Detecting burrows and trapping for mulgaras (Dasycercus cristicauda and D. blythi) can be difficult
Graham G. Thompson A B C and Scott A. Thompson AA Terrestrial Ecosystems, 10 Houston Place, Mt Claremont, WA 6010, Australia.
B Business School, Edith Cowan University, WA 6027, Australia.
C Corresponding author. Email: graham@terrestrialecosystems.com
Australian Mammalogy 36(1) 116-120 https://doi.org/10.1071/AM13031
Submitted: 30 September 2013 Accepted: 15 January 2014 Published: 12 March 2014
Abstract
Mulgaras (Dasycercus cristicauda and D. blythi) are protected by state and commonwealth environmental statutes; as a consequence, land developers and mining companies have an obligation to avoid, mitigate or minimise impacts on these species when they occur in their area of operation (i.e. to implement trapping and translocation programs). Here we assess the effectiveness of searching and trapping programs for mulgaras in four case studies and provide management recommendations to improve outcomes for these species.
Additional keywords: fauna survey and methodology, searches.
Introduction
The crest-tailed mulgara (Dasycercus cristicauda, previously known as D. hillieri or ampurta: Woolley 2005) is currently listed as a vulnerable species under the Environment Protection and Biodiversity Conservation Act 1999, and the brush-tailed mulgara (Dasycercus blythi, previously known as D. cristicauda: Woolley 2005) is listed as a Priority 4 species with the Western Australian Department of Parks and Wildlife. In May 2012, the Department of Sustainability, Environment, Water, Population and Communities (2012) released a consultation paper recommending the delisting of D. hillieri and the listing of D. blythi, presumably as a vulnerable species.
Both species have a wide and overlapping distribution in arid Australia and can be sympatric (Woolley 2005; Pavey et al. 2011; Department of Sustainability, Environment, Water, Population and Communities 2012; Woolley et al. 2013). Currently, there are insufficient data to identify differences in the spatial ecology, burrows and reproductive biology of D. blythi and D. cristicauda. However, there appears to be differences in their preferred habitat, as Pavey et al. (2011) reported that D. cristicauda is confined to crests and slopes of sand ridges, whereas D. blythi is mostly found on gibber and sand plains that generally lack spinifex cover. Masters and Dickman (2012) reported that D. blythi near the Uluru Kata Tjuta National Park live on red clayey sand between a mulga woodland and a low dune field with the dominant plant species being hard spinifex (Triodia basedowii) and in the Tanami Desert on red sandy soil in a saline drainage dominated by Triodia pungens. In the Pilbara, mulgaras are found on sandy plains vegetated with shrubs and spinifex generally up to 1 m high, but we have also caught them in sparsely vegetated stony areas.
Because of the conservation status of mulgaras, fauna management plans that are approved by the Department of the Environment, the Western Australian Department of Parks and Wildlife or the Western Australian Environmental Protection Authority for areas that support mulgaras often require that they are captured and translocated from the impact area before the vegetation clearing (e.g. implementation of the EPBC Approval Decision 2010/5424; available from http://www.environment.gov.au/epbc/notices/assessments/2010/5424/approval.pdf).
Three strategies are generally deployed to detect the presence of mulgaras in an area or to translocate them before vegetation clearing:
-
the area is searched for active or recently active burrows and, if found, they are dug out and the inhabitants caught;
-
the area is trapped; or
-
a combination of these two if the burrows are difficult to locate or are abundant.
Our aim in presenting the following four case studies is to provide guidelines to improve searching and trapping for mulgaras when knowledge of their presence or absence in an area is required or when they are to be translocated from an area before vegetation clearing. We do not judge the success or otherwise of the translocations.
Methods and Results
Case Study 1: Rail corridor
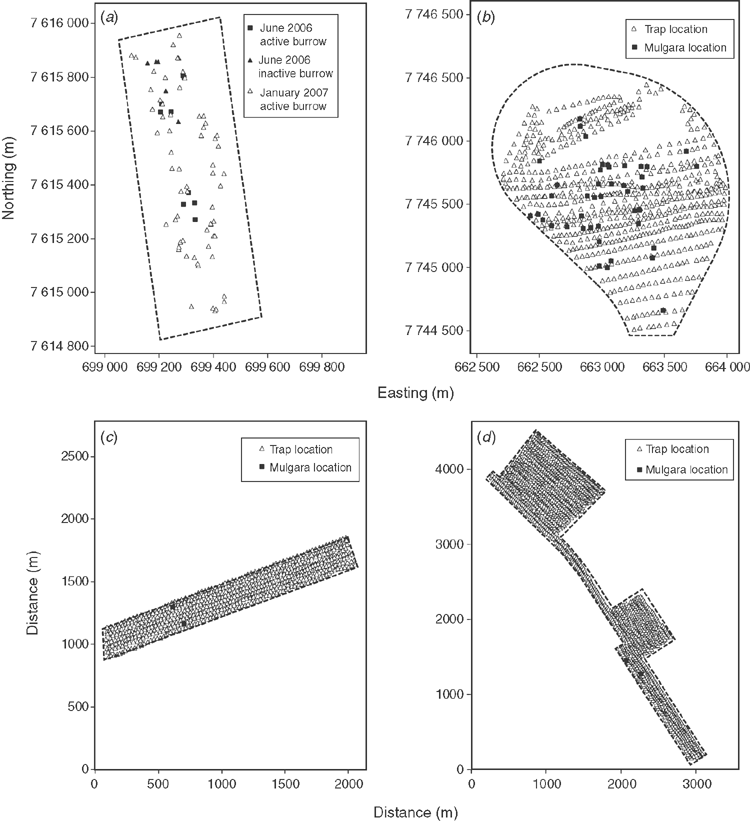
The study area (~1100 m × 400 m) (Fig. 1a) originally contained low shrubs and spinifex (Triodia sp.; >50% cover) to ~600 mm high with some taller shrubs on a substrate of gritty red sand when it was first surveyed in June 2006. Five people walking in parallel lines ~20 m apart up and down the site on four occasions for a total of 22 person-hours systematically searched for mulgara burrows, diggings and scats. The location of all active, potentially active and inactive burrows, diggings and scats were recorded with a GPS. An active or potentially active burrow was one that had no spider webs across the entrance, any loose vegetation or ash in the entrance or there was at least some evidence of ground disturbance at the entrance to indicate that an animal had moved in and out of one of the entrances. If there were multiple burrow entrances in a small area, then this was deemed to be a single burrow complex and recorded once.
Between July and December 2006 the area was burnt leaving small isolated patches of spinifex and dead burnt shrub stems. During 5–10 January 2007, 150 aluminium treadle-activated box traps (325 × 100 × 100 mm) baited with a bolus of oats, peanut butter and sardines (universal bait) were deployed around the 44-ha site. These traps were left open for five nights for a total of 750 trap-nights. The bait was replenished every second day or, if an animal was caught, at the time the trap was reset. During the same period the entire area was searched on foot by up to four people looking for mulgara burrows. All burrows were carefully excavated.
During the search of the area in June 2006, nine burrows were found that were active or potentially active and another five that were inactive (Fig. 1a). When searched again in January 2007, after the area had been burnt, 65 burrows were found and dug out.
Four adult mulgaras (D. cristicauda or D. blythi) were trapped and five adults were dug from burrows. None of the burrows in which mulgaras were recorded were in the remaining small patches of unburnt spinifex. The trapped mulgaras were caught on Nights 1, 2, 3 and 4. Other vertebrates caught included a Varanus gouldii, two Egernia striata, five Nephrurus levis and one Varanus eremius.
Case Study 2: Loop
The northern terminus loop (~50 m wide) of a railway line had been cleared leaving an area of ~210 ha in the centre (Fig. 1b). The loop was relatively flat and vegetated with Acacia stellaticeps low shrubland with scattered Hakea lorea and Corymbia hamersleyana over dense Triodia epatica and T. schinzii. The low vegetation cover ranged from 60–90%. Mulgara burrows were found during a search of the southern section of the loop in September 2006.
During 30 January to 2 February 2007, 600 baited aluminium treadle-activated box traps (325 × 100 × 100 mm) were deployed for a total of 2100 trap-nights in the southern section of the loop. During 8–12 February 2007, 600 aluminium box traps were deployed for a total of 2400 trap-nights, again in the southern section of the loop. During 12–22 June these same 600 aluminium box traps were deployed for a total of 5400 trap-nights in the northern section of the loop. Most of the traps were moved at least once during this period. The total trapping effort equated to 9900 trap-nights. The universal bait was used and trap locations are shown in Fig. 1b. The non-trapped areas shown in Fig. 1b were mostly devoid of Triodia sp., grasses and low shrubs. The bait was replenished every second day or, if an animal was caught, at the time the trap was reset.
Fifty D. blythi were caught (see Fig. 1b), along with 395 Dasykaluta rosamondae, 1 Notomys alexis, 27 Pseudomys desertor, 179 Mus musculus, 1212 Pseudomys hermannsburgensis, 11 Ctenotus grandis, 25 Ctenotus helenae, 2 Ctenotus pantherinus, 13 Ctenotus serventyi, 7 Tiliqua multifasciata, 29 Varanus acanthurus, 2 V. eremius and 3 V. gouldii. All D. blythi, D. rosamondae, P. desertor and the reptiles that were caught were translocated to similar habitat in an undisturbed area ~5 km west of the project area. There was no particular pattern or habitat variation that appeared to be linked to the location of D. blythi, and some were caught in an area that had been trapped for more than three or four nights.
Case Study 3: Pipeline
An area of ~2070 m × 250 m, ~10 km south-west of South Hedland was to be cleared for a pipeline (Fig. 1c). Two broad habitat types were present: spinifex and shrubs to 1.2 m with a 70–80% cover over red clay–sand or spinifex to 500 mm with a 40–50% cover on red clay–sand. In total, 605 baited aluminium treadle-activated box traps (325 × 100 × 100 mm) were set in the proposed pipeline area (Fig. 1c). Traps containing the universal bait were set ~25 m apart on either 16 or 17 May 2013 and were closed five or eight days later (3367 trap-nights). The bait was replenished on Day 3, and every third day if they remained open longer than for five days, or, if an animal was caught, at the time the trap was reset.
Traps were laid out and cleared daily using all-terrain vehicles (ATVs) (2 × Yamaha 500 Grizzly, Yamaha 750 Rhino), and all mammals and reptiles caught were translocated to similar habitat in an undisturbed area ~5 km away.
Two D. blythi were captured, one each on Nights 3 and 5 (see Fig. 1c). In addition, 23 P. desertor, 24 M. musculus, 30 D. rosamondae, 3 C. pantherinus, 46 P. hermannsburgensis and 26 N. alexis were caught and translocated from the area. While clearing traps daily, three burrows were recorded that could have belonged to mulgaras or spinifex hopping mice (N. alexis).
Case Study 4: Easement
Mulgaras were to be caught and removed from a 5 km easement in the Pilbara. The easement was 500 m wide, and there were three substantial areas (~1250 m × 800 m; ~750 m × 425 m; ~1750 m × 120 m) adjacent to the easement that were to be cleared (Fig. 1d). The project area was vegetated with either a mixture of spinifex and low shrubs to ~1 m high or predominantly spinifex to 600 mm high, both on a red gritty sandy substrate. Vegetation cover was estimated to be 60–90%.
In total, 1881 baited aluminium treadle-activated box traps (325 × 100 × 100 mm) were set in the easement, and adjacent areas (Fig. 1d). Traps containing the universal bait were set ~25 m apart during 18–30 May 2013 and were closed after a minimum of five but up to 10 days later (9740 trap-nights). The bait was replenished on Day 3, and every third day if they remained open for longer than five days, or, if an animal was caught, at the time the trap was reset. Traps were laid out and cleared daily using ATVs.
Two D. blythi were captured, one each on Nights 6 and 7 (see Fig. 1d). In addition, 3 C. helenae, 1 C. pantherinus, 19 D. rosamondae, 1 Felis catus, 25 M. musculus, 430 N. alexis, 17 P. desertor, 196 P. hermannsburgensis, 3 T. multifasciata and 2 V. acanthurus were caught and translocated to similar habitat in an adjacent area. Both D. blythi were caught in an area vegetated with mature low shrubs and spinifex on a gritty red sand substrate. Three N. alexis burrows were found.
Discussion
A search for burrows and/or a trapping program is typically implemented to determine the presence/absence of mulgaras in an area and to catch and translocate them. It is our contention that many of these searches are not finding burrows and the trapping effort is insufficient to record their presence or to catch all of the mulgaras in an area, with the result that the remaining mulgaras are inevitably being killed in their burrows during the vegetation-clearing program.
Historical changes
In the past two decades the survey effort to detect mulgaras has generally increased and the approach diversified. For example, Ecologia Environmental Consultants (1995a, 1995b, 1996, 1997; 1998, 2004) undertook multiple surveys using baited aluminium box traps over a decade at the Jundee gold mine to determine the population and spatial distribution of mulgaras and the translocation of individuals out of an impact area. The initial confirmation study for mulgaras at Jundee surveyed nine sites and used between three and 40 baited aluminium box traps at each site set for a period of five nights. In the largest area there were four lines of 10 traps with 50 m between lines and 50 m between traps. In subsequent years a monitoring program that used five lines of 10 baited traps with 50 m between lines and 50 m between traps for five nights was used. In addition, in 1995 a more intensive trapping program was implemented around five burrow complexes, each with 10–40 traps for 3–5 nights in July 1995. Martinick and Associates Pty Ltd (1996) undertook a similar trapping program of the Nimary gold project area (adjacent to the Jundee mine) in 1995. Traps were deployed in grids of ~100 × 100 m, each with five lines of five traps. Biota Environmental Sciences (2004) undertook a visual survey for mulgara burrows in the Cliffs tenement, which is immediately south of the Mt Keith mining village in the Goldfields. ATA Environmental (2005), using 1840 aluminium box trap-nights surveyed the Western Mining Corporation’s Yakabindie mine in 2005 for mulgaras. In 2006, Biota Environmental Sciences (2006) undertook visual searches for burrows, tracks and scats at three locations on the Mt Keith mine site. ATA Environmental (2007) used 4800 trap-nights in July and 2000 trap-nights in October to trap mulgaras in areas densely vegetated with spinifex on a sandy substrate at the Honeymoon Well mine site, south of Wiluna in the Goldfields. Ninox Wildlife Consulting (2010) surveyed for mulgaras at the proposed Mulga Rock mine site in the Great Victoria Desert by increasing the number of baited aluminium box traps from 10 to 16 at each of their 10 general sampling sites. Coffey Environments (2011) surveyed for mulgaras along the proposed Fortescue Metals Group rail corridor in 2.5 ha and 7.1 ha and deployed 50 and 45 baited aluminium box traps respectively for a period of seven nights.
Burrow searches
Mulgaras were initially considered to display strong site fidelity (Masters 1998, 2003; Dickman et al. 2001), however, more recent literature reports that male D. blythi are seldom resident in an area for more than six months, and females up to 18 months in Uluru Kata Tjuta National Park (Masters and Dickman 2012). The consequence of this reported site fidelity is that it is now more common to undertake a search for burrows, scats and tracks to determine the presence of mulgaras in a particular habitat than it was during 1990–2000. However, burrows can be easily confused with those of other animals, particularly N. alexis in the Pilbara.
In Case Study 1, in June 2006, 22 person-hours were spent searching 44 ha in a relatively open spinifex meadow to find 14 burrows. In January 2007 after most of the vegetation had been removed by fire, 65 burrows were recorded. Masters (2003) reported that both males and females use 2–9 burrows, but averaged ~3, whereas, Körtner et al. (2007) reported that mulgaras used up to 15 burrows, with this number still increasing at the end of the survey. Only three burrows (all of N. alexis) were detected in each of Case Studies 3 and 4, yet we know that each N. alexis has a burrow with multiple entrances (Thompson and Thompson 2007); so a very large number of burrows went undetected in Case Studies 1, 3 and 4. In a subsequent radio-tracking program of translocated mulgaras, we recorded animals in burrows that would generally not be considered to be active mulgara burrows. Some of these appeared to have been dug, or at least used, by other animals, which raises the question: do mulgara always dig their own burrows or do they sometimes modify and use existing burrows?
In Case Study 1, it could be concluded that either our search effort was inadequate (i.e. 1 person (2 ha)–1 h–1) or burrows are difficult to locate, or both. We could find no records of the quantification of search efforts (e.g. persons ha–1 h–1) in any other targeted fauna assessments for mulgaras, or other dasyurids to use for comparison; however, our general impression is that environmental consultants in Western Australia are deploying less than 1 person (2 ha)–1 h–1. These data indicate that a search for burrows will often result in many false negatives in areas of mature, high, dense spinifex or shrubs, which is of concern if the purpose of a search is to detect their presence and/or to prevent mulgaras from being injured or killed in vegetation clearing.
Trapping effort
Masters (2003) using the minimum convex polygon method and the 90% contour indicated that home ranges varied in size from 1.0 to 14.4 ha (mean 6.5 ha), with some overlap. In contrast, Körtner et al. (2007), using the same minimum convex polygon method but with the 100% contour, reported home ranges of ~10.8 ha for females and ~25.5 ha for males; however, these estimates were still increasing at the end of the radio-tracking program, and were therefore underestimates. These data indicate that mulgaras are foraging substantial distances from their burrows, probably on a frequent and nightly basis.
Four mulgaras were caught in 44 ha from 750 trap-nights in Case Study 1 in an area that was almost devoid of vegetation and another five mulgaras were dug from burrows, indicating an inadequate trapping effort to catch all mulgaras in that area. It took 9900 traps-nights in Case Study 2 to catch 50 mulgaras, which equates to ~200 trap-nights per individual, which is similar to the result in Case Study 1, which resulted in less than half of the individuals being caught. In Case Study 1, the burn resulted in all burrow entrances being exposed, ensuring that all burrows could be dug up and all the mulgaras in the area were able to be translocated. This suggests that additional trapping and digging out of burrows in Case Study 2 may have resulted in more mulgaras being caught. Pavey et al. (2011) reported that captures for D. cristicauda varied seasonally but peaked in June at 1 capture per 100 trap-nights, whereas the capture rate for D. blythi was also seasonably variable (highest in June), but with a higher peak rate of 1.78 per 100 trap-nights, both of which are substantially more efficient than that experienced in the Pilbara surveys reported here. The trapping effort deployed by environmental consultants in Western Australia is typically less than 10 traps ha–1 and they are seldom set for more than five nights and in many cases the trapping effort would be less. These data would then suggest that surveys for mulgaras could be failing to record their presence or to catch all the mulgaras in an area due to a low survey effort.
Trap avoidance
In Case Studies 1, 3 and 4, mulgaras were caught on Nights 1, 2, 3 and 4, 3 and 5, and 6 and 7 respectively, so it is likely that these caught individuals passed baited traps on multiple occasions on multiple nights before being trapped. So, on the basis of this information and the location of the traps, it is clear that some mulgaras are difficult to trap, and will not necessarily be caught in the first couple of baited traps that they pass or on the first or successive nights that they encounter a baited trap. Of interest, Pavey et al. (2011) reported Dasycercus tracks and scats or burrows on 10 sand ridges in the Simpson Desert that they sampled (5 × 5 baited box traps) but did not catch mulgara at these locations. This trap-avoidance behaviour has obvious implications for the trapping effort required to determine presence/absence or their removal from an area before vegetation clearing.
Summary and recommendations
On the basis of the available information, we conclude that:
-
searching in mature, high or dense spinifex and low shrubs results in many burrows not being detected;
-
it is relatively easy to misidentify active and potentially active burrows;
-
a minimum of 200 trap-nights per individual in an area of relatively high mulgara abundance is necessary to capture most of the mulgaras; and
-
five nights of trapping is not sufficient when traps are placed at 25-m centres (16 traps ha–1) to catch all mulgaras in an area when they are relatively abundant.
It is therefore suggested that:
-
searches to record the presence of mulgaras in an area should be conducted only when the height of spinifex or shrubs is less than 500 mm, the vegetation cover is less than 40% and the search effort is greater than 2 persons ha–1 h–1;
-
in areas where the height of spinifex or shrubs is greater than 500 mm and/or the vegetation cover is greater than 40%, then the area should be trapped;
-
the minimum trapping effort to detect or remove all mulgaras from an area should be 16 traps ha–1 with traps set for a minimum of seven nights; and
-
when the purpose of the trapping program is to catch and/or record all mulgaras in an area, trapping should cease when no mulgaras have been caught within 400 m of the trap for three consecutive nights.
References
ATA Environmental (2005). Mulgara (Dasycercus cristicauda) fauna assessment. Western Mining Corporation, Yakabindie. Unpublished report for Sinclair Knight Merz, Perth.ATA Environmental (2007). Level 2 fauna assessment. Honeymoon Well, Wiluna. Unpublished report for MPI Nickel Pty Ltd, Perth.
Biota Environmental Sciences (2004). An assessment of the distribution of the mulgara Dasycercus cristicauda within the Cliffs Tenement. Unpublished report for Sinclair Knight Merz, Perth.
Biota Environmental Sciences (2006). Northern Nickel Projects mulgara (Dasycercus cristicauda) survey and management plan. Unpublished report for Nickel West Operations, Mt Keith.
Coffey Environments (2011). Targeted surveys – northern quolls, mulgara and pilbara olive pythons. Solomon Rail Project. Unpublished report for Fortescue Metals Group, Perth.
Department of Sustainability, Environment, Water, Population and Communities (2012). Invitation to comment on the proposed delisting of Dasycercus hillieri and listing Dasycercus blythi under the Environment Protection and Biodiversity Conservation Act 1999 (EPBC Act). Department of Sustainability, Environment, Water, Population and Communities, Canberra.
Dickman, C. R., Haythornthwaite, A. S., McNaught, G. H., Mahon, P. S., Tamayo, B., and Letnic, M. (2001). Population dynamics of three species of dasyurid marsupials in arid central Australia: a 10-year study. Wildlife Research 28, 493–506.
| Population dynamics of three species of dasyurid marsupials in arid central Australia: a 10-year study.Crossref | GoogleScholarGoogle Scholar |
Ecologia Environmental Consultants (1995a). Mulgara Dasycercus cristicauda 1995 monitoring programme. Unpublished report for Great Central Mines, N.L., Perth.
Ecologia Environmental Consultants (1995b). Mulgara Dasycercus cristicauda confirmation survey. Unpublished report for Great Central Mines, N.L., Perth.
Ecologia Environmental Consultants (1996). Mulgara Dasycercus cristicauda 1995 monitoring programme. Unpublished report for Great Central Mines, N.L., Perth.
Ecologia Environmental Consultants (1997). Mulgara Dasycercus cristicauda 1997 monitoring programme. Unpublished report for Great Central Mines, N.L., Perth.
Ecologia Environmental Consultants (1998). Wanjarri Nature Reserve mulgara Dasycercus cristicauda translocation. Unpublished report for Great Central Mines, N.L., Perth.
Ecologia Environmental Consultants (2004). Jundee Mulgara Dasycercus cristicauda assessment. Unpublished report for Newmont Australia, Perth.
Körtner, G., Pavey, C. R., and Geiser, F. (2007). Spatial ecology of the mulgara in arid Australia: impact of fire history on home range size and burrow use. Journal of Zoology 273, 350–357.
| Spatial ecology of the mulgara in arid Australia: impact of fire history on home range size and burrow use.Crossref | GoogleScholarGoogle Scholar |
Martinick and Associates Pty Ltd (1996). Mulgara survey at the Nimary Gold Project north east of Wiluna. Unpublished report for Eagle Mining Corp. N.L., Perth.
Masters, P. (1998). The mulgara Dasycercus cristicauda (Marsupialia: Dasyuridae) at Uluru National Park, Northern Territory. Australian Mammalogy 20, 403–404.
Masters, P. (2003). Movement patterns and spatial organisation of the mulgara, Dasycercus cristicauda (Marsupialia: Dasyuridae), in central Australia. Wildlife Research 30, 339–344.
| Movement patterns and spatial organisation of the mulgara, Dasycercus cristicauda (Marsupialia: Dasyuridae), in central Australia.Crossref | GoogleScholarGoogle Scholar |
Masters, P., and Dickman, C. R. (2012). Population dynamics of Dasycercus blythi (Marsupialia: Dasyuridae) in central Australia: how does the mulgara persist? Wildlife Research 39, 419–428.
| Population dynamics of Dasycercus blythi (Marsupialia: Dasyuridae) in central Australia: how does the mulgara persist?Crossref | GoogleScholarGoogle Scholar |
Ninox Wildlife Consulting (2010). A fauna survey of the proposed Mulga Rock project area, Great Victoria Desert, Western Australia. Unpublished report for Energy and Minerals Australia Ltd, Perth.
Pavey, C. R., Nano, C. E. M., Cooper, S. J. B., Cole, J. R., and McDonald, P. J. (2011). Habitat use, population dynamics and species identification of mulgara, Dasycercus blythi and D. cristicauda, in a zone of sympatry in central Australia. Australian Journal of Zoology 59, 156–169.
| Habitat use, population dynamics and species identification of mulgara, Dasycercus blythi and D. cristicauda, in a zone of sympatry in central Australia.Crossref | GoogleScholarGoogle Scholar |
Thompson, G. G., and Thompson, S. A. (2007). Are backfilled burrows a predator protection strategy for the spinifex hopping mouse. Journal of the Royal Society of Western Australia 90, 111–113.
Woolley, P. A. (2005). The species of Dasycercus Peters, 1875 (Marsupialia: Dasyuridae). Memoirs of Museum Victoria 62, 213–221.
Woolley, P. A., Haslem, A., and Westerman, M. (2013). Past and present distribution of Dasycercus: toward a better understanding of the identity of specimens in cave deposits and the conservation status of the currently recognised species D. blythi and D. cristicauda (Marsupialia: Dasyuridae). Australian Journal of Zoology 61, 281–290.
| Past and present distribution of Dasycercus: toward a better understanding of the identity of specimens in cave deposits and the conservation status of the currently recognised species D. blythi and D. cristicauda (Marsupialia: Dasyuridae).Crossref | GoogleScholarGoogle Scholar |