Energy intake and nutritional balance of roe deer (Capreolus capreolus) in special Bavarian landscapes in southern Germany
Andreas König A * , Sarah-Alica Dahl
A * , Sarah-Alica Dahl  A and Wilhelm Windisch B
A and Wilhelm Windisch B
A Technical University of Munich, Wildlife Biology and Management Unit, Hans-Carl-von-Carlowitz Platz 2, Freising D-85354, Germany.
B Technical University of Munich, Chair of Animal Nutrition, Liesel-Beckmann-Strasse 2, Freising D-85354, Germany.
Animal Production Science 63(16) 1648-1663 https://doi.org/10.1071/AN23034
Submitted: 24 January 2023 Accepted: 7 July 2023 Published: 31 July 2023
Abstract
Irrespective of the fact that the European roe deer (Capreolus capreolus) occurs from the Mediterranean to north of the Arctic Circle and is one of the most abundant and widespread species, roe deer are fed in winter in Germany and Austria. Hunters justify the supplementary feeding with the argument that roe deer do not find sufficient high-quality food in our landscape and they would have to starve in winter.
Our aim was to measure the quality and energy content of the browsed roe deer diet (in terms of metabolisable energy, ME) and the daily energy intake by roe deer.
Between 2017 and 2019, rumenreticula of 629 roe deer were collected from five extreme habitats in Bavaria. Samples were examined by standard methods of dietary analysis, such as in vitro ruminal fermentation, crude nutrient analysis and the analysis of neutral/acid detergent fibre and acid detergent lignin. In addition, data on body condition and age were collected for each roe deer.
The diet consumed by roe deer has an energy density between 5.1 MJ/kg dry matter (DM) and 6.1 MJ/kg DM on average. Crude fibre contents in the diet varied between 20% and 38% DM. Roe deer compensate for lower energy densities in vegetation by consuming more diet. Across all habitats, adult consumed an annual mean of between 10.6 and 12.9 MJ ME/day.
Roe deer find sufficient high-energy food in all landscape types. They consume and can utilise raw fibre to the same extent as do red deer or mouflon. Differences in energy density among habitats result from carbohydrate content and are compensated for by more food intake. Energy deficits in roe deer could not be detected in any habitat at any time.
Roe deer do not need supplementary feeding in any habitat. Supplementary feeding leads to browsing damage because of the lack of raw fibre in the feed.
Keywords: crude protein, fibre, habitat, metabolisable energy, NfE, nutritional balance, optimal foraging, rumen, selector.
Introduction
The roe deer (Capreolus capreolus) is the most common and important deer and wild ruminant species in Germany. Since roe deer colonise all European habitats from the Mediterranean to north of the Arctic Circle (Sempere et al. 1996), they occur in all hunting grounds in Germany, regardless of whether these are located in original forest habitats or cultivated landscapes (Stubbe 1997). This prominent role as a huntable species is reflected by the 1.3 million roe deer that were shot in the hunting year 2020/2021 (DJV 2022). Accordingly, all hunters in Germany learn about the biology of roe deer as part of their training (Seibt 2017). Although the range of the roe deer extends far into northern Scandinavia, and it is thus very well adapted to long, cold winters with food of low quality (Onderscheka and Jordan 1976), roe deer are fed by hunters in winter in all habitats in Germany and, indeed, in many areas of Europe (Ossi et al. 2017). Justifications given for this behaviour are Hofmann’s theories and his classification of wild ruminants into the three rumen classifications ‘concentrate selector or browser’, ‘intermediate, opportunistic mixed feeders’, and ‘grass and roughage eaters or grazers’ (Hofmann 1989). Hofmann and others combined this classification, on the basis of rumen morphology, with the attribution of dietary habits to species (Pérez-Barbería et al. 2004). This classification of Hofmann (1989) is associated with the dietary habit of selecting their browsing for high protein content, and avoiding high-fibre food (Drescher-Kaden 1984; Hofmann 1989). This is supposedly because they lack lignin- and cellulose-decomposing microorganisms such as cellulolytic bacteria or fungi in the rumen (Drescher-Kaden and Seifelnasr 1977a; Duncan et al. 1998).
On the basis of these theories of Hofmann (1989), it is assumed that roe deer find little suitable food in the cultivated landscape (Bauer 2007; Weibora 2020), and may therefore build up insufficient fat reserves for the winter, which is why they have to be fed in winter. Another thesis has recently emerged, that the food quality of roe deer is deteriorating as a result of climate change (Wipf et al. 2009). This thesis is also used as justification for the feeding of roe deer in winter (Ossi et al. 2017; Weibora 2020). Mixtures of cereals such as oats, barley and wheat or maize, with an energy density of ~11 MJ ME/kg DM and a crude fibre content of 14% DM to 15% DM, are recommended as species-appropriate winter feed for deer in winter (Weibora 2020).
This rigid adherence to Hofmann’s theses in connection with the feeding of roe deer is astonishing, because the supposed correlations between rumen morphology and dietary habit have been refuted, on the basis of data, in numerous publications on wild ruminants all over the world (Woodall 1992; Behrend et al. 2004; Lechner et al. 2009; Clauss et al. 2010).
Specifically, recent studies on roe deer in two typical landscapes of southern Germany have shown that not only do roe deer voluntarily consume high fibre contents of between 24% and 38% DM, but that today’s cultivated landscape in particular offers roe deer a diet with a significantly higher energy density than that of the natural landscape (König et al. 2020). With their microbiome, the roe deer were also well adapted to these high fibre contents (König et al. 2016; Dahl et al. 2020), so that at no time in either of the study areas did they have a shortfall in energy, and there was thus no need for feeding at all (König et al. 2020). This study analysed two typical habitats corresponding to the Bavarian average. The question now arises as to what quality and energy the vegetation in non-average habitats offers for roe deer, and whether there is not a need for supplementary feeding of roe deer in March/April after all. Only in these 2 months of the year is there the potential for a shortfall in energy for wild ruminants in the northern hemisphere (Hofmann and Kirsten 1982; Arnold et al. 2004). This situation would arise if their fat and body mass reserves were depleted, their metabolism increased as a result to more sun hours per day, and the vegetation were not sufficiently developed at the same time to cover this energy requirement.
According to the ‘optimal foraging theory’, to avoid energy deficits, roe deer would either have to select the optimum plants for their needs within their habitat, or seek out areas/habitats with suitable plants (Morrison et al. 1992) so as to maximise their energy intake (Felton et al. 2016). Although roe deer also migrate, especially in autumn because of the poorer quality of the forage available (Peters et al. 2019), the proportion of individual animals that do migrate is much lower than, for example, in red deer (Mysterud et al. 2012; Peters et al. 2019). Reasons for this include the seasonal territoriality of the roe deer and the generally small sizes of the territories they frequent (Gentsch et al. 2016). Since the nutritional value of the vegetation also decreases strongly in winter (Onderscheka and Jordan 1976), the question arises as to whether and how roe deer react to lower energy concentrations in the vegetation.
However, if we assume that roe deer can absorb sufficient energy in all habitats, as in König et al. (2020), the question arises as to whether, in accordance with the ‘nutritional balance theory’ (Felton et al. 2016, 2018, 2021), roe deer also gear their food intake to a balanced supply of raw nutrients. In the populations of roe deer studied by König et al. (2020), there were thus large differences in the crude protein (CP) content of the browsing intake in a monthly comparison, but not in the annual average, in which the CP content of the browsing intake was 28% DM in both habitats. Crude fibre (CF) was not only ingested by roe deer and exploited with the corresponding microbiome (Dahl et al. 2020), but, in fact, its value did not drop below values of 24% DM in a seasonal comparison or below 21% DM in a monthly comparison. The maximum values reached in the seasonal comparison were 34% DM and in the monthly comparison 38% DM (König et al. 2020). In accordance with the nutritional balance theory, the roe deer thus actively endeavoured to consume an appropriate proportion of crude fibre.
On the basis of the optimal foraging theory and the nutritional balancing theory, the following working hypotheses can be formulated:
H1. In accordance with the nutritional balancing theory, roe deer in all habitats try to maintain a certain balance between the different raw nutrients.
H2. In accordance with the foraging theory, roe deer do their best to optimise their energy intake.
H3. Because of the optimisation of their energy intake, roe deer do not have energy deficits over the course of the year and do not require feeding.
To test the working hypotheses, 679 roe deer samples were collected in five special and partially extreme habitats over a period of 12 months.
Materials and methods
Site selection
In contrast to the study by König et al. (2020), this study targeted roe deer in extreme and special habitats. These were situated in the state of Bavaria in the south-east of Germany. The habitats selected were characterised by extreme climates such as those in mountainous locations and/or subject to extreme temperature or precipitation conditions, and the associated vegetation types (Table 1).
| Study area | Altitude above sea level (m) | Precipitation (mm) | Annual mean temperature (°C) | Mean temperature in growing season (°C) | Growing days | |
|---|---|---|---|---|---|---|
| Bavaria Ø | 750–800 | 7.5 | 14.5 | 150 | ||
| Beech forest (BF) | 350–590 | 680–1170 | 9.0 | 15.0 | 160 | |
| Agriculture with beech–oak forest (ABF) | 250–410 | 650–720 | 8.0–7.5 | 15.0 | 160–140 | |
| Pine forest (PF) | 350–400 | 640–810 | 7.5 | 15.0 | 150 | |
| Grassland with spruce–beech forest (GSF) | 730–800 | 1540 | 6.5–4.0 | 13–10 | 130–110 | |
| Alpine mountain forest (AMF) | 450–1800 | 1640–2430 | 8.0–6.5 | 15–14 | 160–110 |
The selection was made regarding differences in sea level, precipitation, temperature and growing days.
The ‘Beech Forest’ habitat (BF) is located in north-western Bavaria, in the Bavarian part of the ‘Spessart’ region, a low mountain range (Fig. 1). The samples are from the Rothenbuch and Heigenbrücken Bavarian state forestry enterprises. The habitat is dominated by the acidic soil of woodrush–beech forests (Luzulo–Fagetum), with common beech (Fagus sylvatica) as the main species, and proportions of common oak (Quercus robur) and sessile oak (Quercus petraea). The herb layer of ground vegetation is often relatively poor in terms of the number of species. The species are acid-tolerant. Red deer (Cervus elaphus) and wild boar (Sus scrofa) occur in the area as well as roe deer.
Location of the study areas in the federal state of Bavaria, southern Germany (source: openstreetmap).
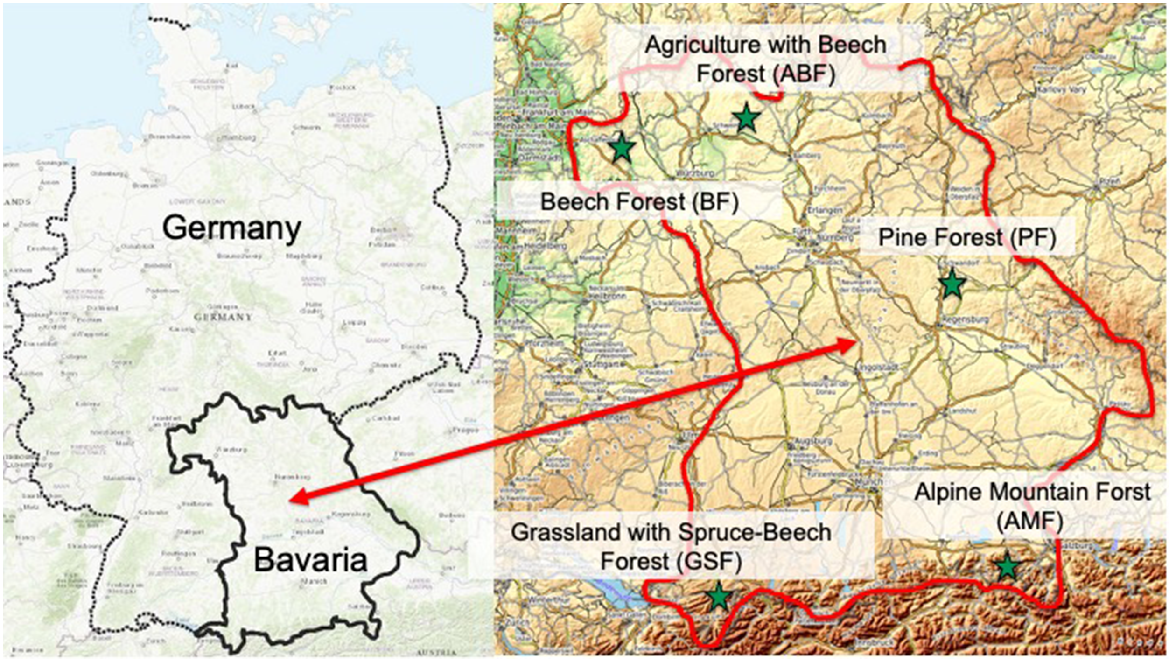
The ‘agriculture with beech–oak forest’ habitat (ABF) is composed of agricultural land and parts of the Sailershausen forest, which is managed by the forestry enterprise of the University of Würzburg. The forest is dominated by beech–hornbeam and oak–hornbeam forest types. In addition to the common beech (F. sylvatica), sessile oak (Q. petraea) and common oak (Q. robur) dominate, along with the European hornbeam (Carpinus betulus). The herb layer is diverse and rich in structure. Cereals and maize were being grown on the agricultural land during the study period. Wild boar occur in the habitat as well as roe deer.
The ‘pine forest’ habitat (PF) is located in the ‘Upper Palatine Basin’ region, and is managed by the Burglengenfeld and Roding Bavarian state forestry enterprises. The forests consist of scots pine (Pinus silvestris) forests without other species, and scots pine forests with a low proportion of admixture tree species, as well as bog forests. The dominant forest societies are leucobryum moss–pine forests (Leucobryo–Pinetum) and pine bog forests (Vaccinio uliginosi–Pinetum sylvestris), with scots pine (Pinus sylvestris) as the main tree species. The forest societies are relatively poor in terms of the number of species. On the sandy and gravelly soils, which are lower in nutrients, dwarf shrubs such as blueberry (Vaccinium myrtillus) or cranberry (Vaccinium vitis-idea) dominate in the pine bog forest, alongside the Scots pine (P. silvestris), and mosses and bog berries. In addition to roe deer, wild boar occurs as a resident game species, and red deer rarely pass through.
The ‘grassland with spruce–beech forest’ habitat (GSF) is located among the molasse deposits of the foothills of the Bavarian Alps in the Upper Allgau region, and is characterised by grassland farming and small patches of forest. The forests are managed by the Bavarian state forestry enterprise of Sonthofen, and private forest owners or farmers. Mixed forests dominate, with Norway spruce (Picea abies) as the main species, mixed with common beech (F. sylvatica) and a few individual European silver firs (Abies alba) and deciduous admixture species. The red deer occurs as an alternative game species alongside the roe deer. Wild boar are rare. The state forest is hunted by state foresters themselves, while the privately owned forests and grassland are hunted by private hunters (hunting ground administered by the municipality of Rettenberg).
Samples of the ‘alpine mountain forest’ habitat (AMF) were collected for the project in the eastern part of the Bavarian Alps. The forests and hunting grounds are managed by the Ruhpolding Bavarian state forestry enterprise. Geologically the habitat is characterised by flysch and the Bavarian Northern Limestone Alps. The sites are covered with mixed mountain forests, with Norway spruce as the main species (P. abies), and varying proportions of European silver fir (A. alba) and common beech (F. sylvatica). The forests in flysch areas are predominantly categorised as mountain woodruff–beech forests (Galio Fagetum), while those in the Limestone Alps are typical mixed mountain forests (Aposiderido Fagetum). In limestone alpine areas, the mixed mountain forests are replaced at higher altitudes by Norway spruce forests (Adenostylo glabrae–Piceetum) and then by subalpine mountain pine scrub (Rhododentro hirsuti–Pinetum mughi). Red deer (C. elaphus) and chamois (Rupicapra rupicapra) occur as well as roe deer, and wild boar (S. scrofa) occasionally appear in the valley areas. The red deer winters at feeding stations and in winter enclosures here in the Alpine region. It is therefore predominantly absent from the area in winter and thus not a food competitor for the roe deer (König 2018).
Sample selection
Between September 2017 and September 2018, 619 roe deer were bagged in the study areas (Table 2). Roe deer were not actively fed during the winter months in any of the study areas. Most of the roe deer were harvested in the course of regular hunting activities. To allow any shortfall of energy for roe deer in the period from January to April (Hofmann and Kirsten 1982) to be determined, the local hunting authorities suspended the closed season for bucks and fawns between 15 January and 1 May 2018 for scientific purposes, in accordance with Art 33 BayJG (Leonhardt and Pießkalla 2021; Reference: 41.3-7533; I/2-751/1-1; 4.14-750/120327; 4.14-750/120329; 5.351-7512-860; LRA OA-7512-WE/E).
| Habitat | Total number of samples | Number of samples for Rr volume and content | Number of samples for crude nutrients | Number of samples for fibre fraction | |
|---|---|---|---|---|---|
| BF | 107 | 70 | 73 | 47 | |
| ABF | 206 | 154 | 83 | 46 | |
| PF | 80 | 71 | 55 | 41 | |
| GSF | 86 | 52 | 38 | 36 | |
| AMF | 200 | 144 | 70 | 58 | |
| Total | 679 | 491 | 319 | 228 |
Once bagged, roe deer were weighed (liveweight, lw) before and after field dressing (fdw) (Table 3). The weights of the fawns (lw P = 0.004, fdw P < 0.001), subadults (lw P < 0.001, fdw P < 0.001) and adult roe deer (lw P = 0.002, fdw P < 0.001) are not identical across habitats.
| Habitat | Age class | Number | Living weight (kg) | Weight field dressed (kg) | |||
|---|---|---|---|---|---|---|---|
| Mean | s.d. | Mean | s.d. | ||||
| BF | Fawn | 41 | 13.1 | 2.46 | 8.8 | 1.81 | |
| Yearling | 29 | 16.23 | 3.05 | 11.6 | 2.38 | ||
| Adult | 35 | 20.69 | 2.57 | 14.49 | 1.68 | ||
| ABF | Fawn | 77 | 13.44 | 2.14 | 9.3 | 1.66 | |
| Yearling | 39 | 19.66 | 3.23 | 14.06 | 2.47 | ||
| Adult | 85 | 21.40 | 2.71 | 15.56 | 2.12 | ||
| PF | Fawn | 23 | 13.28 | 2.27 | 9.27 | 1.77 | |
| Yearling | 32 | 18.09 | 2.53 | 13.06 | 1.96 | ||
| Adult | 22 | 21.24 | 3.2 | 15.09 | 2.23 | ||
| GSF | Fawn | 30 | 14.97 | 2.88 | 11.16 | 1.72 | |
| Yearling | 37 | 18.85 | 3.14 | 13.93 | 2.62 | ||
| Adult | 16 | 21.19 | 2.13 | 15.42 | 2.07 | ||
| AMF | Fawn | 73 | 14.31 | 2.35 | 10.06 | 1.89 | |
| Yearling | 78 | 18.87 | 2.86 | 13.83 | 2.30 | ||
| Adult | 47 | 22.98 | 2.39 | 16.63 | 1.84 | ||
| Over all | Fawn | 244 | 13.83 | 2.42 | 9.72 | 1.77 | |
| Yearling | 215 | 18.34 | 2.97 | 13.3 | 2.36 | ||
| Adult | 205 | 21.50 | 2.60 | 15.44 | 1.99 | ||
Additionally, the length of the right hind leg and, after boiling of the skull, the length of the lower jaw were recorded as measurements of the animal’s constitution (Langvatn 1977). Subsequently, the carcasses were field-dressed, and the entire removed contents of the abdomen, including all organs, were frozen locally at −20°C.
The age of the roe deer was determined by examining the cement layers in the first molar M1 (Habermehl 1961), and the animals were grouped according to sex and age in the categories fawn, yearling and adult. The sex ratio of taken roe deer was 1:0.98 male:female.
Sample preparation
The samples were prepared for further analyses in accordance with König et al. (2020). So as to determine the reticulorumen (RR) volume, the ingesta, omasum and abomasum (stomach) were separated. The ingesta in the RR were subsequently removed and weighed to determine the reticulorumen content (kg). The empty reticulorumen was filled with water to determine its volume (L) on the basis of water displacement.
The ingesta were first homogenised, and then ~150 g was removed, freeze-dried and ground to 1 mm for further analyses. After drying, the sample was weighed again to determine the proportion of dry mass (DM) to fresh mass (FM) (König et al. 2020).
Nutritional analysis
The freeze-dried samples were analysed using the standard procedures (AOAC 2019) with regard to their composition, the crude nutrients and fibre fractions (NDF) (Van Soest et al. 1991; König et al. 2020).
We determined the following crude nutrients (König et al. 2020):
determination of the dry matter (DM) through heat-drying at 103°C
lipids (%DM)
crude fibre (CF; DM 5)
crude protein (CP; %DMNFE)
ash (%DM)
nitrogen-free extract (NfE; %DM)
neutral detergent fibre (NDF; %DM)
acid detergent fibre (ADF; %DM)
acid detergent lignin (ADL; %DM)
water soluble carbohydrates (WSC; %DM)
Hemicellulose, cellulose and lignin were calculated using NDF, ADF and ADL, as follows:
The use of the crude nutrients allows comparison with older studies. NfE with crude fibre (CF) or WSC (sugars, starches and pectins) with NDF (lignin, cellulose and hemicellulose) represent carbohydrates in the browsing material.
Metabolisable energy estimation of the ingested diet
Metabolisable energy (ME) was determined using the in vitro gas-production method according to Menke and Steingass (1988). For this, ground-up ingesta samples for each dead roe deer were mixed with rumen fluid from a ram, and the gas production after 8 and 24 h was recorded. The analyses for each rumen sample were performed four times.
The metabolisable energy (ME) was calculated using the following formula (Menke and Steingass 1988):
Total ME estimation (MJ ME/roe deer.day) (König et al. 2020)
The daily energy intake of the deer was calculated according to König et al. (2020). Time the food remains in the rumen is very short in roe deer, which is why the roe deer has 8–12 browsing periods per day and fills the rumen twice (Bubeník and Lochman 1956). At the time of sampling, 1/3 to 1/4 of the rumen content consisted of undigested, energy-rich food matter. To estimate the energy intake over the whole day, the energy concentrations of the ingested diet determined per rumen sample (DM) were converted to fresh matter (FM) values and multiplied with the weight of the ingesta found in the rumen. To convert the fresh weight to dry weight, we determined the proportion of dry mass in the fresh mass for each sample. This value was used for the correction and was, on average, ~0.223 kg DM/kg FM (±standard deviation 0.036). This corresponds approximately to the value of 0.2 kg dry weight/kg fresh weight in the literature (Stubbe 1997). The estimated energy intake per rumen was then corrected by 1/3 or 1/4 per rumen content (kg) and multiplied by 2 to get the daily energy estimation for the two rumen fillings per day. These two values are used below as minimum (Eqn 3) and maximum (Eqn 4) estimates of daily energy intake. Minimum and maximum estimations of the energy consumed per day form the frame in which the energy consumed by the roe deer lies.
MJ (ME/day) was calculated according to the following equations:
Statistics
Mean values are calculated by forming monthly and/or seasonal mean values. For identifying normal distribution, Kolmogorov–Smirnov and Shapiro–Wilk test was used. Student’s t-tests for equal and unequal variances were used to back up the statistical differences between means. The decision on whether to use a t-test for equal or unequal variances was taken after using Levene’s test for equality of variances. For non-normal distributions of data, the median test with Bonferroni correction (adjusted significance, adj. sig.) was used to back up the statistical differences between medians. For comparisons of the means of the five habitats and the three age classes, one-factor ANOVA with Bonferroni correction (adjusted significance, adj. sig.) was used. The calculations were performed using IBM SPSS Statistics for Windows, Version 29.0. Armonk, NY: IBM Corp.
Results
Quality of the diet
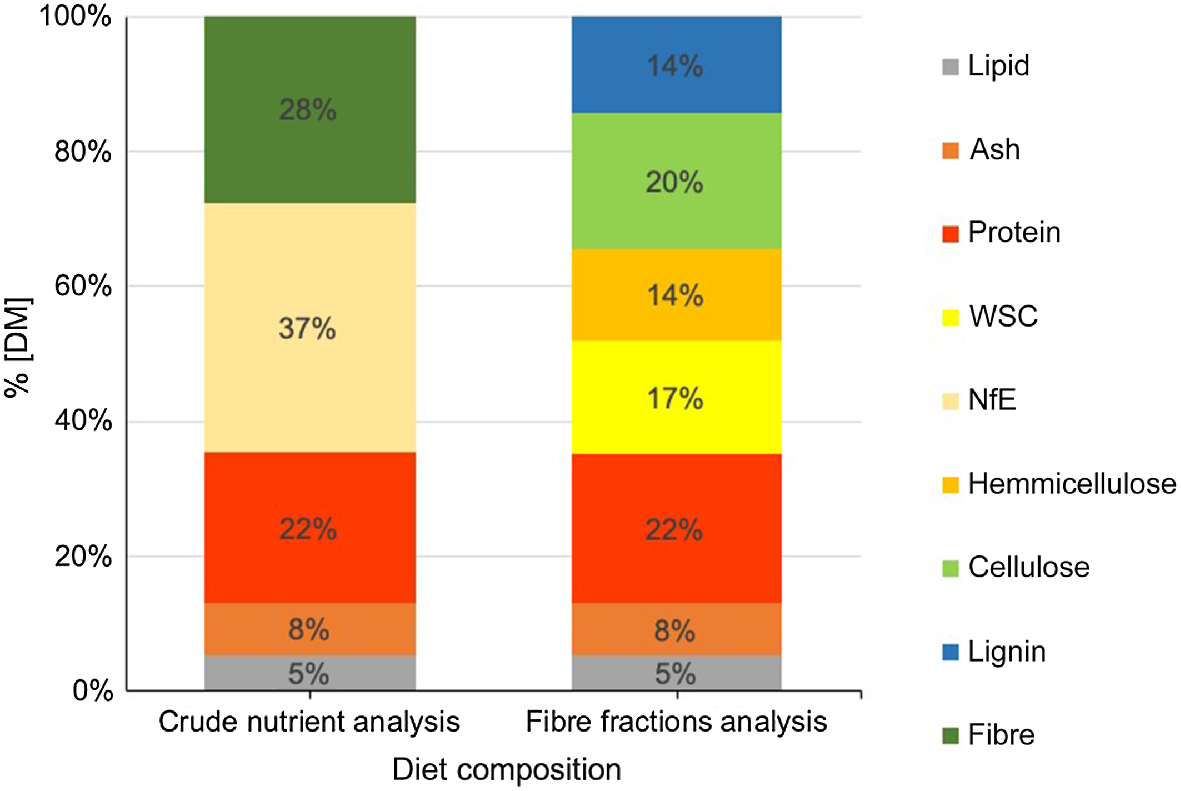
For the analysis of the quality of the roe deer ingested diet, we analysed the crude nutrients and the fibre fractions. With the exception of the lipids, all the examined crude nutrients differed significantly among the sites (Table 4). Across all sites, the CF value was 28% DM and the NfE value was 37% DM. According to the fibre fraction analysis, these values were broken down into 48% NDF (14% DM lignin, 20% DM cellulose, 14% DM hemicellulose), and 17% DM WSC. Ash was, on average, 8% DM and fat 5% DM. The average CP value across all habitats was 22% DM (Fig. 2) and it ranged between 20.1% DM and 23.1% DM (Table 4).
| Parameter | Habitat number | Fibre fraction analysis | Crude nutrients | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NDF 232 | Lignin 232 | Cellulose 232 | Hemicellulose 232 | WSC 232 | CF 323 | Ash 323 | Lipid 323 | CP 323 | NfE 323 | |||
| Spring | BF | 54.7 (6.1) | 14.9 (2.4) | 23.9 (4.2) | 15.9 (4.4) | 10.0 (4.5) | 30.5 (3.8) | 8.1 (2.1) | 4.9 (0.7) | 22.7 (4.1) | 34.0 (4.6) | |
| ABF | 42.8 (9.0) | 11.4 (4.8) | 18.6 (4.3) | 12.8 (4.7) | 15.3 (7.9) | 22.0 (5.5) | 8.5 (1.5) | 5.9 (1.9) | 26.4 (7.1) | 37.2 (8.2) | ||
| PF | 59.2 (6.1) | 16.6 (4.5) | 22.9 (4.2) | 19.8 (6.0) | 11.1 (3.8) | 30.6 (7.1) | 6.2 (1.0) | 5.4 (1.6) | 21.6 (6.8) | 36.1 (3.9) | ||
| GSF | 40.9 (8.4) | 13.0 (4.7) | 19.9 (4.6) | 13.6 (5.9) | 19.8 (6.3) | 23.9 (4.3) | 7.8 (0.8) | 6.0 (1.4) | 26.2 (5.5) | 35.7 (3.9) | ||
| AMF | 43.3 (7.7) | 13.0 (4.7) | 17.8 (3.5) | 12.5 (5.2) | 17.4 (4.6) | 23.9 (4.4) | 7.8 (0.9) | 5.4 (1.3) | 25.6 (5.2) | 36.9 (3.2) | ||
| Total | 48.2 (10.2) | 13.7 (4.5) | 19.9 (4.6) | 13.6 (5.9) | 14.7 (6.4) | 26.2 (6.2) | 7.7 (1.5) | 5.5 (1.4) | 24.5 (5.9) | 36.0 (4.7) | ||
| Summer | BF | 44.8 (7.9) | 14.6 (6.3) | 20.2 (4.0) | 11.6 (3.8) | 17.5 (4.6) | 28.6 (7.5) | 7.8 (2.2) | 5.1 (1.4) | 25.1 (4.4) | 33.4 (5.1) | |
| ABF | 51.4 (7.4) | 12.7 (4.5) | 21.7 (5.2) | 17.0 (4.2) | 15.0 (2.8) | 28.1 (7.6) | 7.7 (2.0) | 5.1 (1.2) | 20.7 (5.2) | 38.4 (4.8) | ||
| PF | 45.6 (7.0) | 12.6 (2.9) | 17.5 (3.3) | 15.5 (4.7) | 17.5 (7.5) | 25.8 (4.6) | 6.8 (1.3) | 6.5 (1.8) | 23.6 (4.1) | 37.3 (6.6) | ||
| GSF | 44.2 (7.0) | 13.5 (2.0) | 21.1 (5.1) | 9.6 (2.0) | 18.0 (2.0) | 28.0 (4.5) | 8.4 (1.1) | 6.0 (1.3) | 23.4 (4.3) | 34.2 (3.5) | ||
| AMF | 41.0 (5.5) | 11.0 (4.0) | 17.6 (2.8) | 12.4 (2.7) | 20.0 (3.5) | 24.4 (6.1) | 8.6 (1.8) | 6.0 (1.0) | 24.7 (3.7) | 36.4 (4.5) | ||
| Total | 45.4 (7.7) | 12.8 (4.6) | 19.4 (4.3) | 13.5 (4.4) | 17.6 (4.8) | 27.0 (6.6) | 7.8 (1.9) | 5.7 (1.4) | 23.5 (4.6) | 36.0 (5.3) | ||
| Autumn | BF | 48.7 (6.3) | 12.9 (3.1) | 21.2 (2.9) | 14.6 (4.1) | 15.4 (5.2) | 26.8 (6.1) | 8.6 (1.9) | 5.5 (1.2) | 22.9 (3.0) | 35.9 (5.2) | |
| ABF | 42.8 (5.8) | 12.5 (3.2) | 16.1 (5.1) | 14.3 (5.9) | 22.3 (11.7) | 22.2 (4.7) | 8.7 (2.8) | 5.3 (1.1) | 21.5 (3.8) | 42.3 (6.7) | ||
| PF | 54.2 (7.7) | 16.4 (5.4) | 10.2 (5.8) | 17.6 (5.6) | 13.9 (3.8) | 27.8 (5.5) | 6.2 (1.3) | 5.4 (1.0) | 19.8 (4.8) | 40.9 (5.5) | ||
| GSF | 46.7 (7.1) | 15.5 (4.2) | 20.3 (2.6) | 11.0 (3.5) | 18.4 (5.8) | 33.8 (10.8) | 7.8 (1.6) | 5.6 (1.2) | 22.3 (2.7) | 30.6 (10.0) | ||
| AMF | 42.7 (5.9) | 12.0 (2.9) | 19.7 (3.1) | 11.0 (2.7) | 20.2 (3.5) | 30.2 (11.1) | 8.2 (1.3) | 6.0 (1.1) | 22.6 (2.7) | 33.1 (9.9) | ||
| Total | 47.0 (7.5) | 13.6 (4.0) | 20.0 (4.2) | 13.4 (4.8) | 18.0 (6.9) | 28.2 (8.2) | 7.9 (2.2) | 5.5 (1.1) | 21.8 (3.6) | 36.6 (8.5) | ||
| Winter | BF | 53.9 (8.1) | 15.8 (2.9) | 21.5 (4.4) | 16.6 (4.0) | 13.2 (3.8) | 30.2 (4.9) | 7.5 (1.6) | 4.4 (1.5) | 20.9 (4.1) | 36.8 (3.7) | |
| ABF | 46.5 (8.4) | 13.8 8 (3.9) | 21.7 (5.2) | 17.0 (4.2) | 19.1 (5.6) | 24.3 (5.1) | 8.4 (2.2) | 4.9 (1.3) | 21.6 (4.1) | 40.8 (5.3) | ||
| PF | 60.0 (7.4) | 17.8 (3.4) | 24.9 (4.3) | 17.3 (3.0) | 14.3 (5.9) | 35.2 (5.6) | 5.6 (4.5) | 4.5 (1.1) | 15.5 (2.0) | 39.1 (6.5) | ||
| GSF | 50.6 (7.0) | 17.3 (3.2) | 21.3 (4.5) | 12.0 (2.6) | 15.8 (3.9) | 33.8 (10.7) | 7.7 (0.9) | 5.0 (0.7) | 21.0 (4.0) | 32.5 (9.5) | ||
| AMF | 50.2 (6.8) | 16.3 (4.9) | 21.4 (3.4) | 12.5 (3.0) | 17.9 (4.3) | 29.7 (5.6) | 7.6 (1.1) | 4.9 (0.9) | 19.6 (3.8) | 38.3 (2.8) | ||
| Total | 52.1 (8.6) | 16.2 (4.0) | 21.6 (4.4) | 14.3 (4.1) | 16.1 (5.6) | 30.7 (7.3) | 7.4 (1.8) | 4.7 (1.2) | 19.7 (4.2) | 37.5 (6.0) | ||
| Total | BF | 49.7 (8.1) | 14.5 (4.3) | 21.4 (3.9) | 14.3 (4.4) | 14.8 (5.2) | 28.8 (5.9) | 8.0 (1.9) | 5.0 (1.3) | 23.0 (4.0) | 35.2 (4.8) | |
| ABF | 46.4 (8.3) | 12.7 (4.1) | 19.1 (4.9) | 14.5 (5.0) | 17.9 (7.6) | 23.9 (5.9) | 8.4 (2.3) | 5.2 (1.3) | 22.0 (4.9) | 40.5 (6.3) | ||
| PF | 54.6 (9.1) | 15.8 (4.4) | 21.4 (5.2) | 17.4 (4.8) | 14.5 (6.5) | 30.0 (6.8) | 6.2 (1.1) | 5.4 (1.5) | 20.1 (5.6) | 38.4 (5.8) | ||
| GSF | 46.0 (8.1) | 15.1 (3.9) | 20.2 (4.0) | 10.4 (3.2) | 17.9 (5.1) | 30.4 (9.6) | 7.8 (1.1) | 5.6 (1.2) | 23.1 (4.5) | 32.9 (8.0) | ||
| AMF | 44.3 (7.3) | 13.1 (4.6) | 19.1 (3.5) | 12.1 (3.5) | 18.9 (4.1) | 27.1 (7.7) | 8.0 (1.3) | 5.5 (1.2) | 23.1 (4.5) | 36.2 (6.1) | ||
| Total | 48.1 (8.9) | 14.1 (4.4) | 20.2 (4.4) | 13.8 (4.8) | 16.8 (6.0) | 27.5 (7.3) | 7.7 (1.9) | 5.3 (1.3) | 22.2 (4.8) | 36.6 (6.6) | ||
| Between habitat | P-value | <0.001 | 0.002 | 0.010 | <0.001 | <0.001 | <0.001 | <0.001 | 0.075 | 0.002 | <0.001 | |
| Between season | P-value | <0.001 | <0.001 | 0.016 | 0.769 | 0.050 | 0.020 | 0.142 | <0.001 | <0.001 | 0.106 | |
Values are mean %DM (s.d. is given in parentheses). ANOVA was used to investigate statistical differences (at P = 0.005) among seasons. NDF, neutral detergent fibre (lignin + cellulose + hemicellulose).
Average proportion (%DM) of crude nutrients and fibre fractions across all habitats (Van Soest et al. 1991; AOAC 2019).
With the CP, the significant (P = 0.002) differences amon habitats were due to the CP values in the PF habitat. While the annual average values ranged between 22% DM and 23% DM in four of the five habitats, the CP value in the PF habitat was only 20% DM and differed significantly from the CP values of the other habitats (BF, P = 0.006; GSF, P = 0.025; and AMF, P = 0.003).
Across all habitats, the lowest CP values were found in the ingested browsing in winter, at just 19.7% DM (Table 4). This value was again determined by the very low CP values in the ingested browsing in the PF habitat, which were, on average, just 15.5% DM in winter.
Differentiated by season, the lowest crude fibre (CF) values in the browsing were found in spring, with an average of 26.2% DM across all habitats. The variation in CF values over the course of the year in the browsing was not the same in all habitats. Especially in spring, the LMF and PF habitats, each with CF values of 30% DM, stood out from the other habitats due to the relatively high values. As expected, the roe deer browsing had the highest CF values across all habitats in winter, with a value of 31% DM. In this season, the PF habitat stood out for having the highest crude fibre content of 35% DM, and the ABF habitat stood out for having the lowest crude fibre content of 24% DM (P < 0.001). According to the fibre fraction analysis, the fibre-bound carbohydrates could be divided into lignin, cellulose and hemicellulose. The ANOVA across all habitats showed no significant differences among the values of the habitats for lignin and cellulose. The highest and significantly different hemicellulose percentages in the ingested browsing were found in the PF habitat (17.4% DM), and the lowest in the GSF habitat (10.4% DM), whereby the values from the GSF habitat did not differ significantly from those of the AMF habitat.
Over the course of the year, the NfE values vary between 31% DM (GSF) and 41% DM (AMF). The variation in the value of this nutrients also showed a completely different annual course among the habitats. On an annual average, the lowest NfE values of 30.6% DM were found in autumn in the GSF habitat, and the highest proportions of 41% DM are found in winter in the ABF habitat. The NfE values in the roe deer diet were significantly (P < 0.001) lower in the GSF habitat than in the PF and ABF habitats. The annual mean values of all habitats differ significantly (P < 0.001).
If we consider the easily soluble carbohydrates (WSC), their value in the browsing intake was 16.8% as an annual average. The lowest values occured in spring, at 14.7% DM. In summer, the WSC values rose to 17.6% DM, before reaching the highest values in autumn at 18.0% DM. The WSC value in winter, at 16.1% DM, was lower the summer and autumn values again. The WSC values did not differ significantly among seasons across all habitats, but did differ among habitats. The browsing in the AMF habitat provided significantly more WSC than did the browsing in the BF (P = 0.002) and PF (P = 0.002) habitats.
Energy concentration
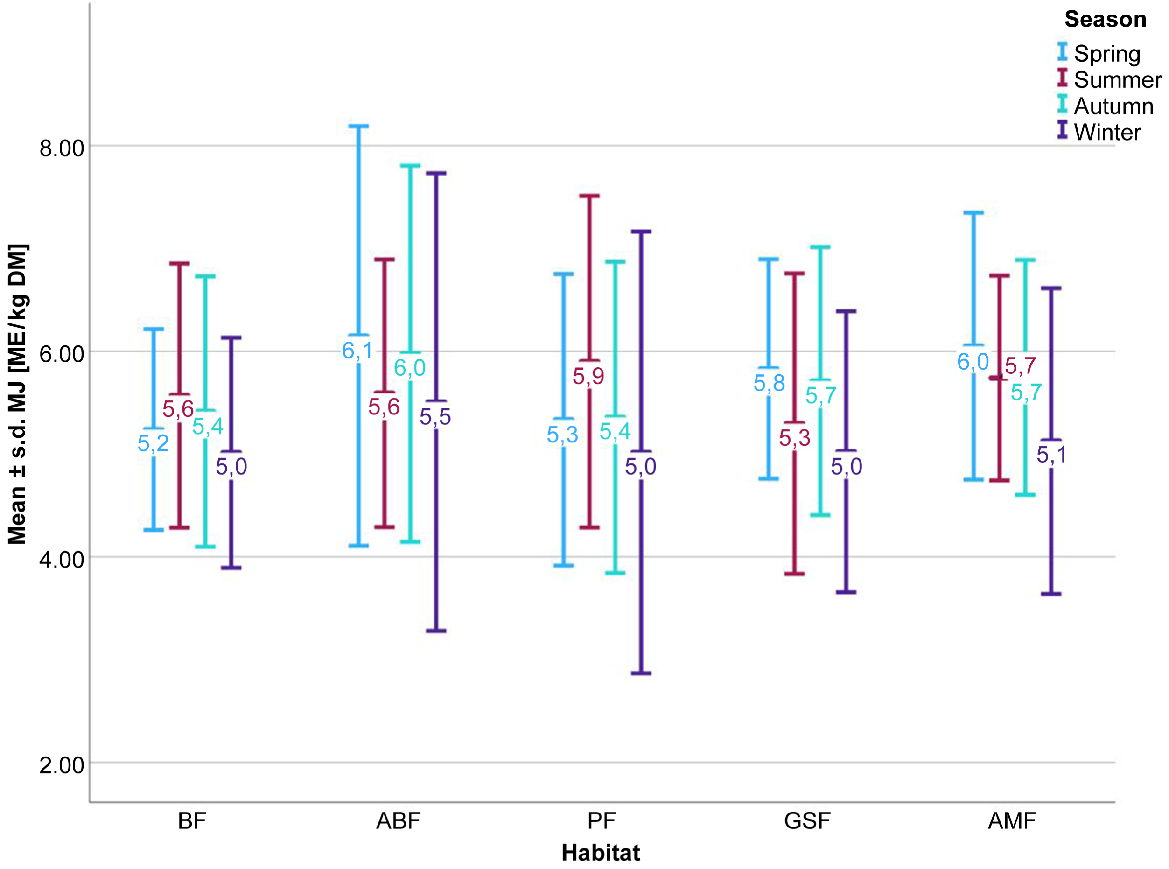
On the basis of ANOVA, the null hypothesis was rejected, with P = 0.007, which means that the energy densities in the different habitats were not the same. On an annual average, the roe deer browsing material in the ABF habitat has the highest energy density at 5.8 MJ ME/kg DM, which is significantly higher than the energy density in the BF habitat, at 5.3 MJ ME/kg DM (P = 0.017).
The highest energy densities in the browsing are thus found in the ABF, GSF and AMF habitats in spring, whereas the highest energy densities in the LMF and PF habitats are measured in summer (Fig. 3). Overall, the energy density in winter is significantly lower than in the other seasons across all habitats (winter to spring, summer and autumn, each P < 0.001).
Reticulorumen (RR) volume of subadult and adult roe deer
For the calculation of the reticulorumen (RR) volumes, median values per season were calculated with the data of the subadult and adult animals, as the mean values of the subadults and adults were not significantly (P = 0.455) different. Taking all sites together, the lowest RR volume was found in winter in subadult and adult roe deer, with an average of 3.7 L (Fig. 4). Towards spring, the volume increased to 3.9 L and remained at the level of 3.8 L in summer and autumn. The volume droped to the lowest rumen volume of 3.7 L in winter.
Reticulorumen content
RR content increased in the median of all habitats (Fig. 5) from spring, with a content of 1.1 kg, to autumn with a content of 1.4 kg (P < 0.001), only to decrease again in winter to 1.3 kg (P = 0.058), whereby the RR content in winter was still significantly higher than that in spring (P = 0.032). Across all habitats and seasons, the median RR content was 1.3 kg.
Seasonal variation in RR content (median + 25th and 75th percentile kg FM) of subadult and adult deer across all habitats (P < 0.001).

The median RR content of subadult and adult roe deer in the BF habitat was significantly higher than in the ABF (P < 0.001) and GSF (P < 0.001) habitats. The RR content of the roe deer (subadult and adult) in habitat AMF was also significantly higher than that of the roe deer in habitats ABF (P < 0.001) and GSF (P = 0.001). There were no significant difference in the RR content of the roe deer among the BF, AMF and PF habitats.
If we distinguish among age classes and habitats (Table 5), the quantity of browsing consumed per age class in the habitats differs significantly (P = 0.050).
| Season | BF | ABF | PF | GSF | AMF | Mean | P-value | |
|---|---|---|---|---|---|---|---|---|
| Fawns | 1.2 (0.37) | 0.8 (0.024) | 0.9 (0.19) | 1.0 (0.31) | 1.12 (0.35) | 1.01 (0.35) | <0.001 | |
| Yearlings | 1.3 (0.39) | 1.2 (0.28) | 1.2 (0.37) | 1.07 (0.28) | 1.38 (0.44) | 1.25 (0.39) | 0.004 | |
| Adults | 1.6 (0.54) | 1.2 (0.38) | 1.3 (0.44) | 1.40 (0.43) | 1.57 (0.48) | 1.36 (0.48) | <0.001 |
Daily total estimated energy intake
The daily energy intake was estimated (MJ ME/roe deer.day) on the basis of the RR content and energy density (Table 6). Subadult and adult roe deer consumed a median of between 11 (s.d. 3.8) and 13 (s.d. 4.6) MJ ME/roe deer.day, and fawns between 8.1 (s.d. 3.6) and 9.8 (s.d. 3.7) MJ ME/roe deer.day of energy (Table 6). Fawns consumed significantly less energy than did yearlings (P < 0.001) and adult roe deer (P < 0.001). The median energy intake of the subadult and adult roe deer did not differ significantly (P = 1.000).
| Item | Number | Median MJ ME/roe deer.day | Adj. sig. (P) | ||
|---|---|---|---|---|---|
| Min | Max | ||||
| Age class | |||||
| FawnA | 100 | 8.1 | 9.8 | ||
| YearlingB | 96 | 10.6 | 12.9 | <0.001BA | |
| AdultC | 97 | 10.6 | 12.9 | <0.001CA | |
| Total | 293 | ||||
| Season | |||||
| SpringA | 54 | 10.3 | 12.5 | 0.007AC | |
| SummerB | 65 | 10.6 | 12.9 | 0.008BC | |
| AutumnC | 44 | 12.8 | 15.6 | ||
| WinterD | 30 | 9.9 | 11.9 | 0.001DC | |
| Total | 193 | 10.9 | 13.2 | ||
| Habitat | |||||
| BF | 42 | 11.6 | 14.1 | ||
| ABF | 43 | 9.7 | 11.8 | ||
| PF | 36 | 9.5 | 11.5 | ||
| GSF | 22 | 9.3 | 11.3 | ||
| AMF | 50 | 12.4 | 14.9 | ||
| Total | 193 | 10.6 | 12.8 | ||
Across all habitats, subadult and adult roe deer showed an increase in energy intake (median MJ ME/kg roe deer.day) starting in spring with 10.3–12.5 MJ ME/kg roe deer.day, and increasing by autumn to 12.8–15.6 MJ ME/roe deer.day (Table 6). In winter, the amount of ME taken in dropped again. The energy intake in autumn was significantly higher than that in spring. Overall, the medians of the ingested energy densities were significantly different across the age classes (P < 0.001) and seasons (P = 0.012).
If we look at the energy intake by habitat over the course of the year, it did not follow the same course in the different habitats. Fig. 6 shows the medians of the energy intake of the subadult and adult roe deer, using the minimum (Eqn 3) energy intake as an example. Roe deer in the ABF habitat thus had the highest median energy intake in spring, whereas the energy intake increased from spring to autumn in the other habitats. Similarly, energy intake dropped in winter in all habitats, except the GSF habitat.
Seasonal habitat-specific cycle of estimated minimum energy intake of roe deer in the five habitats (median ME, MJ ME/roe deer.day). Total medians per habitat are not significantly different.

The energy intake in the ABF habitat was significantly (P = 0.44) higher in spring than that in the GSF habitat and significantly (P = 0.33) lower in winter than that in the BF habitat.
Discussion
This project aimed to answer various hypotheses on the nutritional ecology of roe deer. It was based on roe deer samples collected in five special habitats in Bavaria. Because of their particular vegetation form, location or climate, these habitats are not typical for Bavaria. Habitats that are typical for Bavaria, namely, a near-natural spruce–beech ecosystem and an ecosystem characterised by intensive agriculture, were analysed in a study that was conducted between 2011 and 2014 (König et al. 2020). In both studies, samples were taken in all 12 months of the year, so as to detect any shortfall in energy in the transition between winter and spring, especially in the months of March and April. In these months, the energy demand of the animals increases, because the hourly light amount per day increases and thus the energy requirement also increases. If, at the same time, the animals’ fat reserves are depleted and the vegetation does not provide sufficient energy, wild ruminants could experience an energy shortage during these 2 months (Hofmann 1981; Hofmann and Kirsten 1982; Arnold et al. 2004).
Overall, the results together showed that roe deer are very flexible in adapting to the natural environment in terms of local climate and vegetation conditions, as well as their ability to optimise their energy and nutrient intake.
The raw nutrients are of particular importance for the answering of our questions, as the energy supply of the roe deer depends on them. CF and CP are especially important for roe deer. It is said on the one hand that roe deer cannot utilise crude fibre, especially lignin, and so they avoid crude fibre and lignin (Hofmann 1989; Duncan et al. 1998). On the other hand, it is assumed that deer select their browsing specifically for CP (Drescher-Kaden and Seifelnasr 1977b; Duncan et al. 1998). In wildlife ecology, and independently of roe deer, the presence of proteins in the food and/or excreted with the faeces is generally regarded as a sign of qualitative differences in the diet. This is used to explain different bodyweights and also the spatial behaviour of wild ruminants (Kamler and Homolka 2005; Verheyden et al. 2011). Across all sites, the CP content decreases continuously from 24.5% DM in spring to 19.7% DM in winter (Table 4), thus reflecting plant growth with its cell deposits over the course of the vegetation phases (Schwartz and Hobbs 1985). This annual pattern in the CP concentrations in the ingested diet has already been described in many other studies (Drodz and Osiecki 1973; Serrano Ferron et al. 2012; König et al. 2020) and is consistent with their results. In the study of roe deer from 2011 to 2014, the CP value also decreased from an average of 33% in spring to 23% in winter (König et al. 2020), a phenomenon already described for roe deer by Brüggemann et al. (1967). Although in the study by König et al. (2020) the CP intake did not differ significantly between the two habitats, there are significant differences in this study between the PF habitat and the BF, GSF and AMF habitats. The significant differences arise from the very low mean value in the PF habitat of 15.2% DM CP in winter (Table 4), which is significantly lower than in all other habitats. If we compare the CP between the habitats differentiated according to season, there are no significant differences between the habitats in the proportions of CP in spring, summer and autumn. Therefore, especially after winter, migratory movements of roe deer in spring cannot be explained by differences in CP contents, especially if these originate from the rumen or faeces of the roe deer.
The monthly mean of CP across all habitats ranged from 18.7% DM in February to 28.2% DM in May. These maximum CP values in May are found across all habitats. The highest value is reached in the AMF habitat, at 30% DM. However, in the PF habitat, very low CP values of 15.5% were found over a much longer period, starting in December, which explains the significantly lower mean value for winter. Comparing the seasons across all habitats, the CP values found in this study, with the exception of those in the PF habitat in winter, are within the range of values found in other studies, with a minimum in winter of 20% DM, and a maximum of 25% DM in spring. In the other studies, the CP values range between 20% DM and 36% DM (Brüggemann et al. 1967; Djordjević et al. 2006; Popović et al. 2009; Beuković et al. 2022).
At the same time, in the winter half-year, the PF habitat has the highest mean values for CF, at 35.2% DM. These are caused by the highest proportions of lignin (15.8% DM) and cellulose (24.9% DM). Overall, the CF fractions range between 22.2% DM and 35.2% DM on a seasonal average across the five habitats. This is consistent with the findings of König et al. (2020) and Dahl et al. (2020), who also found values ranging from 21% DM to 38% DM for CF values, with an overall mean of 27.5% DM (Dahl et al. 2020; König et al. 2020), compared with an overall mean of 28% DM in this study. The theses of Hofmann and others (Drescher-Kaden 1984; Hofmann 1989, 2011) that deer do not ingest and utilise CF were thus also refuted by this study, even if only a small part of the CF can be utilised. Thus, Dahl et al. (2020) found a non-insignificant number of Ruminococcus species in their study on the roe deer in typical habitats in Bavaria (Dahl et al. 2020). These have a cellulolytic function and have been detected in roe deer before (Brüggemann et al. 1967), at a time when the fibre fraction was usually only described as CF and not divided into lignin, cellulose and hemicellulose. This conclusion is in line with the large number of studies on ingested plants in roe deer, which not only show a very high level of adaptation of roe deer to the local vegetation, but also the great importance of conifers in the diet of roe deer (Tixier and Duncan 1996; Duncan et al. 1998; Abbas et al. 2011; Serrano Ferron et al. 2012), which is associated with a high proportion of CF in the diet. In ABF and GSF habitats, roe deer could also have avoided CF and lignin respectively, in their diet because they had access to meadows and arable land.
In fact crude fibres such as lignin play an important role in maintaining rumen functionality (Ulbrich et al. 2004) and in keeping the pH in the rumen at a level that does not fall below the critical value of pH 5.0, at which point acute rumen acidosis occurs in roe deer (Dissen and Hartfiel 1985). Forage containing grasses, herbs, leaves and shoots is rich in lignin and/or cellulose and has a low starch content. This means that it is mainly acetate that develops in the rumen as a result of fermentation, and the pH value remains relatively high. However, if more starch and soluble sugar are ingested, more propionate and lactate are formed. This causes a further drop in the pH value in the rumen (Millen et al. 2016). The pH value in the rumen of roe deer increases with an increasing CF or lignin content in the forage, and decreases with increasing proportions of NfE or WSC (König et al. 2016). This correlation is supported by data from Austria, where roe deer are fed traditional diets, i.e. feed with a high proportion of cereals and a crude fibre content of less than 15% DM, had a lower pH of pH 5.5 and were thus on the verge of chronic rumen acidosis, whereas unfed deer had a pH of 5.7 (Ritz et al. 2013).
In Bavaria, the pH values of unfed roe deer in the forest habitat were pH 6.3 and in roe deer from agricultural landscapes pH was 6.1 (König et al. 2016), and higher than the results of Ritz et al. (2013). Rumen pH values below pH 5.0 were not found, although some were found to be below pH 5.5, which could indicate chronic rumen acidosis. However, it cannot be ruled out that the animals would have ingested more fibre if they had not been shot.
If we continue to compare the results for the CF and lignin values in this study with the results of König et al. (2020), we find almost identical mean values of CF at 27.51% DM, and lignin at 14.61% DM in the earlier study (Dahl et al. 2020; König et al. 2020), and CF values of 27.55% DM, and lignin vales of 14.13% DM in Table 4 in the current study. This concurrence is also evident in the 25th percentiles, at CF 22% DM and 11% DM lignin respectively in this study, and at CF 22.6% DM and lignin 11.4% DM in the older study (König et al. 2016).
A similar overlap of the results as for lignin in the two studies from Bavaria can be seen in the energy intake (MJ ME/roe deer.day) of the roe deer. The median minimum energy intake of the subadult and adult roe deer ranged from 9.3 to 12.4 MJ ME/roe deer.day (Table 6), and from 8.9 to 9.8 MJ ME/roe deer.day in the agricultural and forest habitats (König et al. 2020). The energy intake differs among the habitats, but not significantly, so it is therefore random and within a similar range.
In summary, it can be stated that the roe deer in the habitats presented here and in König et al. (2020) tried to keep both nutrients and energy intake in balance, so that H1 can be affirmed. It has also been shown that moose in Scandinavia try to achieve a balance across the different nutrients (Felton et al. 2016, 2021). The same is also known from livestock farming (Ulbrich et al. 2004).
The found energy density of the rumen content does not reflect the energy density of the ingested vegetation, because the material was partly already digested. For this reason, the energy density found is multiplied to calculate the energy intake (König et al. 2020). However, since the energy densities found here are reduced across all habitats in the same species, the differences found in the energy density among the habitats can be compared well.
The energy densities are different after the Bonferroni correction only between the ABF and BF habitats (Fig. 3). In accordance with the ‘foraging theory’ (Morrison et al. 1992), the animals would have to optimise their energy intake, something which migratory species achieve through migration. This is a behaviour that roe deer do not use or rarely want to use, because of their territoriality and the associated relatively low levels of migration (Mysterud 1999; Peters et al. 2019). Instead, roe deer showed a high degree of flexibility and adaptability in the amount of diet consumed (Table 7). Subadult and adult roe deer in the BF habitat consumed significantly more dietary material (1.57 kg RR content) than did roe deer in the ABF, PF and GSF habitats (1.2 kg, 1.2 kg and 1.2 kg respectively). Furthermore, the RR content did not decrease as much as expected in winter in comparison with the RR volume. In this season, the roe deer also compensated for the lower energy density of the vegetation by browsing more. Instead of seeking out areas with higher energy densities, the roe deer reacted with a higher foraging intake to compensate for energetic deficits in the vegetation. Roe deer displayed the same behaviour in a typical agricultural and forest habitat in an earlier study (König et al. 2020). In this case, the roe deer consumed 600 g more foraging material per day in the winter half-year than in the agricultural habitat (König et al. 2020). This flexible adaptation of their food intake was also shown by roe deer in Scandinavia, which also increased their food intake in winter. The foraging intake in Scandinavia was 1.9 kg in winter, as opposed to 1.4 kg and 1.6 kg in summer and autumn respectively, while the RR volume decreased at the same time from 4.0 L to 3.7 L in winter (Holand et al. 1998). On the basis of the results on RR content, the passage rate through the RR of roe deer should also be analysed in further research so as to clarify how they cope with these large amounts of food and high proportions of CF or lignin. In line with our Working Hypothesis H2, it can be stated that, in line with the optimum foraging theory, roe deer adapt their foraging intake instead of migrating to better vegetation patches.
| Age class | kg | SMRA 0.293 MJ/kg0.75 | Energy requirenment | Energy uptake | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SummerB | WinterB | SummerC | WinterC | WinterD | YearE | Winter | ||||||||||
| MJ RMR/kg0.75 | MJ RMR/kg0.75 | MJ SMR/kg0.75 | MJ SMR/kg0.75 | MJ NE/kg0.75 | Min MJ ME/kg0.75 | Max MJ ME/kg0.75 | Min MJ NE/kg0.75 | Max MJ NE/kg0.75 | Min MJ ME/kg0.75 | Max MJ ME/kg0.75 | Winter Min MJ NE/kg0.75 | Winter Max MJ NE/kg0.75 | ||||
| Fawn | 13.8 | 2.1 | 2.7 | 2.7 | 2.8 | 8.2 | 10.0 | 5.8 | 7.0 | 7.8 | 9.5 | 5.6 | 6.8 | |||
| Yearling | 18.3 | 2.6 | 4.0 | 3.4 | 4.8 | 4.2 | 3.5 | 10.9 | 13.2 | 7.7 | 9.3 | 10.1 | 12.2 | 7.3 | 8.8 | |
| Adult | 21.5 | 2.9 | 4.5 | 3.8 | 5.4 | 4.8 | 3.9 | 10.9 | 13.2 | 7.7 | 9.3 | 10.1 | 12.2 | 7.3 | 8.6 | |
The third working hypothesis was to test whether a shortfall in food can occur in these extreme study habitats. For this, Table 7 provides an overview of the energy intake and the energy requirement known from the standard literature.
Depending on the reference value chosen (Table 7), namely, the standard metabolism rate (SMR), resting metabolism rate (RMR), and net energy (NE) requirement (Weiner 1977; Bubenik 1984; Kirchgeßner et al. 2008; Kamphues et al. 2009; Dryden 2011), the roe deer require between 2.7 MJ NE/kg0.75 and 5.4 MJ NE/kg0.75, depending on their age. If we convert the ME intake into NE in accordance with Kirchgeßner et al. (2008), then roe deer in this study consumed between 5.8 and 9.3 MJ NE/kg0.75, or between 8.2 and 13.2 MJ ME/kg0.75. By subtracting the heat production from the convertible energy (ME), we obtained the NE, which can flow into sustenance, growth, lactation, etc. (Kirchgeßner et al. 2008; Kamphues et al. 2009; Dryden 2011). Because roe deer have a thermo-neutral range that goes as low as −10°C (Bubenik 1984), almost 95% of the convertible energy can be used to sustain a roe deer in a normal winter, except during a few days in January/February. From the Bavarian mountains (BF) to the drought areas with pine forests (PF), we thus did not detect any shortage of energy for roe deer! We can thus confirm Working Hypothesis H3, that it is not necessary to feed the roe deer in the studied habitats to compensate for an emergency period caused by a shortfall in food or energy, and that, given the climate-change situation, this will probably not be necessary in the future either (Ossi et al. 2017). This finding is supported by the work of König et al. (2020). König et al. (2020) found that there was no energy shortfall for roe deer in typical habitats in Bavaria, and thus no need for winter feeding, which in Bavaria is permitted only in times of need. Times of need are when roe deer of average condition cannot survive the winter, so that the continued existence of the game population is endangered (Leonhardt and Pießkalla 2021). As a result of climate warming, this situation is no longer to be expected in large parts of Europe, because the vegetation period, and thus the time to build up energy reserves, has lengthened considerably (Menzel et al. 2006).
If we consider the recommendations for feeding, the recommended feed (Onderscheka 1999; Weibora 2020) has a low fibre content (<15% DM) and a high proportion of cereals with correspondingly high proportions of starch, which reduce the RR pH value. In general, the consequences of feeding deer are increased environmental capacity (Ossi et al. 2017), associated with undermining natural selection, and browsing of plants by deer to increase pH in their rumen through fibre intake. Against this background and the lack of any necessity to feed roe deer to maintain their population in central Europe, we must call into question the feeding of roe deer, and its necessity at all. In fact, it is to be expected that where roe deer are fed, they are more likely to cause browsing damage to forest vegetation.
Conclusions
Deer can use vegetation efficiently in all habitats in Bavaria.
The CF content of the ingesta is, on average, 27.5% DM and does not fall below 22% DM.
According to the nutritional balance theory, roe deer try to keep CF and CP as well as the ingested energy in a balanced ratio.
Carbohydrates (NfE/WSC) are an important energy resource.
A lower energy concentration in the diet is compensated by more food intake to optimise energy intake according to the optimal foraging theory.
There were no deficits because of a lack of energy.
To survive the winter, deer do not need supplemental feeding in any habitat in Bavaria.
Roe deer are selectors or browsers, but not ‘concentrate selectors’, because they take in and exploit CF to a similar extent as do ruminants classed as grazers or intermediate feeders.
Acknowledgements
For their practical support in the field, we express our sincere thanks to the colleagues of the Bavarian State Forests (FoB Rothenbuch, FoB Heigenbrücken, FoB Burglengenfeld, FoB Roding, FoB Ruhpolding and FoB Sonthofen), as well as the University Forestry Office Sailershausen and Kai Bomanns in Oberallgäu, and all the students involved. For the professional translation of the manuscript by a native speaker we thank Ms Tessa Feller.
References
Abbas F, Morellet N, Hewison AJM, Merlet J, Cargnelutti B, Lourtet B, Angibault J-M, Daufresne T, Aulagnier S, Verheyden H (2011) Landscape fragmentation generates spatial variation of diet composition and quality in a generalist herbivore. Oecologia 167, 401-411.
| Crossref | Google Scholar |
Arnold W, Ruf T, Reimoser S, Tataruch F, Onderscheka K, Schober F (2004) Nocturnal hypometabolism as an overwintering strategy of red deer (Cervus elaphus). American Journal of Physiology-Regulatory, Integrative and Comparative Physiology 286, R174-R181.
| Crossref | Google Scholar |
Behrend A, Lechner-Doll M, Streich WJ, Clauss M (2004) Seasonal faecal excretion, gut fill, liquid and particle marker retention in mouflon Ovis ammon musimon, and a comparison with roe deer Capreolus capreolus. Acta Theriologica 49, 503-515.
| Crossref | Google Scholar |
Beuković D, Obranović I, Vukadinović M, Popović Z, Horvatović MP, Lavadinović V, Beuković M (2022) The quality of roe deer (Capreolus Capreolus) diet in the winter and spring periods based on rumen contents. Contemporary Agriculture 71, 137-140.
| Crossref | Google Scholar |
Brüggemann J, Giesecke D, Walser-Kärst K (1967) Beiträge zur Wildbiologie und vergleichenden Tierphysiologie. II. Mikroorganismen im Pansen von Rothirsch und Reh. Zeitschrift für Tierphysiologie Tierernährung und Futtermittelkunde 23, 143-151.
| Crossref | Google Scholar |
Bubeník A, Lochman J (1956) Futterverbrauch und Tagesrhythmus der Futteraufnahme bei Reh- und Rotwild. Zeitschrift für Jagdwissenschaft 2, 112-118.
| Crossref | Google Scholar |
Clauss M, Hume ID, Hummel J (2010) Evolutionary adaptations of ruminants and their potential relevance for modern production systems. Animal 4, 979-992.
| Crossref | Google Scholar |
Dahl S-A, Hudler M, Windisch W, Bolduan C, Brugger D, König A (2020) High fibre selection by roe deer (Capreolus capreolus): evidence of ruminal microbiome adaption to seasonal and geographical differences in nutrient composition. Animal Production Science 60, 1303-1314.
| Crossref | Google Scholar |
Dissen J, Hartfiel W (1985) Beobachtungen zum Äsungsverhalten sowie Untersuchungen zur Nährstoffverdaulichkeit von Rehwild. Zeitschrift für Jagdwissenschaft 31, 83-91.
| Crossref | Google Scholar |
Djordjević N, Popović Z, Grubić G (2006) Chemical composition of the rumen contents in roe deer (Capreolus capreolus) as potential quality indicator of their feeding. Journal of Agricultural Sciences 51, 133-140.
| Crossref | Google Scholar |
Drescher-Kaden U, Seifelnasr EA (1977a) Untersuchungen am Verdauungstrakt von Reh, Damhirsch und Mufflon. Mitteilungen 3: Mikroorganismen im Pansen von Reh, Damhirsch und Mufflon. Zeitschrift fur Jagdwissenschaft 23, 64-69.
| Crossref | Google Scholar |
Drescher-Kaden U, Seifelnasr EA (1977b) Untersuchungen am Verdauungstrakt von Rehwild, Damhirsch und Mufflon. Mitteilung 2: Rohnährstoffe im Panseninhalt von Reh, Damhirsch und Mufflon. Zeitschrift für Jagdwissenschaft 23, 6-11.
| Google Scholar |
Drodz A, Osiecki A (1973) Intake and digestibility of natural feeds by roe-deer. Acta Theriologica 18, 81-91.
| Google Scholar |
Dryden GM (2011) Quantitative nutrition of deer: energy, protein and water. Animal Production Science 51, 292-302.
| Crossref | Google Scholar |
Felton AM, Felton A, Raubenheimer D, Simpson SJ, Krizsan SJ, Hedwall P-O, Stolter C (2016) The nutritional balancing act of a large herbivore: an experiment with captive moose (Alces alces L). PLoS ONE 11, e0150870.
| Crossref | Google Scholar |
Felton AM, Wam HK, Stolter C, Mathisen KM, Wallgren M (2018) The complexity of interacting nutritional drivers behind food selection, a review of northern cervids. Ecosphere 9, e02230.
| Crossref | Google Scholar |
Felton AM, Wam HK, Felton A, Simpson SJ, Stolter C, Hedwall P-O, Malmsten J, Eriksson T, Tigabo M, Raubenheimer D (2021) Macronutrient balancing in free-ranging populations of moose. Ecology and Evolution 11, 11223-11240.
| Crossref | Google Scholar |
Gentsch R, Heurich M, König A (2016) Raumverhalten von Rehen (Capreolus capreolus) in der Kulturlandschaft des Bayerischen Waldes. Homeranges, Tagesablauf und Habitatbestimmung. In ‘Wildtiere in einer sich wandelnden Umwelt (2014 in Freising), Große Pflanzenfresser, Große Karnivoren, Große Schutzgebiete (2016 in Trippstadt). Vol. 2’. (Eds A König, U Hohmann, C Ebert, J Mitschke) pp. 55–73. (Verlag Kessel: Freiburg, Germany)
Hofmann RR (1989) Evolutionary steps of ecophysiological adaptation and diversification of ruminants: a comparative view of their digestive system. Oecologia 78, 443-457.
| Crossref | Google Scholar |
Kamler J, Homolka M (2005) Faecal nitrogen: a potential indicator of red and roe deer diet quality in forest habitats. Folia Zoologica 54, 89-98.
| Google Scholar |
König A, Hudler M, Dahl S-A, Bolduan C, Brugger D, Windisch W (2020) Response of roe deer (Capreolus capreolus) to seasonal and local changes in dietary energy content and quality. Animal Production Science 60, 1315-1325.
| Crossref | Google Scholar |
Lechner I, Barboza P, Collins W, Günther D, Hattendorf B, Hummel J, Clauss M (2009) No ‘bypass’ in adult ruminants: passage of fluid ingested vs. fluid inserted into the rumen in fistulated muskoxen (Ovibos moschatus), reindeer (Rangifer tarandus) and moose (Alces alces). Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology 154, 151-156.
| Crossref | Google Scholar |
Menke KH, Steingass H (1988) Estimation of the energetic feed value obtained from chemical analysis and in vitro gas production using rumen fluid. Animal Research and Development 28, 7-55.
| Google Scholar |
Menzel A, Sparks TH, Estrella N, Koch E, Aasa A, Ahas R, Alm-Kübler K, Bissolli P, Braslavská OG, Briede A, Chmielewski FM, Crepinsek Z, Curnel Y, Dahl Å, Defila C, Donnelly A, Filella Y, Jatczak K, Måge F, Mestre A, Nordli Ø, Peñuelas J, Pirinen P, Remišová V, Scheifinger H, Striz M, Susnik A, Van Vliet AJH, Wielgolaski F-E, Zach S, Zust A (2006) European phenological response to climate change matches the warming pattern. Global Change Biology 12, 1969-1976.
| Crossref | Google Scholar |
Mysterud A (1999) Seasonal migration pattern and home range of roe deer (Capreolus capreolus) in an altitudinal gradient in southern Norway. Journal of Zoology 247, 479-486.
| Crossref | Google Scholar |
Mysterud A, Bischof R, Loe LE, Odden J, Linnell JDC (2012) Contrasting migration tendencies of sympatric red deer and roe deer suggest multiple causes of migration in ungulates. Ecosphere 3, 1-6.
| Crossref | Google Scholar |
Onderscheka K, Jordan HR (1976) Einfluß der Jahreszeit, des Biotops und der Äsungskonkurrenz auf die botanische Zusammensetzung des Panseninhaltes beim Gams-, Reh-, Muffel- und Rotwild. Die Bodenkultur 27, 202-217.
| Google Scholar |
Ossi F, Gaillard J-M, Hebblewhite M, Morellet N, Ranc N, Sandfort R, Kroeschel M, Kjellander P, Mysterud A, Linnell JDC, Heurich M, Soennichsen L, Sustr P, Berger A, Rocca M, Urbano F, Cagnacci F (2017) Plastic response by a small cervid to supplemental feeding in winter across a wide environmental gradient. Ecosphere 8, e01629.
| Crossref | Google Scholar |
Pérez-Barbería FJ, Elston DA, Gordon IJ, Illius AW (2004) The evolution of phylogenetic differences in the efficiency of digestion in ruminants. Proceedings of the Royal Society of London. Series B: Biological Sciences 271, 1081-1090.
| Crossref | Google Scholar |
Peters W, Hebblewhite M, Mysterud A, Eacker D, Hewison AJM, Linnell JDC, Focardi S, Urbano F, De Groeve J, Gehr B, Heurich M, Jarnemo A, Kjellander P, Kröschel M, Morellet N, Pedrotti L, Reinecke H, Sandfort R, Sönnichsen L, Sunde P, Cagnacci F (2019) Large herbivore migration plasticity along environmental gradients in Europe: life-history traits modulate forage effects. Oikos 128, 416-429.
| Crossref | Google Scholar |
Popović Z, Đorđević N, Đorđević M, Grubić G, Stojanović B (2009) Estimation of the quality of the nutrition of roe deer based on chemical composition of the rumen content. Acta Veterinaria 59, 653-663.
| Google Scholar |
Ritz J, Hofer K, Hofer E, Hackländer K, Immekus D, Codron D, Clauss M (2013) Forestomach pH in hunted roe deer (Capreolus capreolus) in relation to forestomach region, time of measurement and supplemental feeding and comparison among wild ruminant species. European Journal of Wildlife Research 59, 505-517.
| Crossref | Google Scholar |
Sempere AJ, Sokolov VE, Danilkin AA (1996) Capreolus capreolus. Mammalian Species 538, 1-9.
| Crossref | Google Scholar |
Serrano Ferron E, Verheyden H, Hummel J, Cargnelutti B, Lourtet B, Merlet J, González-Candela M, Angibault JM, Hewison AJM, Clauss M (2012) Digestive plasticity as a response to woodland fragmentation in roe deer. Ecological Research 27, 77-82.
| Crossref | Google Scholar |
Tixier H, Duncan P (1996) Are European roe deer browsers? A review of variations in the composition of their diets. Revue d’Écologie 51, 3-17.
| Crossref | Google Scholar |
Van Soest PJ, Robertson JB, Lewis BA (1991) Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. Journal of Dairy Science 74, 3583-3597.
| Crossref | Google Scholar |
Verheyden H, Aubry L, Merlet J, Petibon P, Chauveau-Duriot B, Guillon N, Duncan P (2011) Faecal nitrogen, an index of diet quality in roe deer Capreolus capreolus? Wildlife Biology 17, 166-175.
| Crossref | Google Scholar |
Weibora S (2020) Rehwildfütterung im BJV-Lehr-und Forschungsrevier Wunsiedel. Jagd in Bayern 7-9.
| Google Scholar |
Weiner J (1977) Energy Metabolism of the Roe Deer. Acta Theriologica 22, 3-24.
| Crossref | Google Scholar |
Wipf S, Stoeckli V, Bebi P (2009) Winter climate change in alpine tundra: plant responses to changes in snow depth and snowmelt timing. Climatic Change 94, 105-121.
| Crossref | Google Scholar |
Woodall PF (1992) An evaluation of a rapid method for estimating digestibility. African Journal of Ecology 30, 181-185.
| Crossref | Google Scholar |