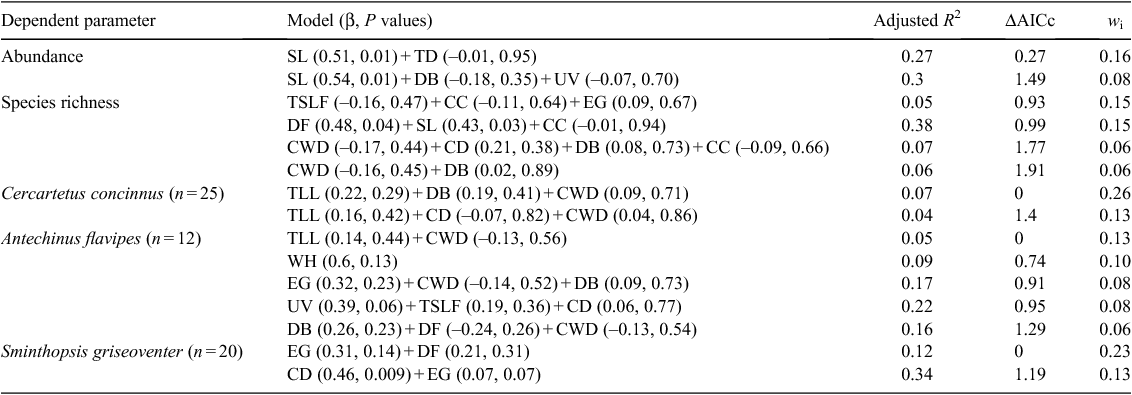
Does woodland condition influence the diversity and abundance of small mammal communities?
T. L. Moore A D , L. E. Valentine A C , M. D. Craig B , G. E. St J. Hardy B and P. A. Fleming AA Western Australian Centre of Excellence for Climate Change, Woodland and Forest Health, School of Veterinary and Biomedical Sciences, Murdoch University, Murdoch, WA 6150, Australia.
B Western Australian Centre of Excellence for Climate Change, Woodland and Forest Health, School of Biological Sciences and Biotechnology, Murdoch University, Murdoch, WA 6150, Australia.
C Present address: ARC Centre of Excellence for Environmental Decisions, School of Plant Biology, University of Western Australia, Nedlands, WA 6009, Australia.
D Corresponding author. Email: tracey.moore@dpaw.wa.gov.au
Australian Mammalogy 36(1) 35-44 https://doi.org/10.1071/AM13007
Submitted: 15 March 2013 Accepted: 16 August 2013 Published: 8 November 2013
Abstract
Loss of mammal species in Australia in the last 200 years has been attributed to many factors including habitat removal and altered fire regimes. Decline in tree condition could contribute further to the ongoing decline of mammals. Eucalyptus wandoo trees are currently undergoing a decline in condition that can result in a loss of canopy and other changes to the habitat. This paper examines the relationships between E. wandoo tree condition, habitat characteristics and small mammal species richness and abundance. Live-capture trapping was conducted at 24 E. wandoo sites at Dryandra State Forest and Wandoo Conservation Park, Western Australia. Condition and microhabitat variables of E. wandoo were recorded for each site. Generalised additive mixed models revealed a range of habitat and tree condition characteristics that influenced small mammal abundance and species richness, including site litter cover, crown dieback, understorey vegetation cover and tree density. The availability of coarse woody debris played a large role in explaining the abundance of Cercartetus concinnus and Antechinus flavipes, along with other microhabitat and tree condition variables, such as tree leaf litter and crown dieback. Epicormic growth, crown density and the distance to the drift fence from E. wandoo trees were the common variables in the best model for the abundance of Sminthopsis griseoventer. The decline in condition of E. wandoo and the subsequent modifications to the microhabitat are correlated with changes in the small mammal community. A better understanding of how the decline of E. wandoo impacts small mammal communities could improve management practices in E. wandoo woodlands.
Additional keywords: Antechinus, Cercartetus, Eucalyptus wandoo, microhabitat, Sminthopsis.
Introduction
The distribution and abundance of small mammals is often linked to habitat structure, including crown density, understorey cover, coarse woody debris and leaf litter cover (Knight and Fox 2000; Catling et al. 2001; Holland and Bennett 2007). These habitat structures contribute to overall habitat complexity and provide small mammals with nesting and foraging resources (McElhinny et al. 2006; Mac Nally and Horrocks 2008) and refuge from predators and extreme weather conditions (Bos and Carthew 2003). An emerging phenomenon that is altering habitat structure of forests and woodlands globally is the decline in tree condition (Allen et al. 2010). Although tree decline can be attributed to multiple causes (e.g. drought: Allen et al. 2010; pathogens: Stone et al. 1998), the death of upper portions of the tree canopy is an increasingly common phenomenon in forests and woodlands (Manion and Lachance 1981; Close and Davidson 2004). Loss of the foliage creates an open canopy, directing more sunlight to the understorey, altering herb communities, bare ground, leaf litter and understorey vegetation density (due to greater isolation, changes in soil moisture, and pH). These changes will alter available habitat for small mammals (Loyn and Middleton 1980; Jurskis 2005). The effects of tree decline on small mammals are not well understood, though a decline in tree condition could result in a major loss of habitat and resources for them (Catling and Burt 1995; Williams et al. 2002; McKenzie et al. 2007).
Of all continents, Australia has recorded the most mammal extinctions over recent centuries. Seventeen Australian mammal species have been lost over the last 200 years, which is approximately half the total number of mammal species extinctions worldwide (Short and Smith 1994; Cardillo and Bromham 2001; Woinarski et al. 2010). Mammal decline and extinction in Australia are highest in Western Australia’s farming and cropping area (Burbidge and McKenzie 1989), where land clearing has led to a loss of more than 90% of native vegetation (Hobbs 1993). During the early colonisation of Western Australia, 43 mammal species (excluding bat species) were present in the farming region. However, by the 1970s, only 12 species were moderately common in the region (Kitchener et al. 1980). Today a large number of threatened mammals persist in patches of remnant vegetation (Yates et al. 2000) and a decline in the condition of remnant vegetation may contribute to further regional extinctions. Eucalyptus wandoo, a smooth-barked tree, once covered most of the greater agricultural area of the south-west region of Western Australia. Following clearing of the wheatbelt area, only 40% of E. wandoo–dominated woodlands remain, largely as small remnant patches (Mattiske Consulting Pty Ltd and Havel Land Consultants 1998; Wandoo Recovery Group 2006). Ten years ago it was noted that trees within two of the three largest patches, the Wandoo Conservation Park and the Dryandra State Forest, were showing signs of decline with symptomatic retraction or loss of canopy (Wandoo Recovery Group 2006; Brouwers et al. 2013). Declines of this eucalypt occur heterogeneously, where healthy trees can be adjacent to declining trees (Brouwers et al. 2013; Moore et al. 2013), differing from other eucalypt decline where large-scale canopy loss occurs (e.g. Eucalyptus marginata, jarrah) (Matusick et al. 2012).
Alteration in vegetation structure and microhabitat availability caused by a decline in tree condition of E. wandoo–dominated woodlands may affect the availability of resources for small mammals and influence their presence and abundance. This study explores the influence of decline in E. wandoo canopy condition and associated structural and microhabitat changes on mammalian species richness and abundance. We ask two questions: (1) is small mammal abundance and species richness associated with E. wandoo canopy condition; and (2) what other habitat characteristics (e.g. time since last fire, site litter cover and understorey density), potentially associated with canopy decline, are reflected in the abundance and species richness of small mammals in E. wandoo woodlands.
Methods
Site description
Study sites were located in Dryandra State Forest (32°48′S, 116°53′E) and Wandoo Conservation Park (31°54′S, 116°27′E) 160 km south-east and 75 km east of Perth, respectively. E. wandoo woodlands within these reserves have an open canopy (<30% canopy cover), a grassy herb layer and a patchy understorey of small shrubs including Gastrolobium spp., Macrozamia riedlei and Xanthorrhoea preissii (although sites with high X. preissii numbers were avoided as they are classified as another habitat type) (Mercer 1991). These two reserves were chosen as they are some of the largest areas of remnant E. wandoo woodlands in the Western Australian farming region. Despite their differences in tenure (Department of Conservation and Land Management 1980), the two reserves have similar histories of land clearing, stock grazing, timber harvesting, and prescribed fire management. A total of 24 sites were chosen within these reserves using Landsat imagery (1990–2009) and Vegmachine (CSIRO 2010) (vegmachine calculates the changes in vegetation cover over a landscape using Landsat imagery) to identify sites with predominately healthy or declining E. wandoo trees (12 of each condition, six of each per reserve) at least 500 m away from the edges of remnant native vegetation. Initially, this project was not focussed on the fire history of the reserves and therefore matching fire ages were not considered in the planning stages of the research project. Similarly, the sites varied in terms of understorey vegetation, litter cover, tree density and the density of coarse woody debris.
Trapping design
Trapping grids consisted of three 20-L buckets, two 45-cm lengths of PVC pipe and four Elliott traps. Buckets and PVC tubes were arranged along a 29-m drift fence with ~6 m between traps, beginning and ending 3 m from each end of the drift fence. Elliott traps were located in the understorey 5 m diagonally from the end of each drift fence and baited with universal bait (i.e. peanut butter, sardines and rolled oats). Styrofoam trays and leaf litter were placed inside the buckets and PVC tubes and Elliot traps were lined with shredded tissue paper as shelter and nesting for captured animals. Buckets and PVC tubes were installed in late August and early September to allow the traps to settle in the ground before trapping commenced. Twelve sites were trapped over four consecutive nights in September/October 2009, November 2009, December 2009 and March/April 2010 within each location (12 sites in both Dryandra State Forest and Wandoo Conservation Park). A total of 16 nights trapping per site gave a total of 3456 trap-nights (24 sites × 16 nights × 9 traps per site). All 24 sites could not be monitored simultaneously due to distance and logistics. All Elliot traps were set, baited and positioned, and buckets and PVC tube lids opened on the afternoon preceding the four trapping nights, checked morning and afternoon for the four days and all traps were closed or removed on the last morning. All animals captured were weighed, sexed, ear notched and head–body length measured and released immediately after processing.
Measuring tree condition and other habitat characteristics
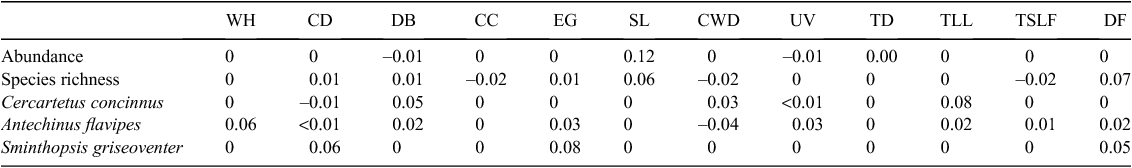
Seven characteristics (Table 1) were measured on six trees (termed ‘site trees’) at each of the 24 sites. Site trees were those (diameter at breast height >20 cm) closest to the trapping line. Whitford tree condition index is a semiqualitative measure that rates tree canopy condition holistically on a pictorial scale to provide a categorical value. USDA tree condition index (Schomaker et al. 2007) includes a range of tree characteristics originally designed for use by USA foresters assessing tree condition of Pinus spp. Some of these indices can be adapted to measure a range of tree types depending on the growth form. Those that were the most appropriate indices for E. wandoo were crown density, crown dieback and uncompacted live crown ratio. Lastly, epicormic growth, canopy cover and the percentage of dead branches were recorded for each tree as the individual characteristics have been deemed important in other studies that have investigated tree condition and fauna (Wentzel 2010). In addition to these tree characteristics and indices, six habitat characteristics were recorded for each site (Table 2). One sampling characteristic, distance to the drift fence, was recorded as trapping grids were opportunistically installed in more open areas and subsequently there were differences in the distance from trees to the trapping grids between sites (Table 2). All percentage cover values were arcsine-square-root transformed and tree density values were log-transformed to meet the assumptions of ANOVA and Pearson’s correlations (Zar 1998).
|
Analysis
Mammal variables of interest were mammal species richness, overall mammal abundance and abundances of individual mammal species. These variables are measures of individual captures and do not include recaptures. Data from 16 trap-nights from all three trap types were pooled for each site to estimate these dependent variables and then square-root-transformed to meet the assumptions of parametric statistics (Zar 1998).
Generalised additive mixed models (GAMMs) are powerful statistical analyses that allow blending of generalised linear models and additive models (non-parametric models). Generalised additive mixed models (REML – restricted maximum likelihood) were used to explore the relationships between the dependent and independent variables. The dependent variables were mammal species richness, mammal abundance (number of individuals) and the abundance of the three most common mammal species (Cercartetus concinnus, Antechinus flavipes and Sminthopsis griseoventer); independent variables were tree condition and habitat characteristics (Tables 1 and 2). Location (Dryandra State Forest or Wandoo Conservation Park) was present in all models as a random factor. The number of independent variables in each model ranged from 1 to 5 to avoid overfitting models (Burnham and Anderson 2002).
A total of 447 models were created for each dependent variable, to capture combinations of the independent variables. To avoid autocorrelation between variables, a Pearson’s correlation matrix of tree and habitat characteristics was constructed (Microsoft Excel) and variables that were correlated (r ≥ 0.35) were not included in the same models. Independent variables included time since last fire (years), site litter cover, understorey vegetation <1 m cover, tree litter cover, coarse woody debris density, tree density, crown density, crown dieback, uncompacted live crown ratio, dead branches, epicormic growth, canopy cover, Whitford tree condition index, since each species could respond to one aspect of tree condition independently of other indices. The only relationship found was between time since last fire and tree litter (r = 0.57, P < 0.05), so these two variables were not included in the same model.
GAMMs were fitted using the GAM function of the MGCV package in R (Tinn-R and R 1.12.1) (R Development Core Team 2011). Akaike Information Criterion adjusted for small sample size (AICc) and adjusted R2 values were used to rank models within each model-set. Each parameter within the model produced standardised β coefficients and P values. The standardised β coefficients are the regression slopes obtained if all variables were first standardised to a mean of 0 and a standard deviation of 1. Thus, the standardised β value allows direct assessment of the relative contribution of each independent variable (i.e. tree and habitat characteristics) in the prediction of the dependant variable (i.e. mammal abundance and species richness). Where the standardised β value yielded P > 0.05 in a model, the model was compared against the same model, but excluding that variable. The AICc model weight (wi) was calculated for each of the 447 models created; wi values indicate the likelihood that each model is the model that best describes the data. Model-averaged β values (β·wi) (Burnham and Anderson 2002) were summed to determine the importance of each habitat variable in the prediction of the dependent variable.
Results
Captures
Six mammal species were captured over 3456 trap-nights: Cercartetus concinnus, S. griseoventer, Sminthopsis gilberti, A. flavipes, Phascogale calura and Mus musculus. Numbers of total captures, average body mass, sex ratios and average head–body lengths for each species are listed in Appendix 1.
Relationship between tree and microhabitat characteristics and small mammal occurrence.
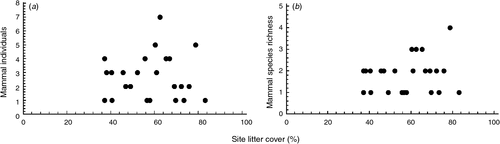
Several models to describe the relationships between the tree/habitat characteristics and mammal abundance and species richness were well supported (<2 ΔAICc) (Table 3); model-weighted β values are listed in Table 4. There were two well supported models for mammal abundance, which displayed positive relationships with site litter cover (Fig. 1a) and negative relationships with crown dieback, understorey vegetation cover, and tree density (Table 3). There were four well supported models for mammal species richness (Tables 3 and 4); these included positive relationships with site litter cover (Fig. 1b), distance to drift fence, crown density, crown dieback and epicormic growth, but negative relationships with canopy cover, coarse woody debris and time since last fire (Table 4). Some tree condition variables were not included in any of the best models, including percentage of dead branches and uncompacted live crown ratio.
|
Relationships between tree and microhabitat characteristics and individual species
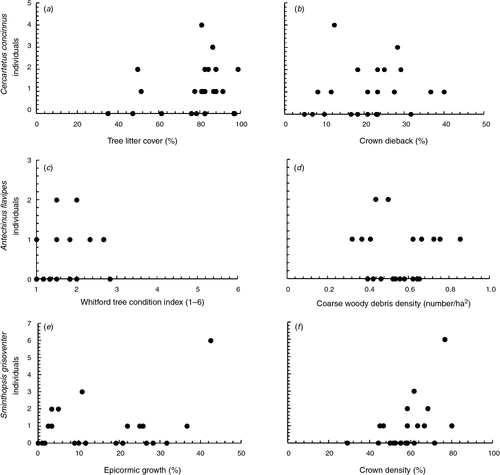
There were two well supported models for the abundance of C. concinnus (n = 25 individuals) (Table 3), which included positive relationships with tree litter cover (Fig. 2a), crown dieback (Fig. 2b) and coarse woody debris and a negative relationship with crown density. The abundance of A. flavipes (n = 12 individuals) had five well supported models that included a range of variables: positive relationships with Whitford tree condition indices (Fig. 2c), understorey cover, epicormic growth, crown dieback, tree litter cover, time since last fire, distance to drift fence, crown density and negative relationships with coarse woody debris (Fig. 2d; Tables 3 and 4). However, the model weights of the five models were low, indicating that each model has little explanatory power in terms of the abundance of A. flavipes. The abundance of S. griseoventer (n = 20 individuals) was best explained by two models that included positive relationships with epicormic growth (Fig. 2e), crown density (Fig. 2f) and distance to drift fence (Tables 3 and 4). Model weights for all other measures of the mammal community had adequate explanatory power.
|
Discussion
This study investigated the relationships between small mammals, E. wandoo decline and the microhabitat. Relationships indicated that not only E. wandoo condition, but also understorey habitat, were strongly correlated with the small mammal community, highlighting that management should include preservation of E. wandoo trees and the understorey microhabitat necessary for shelter and food resources by small mammals, as seen in other studies (Stephens et al. 2012). It should be noted that generalising results over the two locations can be difficult; however, the heterogeneous nature of the E. wandoo decline (and the microhabitat) (Brouwers et al. 2013; Moore et al. 2013) meant there was habitat variability within a site, as well as a location.
Indices of tree canopy condition were retained in the best models describing both mammal abundance and species richness. Less crown dieback, more epicormic foliage and overall healthier woodlands were related to increased mammal captures in E. wandoo woodlands. However, mammal species richness was negatively correlated with E. wandoo canopy cover, perhaps a result of the naturally open E. wandoo canopy (<30% canopy cover: Mercer 1991) and therefore low validity of this measure. Decline of E. wandoo alters the surrounding microhabitat, including changes in the build-up of litter cover and changes in the understorey vegetation from a loss of overhead foliage (Jurskis 2005); both of these factors are also correlated with overall mammal community composition. Site litter cover was the strongest predictor of small mammal abundance and species richness in this study, with the greatest abundance and diversity of mammals located at sites with more litter cover. Leaf litter is a productive foraging substrate and provides nesting sites and nesting material for small mammals (Mac Nally and Brown 2001; Mac Nally et al. 2001; McElhinny et al. 2006; Gresser 2009). Links between E. wandoo condition, the microhabitat and mammal abundance and species richness reinforce general findings in the literature that small mammal communities are strongly related to their habitat (Bowers and Dooley 1993; Lagos et al. 1995; Bos and Carthew 2003; Stokes et al. 2004; Torre and Diaz 2004; Stephens et al. 2012) and point towards a greater number and diversity of mammals at sites with healthier E. wandoo trees.
Cercartetus concinnus feeds on nectar and pollen, as well as insects, within both arboreal and terrestrial habitats (Pestell and Petit 2007; Morrant et al. 2010). In the present study, the abundance of C. concinnus was linked to the loss of canopy foliage and build-up of coarse woody debris and leaf litter, resulting from E. wandoo decline. A preference for open, declining E. wandoo canopies may reflect the use of terrestrial resources by these animals, since Cercartetus spp. use a range of microhabitat features for nesting and foraging (for invertebrate food resources), including woody debris (Sutherland et al. 2004; Tulloch 2004; Short et al. 2009) and leaf litter at the base of trees (Kemp and Carthew 2004; Tulloch 2004).
A range of variables were retained in the best models describing abundance of A. flavipes, including understorey vegetation, epicormic growth, crown dieback, tree litter cover, time since last fire and distance to drift fence. We expected the abundance of A. flavipes to be related to a dense canopy, more coarse woody debris and leaf litter cover (for invertebrate prey, nesting sites and protection from predation) as other studies have noted (Braithwaite 1979; Newell 1998; Mac Nally et al. 2001; Holland and Bennett 2007; Lada et al. 2007, 2008; Armistead 2008). However, the strongest relationships for A. flavipes were with declining E. wandoo trees, sparse canopies and less coarse woody debris. Low model weights for A. flavipes abundance suggest that it may be responding to additional microhabitat features than those captured by this study.
Sminthopsis spp. forage nocturnally for insects in structurally complex terrestrial microhabitats (Stokes et al. 2004; Wilson and Aberton 2006; Finlayson et al. 2008). Abundance of S. griseoventer was related to changes in the canopy of E. wandoo, but, surprisingly, no links were seen with terrestrial microhabitat variables. Positive links between abundance of S. griseoventer and crown density, epicormic growth and distance from the drift fence to E. wandoo trees were noted in this study. Epicormic growth generally has a higher invertebrate load than older foliage on the same tree (Landsberg and Wylie 1983; Landsberg 1988, 1990). Although S. griseoventer is not an arboreal species, it is possible that the higher insect load of a recovering E. wandoo tree is not restricted to the canopy, and terrestrial abundance of insects and food resources for the small mammal may also be higher. Links between the abundance of S. griseoventer and higher crown densities may also reflect a response to the higher predation pressure in open canopy woodlands, since previous research (Bowers and Dooley 1993; Lagos et al. 1995) shows that a less complex habitat makes small mammals more susceptible to predation.
This study tested the relevance of a range of microhabitat measures to small mammal abundance and diversity. The holistic tree condition index (Whitford Index) was not common in the best supported models when compared with individual tree condition measures, highlighting that small mammals are likely to be responding to changes in microhabitat availability, rather than the decline of the woodland as a whole. Dead branches and uncompacted live crown ratio were also not included in any of the best models for the small mammal community and individual species. Small mammals are not related to the loss of individual branches in the canopy, as seen by the lack of dead branches in the GAMMs, but rather the loss of canopy (branches, foliage, flowers and bark), as measured by crown density and crown dieback. Uncompacted live crown ratio is a measure of tree size (trunk to canopy ratio). Perhaps as long as E. wandoo trees are still present in the woodlands, small mammals are not influenced by the size of the trees themselves.
Understanding relationships between small mammals and their habitat is important for reserve management (Flynn et al. 2011). This study demonstrated strong links between the small mammal community and their habitat, thus management actions, such as mosaic burning, should ensure the availability of healthy E. wandoo trees and microhabitat for small mammal shelter, nesting and food resources. Particularly, warmer temperatures and reduced rainfall are likely to exacerbate the decline of E. wandoo, further altering microhabitats for small mammals. In conclusion, future management needs to consider the decline in condition of E. wandoo and woodland microhabitats in the conservation of small mammal communities.
Acknowledgements
We thank the Holsworth Research Endowment, Wildlife Preservation Society Australia, State Centre for Excellence of Climate Change, Woodland and Forest Health and Murdoch University for funding this research. TLM was the recipient of a Murdoch University Research Scholarship for the duration of this research. Field assistance was gratefully received from many volunteers, particularly Bryony Palmer, Liz Manning, Shannon Dundas, Tegan Douglas and Kathryn Napier. This work was carried out with a Murdoch University’s Animal Ethics Committee permit (R2270/09) and Department of Environment and Conservation permits (Regulation 17: SF007629).
References
Allen, C. D., Macalady, A. K., Chencouni, H., Bachelet, D., Mc Dowell, N., Vennetier, M., Kitzberger, T., Rigling, A., Breshears, D. D., Hogg, E. H., Gonzalez, P., Fensham, R., Zhang, Z., Casto, J., Demidova, N., Lim, J. H., Allard, G., Running, S. W., Semerci, A., and Cobb, A. (2010). A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. Forest Ecology and Management 259, 660–684.| A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests.Crossref | GoogleScholarGoogle Scholar |
Armistead, R. (2008). The impact of Phytophthora cinnamomi on the yellow-footed antechinus (mardo) (Antechinus flavipes leucogaster) (Marsupialia: Dasyuridae). Ph.D. Thesis, Murdoch University.
Barbour, M.G., Burk, J.H., and Pitts, W.D. (1987) ’Terrestrial Plant Ecology ’ Second Edition edn. (Benjamin/Cummings Publishing Company: San Francisco, CA)
Bos, D. G., and Carthew, S. M. (2003). The influence of behaviour and season on habitat selection by a small mammal. Ecography 26, 810–820.
| The influence of behaviour and season on habitat selection by a small mammal.Crossref | GoogleScholarGoogle Scholar |
Bowers, M. A., and Dooley, J. L. (1993). Predation hazard and seed removal by small mammals: microhabitat versus patch scale effects. Oecologia 94, 247–254.
| Predation hazard and seed removal by small mammals: microhabitat versus patch scale effects.Crossref | GoogleScholarGoogle Scholar |
Braithwaite, R. W. (1979). Social dominance and habitat utilization in Antechinus stuartii (Marsupialia). Australian Journal of Zoology 27, 517–528.
| Social dominance and habitat utilization in Antechinus stuartii (Marsupialia).Crossref | GoogleScholarGoogle Scholar |
Brouwers, N. C., Mercer, J., Lyons, T., Poot, P., Veneklaas, E., and Hardy, G. (2013). Climate and landscape drivers of tree decline in a Mediterranean ecoregion. , .
| Climate and landscape drivers of tree decline in a Mediterranean ecoregion.Crossref | GoogleScholarGoogle Scholar |
Burbidge, A. A., and McKenzie, N. L. (1989). Patterns in the modern decline of Western Australia’s vertebrate fauna: causes and conservation implications. Biological Conservation 50, 143–198.
| Patterns in the modern decline of Western Australia’s vertebrate fauna: causes and conservation implications.Crossref | GoogleScholarGoogle Scholar |
Burnham, K. P., and Anderson, D. R. (2002). ‘Model Selection and Multimodel Inference. A Practical Information-Theoretic Approach.’ 2nd edn. (Springer-Verlag: New York.)
Cardillo, M., and Bromham, L. (2001). Body size and risk of extinction in Australian mammals. Conservation Biology 15, 1435–1440.
| Body size and risk of extinction in Australian mammals.Crossref | GoogleScholarGoogle Scholar |
Catling, P. C., and Burt, R. J. (1995). Studies of ground-dwelling mammals of eucalypt forests in south-eastern New South Wales: the effect of habitat variables on distribution and abundance. Wildlife Research 22, 271–288.
| Studies of ground-dwelling mammals of eucalypt forests in south-eastern New South Wales: the effect of habitat variables on distribution and abundance.Crossref | GoogleScholarGoogle Scholar |
Catling, P. C., Coops, N. C., and Burt, R. J. (2001). The distribution and abundance of ground dwelling mammals in relation to time since wildfire and vegetation structure in south-eastern Australia. Wildlife Research 28, 555–564.
| The distribution and abundance of ground dwelling mammals in relation to time since wildfire and vegetation structure in south-eastern Australia.Crossref | GoogleScholarGoogle Scholar |
Close, D. C., and Davidson, N. J. (2004). Review of rural tree decline in a changing Australian climate. Tasmanian Forests 15, 1–18.
CSIRO (2010). Vegmachine 2.0: State of Queensland, Department of Employment, Economic Development and Innovation.
Department of Conservation and Land Management (1980). Dryandra Management Plan. Western Australia Department of Conservation and Land Management, Perth.
Finlayson, G. R., Vieira, E. M., Priddel, D., Wheeler, R., Bentley, J., and Dickman, C. R. (2008). Multi-scale patterns of habitat use by re-introduced mammals: a case study using medium-sized marsupials. Biological Conservation 141, 320–331.
| Multi-scale patterns of habitat use by re-introduced mammals: a case study using medium-sized marsupials.Crossref | GoogleScholarGoogle Scholar |
Flynn, E. M., Jones, S. M., Jones, M. E., Jordan, G. J., and Munks, S. A. (2011). Characteristics of mammal communities in Tasmanian forests: exploring the influence of forest type and disturbance history. Wildlife Research 38, 13–29.
| Characteristics of mammal communities in Tasmanian forests: exploring the influence of forest type and disturbance history.Crossref | GoogleScholarGoogle Scholar |
Gresser, M. (2009). Effects of thinning and burning rehabilitation on Phytophthora cinnamomi and small mammal populations. Honours Thesis, Murdoch University, Perth.
Hobbs, R. J. (1993). Effects of landscape fragmentation on ecosystem processes in the Western Australian wheatbelt. Biological Conservation 64, 193–201.
| Effects of landscape fragmentation on ecosystem processes in the Western Australian wheatbelt.Crossref | GoogleScholarGoogle Scholar |
Holland, G. J., and Bennett, A. F. (2007). Occurrence of small mammals in a fragmented landscape: the role of vegetation heterogeneity. Wildlife Research 34, 387–397.
| Occurrence of small mammals in a fragmented landscape: the role of vegetation heterogeneity.Crossref | GoogleScholarGoogle Scholar |
Jurskis, V. (2005). Eucalypt decline in Australia and a general concept of tree decline and dieback. Forest Ecology and Management 215, 1–20.
| Eucalypt decline in Australia and a general concept of tree decline and dieback.Crossref | GoogleScholarGoogle Scholar |
Kemp, L. F., and Carthew, S. M. (2004). Nest site selection by the western pygmy-possum Cercartetus concinnus. In ‘The Biology of Australian Possums and Gliders’. (Eds R. L. Goldingay, and S. M. Jackson.) pp. 237–245. (Surrey Beatty: Sydney.)
Kitchener, D. J., Chapman, A., and Muir, B. G. (1980). The conservation value for mammals of reserves in the Western Australian wheatbelt. Biological Conservation 18, 179–207.
| The conservation value for mammals of reserves in the Western Australian wheatbelt.Crossref | GoogleScholarGoogle Scholar |
Knight, E. H., and Fox, B. J. (2000). Does habitat structure mediate the effects of forest fragmentation and human-induced disturbance on the abundance of Antechinus stuartii? Australian Journal of Zoology 48, 577–595.
| Does habitat structure mediate the effects of forest fragmentation and human-induced disturbance on the abundance of Antechinus stuartii?Crossref | GoogleScholarGoogle Scholar |
Lada, H., Thomson, J. R., Mac Nally, R., Horrocks, G., and Taylor, A. C. (2007). Evaluating simultaneous impacts of three anthropogenic effects on a flood-plain dwelling marsupial Antechinus flavipes. Biological Conservation 134, 527–536.
| Evaluating simultaneous impacts of three anthropogenic effects on a flood-plain dwelling marsupial Antechinus flavipes.Crossref | GoogleScholarGoogle Scholar |
Lada, H., Mac Nally, R., and Taylor, A. C. (2008). Responses of a carnivorous marsupial (Antechinus flavipes) to local habitat factors in two forest types. Journal of Mammalogy 89, 398–407.
| Responses of a carnivorous marsupial (Antechinus flavipes) to local habitat factors in two forest types.Crossref | GoogleScholarGoogle Scholar |
Lagos, V. O., Contreras, L. C., Meserve, P. L., Gutierrez, J. R., and Jaksie, F. M. (1995). Effects of predation risk on space use by small mammals: a field experiment with a Neotropical rodent. Oikos 74, 259–264.
| Effects of predation risk on space use by small mammals: a field experiment with a Neotropical rodent.Crossref | GoogleScholarGoogle Scholar |
Landsberg, J. (1988). Dieback of rural eucalypts: tree phenology and damage caused by leaf-feeding insects. Australian Journal of Ecology 13, 251–267.
| Dieback of rural eucalypts: tree phenology and damage caused by leaf-feeding insects.Crossref | GoogleScholarGoogle Scholar |
Landsberg, J. (1990). Dieback of rural eucalypts: response of foliar dietary quality and herbivory to defoliation. Australian Journal of Ecology 15, 89–96.
| Dieback of rural eucalypts: response of foliar dietary quality and herbivory to defoliation.Crossref | GoogleScholarGoogle Scholar |
Landsberg, J., and Wylie, F. R. (1983). Water stress, leaf nutrients and defoliation: a model of dieback of rural eucalypts. Australian Journal of Ecology 8, 27–41.
| Water stress, leaf nutrients and defoliation: a model of dieback of rural eucalypts.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL2cXot12kuw%3D%3D&md5=7670a4f7f80867dbcefa899ef6eb8eceCAS |
Loyn, R. H., and Middleton, W. G. D. (1980). Eucalypt decline and wildlife in rural areas. In ‘Eucalypt Dieback in Forests and Woodlands’. (Eds K. M. Old, G. A. Kile, and C. P. Ohmart.) pp. 95–111. (CSIRO: Melbourne.)
Mac Nally, R., and Brown, G. W. (2001). Reptiles and habitat fragmentation in the box–ironbark forests of central Victoria, Australia: predictions, compositional change and faunal nestedness. Oecologia 128, 116–125.
| Reptiles and habitat fragmentation in the box–ironbark forests of central Victoria, Australia: predictions, compositional change and faunal nestedness.Crossref | GoogleScholarGoogle Scholar |
Mac Nally, R., and Horrocks, G. (2008). Long-term responses of a flood-plain dwelling marsupial to experimental manipulation of fallen timber loads. Basic and Applied Ecology 9, 458–465.
| Long-term responses of a flood-plain dwelling marsupial to experimental manipulation of fallen timber loads.Crossref | GoogleScholarGoogle Scholar |
Mac Nally, R., Parkinson, A., Horrocks, G., Conole, L., and Tzaros, C. (2001). Relationships between terrestrial vertebrate diversity, abundance and availability of coarse woody debris on south-eastern Australian floodplains. Biological Conservation 99, 191–205.
| Relationships between terrestrial vertebrate diversity, abundance and availability of coarse woody debris on south-eastern Australian floodplains.Crossref | GoogleScholarGoogle Scholar |
Manion, P. D., and Lachance, P. D. (1981). ‘Tree Disease Concepts: An Overview.’ (Prentice Hall: Englewood Cliffs, NJ.)
Mattiske Consulting Pty Ltd & Havel Land Consultants (1998). Vegetation mapping in the south west of Western Australia. Prepared for Environment Australia and the Department of Conservation and Land Management.
Matusick, G., Ruthrof, K. X., Brouwers, N., Dell, B., and Hardy, G. E. S. J. (2012). Sudden forest canopy collapse corresponding with extreme drought and heat in a mediterranean-type forest in southwestern Australia. Landscape Ecology 28, 69–80.
McElhinny, C., Gibbons, P., Brack, C., and Bauhus, J. (2006). Fauna–habitat relationships: a basis for identifying key stand structural attributes in temperate Australian eucalypt forests and woodlands. Pacific Conservation Biology 12, 89–110.
McKenzie, M.L., Burbidge, A.A., Baynes, A., Brereton, R.N., Dickman, C.R., Gordon, G., Gibson, L.A., Menkhorst, P.W., Robinson, A.C., Williams, M.R., and Woinarsi, J.C.Z. (2007). Analysis of factors implicated in the recent decline of Australia’s mammal fauna. Journal of Biogeography 34, 597–611.
| Analysis of factors implicated in the recent decline of Australia’s mammal fauna.Crossref | GoogleScholarGoogle Scholar |
Mercer, J. (1991). The decline of Eucalyptus wandoo Blakely in the Western Australian wheatbelt area. Honours Thesis, Murdoch University.
Moore, T. L., Valentine, L. E., Craig, M. D., Hardy, G. E. S. J., and Fleming, P. A. (2013). Is the reptile community affected by Eucalyptus wandoo tree condition? Wildlife Research , .
| Is the reptile community affected by Eucalyptus wandoo tree condition?Crossref | GoogleScholarGoogle Scholar |
Morrant, D. S., Petit, S., and Schumann, R. (2010). Floral nectar sugar composition and flowering phenology of the food plants used by the western pygmy possum, Cercatetus concinnus, at Innes National Park, South Australia. Ecological Research 25, 579–589.
| Floral nectar sugar composition and flowering phenology of the food plants used by the western pygmy possum, Cercatetus concinnus, at Innes National Park, South Australia.Crossref | GoogleScholarGoogle Scholar |
Newell, G. R. (1998). Characterisation of vegetation in an Australian open forest community affected by cinnamon fungus (Phytophthora cinnamomi): implications for faunal habitat quality. Plant Ecology 137, 55–70.
| Characterisation of vegetation in an Australian open forest community affected by cinnamon fungus (Phytophthora cinnamomi): implications for faunal habitat quality.Crossref | GoogleScholarGoogle Scholar |
Pestell, A. J. L., and Petit, S. (2007). Diet of the western pygmy possum (Cercartetus concinnus) (Marsupialia: Burramyidae), at Innnes National Park, South Australia, and evaluation of diet sampling methods. Australian Journal of Zoology 55, 275–284.
| Diet of the western pygmy possum (Cercartetus concinnus) (Marsupialia: Burramyidae), at Innnes National Park, South Australia, and evaluation of diet sampling methods.Crossref | GoogleScholarGoogle Scholar |
Podger, F.D. (1980) Some difficulties in the diagnosis of drought as a cause of dieback. In ’Eucalypt dieback in forests and woodlands.’ (Eds. K. M. Old, G.A. Kile, C.P. Ohmart.) pp. 167–173. (CSIRO Publishing: Melbourne).
R Development Core Team (2011). ‘R: A Language and Environment for Statistical Computing.’ (R Foundation for Statistical Computing: Vienna.) URL: http://www.R-project.org/.
Schomaker, M.E., Zarnoch, S.J., Bechtold, W.A., Latelle, D.J., Burkman, W.G., and Cox, S.M. (2007) Crown-condition classification: A guide to data collection and analysis. United States Department of Agriculture, Forest Service, Southern Research Station, Asheville.
Short, J., and Smith, A. (1994). Mammal decline and recovery in Australia. Journal of Mammalogy 75, 288–297.
| Mammal decline and recovery in Australia.Crossref | GoogleScholarGoogle Scholar |
Short, M., Smith, F. P., and Van Etten, E. (2009). Oil mallees provide foraging habitat for the western pygmy possum (Cercartetus concinnus) in the wheatbelt of Western Australia. Ecological Management & Restoration 10, 233–236.
| Oil mallees provide foraging habitat for the western pygmy possum (Cercartetus concinnus) in the wheatbelt of Western Australia.Crossref | GoogleScholarGoogle Scholar |
Stephens, H. C., Baker, S. C., Potts, B. M., Munks, S. A., Stephens, D., and O’Reilly-Wapstra, J. M. (2012). Short-term responses of native rodents to aggregated retention in old growth wet Eucalyptus forests. Forest Ecology and Management 267, 18–27.
| Short-term responses of native rodents to aggregated retention in old growth wet Eucalyptus forests.Crossref | GoogleScholarGoogle Scholar |
Stokes, V. L., Pech, R. P., Banks, P. B., and Arthur, A. D. (2004). Foraging behaviour and habitat use by Antechinus flavipes and Sminthopsis murina (Marsupiala: Dasyuridae) in response to predation risk in eucalypt woodland. Biological Conservation 117, 331–342.
| Foraging behaviour and habitat use by Antechinus flavipes and Sminthopsis murina (Marsupiala: Dasyuridae) in response to predation risk in eucalypt woodland.Crossref | GoogleScholarGoogle Scholar |
Stone, C., Simpson, J. A., and Gittins, R. (1998). Differential impact of insect herbivores and fungal pathogens on the Eucalyptus subgenera Symphyomyrtus and Monocalyptus and genus Corymbia. Australian Journal of Botany 46, 723–734.
| Differential impact of insect herbivores and fungal pathogens on the Eucalyptus subgenera Symphyomyrtus and Monocalyptus and genus Corymbia.Crossref | GoogleScholarGoogle Scholar |
Stone, C. (1999). Assessment and monitoring of decline and dieback of forest eucalypts in relation to ecologically sustainable forest management; a review with a case study. Australian Forestry 62, 51–58.
Sutherland, E. F., Lunney, D., Matthews, A., and Recher, H. F. (2004). Post fire observations of the eastern pygmy-possum Cercartetus nanus in Nadgee Nature Reserve and Ku-ring-gai Chase National Park, New South Wales. In ‘The Biology of Australian Possums and Gliders’. (Eds R. L. Goldingay, and S. M. Jackson.) pp. 230–236. (Surrey Beatty: Sydney.)
Torre, I., and Diaz, D. (2004). Small mammal abundance in Mediterranean post-fire habitats: a role for predators? Acta Oecologica 25, 137–142.
| Small mammal abundance in Mediterranean post-fire habitats: a role for predators?Crossref | GoogleScholarGoogle Scholar |
Tulloch, A. I. (2004). The importance of food and shelter for habitat use and conservation of the burramyids in Australia. In ‘The Biology of Australian Possums and Gliders’. (Eds R. L. Goldingay, and S. M. Jackson.) pp. 268–284. (Surrey Beatty: Sydney.)
Wandoo Recovery Group. (2006). Wandoo crown decline. Situation statement, July 2006.
Wentzel, J. J. (2010). Is tuart (Eucalyptus gomphocephala) decline detrimental for fauna? Ph.D. Thesis, Murdoch University.
Whitford, K., Manning, L., and Wills, A. (2008) Wandoo crown condition. Report of wandoo crown decline surveys, 2008. Department of Environment and Conservation and Wandoo Recovery Group, Perth.
Williams, S. E., Marsh, H., and Winter, J. (2002). Spatial scale, species diversity, and habitat structure: small mammals in Australian tropical rain forest. Ecology 83, 1317–1329.
| Spatial scale, species diversity, and habitat structure: small mammals in Australian tropical rain forest.Crossref | GoogleScholarGoogle Scholar |
Wilson, B. A., and Aberton, J. G. (2006). Effects of landscape, habitat and fire on the distribution of the white-footed dunnart Sminthopsis leucopus (Marsupialia:Dasyuridae) in the eastern Otways, Victoria. Australian Mammalogy 28, 27–38.
| Effects of landscape, habitat and fire on the distribution of the white-footed dunnart Sminthopsis leucopus (Marsupialia:Dasyuridae) in the eastern Otways, Victoria.Crossref | GoogleScholarGoogle Scholar |
Woinarski, J. C. Z., Armstrong, M., Brennan, K., Fisher, A., Griffiths, A. D., Hill, B., Milne, D. J., Palmer, C., Ward, S., Watson, M., Winderlich, S., and Young, S. (2010). Monitoring indicates rapid and severe decline of native small mammals in Kakadu National Park, northern. Australian Wildlife Research 37, 116–126.
| Monitoring indicates rapid and severe decline of native small mammals in Kakadu National Park, northern.Crossref | GoogleScholarGoogle Scholar |
Yates, C. J., Hobbs, R. J., and True, D. T. (2000). The distribution and status of eucalypt woodlands in Western Australia. In ‘Temperate Eucalypt Woodlands in Australia. Biology, Conservation, Management and Restoration’. (Eds R. J. Hobbs, and C. J. Yates.) pp. 86–106. (Surrey Beatty: Sydney.)
Zar, J. H. (1998). ‘Biostatistical Analysis.’ 4th edn (Prentice Hall: New Jersey.)