Seasonal variation in the nitrogen nutrition and carbon assimilation in wild and cultivated Aspalathus linearis (rooibos tea)
Daleen Lötter A B , Emma Archer van Garderen A D , Mark Tadross B E and Alexander J. Valentine C FA CSIR, Natural Resources and the Environment, PO Box 320, Stellenbosch 7602, South Africa.
B Climate Systems Analysis Group, Department of Environmental and Geographical Science, University of Cape Town, Private Bag, Rondebosch 7701, Republic of South Africa.
C Botany and Zoology Department, University of Stellenbosch, Private Bag X1, Matieland 7602, South Africa.
D CSIR Natural Resources & the Environment, Bldg 1, cnr Carlow & Rustenburg Roads, Emmarentia 2193/School of Geography, Archaeology and Environmental Studies, University of the Witwatersrand, 1 Jan Smuts Avenue, Braamfontein 2000, South Africa.
E Present address: United Nations Development Programme (UNDP–GEF), Energy and Environment Group, BDP 304 East 45th Street, 9th Floor, New York, NY 10017, USA.
F Corresponding author. Email: alexvalentine@mac.com
Australian Journal of Botany 62(1) 65-73 https://doi.org/10.1071/BT13237
Submitted: 28 September 2013 Accepted: 13 March 2014 Published: 28 April 2014
Abstract
The Fynbos Biome of southern Africa is a Mediterranean-climate ecosystem with highly infertile soil. It is home to the endemic leguminous shrub Aspalathus linearis (rooibos tea), which is both an invaluable wild resource and commercially cultivated plant. Wild rooibos has a narrow geographic range and is confined to mountain ranges of the Cederberg Region. Under projected climate change, warmer and more arid conditions may place additional pressure on these range-restricted plants to survive in an already resource-limited environment. To understand the adaptive strategies that may allow rooibos to persist in its habitat under future climate change, the present study evaluated changes in the photosynthetic activity and nutrient cycling of wild and cultivated A. linearis, at the temperature and rainfall extremes of summer and winter. Wild and cultivated rooibos tea had different methods of adapting to nitrogen (N) nutrition and carbon (C) assimilation during wet and dry seasons. In particular, the wild plants were better able to tolerate summer drought by increased water use efficiency and maintaining higher levels of biological N2 fixation than was the cultivated tea.
Additional keywords: Cape Fynbos, legume, mineral nutrition.
Introduction
Aspalathus linearis (Burm.F.) Dahlg., Fabaceae, is an evergreen leguminous shrub indigenous to the Fynbos Biome of the Cape Floristic Region (Dahlgren 1968) and one of the most successful commercially propagated endemic crops of South Africa. The Fynbos Biome is one of five Mediterranean climate regions of the world, which are all recognised as biodiversity hotspots (Cowling et al. 1996). There is a particularly remarkable ecological convergence between two of the five, namely the Cape Floristic Region and the kwongan of South-western Australia (Cowling et al. 1996; Wisheu et al. 2000). Both fynbos and kwongan occur on severely nutrient-impoverished soils, display similar fire regimes, a remarkable convergence in growth forms and species diversity at different spatial scales (Cowling and Wikowski 1994; Cowling et al. 1996; Wisheu et al. 2000). These areas are particularly species rich, with high levels of rarity and endemism, where 68–75% of plants are endemic as opposed to 23–50% in other Mediterranean-climate regions (Cowling et al. 1996). In addition, several of the fynbos species are restricted to extremely small distribution ranges and face similar threats in terms of habitat fragmentation and climate change (Bomhard et al. 2005).
The Mediterranean climate is characterised by a strong seasonal pattern of rainfall, with marked aridity during summer months and most of the annual rainfall falling predominantly during the winter period. The water-deficit conditions during summer months are generally considered to be the main environmental constraint to plant growth and productivity in Mediterranean-type vegetation (Larcher 2000). Low availability of soil nutrients is a further limiting factor in Mediterranean ecosystems (Kruger et al. 1983; Sardans et al. 2006). Fynbos soils are sandstone-derived and characterised by high acidity and poor nutrient availability and are especially deficient in nitrogen (N), phosphorus (P) and calcium (Ca) (Kruger et al. 1983; Muofhe and Dakora 2000). N and P are known to be limiting nutrients for plant productivity in Mediterranean-type ecosystems, and the concentrations thereof in the ecosystem may also change in response to warming and drought (Lloret et al. 1999; Sardans et al. 2008). Plant growth and fitness in such an environment depend largely on a species ability to optimally utilise available resources. Many species possess certain eco-physiological traits that enable them to adapt to the existing environmental constraints. One such important trait is biological N2 fixation. Being a legume, rooibos has a proven ability to fix N2 and is known to be able to fix well over 100 kg N ha−1 annually (Sprent et al. 2009). According to Lamont (1983) and Mitchell et al. (1986), several plants growing in Mediterranean ecosystems have developed specialised nutrient-uptake and internal nutrient-cycling strategies. An important mechanism by which critical nutrients are maintained is via nutrient reabsorption, in which nutrients are mobilised from senescing leaves and transported to other plant tissues (Killingbeck 1996). Another functional strategy to deal with restrictive environments is sclerophylly, which is associated with a high ratio of C to N in plant leaves (Rundell 1988). Sclerophylly is a typical characteristic of fynbos vegetation and it has been suggested to be an adaptation to specific stresses such as seasonal water deficits (Bussotti et al. 1998) and low-nutrient soils (Gutschick 1999). Apart from nutrition, plants may also adapt via photosynthetic and water-relation adjustments (Chaves et al. 2002, 2003; Vitale et al. 2012). It has been observed in field-grown plants that as summer drought progresses, leaf photosynthesis is increasingly limited because of a decline in stomatal conductance (Chaves et al. 2002). This leads to a reduction in water loss and an increase in water use efficiency (the ratio of water loss to biomass gained).
Climate change, which is predicted to cause an increase in aridity in the western coast of South Africa by the end of the century (Hewitson and Crane 2006; IPCC 2007; Engelbrecht et al. 2009) may place additional pressure on plants to survive in an already resource-limited environment. Previous research (Archer et al. 2008) has suggested that these changing climate conditions might have a severe impact on rooibos tea. It is, therefore, essential to understand the eco-physiological behaviour of these plants in relation to current climatic and soil constraints. However, not much is known about the nutritional and water-relation physiology of A. linearis under drought stress in its natural habitat. Local knowledge suggests that wild rooibos shrubs may have higher drought resistance than does the cultivated type (Louw 2006). A. linearis is one of the relatively few economically important fynbos species that has successfully made the transition from wild resource to an agriculturally important plant in the 20th century. Wild rooibos was historically used by local inhabitants of the Cederberg Region to produce tea, as well as for its medicinal and health properties (Dahlgren 1968). On the basis of growth form, several wild ecotypes of A. linearis have previously been identified (Malgas et al. 2007). Preliminary genetic testing has suggested a high level of genetic diversity within A. linearis (Van der Bank et al. 1995, 1999; Malgas et al. 2009). The cultivated type is thought to have originated from a wild type found in the Pakhuis area of the southern Cederberg during the 1930s (Cheney and Scholz 1963; Morton 1983). The entire commercial industry therefore hinge on one A. linearis selection made ~80 years ago. This selection was based on qualities such as growth rate, seed production and especially taste.
A useful approach to gain information on plant physiological performance is to study seasonal patterns in nutrient cycling and gas exchange (Llorens et al. 2003). Hence, the aim of the present study was to determine how the wet and dry seasons affect the photosynthetic gas exchange, nutrient cycling and water use efficiencies of wild and cultivated A. linearis. This will allow us to better understand the ability of A. linearis to persist in its habitat under future climate change.
Materials and methods
Site and plant selection
The study was conducted on communal land outside Heuningvlei (32.2°S, 19.13°E, 858 m asl), which is an outpost of the Moravian church village of Wupperthal in the Cederberg mountains, Western Cape, South Africa (Fig. 1). The soils in the area comprise mainly quartzitic sandstones of the Table Mountain Group that are well leached nutrient-poor and acidic soils. Cederberg sandstone fynbos vegetation predominates in this area and contains numerous endemic plant species, of which rooibos is one. The study site included a plantation of cultivated tea and a nearby site with wild rooibos growing among other fynbos plants. Wild populations of A. linearis have a narrow geographic range within the fynbos biome and are confined to mountain ranges of the Cedarberg Region (Dahlgren 1968). The wild plants occur naturally between 450-m and 900-m asl, whereas cultivated rooibos is grown over a wider geographical range than is the natural distribution. Cultivated seedlings are normally grown in seedbeds for 6 months under fertilisation and irrigation conditions. Thereafter, the seedlings are transplanted to the field, where they receive no additional fertiliser or irrigation. In the present study, the cultivated plants have been grown for 10 years under field conditions. Similarly, the wild tea plants have been growing in the area for at least 10 years as well. In both sites, there have been no fire events during the past 10 years, and plants have not been subjected to a harvesting cycle during the 2 years of the present study.

|
The study site was therefore chosen on the basis of the following three criteria: (1) an area where cultivated and a wild rooibos co-occurred closely, (2) accessibility and (3) known fire history and harvesting practices. Precipitation and air temperatures during the sampling period were obtained from the nearest weather station Mertenhof (32.14°S, 1919°E), which is 10 km from the study site, and meteorological data are reported in Fig. 2. Climate conditions were typical for a Mediterranean-type climate, with hot and dry summers, relatively cold winters, and rainfall periods concentrated in May until September. During the experimental period, summer drought occurred from November to March, when monthly rainfall ranged between 1 and 10 mm, whereas maximum daily temperatures averaged over 30°C. Total rainfall during winter (JJA) was 145 mm and minimum temperatures ranged between 3.5°C and 4.4°C.

|
Plant and soil sampling
Data were collected from two sites where wild and cultivated A. linearis populations occurred separately. The first site included cultivated rooibos tea, where plants were grown in rows, set 1.5 m apart on an area of ~50 × 60 m. The second site featured wild rooibos plants growing among other fynbos species, and was located ~100 m from the first site.
Ten mature shrubs of similar age and size were randomly selected in both sites; thus, 20 shrubs in total were sampled. Sampling was conducted during late summer months (dry season) as well as during the late winter months (wet season). The shrubs were numbered and the same shrubs were sampled in each season.
For the plant samples, a 20-cm portion of disease-free and undamaged branch was cut off the top of each shrub and placed in paper bags. Recent leaf litter was collected at the base of each selected plant (litter deposited most recently according to the local farmers was taken). Plant material was clearly marked and dried in an oven at 60°C for 72 h. After drying, the plant material was finely ground for C and N stable isotope analysis. For the soil samples, three samples (each 10 cm deep) were taken from underneath each shrub, equally spaced from each other. These three samples were pooled to form one bulk sample for each shrub. Another three soil samples (each 10 cm deep) were taken 1 m away from each shrub, equally spaced from each other. The samples were once again combined to form a single sample for each plant. Soil samples were kept at 15°C until analysed.
Calculations of N-reabsorption efficiency, δ15N and δ13C
Nitrogen-reabsorption efficiency was calculated as the difference between maximum N pools in green leaves and N pools in senescent leaves, as described by Killingbeck (1996), as follows:

where XGr is the N concentration of green foliage and XSen is the N concentration of senescent leaves. Calculations were made separately for each plant before statistical analysis.
The stable isotope analyses were carried out at the Archaeology Department, University of Cape Town. Values were expressed relative to the Pee-Dee Belemnite (PDB) standard for δ13C and relative to atmospheric N for δ15N, as (%), according to the following equation:

where Z is the heavy isotope of either N or C, and R is the ratio of heavier to lighter isotope for the sample and standard (13C/12C or 15N/14N).
The oven-dried plant components were milled in a Wiley mill (Thomas Scientific, Swedesboro, NJ, USA), after which the samples were combusted in a Flash 2000 organic elemental analyser. Three in-house standards (Merck Gel, Lentil, Acacia saligna) were used and calibrated against the standards of International Atomic Energy Agency (IAEA). N is expressed in terms of its value relative to atmospheric N, whereas C is expressed in terms of its value relative to Pee-Dee Belemnite.
Gas-exchange measurements
Maximum photosynthetic rates (Pn), stomatal conductance (Gs) and transpiration rates (E) were measured using a using a LI-6400 portable infrared gas analyser (Li-Cor, Lincoln, NE, USA). The reference CO2 concentration was maintained at 0.04% CO2 in air, flow rate was 500 µmol s–1, whereas light intensity inside the chamber was set at 1500 µmol photons m–2 s–1 (light-saturated value derived from light-saturation curves). Measurements were made during mid-morning on clear, sunny days. The measured leave material was removed, oven-dried and weighed to express gas-exchange rates on a leaf dry-mass (g) basis.
Instantaneous and integrated water-use efficiency
Instantaneous (photosynthetic) water use efficiency (WUEi) was calculated from gas exchange, to assess the efficiency of water usage during photosynthesis. The WUEi was estimated by dividing the photosynthetic rate (Pn) by the transpiration rate (E), at the same light level, and is expressed as net photosynthetic rate/transpiration rate. Integrated WUE describes leaf WUE (based on stomatal behaviour) over the whole growing season and can be estimated using C isotope discrimination, whereby plants discriminate against 13C during photosynthesis.
Statistical analysis
A two-way ANOVA with season and type (wild and cultivated rooibos) as a fixed factor was performed on gas exchange, stable isotope composition and N reabsorption, to detect differences in responses within seasons and across types. The normality of data and homogeneity of variance were tested by the Shapiro–Wilk and Levene tests, respectively, before ANOVA, to ensure that the assumptions of ANOVA were met. The different variables and their interactions were tested to detect significant factors (Kaleidagraph, Synergy Software, Reading, PA, USA). Where the ANOVA revealed significant differences among treatments, the means (n = 10) were separated using a post hoc Tukey’s HSD, a multiple-range test based on a significance level of P = 0.05.
Results
Plant nutrient and isotope analysis
Leaf N concentrations displayed a marked difference between wild and cultivated rooibos, being significantly lower for wild tea. These differences were observed in both summer and winter. Both wild and cultivated rooibos types displayed the highest leaf N concentrations during winter, with values ranging between 0.91% and 1.53% (Fig. 3).
The nutrient reabsorption of N followed a seasonal trend similar to that for the foliar N concentration. Absorption of N from senescing material was highest during winter when 32% of cultivated- and 23% of wild-plant N was reabsorbed (Fig. 3). Cultivated tea displayed a significantly higher N reabsorption than did wild tea in both seasons. The greatest difference between wild and cultivated rooibos was observed during the summer period.
In contrast to the N concentration, the leaf C values were higher in wild tea than in the cultivated tea, although this was significant only during the winter period. Seasonally, leaf C content was significantly lower during winter than in summer in cultivated tea. The C : N ratio was significantly higher in the wild plants than in cultivated plants. During summer, wild plants showed a comparatively higher (10%) C : N ratio (Fig. 3). The C : N ratio followed a similar trend for both wild and cultivated plants, with the highest values during summer and lowest in winter.
There was a distinct difference in the ability of wild and cultivated rooibos to fix atmospheric N via biological N2 fixation (BNF). On average, BNF in wild rooibos plants was 2.3 times higher than that in the cultivated plants (Fig. 4). Differences between wild and cultivated rooibos were most prominent during summer when BNF in wild tea was approximately nine times higher. However, the lower BNF rates in the cultivated plants were not accompanied by similar decreases in foliar N concentrations. Both types displayed the same seasonal pattern, with significantly higher BNF during winter.
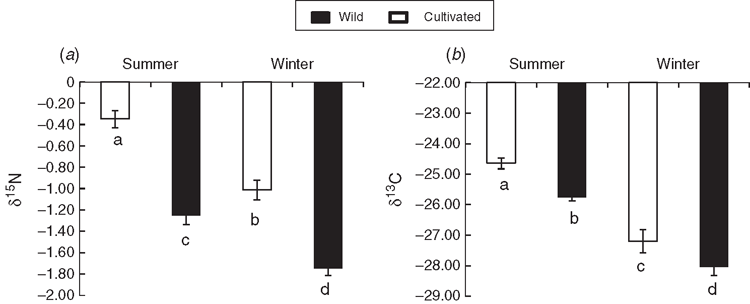
Wild and cultivated rooibos revealed substantial differences in δ13C values, with wild tea showing significantly lower values. On average, wild tea had 1.04 times lower δ13C values than did cultivated tea, with the most significant difference being observed during summer (Fig. 4). Both wild and cultivated plants displayed the same pattern, with the lowest δ13C values occurring during winter and the maximum in summer.
Photosynthesis and water relations
There was a significant difference in photosynthetic rates between seasons and between types (wild and cultivated A. linearis). The lowest photosynthetic assimilation rates were recorded during the summer period for both wild and cultivated plants (Fig. 5). The wild type, however, had a higher photosynthetic rate than did the cultivated type during the summer period, whereas this was not the case during the winter. Cultivated tea had higher photosynthetic and transpiration rates in winter. In both wild and cultivated rooibos, transpiration rates were higher in winter than in summer. Increases in photosynthetic rates were mostly accompanied by concomitantly higher stomatal conductances.
Both wild and cultivated rooibos exhibited lower WUEi during the wet season. This coincided with the δ13C-based integrated-WUE variation between winter and summer. There was, however, no difference in instantaneous WUE between wild and cultivated rooibos during winter, although the integrated WUE did show significantly higher WUE in cultivated plants. During the dry summer period, the wild plants demonstrated a significantly higher instantaneous WUE.
Soil N data revealed a significant difference in soil N concentration between wild and cultivated sites. Although the cultivated site had higher soil N than did the wild site, seasonal differences were not significant (Fig. 6). Soil N displayed a seasonal pattern similar to that of plant N, being highest during late winter. Soil C was significantly higher at the wild site than at the cultivated site during both seasons. Soil P, however, was significantly higher at the cultivated site. There were no significant differences between seasons for either soil C or P for the respective sites.
Discussion
Dry and wet seasonal variations in N cycling and gas exchange of wild and cultivated A. linearis legumes were investigated. It was found that the two types of rooibos tea had different methods of adapting to N nutrition and C assimilation during wet and dry seasons.
The δ13C comparison between the wet and dry season indicated that both wild and cultivated rooibos experienced water stress during the dry period of the year. Increasing aridity may cause stomatal closure, which can result in less discrimination against 13C during photosynthesis, with associated elevated δ13C values (Ehleringer 1994; Swap et al. 2004). However, on the basis of the significantly lower δ13C values of the wild tea type than the cultivated type during the dry period, it appears that the wild type was less water stressed.
This improved resistance of the wild type to drought may be related to the increased C : N ratio, as an indication of developing schlerophylly. Schlerophylly is often associated with dry climates as an adaptation to low water levels, but also to low nutrient availability (Groom and Lamont 1999; Gutschick 1999; Sardans et al. 2008). Bussotti et al. (1998, 2000) found that the level of sclerophylly increases during the summer months in response to adverse environmental field conditions. In this regard, both types had lower C : N during winter. Seasonal changes may also cause differences in nutrient availability, and this may be enhanced in nutrient-poor ecosystems such as the fynbos.
This effect is evident in the leaf N nutrition of both plants during the dry and wet seasons. The highest N concentrations were found in cultivated tea during winter (1.27%), whereas the wild tea during the arid summer had the lowest N concentrations (0.85%). These values are well within the range of other evergreen sclerophyllous mountain fynbos plants (Herppich et al. 2002). It has been shown that plants in Mediterranean regions take up N during the winter rainy season, before any above-ground growth, and that these minerals are stored in the old evergreen leaf tissue before being translocated to new growth in spring (Mooney and Rundel 1979). Similar seasonal patterns have been reported for other endemic fynbos species (Stock et al. 1987; Witkowski et al. 1990; Herppich et al. 2002). Maier et al. (1995) suggested that the mature leaves may function as sink organs during times of high nutrient availability and eventually reallocate this shared N to the newly formed sinks during spring growth.
Because cultivated tea plants were less reliant on BNF, they may have been able to conserve more N from their leaves through reabsorption, before abscission. In contrast, the wild plants relied more heavily on BNF and resorbed less N from leaves. This reliance of wild plants on BNF was particularly important in winter, when the soil N was poorer than in cultivated plant plots. Reabsorption from senescing material is primarily a N-conservation strategy (Eckstein et al. 1999; Aerts and Chapin 1999) and essential in assisting in alleviating nutrient scarcity. Although the values reported for N reabsorption in other fynbos species (35–70%) (Stock et al. 1987; Witkowski 1991) are somewhat higher, the reabsorption of N, coupled with the ability to fix N, is an important mechanism to overcome nutrient deficiency in this nutrient-constrained environment.
The effect of the dry season on symbiotic N2 fixation was more evident in the cultivated than the wild plants, evidenced by the greater percentage decline in BNF during summer relative to winter, in the cultivated plants. Because these plants did not show a proportional increase in the reabsorption of leaf N, the remaining N had to be acquired from soil uptake via the roots. This increased reliance on soil N in the cultivated plants during summer may be the result of the lower energy costs associated with soil mineral uptake of N. N2-fixing plants incur additional C costs for the synthesis of roots and nodules and maintenance of mycorrhizal fungi (Vance and Heichel 1991). Nodule initiation, growth, and activity are also more sensitive to moisture stress than are general root and shoot metabolism (Albrecht et al. 1984). During the summer stress, this ability to switch N sources may be a distinct advantage.
The seasonal pattern in net assimilation rates, observed in A. linearis, coincided with the results obtained in other studies investigating schlerophyllous shrubs from Mediterranean climate regions (van der Heyden and Lewis 1989; Flexas et al. 2001). These studies showed lowest rates of photosynthesis during summer when photosynthesis is reduced by stomatal limitation, triggered by low water availability. According to Louw (2006), drought limits biomass production in A. linearis, as was confirmed in our study, by the lower photosynthetic rates of both wild and cultivated rooibos during the late summer months when aridity is most pronounced. However, during late winter, under favourable soil moisture conditions, cultivated tea had elevated gas-exchange rates compared with the wild tea. Conversely, during the dry summer months, the wild tea exhibited an increased ability to adapt to the limited soil-moisture conditions by sustaining higher photosynthetic rates. This was also accompanied by their ability to maintain higher WUEs, facilitated by effective control of transpirational water loss. This enhanced ability to adapt to dry conditions may be related to their higher sclerophylly index, which Turner (1994) argues to be an important protective mechanism to resist extreme climatic events. Bussotti et al. (2002) demonstrated that leaves with a higher level of sclerophylly are able to maintain their internal moisture status, even in extreme drought conditions.
The results of our study suggest that, under the present climate conditions, the wild plants are better able to tolerate summer drought, with greater water economy, and more reliance on BNF for N nutrition. These wild plants may therefore be better adapted to tolerate the warmer and drier conditions predicted for the next decades, which will underpin the survival of this legume species under current models of climate change.
Acknowledgements
This study was funded by the Volkswagen Foundation. We thank the University of Cape Town and the University of Stellenbosch for the use of their facilities.
References
Aerts R, Chapin FS (1999) The mineral nutrition of wild plants revisited: a re-evaluation of processes and patterns. Advances in Ecological Research 30, 1–67.| The mineral nutrition of wild plants revisited: a re-evaluation of processes and patterns.Crossref | GoogleScholarGoogle Scholar |
Albrecht SL, Bennett JM, Boote KJ (1984) Relationship of nitrogenase activity to plant water stress in field grown soybeans. Field Crops Research 8, 61–71.
| Relationship of nitrogenase activity to plant water stress in field grown soybeans.Crossref | GoogleScholarGoogle Scholar |
Archer ERM, Oettle' NMO, Louw R, Tadross MA (2008) ‘Farming on the Edge’ in arid western South Africa: adapting to climate change in marginal environments. Geography 93, 98–107.
Bomhard B, Richardson DM, Donaldson JS, Hughes GO, Midgley GF, Raimondo DC, Rebelo AG, Rouget M, Thuiller W (2005) Potential impacts of future land use and climate change on the Red List status of the Proteaceae in the Cape Floristic Region, South Africa. Global Change Biology 11, 1452–1468.
| Potential impacts of future land use and climate change on the Red List status of the Proteaceae in the Cape Floristic Region, South Africa.Crossref | GoogleScholarGoogle Scholar |
Bussotti F, Gravano E, Grossoni P, Tani C (1998) Occurrence of tannins in leaves of beech trees (Fagus sylvatica) along an ecological gradient, detected by histochemical and ultra-structural analyses. New Phytologist 138, 469–479.
| Occurrence of tannins in leaves of beech trees (Fagus sylvatica) along an ecological gradient, detected by histochemical and ultra-structural analyses.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXivVSqsL4%3D&md5=3a07b06e321208f12f95a8ca189615dcCAS |
Bussotti F, Borghini F, Celesti C, Leonzio C, Bruschi P (2000) Leaf morphology and macronutrients in broadleaved trees in central Italy. Trees 14, 361–368.
| Leaf morphology and macronutrients in broadleaved trees in central Italy.Crossref | GoogleScholarGoogle Scholar |
Bussotti F, Bettini D, Grossoni P, Mansuino S, Nibbi R, Soda C, Tani C (2002) Structural and functional traits of Quercus ilex in response to water availability. Environmental and Experimental Botany 47, 11–23.
| Structural and functional traits of Quercus ilex in response to water availability.Crossref | GoogleScholarGoogle Scholar |
Chaves MM, Pereira JS, Maroco J, Rodrigues ML, Ricardo CPP, Osório ML, Carvalho I, Faria T, Pinheiro C (2002) How plants cope with water stress in the field: photosynthesis and growth. Annals of Botany 89, 907–916.
| How plants cope with water stress in the field: photosynthesis and growth.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XlsVeitb4%3D&md5=b99c6156215899dc8d3ee44c4c4d2e66CAS | 12102516PubMed |
Chaves MM, Maroco JP, Pereira JS (2003) Understanding plant responses to drought – from genes to the whole plant. Functional Plant Biology 30, 239–264.
| Understanding plant responses to drought – from genes to the whole plant.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXjtVKlt7o%3D&md5=b615f4a799dad07d27c9fa3099eddd64CAS |
Cheney RH, Scholtz E (1963) Rooibos tea, a South African contribution to world beverages. Economic Botany 17, 186–194.
| Rooibos tea, a South African contribution to world beverages.Crossref | GoogleScholarGoogle Scholar |
Cowling RM, Witkowski ETF (1994) Convergence and non-convergence of plant traits in climatically and edaphically matched sites in mediterranean Australia and South Africa. Australian Journal of Ecology 19, 220–232.
Cowling RM, Rundel PW, Lamont BB, Arroyo MK, Arianoutsou M (1996) Plant diversity in mediterranean-climate regions. Trends in Ecology & Evolution 11, 362–366.
| Plant diversity in mediterranean-climate regions.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3M7itFGjtg%3D%3D&md5=9cbef34ad5592af72b7ef0bc46e557a6CAS |
Dahlgren R (1968) Revision of the genus Aspalathus II: the species with ericoid and pinoid leaves. 7 subgenus Nortieria, with remarks on rooibos cultivation. Botaniska Notiser 121, 165–208.
Eckstein RL, Karlsson PS, Weih M (1999) Leaf life span and nutrient reabsorption as determinants of plant nutrient conservation in temperate–arctic regions. New Phytologist 143, 177–189.
| Leaf life span and nutrient reabsorption as determinants of plant nutrient conservation in temperate–arctic regions.Crossref | GoogleScholarGoogle Scholar |
Ehleringer JR (1994) Variation in gas exchange characteristics among desert plants. In ’Ecophysiology of photosynthesis’. Ecological studies series. (Eds E–D Schulze, MM Caldwell) pp. 361–392. (Springer Verlag: New York)
Engelbrecht FA, McGregor JL, Engelbrecht CJ (2009) Dynamics of the conformal-cubic atmospheric model projected climate-change signal over southern Africa. International Journal of Climatology 29, 1013–1033.
| Dynamics of the conformal-cubic atmospheric model projected climate-change signal over southern Africa.Crossref | GoogleScholarGoogle Scholar |
Flexas J, Gulías J, Jonasson S, Medrano H, Mus M (2001) Seasonal patterns and control of gas exchange in local populations of the Mediterranean evergreen shrub Pistacia lentiscus L. Acta Oecologica 22, 33–43.
| Seasonal patterns and control of gas exchange in local populations of the Mediterranean evergreen shrub Pistacia lentiscus L.Crossref | GoogleScholarGoogle Scholar |
Groom PK, Lamont BB (1999) Which common indices of sclerophylly best reflect differences in leaf structure? Ecoscience 6, 471–474.
Gutschick VP (1999) Biotic and abiotic consequences of differences in leaf structure. New Phytologist 143, 3–18.
| Biotic and abiotic consequences of differences in leaf structure.Crossref | GoogleScholarGoogle Scholar |
Herppich H, Herppich WB, von Willert DJ (2002) Leaf nitrogen content and photosynthetic activity in relation to soil nutrient availability in coastal and mountain fynbos plants (South Africa). Basic and Applied Ecology 3, 329–337.
| Leaf nitrogen content and photosynthetic activity in relation to soil nutrient availability in coastal and mountain fynbos plants (South Africa).Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XpsV2ntLs%3D&md5=9416c48a3ae1ebd3b09beed064b884a1CAS |
Hewitson BC, Crane RG (2006) Consensus between GCM climate change projections with empirical downscaling: precipitation downscaling over South Africa. International Journal of Climatology 26, 1315–1337.
| Consensus between GCM climate change projections with empirical downscaling: precipitation downscaling over South Africa.Crossref | GoogleScholarGoogle Scholar |
IPCC (2007) In ‘Climate change 2007: synthesis report’. (Eds RK Pachauri, A Reisinger) (IPCC: Geneva, Switzerland)
Killingbeck KT (1996) Nutrients in senesced leaves: keys to the search for potential reabsorption and reabsorption proficiency. Ecology 77, 1716–1727.
| Nutrients in senesced leaves: keys to the search for potential reabsorption and reabsorption proficiency.Crossref | GoogleScholarGoogle Scholar |
Kruger FJ, Mitchell DT, Jarvis JUM (1983) ‘Mediterranean-type ecosystems. The role of nutrients.’ (Springer Verlag: Berlin)
Lamont BB (1983) Strategies of maximizing nutrient uptake in two Mediterranean ecosystems of low nutrient status. In ‘Mediterranean-type ecosystems. The role of nutrients’. (Eds FJ Kruger, DT Mitchell, JUM Jarvis) pp. 246 273. (Springer Verlag: Berlin)
Larcher W (2000) Temperature stress and survival ability of Mediterranean sclerophyllous plants. Plant Biosystems 134, 279–295.
| Temperature stress and survival ability of Mediterranean sclerophyllous plants.Crossref | GoogleScholarGoogle Scholar |
Llorens L, Peñuelas J, Estiarte M (2003) Ecophysiological responses of two Mediterranean shrubs, Erica multiflora and Globularia alypum, to experimentally drier and warmer conditions. Physiologia Plantarum 119, 231–243.
| Ecophysiological responses of two Mediterranean shrubs, Erica multiflora and Globularia alypum, to experimentally drier and warmer conditions.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXnvVyhsbc%3D&md5=3e7e4471f614d51509c24961d0378508CAS |
Lloret F, Casanovas C, Peñuelas J (1999) Seedling survival of Mediterranean shrubland species in relation to root : shoot ratio, seed size and water and nitrogen use. Functional Ecology 13, 210–216.
| Seedling survival of Mediterranean shrubland species in relation to root : shoot ratio, seed size and water and nitrogen use.Crossref | GoogleScholarGoogle Scholar |
Louw RR (2006) Sustainable harvesting of wild rooibos (Aspalathus linearis) in the Suid Bokkeveld, Northern Cape. MSc(Bot.) Thesis, Leslie Hill Institute for Plant Conservation, Botany Department, University of Cape Town, Cape Town South Africa.
Maier NA, Barth GE, Cecil JS, Chvyl WL, Bartetzko MN (1995) Effect of sampling time and leaf position on leaf nutrient composition in Protea ‘Pink Ice’. Australian Journal of Experimental Agriculture 35, 275–283.
| Effect of sampling time and leaf position on leaf nutrient composition in Protea ‘Pink Ice’.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2MXnsF2ltr4%3D&md5=ac101608c853f8ec4fa147b58dd6417cCAS |
Malgas R, Oettle N (2007) Die volhoubare oes van rooibos-veldtee. ‘n Handleiding vir die noordelike sederberge en die bokkeveld-plato.’ (Environmental Monitoring Group, Paarl Print: South Africa)
Mitchell DT, Coley PGF, Webb S, Allsopp N (1986) Litter fall and decomposition processes in the coastal fynbos vegetation, south-western Cape, South Africa. Journal of Ecology 74, 977–993.
| Litter fall and decomposition processes in the coastal fynbos vegetation, south-western Cape, South Africa.Crossref | GoogleScholarGoogle Scholar |
Mooney HA, Rundel PW (1979) Nutrient relations of the evergreen shrub, Adenostoma fasciculatum, in the Californian chaparral. Botanical Gazette 140, 109–113.
| Nutrient relations of the evergreen shrub, Adenostoma fasciculatum, in the Californian chaparral.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaE1MXktlyhsro%3D&md5=c54b61a30709c58172b4b73bed7b967cCAS |
Morton JF (1983) Rooibos tea, Aspalathus linearis, a caffeineless, low-tannin beverage. Economic Botany 37, 164–173.
| Rooibos tea, Aspalathus linearis, a caffeineless, low-tannin beverage.Crossref | GoogleScholarGoogle Scholar |
Muofhe ML, Dakora FD (2000) Modification of rhizosphere pH by the symbiotic legume Aspalathus linearis growing in a sandy acidic soil. Australian Journal of Plant Physiology 27, 1169–1173.
Rundell PW (1988) Leaf structure and nutrition in Mediterranean climate sclerophylls. In ‘Mediterranean-type ecosystems’. (Ed. RL Specht) pp. 157–167. (Kluwer Academic: Dordrecht, The Netherlands)
Sardans J, Peñuelas J, Rodà F (2006) The effects of nutrient availability and removal of competing vegetation on resprouter capacity and nutrient accumulation in the shrub Erica multiflora. Acta Oecologica 29, 221–232.
| The effects of nutrient availability and removal of competing vegetation on resprouter capacity and nutrient accumulation in the shrub Erica multiflora.Crossref | GoogleScholarGoogle Scholar |
Sardans J, Peñuelas J, Estiarte M, Prieto P (2008) Warming and drought alter C and N concentration, allocation and accumulation in a Mediterranean shrubland. Global Change Biology 14, 2304–2316.
| Warming and drought alter C and N concentration, allocation and accumulation in a Mediterranean shrubland.Crossref | GoogleScholarGoogle Scholar |
Sprent JI, Odee DW, Dakora FD (2009) African legume: a vital but underutilized resource. Journal of Experimental Botany 25, 1–9.
Stock WD, Sommerville JEM, Lewis OAM (1987) Seasonal allocation of dry mass and nitrogen in a fynbos endemic Restionaceae species, Thamnochortus punctatus Pill. Oecologia 72, 315–320.
| Seasonal allocation of dry mass and nitrogen in a fynbos endemic Restionaceae species, Thamnochortus punctatus Pill.Crossref | GoogleScholarGoogle Scholar |
Swap RJ, Aranibar JN, Dowty PR, Gilhooly WP, Macko SA (2004) Natural abundance of 13C and 15N in C3 and C4 vegetation of southern Africa: patterns and implications. Global Change Biology 10, 350–358.
| Natural abundance of 13C and 15N in C3 and C4 vegetation of southern Africa: patterns and implications.Crossref | GoogleScholarGoogle Scholar |
Turner JM (1994) Sclerophylly: primarily protective? Functional Ecology 8, 669–675.
| Sclerophylly: primarily protective?Crossref | GoogleScholarGoogle Scholar |
Van Der Bank M, Van Wyk BE, Van Der Bank H (1995) Biochemical genetic variation in four wild populations of Aspalathus linearis (rooibos tea). Biochemical Systematics and Ecology 23, 257–262.
Van Der Bank M, Van Der Bank H, Van Wyk BE (1999) Evolution of sprouting versus seeding in Aspalathus linearis. Plant Systematics and Evolution 219, 27–38.
van der Heyden F, Lewis OAM (1989) Seasonal variation in photosynthetic capacity with respect to plant water status of five species of the Mediterranean climate region of South Africa. South African Journal of Botany 55, 509–515.
Vance CP, Heichel GH (1991) Carbon in N2 fixation: limitation or exquisite adaptation. Annual Review of Plant Physiology and Plant Molecular Biology 42, 373–390.
| Carbon in N2 fixation: limitation or exquisite adaptation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3MXltFSms78%3D&md5=af5defaf6e405961a832d5396f068d5dCAS |
Vitale L, Arena C, De Santo AV (2012) Seasonal changes in photosynthetic activity and photochemical efficiency of the Mediterranean shrub Phillyrea angustifolia L. Plant Biosystems 146, 443–450.
| Seasonal changes in photosynthetic activity and photochemical efficiency of the Mediterranean shrub Phillyrea angustifolia L.Crossref | GoogleScholarGoogle Scholar |
Wisheu IC, Rosenzweig ML, Olsvig-Whittaker L, Shmida A (2000) What makes nutrient-poor Mediterranean heathlands so rich in plant diversity? Evolutionary Ecology Research 2, 935–955.
Witkowski ETF (1991) Effects of invasive alien acacias on nutrient cycling in the coastal lowlands of the Cape fynbos. Journal of Applied Ecology 28, 1–15.
| Effects of invasive alien acacias on nutrient cycling in the coastal lowlands of the Cape fynbos.Crossref | GoogleScholarGoogle Scholar |
Witkowski ETF, Mitchell DT, Stock WD (1990) Responses of a Cape fynbos ecosystem to nutrient additions: shoot growth and nutrient contents of a proteoid (Leucospermum parile) and an ericoid (Phylica cephalantha) evergreen shrub. Acta Ecologica 11, 311–326.