A review of the classification and taxonomic and geographic distribution of cleistogamy in Australian grasses
E. J. Thompson A *
A *
A Queensland Herbarium, Department of Environment and Science, Brisbane Botanic Gardens, Mt Coot-tha Road, Toowong, Qld 4066, Australia.
Australian Journal of Botany 70(1) 63-101 https://doi.org/10.1071/BT20114
Submitted: 3 September 2020 Accepted: 15 August 2021 Published: 22 December 2021
© 2022 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Cleistogamy, self-fertilisation within a closed flower, was found in 135 Australian grass species from 46 genera within 5 subfamilies representing 14% of the species and 30% of the genera. This represents an increase from 4% of species and 12% of genera from previous records. Expressions of cleistogamy were classified into three main categories on the basis of: presence or absence of anther dimorphism, presence of amphigamy with or without spikelet peculiarities, and chasmogamous and cleistogamous spikelets on separate plants. One category of these dimorphisms involves species that have differing terminal and axillary inflorescences (amphigamy) with corresponding spikelets so different that the axillary ones appear to belong to a different genus. Dimorphisms within cleistogamous species were found in inflorescences, spikelets, florets, anthers and caryopses. The highest concentration of Australian cleistogamous grasses occurs in the subtropical climatic zone and more than three-quarters of the species are chloridoid and panicoid with nearly equal proportions. Of Australian cleistogamous grasses, 33% have C3 photosynthetic pathway and 67% have C4, and the largest taxonomic groups are panicoid with 38% and chloridoid with 39%.
Keywords: amphigamy, cleistogamy, dimorphic spikelets, grasses, plant reproduction, Poaceae, reproductive dimorphism.
Introduction
Grasses occupy a diverse range of plant communities, from grasslands to rainforests, throughout the world where they have varying significance in their contribution to ecosystem dynamics, physiognomic structure, floristic diversity, biomass, conservation and economic values, and they play important roles in managing fire and grazing. Management of grassy ecosystems requires understanding of grass biology that includes their breeding systems. Grasses display a broad spectrum of breeding systems, inflorescence types and physiology (Campbell et al. 1983; Quiroga et al. 2010; Christin et al. 2012; Reinheimer et al. 2013a, 2013b). Breeding systems in grasses include asexual reproduction, both vegetative and by seeds (apomixis), and types of pollination ranging from outcrossing with self-incompatibility to self-compatible (Connor 1979; Richards 1990; Gibson 2009). Breeding systems influence both genetic structure and genetic diversity of grasses, with self-pollinating species having more genetic divergence than mixed-mating or outcrossing species (Godt and Hamrick 1998).
The predominant breeding system in grasses involves hermaphrodite florets that open briefly, mostly by the action of lodicules, to release the anthers followed by the emergence of stigmas permitting cross-pollination and thus gene flow, chasmogamy (CH; Connor 1979; Clayton 1990; Richards 1990; Chapman 1996; Groves and Whalley 2002; Gibson 2009; Kirchoff and Claßen-Bockhoff 2013; Reinheimer et al. 2013b). Autogamy is common in grasses where fertilisation results from pollen transfer from other spikelets on the same plant, from the same spikelet, and self-pollinating before the flower opens (preanthesis cleistogamy; bud pollination) (Stebbins 1957; Ornduff 1969; Frankel and Galun 1977; Connor 1979; Lord 1981; Cheplick and Quinn 1986; Lloyd and Schoen 1992; Whalley et al. 2013). A further component in the self-pollination spectrum is a system where the flower remains closed but produces viable seeds, cleistogamy (CL) (Darwin 1877; Uphof 1938; Campbell et al. 1983).
Self-pollination is viewed as an evolutionary adaptation to environmental influences (Lord 1981; Lloyd and Schoen 1992; Quiroga et al. 2010; Sicard and Lenhard 2011; Reinheimer et al. 2013a, 2013b). Furthermore, the presence, abundance and, in some cases, the nature of the expression of CL, are affected by ecological, morphological and physiological factors (Lindauer and Quinn 1972; Clay 1982; Campbell et al. 1983; Clay and Antonovics 1985; Philipson 1986). CL has been reported as a response to various environmental influences, including herbivory, crowding, soil moisture and fertility, temperature, daylength and light intensity (Uphof 1938; Lord 1981; Campbell et al. 1983; De Jong and Klinkhamer 2005; Culley and Klooster 2007; Gibson 2009). Nevertheless, both facultative and obligate forms of CL are genetically controlled, although sometimes there can be incidental lodicule abortion resulting in failure of the floret to open (Frankel and Galun 1977; Campbell et al. 1983; Clayton 1990; Groves and Whalley 2002).
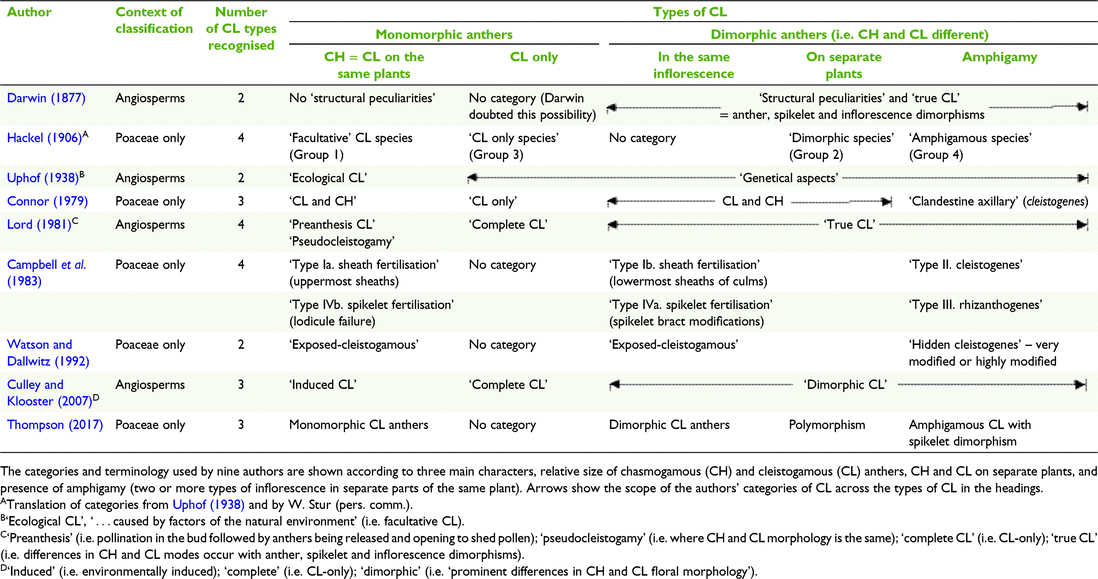
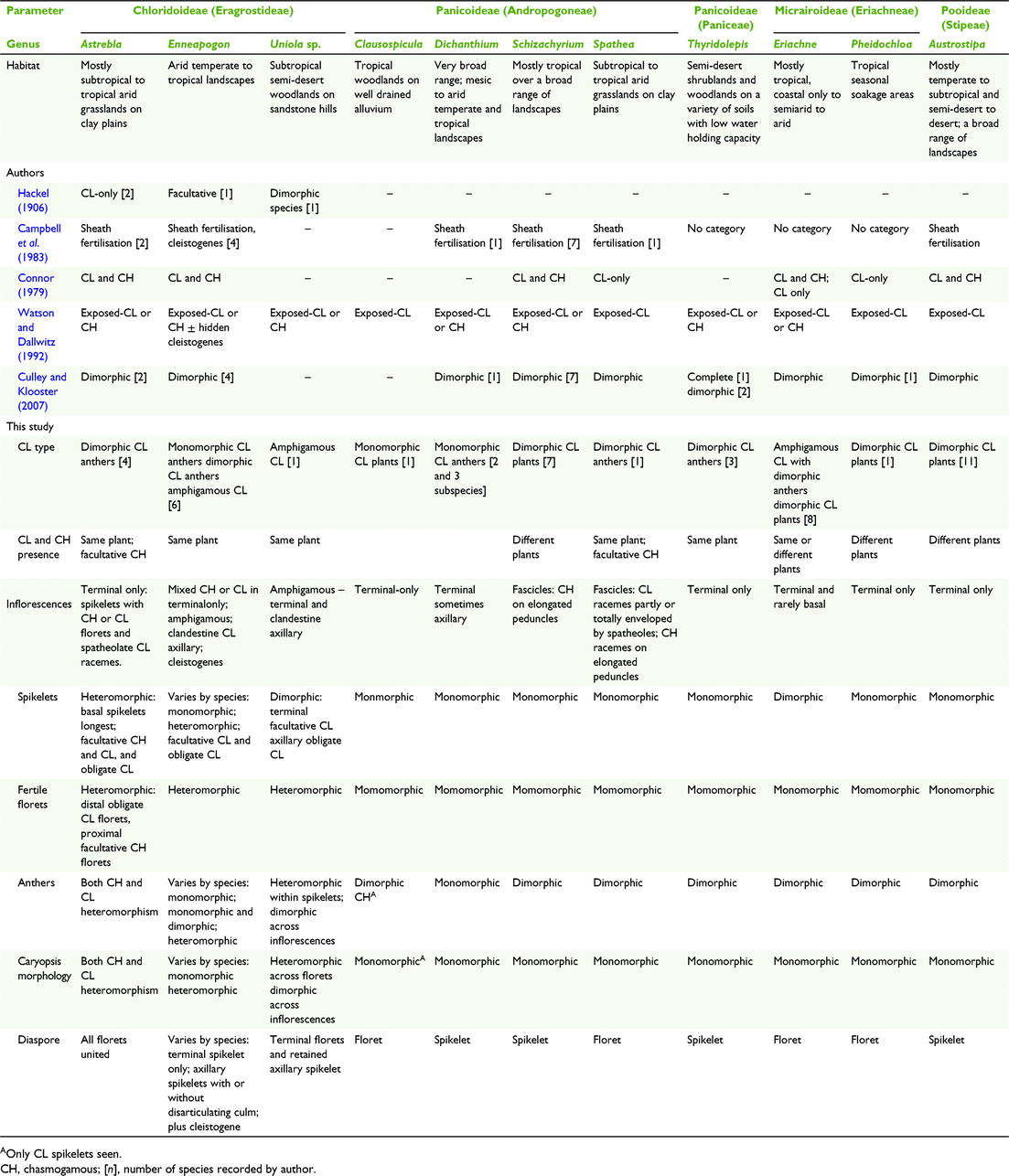
A study of the history of the classification of CL for this paper elucidated a range of terminology and criteria used to define categories of CL, with some of the work on Poaceae imbedded in the broader context of studies of angiosperm CL (Table 1). The categories used by authors including Darwin (1877), Uphof (1938), Lord (1981) and Culley and Klooster (2007) to classify CL in angiosperms when applied to grass CL are informative, despite the broader scope of taxa and morphology. Also, Connor (1979) provided categories of CL in a study of the broad spectrum of breeding systems in grasses. From this background research, it was considered for the current study that most of the diversity in grass CL is captured in the categories used by Hackel (1906) and Campbell et al. (1983) (Table 1).
|
The predominant expression of CL is in the terminal inflorescences on the same plant as interspersed CL and CH spikelets that have similar overall morphology, except in some species the anthers are the same (monomorphic) and in others they are different (dimorphic) (Campbell et al. 1983; Freckmann and Lelong 2003; Culley and Klooster 2007; Morrone et al. 2008). However, CL dimorphism extends beyond anther dimorphism and Darwin (1877) referred to it as ‘structural peculiarities’ in what he perceived to be ‘true cleistogamy’ (Table 1). Connor (1998) considered that the ultimate grass CL syndrome can be expressed in the structural peculiarities of six characters relating to the CL spikelets, rarely all coinciding. The structural peculiarities include the following:
-
Lodicules (absence or reduced size),
-
Anthers and stigma–styles (reduction in size or number),
-
Pollen germination through the anther walls,
-
Floret morphology different from the CH spikelets, and the presence of cleistogenes or rhizanthogenes, i.e. highly modified obligately CL spikelets hidden in basal leaf sheaths or subterranean respectively. Other CL structural peculiarities found in grasses include types of inflorescences (including amphigamy, different inflorescences in different places on the same plant (Chase 1918; Uphof 1938; Campbell et al. 1983; Thompson and Fabillo 2021; Fig. 1); CH and CL in different positions in the same terminal inflorescence; synchronicity of the CH and CL flowering; CH and CL on different plants, variations in diaspore morphology and dispersal mechanisms, caryopses (larger size, dormancy, germination) and greater seedling adaptability (Hackel 1906; Laude 1949; Clay 1982, 1983a, 1983b; Campbell et al. 1983; Clay and Antonovics 1985; Clay and Antonovics 1986; Bartholomew 1987; Adkins et al. 2002). Chase (1918) considered the differences in the morphology of the terminal spikelets and the cleistogenes in some North American chloridoid and danthonioid species (Table 2) to be so great that ‘Often, if their source was unknown, they would not be placed in the same tribe’ (p. 255). The CH and CL morphs can occur on separate plants of the same species (Hackel 1906; Uphof 1938; Auquier and Stace 1980).
|
Another category of CL involves species thought to have only CL spikelets without any CH flowers, referred to as ‘CL only’ and ‘complete’ (Table 1). Darwin (1877) believed that plants considered to be CL-only will ultimately produce seeds from CH flowers. Other authors have also expressed doubt about the existence of CL-only. For example, Campbell et al. (1983) in their classification of CL in grasses, did not provide a category for CL-only. Where CL-only is presumed, it has been suggested that intensive survey of the species should be conducted over its natural habitat to verify the existence of CH (Darwin 1877; Lord 1981; Campbell et al. 1983; Culley and Klooster 2007). For example, low frequency of the CH morph in the natural habitat of some grasses offers an explanation for apparent CL-only (Beddows 1931; Kannenberg and Allard 1967; Connor 1998).
It is considered that the presence of apomixis, seed production without meiosis, should be considered in all studies of CL (Connor 1979; Philipson 1986; Bicknell and Koltunow 2004). Apomixy is difficult to assess, requiring studies of progeny, cytology and embryology (Philipson 1986; Bashaw and Hanna 1990; Yu et al. 2000, 2003). There is little or no morphological distinction between the apomictic and sexual equivalents including some types of CL such as monomorphic CL anthers, although in the latter the anthers remain in the floret (Bashaw and Hanna 1990; Kellogg 1990; Thompson 2017). The capacity for sexual reproduction is retained for most, if not all, apomicts as exemplified by oat grasses (Danthonia DC.) from North America (Philipson 1986; Kellogg 1990; Carman 1997; Klingenberg and Gidaszewski 2010).
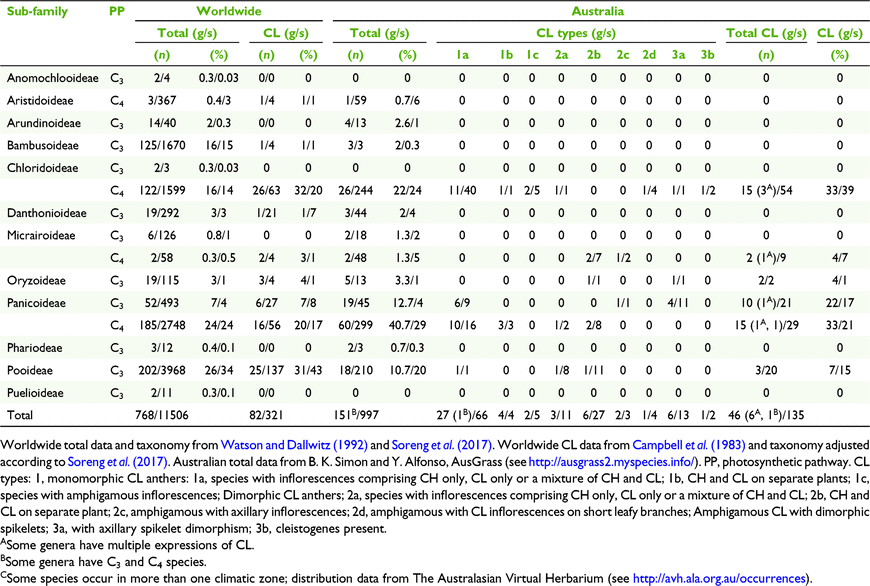
Of the twelve subfamilies of Poaceae worldwide, nine manifest some form of CL and of the nine subfamilies occurring in Australia, five have species with CL (Campbell et al. 1983; Culley and Klooster 2007; Soreng et al. 2017). Since the work on classification of grass CL by Campbell et al. (1983) and Culley and Klooster (2007), more information has been gathered about CL in Australian grasses. Until now ∼46 native grasses within 18 genera (∼12%) have been recorded with CL representing ∼4% of the native species in Australia compared to ∼3% of species and 10% of genera worldwide (Campbell et al. 1983; Watson and Dallwitz 1992; Culley and Klooster 2007).
The aim of this study is to review the classification of CL expressed in grasses by examining the morphological and physiological variation, and geographic and taxonomic distributions of Australian species. The study also investigates possible morphological relationships of the expressions of the CL types.
Materials and methods
Information gathered for this study and presented in Table 3 came from five main sources viz., herbarium specimens, images of herbarium specimens on JSTOR Global Plants (http://plants.jstor.org/, accessed March 2020), field observations, plants cultivated by the author, and the literature. Most of the information provided in Table 3 was obtained from examination of numerous herbarium specimens. Multiple spikelets of several specimens for each species were examined to determine frequency, abundance, distribution on the plant, and characteristics of the expression of CL. Targeting of taxa for detailed inspection was based on the records of CL in grass genera by Campbell et al. (1983) and Culley and Klooster (2007). For example, few specimens of species of Aristida L. were examined because of the absence of records of CL in Australian species. However, extensive searches of specimens were made in subtribes such as Andropogoninae (subfamily Panicoideae, tribe Andropogoneae) where several taxa have been recorded with CL and the expression of CL is potentially facultative or has sporadic occurrence.
|
Taxonomic classification and nomenclature are based on Brown and Bostock (see http://data.qld.gov.au/dataset/censusof-the-queensland-flora-2020/, accessed 16 June 2021) and IPNI (https://anbg.gov.au/apni/, accessed 14 May 2021) for genera and species and Soreng et al. (2017) for higher-order taxa including tribes and subtribes (Table 3). Characters used in the identification of tribes and subtribes as defined by McClusker (2002) have been noted.
Terminology follows Tothill and Hacker (1983), Jacobs et al. (2008), Gibson (2009) and Beentje (2010). The term spikelet defines the basic inflorescence of a grass. Spikelets consist of one or more florets which can be sterile, male-only, female-only or composed of a pistil and usually three stamens enveloped by one or two bracts (lemma with or without palea) subtended by one or two additional bracts (glumes). The spikelet equates to a spike in the broader context of inflorescences (Kellogg 2006; Endress 2010). Terminology used for general types of inflorescences follows Tothill and Hacker (1983). Some of the less commonly used terminology that has been used in relation to grass CL is presented in Fig. 1. Heteromorphism is used in this study to refer to variation in the elements of a compound character (Fig. 1) (Schoen and Lloyd 1984; Maxwell et al. 1994; Culley and Klooster 2007; Lerner et al. 2008). Other authors referred to this variation as somatic polymorphism (Harper et al. 1970; Sorensen 1978; Dore and McNeill 1980; Silvertown 1984; Maxwell et al. 1994).
CL was confirmed by the presence of both anthers and stigmas enclosed within the spikelet accompanied by a partially to fully developed caryopsis. Chasmogamy was determined by either of four conditions, viz. the presence of exserted anthers alone, exserted anthers and stigmas, stigmas alone in the absence of anthers (filaments present), or caryopsis present with absence of anthers and stigmas. Length of CH and CL anthers was measured and recorded for all CL species so as to fill gaps in the literature (Table 3). Anther lengths were measured for each fertile floret in the case of some multi-floreted chloridoid species (Fig. 1) and some panicoid species with staminate lower floret or pedicellate spikelet. Stigma size was also observed. Field collections were conducted so as to order to provide sufficient flowering and fruiting material for some species, as mentioned below.
Ex situ plants were cultivated and plants were also propagated from caryopses for some species. The caryopses were acquired from herbarium specimens and field collections. For most species, caryopses were manually de-husked and scarified by scraping off a small area of pericarp just above the apex of the scutellum. Plants were cultivated in pots under nursery conditions with daily watering. Some C3 and C4 species were cultivated in both full sun and partial shade to examine potential impact of light conditions on the presence and abundance of CL.
The 54 species listed in Table 3 were cultivated to observe a possible influence of environmental conditions on the presence and type of CL, and spikelet and inflorescence morphology. Plants of most species were maintained over a period of 5 years but some annual species of Schizachyrium Nees were re-propagated from caryopses for only two successive years. Several trials were conducted with CL-only plants by varying conditions, including full sun v. shaded and strongly pruned v. unpruned.
Field surveys of Astreba F.Muell. and Spathia Ewart and Davies were conducted near Camooweal and Winton, north-western Queensland in April and November 2016.
CL classification for this study was based on categories defined by other authors (Table 1) and CL morphology observed from herbarium specimens and cultivated plants. The morphological characters used to define the CL types include the following:
-
CL and CH anthers equal size in the case of monomorphic CL anthers or unequal size in the case of dimorphic CL anthers.
-
CH and CL spikelets in the same inflorescence.
-
Presence of amphigamy, i.e. different inflorescences in different places on the same plant.
-
CH and CL spikelet differences such as reported by Hubbard (1933a, 1933b), Blake (1941), Campbell et al. (1983) and Connor (1998).
-
CH and CL spikelets on different plants.
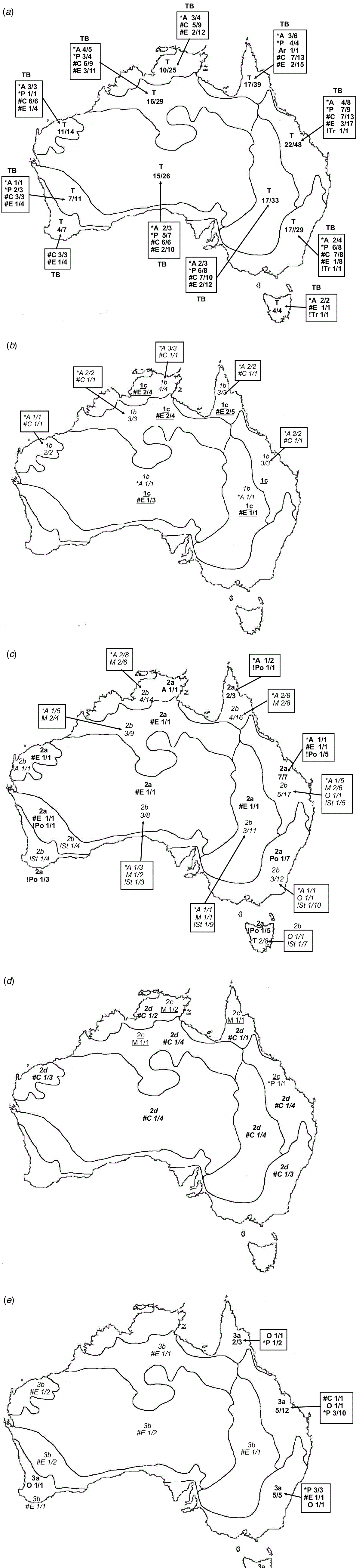
For each CL species, the following were recorded: taxonomic distribution, life cycle, photosynthetic pathway according to Watson and Dallwitz (1992), type of terminal inflorescence, and geographic distribution by climate zones, modified from climate classification of Australia by the Bureau of Meteorology (BOM; http://www.bom.gov.au/climate/how/newproducts/images/zones.shtml, accessed 27 February 2020; Table 3, Fig. 2). Three of the climatic zones were subdivided in order to accommodate biogeographic diversity in Australian grasses found during this study, viz. tropical: western (T1) and eastern (T2); semi-desert: northern (Ds1), eastern (Ds2), southern (Ds3) and western (Ds4); and temperate: eastern (M1), Tasmania (M2) and western (M3). Maps of the frequency of genera and species by subfamily and tribe were developed from The Australasian Virtual Herbarium (see http://avh.ala.org.au/occurrences, accessed January 2021) and BRI herbarium specimen records (Fig. 3 and 4). Distribution records for each species were tallied for each of the climatic zones and are summarised in Fig. 3.
|
|
Diagrammatic representations of hypothetical relationships of the CL types were developed for the taxa within each of the five Australian subfamilies (Appendix 1). The individual diagrams were used to establish an overall CL relationship diagram (Fig. 5).
In total, 33 species from 11 genera in 5 sub-families were chosen as representative examples of the types of CL discovered and are described below (Table 4). The observations made from herbarium specimens and cultivated plants for these examples provide the framework for the model of relationships between the CL types (Fig. 5 and Appendix 1). Information relating to amphigamous species worldwide was gathered from examination of herbarium specimens and the literature (Table 2). The taxonomic distribution of grass CL worldwide was gathered from the literature, mainly from Campbell et al. (1983) and Watson and Dallwitz (1992) (Table 5).
|
|
Results
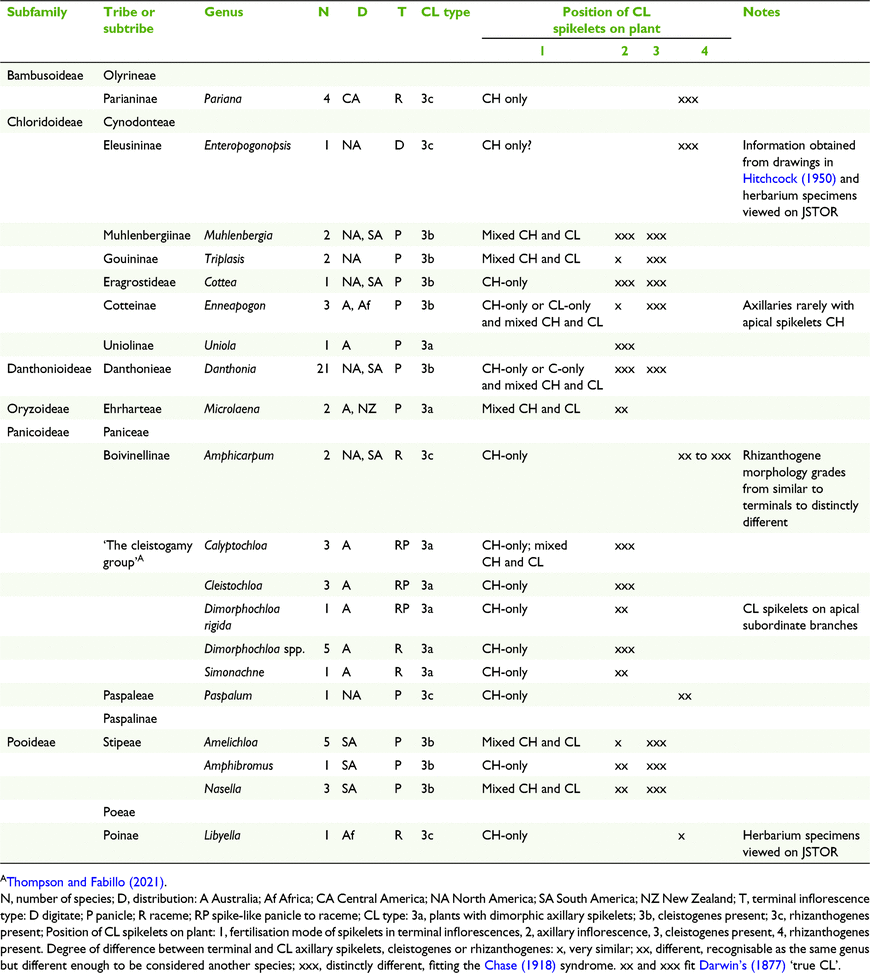
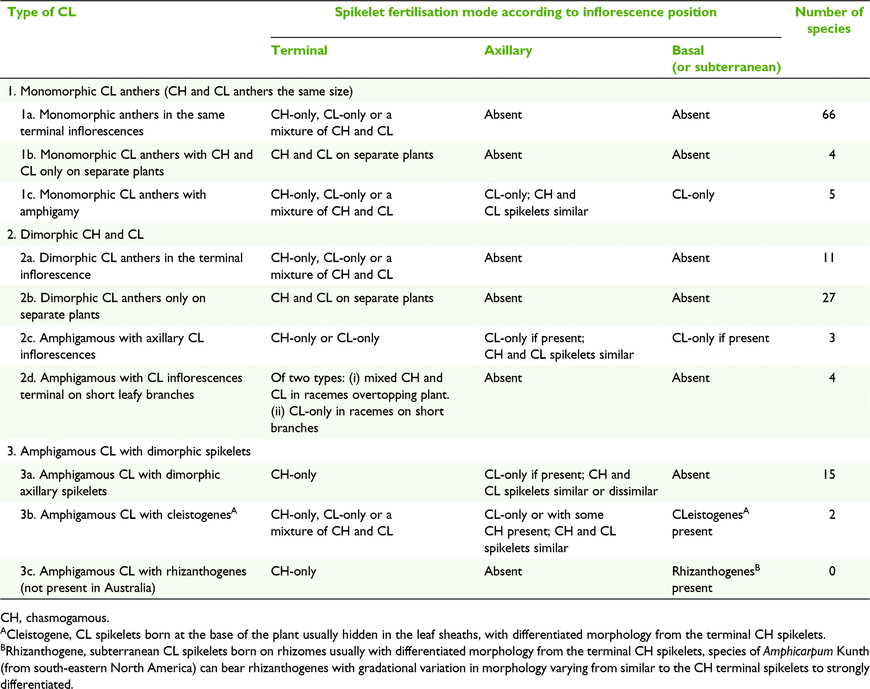
Three broad groups of CL types were defined, viz. Type 1 monomorphic CL anthers, Type 2 dimorphic CH and CL anthers and Type 3, amphigamous CL with dimorphic spikelet, and with ten subgroups (Table 6). Amphigamy in Australian grasses is represented by several combinations of inflorescence types (Fig. 1). Terminal inflorescences can vary from open panicles or reduced panicles to racemes in combination with axillary inflorescences varying from reduced panicles to few-flowered racemes. Basal inflorescences are rare in Australian grasses and are possessed by two species of Enneapogon Desv. ex P.Beauv. and one species of Eriachne R.Br having single- to few-flowered racemes and few-flowered racemes to reduced panicles respectively.
|
Fig. 5 shows hypothetical morphological relationships for the 10 subgroups of CL types for the twelve subfamilies represented in Australia. The core of the relationships is based on plants that are CH-only, CL-only and combined CH and CL on different plants of the same species. In its simplest expression, the CH and CL anthers are the same size (CL Type1).
Of the 997 native grasses from 151 genera recorded in Australia, 135 species from 46 genera were found to have some form of CL (Tables 3 and 5). These figures represent 14% of all species there and 30% of the genera, compared to 3 and 11% worldwide respectively (Table 5). However, there are disproportions in the distribution of taxonomic, geographic, life cycle, physiological and morphological aspects of CL species (Tables 5 and 7). In total, grasses worldwide and in Australia are placed predominantly in three subfamilies, viz. Chloridoideae, Panicoideae and Pooideae, in approximately similar proportions (Table 5). The taxonomic distribution of CL is also predominated by these subfamilies, but worldwide proportionately more CL species occur in Pooideae (Table 5).
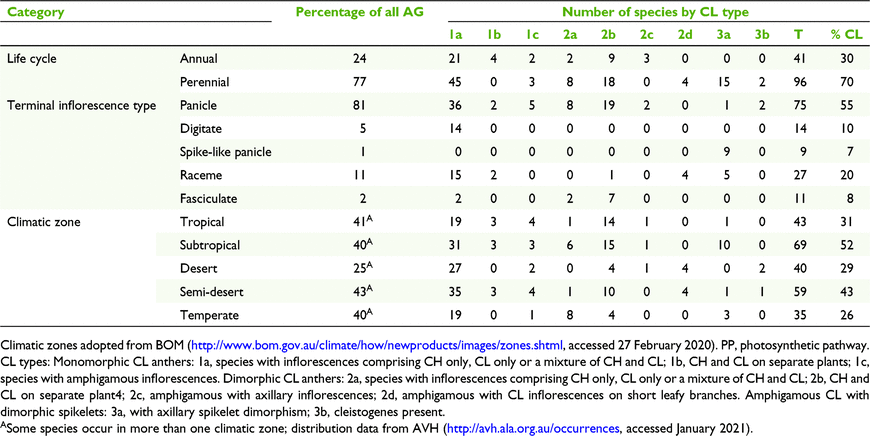
|
More than half of all CL Australian grasses have CL Type 1. Choridoid and panicoid grasses represent 77% of all Australian CL species in nearly equal proportions compared to both groups representing 24 and 37% of all Australian Poaceae respectively (Table 5). Chloridoid and panicoid grasses have the broadest range of CL types, with some of the types overlapping (Table 5). Geographic distribution and taxonomic diversity of CL types are highest in the subtropics and semi-desert of Australia (Fig. 3 and 4, Table 7). Two CL types, 2d and 3b, are found only in semi-desert and desert and are represented by the chloridoid genera, Astrebla F.Muell. and Enneapogon Desv. ex P.Beauv. and the latter is the only Australian genus known with species that exhibit differentiated basal CL spikelets hidden in the leaf sheaths, cleistogenes.
The taxonomic distribution of Australian CL species within the tribes and subtribes of subfamilies Chloridoideae and Panicoideae is restricted (Soreng et al. 2017; Table 5). In the Australian Chloridoideae, three of the four tribes and five of the ten subtribes contain CL species. Most of the CL in the Australian Chloridoideae are found in the tribes Cynodonteae and Eragrostoideae representing respectively 14 and 30% of all the Australian species in those tribes. There is a similar pattern in the Australian Panicoideae. Three of the four panicoid tribes and nine of the eighteen subtribes have CL species. The panicoid tribes Andropogoneae and Paniceae contain most of the CL species and represent respectively 18 and 13% of all the species in the two tribes.
CL in axillary and basal inflorescences can be obligate or facultative. Where it occurs in CL Type 3 it is obligate with few exceptions as found in Enneapogon. In CL Types 1 and 2, the axillary spikelets have facultative CL.
The geographic distribution of the taxa within Australian Poaceae shows distinct trends with highest frequencies along the tropical eastern coast (36% of species), subtropical eastern coast (46% of species) and temperate eastern coast (38% of species) (Fig. 3). The distribution of CL grasses follows a similar trend with 50% of species occurring in the tropical zone, 71% in the subtropical zone and 42% in the temperate zone (Fig. 3 and 4). Nearly 80% of all Australian CL species occur in the eastern coastal subtropical to tropical zones within the latitude range 10–30°S.
The composition of grass floras worldwide and in Australia with respect to photosynthetic pathway is distinctly different (Table 7). Australia has a greater proportion of C4 grasses than worldwide. However, the proportions of CL species that are C3 and C4 are similar to those for the totals of C3 and C4 species for the world and Australia.
Of Australian CL grasses, more than twice as many are perennial as are annual. Further research is required to determine the relative proportions of these life cycles in all grasses in Australia and worldwide.
The distribution of types of terminal inflorescences found in types of CL grasses also shows disproportionalities (Table 7). CL grasses with a reduced inflorescence such as a raceme represent 27% of all CL Australian species, whereas racemes are represented by 11% of Australian grasses overall. By contrast, 75% of CL species have panicles and 81% of all Australian species have panicles.
Cultivated plants from CL caryopses of species of Austrostipa S.W.L.Jacobs and J.Everett and Schizachyrium that have CL Type 2b produced CL-only inflorescences; however, it is unknown whether the CH caryopses from these species produce CH-only plants. Both CH and CL caryopses from species with CL Types 1a and 2a appear from cultivation trials to produce plants with inflorescences of all three possibilities, viz. spikelets CH-only, CL-only or a mixture of CH and CL.
The light condition trial using cultivated plants provided mixed results. C3 genera including Calytptochloa and Dimorphochloa were similar in producing CL inflorescences in both full sun and partial shade. The C4 species of Enteropogon differed by the partial-shade plants mostly producing fewer inflorescences. The C4 grasses Arthraxon, Clausospicula and Spathia strongly favoured full-sun conditions.
Discussion
The three broad categories of CL types, viz. (1) monomorphic CL anthers, (2) dimorphic CL anthers and (3) amphigamy with dimorphic spikelets, with the ten subtypes are discussed below using examples representative of each type. These examples contribute new information relating to grass CL and provide the foundation for this study. The examples also enable comparison with other authors’ classifications of CL types presented in Table 4, thereby permitting an understanding of the impact of the new information on the review of classification of grass CL in Australia.
(1) Monomorphic CL anthers
This category is defined by species having CH and CL anthers of the same size. Monomorphic CL anthers are usually found in species with only terminal inflorescences, but several species are amphigamous. The CL spikelets are usually interspersed with CH spikelets in the same inflorescence in various proportions, sometimes influenced by environmental factors (Campbell et al. 1983; Bell and Quinn 1985; Philipson 1986; Bell and Quinn 1987; Culley and Klooster 2007). Soil moisture, light intensity and time of year have been found to influence the presence of CL in Dichanthelium clandestinum (L.) Gould. CH and CL spikelets can occur without apparent pattern in the same inflorescence or in different parts of the same inflorescence (Hitchcock and Chase 1915; Burbidge 1941; Campbell et al. 1983; Bell and Quinn 1985; Philipson 1986; Bell and Quinn 1987; Morrone et al. 2007). Hackel (1906) referred to this category as ‘facultative cleistogamous species’.
Of the 135 Australian grasses recorded with CL ∼55% have monomorphic CL anthers (Table 5). Of the new records of CL since Culley and Klooster (2007), 45% relate to species with monomorphic CL anthers, although Culley and Klooster (2007) did not use this category. Discussed below are examples of the three subtypes of monomorphic CL anthers where the CH and CL spikelets are: (a) in the same inflorescence, e.g. Dichanthium Willmet, Enneapogon, Spathia and Thyridolepis (Nees) S.T.Blake; (b) on separate plants, e.g. Clausospicula Lazarides; (c) with amphigamy, e.g. Ectrosia R.Br. and Eragrostis N.M.Wolf.
(1a) Monomorphic anthers in the same terminal inflorescences
Dichanthium
Dichanthium comprises ∼16 species worldwide, with five native to Australia, one with three subspecies (Watson and Dallwitz 1992; Simon 2002). Dichanthium sericeum (R.Br.) A.Camus. is the only species that has been recorded with CL until now, but without mention of subspecies (Campbell et al. 1983; Yu et al. 2000). Whalley et al. (2013) recorded 65% CL for D. sericeum subsp. sericeum. The latter subspecies is a short-lived perennial growing in a variety of habitats, whereas the other two subspecies have been recorded as annuals mostly found in grasslands on heavy clay soils. Dichanthium queenslandicum B.K.Simon, a rare perennial, has not been recorded with CL before this study and also mostly inhabits grasslands on clayey soils.
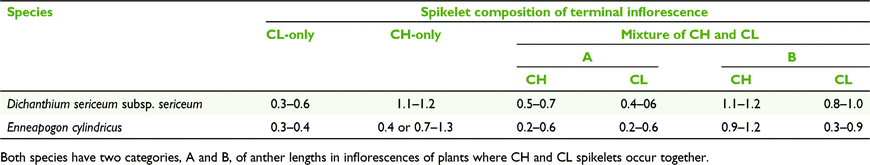
Inflorescences of plants of the CL species of Dichanthium can occur in either of three states of spikelets, viz. CL-only, CH-only, or a mixture of both CH and CL (Table 8). Furthermore, in mixed CH and CL panicles, two sets of ranges for CH and CL anthers were found and these ranges more or less match the ranges for CH-only and CL-only (Table 9).
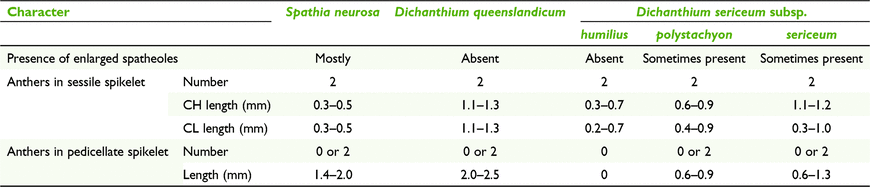
|
Enneapogon
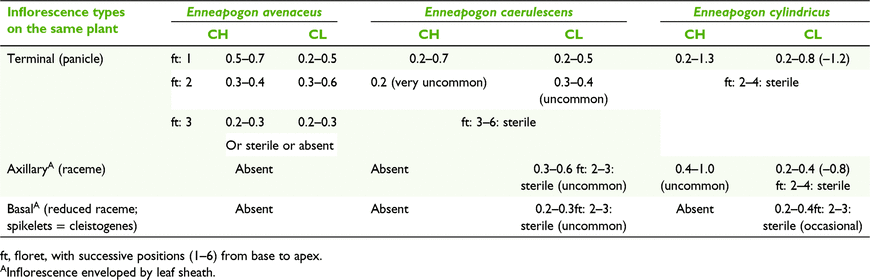
Enneapogon consists of 30 species worldwide (Watson and Dallwitz 1992). Fifteen are endemic to Australia and one Australian species extends into New Guinea (Weiller and Lazarides 2005). Four Australian species have been reported with CL (Hubbard 1937; Burbidge 1941).
Three types of CL were recorded for the five Australian species of Enneapogon with CL (Tables 2 and 10). Two species have monomorphic CL anthers, viz. Enneapogon asperatus C.E.Hubb., and Enneapogon eremophilus Kakudidi. E. asperatus, as reported by Burbidge (1941), has CL in the proximal primary branch of the terminal inflorescence enveloped by the leaf sheath with the remainder of the inflorescence exserted, and CH. This specific form of CL is very rare in grasses, although Campbell et al. (1983) in the broad sense, referred to it as ‘Type 1. Sheath fertilisation’, where the inflorescence or spikelets, at least initially, are held within the leaf sheaths of the upper portions of inflorescence culms. A variation on clandestine CL inflorescences can be in found in the panicoid genus Dichanthelium (Hitch. and Chase) Gould from North America where the CL spikelets with dimorphic anthers are produced periodically and hiddened in the leaf sheaths (Freckmann and Lelong 2003).
|
Spathia
Spathia is a monospecific genus with close affinity to Dichanthium, differing by the slightly thinner texture of the glumes and the colour of their indumentum (Blake 1944a). S. neurosa has been reported as an annual with a restricted occurrence mostly in north-western portions of the black soil plains of the Mitchell Grass Downs in the hot arid tropics of Australia (Lazarides 1970; Silcock et al. 2014).
The circumscription of S. neurosa was based on one specimen (Ewart and Davies 1917; Blake 1944a). Since then, 27 additional accessions, including seven by Blake, have been contributed to Australian herbaria. Observations from these specimens support the description by Blake (1944a) in terms of the anthers being only CL and 0.5 mm long, except for three specimens that Blake did not see. Two had male pedicellate spikelets with large anthers and one had most racemes enveloped by spatheoles as well as a single elongated inflorescence culm, but the raceme had disarticulated. By contrast, plants cultivated for this study from CL caryopses produced three kinds of racemes on:
-
Short inflorescence culms enveloped by the spatheoles with sessile spikelets CL-only, anthers 0.3–0.6 mm long, and pedicellate spikelets empty with or without a lemma,
-
Short or partially elongated inflorescence culms with sessile spikelets mostly CL with pedicellate spikelets male, anthers 1.4–2.0 mm long, and
-
Usually elongated inflorescence culms exserted from the spatheoles with sessile spikelets with monomorphic CH and CL anthers, and male pedicellate spikelets male with large anthers and the lemma present (Table 8).
Thyridolepis
Thyridolepis is an Australian endemic genus consisting of three perennial species from semiarid to arid parts of Australia (Blake 1972; B. K. Simon and Y. Alfonso, AusGrass: Grasses of Australia, ver. 1.0, see http://ausgrass2.myspecies.info/, accessed 15 June 2020). Blake (1972) recorded two species with CL.
Species of Thyridolepis have spikelets with male or empty lower florets and hermaphrodite upper florets that are mostly CL. CH and CL anthers in the upper florets have a similar size range, whereas anthers in the lower florets are much larger. Cultivated plants of Thyridolepis xerophylla (Domin) S.T.Blake produced spikelets with CH upper florets, with anthers of two sizes, 1.2–1.6 mm long and 0.3–0.7 mm long, the former being similar to the lower floret and the latter being similar to the CL anthers.
Other genera
Heslop-Harrison (1961) reported the role of the glume pit in control of CL in Bothriochloa decipiens (Hack.) C.E.Hubb. By contrast, other grasses with pitted glumes, including the naturalised species, Bothriochloa insculpta (Hochst. ex A.Rich) A.Camus and Bothriochloa pertusa (L.) A.Camus, have not been observed with CL. Furthermore, examination of herbarium specimens for this study showed that the two varieties of B. decipiens differ in the abundance of CL-only inflorescences and the ratio of CL:CH in mixed CH and CL inflorescences. In Bothriochloa decipiens var. decipiens (Hack.) C.E. Hubb, CL is common, whereas in Bothriochloa decipiens var. cloncurrensis (Domin) C.E.Hubb., CL is uncommon.
Cymbopogon Spreng. is a tropical and subtropical genus of ∼40 species with no CL having been recorded before this study (Soenarko 1977; Campbell et al. 1983; Culley and Klooster 2007). CL has been recorded for the first time in Cymbopogon gratus Domin and Cymbopogon procerus (R.Br.) Domin. CL was found to be an occasional occurrence mixed with CH in the same inflorescence in some herbarium specimens of both species.
Field collections of Entolasia whiteana C.E.Hubb. following a period of erratic rainfall showed some differences in inflorescences on plants in the same population. The affected panicles were much reduced in size and all spikelets were CL.
Australian species in the chloridoid genera Tripogon Roem. and Schult. and Sporobolus R.Br. were also found for this study to have monomorphic cleistogamous anthers in the same inflorescence. Tripogon loliiformis (F.Muell.) C.E.Hubb., known by the common name five-minute grass, and other members of the genus are known as resurrection plants for their ability to recover after complete dehydration (Gaff and Latz 1978; Tothill and Hacker 1983; Lazarides 1992). Sporobolus disjunctus R.Mills ex B.K.Simon is the only known Australian species in the genus to be CL. Some North American species of Sporobolus have been recorded with amphigamy and CL (Hitchcock and Chase 1915; Campbell et al. 1983). Specimens of Sporobolus cryptandrus (Torr.) A.Gray and S. vaginiflorus (Torr. ex A.Gray) Alph. Wood examined for this study included CL-only and CH-only on separate plants. Further research is required to determine if these American species match CL Type 1b.
(1b) Monomorphic CL anthers with CH-only and CL-only on separate plants
Three annuals were recorded with monomorphic CH and CL anthers on separate plants, viz. two andropogonoid, Clausospicula extensa and Vacoparis laxiflorum (Bailey) Sprangler., and one chloridoid, Acrachne racemosa. No specimens of A. racemosa and C. extensa were observed with CH anthers and some specimens of A. racemosa had reduced axillary CL panicles. It is considered to be likely that C. extensa and A. racemosa are species with a rare incidence of CH plants, as is the case for Pheidochloa gracillima S.T.Blake discussed below. Further research on the breeding system of A. racemosa and C. extensa is required, including field collections and observations from cultivated plants.
Clausospicula
Clausospicula is a monotypic genus with affinities to Cymbopogon Spreng, Dichanthium, Schizachyrium and Spathia Ewart and Archer (Soreng et al. 2017). Lazarides et al. (1991) considered C. extensa to be solely CL from the four specimens cited. Eleven accessions from Australian herbaria were examined for this study and all were found to be CL-only. Plants cultivated over a 4-year period were found to be CL-only with anthers of two sizes, namely one large and two smaller, 0.8–1.2 and 0.5–0.6 mm long respectively.
C. extensa has been temporarily placed in this category, pending further evidence.
(1c) Monomorphic CL anthers with amphigamy
Several species with monomorphic CL anthers in the terminal inflorescences have axillary or basal inflorescences that are CL-only or CL and CH. Species with CL-only basal inflorescences include Eragrostis concinna (R.Br.) Steud, and Eragrostis fallax M.Lazarides. Species with basal or axillary inflorescences with mixed CH and CL include Ectrosia danesii Domin, Eragrostis basedowii Jedwabnick and Eragrostis stenostachya (R.Br.) Steud.
(2) Dimorphic CL anthers
Campbell et al. (1983) recognised various types of dimorphism, with categories being based on what prevents the grass floret from opening, including the leaf sheath (where the inflorescence is enveloped by the sheath), spikelet modifications, or the soil. Culley and Klooster (2007) used a single category of ‘dimorphic’ representing ∼87% of total CL in grasses; however, their category of dimorphic CL includes other types of CL as defined in the current study (Table 1). Dimorphic CL anthers as defined here refer to CL and CH on the same or different plants of the same species where the CH and CL anthers have differences, including length, width or shape. Nine genera within four subfamilies with dimorphic CL plants are represented in Australia. Four subtypes are recognised on the basis of CH and CL on separate plants or the position of amphigamous inflorescences on the same plant. Astrebla F.Muell., Austrostipa, Eriachne, Pheidochloa S.T.Blake and Schizachyrium are discussed below.
(2a) Dimorphic CL anthers in the terminal inflorescence
Terminal inflorescences of species with dimorphic CL anthers can comprise CH-only, CL-only or a mixture of CH and CL spikelets. Three genera were recorded for this category, viz. Dichelachne Endl., Elionurus Humb. and Bonpl. and Enneapogon.
Enneapogon
Enneapogon avenaceus (Lindl.) C.E.Hubb. had not been recorded with CL before this study. Spikelets have up to three (usually two) heteromorphic fertile florets, with diminishing size of anthers, stigmas and caryopses from proximal to distal florets (Table 10). The proximal florets have dimorphic CL and the distal florets have monomorphic CL. Spikelets disarticulate readily at maturity and the diaspore is composed of the united florets as are the spikelets in the terminal inflorescences in other species of Enneapogon.
(2b) Dimorphic CL anthers only on separate plants
Four genera with dimorphic CL anthers on separate plants are discussed below. Typically, herbarium specimens examined comprised CL-only plants, and CH plants were usually very uncommon.
Austrostipa
CL has been recorded for 11 of the 62 predominantly perennial species of Austrostipa (syn. Stipa L.) (Townrow 1978; Vickery et al. 1986; Jacobs et al. 1989; Jacobs and Everett 1996). The information provided by Townrow (1978) indicates that the breeding systems of the Tasmanian species can range from CH-only, to partially CL to whole inflorescences CL or CH. The Tasmanian species have CL and CH anthers with two smaller than the other and the CH larger than the CL (Townrow 1978). Examination of herbarium specimens showed that at least some CL species have whole inflorescences CH or CL. However, it is not known what ratio of CH:CL occurs in a natural population and whether the same plants have the capacity to switch breeding system in response to environmental influences.
Austrostipa scabra (Lindley) S.W.L.Jacobs and J.Everett was propagated from CL caryopses and cultivated over several generations. Plants were found to be CL-only. A. scabra differs from most other Austrostipa by the caryopsis elongating beyond the apex of the palea thereby exposing the stylar base with the CL anthers entangled in the stigmas that subsequently detaches.
Eriachne
Eriachne R.Br. is a genus of 48 species in Australia, of which 3 were reported with CL by Lazarides et al. (2005) and now updated to 9 species (Table 3). Six species of Eriachne were assessed as having dimorphic CL plants, although several species need further investigation pending examination of additional specimens.
Pheidochloa
Pheidochloa S.T.Blake is a monospecific endemic genus from northern tropical Australia (Blake 1944b; Weiller 2005). Pheiochloa gracilis S.T.Blake is a small delicate annual mostly found in damp habitats. Blake (1944b) considered P. gracilis to be ‘very evidently cleistogamous’ and contextualised this condition with the small anthers found in three species of the allied genus, Eriachne.
Of the many herbarium specimens examined, most consisted of numerous CL plants with anthers 0.1 mm long, and only a few sheets included some CH plants with anthers 0.8 mm long.
Schizachyrium
Schizachyrium is a genus of ∼60 tropical species with seven recorded for Australia (Simon 1989; Watson and Dallwitz 1992; B. K. Simon nd Y. Alfonso, AusGrass, see http://ausgrass2.myspecies.info/). Sixteen species of Schizachyrium have been recorded with CL worldwide (Campbell et al. 1983). Blake (1974) provided descriptions of seven Australian species and in his generic diagnosis reported sessile spikelets to be commonly CL. He described the CH spikelets of Schizachyrium dolosum S.T.Blake as having larger anthers on long inflorescence culms exserted from tightly convolute spatheoles.
All of the Australian species of Schizachyrium have dimorphic CL plants with dimorphic anthers. Inflorescences are fascicles consisting of spatheolate terminal racemes on numerous short branches. Most specimens examined had racemes comprising CL spikelets on short inflorescence culms completely to partially enveloped by the spatheole, often gaping at maturity for some species. Conversely, CH-only racemes were found on elongated inflorescence culms and exserted from the spatheole. However, some specimens of species such as Schizachyrium occultum S.T.Blake had CL spikelets in racemes on elongated inflorescence culms exserted from the spatheole. This syndrome of CH racemes on elongating inflorescence culms was also found in Astrebla and Spathia.
Five species of Schizachyrium were cultivated for this study from CL caryopses and all of the numerous plants propagated from 2016 to 2020 had racemes enveloped by the spatheole with CL-only spikelets. A hypothesis from this finding and from cultivation of Austrostipa species is that caryopses from dimorphic CL plants produce only CL plants. No CH caryopses were found on herbarium specimens to be able to test a similar hypothesis with CH-only plants because the CH spikelets disarticulate freely as soon as they are ripe, unlike the CL spikelets that are usually retained until the spatheole gapes during drying.
(2c) Amphigamous with axillary CL inflorescences
Two Australian grasses, viz. Eriachne basalis Lazarides and Entolasia sp. aff. whiteana, have been recorded with amphigamous CL without or with only minor spikelet dimorphism. E. basalis has a mixture of CH and CL spikelets in the terminal panicles and reduced basal panicles or single CL spikelets. E. sp. aff. whiteana is an undescribed species known from a single location in semiarid central Queensland. The terminal spikelets are CH-only and a little shorter than the axillary CL spikelets.
(2d) Amphigamous with CL inflorescences terminal on short leafy branches
Astrebla
Astrebla (Mitchell grasses) is an Australian endemic genus of four perennial species from semi-arid to arid grasslands considered to be an important asset to the grazing industry (Breakwell 1923; Everist 1935; Orr 1975; Nightingale and Weiller 2005). Astrebla spp. and Mitchell grasslands have been studied and written about more extensively than any other Australian grasses. Mitchell grasslands grow on heavy cracking clay soils prone to drying out rapidly in a tropical to subtropical climate with erratic summer rainfall and high evaporation rate, and frequent drought. Growth and flowering responses to rain, including at particular periods of the year, have been reported in several studies (Everist 1935, 1951; Roe 1941; Allen 1963; Everist 1964; Jozwik 1970; Jozwik et al. 1970; Williams and Roe 1975; Scanlan 1983; Hunter 1989; Orr 1991; Orr and Evenson 1991a, 1991b). Mitchell grasses produce two kinds of shoots in response to rain, innovation shoots following rainfall events of >50 mm and only short leafy branches on tillers generated following rain of ∼15 mm (Everist 1964; Jozwik et al. 1970).
There has been little documentation on the breeding system of Astrebla. Hackel (1906) recorded two species of Astrebla, viz. Astrebla pectinata (Lind.) F.Muell. ex Benth and Astrebla lappacea (Lind.) Domin (Hackel (1906) as Astrebla triticoides Muell.), as being CL-only. Three other publications used the information from Hackel (1906) to classify CL in Astrebla spp. in different ways (Connor 1979; Campbell et al. 1983; Culley and Klooster 2007). Bailey (1878) referred to ‘the palea empty or with rudimentary flower’ for A. pectinata. Orr (1998) referred to Astrebla as autogamous. Nightingale and Weiller (2005) did not classify the form of CL but considered three of the species to have it, with Astrebla squarrosa C.E.Hubb. being the exception. Whalley et al. (2013) considered the breeding system of A. lappacea to be ‘mostly inbreeding with a small degree of cross-breeding’. Jozwik (1970) speculated that apomixis could be present in Astrebla, although Whalley et al. (2013) considered it unlikely.
In this study, the racemes of the four species of Astrebla can be totally or partially CL, depending on rainfall. CH occurs in the racemes produced on innovative shoots. Plants continue to flower as the soil dries out and then produce CL racemes, or only CL racemes are present following rainfall events of ∼15 mm. Plants of A. lappacea and A. pectinata were found to bear two types of racemes, usually relatively long ones on the innovation shoots with elongated inflorescence culms and shorter ones on lateral branch shoots with short inflorescence culms. This dimorphism in racemes size and position represents an expression of facultative amphigamy. The shorter racemes were found to be less common for A. squarrosa and A. elymoides. The long racemes exceed the perimeter of the foliage, with the inflorescence culm exserted from the spatheoles and at least apical spikelets bearing proximal CH florets. The short racemes are produced at the perimeter of the foliage on the same plants as the long racemes or can occur alone. The short racemes are CL-only and are at first enveloped by the spatheoles and then gape at maturity. Inflorescences on herbarium specimens of species of Astrebla commonly consist entirely of CL-only racemes. It is therefore understandable why Hackel (1906) thought that the two species of Astrebla he cited were CL-only, given that he saw only the shorter racemes.
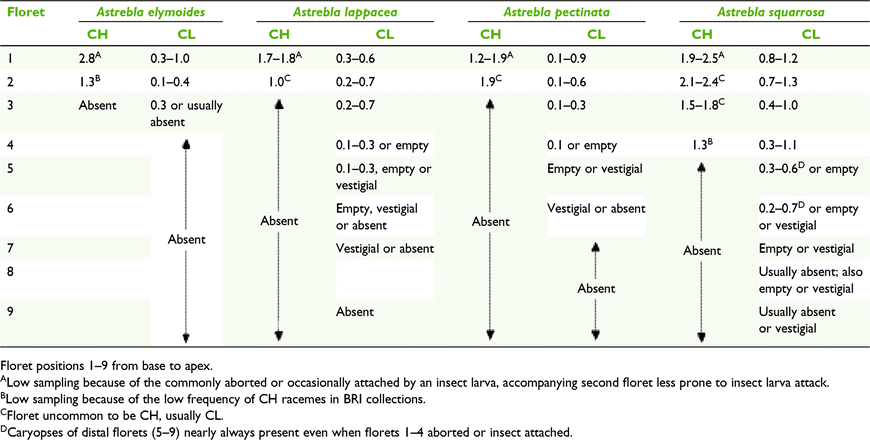
Astrebla species display both anther dimorphism and heteromorphism (Fig. 1, 6 and 7). All racemes consist of heteromorphic spikelets, with the longest ones at the base. Racemes that bear CH florets have obligately CL spikelets at least in the lower third of the raceme. Also, CH-bearing racemes frequently have some spikelets with up to three of the proximal florets aborted or sometimes damaged by an insect larva. Both CH and CL spikelets comprise several heteromorphic florets with heteromorphic anthers and caryopses smallest in the apical floret (Fig. 6, Table 11). Proximal florets can be CH or CL but the distal florets are obligately CL (Table 11). Anther size also varies within an individual floret, with one of the anthers being ∼15–20% longer than the other two (Fig. 7). The morphology of spikelets from racemes exserted from the spatheoles of Astrebla spp. has similarities to Enneapogon avenaceus (Table 10).
|
Field survey showed that racemes on short tillers bearing CL-only spikelets can be continuously produced through the growing season or in a few more or less even-aged groups, presumably dependent on the suitability of soil moisture levels. Plants of A. lappacea and A. pectinata observed in the Camooweal area, north-western Queensland, had three age groups of CL-only racemes, two of the age groups coinciding with rainfall of 204 mm in December 2015 (annual average 63 mm) and 157 mm in March 2016 (annual average 56 mm; http://www.bom.gov.au/climate/data/, accessed 27 February 2020), and the third group, represented by relatively few racemes, from the previous season. The three age groups were distinguishable by the colour of the spikelets, green, straw-coloured and greyish ones from the previous season. The green racemes had immature caryopses with anthers 0.2–0.3 mm long and the mature ones had anthers 0.4–0.7 mm long (Fig. 6). No CH spikelets were seen in the collections of A. pectinata at that time. Similar observations were made from specimens collected from the Winton area following major rainfall events in March and July 2016.
Field observations also showed that dispersal of diaspores in Astrebla is most commonly shortly after maturity or sometimes release of the diaspores can be delayed. In Astrebla, the diaspore consists of the united florets, unlike many other chloridoid grasses that disarticulate between the florets. Release of CL spikelets can be inhibited by the spatheole and spikelets can be retained on the plants for several months after maturity, providing an aerial component to the seed bank. In specimens examined for this study, the retained spikelets had CL caryopses in the most distal one to three fertile florets and the proximal florets were empty.
From limited pot-cultivation nursery trials with plants propagated from CL caryopses, plants of A. lappacea and A. pectinata produced a few racemes with some CH spikelets and CL-only respectively, and A. elymoides and A. squarrosa did not produce any inflorescences.
(3) Amphigamy with dimorphic CL spikelets
This category refers to species that have two or more types of inflorescences in different positions (terminal, axillary or basal, or subterranean) on the same plant, with corresponding spikelet dimorphism (Quinn 1998). Spikelets in terminal inflorescences can be CH-only, CL-only or a combination of CH and CL; axillary spikelets are predominantly CL-only, with inflorescences consistently reduced compared to the terminals; and spikelets at the base and subterranean are CL-only, with inflorescences comprising a single spikelet or raceme of up to several spikelets. Chase (1918) referred to the CL spikelets at the base of the plant as cleistogenes and compared them to the spikelets in the terminal inflorescences of some North American grasses. However, there is variation in the degree of differentiation between the spikelets from the three types of inflorescences (Table 10).
Approximately 65 species, less than 0.5% of grasses worldwide, have been recorded as amphigamous with CL anther dimorphism (Table 2). This breeding system has been referred to variously, including cleisto-chasmogamic floral dimorphism (Frankel and Galun 1977), multiple strategies (Lloyd 1984), CL heteromorphic reproductive pattern (Cheplick and Clay 1989), structural dimorphism (Plitmann 1995), mixed mating (Goodwillie et al. 2005; Oakley et al. 2007), reproductive dimorphism (Cheplick 2007) and dimorphic CL (Albert et al. 2011).
In this study, species with amphigamous dimorphic CL have been subdivided into three categories, viz. with dimorphic axillary spikelets without cleistogenes, with cleistogenes, or with rhizanthogenes. In all cases known, cleistogenes and rhizanthogenes are obligately CL and in most cases axillary CL is obligate whereas CL in terminal inflorescences is facultative or absent. Phenology of CL axillary inflorescences usually precedes terminal inflorescences.
(3a) Amphigamous CL with dimorphic axillary spikelets
Six Australian grass genera, one chloridoid (Uniola sp.), one oryzoid (Microlaena R.Br.) and four panicoid (‘the cleistogamy group’) have been recorded with dimorphic axillary CL spikelets (Clifford 1962; Connor and Matthews 1977; Thompson and Fabillo 2021) (Tables 5 and 7). The degree of difference between the terminal and axillary spikelets of the taxa varies from relatively small to distinctive (Table 2). Species in ‘the cleistogamy group’ share unique morphology and the group consists of 11 putative species from Calyptochloa C.E.Hubb., Cleistochloa s.l., Dimorphochloa s.l. and Ancistrachne maidenii (Hamilton) Vickery that all occur predominantly in shady habitats, mostly on shallow sandy soils derived from sandstone (Thompson and Fabillo 2021; Table 3). The species occur within the latitude range 5–36°S, covering tropical, subtropical and temperate climate zones, with only Cleistochloa subjuncea C.E.Hubb. occurring in all three zones. Calyptochloa, Cleistochloa, Dimorphochloa and A. maidenii rarely produce caryopses in the terminal inflorescences and when present have always been found to be CH. From observation of cultivated plants, species in Cleistochloinae always produce axillary CL spikelets before the terminal inflorescences appear mostly in summer and autumn and in some species only axillary spikelets were produced.
The proportion of caryopses produced in terminal and axillary inflorescences varies with the taxa. Uniola sp. (Palm Grove P.I. Forster PIF23666) produces most caryopses in the terminal inflorescences. Herbarium specimens and cultivated plants of Microlaena stipoides (Labill.) R.Br. from Queensland were rarely found with axillary spikelets.
Uniola sp. (Palm Grove P.I. Forster PIF23666)
Uniola L. s.s. consists of two species from North America and West Indies growing on coastal sand dunes (Yates 1966, 2003; Watson and Dallwitz 1992). Uniola sp. (Palm Grove P.I. Forster PIF23666) is a caespitose perennial growing in woodlands on well-drained soils derived from sandstone hills in central Queensland and known from five collections at BRI. The taxonomy of this rare species is uncertain, but it has affinity with Uniola s.s., with the laterally compressed spikelets and the distinctive paleas being two-keeled with wings. Notable differences between Uniola sp. (Palm Grove P.I. Forster PIF23666) and Uniola s.s. relate to the breeding system (amphigamous CL v. dimorphic CL plants), inflorescence type (raceme v. panicle) and diaspore dispersal mechanism (terminal spikelets disarticulating and axillary spikelets retained in sheath v. falling entire (the latter reported by Watson and Dallwitz 1992).
The inflorescences of Uniola sp. (Palm Grove P.I. Forster PIF23666) comprise terminal racemes and single axillary spikelets different from the terminal ones. The terminal spikelets have up to three heteromorphic florets, of which the first and second are fertile with heteromorphic anthers and caryopses (Fig. 8). The first floret is CH or CL with dimorphic anthers, 1.6–1.8 and 0.4 mm long respectively, and the second floret is CL-only with anthers 0.2 mm long. The axillary spikelets occur at several nodes where they are hidden within the leaf sheath and are obligate CL with anthers 0.2 mm long. Axillary spikelets typically consist of a single fertile floret but have also been found to be multi-floreted. This type of variation in the axillary spikelet resembles that found in Danthonia spicata (L.) Beauv., also having spikelets with a single floret, or alternatively, an elongated rachilla bearing several florets (Weatherwax 1928).
Uniola sp. (Palm Grove P.I. Forster PIF23666) overlaps in geographic distribution and similarity of landscape type with some species in ‘the cleistogamy group’ of panicoid grasses that have the CL Type 3a syndrome.
(3b) Amphigamous CL with cleistogenes
Enneapogon
Enneapogon caerulescens (Gaudich.) N.T.Burbidge and Enneapogon cylindricus N.T.Burbidge have three types of inflorescences; namely, terminal, axillary and basal. Both species have terminal panicles with three states of spikelets, CH-only, CL-only or a mixture of both CH and CL, similar to Dichanthium sericeum subsp. sericeum (Table 9). E. cylindricus differs from E. caerulescens by having dimorphic CH and CL anthers in the terminal panicles (Table 10). The axillary racemes, when present, are mostly enveloped by the leaf sheath but E. cylindricus occasionally has exposed apical spikelets that are CH. E. caerulescens and E. cylindricus are the only Australian grasses recorded with cleistogenes (Burbidge 1941; Connor 1979; Campbell et al. 1983; Watson and Dallwitz 1992; Weiller and Lazarides 2005; Culley and Klooster 2007). Clandestine axillary spikelets and cleistogenes are obligately CL. Cleistogenes are commonly absent or of low frequency in herbarium specimens of these two species, suggesting that their presence may be influenced by environmental factors. However, it is possible that cleistogenes are produced some time after the terminal inflorescences as is case in North American species of Danthonia, as reported by Chase (1918). Further field collections would be required to investigate the possibility of this temporal succession of CL spikelet production. The cleistogenes of species of Enneapogon differ from the terminal and axillary spikelets by having fewer florets, shorter lemma awns, smaller anthers and larger caryopses (Fig. 9). Despite these differences, these two Australian species of Enneapogon do not fit Chase’s (1918) conundrum (postulates that the cleistogenes have morphology that gives them the appearance that they below to species from another tribe) because both species have cleistogenes that are readily recognisable as belonging to Enneapogon but, speculatively, a different species.
Enneapogon caerulescens and E. cylindricus each have three types of diaspores corresponding to the different inflorescences. As for Enneapogon desvauxii Beauv. from Africa, the Americas and Asia, the axillary spikelets of E. cylindricus and E. caerulescens mostly remain trapped in the leaf sheath. However, in E. cylindricus and E. desvauxii, the tillers disarticulates at the lowest nodes, whereas the tillers do not disarticulate in E. caerulescens (Hitchcock 1936; Burbidge 1941; Chippindall 1955; Van Oudtshoorn 1999). The cleistogenes of the species of Enneapogon can potentially remain for some years at the base of the mother plant confined by multiple layers of old leaf sheaths or be released to disperse after disintegration of the mother plant. The cleistogenes of Enneapogon and other genera have been reported to germinate in situ, and thus grow out of the old plant (Poulter 1932; Chippindall 1955; Gibbs Russell et al. 1990). From the present study, it appears that cleistogenes continue to enlarge while attached to the living mother plant.
The occurrence of disarticulating tillers that function as a CL diaspore is very uncommon in Poaceae. Besides Enneapogon, it is known from the panicoid grass Calyptochloa gracillima (C.E.Hubb.) E.J.Thomps. and B.K.Simon from Australia and American species of Danthonia (Darbyshire 2003; Thompson and Simon 2012). Most North American species of Danthonia produce three types of inflorescences that are all differentiated with corresponding spikelet dimorphisms. The terminal inflorescences have facultative CL. Cultivation trials by the author with Danthonia sericea over several years resulted in plants producing only terminal inflorescences that were CL-only or occasionally with a few CH spikelets. Axillary spikelets and cleistogenes were never produced, indicating the possibility of environmental influence.
(3c) Amphigamous CL with rhizanthogenes
Grasses that have amphigamy with subterranean spikelets, i.e. rhizanthogenes, are not represented in Australia. Nine species within five genera across four subfamilies have been recorded with rhizanthogenes and most species occur in relatively close geographical proximity in or near central America including Florida and Cuba, except Libyella Pamp., which occurs in northern Africa (Hitchcock 1950; Campbell et al. 1983; Clayton 1990; Watson and Dallwitz 1992; Table 2).
Overview
The three main categories of grass CL defined in this study have a strong overlap with those defined by other authors (Table 1). The study also concurs with other authors who did not accept that a species is likely to have CL-only as its only pollination system. It was found, during this study, that some Australian species have a very low occurrence of CH. However, species such as Clausospicula extensa Lazarides, not listed by Culley and Klooster (2007), and Acrachne racemosa (B.Heyne ex Roem. and Schult.) Ohwi, have been thought to be CL-only (Lazarides et al. 1991; Culley and Klooster 2007). Only CL-only material of C. extensa was observed in the relatively small number of specimens in herbarium collections and plants cultivated for this study. A. racemosa was also found to be CL-only in herbarium specimens. It is expected that cultivated plants propagated from CL-only plants would produce CL-only spikelets. Furthermore, it is considered likely that intensive field investigation would show CH-only plants in both of these species.
CL Types 1a and 2a include species with plants that bear inflorescences composed of spikelets in either of three modes, viz. CH-only, CL-only or a mixture of CH and CL. Observations from cultivated plants and herbarium specimens indicate that these modes are facultative. The three modes are central to understanding the relationships of the types of CL in Australian grasses (Fig. 5). Furthermore, the spectrum of expressions of CL represented in Australian species of the chloridoid genus Enneapogon provides an example of supporting evidence for the model. Australian species of Enneapogon exhibit several types of CL, including the following four occurrences:
-
The three modes in terminal inflorescences with or without anther dimorphism,
-
CL in the terminal inflorescence hidden in the basal branch that is enveloped by the leaf sheath,
-
Axillary racemes that can be CL-only or sometimes mixed CH and CL, and
-
Cleistogenes with modified spikelet characters (Table 10).
Both CH and CL morphs in Australian CL grasses can be obligate or facultative. The favourable conditions of the nursery in this study stimulated CH spikelets in Spathia previously thought to be CL-only. Similarly, field plants of Astrebla following good rainfall produce innovative shoots that bear spikelets with CH proximal florets, but the distal florets are always CL. Conversely, the nursery environment did not stimulate CH plants in species of Schizachyrium and Austrostipa propagated from CL caryopses. Also, species in ‘the cleistogamy group’ rarely produce caryopsis in the terminal inflorescences, with most being found in the axillary CL spikelets under field conditions as well as in the nursery.
Fifteen Australian grasses possess CL Type 3 with dimorphisms that correspond with the category of ‘true CL’ of Darwin (1877). The CL characters include dimorphisms in anthers, spikelets and inflorescences, but the degree of difference in the CH–CL characters can vary (Table 6). Two of these Australian species with Type 3 CL bear cleistogenes, but these basal CL spikelets do not completely match the syndrome described by Chase (1918) where the cleistogenes differ distinctly from the CH ones. Instead, in the Australian species with CL Type 3a, a different form of the Chase syndrome is expressed in that the spikelet peculiarities are found in terminal and axillary inflorescences (Fig. 1). The Australian CL Type 3 species have narrow taxonomic distribution, occurring in eight genera in subfamilies Chloridoideae, Oryzoideae and Panicoideae with 13 species in the latter. Fourteen species have C3 photosynthetic pathway and predominantly occur in subtropical eastern Australia in woodlands on landscapes with shallow to skeletal soils. The three C4 species are all chloridoid, of which two occur in open habitats in the desert and the third one in woodlands in the subtropics.
Aristida also presents anomalies in the geographic and environmental distribution of CL. Australian species of Aristida occupy a broad range of habitats, including extremely arid habitats; however, no form of CL has been recorded for any of the species. By contrast, four species in North America display CL including two species with amphigamous CL without spikelet dimorphism (Hitchcock 1924; Henrard 1929; Hitchcock 1950; Campbell et al. 1983).
This study also showed peculiarities in CH inflorescences more commonly associated with CL, especially with respect to the occurrence of axillary racemes and clandestine spikelets. Clandestine racemes or single spikelets are characteristic of dimorphic CL species, but they are very rare with CH-only grasses, e.g. Cenchrus clandestinus (Hochst. ex Chiov.) Morrone. CH axillary racemes occur in Rottboellia cochinchinensis, Ischaemum fragile R.Br. and some species of Thaumastochloa, although some herbarium specimens of these species also have short lateral branches terminated by a raceme. Furthermore, some herbarium specimens of Thaumastochloa major S.T.Blake and Thaumastochloa pubescens (Benth.) C.E.Hubb. have dimorphic racemes with differentiated spikelets on the same plants. This type of raceme dimorphism represents a form of amphigamy being somewhat parallel to that found in Astrebla. De Koning et al. (1983) reported this dual inflorescence condition as ‘heteromorphic spikes’ for T. major. Cultivation trials over two growing seasons have shown a temporal aspect to the occurrence of these dimorphic inflorescences, i.e. they begin to appear towards the end on the growing season in autumn.
A further expression of diversity in taxa and adaptation to aridity occurs in resurrection grasses (Gaff and Latz 1978; Lazarides 1992). However, of the Australian genera, viz. Eragrostiella Bor, Sporobolus R.Br. and Tripogon (subfamily Chloridoideae), and Micraira F.Muell. (subfamily Micrairoideae), only Sporobolus and Tripogon are known to have species with CL.
CL morphology has played a role in the taxonomy of grasses. CL characters have been used in generic keys (Webster 1987; Simon 2002; Barkworth 2003). Presence of cleistogenes has been used to separate species of Enneapogon (Weiller and Lazarides 2005). CL morphology has also been shown to discriminate groups of genera (Thompson and Fabillo 2021).
Habitat specificity, combined with structural peculiarities in inflorescences, spikelets and florets, also occurs in relation to other types of breeding systems in Poaceae. The Australian dioecious genera Pseudochaetochloa A.Hitch., Spinifex L. and Zygochloa S.T.Blake express various degrees of dimorphism in male and female terminal inflorescences and spikelets, and occupy xeric habitats including rocky environments of various geology, coastal sand dunes and inland dunes respectively. A further example includes Chionachne R.Br., Hygrochloa Lazarides and Thaurea Pers., which have dimorphic spikelets in the same inflorescence on the same plant and usually a high level of habitat specificity, viz. clayey soil, wetlands and coastal dunes respectively.
Although the evolution of each of the CL types may have occurred independently, especially across the different taxa, the relationship model presented in Fig. 5 provides a starting point for understanding the taxonomic distribution of the diversity of CL morphology in grasses. In the absence of fossil records, the present-day geographic and taxonomic distributions of the CL types must form the basis for investigation of the evolution of grass CL (Fig. 3). Accordingly, one plausible driving force for some grass CL evolution is reproductive diversification in response to a climate with erratic rainfall with C3 grasses more prevalent in shady habitats and C4 grasses dominant in open habitats especially in the tropics and sub-tropics (Christie 1981; Hattersley 1983, 1992; Conover and Sovonick-Dunford 1989; Osborne and Freckleton 2008). However, the presence, absence, range of CL types and geographic range of CL across species in some genera and complete absence of CL in other genera with sympatric species pose paradoxes about the evolutionary driving forces of CL in Australian grasses. The genus Enneapogon displays a spectrum of CL types, as mentioned above, but most of the species are CH-only and some species inhabit arid environments that overlap with the CL species. Furthermore, the chloridoid genus Triodia R.Br. comprises species, mostly from arid habitats with low water holding-capacity soils, that rarely produce seed and are CH-only, as was found by the author from examination of herbarium specimens and field observation (Burbidge 1953; Wright et al. 2014). Another example involves two C4 andropogonoid genera that are frequently sympatric in semi-arid and tropical regions of Australia, viz. Thaumastochloa C.E.Hubb. with CH-only species and Schizachyrium with species having dimorphic CL on separate plants (Type 2b).
Conclusions
This study has provided further understanding of the taxonomic and geographic distributions of CL in Australian grasses. Information on CL breeding systems presented here broadens understanding of the taxonomy, ecology and management of several species, in particular Mitchell grasses that are important to the grazing industry. Furthermore, for most species that appear to be CL-only, it is likely that there is very low incidence of CH in the natural population or it is possible that localised environmental conditions trigger it. For some species the breeding system involves plants that are CH-only, CL-only or combined CH and CL. This information has enabled insight into hypothetical relationships of the types of CL in Australian grasses and has provided a new explanation for the possible origin of some CL categories. Responses to varying degrees of erratic rainfall offer a probable evolutionary driving force for at least some of the CL types found across the variation in taxonomic and geographic distributions and physiology of the species. By contrast, sympatric occurrences of CL and CH-only species in arid habitats provide a paradox for this notion. Besides this, the findings from this study have implications about the possible need for further investigation of grass CL in other parts of world.
Data availability
Data used in this manuscript are presented in Table 3.
Conflicts of interest
The author declares that he has no conflicts of interest.
Declaration of funding
This research was supported by the Queensland Herbarium but did not receive any specific funding.
Acknowledgements
I am most thankful to Guillame Lannuzel and Bruno Fogliani, Axe II ‘Diversités biologique et fonctionnelle des écosystèmes terrestres’, Paita, New Caledonia for providing material of Ancistrachne numaeensis. Thanks go to the curators at CANB, K, MEL, MO, NSW, PERTH, NT, RBG, RSA, UCR, US and UTC for the loan of material. I greatly appreciate help from Dr Werner Stur with translation of some of the German text from Hackel (1906). Many thanks go to Dr Gordon Guymer for his continuous support at BRI. I am very thankful for the comments and suggestions provided by the publisher’s reviewers of a draft of this paper.
References
Adkins SW, Bellairs SM, Loch DS (2002) Seed dormancy mechanisms in warm season grass species. Euphytica 126, 13–20.| Seed dormancy mechanisms in warm season grass species.Crossref | GoogleScholarGoogle Scholar |
Albert LP, Campbell LG, Whitney KD (2011) Beyond simple reproductive assurance: cleistogamy allows adaptive plastic responses to pollen limitation. International Journal of Plant Sciences 172, 862–869.
| Beyond simple reproductive assurance: cleistogamy allows adaptive plastic responses to pollen limitation.Crossref | GoogleScholarGoogle Scholar |
Allen GH (1963) Moisture penetration and plant growth in a Mitchell Grass community following summer rain. Field Station Record, Division of Plant Industry, CSIRO 2, 55–57.
Auquier P, Stace CA (1980) Variation in flowering behaviour in Vulpia (Poaceae). Plant Systematics and Evolution 136, 47–52.
| Variation in flowering behaviour in Vulpia (Poaceae).Crossref | GoogleScholarGoogle Scholar |
Bailey FM (1878) ‘Flora of Queensland.’ (Government Press: Brisbane, Qld, Australia)
Barkworth ME (2003) 25. Paniceae R.Br. In ‘Flora of North America, North of Mexico, Vol. 25, Magnoliophyta: Commelinidae (in part): Poaceae, part 2’. (Eds ME Barkworth, KM Capels, S Long, MB Piep) pp. 353–358. (Oxford University Press: New York, NY, USA)
Bartholomew SE (1987) An overview of the selective advantage of cleistigamy in the Poaceae. Erigenia 7, 21–31.
Bashaw EC, Hanna WW (1990) Apomictic reproduction. In ‘Reproductive versatility in the grasses’. (Ed. P Chapman) pp. 100–130. (Cambridge University Press: New York, NY, USA)
Beddows AR (1931) Triodia decumbens, Beauv. (Sieglingia decumbens, Bernh.). Annals of Botany 45, 443–451.
| Triodia decumbens, Beauv. (Sieglingia decumbens, Bernh.).Crossref | GoogleScholarGoogle Scholar |
Beentje H (2010) ‘The Kew Plant Glossary an illustrated dictionary of plant terms.’ (Kew Publishing: Kew, UK)
Bell TJ, Quinn JA (1985) Relative importance of chasmogamously and cleistogamously derived seeds of Dichanthelium clandestinum (L.) Gould. Botanical Gazette 146, 252–258.
| Relative importance of chasmogamously and cleistogamously derived seeds of Dichanthelium clandestinum (L.) Gould.Crossref | GoogleScholarGoogle Scholar |
Bell TJ, Quinn JA (1987) Effects of moisture and light intensity on the chasmogamous and cleistogamous components of reproductive effort of Dichanthelium clandestinum populations. Canadian Journal of Botany 65, 2243–2249.
| Effects of moisture and light intensity on the chasmogamous and cleistogamous components of reproductive effort of Dichanthelium clandestinum populations.Crossref | GoogleScholarGoogle Scholar |
Bicknell RA, Koltunow AM (2004) Understanding apomixis: recent advances and remaining conundrums. The Plant Cell 16, S228–S245.
| Understanding apomixis: recent advances and remaining conundrums.Crossref | GoogleScholarGoogle Scholar | 15131250PubMed |
Blake ST (1941) New genera of Australian grasses. University of Queensland, Papers Department of Biology 1, 1–12.
Blake ST (1944a) Monographic studies in the Australian Andropogoneae, Part I. Revisions of the genera Bothriochloa, Capillepedium, Chrysopogon and Spathia. University of Queensland Papers, Department of Biology 2, 1–62.
Blake ST (1944b) On Streptachne R. Brown and Pheidochloa genus novum, two genera of grasses from Queensland. Proceedings of the Royal Society of Queensland 56, 11–22.
Blake ST (1972) Neurachne and its allies (Gramineae). Contributions from the Queensland Herbarium 13, 1–53.
Blake ST (1974) Revision of the genera Cymbopogon and Schizachyrium (Gramineae) in Australia. Contributions from the Queensland Herbarium 17, 1–70.
Bouchenak-Khelladi Y, Slingsby JA, Verboom GA, Bond WJ (2014) Diversification of C4 grasses (Poaceae) does not coincide with ecological dominance. American Journal of Botany 101, 300–307.
| Diversification of C4 grasses (Poaceae) does not coincide with ecological dominance.Crossref | GoogleScholarGoogle Scholar | 24509796PubMed |
Breakwell E (1923) ‘Grasses and fodder plants of New South Wales.’ (Government Press: Sydney, NSW, Australia)
Burbidge NT (1941) A revision of the Australian species of Enneapogon Desv. Proceedings of the Linnean Society of London 153, 52–91.
| A revision of the Australian species of Enneapogon Desv.Crossref | GoogleScholarGoogle Scholar |
Burbidge NT (1953) The genus Triodia R.Br. (Gramineae). Australian Journal of Botany 1, 121–184.
| The genus Triodia R.Br. (Gramineae).Crossref | GoogleScholarGoogle Scholar |
Campbell CS, Quinn JA, Cheplick GP, Bell TJ (1983) Cleistogamy in grasses. Annual Review of Ecology and Systematics 14, 411–441.
| Cleistogamy in grasses.Crossref | GoogleScholarGoogle Scholar |
Carman JG (1997) Asynchronous expression of duplicate genes in angiosperms may cause apomixis, bispory, tetraspory, and polyembtryony. Biological Journal of the Linnean Society 61, 51–94.
| Asynchronous expression of duplicate genes in angiosperms may cause apomixis, bispory, tetraspory, and polyembtryony.Crossref | GoogleScholarGoogle Scholar |
Chapman GP (1996) ‘The biology of grasses.’ (CAB International: Wallingford, UK)
Chase A (1918) Axillary cleistogenes in some American grasses. American Journal of Botany 5, 254–258.
| Axillary cleistogenes in some American grasses.Crossref | GoogleScholarGoogle Scholar |
Cheplick GP (2007) Plasticity of chasmogamous and cleistogamous reproductive allocation grasses. Aliso 23, 286–294.
| Plasticity of chasmogamous and cleistogamous reproductive allocation grasses.Crossref | GoogleScholarGoogle Scholar |
Cheplick GP, Clay K (1989) Convergent evolution of cleistogamy and seed heteromorphism in two perennial grasses. Evolutionary Trends in Plants 3, 127–136.
Cheplick GP, Quinn JA (1986) Self-fertilization in Amphicarpum purshii: its influence on fitness and variation of progeny from aerial panicles. American Midland Naturalist 116, 394–402.
| Self-fertilization in Amphicarpum purshii: its influence on fitness and variation of progeny from aerial panicles.Crossref | GoogleScholarGoogle Scholar |
Chippindall LKA (1955) ‘The grasses and pastures of South Africa. Part 1. A guide to the identification of grasses of South Africa.’ (Trustees of the Grasses and Pastures of South Africa Book Fund: Cape Town, South Africa)
Christie EK (1981) Biomass and nutrient dynamics in a C4 semi-arid Austraian grassland community. Journal of Applied Ecology 18, 907–918.
| Biomass and nutrient dynamics in a C4 semi-arid Austraian grassland community.Crossref | GoogleScholarGoogle Scholar |
Christin P-A, Edwards EJ, Besnard G, Boxall SF, Gregory R, Kellogg EA, Harwell J, Osborne CP (2012) Adaptive evolution of C4 photosynthesis through recurrent lateral gene transfer. Current Biology 22, 445–449.
| Adaptive evolution of C4 photosynthesis through recurrent lateral gene transfer.Crossref | GoogleScholarGoogle Scholar | 22342748PubMed |
Clay K (1982) Environmental and genetic determinants of cleistogamy in a natural population of the grass Danthonia spicata. Evolution 36, 734–741.
| Environmental and genetic determinants of cleistogamy in a natural population of the grass Danthonia spicata.Crossref | GoogleScholarGoogle Scholar | 28568236PubMed |
Clay K (1983a) The differential establishment of seedlings from chasmogamous and cleistogamous flowers in natural populations of the grass Danthonia spicata (L.) Beauv. Oecologia 57, 183–188.
| The differential establishment of seedlings from chasmogamous and cleistogamous flowers in natural populations of the grass Danthonia spicata (L.) Beauv.Crossref | GoogleScholarGoogle Scholar | 28310174PubMed |
Clay K (1983b) Variation in the degree of cleistogamy within and among species of the grass Danthonia. American Journal of Botany 70, 835–843.
| Variation in the degree of cleistogamy within and among species of the grass Danthonia.Crossref | GoogleScholarGoogle Scholar |
Clay K, Antonovics J (1985) Quantitative variation of progeny from chasmogamous and cleistogamous flowers in the grass Danthonia spicata. Evolution 39, 335–348.
| Quantitative variation of progeny from chasmogamous and cleistogamous flowers in the grass Danthonia spicata.Crossref | GoogleScholarGoogle Scholar | 28564219PubMed |
Clay K, Antonovics J (1986) Demographic genetics of the grass Danthonia spicata: success of progeny from chasmogamous and cleistogamous flowers. Evolution 39, 205–210.
Clayton WD (1990) The spikelet. In ‘Reproductive versatility in the grasses’. (Ed. P Chapman) pp. 32–51. (Cambridge University Press: Cambridge, UK)
Clifford HT (1962) Cleistogamy in Microlaena stipoides (Labill.) R.Br. University of Queensland Papers, Department of Botany 4, 63–72.
Connor HE (1979) Breeding systems in the grasses: a survey. New Zealand Journal of Botany 17, 547–574.
| Breeding systems in the grasses: a survey.Crossref | GoogleScholarGoogle Scholar |
Connor HE (1998) Breeding systems in New Zealand grasses XII. Cleistogamy in Festuca. New Zealand Journal of Botany 36, 471–476.
| Breeding systems in New Zealand grasses XII. Cleistogamy in Festuca.Crossref | GoogleScholarGoogle Scholar |
Connor HE, Matthews BA (1977) Breeding systems in New Zealand grasses VII. Cleistogamy in Microlaena. New Zealand Journal of Botany 15, 531–534.
| Breeding systems in New Zealand grasses VII. Cleistogamy in Microlaena.Crossref | GoogleScholarGoogle Scholar |
Conover DG, Sovonick-Dunford SA (1989) Influence of water deficits on the water relations and growth of Echinchloa turneriana, Echinochloa crus-galli, and Pennisetum americanum. Australian Journal of Plant Physiology 16, 291–304.
Culley TM, Klooster MR (2007) The cleistogamous breeding system: a review of its frequency, evolution, and ecology in angiosperms. The Botanical Review 73, 1–30.
| The cleistogamous breeding system: a review of its frequency, evolution, and ecology in angiosperms.Crossref | GoogleScholarGoogle Scholar |
Darbyshire SJ (2003) Danthonia D.C. In ‘Flora of North America. Vol. 25’. (Eds ME Barkworth, KM Capels, S Long, MB Piep) pp. 301–305. (Oxford University Press: New York, NY, USA)
Darwin C (1877) ‘The different forms of flowers on plants of the same species.’ (John Murray: London, UK)
De Jong T, Klinkhamer P (2005) ‘Evolutionary ecology of plant reproductive strategies.’ (Cambridge University Press: Cambridge, UK)
De Koning R, Sosef MSM, Veldkamp JF (1983) A revision of Heteropholis and Thaumastochloa (Gramineae). Gardens’ Bulletin (Singapore) 36, 137–162.
Dore WG, McNeill J (1980) ‘Grasses of Ontario.’ (Canadian Government Publishing Centre: Quebec, QC, Canada)
Endress PK (2010) Disentangling confusions in inflorescence morphology: patterns and diversity of reproductive shoot ramification in angiosperms. Journal of Systematics and Evolution 48, 225–239.
| Disentangling confusions in inflorescence morphology: patterns and diversity of reproductive shoot ramification in angiosperms.Crossref | GoogleScholarGoogle Scholar |
Everist SL (1935) Inland pastures part II. Response during 1934 season of Mitchell and other grasses in western and central Queensland. Queensland Agricultural Journal 43, 374–387.
Everist SL (1951) Notes on some plants of western Queensland. Queensland Naturalist 14, 52–55.
Everist SL (1964) The Mitchell grass country. Queensland Naturalist 17, 45–50.
Ewart AJ, Davies OB (1917) ‘Flora of the Northern Territory.’ (McCarron, Bird & Co: Melbourne, Vic., Australia)
Frankel R, Galun E (1977) ‘Pollination mechanisms reproduction and plant breeding.’ (New York Springer Verlag: New York, NY, USA)
Freckmann RW, Lelong MG (2003) Dichanthelium (Hitch. & Chase) Gould. In ‘Flora of North America, Vol. 24’. (Eds MEK Barkworth, KM Capels, S Long, MB Piep) pp. 406–450. (Oxford University Press: New York, NY, USA)
Gaff DF, Latz PK (1978) The occurrence of resurrection plants in the Australian flora. Australian Journal of Botany 26, 485–492.
| The occurrence of resurrection plants in the Australian flora.Crossref | GoogleScholarGoogle Scholar |
Gibbs Russell GE, Watson L, Koekemoer M, Smook L, Barker NP, Anderson HM, Dallwitz MJ (1990) ‘Grasses of southern Africa. Memoirs of the Botanical Survey of South Africa Number 58. (National Botanic Gardens, Botanical Research Institute: Pretoria, South Africa)
Gibson DJ (2009) ‘Grasses and grassland ecology.’ (Oxford University Press: Oxford, UK)
Godt MJW, Hamrick JL (1998) Allozyme diversity in the grasses. In ‘Population biology of the grasses’. (Ed. GP Cheplick) pp. 11–29. (Cambridge University Press: Cambridge, UK)
| Crossref |
Goodwillie C, Kalisz S, Eckert CG (2005) The evolutionary enigma of mixed mating systems in plants: occurrence, theoretical explanations, and empirical evidence. Annual Review of Ecology, Evolution, and Systematics 36, 47–79.
| The evolutionary enigma of mixed mating systems in plants: occurrence, theoretical explanations, and empirical evidence.Crossref | GoogleScholarGoogle Scholar |
Groves RH, Whalley RDB (2002) Grass and grassland ecology. In ‘Flora of Australia. Poaceae: introduction and atlas. Vol. 43’. (Eds K Mallett, AE Orchard) pp. 157–182. (ABRS: Canberra, ACT, Australia; and CSIRO Publishing: Melbourne, Vic., Australia)
Hackel E (1906) Über Kleistogamie bei den Gräsern. Österreichische Botanicheskii Zhurnal 56, 81–88.
| Über Kleistogamie bei den Gräsern.Crossref | GoogleScholarGoogle Scholar |
Harper JL, Lovell PH, Moore KG (1970) The shapes and sizes of seeds. Annual Review of Ecology and Systematics 1, 327–356.
| The shapes and sizes of seeds.Crossref | GoogleScholarGoogle Scholar |
Hattersley PW (1983) The distribution of C3 and C4 grasses in Australia. Oecologia 57, 113–128.
| The distribution of C3 and C4 grasses in Australia.Crossref | GoogleScholarGoogle Scholar | 28310164PubMed |
Hattersley P (1992) C4 photosynthetic pathway variation in grasses (Poaceae): its significance for arid and semi-arid lands. In ‘Dersertified grasslands’. (Ed. GP Chapman) pp. 181–212. (Academic Press: London, UK)
Henrard JT (1929) A monograph of the genus Aristida First Volume. Mededeelingen van’s Rijks Herbarium 58, 1–153.
Heslop-Harrison J (1961) The function of the glume pit and the control of cleistogamy in Bothriochloa decipiens (Hack.) C.E.Hubbard. Phytomorphology 11, 378–383.
Hitchcock AS (1924) The North American species of Aristida. Contributions from the United States National Herbarium 22, 517–586.
Hitchcock AS (1936) ‘The genera of grasses of the United States with special reference to the economic species.’ (United States Department of Agriculture: Washington, DC, USA)
Hitchcock AS (1950) ‘Manual of the grasses of the United States’, 2nd edn. (United States Government Printing Office: Washington, DC, USA)
Hitchcock AS, Chase A (1915) Tropical North American species of Panicum. Contributions from the United States National Herbarium 17, 459–539.
Hubbard CE (1933a) Cleistochloa subjuncea C.E.Hubbard. Hooker’s Icones Plantarum 33, 3209
Hubbard CE (1933b) Calyptochloa gracillima C.E.Hubbard. Hooker’s Icones Plantarum 33, 3210
Hubbard CE (1937) Enneapogon asperatus C.E.Hubbard. Hooker’s Icones Plantarum 37, 3337
Hunter DM (1989) The response of Mitchell grasses (Astrebla spp.) and button grass (Dactyloctenium radulans (R.Br.)) to rainfall and their importance to the survival of the Australian plague locust, Chortoicetes terminifera (Walker), in the arid zone. Australian Journal of Ecology 14, 467–471.
| The response of Mitchell grasses (Astrebla spp.) and button grass (Dactyloctenium radulans (R.Br.)) to rainfall and their importance to the survival of the Australian plague locust, Chortoicetes terminifera (Walker), in the arid zone.Crossref | GoogleScholarGoogle Scholar |
Jacobs SWL, Everett J (1996) Austrostipa, a new genus, and new names for Australian species formerly included in Stipa (Gramineae). Telopea 6, 579–595.
| Austrostipa, a new genus, and new names for Australian species formerly included in Stipa (Gramineae).Crossref | GoogleScholarGoogle Scholar |
Jacobs SWL, Everett J, Connor HE, Edgar E (1989) Stipoid grasses in New Zealand. New Zealand Journal of Botany 27, 569–582.
| Stipoid grasses in New Zealand.Crossref | GoogleScholarGoogle Scholar |
Jacobs SWL, Whalley RDB, Wheeler DJB (2008) ‘Grasses of New South Wales.’ (University of New England: Armidale, NSW, Australia)
Jozwik FX (1970) Response of Mitchell grasses (Astrebla F. Muell.) to photoperiod and temperature. Australian Journal of Agricultural Research 21, 395–405.
| Response of Mitchell grasses (Astrebla F. Muell.) to photoperiod and temperature.Crossref | GoogleScholarGoogle Scholar |
Jozwik FX, Nicholls AO, Perry RA (1970) Studies on the Mitchell grasses (Astrebla F. Muell.). In ‘Proceedings of the XI international grassland congress’, 13–23 April 1970, Surfers Paradise, Qld, Australia. (Ed. MJT Norman) pp. 48–51. (Australian National University Press: Canberra, ACT, Australia)
Kannenberg LW, Allard RW (1967) Population studies in predominantly self-pollinated species. VIII. Genetic variability in the Festuca microstachys complex. Evolution 21, 227–240.
| Population studies in predominantly self-pollinated species. VIII. Genetic variability in the Festuca microstachys complex.Crossref | GoogleScholarGoogle Scholar | 28556141PubMed |
Kellogg EA (1990) Variation and species limits in agamospermous grasses. Systematic Botany 15, 112–123.
| Variation and species limits in agamospermous grasses.Crossref | GoogleScholarGoogle Scholar |
Kellogg EA (2006) Beyond taxonomy: prospects for understanding morphological diversity in the grasses (Poaceae). Darwiniana 44, 7–17.
Kirchoff BK, Claßen-Bockhoff R (2013) Inflorescences: concepts, function, development and evolution. Annals of Botany 112, 1471–1476.
| Inflorescences: concepts, function, development and evolution.Crossref | GoogleScholarGoogle Scholar | 24383103PubMed |
Klingenberg CP, Gidaszewski NA (2010) Testing and quantifying phylogenetic signals and homoplasy in morphometric data. Systematic Biology 59, 245–261.
| Testing and quantifying phylogenetic signals and homoplasy in morphometric data.Crossref | GoogleScholarGoogle Scholar | 20525633PubMed |
Laude HM (1949) Delayed germination of California oatgrass, Danthonia californica. Agronomy Journal 41, 404–408.
| Delayed germination of California oatgrass, Danthonia californica.Crossref | GoogleScholarGoogle Scholar |
Lazarides M (1970) ‘The grasses of central Australia.’ (Australian National University Press: Canberra, ACT, Australia)
Lazarides M (1992) Resurrection grasses (Poaceae) in Australia. In ‘Dersertified grasslands’. (Ed. GP Chapman) pp. 213–234. (Academic Press: London, UK)
Lazarides M, Lenz J, Watson L (1991) Clausospicula, a new Australian genus of grasses (Poaceae, Andropogoneae). Australian Systematic Botany 4, 391–405.
| Clausospicula, a new Australian genus of grasses (Poaceae, Andropogoneae).Crossref | GoogleScholarGoogle Scholar |
Lazarides M, Van den Borre A, Weiller CM (2005) Eriachne. In ‘Flora of Australia. Poaceae 3. Vol. 44B’. (Ed. K Mallett) pp. 132–175. (ABRS: Canberra, ACT, Australia; and CSIRO Publishing: Melbourne, Vic., Australia)
Lerner PD, Bai Y, Morici EFA (2008) Does seed heteromorphism have different roles in the fitness of species with contrasting life history strategies? Botany 86, 1404–1415.
| Does seed heteromorphism have different roles in the fitness of species with contrasting life history strategies?Crossref | GoogleScholarGoogle Scholar |
Lindauer LL, Quinn JA (1972) Germination ecology of Danthonia sericea populations. American Journal of Botany 59, 942–951.
| Germination ecology of Danthonia sericea populations.Crossref | GoogleScholarGoogle Scholar |
Lloyd DG (1984) Variation stragegies of plants in hetergeneous environments. Biological Journal of the Linnean Society 21, 357–385.
| Variation stragegies of plants in hetergeneous environments.Crossref | GoogleScholarGoogle Scholar |
Lloyd DG, Schoen DJ (1992) Self- and cross-fertilization in plants. I. Functional dimensions. International Journal of Plant Sciences 153, 358–369.
| Self- and cross-fertilization in plants. I. Functional dimensions.Crossref | GoogleScholarGoogle Scholar |
Lord EM (1981) Cleistogamy: a tool for the study of floral morphogenesis, function and evolution. Botanical Review 47, 421–449.
| Cleistogamy: a tool for the study of floral morphogenesis, function and evolution.Crossref | GoogleScholarGoogle Scholar |
Maxwell CD, Zobel A, Woodfine D (1994) Somatic polymorphism in the achenes of Tragopogon dubius. Canadian Journal of Botany 72, 1282–1288.
| Somatic polymorphism in the achenes of Tragopogon dubius.Crossref | GoogleScholarGoogle Scholar |
McClusker A (2002) Key to tribes of Australian Grasses. In ‘Flora of Australia. Poaceae 1. Vol. 43’. (Ed. K Mallett) pp. 249–252. (ABRS: Canberra, ACT, Australia; and CSIRO Publishing: Melbourne, Vic., Australia)
Morrone O, Scataglini MA, Zuloaga FO (2007) Cyphonanthus, a new genus segregated from Panicum (Poaceae: Panicoideae: Paniceae) based on morphological, anatomical and molecular data. Taxon 56, 521–532.
| Cyphonanthus, a new genus segregated from Panicum (Poaceae: Panicoideae: Paniceae) based on morphological, anatomical and molecular data.Crossref | GoogleScholarGoogle Scholar |
Morrone O, Denham SS, Aliscioni SS, Zuloaga FO (2008) Parodiophyllochloa, a new genus segregated from Panicum (Paniceae, Poaceae) based on morphological and molecular data. Systematic Botany 33, 66–76.
| Parodiophyllochloa, a new genus segregated from Panicum (Paniceae, Poaceae) based on morphological and molecular data.Crossref | GoogleScholarGoogle Scholar |
Nightingale ME, Weiller CM (2005) Astrebla. In ‘Flora of Australia. Poaceae 3. Vol. 44B’. (Ed. K Mallett) pp. 452–457. (ABRS: Canberra, ACT, Australia; and CSIRO Publishing: Melbourne, Vic., Australia)
Oakley CG, Moriuchi KS, Winn AA (2007) The maintenance of outcrossing in predominantly selfing species: ideas and evidence from cleistogamous species. Annual Review of Ecology, Evolution, and Systematics 38, 437–457.
| The maintenance of outcrossing in predominantly selfing species: ideas and evidence from cleistogamous species.Crossref | GoogleScholarGoogle Scholar |
Ornduff R (1969) Reproductive biology in relation to systematics. Taxon 18, 121–133.
| Reproductive biology in relation to systematics.Crossref | GoogleScholarGoogle Scholar |
Orr DM (1975) A review of Astrebla (Mitchell grass) pastures in Australia. Tropical Grasslands 9, 21–36.
Orr DM (1991) Trends in the recruitment of Astreba spp. in relation to seasonal rainfall. The Rangeland Journal 13, 107–117.
| Trends in the recruitment of Astreba spp. in relation to seasonal rainfall.Crossref | GoogleScholarGoogle Scholar |
Orr DM (1998) A life cycle approach to the population ecology of two tropical grasses in Queensland, Australia. In ‘Population biology of grasses’. (Ed. GP Cheplick) pp. 366–389. (Cambridge University Press: Cambridge, UK)
Orr DM, Evenson CJ (1991a) Effect of single clipping on the seed production response of Astrebla spp. The Rangeland Journal 13, 57–60.
| Effect of single clipping on the seed production response of Astrebla spp.Crossref | GoogleScholarGoogle Scholar |
Orr DM, Evenson CJ (1991b) Effects of sheep grazing Astrebla grasslands in central western Queensland. III. Dynamics of Astrebla spp. under grazing and exclosure between 1975 and 1986. The Rangeland Journal 13, 36–48.
| Effects of sheep grazing Astrebla grasslands in central western Queensland. III. Dynamics of Astrebla spp. under grazing and exclosure between 1975 and 1986.Crossref | GoogleScholarGoogle Scholar |
Osborne CP, Freckleton RP (2008) Ecological selection pressures for C4 photosynthesis in the grasses. Proceedings of the Royal Society of London – B. Biological Sciences 276, 1753–1760.
| Ecological selection pressures for C4 photosynthesis in the grasses.Crossref | GoogleScholarGoogle Scholar |
Philipson MN (1986) A re-assessment of the form of reproduction in Danthonia spicata (L.) Beauv. New Phytologist 103, 231–243.
| A re-assessment of the form of reproduction in Danthonia spicata (L.) Beauv.Crossref | GoogleScholarGoogle Scholar |
Plitmann U (1995) Distribution of dimorphic flowers as related to other elements of the reproductive strategy. Plant Species Biology 10, 53–60.
| Distribution of dimorphic flowers as related to other elements of the reproductive strategy.Crossref | GoogleScholarGoogle Scholar |
Poulter AA (1932) Occurrences of ‘cleistogenes’ in certain grasses. Nature 129, 690–691.
| Occurrences of ‘cleistogenes’ in certain grasses.Crossref | GoogleScholarGoogle Scholar |
Quinn JA (1998) Ecological aspects of sex expression in grasses. In ‘Population biology of grasses’. (Ed. GP Cheplick) pp. 136–154. (Cambridge University Press: Cambridge, UK)
Quiroga RE, Golluscio RA, Blanco LJ, Fernandez RJ (2010) Aridity and grazing as convergent selective forces: an experiment with an Arid Chaco bunchgrass. Ecological Applications 20, 1876–1889.
| Aridity and grazing as convergent selective forces: an experiment with an Arid Chaco bunchgrass.Crossref | GoogleScholarGoogle Scholar | 21049876PubMed |
Reinheimer R, Amsler A, Vegetti AC (2013a) Insights into panicoid inflorescence evolution. Organisms, Diversity & Evolution 13, 97–110.
| Insights into panicoid inflorescence evolution.Crossref | GoogleScholarGoogle Scholar |
Reinheimer R, Vegetti AC, Rua GH (2013b) Macroevolution of panicoid inflorescences: a history of contingency and order of trait acquisition. Annals of Botany 112, 1613–1628.
| Macroevolution of panicoid inflorescences: a history of contingency and order of trait acquisition.Crossref | GoogleScholarGoogle Scholar | 23478945PubMed |
Richards AJ (1990) The implications of reproductive versatility for the structure of grass populations. In ‘Reproductive versatility in the grasses’. (Ed. PG Chapman) pp. 131–153. (Cambridge University Press: Cambridge, UK)
Roe R (1941) Studies on the Mitchell grass association in south-western Queensland. 1. Some observations on the response of Mitchell grass pastures to good summer rains following the 1940 drought. Journal of the Council of Scientific and Industrial Research 14, 253–259.
Scanlan JC (1983) Changes in tiller and tussock characteristics of Astrebla lappacea (curly Michell grass) after burning. Australian Rangeland Journal 5, 13–19.
| Changes in tiller and tussock characteristics of Astrebla lappacea (curly Michell grass) after burning.Crossref | GoogleScholarGoogle Scholar |
Schoen DJ, Lloyd DG (1984) The selection of cleistogamy and heteromorphic diaspores. Biological Journal of the Linnean Society 23, 303–322.
| The selection of cleistogamy and heteromorphic diaspores.Crossref | GoogleScholarGoogle Scholar |
Sicard A, Lenhard M (2011) The selfing syndrome: a model for studying the genetic and evolutionary basis of morphological adaptation in plants. Annals of Botany 107, 1433–1443.
| The selfing syndrome: a model for studying the genetic and evolutionary basis of morphological adaptation in plants.Crossref | GoogleScholarGoogle Scholar | 21303786PubMed |
Silcock JL, Healy AJ, Fensham RJ (2014) Lost in time and space: re-assessment of conservation status in an arid-zone flora through targeted field survey. Australian Journal of Botany 62, 674–688.
| Lost in time and space: re-assessment of conservation status in an arid-zone flora through targeted field survey.Crossref | GoogleScholarGoogle Scholar |
Silvertown JW (1984) Phenotypic variety in seed germination behaviour: the ontogeny and evolution of somatic polymorphism in seeds. The American Naturalist 124, 1–16.
| Phenotypic variety in seed germination behaviour: the ontogeny and evolution of somatic polymorphism in seeds.Crossref | GoogleScholarGoogle Scholar |
Simon BK (1989) Studies in Australian grasses: 4. Taxonomic and nomenclatural studies in Australian Andropogoneae. Austrobaileya 3, 79–99.
Simon BK (2002) Key to genera of Australian grasses. In ‘Flora of Australia. Poaceae 1: introduction and atlas. Vol. 43’. (Eds K Mallett, AE Orchard) pp. 263–277. (ABRS: Canberra, ACT, Australia; and CSIRO Publishing: Melbourne, Vic., Australia)
Soenarko S (1977) The genus Cymbopogon. Reinwardtia 9, 225–237.
Soreng RJ, Peterson PM, Romaschenko K, Davidse G, Teisher JK, Clark LG, Barberá P, Gillespie LJ, Zuloaga FO (2017) A worldwide phylogenetic classification of the Poaceae (Gramineae) II: an update and a comparison of the two 2015 classifications. Journal of Systematics and Evolution 55, 259–290.
| A worldwide phylogenetic classification of the Poaceae (Gramineae) II: an update and a comparison of the two 2015 classifications.Crossref | GoogleScholarGoogle Scholar |
Sorensen AE (1978) Somatic polymorphism and seed dispersal. Nature 276, 174–176.
| Somatic polymorphism and seed dispersal.Crossref | GoogleScholarGoogle Scholar |
Stebbins GL (1957) Self fertilisation and population variability in the higher plants. The American Naturalist 91, 337–354.
| Self fertilisation and population variability in the higher plants.Crossref | GoogleScholarGoogle Scholar |
Thompson EJ (2017) Elionurus purpureus (Panicoideae: Andropogoneae: Rottboelliinae), a new species for Queensland: circumscription and breeding system. Austrobaileya 10, 139–162.
Thompson EJ, Fabillo M (2021) The impact of multiple molecular and morphological data sets on the phylogenetic reconstruction of subtribe Neurachninae (Poaceae: Panicoideae: Paniceae). Australian Systematic Botany 34, 227–251.
| The impact of multiple molecular and morphological data sets on the phylogenetic reconstruction of subtribe Neurachninae (Poaceae: Panicoideae: Paniceae).Crossref | GoogleScholarGoogle Scholar |
Thompson EJ, Simon BK (2012) A revision of Calyptochloa C.E.Hubb. (Poaceae), with two new species and a new subspecies. Austrobaileya 8, 634–652.
Tothill JC, Hacker JB (1983) ‘The grasses of Southern Queensland.’ (University of Queensland Press: Brisbane, Qld, Australia)
Townrow JES (1978) The genus Stipa L. in Tasmania. Part 3 – revised taxonomy. Papers and Proceedings of the Royal Society of Tasmania 112, 227–287.
| The genus Stipa L. in Tasmania. Part 3 – revised taxonomy.Crossref | GoogleScholarGoogle Scholar |
Uphof JCT (1938) Cleistogamic flowers. Botanical Review 4, 21–49.
| Cleistogamic flowers.Crossref | GoogleScholarGoogle Scholar |
Van Oudtshoorn F (1999) ‘Guide to grasses of Southern Africa.’ (Briza Publications: Pretoria, South Africa)
Vickery JW, Jacobs SWL, Everett J (1986) Taxonomic studies in Stipa (Poaceae) in Australia. Telopea 3, 1–132.
| Taxonomic studies in Stipa (Poaceae) in Australia.Crossref | GoogleScholarGoogle Scholar |
Watson L, Dallwitz MJ (1992) ‘The grass genera of the world.’ (University Press: Cambridge, UK)
Weatherwax P (1928) Cleistogamy in two species of Danthonia. Botanical Gazette 85, 104–109.
| Cleistogamy in two species of Danthonia.Crossref | GoogleScholarGoogle Scholar |
Webster RD (1987) ‘The Australian Paniceae (Poaceae).’ (J. Cramer: Berlin, Germany)
Weiller CM (2005) Pheidochloa. In ‘Flora of Australia. Poaceae 3. Vol. 44B’. (Ed. K Mallett) pp. 175–176. (ABRS: Canberra, ACT, Australia; and CSIRO Publishing: Melbourne, Vic., Australia)
Weiller CM, Lazarides M (2005) Enneapogon. In ‘Flora of Australia. Poaceae 3. Vol. 44B’. (Ed. K Mallett) pp. 188–202. (ABRS: Canberra, ACT, Australia; and CSIRO Publishing: Melbourne, Vic., Australia)
Whalley RDB, Chivers IH, Waters CM (2013) Revegetation with Australian native grasses – a reassessment of the importance of using local provenances. The Rangeland Journal 35, 155–166.
| Revegetation with Australian native grasses – a reassessment of the importance of using local provenances.Crossref | GoogleScholarGoogle Scholar |
Williams DB, Roe R (1975) Management of arid grasslands for sheep: plant demography of six grasses in relation to climate and grazing. Proceedings of the Ecological Society of Australia 9, 142–156.
Wright BR, Zuur AF, Chan GCK (2014) Proximate causes and possible adaptive functions of mast seeding and barren flower shows in spinifex grasses (Triodia spp.) in arid regions of Australia. The Rangeland Journal 36, 297–308.
| Proximate causes and possible adaptive functions of mast seeding and barren flower shows in spinifex grasses (Triodia spp.) in arid regions of Australia.Crossref | GoogleScholarGoogle Scholar |
Yates HO (1966) Morphology and cytology of Uniola (Gramineae). The Southwestern Naturalist 11, 145–189.
| Morphology and cytology of Uniola (Gramineae).Crossref | GoogleScholarGoogle Scholar |
Yates HO (2003) 17.02 Uniola L. In ‘Flora of North America. Vol. 25’. (Eds ME Barkworth, KM Capels, S Long, MB Piep) pp. 22–24. (Oxford University Press: New York, NY, USA)
Yu P, Prakash N, Whalley RDB (2000) Comparative reproduction biology of the vulnerable common grasses in Bothriochloa and Dichanthium. In ‘Grasses: systematics and evolution’. (Eds SWL Jacobs, JE Everett) pp. 307–315. (CSIRO Publishing: Melbourne, Vic., Australia)
Yu P, Prakash N, Whalley RDB (2003) Sexual and apomictic seed development in the vulnerable grass Bothriochloa biloba. Australian Journal of Botany 51, 75–84.
| Sexual and apomictic seed development in the vulnerable grass Bothriochloa biloba.Crossref | GoogleScholarGoogle Scholar |