Reproductive biology of the threatened and highly fragmented shrub Persoonia hirsuta (Proteaceae)
Nathan J. Emery A * and Catherine A. Offord A
A * and Catherine A. Offord A
A The Australian PlantBank, Australian Institute of Botanical Science, Australian Botanic Garden, Mount Annan, NSW 2567, Australia.
Australian Journal of Botany 70(1) 56-62 https://doi.org/10.1071/BT21068
Submitted: 1 June 2021 Accepted: 10 November 2021 Published: 7 December 2021
© 2022 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
The Australian Proteaceous genus Persoonia is known to be pollinated by bees and have variable breeding systems that range from strictly self-incompatible to differing levels of self-compatibility. The endangered Persoonia hirsuta (Hairy Geebung) is a species currently in decline throughout its distribution, with many populations occurring in fragmented habitats comprising fewer than 10 plants or a single isolated individual. Despite its threatened status and recent population decline, the reproductive biology of P. hirsuta is unknown. In this study, we surveyed floral visitors and their foraging behaviour, and investigated the breeding system of P. hirsuta by conducting experimental hand-pollinations at two of the largest known populations. P. hirsuta was almost exclusively visited by native bees, including Leioproctus, Megachile and Tetragonula species. This study was the first to report Xylocopa bees and Zizinia butterflies visiting Persoonia flowers. On average, both foraging time per flower and the number of flowers visited per plant varied significantly among insect genera. Notably, Xylocopa bees visited more flowers per plant than did any other pollinator (22.1 ± 10.8 flowers). P. hirsuta has a breeding system that displays partial self-incompatibility with significantly higher fruit set in the cross- and open-pollination treatments at both populations (19.4 ± 10.8 to 44.8 ± 8.2%) than in the autogamy and selfed treatments (0.6 ± 0.6 to 9.0 ± 5.1%). The results of this study are critical to the future management of P. hirsuta, and suggest that its small and isolated populations may produce very few viable fruits in the absence of outcrossing.
Keywords: bees, breeding system, habitat fragmentation, hairy geebung, Leioproctus, Megachile, pollination, self-incompatibility, Xylocopa.
Introduction
Habitat fragmentation owing to anthropogenic pressures is a major cause of plant rarity by increasing population isolation and reducing population size, and has knock-on effects on ecosystem processes such as plant–pollinator interactions (Wilcock and Neiland 2002; Field et al. 2005; Aguilar et al. 2006; Schurr et al. 2019). Pollinator behaviour varies depending on the spatial arrangement of plants and their flowers as smaller populations are typically less attractive to local pollinators, resulting in fewer between-plant visits and more within-plant visits, than larger populations with greater floral displays (Lamont et al. 1993; Mustajärvi et al. 2001; Field et al. 2005; Grobler and Campbell 2020). Small populations may not contain enough resources for pollinators, in which case some pollinators may travel further for additional resources and eventually result in between-population crossing (Dick et al. 2008). The impact of fragmented habitats on reproductive success also depends on its pollinator community. For insect-pollinated plants, there may be an overall reduction in reproductive success, an increase in inbreeding or reduced gene flow (Aguilar et al. 2008). Fragmentation size is also an important consideration in plant–pollinator interactions because bee communities can have a sustained persistence in some habitat fragments (Cane 2001). Therefore, plant–pollinator interactions are of particular interest to conservation biology because rare and threatened plants often occur in small populations that might be at risk of local extinction if pollinator behaviour negatively affects reproductive success.
Quantifying how changes to pollinator behaviour might affect reproductive success also depends on the breeding system of the plant. For self-compatible or autogamous species, pollen limitation owing to reduced pollinator visitation may not negatively affect fruit set, but could reduce fitness because of inbreeding (Charlesworth and Charlesworth 1987; Aguilar et al. 2006). By contrast, pollen limitation and self-pollination are likely to have significant negative effects on self-incompatible plant species by potentially causing reproductive failure (Kunin 1997; Aizen et al. 2002; Aguilar et al. 2006). This effect may be exacerbated in obligate-seeding species that produce small numbers of viable seeds or have soil seedbanks that rapidly lose viability. Importantly, there are also species with partial self-compatible breeding systems, and the level of self-compatibility in other species can vary among populations (Eckert 2002). Consequently, partially self-incompatible rare species that have fragmented distributions may require direct intervention using population supplementation to lower the risk of inbreeding and reproductive failure (Auld et al. 2018). Therefore, understanding the breeding system and pollinator community of these species is important in the context of conservation management.
Persoonia is an Austalian genus of some 99 species that occur in 64 of the country’s 87 bioregions (Emery and Offord 2018), and is one of the few Proteaceae groups that have small, singular and inconspicuous flowers that lack a pollen presenter. Reproductive biology is an important consideration for the ongoing management of Persoonia species because plants have a very low rate of fruit set that may also vary significantly within a population (Collins and Rebelo 1987; Chia et al. 2015; Emery and Offord 2019), and several species have soil seedbanks that rapidly lose viability in the short term (Auld et al. 2000; McKenna 2007; Nield et al. 2015). Persoonia species are most frequently pollinated by a diversity of native bees as well as the European honeybee (Apis mellifera; Bernhardt and Weston 1996). However, the breeding system has been studied only in ∼10% of Persoonia species, and many were reported as partially self-incompatible (Emery and Offord 2018, 2019; Tierney et al. 2020). In addition, nine Persoonia species are currently rare or threatened under State and Federal legislation, having experienced population decline, range contraction and habitat fragmentation as a result of fire or urban development (Emery and Offord 2018). In a study that investigated the reproductive success of two rare and two common Persoonia species, the common species (P. mollis subsp. nectens and P. lanceolata) had three times more native bee visits than did the rare species (P. glaucescens and P. mollis subsp. maxima; Rymer et al. 2005). Consequently, there was a significantly lower fruit set in the open-pollination treatment when comparing rare and common species (18 v. 35–41%), suggesting that pollen limitation may be contributing to reduced reproduction in the rare species. Rare Persoonia species with partial self-incompatible breeding systems are also at a greater risk of inbreeding depression when occurring in small, sparse populations because of increased rates of self-pollen loads and geitonogamous pollination (Field et al. 2005).
In this study, we examined the pollinator composition and behaviour as well as the breeding system of the endangered Persoonia hirsuta Pers. (hairy geebung), a long-lived shrub species with a highly fragmented distribution confined to the Sydney region of New South Wales (NSW). Despite a large area of occurrence, almost all wild populations have declined over the past two decades, with many surviving as fewer than 10 adult plants or as an isolated individual. Following recent field surveys, 12 surviving populations and isolated plants remained, down from 21 populations a little over a decade ago. Populations are thought to have declined from inappropriate fire management, coupled with increasingly fragmented populations as a result of land clearing and urban development. Recruitment events among populations are rare but may be triggered by disturbance events such as fire or grading fire trails (N. J. Emery, pers. obs.). A recent genetic study of three P. hirsuta populations using microsatellite markers showed similar allelic richness but with little differentiation, suggesting that these isolated populations were once connected (Ayre et al. 2021). The authors suggested that the species is likely to have a mixed or outcrossed breeding system. Because pollinator surveys and experimental hand-pollinations had not been conducted, this study was an opportunity to determine the essential pollinators of P. hirsuta and whether the breeding system shows self-incompatibility.
Materials and methods
Study species
Persoonia hirsuta is a spreading to decumbent shrub growing between 0.3 and 1.5 m tall and is most notably distinguished from other Persoonia species by its hairiness (i.e. hirsute), with young branchlets being moderately to densely hairy (Benson and McDougall 2000). The species is currently listed as Endangered under the Federal Environment Protection and Biodiversity Conservation Act 1999 and NSW Biodiversity Conservation Act 2016. The characteristic tubular yellow flowers are hermaphroditic, ∼1 cm long and hairy, and occur randomly in the axils of new growth. Peak flowering is from November to January, with larger plants having several hundred open flowers during this time (N. J. Emery, pers. obs.). Mature fruits drop from the maternal plants approximately 8–9 months after flowering. Plants often occur on ridge tops or mid-slopes in dry sclerophyll forests and woodlands on acidic sandy to stony soils derived from sandstone. The largest historical populations occurred along roadside easements and edges of fire tails, and under powerlines. Like for other reported Persoonia species, it was thought that native bees and honeybees were the primary pollinators of P. hirsuta (Bernhardt and Weston 1996), but no surveys had been conducted prior to this study. The study populations comprised 20 adult plants along two adjoining roadsides in Glenorie, and 18 adult plants in a cleared area underneath a transmission tower in Parr State Conservation Area (hereafter called ‘Parr’). The populations were approximately 25 km apart from one another and both occurred north-west of Sydney. At the time of this study, these sites contained the largest extant populations, and the only two sites with enough plants to feasibly conduct hand-pollinations. Owing to asynchronous flowering among the two populations, coupled with the significant, widespread bushfires across NSW in summer 2019–2020, experimental work at each site was conducted in separate years.
Pollinator surveys
To determine the diversity, abundance and effectiveness of insect visitors, we recorded observations on the behaviour and movement of individual insects on P. hirsuta plants. Pollinators were observed for a period of 60 min between 1000 and 1500 hours on 3 days over November and December at both Glenorie in 2019 and Parr in 2020. We recorded the time (in seconds) an insect spent foraging on a flower, and whether it contacted the stigma and the number of flowers it visited on the plant before it flew away or to another plant. Between-plant tracking of insects was not possible because of the very low density of P. hirsuta plants at both sites. Two native bees were identified to species level in the field, but the remaining insect visitors could be identified only to genus level.
Data for pollinator foraging time and the number of flowers visited per plant showed a Poisson distribution and analyses were conducted as generalised linear mixed effects models by using the lme4 package (Bates et al. 2015) in R (ver. 3.5.0, R Foundation for Statistical Computing, Vienna, Austria, see https://www.r-project.org/). Data from each population was analysed separately. We used invertebrate genus for consistency among visitors as a fixed effect and survey day nested within site was included as a random factor in both models. Likelihood ratio tests using an ANOVA were performed to determine significance of invertebrate genera, and a parametric bootstrap was also run to examine random effects. Pairwise comparisons between invertebrate genera were made using Tukey’s test where applicable, by using the emmeans package (ver. 1.6.0, R. V. Length, see https://CRAN.R-project.org/package=emmeans).
Breeding system
Experimental pollinations were conducted to characterise the breeding system at Glenorie in November 2019 and at Parr in November 2020. Five large plants at least 5 m apart from one another were selected for hand-pollinations. Of the available flowering branches at least 20 cm in length and with more than five floral buds, four were randomly selected per plant and assigned to one of the following treatments:
-
autogamy: the branch was bagged prior to flowers opening and not manipulated further;
-
self: the branch was bagged prior to flowering; anthers were removed from flowers that opened within the bag, self-pollen was applied to the stigma, treated flowers were tagged and then the branch was re-bagged;
-
cross: as per the self-treatment (2), except pollen from a mixture of at least three conspecific plants at least 5 m away was applied to the stigma of treated flowers;
-
open: the branch and floral buds were tagged and left to allow pollinators to visit.
The number of tagged flowers were counted for all treated branches. We removed any insect-damaged buds, as well as any flowers that were already open or had senesced before bagging. Pollinators were excluded using large organza bags with a mesh size sufficient to exclude visitors, while allowing air and moisture to pass through. Treated flowers were tagged with 2-mm rubber bands placed over and immediately below the flowers or liquid nail polish painted on the stem below the flowers (depending on the most feasible method per treated flower). Pollen was collected and applied to treated flowers following the method described by Rymer et al. (2005). Receptive flowers (i.e. visibly glistening stigma) were pollinated over 4–7 days, until between 5 and 36 flowers were treated per branch. Fruit set was recorded in February 2020 (Glenorie) and March 2021 (Parr) when fruit size was ∼5 mm in length (i.e. fruit initiation).
Fruit set was calculated as the proportion of the total flowers treated and was the dependent variable in a single-factor binomial generalised linear model (GLM). Each population was analysed separately. Pairwise comparisons between pollination treatments were made using Tukey’s test by using the emmeans package (ver. 1.6.0, see https://CRAN.R-project.org/package=emmeans). Analyses were run in R (ver. 3.5.0, R Foundation for Statistical Computing).
Results
Pollinator surveys
In total, 43 pollinators from 6 genera and 112 pollinators from 5 genera were recorded over 3 survey days at Glenorie and Parr respectively. All pollinators were either native bees or blue butterflies (Zizina), the latter being recorded in Parr only (Fig. 1, Table 1). One species was identified for both Hylaeus (H. perhumilis) and Tetragonula (T. carbonaria). Behavioural observations indicated different foraging strategies among insect genera and were reflected in significantly different foraging times per flower (P < 0.001). Tetragonula and Zizina species often landed on tepals, moved around the anthers to collect pollen and very rarely contacted the stigma, and foraged on flowers for significantly longer than did other pollinators (21.9 ± 3.2 and 21.5 ± 4.2 s respectively; Table 1). Leioproctus and Xylocopa bees also foraged on flowers for significantly longer than did other bees (5.9 ± 0.3 and 4.1 ± 0.2 s respectively). Leioproctus bees most frequently contacted the stigma and anthers because individuals tended to bend the latter backwards while foraging for pollen and nectar. Xylocopa bees often contacted the tips of anthers and the stigma owing to their large size (Fig. 1). Amegilla, Hylaeus and Megachile species often foraged from the outside of flowers and only occasionally contacted the stigma (Fig. 1). Pollinators also significantly differed in the number of flowers visited per plant (P < 0.001). On average, Xylocopa bees typically visited more flowers per plant than did any other pollinator (22.1 ± 10.8 flowers; Table 1). Tetragonula and Zizina species were often observed visiting one or two flowers per plant before flying away. Pollinator foraging time and number of flower visits per plant were similar between sites (P = 0.535; Table 1). There was a significant effect of survey days within sites on both pollinator forage time and the number of flowers visited (P < 0.001).
|
|

|
Breeding system
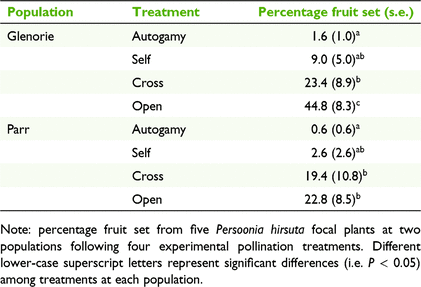
Pollination treatments had a significant effect on percentage fruit set at both populations (P < 0.001). Our results showed that fruit set success among treatments within each population had a similar trend (Table 2). On average, more fruits set from flowers left open to pollinators than from bagged treatments at both Glenorie (44.8% compared with 1.6–23.4%) and Parr (22.8% compared with 0.6–19.4%; Table 2). Fruit set in the open treatment at Glenorie was significantly higher than the crossed treatment (P < 0.001), but these treatments were statistically similar at Parr (P = 0.937). Both autogamy and selfing treatments produced comparably few fruits at Glenorie and Parr (P = 0.220 and 0.190 respectively), and the latter was not statistically different from the cross-pollination treatment at both populations (P = 0.085 and P = 0.140; Table 2). The few set fruits in the autogamy and selfing treatments relative to the other treatments suggests that P. hirsuta has partial self-incompatibility.
|
Discussion
This study demonstrated that native bees are the main floral visitors to Persoonia hirsuta. In particular, Xylocopa, Leioproctus, Megachile and Tetragonula carbonaria were some of common native bees observed. Xylocopa and Leioproctus bees were the most active visitors, foraging on flowers for longer and contacting the stigma more frequently than did other bee species. The reliance on these biotic pollen vectors is highlighted by the partially self-incompatible breeding system of P. hirsuta illustrated by our pollination experiments at the two largest extant populations, and supports recent genetic evidence (Ayre et al. 2021). Our results showed that P. hirsuta is a preferential outcrosser with more fruits being initiated in the crossed (19.4–23.4%) and open (22.8–44.8%) pollination treatments than following self- or autogamous pollination (<10%).
Leioproctus (Cladocerapis) bees are reported as the most common and efficient pollinators of Persoonia (Bernhardt and Weston 1996; Wallace et al. 2002; Emery and Offord 2019), and were the most frequent genera observed visiting P. hirsuta flowers (Table 1). Maynard (1995) outlined several anatomical features of Leioproctus bees, such as a flattened clypeus that facilitates effective pollen and nectar gathering from Persoonia flowers. Leioproctus has been reported as a common flower visitor for several rare and common, obligate-seeding Persoonia species (Wallace et al. 2002; Field et al. 2005; Rymer et al. 2005; Emery and Offord 2019). Notably, this study is the first to report Xylocopa bees and Zizina butterflies visiting Persoonia flowers. On average, Xylocopa bees visited more flowers per plant than did any other recorded visitor and, because of their large size, frequently contacted the stigma and anthers while foraging. Several other native bee genera are also known to visit Persoonia species, including Exoneura, Amegilla, Tetragonula and Hylaeus (Bernhardt and Weston 1996), and several of these bee genera were observed visiting P. hirsuta flowers (Table 1). Importantly, only A. mellifera, Exoneura spp. and Leioproctus spp. have been observed to collect large amounts of pollen and regularly contact the stigmas of Persoonia flowers (Bernhardt and Weston 1996; Wallace et al. 2002). This was also the case in this study, because Amegilla, Hylaeus and Megachile species occasionally accessed nectar from the outside of flowers, often landing on the tepals without contacting the stigma. Furthermore, A. mellifera is thought to be an ineffecient pollinator because individuals often mix pollen with nectar on their bodies, causing premature pollen germination (Paton 1997). Interestingly, A. mellifera was not observed visiting P. hirsuta flowers at either population, and was also not observed visiting dominant, co-flowering species, including Grevillea buxifolia and Angophora hispida.
Our results showed that selfing within the two Persoonia hirsuta populations was negliable as fruit set occurred in less than 10% of flowers with self-pollen. Persoonia species have contrasting breeding systems that range from being highly outcrossed with almost no selfed fruits in P. mollis (Krauss 1994) to no evidence of self-incompatibility in P. rigida (Trueman and Wallace 1999). More commonly reported is partial self-incompatibility in other obligate-seeding species, including P. bargoensis, P. glaucescens, P. lanceolata, P. mollis subsp. maxima, P. mollis subsp. nectens, P. pauciflora and P. virgata (Wallace et al. 2002; Field et al. 2005; Rymer et al. 2005; Emery and Offord 2019). Fruit set following open pollination at Glenorie (44.8%) is comparable with that in many previous studies of other Persoonia species that occur in the Sydney region (Cadzow and Carthew 2000; Wallace et al. 2002; Field et al. 2005; Rymer et al. 2005), and was higher than in the manual cross-pollination treatment. Fruit set success in the open-pollination treatment at Parr was much lower (22.8%) but similar to previous results in other rare Persoonia species (Krauss 1994; Tierney et al. 2020). The proportion of P. hirsuta fruits set in the open-pollination treatment could not be matched by the crossed hand-pollination treatment at Glenorie. This contrasts with previous reports of other Persoonia species where cross- and open-pollination treatments produced comparable fruit sets (Wallace et al. 2002; Field et al. 2005; Rymer et al. 2005). There are two possible explanations. First, that local pollinators were not limited, and the abundance and density of P. hirsuta plants at this population may facilitate a higher likelihood of outcrossed pollination and attractiveness of plants to potential pollinators. This is further supported by the cross- and open-pollination treatments at Parr being similar. However, both sites had similar numbers of plants (18–20 mature plants), which were considerbly fewer and more sparse than other reports of rare Persoonia species from the Sydney region where pollen was limited (Field et al. 2005; Rymer et al. 2005). Second, this result could be explained by high pollinator effectiveness at Glenorie because approximately half of the surveyed floral visitors (51.2%) comprised Leioproctus and Xylocopa bees that frequently contacted stigmas and anthers, compared with approximately one-third of visits (38.4%) at Parr. This may also be compounded if bagging branches had a detrimental effect on fruit set; however, this might be unlikely because both the crossed and open treatments produced similar numbers of initiated fruits at Parr. Additionally, it is plausible that the efficiency of hand-pollinating flowers was more variable within or among plants and populations than was that of leaving flowers open to pollinators. It is suggested that future studies include a supplementary hand-pollination treatment to determine whether pollen is limited. Repeat breeding system manipulations will also help determine possible negative effects of hand-pollination.
In this study, we reported on fruit set as initiated fruits that had swollen gynoeciums 81 and 109 days after pollination treatment at Glenorie and Parr respectively. Although this method has been used in pollination studies of other Persoonia species (Wallace et al. 2002), it is possible that the proportion of set fruits in this study are not carried through to fruit maturity. Persoonia fruits require up to 12 months to reach maturity and disperse from the maternal plant, and other studies have suggested that several species have a post-zygotic genetic mechanism that recognises selfed fruits and terminates most of these during the maturing process (Krauss 1994; Cadzow and Carthew 2000; Emery and Offord 2019). For example, a proportion of early set fruits of P. pauciflora were terminated over 6 months, resulting in an overall decline in fruit production (Emery and Offord 2019). Importantly, fruit termination in P. pauciflora plants was more pronounced in the three ‘selfing’ pollination treatments than in the crossed treatment. A similar result was observed in P. juniperina where selfed and crossed fruits aborted over an initial 8-week period, 4 weeks post-pollination (Cadzow and Carthew 2000). It is likely that not all P. hirsuta self-pollinated fruits terminate before maturity because several isolated plants have been observed to produce mature fruits in situ (N. J. Emery, pers. obs.). Additionally, Trueman and Wallace (1999) demonstrated that P. rigida fruits may be terminated at different times throughout the maturation process if branches were defoliated. Further investigations are required to determine the rate of abortion throughout fruit maturation of P. hirsuta.
Significant urban expansion in the Sydney region is thought to have accelerated the fragmentation of P. hirsuta habitat, resulting in increasingly isolated and small populations. Surveys conducted between 2017 and 2019 confirmed several recently extinct populations and ∼50% of the more than 600 historical records were not recorded (N. J. Emery, unpubl. data). More recently, the catastrophic fire season in NSW over spring and summer 2019–2020 has severely affected several P. hirsuta populations at the northern and southern limits of its known distribution. Almost all unaffected populations persist as 20 or fewer individuals spread across several localised sites. As P. hirsuta is a preferential outcrosser, the impacts of habitat fragmentation and modification are likely to be severe and may potentially lead to very limited reproductive success and continued population decline (Aguilar et al. 2006). Given the results of this study, coupled with the mostly small and isolated extant populations, future management actions for this species should consider direct intervention to supplement declining populations with genetically diverse plant material to promote outcrossing.
Data availability
The data that support this study will be shared upon reasonable request to the corresponding author.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Declaration of funding
This work was funded by an Australian Coal Industry ACARP scientific grant (Project C28028).
Acknowledgements
The authors thank Justin Collette, Christie Foster and Lyndle Hardstaff for field assistance.
References
Aguilar R, Ashworth L, Galetto L, Aizen MA (2006) Plant reproductive susceptibility to habitat fragmentation: review and synthesis through a meta-analysis. Ecology Letters 9, 968–980.| Plant reproductive susceptibility to habitat fragmentation: review and synthesis through a meta-analysis.Crossref | GoogleScholarGoogle Scholar | 16913941PubMed |
Aguilar R, Quesada M, Ashworth L, Herrerias-Diego Y, Lobo J (2008) Genetic consequences of habitat fragmentation in plant populations: susceptible signals in plant traits and methodological approaches. Molecular Ecology 17, 5177–5188.
| Genetic consequences of habitat fragmentation in plant populations: susceptible signals in plant traits and methodological approaches.Crossref | GoogleScholarGoogle Scholar | 19120995PubMed |
Aizen MA, Ashworth L, Galetto L (2002) Reproductive success in fragmented habitats: do compatibility systems and pollination specialization matter? Journal of Vegetation Science 13, 885–892.
| Reproductive success in fragmented habitats: do compatibility systems and pollination specialization matter?Crossref | GoogleScholarGoogle Scholar |
Auld TD, Keith DA, Bradstock RA (2000) Patterns in longevity of soil seedbanks in fire-prone communities of south-eastern Australia. Australian Journal of Botany 48, 539–548.
| Patterns in longevity of soil seedbanks in fire-prone communities of south-eastern Australia.Crossref | GoogleScholarGoogle Scholar |
Auld T, Freestone M, Broadhurst L, Zimmer H, Swarts N, Gallagher R (2018) Deciding whether to translocate. In ‘Guidelines for the translocation of threatened plants in Australia’, 3rd edn. (Eds LE Commander, DJ Coates, L Broadhurst, CA Offord, RO Makinson, M Mattes) pp. 9–26. (Australian Network for Plant Conservation: Canberra, ACT, Australia)
Ayre D, Haynes A, Gregory D (2021) Low genetic differentiation despite fragmentation in an endangered fire-sensitive shrub. International Journal of Plant Sciences 182, 229–237.
| Low genetic differentiation despite fragmentation in an endangered fire-sensitive shrub.Crossref | GoogleScholarGoogle Scholar |
Bates D, Mächler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. Journal of Statistical Software 67, 1–48.
| Fitting linear mixed-effects models using lme4.Crossref | GoogleScholarGoogle Scholar |
Benson D, McDougall L (2000) Ecology of Sydney plant species. Part 7b: dicotyledon family Proteaceae to Rubiaceae. Cunninghamia 6, 1016–1202.
Bernhardt P, Weston P (1996) The pollination ecology of Persoonia (Proteaceae) in eastern Australia. Telopea 6, 775–804.
| The pollination ecology of Persoonia (Proteaceae) in eastern Australia.Crossref | GoogleScholarGoogle Scholar |
Cadzow B, Carthew SM (2000) Breeding system and fruit development in Persoonia juniperina (Proteaceae). Cunninghamia 6, 941–950.
Cane JH (2001) Habitat fragmentation and native bees: a premature verdict? Conservation Ecology 5, 3–10.
| Habitat fragmentation and native bees: a premature verdict?Crossref | GoogleScholarGoogle Scholar |
Charlesworth D, Charlesworth B (1987) Inbreeding depression and its evolutionary consequences. Annual Review of Ecology and Systematics 18, 237–268.
| Inbreeding depression and its evolutionary consequences.Crossref | GoogleScholarGoogle Scholar |
Chia KA, Koch JM, Sadler R, Turner SR (2015) Developmental phenology of Persoonia longifolia (Proteaceae, R.Br.) and the impact of fire on these events. Australian Journal of Botany 63, 415–425.
| Developmental phenology of Persoonia longifolia (Proteaceae, R.Br.) and the impact of fire on these events.Crossref | GoogleScholarGoogle Scholar |
Collins BG, Rebelo T (1987) Pollination biology of the Proteaceae in Australia and southern Africa. Australian Journal of Ecology 12, 387–421.
| Pollination biology of the Proteaceae in Australia and southern Africa.Crossref | GoogleScholarGoogle Scholar |
Dick CW, Hardy OJ, Jones FA, Petit RJ (2008) Spatial scales of pollen and seed-mediated gene flow in tropical rain forest trees. Tropical Plant Biology 1, 20–33.
| Spatial scales of pollen and seed-mediated gene flow in tropical rain forest trees.Crossref | GoogleScholarGoogle Scholar |
Eckert CG (2002) Effect of geographical variation in pollinator fauna on the mating system of Decodon verticillatus (Lythraceae). International Journal of Plant Sciences 163, 123–132.
| Effect of geographical variation in pollinator fauna on the mating system of Decodon verticillatus (Lythraceae).Crossref | GoogleScholarGoogle Scholar |
Emery NJ, Offord CA (2018) Managing Persoonia (Proteaceae) species in the landscape through a better understanding of their seed biology and ecology. Cunninghamia 18, 89–107.
Emery NJ, Offord CA (2019) Environmental factors influencing fruit production and seed biology of the critically endangered Persoonia pauciflora (Proteaceae). Folia Geobotanica 54, 99–113.
| Environmental factors influencing fruit production and seed biology of the critically endangered Persoonia pauciflora (Proteaceae).Crossref | GoogleScholarGoogle Scholar |
Field DL, Ayre DJ, Whelan RJ (2005) The effect of local plant density on pollinator behavior and the breeding system of Persoonia bargoensis (Proteaceae). International Journal of Plant Sciences 166, 969–977.
| The effect of local plant density on pollinator behavior and the breeding system of Persoonia bargoensis (Proteaceae).Crossref | GoogleScholarGoogle Scholar |
Grobler BA, Campbell EE (2020) Pollinator activity and the fecundity of a rare and highly threatened honeybush species along a highway in the Cape Floristic Region. International Journal of Plant Sciences 181, 581–593.
| Pollinator activity and the fecundity of a rare and highly threatened honeybush species along a highway in the Cape Floristic Region.Crossref | GoogleScholarGoogle Scholar |
Krauss SL (1994) Preferential outcrossing in the complex species Persoonia mollis R.Br. (Proteaceae). Oecologia 97, 256–264.
| Preferential outcrossing in the complex species Persoonia mollis R.Br. (Proteaceae).Crossref | GoogleScholarGoogle Scholar | 28313937PubMed |
Kunin WE (1997) Population size and density effects in pollination: pollinator foraging and plant reproductive success in experimental arrays of Brassica kaber. Journal of Ecology 85, 225–234.
| Population size and density effects in pollination: pollinator foraging and plant reproductive success in experimental arrays of Brassica kaber.Crossref | GoogleScholarGoogle Scholar |
Lamont BB, Klinkhamer PGL, Witkowski ETF (1993) Population fragmentation may reduce fertility to zero in Banksia goodii – a demonstration of the Allee effect. Oecologia 94, 446–450.
| Population fragmentation may reduce fertility to zero in Banksia goodii – a demonstration of the Allee effect.Crossref | GoogleScholarGoogle Scholar | 28313684PubMed |
Maynard GV (1995) Pollinators of Australian Proteaceae. Flora of Australia 16, 30–36.
McKenna DJ (2007) Demographic and ecological indicators of rarity in a suite of obligate-seeding Persoonia (Proteaceae) shrubs. PhD thesis, Biological Sciences, University of Wollongong.
Mustajärvi K, Siikamäki P, Rytkönen S, Lammi A (2001) Consequences of plant population size and density for plant–pollinator interactions and plant performance. Journal of Ecology 89, 80–87.
| Consequences of plant population size and density for plant–pollinator interactions and plant performance.Crossref | GoogleScholarGoogle Scholar |
Nield AP, Monaco S, Birnbaum C, Enright NJ (2015) Regeneration failure threatens persistence of Persoonia elliptica (Proteaceae) in Western Australian jarrah forests. Plant Ecology 216, 189–198.
| Regeneration failure threatens persistence of Persoonia elliptica (Proteaceae) in Western Australian jarrah forests.Crossref | GoogleScholarGoogle Scholar |
Paton DC (1997) Honey bees Apis mellifera and the disruption of plant–pollinator systems in Australia. Victorian Naturalist 114, 23–29.
Rymer PD, Whelan RJ, Ayre DJ, Weston PH, Russell KG (2005) Reproductive success and pollinator effectiveness differ in common and rare Persoonia species (Proteaceae). Biological Conservation 123, 521–532.
| Reproductive success and pollinator effectiveness differ in common and rare Persoonia species (Proteaceae).Crossref | GoogleScholarGoogle Scholar |
Schurr L, Affre L, Flacher F, Tatoni T, Le Mire Pecheux L, Geslin B (2019) Pollination insights for the conservation of a rare threatened plant species, Astragalus tragacantha (Fabaceae). Biodiversity and Conservation 28, 1389–1409.
| Pollination insights for the conservation of a rare threatened plant species, Astragalus tragacantha (Fabaceae).Crossref | GoogleScholarGoogle Scholar |
Tierney DA, Ahrens C, Rymer P, Auld TD (2020) The interaction of clonality, breeding system and genomics for a highly threatened plant species and the management implications. Biodiversity and Conservation 29, 3009–3029.
| The interaction of clonality, breeding system and genomics for a highly threatened plant species and the management implications.Crossref | GoogleScholarGoogle Scholar |
Trueman S, Wallace H (1999) Pollination and resource constraints on fruit set and fruit size of Persoonia rigida (Proteaceae). Annals of Botany 83, 145–155.
| Pollination and resource constraints on fruit set and fruit size of Persoonia rigida (Proteaceae).Crossref | GoogleScholarGoogle Scholar |
Wallace HM, Maynard GV, Trueman SJ (2002) Insect flower visitors, foraging behaviour and their effectiveness as pollinators of Persoonia virgata R.Br. (Proteaceae). Australian Journal of Entomology 41, 55–59.
| Insect flower visitors, foraging behaviour and their effectiveness as pollinators of Persoonia virgata R.Br. (Proteaceae).Crossref | GoogleScholarGoogle Scholar |
Wilcock C, Neiland R (2002) Pollination failure in plants: why it happens and when it matters. Trends in Plant Science 7, 270–277.
| Pollination failure in plants: why it happens and when it matters.Crossref | GoogleScholarGoogle Scholar | 12049924PubMed |


