and Conservation, Perth, WA, Australia)
Wilson BA, Annett K, Laidlaw WS, Cahill DM, Garkaklis MJ, Zhuang-Griffin L (2020) Long term impacts of Phytophthora cinnamomi infestation on heathy woodland in the Great Otway National Park in south-eastern Australia. Australian Journal of Botany 68, 542-556.
| Crossref | Google Scholar |