Germinating the seeds of three species of Pimelea sect. Epallage (Thymelaeaceae)1
Richard G. Silcock A C and Michael B. Mann BA Agri-Science Queensland, Ecosciences Precinct, Dutton Park, Qld 4102, Australia.
B Formerly Agri-Science Queensland, Leslie Research Centre, Toowoomba, Qld 4350, Australia.
C Corresponding author. Email: richard.silcock@daff.qld.gov.au
Australian Journal of Botany 62(1) 74-83 https://doi.org/10.1071/BT13297
Submitted: 11 December 2013 Accepted: 15 March 2014 Published: 28 April 2014
Abstract
Pimelea trichostachya Lindl., P. simplex F.Muell. and P. elongata Threlfall frequently cause pimelea poisoning of cattle. Fresh seeds of these species, belonging to sect. Epallage (Endl.) Benth. of Pimelea Gaertn. (Thymelaeaceae) are strongly dormant for years when in laboratory storage. Common methods of stimulating germination, such as scarification, dry heat and cold stratification, did not remove much of the dormancy. ‘Smoke water’ stimulated some germination but its effect was unpredictable and many seedlings then grew aberrantly. Exposure of imbibed seeds to gibberellic acid greatly and reliably improved the germination of all three species. However, the manner of application and the concentration of gibberellic acid used had to be appropriate or many young seedlings grew abnormally or died suddenly, limiting successful plant establishment rates. The dormancy type involved is non-deep Type 2 physiological. Ten days of good moisture, in addition to gibberellic acid exposure, is required before appreciable laboratory germination occurs at optimal temperatures. Thus, the mechanism by which gibberellic acid stimulates good germination does not appear to be the same as that which primes seeds for the rapid and prolific germination often seen under natural conditions in arid Australia. Seeds of P. simplex subsp. continua (J.M.Black) Threlfall proved most difficult to germinate and those of P. elongata the easiest.
Additional keywords: dormancy, gibberellic acid, smoke water.
Introduction
The genus Pimelea (Thymelaeaceae) has at least 90 species and is restricted to Australasia. Most species have a dry, single-seeded fruit encased in the dry floral tube (hypanthium) but a few from sect. Pimelea have succulent fruits, e.g. P. microcephala R.Br. (Rye 1990). The genus has been studied primarily for floriculture, e.g. P. rosea R.Br. and P. floribunda Meisn. (Keighery and Dixon 1984; King et al. 1992; Seaton 2005), or as plants that poison cattle, mainly P. trichostachya Lindl. and P. simplex F.Muell. (Everist 1980; Dadswell 1994). Seeds from fleshy fruits of P. arenaria A.Cunn. (Dawson et al. 2005) and P. actea C.J.Burrows (Burrows 2008) can be germinated without much trouble, whereas those from species with dry fruits are often problematic (Burrows 2008). Species used as decorative flowers are propagated vegetatively (Keighery and Dixon 1984; Seaton 2005) because their seeds are difficult to germinate or produce in bulk. Dixon et al. (1995) reported that smoke directly improved germination of some provenances of P. spectabilis Lindl. and P. sylvestris R.Br., whereas Roche et al. (1997) used smoke to stimulate four Pimelea species to earlier germination from a very low base, zero to 23% for P. suaveolens Meisnr.
We required a ready supply of plants of three Pimelea species that cause pimelea poisoning of cattle (Fletcher 2008), namely P. elongata, P. simplex and P. trichostachya, for toxicology studies. They are all members of sect. Epallage that grow widely in inland Australia and have dry fruit (Rye 1990). However, our preliminary attempts to germinate seeds freshly collected in the field were unsuccessful, despite using classical germination-enhancement strategies.
‘Smoke water’ was difficult to obtain commercially and was not tested at that time. The only information initially available for these species reported that the optimal temperature for germination of P. trichostachya was in the range of 15−25°C and that it was not stimulated by light, alternating temperatures or gibberellic acid (Pressland and Dadswell 1992; Dadswell et al. 1994). A related study (Douglas 1992) reported that seeds of P. trichostachya that were stored under silica gel for 6 months could be partially germinated if the seedcoat was manually nicked. We could not reproduce this outcome using nicked fresh seed and it is difficult to do without a high rate of embryo damage. Douglas (1992) also reported that a tetrazolium test for viability of the seeds found that only 12–14% contained a viable embryo, a similar proportion contained ‘incomplete’ embryos and ~75% were empty.
Hence, we conducted new research on ways to increase seed germination of P. elongata, P. simplex and P. trichostachya, so as to grow plants for toxicology studies.
Materials and methods
Seed material
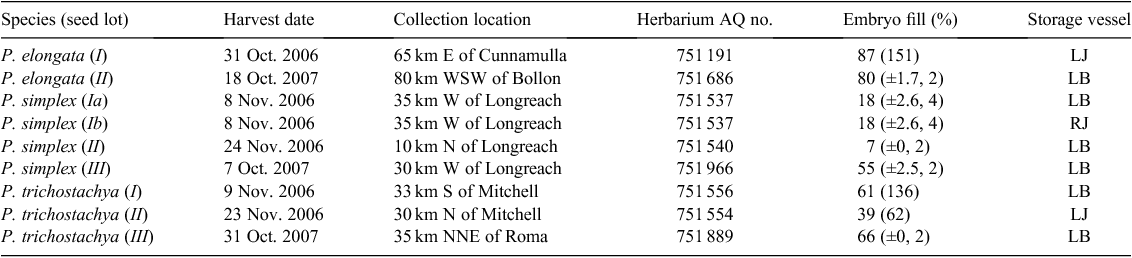
Pimelea seed lots were field collected from various sites in western Queensland during spring of 2006 and 2007 (Table 1). Flowering adult voucher specimens were obtained from each site. The P. simplex type was consistently identified as subspecies continua (J.M.Black) Threlfall. Seasonal conditions were verging on drought in many districts at this time, so finding adequate amounts of good seeds was a problem. Seeds were generally collected directly from the parent plants by either cutting and swathing, banging heads inside plastic buckets or hand-stripping. One line of P. simplex (II) seeds was collected off the ground with a hand-held vacuum device. Seed material (diaspores consisting of a single seed enclosed in a hairy hypanthium) was stored in the laboratory in either sealed jars or paper bags for periods of between 3 and 18 months, before the various germination experiments.
Embryo fill (Table 1) was assessed by cutting diaspores with a scalpel blade, to find the proportion that contained a plump, white embryo. Lots of 20 or 25 seeds were used in some assessments (fewer lots where the count variability was low), whereas in others, a large number of diaspores was sectioned.
Germination tests
Standard germination environment
All germination tests were conducted in a standardised germination environment that would approximate field conditions in inland Australia during rainy spring or autumn weather. This was a 150-L germination cabinet with 12 h fluorescent light daily during the 25°C warm cycle and 12 h darkness at 15°C. Seeds were spread out to germinate on Whatman No. 1 filter paper in 9-cm-diameter Petri dishes with a loosely fitting lid. Three replicates of 50 or 100 diaspores were used for each treatment. The paper was wetted to slight excess with potable town tapwater or reverse-osmosis (RO) water and any moisture loss was corrected daily by a further addition of RO water. Tests ran for ~21 days and germinated seedlings were removed regularly, usually daily, after they appeared. Seeds were rated as germinated if the radicle protruded at least 1 mm from a split seedcoat.
The reported experiments evolved as our knowledge grew, but the quantity of seeds initially available influenced which lots were used in the first studies. In early 2007, we had most seeds of lots with the lowest embryo-fill percentage.
Experiment 1. Differing stimulation agents
This germination test used two lines of seeds known to have a reasonable level of embryo fill and that were over a year old and had exhibited a small degree of germination in prior tests. They were P. elongata (I) and P. trichostachya (I) (Table 1) and they were tested with a range of chemical solutions in the filter paper rather than water. These solutions were 5 mL of
-
100 µg g–1 w/v AR-grade gibberellic acid (GA3 – MWt 346.4) dissolved in RO water,
-
500 µg g–1 w/v AR-grade GA3 dissolved in RO water,
-
0.2% KNO3 in RO water, or
-
RO water.
Experiment 2. Effectiveness of GA3 on P. simplex seed
Having achieved a significant germination benefit in Experiment 1 from including GA3 in the germination substrate, we tested it next on the most difficult species to germinate, P. simplex. Two seed lots of very different ages of P. simplex (Ia and III) were used, being 16 and 5 months old, respectively, at the time. Twenty-four samples of 100 diaspores were counted out. Half the samples were individually pre-soaked in 10 mL of 500 µg g–1 GA3 solution in a vial for 24 h at room temperature and half were not pre-soaked. Then, all 24 lots, 2 ages × 4 treatments × 3 replications, were put at the same time on to the filter paper substrate wetted with either town water or 5 mL of 100 µg g–1 GA3. The pH of the wet substrates was determined with a Merck Universalindikator pH-range dipstick (Merck KGaA, Darmstadt, Germany).
Experiment 3. Effectiveness of ‘smoke water’
In this test, the paper substrate was moistened with 5 mL of a diluted solution of either of two proprietary ‘smoke-water’ products provided by Technica (Australian National Botanical Gardens (ANBG) 2011; Grayson 2011). One was called Smokemaster® and the other Regen Direct®. The concentration used followed label recommendations, namely dilution with water to 10% for both. Seed lots P. elongata (I) and P. trichostachya (I) were used to compare species responses. The RO water data from Experiment 1 served as the control.
Experiment 4. Multi-factorial stimulation of germination
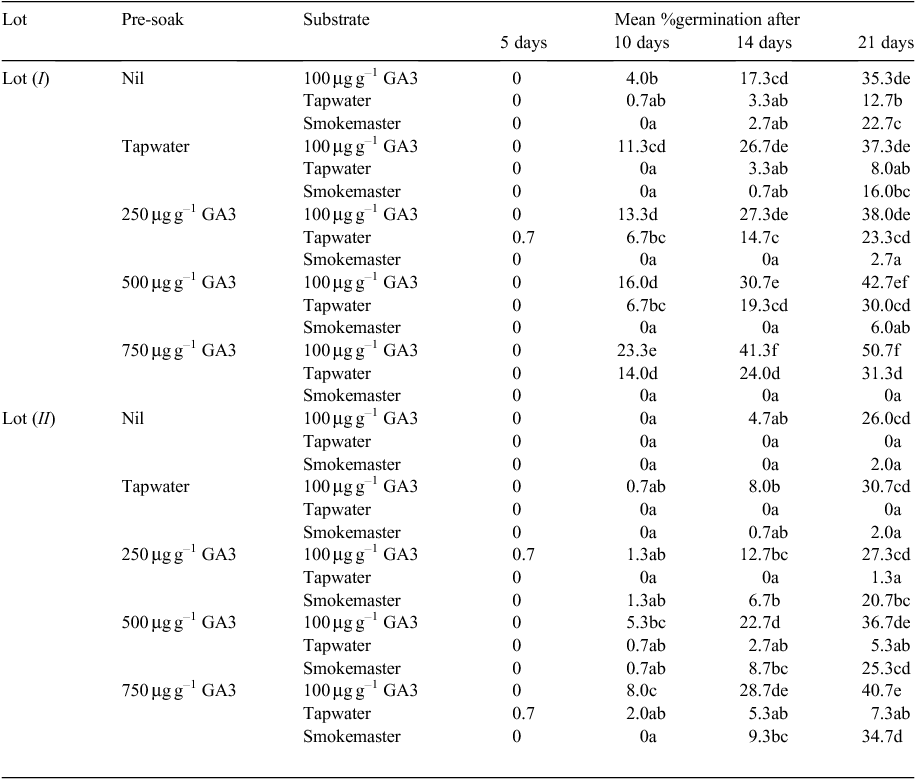
A multi-factorial experiment was then conducted using P. elongata (I and II) seeds that were subjected to a combination of three pre-soaking GA3 concentrations or tapwater or no pre-soaking, and all were then germinated on substrates infused with either 10% ‘Smokemaster’ or 100 µg g–1 GA3. At the time, these seeds were 16 and 5 months old, respectively.
The pre-soaking treatments lasted 24 h and used tapwater or 250 µg g–1, 500 µg g–1 or 750 µg g–1 w/v GA3 solution. Diaspores in groups of 50 were soaked in 20-mL jars containing 5 mL of the respective solution. No wetting agent was added but the groups of furry diaspores, which tend to be hydrophobic, were individually re-stirred and mixed after 6 and 22 h of soaking. Soaked diaspores were then drained, tamped dry with paper towel and placed immediately on pre-wetted filter paper that contained 5 mL of tapwater, 100 µg g–1 GA3 or 10% ‘Smokemaster’. The control seed lots that had not been pre-soaked were put on to the various substrates at the same time. In summary, there were three replicates of 50 diaspores for two seed lots of differing ages, five pre-soaking treatments and three substrate solutions. The pH of the wet substrates was determined with a Merck Universalindikator pH-range dipstick.
Experiment 5. GA3 pre-soaking versus GA3 substrate infusion
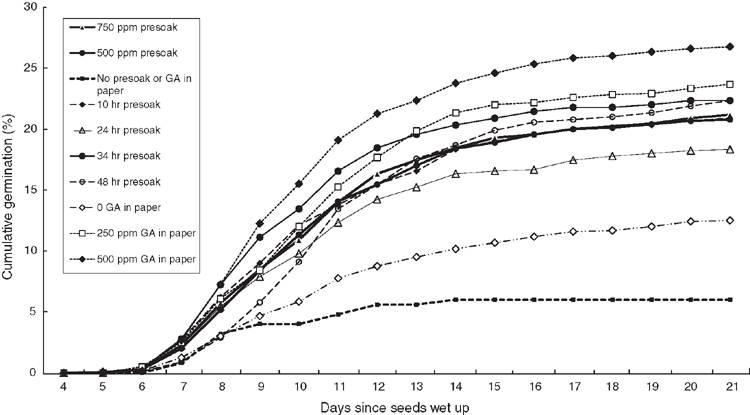
This experiment tested the relative impact of pre-soaking in GA3 versus GA3 infused into the germination substrate. Diaspores of P. trichostachya (I) that were 18 months old were pre-soaked in triplicate in lots of 50 in 5 mL of 750 µg g–1 or 500 µg g–1 GA3 for 10, 24, 34 or 48 h. Because a wetting agent was not used, regular stirring and gentle compaction of the wet seed mass was required to achieve complete early wetting. After soaking, diaspores were drained, excess moisture tamped off with paper towels and diaspores were then spread evenly over filter paper moistened with 5 mL of RO water, 250 µg g–1 GA3 solution or 500 µg g–1 GA3 solution. A control treatment of five replications of 50 diaspores with no pre-soaking and only RO water in the substrate filter paper began when the pre-soaking treatments commenced.
Experiment 6. Optimised GA3-mediated germination of nine seed lots
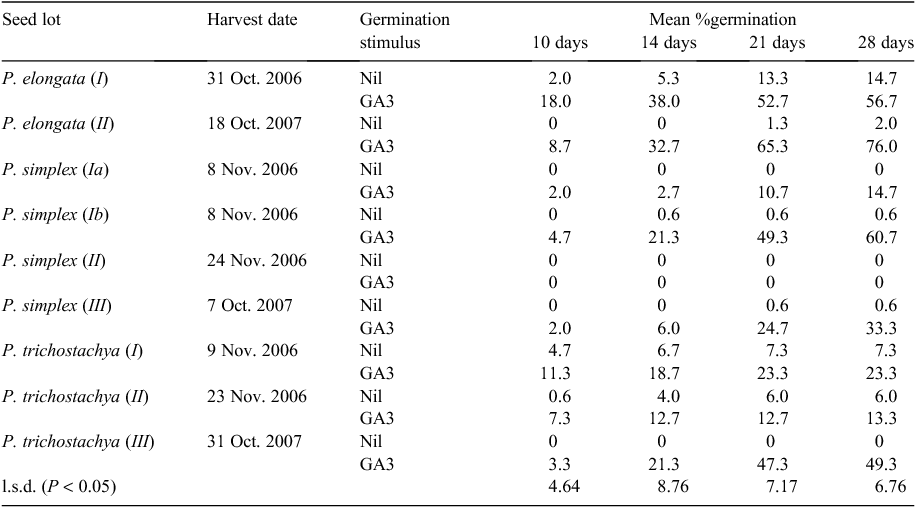
With a big GA3 stimulus of Pimelea germination confirmed and near-optimal concentration and application regime determined so as to minimise aberrant growth after germination, nine lots of seeds, some previously used (Table 1), were now tested simultaneously using the most beneficial agents. Lots of 50 diaspores were germinated on filter paper pre-soaked with 5 mL of either 250 µg g–1 GA3 or tapwater. The diaspores had either been pre-soaked for 24 h in 5 mL of 500 µg g–1 GA3 solution (plus a drop of dilute BS1000 wetting agent to make immersion easier) or left air dry until placed on the filter paper. An extra treatment was added for the P. trichostachya (I) seeds whereby a drop of diluted BS1000 wetting agent was added to the substrate filter paper of both the GA3 and tapwater treatments to hasten the wetting of such hairy seeds.
Germination was assessed for 28 days thereafter, with regular germination counts and observations on fungal infection levels.
Statistical analysis
Results were subjected to statistical analysis using the Genstat v.11 software package (Genstat 2008). Data were entered as counts and an unblocked ANOVA was run on each germination experiment. Fisher’s protected t-test was used to distinguish statistically different treatments.
Results
Embryo fill of diaspores was generally acceptable (Table 1), but two of the P. simplex lots had a low level, namely 7% and 18%. The poorest fill was from material vacuum harvested from off the ground. Fill levels were much higher in the second year for the more hairy diaspores of P. simplex and P. trichostachya, probably because our improved understanding by then of seed development and plant ecology allowed better targeting of the appropriate seed-maturation stage.
The embryo radicle is contained in the narrower section of each seed and extends out from there as the first sign of germination after the seed coat splits longitudinally down both sides under internal pressure after moisture uptake. Some seeds split the seed coat without continuing to germinate during the trial period and were not counted.
Experiment 1. Effect of KNO3 and GA3
Gibberellic acid stimulated the germination of seeds of both species; however, it was a gradual enhancement rather than a switching on of activity (Table 2). By comparison, KNO3 in the substrate had no major effect on germination, compared with RO water. A higher concentration of GA3 in the substrate stimulated germination of P. elongata seeds far more than it did of P. trichostachya seeds from 14 days onward. For both species, the effect of GA3 concentration was significant (P < 0.05) after 22 days. Thus, GA3 caused one-third of the filled P. trichostachya seeds to germinate and almost two-thirds of the filled P. elongata seeds to germinate (Table 1).

|
Fungal growth on the external cover of diaspores was generally more pronounced on P. trichostachya and that too was less pronounced where GA3 was in the substrate than where KNO3 or RO water was used.
Experiment 2. Effect of pre-soaking seeds in GA3
The older lot of P. simplex had hardly any seeds germinate by 21 days and, contrary to Experiment 1, there was no significant response to applied GA3 either by pre-soaking or in the substrate (Table 3). By contrast, the younger lot with a much greater proportion of filled diaspores (Table 1) responded significantly to GA3 in the substrate and to a 24-h soaking in 500 µg g–1 GA3 solution (Table 3). The stimulus was even greater if GA3 was used both in a pre-soaking and in the germination substrate.
Again, the earliness with which germination began was not strongly affected compared with the final enhanced level of germination achieved. Nearly 3 weeks was required for appreciable germination to be recorded in even the most responsive treatments.
Germination on a substrate infused with tapwater was slightly enhanced by pre-soaking in GA3. Fungal activity was less on seeds that had either been pre-soaked in GA3 solution or had GA3 infused into the filter-paper substrate. The pH of the germination medium was ~5–7 for both tapwater and GA3 substrates.
Experiment 3. Effect of ‘smoke water’
The ‘smoke-water’ treatments had a variable effect on the germination of the Pimelea seed lots used. ‘Smokemaster’ significantly improved the level of germination of P. trichostachya seeds after 21 days (P < 0.001) but did not hasten the onset of germination compared with tapwater and GA3 in Experiment 1, which was conducted at the same time. In contrast, ‘Regen Direct’ inhibited the germination of both species at the 10% concentration used (Table 4).

|
An appreciable amount of fungal growth was seen on the paper treated with ‘Smokemaster’ and several of the P. trichostachya seedlings in the ‘Smokemaster’ dishes grew abnormally after the radicle emerged. There was no fungal colonisation of seeds or paper in the ‘Regen Direct’ dishes and the pH of that medium was ~2.5–3, compared with 3.5 for ‘Smokemaster’ and 5–7 for GA3 and tapwater in Experiment 1. Thorough rinsing of the ‘Regen Direct’ dishes with tapwater after 21 days, before returning them to the germination environment for a further 11 days, resulted in five seeds germinating after another 6 days, although most exhibited aberrant growth.
Experiment 4. Multifactorial stimulation
The effect of each of the 15 treatments is shown in Table 5. The best germination after 21 days (50% from Lot I and 40% from Lot II P. elongata diaspores) was achieved by pre-soaking in 750 µg g–1 GA3 and then germinating on paper infused with 100 µg g–1 GA3. Pre-soaking in 500 µg g–1 GA3 also produced good results (43% from Lot I and 37% from Lot II diaspores). At any time for both seed lots, germination on a substrate infused with GA3 was greater from seeds pre-soaked in GA3 and always significantly greater from 750 µg g–1 than from 250 µg g–1 in the pre-soak solution. The worst individual result was zero germination from diaspores that lay on a substrate infused with tapwater or ‘Smokemaster’ after various pre-soaking treatments, including 750 µg g–1 GA3. A complex interaction between ‘Smokemaster’ in the germination substrate and pre-soaking treatments was apparent (Table 5).
The main treatment effects summarised in Table 6 show that the older seed lot (Lot I) had greater germinability (P < 0.001) and that increasing concentrations of GA3 during pre-soaking could slightly increase germination (P < 0.001). However, the greatest improvement was produced by the inclusion of GA3 in the substrate during germination and it greatly out-performed ‘Smokemaster’ and tapwater for wetting the paper (P < 0.001). After 21 days, ‘Smokemaster’ solution in the substrate had no significant effect compared with tapwater (Table 6). Also pre-soaking for 24 h in 250 µg g–1 GA3 did not significantly (P > 0.05) increase germination. However, pre-soaking in 500 or 750 µg g–1 GA3 produced a significant and similar increase (Table 6). The slightly better embryo fill of Lot I diaspores (87 vs 80%) does not fully explain the significant difference in the final germination achieved by the two seed lots.

|
Fungal growth was more vigorous on papers infused with ‘Smokemaster’ than on those infused with tapwater. Fungal activity was slightly less on the diaspores that had either been pre-soaked in GA3 solution or that had GA3 infused into the substrate.
Experiment 5. GA3 stimulation via different methods and concentrations
Significant germination (P < 0.05) of the 14-month-old P. trichostachya seeds did not commence until 7 days after imbibition began and was largely complete by Day 14 (Fig. 1). The greatest rate of germination was by seeds pre-soaked in GA3 and those with GA3 infused into the germination paper (Fig. 1). Seeds soaked longest in GA3 (48 h) were slow to begin germinating but did catch up to most other GA3 treatments by Day 12. Seeds that were not pre-soaked and were germinated on paper moistened by tapwater reached only 6% germination in 21 days, whereas those that received some pre-soaking in GA3 achieved at least twice that level of germination even if they had only tapwater in the germination substrate.
There was no significant (P > 0.05) enhancement of final germination by increasing the length of pre-soaking from 10 h (20.9%) to 34 h (22.3%), with a similar difference at 14 days. Pre-soaking in 750 µg g–1 GA3 achieved a final germination similar to that from 500 µg g–1, of 21.2% and 20.8%, respectively (Fig. 1). There was also only a 3% (P < 0.05) benefit after 21 days from using 500 µg g–1 in the filter paper compared with 250 µg g–1, namely 26.8% versus 23.7%. The best result was achieved by a 34-h pre-soaking in 500 µg g–1 of GA3, followed by germination on paper infused with 500 µg g–1 GA3. However, this was still only 34%, much lower than the 61% embryo fill recorded by destructive sampling (Table 1).
Experiment 6. Nine seed lots with optimal GA3 stimulation
The conservative use of GA3 to stimulate germination of a wide range of Pimelea seed lots was very successful, with one notable exception, P. simplex (II) (Table 7). This line of seeds was the only one collected off the ground with a vacuum device and had the lowest seed fill (7%, Table 1). Adding a wetting agent to the substrate to improve wettability of the hairy seeds was very effective and had no effect on the germination of P. trichostachya (I) seeds (data not shown).
All three species responded to GA3 stimulation and the degree was independent of seed age (Table 7). Use of GA3 increased mean germination after 4 weeks from 3.5% to 36%, whereas at 2 weeks, the advantage was 23% versus 1.8%. The best germination after 28 days in the absence of a GA3 stimulus was 14.7% from the P. elongata (I) lot that was 18 months old and had over 80% seed fill, compared with 57% germination with GA3 in the substrate. An even higher germination of 76% was achieved with the other P. elongata (II) lot. However, in the absence of GA3 stimulation, only 2% of the 7-month-old seeds germinated in 28 days. Generally, there was little gained by continuing the germination test beyond the standard 21 days used in prior tests but, where the GA3 response was strong, a significant further germination was often recorded (Table 7).
The subsample of P. simplex (Ib) seeds that had been stored in a sealed jar in a fridge for over a year germinated much better than did the same lot stored in a paper bag on a laboratory bench (61% vs 15%). The bench seeds did have some insect activity in them, although visual inspection showed that most seeds were intact and that any damage was to the hairy outer hypanthium rather than to the discreet seed within.
Discussion
There was a highly significant (P < 0.001) stimulus of the germination of seeds of three Pimelea species by gibberellic acid in the germination substrate. Dormant seeds of many species do not respond to gibberellic acid stimulus (Kallio and Piiroinen 1959) and often any positive GA3 effect is a marginal (<20%) improvement to germination that is already moderate, i.e. over 30% and often over 60%. Our results differed in that germination of untreated seeds was often negligible, whereas GA3 at an appropriate concentration boosted that to over 50% (Table 7). However, large effects on other responsive species have been reported before (Plummer and Bell 1995; Purbey and Meghwal 2005). Unfortunately, responses can be very species-specific (Commander et al. 2009) even within the same plant family (Bunker 1994) and our results may not apply to other Pimelea species, especially to those in other taxonomic sections of this diverse genus.
The magnitude of our GA3 stimulus (up to >50% germination) was much greater than that achieved from many other treatments tried by us over the previous year under identical conditions of temperature, light and moisture. The best previous germination was 4% from a lot of P. elongata seed and 3% from P. trichostachya seeds. There was probably an ageing effect in play, although it has been very minor – compare the germination of the same seed lots from Experiments 1 and 6 (Tables 2, 7).
Uptake of moisture by our Pimelea seeds was not an important factor in the constraint of their germination (R. G. Silcock, unpubl. data) compared with its large influence on related Stellera chamaejasme L. in northern China (Liu 2010). For that perennial member of the Thymelaeaceae, a combination of seedcoat damage, cold stratification and gibberellic acid stimulation was required to achieve over 50% germination of 1-year-old seeds within 20 days.
Where there was little GA3 response in our trials, seed fill was usually very poor. The two low values, 7% and 18%, were similar to that reported by Douglas (1992) for P. trichostachya, a species where our samples had mostly good seed fill (Table 1). Douglas also reported a significant proportion of ‘incomplete embryos’, which we also found in those poorly filled lots. Our incomplete embryos were thought to be shrivelled embryo sacs from fertilised ovaries that grew rapidly, formed a fully developed, strong dark seedcoat but then aborted or ran out of the moisture needed to grow the embryo to maturity. Hence, poor germination of full-sized, hairy Pimelea diaspores may sometimes be due to a low proportion of embryo fill rather than low embryo viability or seed dormancy.
KNO3
There was a statistically non-significant improvement in P. elongata germination by the use of KNO3 in the substrate (Table 2), although overall germination after 3 weeks was still quite low and not adequate to encourage its use with seeds in controlled growth studies, where even, rapid, moderate levels of germination (>30%) are required.
‘Smoke water’
The ‘Smokemaster’ product stimulated germination (Tables 4, 5), but there was an appreciable amount of abnormal early growth of the seedlings. Its effect was sometimes equivalent to that of 100 µg g–1 GA3 in the substrate or pre-soaking in 250 µg g–1 GA3, e.g. P. elongata (Table 5); however, the effect was much less on other seed lots. There was an interaction with seed lot in Experiment 4 such that the ‘smoke-water’ effect diminished as the pre-soaking concentration of GA3 increased for the older lot (I) seeds, whereas the effect improved as pre-soaking concentration increased for lot (II) seeds (Table 5). The net effect was no significant stimulation of germination across all treatment combinations through having ‘smoke water’ in the substrate (Table 6). The cause of this contrasting response is uncertain.
Smoke has promoted germination of some other Pimelea species (Dixon et al. 1995; Roche et al. 1997), so the positive result here was anticipated. However, field observations by numerous people over many years have not documented an abundance of Pimelea plants on recently burnt country (Fletcher et al. 2009). The other smoke product ‘Regen Direct’ was not a germination stimulator at the concentration used. Perhaps the low pH of the solution was causing the problem; however, we did not investigate this further because even ‘Smokemaster’ was associated with aberrant seedling growth and its effect was inconsistent. The responsive species of Pimelea used by Dixon et al. (1995) and Roche et al. (1997) differed in being from the Heterolaena and Calyptrostegia sections of the genus (Rye 1990), although they are similar to ours from sect. Epallage, in having dry, hairy diaspores. The time required for their smoke treatment to initiate germination, namely 30–45 days, was very long (Roche et al. 1997).
GA3 pre-soaking
Pre-soaking seeds in GA3 solutions stimulated germination (Tables 3, 5, 6) and, generally, the greater the concentration and the longer the soaking period, the greater the stimulus. However, soaking for 48 h seemed to be detrimental (Fig. 1), and an increase in concentration from 250 to 750 µg g–1 caused a slight increase in aberrant seedling growth. Surprisingly, a 24-h soaking in GA3 was not as beneficial eventually as was a 10-h or 34-h soaking in Experiment 5, although it initiated germination just as rapidly after 7–9 days. Operationally, soaking seeds for 24 h is very workable and the use of a lower concentration of GA3, say 250 µg g–1, would reduce costs and probably the extent of abnormal seedling growth.
GA3 infusion of the substrate
The presence of GA3 in the germination substrate had a consistent and often powerful stimulatory effect within 2 weeks, unlike ‘smoke water’. The upward trend in numbers after 14 days, even in the absence of GA3 (Fig. 1, Table 5), seemed to show that other factors may be able to enhance Pimelea germination. The trend of the results from a similar range of substrate treatments was the same for P. elongata seeds (Tables 2, 5) and for P. simplex (Table 3), but the final differences were variable (Table 7). The seedcoats of many Pimelea seeds that were stimulated to germinate by GA3 split open longitudinally on opposite sides, several days before the embryo within expanded. This indicated that some embryo-maturation process was taking several more days to complete, even though moisture was freely available and light and temperature are near optimal.
Response patterns to GA3 stimulation
The onset of germination was slightly sped up by GA3, although germination generally started 7–10 days after moisture was first added. GA3 did not seem to compress the period over which germination occurred, nor hasten the date at which the final germinating seed was observed. There was never any marked increase in germination between Days 14 and 21 when the germination substrate contained only water.
Seed germination started slowly in all treatments irrespective of the additives used and, thereafter, a range of response curves ensued (Fig. 1). In some cases, appreciable germination was evident after 10 days, whereas in other instances, this took 3 weeks to occur. After an initial burst, little more germination occurred in some lots, whereas in others, appreciable extra germination continued until the tests were terminated (Table 7). By then, some seed lots had exceeded 50% germination, whereas those imbibed in tapwater had very few seeds germinated (Tables 2, 5, 7). Gibberellic acid does not promote germination directly but acts by inhibiting the dormancy-enforcing properties of abscisic acid (ABA) (Miransari and Smith 2014). Thus, its effect on our Pimelea species may be to overcome an ABA-enforced dormancy generated to various intensities by differing environments during seed maturation (Rodríguez-Gacio et al. 2009).
The need to maintain seed exposure to GA3 for more than 14 days for best germination indicates that a slow metabolic conversion or adjustment happens. This is typical of physiological dormancy (Baskin and Baskin 2004; Fu et al. 2013). The dormancy type involved seems to be non-deep Type 2 physiological dormancy (PD) because the seedcoat is permeable to moisture, the embryo is fully developed in the ripe seed, dormancy is relieved by gibberellic acid, and germination is greater at lower temperatures when the intensity of dormancy is lowered (Baskin and Baskin 2004). GA3 is routinely reported to stimulate cell elongation (Chacko and Singh 1966; Day 2000) and perhaps what we have sometimes recorded as germination (radicle length >1 mm) might be only elongation of the cells of the embryonic axis. Continued autotrophic growth may not always occur thereafter. The shoot of seedlings resulting from the use of GA3 does turn green in light but is often paler than normal, as reported by Morgan and Mees (1958) and Williams and Arnold (1964). This may signify impaired chlorophyll synthesis or soil nutrient uptake.
Field germination
Fresh seeds do not germinate readily for some months in the field, but once primed to germinate, they do so in about 3 days under ideal conditions (Fletcher et al. 2009). This is much faster than our GA3 method achieved; however, it is what would be required under natural conditions in the unreliable, low-rainfall environments where these plants are found. During prolonged droughts, winter rains of 25–50 mm can produce vast swathes of Pimelea in inland Australia (Fletcher et al. 2009), whereas at other times, significant numbers are mainly found in damp microsites and in disturbed soil, such as along recently graded roadsides and table drains. So, they seem to require prolonged moist conditions and some soil disturbance to induce successful recruitment when rainfall is barely adequate or when most seeds are not primed for germination. There is also a constraint on germination of our Pimelea taxa by high ambient temperatures that minimises germination during summer (Dadswell et al. 1994).
Practicalities
Pre-soaking diaspores is not difficult, whereas sowing them afterwards is made much more difficult by e.g. reduced flowability, softened husks and aggregation. There is probably a practical benefit by not pre-soaking and partially compensating by increasing the concentration of GA3 in the substrate. Hence, using 250 µg g–1 solution in the substrate without pre-soaking is currently recommended.
There is always a high level of fungal activity associated with prolonged moistening of hand-harvested P. simplex and P. trichostachya diaspores. This does not seem to affect healthy germinating seeds, provided fungal growth is not excessive. Gibberellic acid restricted fungal growth in our Petri dishes. Captan® very effectively controlled germination of filamentous fungi in Petri dishes, without any deleterious effects on Pimelea seed germination (R. G. Silcock and M. B. Mann, unpubl. data).
Subsequent seedling growth
Gibberellic acid provides reliable germination and, thus, allows more extensive research into the ecology and agronomy of Pimelea sect. Epallage. However, many seedlings planted out after such stimulated germination showed compromised ability to grow normally in large pots of soil. If seedlings are not quickly planted out once the radicle emerges, they develop thin, twisted stems and roots and do not readily survive. Even early transplanted ones, which grew relatively well for many days, had internodes that were much longer than normal and leaves of pale green colour. Then they often died suddenly for no obvious reason or succumbed slowly to collar rot. Microscopic examination of the radicle of laboratory-germinated seeds stimulated by GA3 often revealed transverse fractures of the phloem that indicated excessive elongation of the stele of the root compared with the sheathing phloem. If this happens, the root then turns brown and ceases to grow, and seems to be readily attacked by watery moulds.
Other studies using seedlings derived from gibberellin-enhanced germination have shown mixed effects on survival and growth. Generally, growth was more etiolated (Ratan and Reddy 2004; Purbey and Meghwal 2005), but survival was rarely reported as worse. Those results arose from 12–24-h pre-soaking of the seeds, compared with a continuous presence in the substrate. The mean impact on seedling growth may then also be less because the stimulus was only on a small proportion of the resultant seedling population, compared with virtually all seeds in our case.
Future research
Although these studies produced a desired improvement in Pimelea seed germination, further refinement is needed to reduce the proportion of seedling losses after pricking out into soil, especially of P. simplex subsp. continua. Perhaps a method of effectively using GA3 in the soil or potting mix might be a better option. A better understanding of the effect of seed lot age and storage conditions on ease of germination of these species would also advance scientific understanding of these native plants that can have a big impact on the Australian beef-cattle industry.
Acknowledgements
Jenny Milson, Mary Fletcher, Bez Berry and Ross McKenzie assisted the studies by collecting seed samples for us. Funding towards this research came from the Commonwealth Government’s Natural Heritage Trust via AgForce Queensland, and is gratefully acknowledged.
References
Australian National Botanical Gardens (ANBG) (2011) ‘The latest research – use of smoke in stimulating germination of seed.’ (Australian National Botanical Gardens; Canberra). Available at www.anbg.gov.au/PROPGATE/latest.htm. [Verified 21 January 2011]Baskin JM, Baskin CC (2004) A classification system for seed dormancy. Seed Science Research 14, 1–16.
| A classification system for seed dormancy.Crossref | GoogleScholarGoogle Scholar |
Bunker KV (1994) Overcoming poor germination in Australian daisies (Asteraceae) by combinations of gibberellin, scarification, light and dark. Scientia Horticulturae 59, 243–252.
| Overcoming poor germination in Australian daisies (Asteraceae) by combinations of gibberellin, scarification, light and dark.Crossref | GoogleScholarGoogle Scholar |
Burrows CJ (2008) Genus Pimelea (Thymelaeaceae) in New Zealand 1. The taxonomic treatment of seven endemic, glabrous-leaved species. New Zealand Journal of Botany 46, 127–176.
| Genus Pimelea (Thymelaeaceae) in New Zealand 1. The taxonomic treatment of seven endemic, glabrous-leaved species.Crossref | GoogleScholarGoogle Scholar |
Chacko EK, Singh RN (1966) The effect of gibberellic acid on the germination of papaya seeds and subsequent seedling growth. Tropical Agriculture 43, 341–346.
Commander LE, Merritt DJ, Rokich DP, Dixon KW (2009) Seed biology of Australian arid zone species: germination of 18 species used for rehabilitation. Journal of Arid Environments 73, 617–625.
| Seed biology of Australian arid zone species: germination of 18 species used for rehabilitation.Crossref | GoogleScholarGoogle Scholar |
Dadswell LP (1994) Epidemiology and ecology of Pimelea poisoning in Queensland. In ‘Plant associated toxins; agricultural, phytochemical and ecological aspects’. (Eds SM Colegate, PR Dorling) pp. 40–44. (CAB International: Wallingford, UK)
Dadswell LP, Graham TG, Newman RD, D’Occhio M, Burton D, Schefe C (1994) Pimelea poisoning in beef cattle: plant ecology, epidemiology, therapeutic control and immunogen feasibility studies. Final report. Part 3: technical report (Project DAQ.072) to Meat and Livestock Australia. Project report QO94021. Queensland Department of Primary Industries: Brisbane.
Dawson PAC, Rapson GL, Robertson AW, Fordham RA (2005) Limitations on recruitment of the rare sand daphne Pimelea arenaria (Thymelaeaceae), lower North Island, New Zealand. New Zealand Journal of Botany 43, 619–630.
| Limitations on recruitment of the rare sand daphne Pimelea arenaria (Thymelaeaceae), lower North Island, New Zealand.Crossref | GoogleScholarGoogle Scholar |
Day JS (2000) The influence of seed pre-treatment and soil conditions on the emergence of sesame seedlings. Australian Journal of Experimental Agriculture 40, 843–848.
| The influence of seed pre-treatment and soil conditions on the emergence of sesame seedlings.Crossref | GoogleScholarGoogle Scholar |
Dixon KW, Roche S, Pate JS (1995) The promotive effect of smoke derived from burnt native vegetation on seed germination of Western Australian plants. Oecologia 101, 185–192.
| The promotive effect of smoke derived from burnt native vegetation on seed germination of Western Australian plants.Crossref | GoogleScholarGoogle Scholar |
Douglas AC (1992) Biology of the toxic weed Pimelea trichostachya. Morphology and factors affecting growth in the field. Department of Agriculture report, University of Queensland: St Lucia, Brisbane.
Everist SL (1980) ‘Poisonous plants of Australia.’ Revised edn. (Angus and Robertson: Sydney)
Fletcher MT (2008) Development of sustainable management priorities of pimelea and pimelea poisoning and development of best practice management guide. Final report of Project 60436 to Natural Heritage Trust Fund and AgForce Queensland. Queensland Department of Employment, Economic Development and Innovation, Brisbane.
Fletcher M, Silcock R, Ossedryver S, Milson J, Chow C (2009) ‘Understanding pimelea poisoning of cattle.’ (Department of Employment, Economic Development and Innovation: Brisbane)
Fu Z, Tan D, Baskin JM, Baskin CC (2013) Seed dormancy and germination of the subalpine geophyte Crocus alatavicus (Iridaceae). Australian Journal of Botany 61, 376–382.
| Seed dormancy and germination of the subalpine geophyte Crocus alatavicus (Iridaceae).Crossref | GoogleScholarGoogle Scholar |
Genstat (2008) ‘Genstat for windows.’ 11th edn. (VSN International: Hemel Hempstead, UK)
Grayson (2011) ‘Regen 2000®. Native and exotic plant germination.’ Available at www.technica.com.au/Regen-overview.html. [Verified 20 January 2011]
Kallio P, Piiroinen P (1959) Effect of gibberellin on the termination of dormancy in some seeds. Nature 183, 1830–1831.
| Effect of gibberellin on the termination of dormancy in some seeds.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaF3c7ls1Olsg%3D%3D&md5=1c4a0b1ae8fa5b243b7adb3fc1ccc7feCAS | 14404144PubMed |
Keighery G, Dixon R (1984) Pimelea species of Western Australia. Australian Plants 12, 287–303.
King RW, Dawson IA, Speer SS (1992) Control of growth and flowering in two Western Australian species of Pimelea. Australian Journal of Botany 40, 377–388.
| Control of growth and flowering in two Western Australian species of Pimelea.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3sXjsVeluw%3D%3D&md5=0105e270a4c087797f55dcf3d2feb836CAS |
Liu W (2010) The biological study of Stellera chamaejasme (Thymelaeaceae). Masters Thesis, Harbin Normal University, Harbin, China. [In Chinese]
Miransari M, Smith DL (2014) Plant hormones and seed germination. Environmental and Experimental Botany 99, 110–121.
| Plant hormones and seed germination.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXislKjurw%3D&md5=71c4bc375efa9eabdece4ddd70134916CAS |
Morgan DG, Mees GC (1958) Gibberellic acid and the growth of crop plants. The Journal of Agricultural Science 50, 49–59.
| Gibberellic acid and the growth of crop plants.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaG1cXmslSntg%3D%3D&md5=bdbf5190f13f0266f42aa487f24860deCAS |
Plummer JA, Bell DT (1995) The effect of temperature, light and gibberellic acid (GA3) on the germination of Australian everlasting daisies (Asteraceae, tribe Inuleae). Australian Journal of Botany 43, 93–102.
| The effect of temperature, light and gibberellic acid (GA3) on the germination of Australian everlasting daisies (Asteraceae, tribe Inuleae).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2MXmtFagsLk%3D&md5=baba9d802e98ecf312e6f52640321905CAS |
Pressland AJ, Dadswell LP (1992) Ecological, epidemiological and management aspects of pimelea poisoning in Queensland. Vet Update 92, 595–613. (University of Queensland, Brisbane)
Purbey SK, Meghwal PR (2005) Effect of pre-sowing seed treatment on seed germination and vigour of aonla seedlings. Research on Crops 6, 560–561.
Ratan PB, Reddy YN (2004) Influence of gibberellic acid on custard apple (Annona squamosa L.) seed germination and subsequent seedling growth. Journal of Research ANGRAU 32, 93–95.
Roche S, Dixon KW, Pate JS (1997) Seed ageing and smoke: partner cues in the amelioration of seed dormancy in selected Australian native species. Australian Journal of Botany 45, 783–815.
| Seed ageing and smoke: partner cues in the amelioration of seed dormancy in selected Australian native species.Crossref | GoogleScholarGoogle Scholar |
Rodríguez-Gacio M, Matilla-Vázquez MA, Matilla AJ (2009) Seed dormancy and ABA signaling: the breakthrough goes on. Plant Signaling & Behavior 4, 1035–1048.
| Seed dormancy and ABA signaling: the breakthrough goes on.Crossref | GoogleScholarGoogle Scholar |
Rye BL (1990) Thymelaeaceae (genus Pimelea). In ‘Flora of Australia, Vol. 18. Podostemaceae to Combretaceae’. (Ed. AS George) pp. 134–210. (Australian Government Publishing Service: Canberra)
Seaton K (2005) Qualup bell (Pimelea physodes) for cut flower production. Farmnote No. 111/99. Department of Agriculture and Food, Perth.
Williams CN, Arnold GW (1964) Winter growth stimulation by gibberellin in differentially grazed pastures of Phalaris tuberosa. Australian Journal of Experimental Agriculture and Animal Husbandry 4, 225–230.
| Winter growth stimulation by gibberellin in differentially grazed pastures of Phalaris tuberosa.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaF2MXhtFyjtQ%3D%3D&md5=70d0e1b7895d9de6af691c16ee7606c6CAS |
1 1©The State of Queensland (through the Department of Agriculture, Fisheries and Forestry Queensland) (2013).