Mercury partitioning in oil and gas production systems - design optimisation and risk mitigation through advanced simulation
Peter Crafts A C and Mark Williams BA Genesis, Aspect 32, Pavilion 3, Prospect Road, Arnhall Business Park, Westhill, AB32 6FE, UK.
B Genesis, 1120 Hay Street, West Perth, WA 6022, Australia.
C Corresponding author. Email: Peter.Crafts@genesisoilandgas.com
The APPEA Journal 60(1) 97-109 https://doi.org/10.1071/AJ19167
Submitted: 16 December 2019 Accepted: 24 January 2020 Published: 15 May 2020
Abstract
Operators are increasingly producing fields with challenging operational environments, including fluids with higher concentrations of mercury. Mercury is harmful to personnel and the environment and contaminates most of the production plant that it contacts through physical adsorption, potentially creating hazardous wastes through the operating lifetime and subsequent decommissioning. The assessment of mercury removal locations requires careful consideration at the design stage. Mercury exists in various chemical forms that readily partition between streams in the production process. Mercury partitioning simulations are an essential step in managing the operational, safety, environmental, production and decommissioning risks associated with mercury. Accurate assessment of mercury species during welltests is an essential step towards successful mercury risk management; providing the basis to model partitioning into vapour, liquid, aqueous and solid phases through production. Adsorption modelling is also necessary to understanding the propagation of mercury through offshore and onshore systems, identifying release points to the environment. Only once form, flow and accumulation locations are understood, can the adequate design of mercury removal facilities be confidently completed. Experience in thermodynamic modelling and verification through laboratory research and plant analysis is required to fully understand modelling limitations, capabilities and applications to proposed or existing infrastructure. Changes in inlet stream conditions may affect propagation of mercury through an operating plant thus the influence of predicted conditions through the full life of the field should be considered.
Keywords: mercury, partitioning, equilibria, risk, environment, modelling, safety, species, research, removal, design, adsorption, aqueous, emissions, thermodynamic, subsea, offshore, onshore, reservoir, sampling, welltest, pressure, temperature, composition, operation, ionic, inorganic, organic, corrosion, materials.
Introduction
Mercury is a trace contaminant found in hydrocarbon reservoir fluids globally and is often overlooked during field development planning and greenfield/brownfield development projects. Genesis has provided mercury consultancy services to the upstream oil and gas industry for over 15 years and shares some lessons learned in this paper.
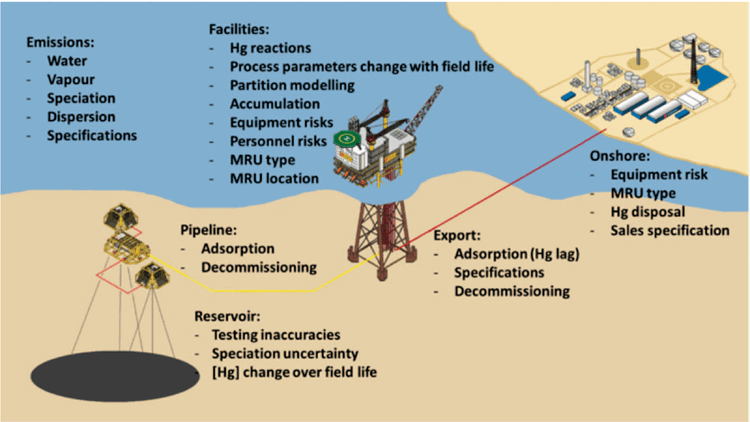
It remains technically challenging to make accurate measurements of mercury concentration and speciation in the field, and project teams are often faced with at least an order of magnitude uncertainty in wellfluid mercury concentrations. Figure 1 shows a summary of issues associated with the measurement and modelling of mercury from reservoir to product.
Industrial awareness of mercury issues has improved over recent years together with improvements in the robustness and reliability of analytical methods, however there is still some way to go before mercury risk management practices are normalised throughout the industry.
Mercury concentrations vary globally with the highest concentrations reported in regions of high volcanic activity, including Northern and Eastern Australia, Indonesia and Malaysia for example.
The most prevalent geological form of mercury is the mineral cinnabar (HgS) and is often regarded as the most likely source of reservoir contamination. Cinnabar is relatively stable at standard conditions, however it may partially decompose in contact with reservoir fluids to liberate elemental mercury. Operators report that mercury concentrations often increase with reservoir depth and temperature; experience suggests that high pressure/high temperature reservoirs often carry the highest risk of mercury contamination within a geographical region.
The Moomba Gas Plant fire in Australia in 2004, and the Skikda Terminal explosion in Algeria in 1977, both highlight the risks of corrosion by mercury-induced liquid metal embrittlement, resulting in potential integrity failures and loss of hydrocarbon containment. Mercury’s high toxicity and persistence in the human body, marine species and the natural environment are further risks that must be managed.
The United Nations Environment Programme’s (UNEP) Minimata convention (http://www.mercuryconvention.org/) came in to force on 16 August 2017, and has now been signed by 98 governments, setting the high-level objective to ‘make mercury history’. The Minimata convention requires governments to identify and quantify potential sources of mercury releases to the environment; identify mercury contaminated sites; improve waste management practices and eliminate the industrial use of mercury and mercury containing products where possible. The Minimata convention is expected to drive future legislation that may affect the oil and gas industry.
Impacts of mercury
Mercury may impact the integrity of hydrocarbon containment systems; equipment design specifications; product specifications; occupational exposure risks and environmental emissions. Mercury specifications are stringent and will often be exceeded, even in global regions considered at low risk for mercury contamination. Current specifications for gas, condensate and produced water are discussed in this section and project operators are strongly advised to consider mercury specifications during early field development planning and before drilling the first test well.
Product specifications
The most common specification for mercury in gas export streams globally is less than 10 nanograms per standard cubic metre (Sm3). This specification is frequently stated as 0.01 μg/Sm3 in more common units of measurement.
Mercury concentrations in reservoir fluids from the Timor Sea region of North Western Australia are typically in the range 100 to 300 μg/Sm3, and gas export concentrations will usually far exceed the mercury gas export specification without offshore removal facilities. The UK North Sea region is often considered to have relatively low levels of mercury contamination, typically in the range between 5 and 50 μg/Sm3. Several recent UK projects have discovered late in development, however, that predicted mercury concentrations in the gas export stream exceed the offshore trunk line entry specification by an order of magnitude, and that mercury removal facilities should be added to the project scopes.
Hydrocarbon liquids are typically subject to a reduced sales price if the total mercury concentrations exceed 30 parts per billion by weight (ppbw) and this criteria is often exceeded when full wellstream concentrations exceed ~100 μg/Sm3. The partitioning of mercury between gas and liquids is dependent on reservoir fluid compositions, and gas to oil/condensate ratios and should be assessed on a case by case basis.
Specifications for mercury in produced water vary globally. The Australian and New Zealand Conservation Council (ANZECC) have published toxicity trigger values for inorganic mercury at three levels of protection under the Australian and New Zealand Guidelines for Fresh and Marine Water Quality (ANZECC and ARMCANZ 2000). The oil and gas industry normally apply the limit of 0.1 μg/L or 0.1 ppbw at the limit of the produced water marine dispersion zone.
In the writer’s experience of detailed mercury partitioning assessments, the elemental mercury concentration in produced water overboard streams is typically in the range of 2 to 4 ppbw, therefore hydrodynamic modelling of the marine dispersion plume is often required to determine if the environmental discharge limits will be met.
Current industry standard methods for mercury partitioning are limited to estimates of elemental mercury species and do not include contributions from ionic mercury species and solid mercury sulphide that may also contaminate produced water streams. For this reason, it is recommended that speciated mercury analysis is completed on welltest liquids to confirm any requirements for additional produced water filtration and mercury removal facilities.
Major Accident Event (MAE) hazard risks
Liquid mercury is corrosive to some common materials of construction used in the oil and gas industry, and is particularly corrosive to aluminium and aluminium alloys used in cold box heat exchangers.
The MAE hazard potential of mercury-induced liquid metal embrittlement (LME) through loss of hydrocarbon containment is illustrated by the 2004 explosion at Skikda Gas Processing Terminal in Algeria; resulting in 27 fatalities, 72 injured and estimated losses of US$30 million.
Liquid mercury in contact with metal substrates where the protective oxide coating is susceptible to damage may allow mercury atoms to form a solid solution/amalgam with the substrate, leading to generalised corrosion and problems of stress-induced cracking. LME refers to a decrease in ductility of the base metal that can occur rapidly when the surface is wetted by liquid mercury. LME may result from a single contamination event, and in severe cases this may result in mechanical failure due to brittle intergranular fracture. LME risks depend on materials and the environment, including the process fluid composition, chemistry and operating temperatures.
Aluminium, zinc, titanium and copper alloys (including Monel) are well known as being susceptible to mercury-induced degradation. Carbon steel and low alloy steels are generally immune to mercury-induced LME, however 304 stainless steel (304 SS) is reported to show reduced ductility under conditions of plastic strain (Wasson et al. 2013).
The presence of 304 SS in an existing flare header, subject to low temperatures, cyclic strains and the risk of liquid mercury drop-out, has recently influenced a project team to select an offshore mercury removal option in preference to an onshore ‘end of pipeline’ removal option. The onshore mercury removal option could not safeguard upstream sections of the reception facilities existing flare header system.
Occupational health risks
Mercury is highly toxic to personnel and may accumulate in the body following repeat exposure. Mercury is particularly harmful to the central and peripheral nervous systems, with symptoms including tremors and cognitive effects that may occur sometime after exposure and may be fatal.
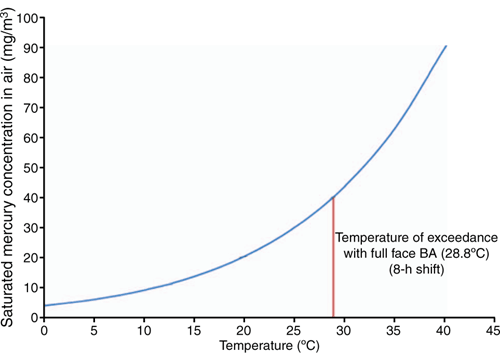
Inhalation is often considered as the primary route for personnel exposure, with ingestion and skin absorption risks increased by poor work hygiene standards and failure to decontaminate both tools and PPE. The 8 h TWA (time weighted average) exposure limit stated in UK HSE EH40/2005 guidance is 0.02 mg/m3 (UK HSE 2018) and the immediately dangerous to health or life (IDHL) stated by the National Institute for Occupational Safety and Health (NIOSH) is 10 mg/m3 (NIOSH 2007). For perspective; evaporation of a 2.1 mm droplet of mercury is sufficient to reach the IDHL in 50 m3 of stagnant air contained in a typical separator vessel; and evaporation of a 0.26 mm droplet is sufficient to reach the 8 h TWA.
A modern air fed on demand full face mask typically has an assigned protection factor of 2000 in use for an 8 h shift and increases the maximum permissible atmospheric concentration to 40 mg/m3. This limit will be exceeded however in stagnant atmospheres for ambient temperatures above 29°C, as shown in Fig. 2.
|
A combination of forced air ventilation, appropriate respiratory protective equipment (RPE), personnel protective equipment (PPE), environmental monitoring and procedural controls is usually required to manage occupational exposure risks.
The release of adsorbed mercury from steel surfaces and residues represents a significant risk during hot work, maintenance and decommissioning activities. Adsorbed mercury is rapidly released at high temperatures and may cause high localised concentrations of mercury in the atmosphere.
Environmental risks
Vapour emissions
Vapour emissions from flare and gas turbine exhausts must be considered against country specific environmental emission limits. Detailed elemental mercury partitioning studies are required to define source terms for environmental emissions modelling and regulatory submissions. In a recent study, it was recommended that a fuel gas feed stream is relocated downstream of the gas mercury removal unit (GMRU) as the best available technology (BAT) solution to minimise mercury in gas turbine exhausts, and that further reductions were possible through re-routing a medium pressure vapour recovery steam to upstream of the same GMRU.
Computational fluid dynamics (CFD) assessment is recommended to confirm the extent of mercury concentrations from vents and fugitive emissions with regards to normally occupied areas. Vapour emissions from cold vents will often disperse to less than the applicable occupational exposure limit within a few tens of metres of the source. The formation of mercury aerosols from some vents has been identified to be more problematic.
Mercury liquid droplet emissions
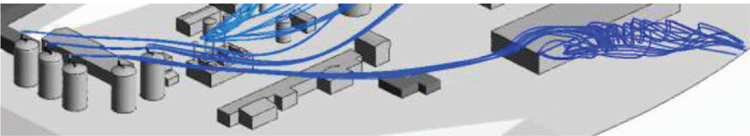
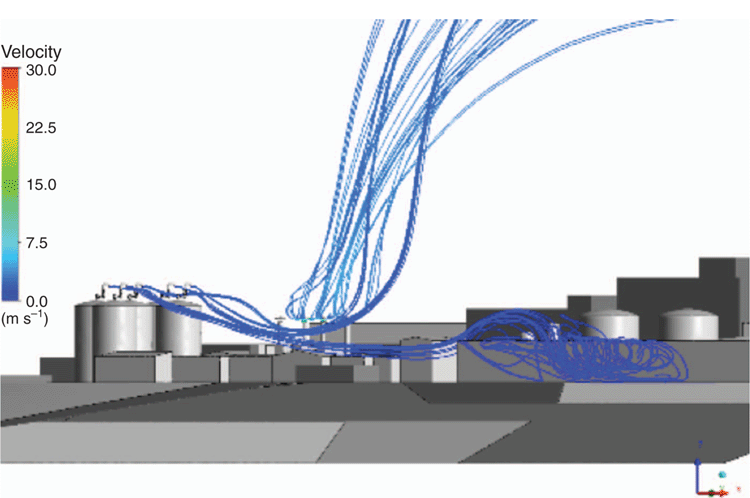
Droplets of liquid mercury may condense in vent headers and cold vents when mercury saturated vapour streams are cooled towards lower ambient temperature. Elemental mercury is only sparingly soluble in aqueous streams and partitioning studies show that cold vents from produced water treatment systems and monoethylene glycol (MEG)/triethylene glycol (TEG) regeneration plants may operate at the limit of mercury saturation at normal process temperatures. Mercury at typical concentrations of 1500 ppbw in cold vents may then precipitate as an aerosol of fine droplets. CFD analysis shows that micron sized droplets of mercury will evaporate very slowly due to mercury’s low vapour pressure and may be carried for significant distances. Figure 3 shows the trajectory of mercury aerosol droplets from a group of onshore MEG storage tank vents estimated by CFD analysis. Evaporation of mercury droplets is expected to take tens of minutes, over which time these droplets may be carried several hundred metres. Figure 3 and Fig. 4 show that mercury aerosols could impact normally occupied buildings with adverse wind direction.
|
Emissions of solid phase mercury
Temperatures below the freezing point of liquid mercury (–39°C) are often expected downstream of blowdown valves and within flare systems. The risk of both liquid and solid phase mercury in blowdown streams for all detailed mercury partitioning assessments must be considered. Blowdown of process equipment located upstream of mercury removal facilities will often result in mercury saturated conditions in the flare header, causing precipitation of solid mercury particulates or liquid mercury droplets. With high velocities present in the flare system, it is also possible that some solid particulate mercury or liquid mercury droplets will be emitted from the flare tip. The fate of mercury particulate emissions should be assessed using CFD to confirm that mercury is fully dispersed before any contact with the ground and potentially occupied areas.
Mercury properties and chemistry
Mercury may occur in several chemical forms through oil and gas processing, for example:
-
Elemental (Hg0); e.g. soluble mercury and metallic liquid mercury;
-
Ionic; e.g. mercuric chloride (HgCl2);
-
Inorganic; e.g. mercuric sulphide (HgS[solid]);
-
Organic; e.g. dimethyl mercury (CH3)2Hg.
Mercury species are often reactive at conditions found in reservoirs, pipelines and through processing. Mercury may transfer between species, depending on the prevailing conditions of temperature, pressure, pH and chemical environment.
Elemental mercury is often regarded as the most prevalent form of mercury in wellfluids entering oil and gas processing. This simplification is broadly correct when considering the overall production process, however ionic and inorganic mercury species are expected to dominate in some areas of the process. Ionic and inorganic mercury species may dominate in condensate and produced water streams, for example.
Partitioning of mercury species
Elemental mercury occurs as a dissolved component in gas, condensate and produced water streams. If concentrations exceed the saturation point, then liquid mercury will precipitate, and at temperatures below the freezing point (–39°C) then solid phase mercury metal will crystallise. Elemental mercury is strongly adsorbed onto solids, including steel surfaces, corrosion products and scale deposits, and may contaminate wax and asphaltene precipitates.
Elemental mercury is readily oxidised to ions in aqueous environments, forming Hg2+ and 2Hg2+ ions. Mercury ions will form ion pairs in aqueous solutions with available counterions (e.g. chloride). Ionic mercury species will normally remain as dissolved components through processing in observed experience, however, solid phase ionic mercury compounds may crystallise in cases where the solubility product is exceeded.
Mercury sulphide is the most common form of inorganic mercury species and occurs naturally as the mineral ‘cinnabar’. Mercury sulphide is a stable crystalline solid at standard conditions and is insoluble in both hydrocarbon liquids and produced water at normal process temperatures. Mercury sulphide, as a fine suspended solid, is a common contaminant in condensate and produced water streams and should be removed by filtration if mercury concentrations exceed specification limits.
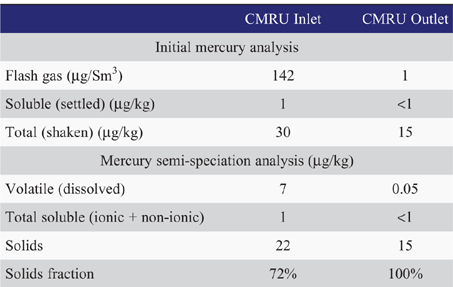
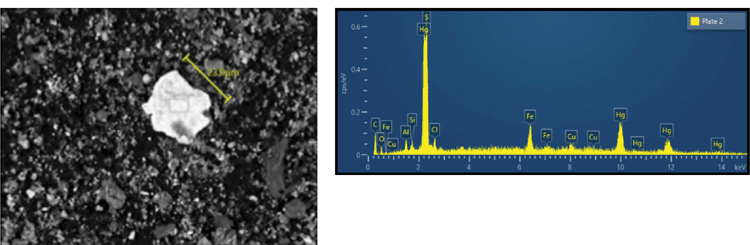
Recent analysis of mercury species through an operating onshore condensate mercury removal unit are shown in Table 1. These results show the majority of mercury in this unstabilised condensate comprises solid phase mercury species. Further scanning electron microscopy/energy dispersive X-ray (SEM/EDX) analysis of the solids showed that mercury sulphide was the dominant species (Fig. 5). The adsorbent installed in condensate MRUs is only intended to remove elemental mercury and is not effective at removing solid mercury species, therefore in this case the installed MRU was not effective in controlling the total mercury concentration.
|
|
Mercury speciation can significantly affect the partitioning behaviour of mercury through oil and gas processing and should be considered in all aspects of mercury risk management to ensure the mercury risk picture is fully understood.
Properties and partitioning behaviour
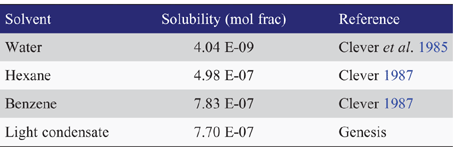
The apparent volatility of elemental mercury is much higher than would be expected from inspection of the pure component vapour pressure alone. Relative volatility is enhanced because elemental mercury does not interact favourably with other bulk components including hydrocarbons, water and glycols. Mercury thus prefers to occupy the vapour phase where intermolecular interactions will be minimised, and prefers to form a separate and almost pure metallic liquid mercury phase at higher concentrations. The solubility of elemental mercury in some pure solvents and a light North Sea condensate are given in Table 2.
|
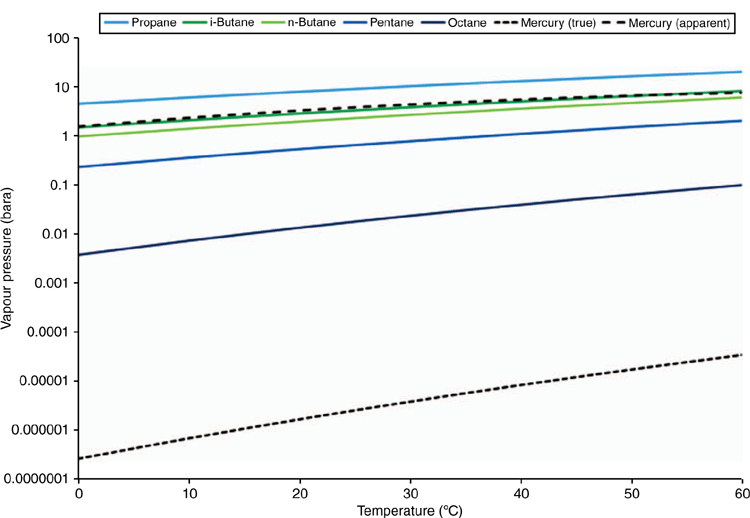
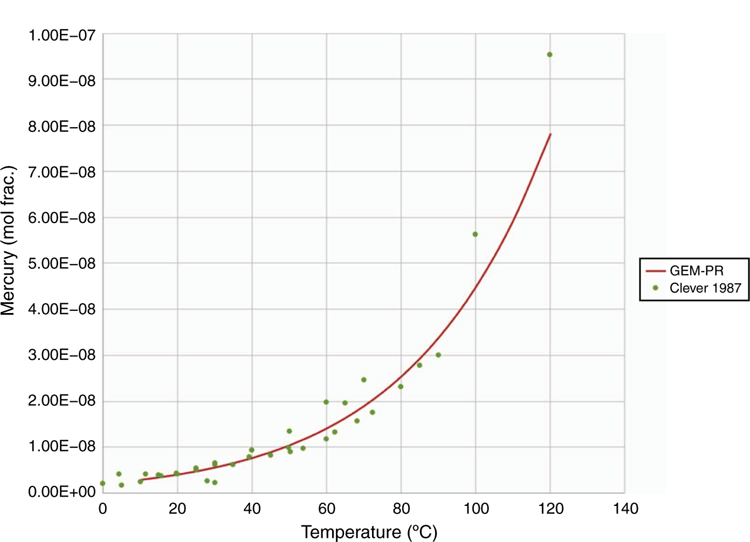
As a ‘rule of thumb’, elemental mercury is often estimated to follow the C4 components through gas processing. This behaviour can be explained by considering the solubility and activity of mercury in pure C5 to C10 hydrocarbon liquids (Clever and Iwamoto 1987). The activity coefficient of mercury in pentane is ~1 × 106, and the apparent vapour pressure of elemental mercury dissolved in light alkanes is therefore enhanced by approximately six orders of magnitude, which brings the apparent vapour pressure close to that of butane as illustrated in Fig. 6. This analysis is simplistic and does not account for the effects of temperature, pressure and composition on fugacity, therefore an equation of state model is recommended for detailed partitioning studies, as discussed in the next section.
Risk management process
When designing facilities which may be affected by the presence of mercury, a study should be undertaken to identify the mercury removal technologies required to reduce safety, health, and environmental risks to ALARP (as low as reasonably practicable). The CAPEX and OPEX costs of commercially proven technology options should both be assessed against the risk reduction benefits provided and capability to achieve regulatory emissions standards.
Safety studies should consider full lifecycle of facilities including decommissioning and the additional requirements for manual handling of hazardous waste versus the removal of contamination and loss of containment risk for equipment downstream of any MRUs in place.
The governing legislation from NOPSEMA relates to the object of the Offshore Petroleum and Greenhouse Gas Storage (Environment) Regulations 2009 (Environment Regulations), as set out in regulation 3, to ensure that any petroleum activity or greenhouse gas activity carried out in an offshore area is carried out in a manner consistent with the principles of ecologically sustainable development (ESD) as set out in section 3A of the Environment Protection and Biodiversity Conservation Act 1999 (EPBC Act), and carried out in a manner by which the environmental impacts and risks of the activity will be reduced to ALARP and acceptable levels (NOPSEMA 2019).
Practically speaking, the requirement to demonstrate an ALARP risk position should include the following:
-
Identify amount and characterisation of mercury species present: well testing via approved methodology to ensure accurate and repeatable estimation of mercury presence. Speciation is required as an input to downstream assessments due to the differences between reactive and solubility nature of mercury species.
-
Perform partitioning studies over field life: required to get a full assessment of boundary mass balances and accumulation for all mercury species.
-
Removal options assessment: assessment of MRU location and type to meet legislative and equipment specifications in each fluid phase.
-
Safeguarding measures: requirements to protect personnel.
Identification of mercury risks early in the design process and include appropriate engineering safeguards inline within an industry accepted risk management hierarchy.
Successful welltests for mercury
Many recent industry welltests have delivered poor quality analytical results for mercury when compared to the reality of production data. This often leaves the development project to carry significant uncertainty regarding the design of mercury removal facilities. Common mercury welltest problems include:
-
Insufficient time to reach steady-state for mercury;
-
Insufficient samples to demonstrate that steady-state was reached for mercury;
-
Analysis of only one stream from the test separator prevents validation using flash analysis;
-
Mercury speciation is not considered in condensate and produced water samples;
-
Mercury is adsorbed onto sample lines and samples are not representative;
-
Mercury precipitates as a separate liquid phase upstream of the sample points;
-
Mercury analysis is shipped to shore allowing time for:
Elemental mercury to react with other species
Mercury to adsorb onto exposed metal surfaces of the sample bombs.
Based on this experience, reservoir development teams should complete a basis of design and welltest mercury analysis programme in advance of all welltests.
The mercury welltest assessment should include the following items as a minimum:
-
A mercury partitioning assessment from perforations through to the test separator and sample points to ensure the welltest is planned where possible to prevent conditions where liquid mercury could drop-out and holdup. This will help to ensure that samples are fully representative of total mercury in the wellfluids.
-
A dynamic mercury adsorption study should be completed for the drill string assembly to estimate the time required to achieve steady-state. The main flow test period should be planned to accommodate this requirement.
-
Mercury sampling should include gas, condensate and produced water phases so that flash analysis can be used to cross check and validate the sampling results.
-
Analysis of flash gas from the condensate samples should be completed to determine the total volatile elemental mercury.
-
Mercury analysis of the drilling mud should be completed to identify potential sources of mercury contamination (e.g. barite).
-
Condensate and produced water samples should include a functional mercury speciation analysis using UOP938–10 (UOP 2010) or similar, to determine the fractions of ionic, inorganic, organic and solid phase mercury species.
-
Duplicate samples should be taken at least during the steady-state flow period to estimate sampling accuracy.
-
All mercury analysis should be completed onboard the drill rig where possible, using a specialist contractor, to avoid common problems with mercury sampling and to avoid issues with reaction and adsorption during storage and shipping to shore.
Mercury adsorption/desorption behaviour
Mercury is adsorbed onto steel surfaces (Chaiyasit et al. 2010) and porous scale deposits in gas transmission pipelines (Wilhelm and Bloom 2000), although there is no evidence for any reaction between mercury and carbon steel, or for the absorption of mercury into carbon steel substrates (Wilhelm and Nelson 2010). Current literature documents several cases where mercury was known to be entering offshore gas transmission pipelines for several years before any significant mercury concentrations were detected at onshore reception facilities (Wilhelm and Bloom 2000). This phenomenon is often referred to as the mercury lag effect.
Genesis has developed a proprietary dynamic pipeline adsorption model to study this behaviour and has previously applied the model successfully for clients operating new and existing pipelines in the North Sea region and Indonesia for both adsorption and desorption cases.
The pipeline adsorption model may be used to advise the following:
-
What is the effect of drill string mercury adsorption in welltests and what flow period is required to achieve steady-state conditions for mercury?
-
How long should sample points be flowed before samples are taken for analysis to ensure that samples are representative of production fluids?
-
What is the effect of GMRU failure compared to the potential adsorption capacity of the gas export pipeline and how does this affect the design of GMRU facilities?
-
What is the potential for mercury pipeline contamination as input to decommissioning strategy.
The mercury pipeline adsorption model is developed from first principles to describe mass transfer processes and physical adsorption of elemental mercury on steel surfaces of pipelines operating with a single dense gas phase.
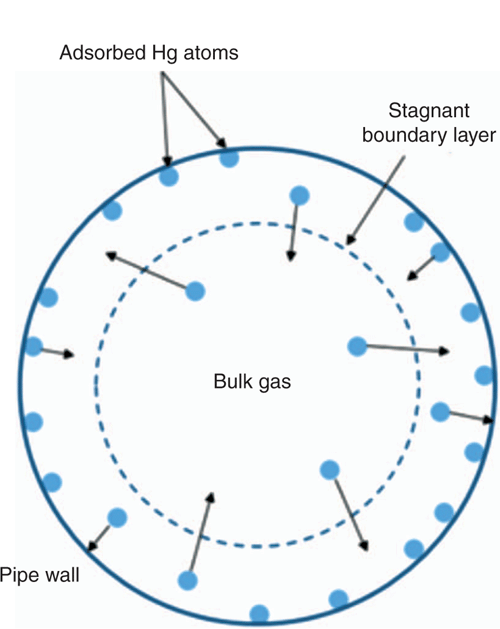
Figure 7 illustrates the rate processes that are important for mercury adsorption and desorption between the bulk gas phase and the internal pipe wall surface. The model is summarised as follows:
-
Bulk gas is well mixed with no radial concentration gradients (assuming turbulent flow regime);
-
All resistance to mercury mass transfer between the bulk gas and pipe internal surface is due to a stagnant boundary layer (dashed line in Fig. 7);
-
The mass transfer coefficient for mercury diffusion through the stagnant boundary layer is calculated using the Chilton-Colburn analogy;
-
Mercury atoms are free to diffuse through the stagnant boundary layer to the pipe wall where a reversible physical adsorption process occurs;
-
A temperature-dependent adsorption isotherm defines the mass of mercury adsorbed on the wall and the equilibrium concentration of mercury in the stagnant boundary layer adjacent to the wall;
-
Mercury atoms are free to desorb from the pipe wall and diffuse back across the stagnant boundary layer into the bulk gas flow. Desorption occurs when the bulk gas concentration is lower than the equilibrium concentration of the adsorption isotherm;
-
The rate of mercury diffusion through the stagnant boundary layer is calculated using Fick’s law of diffusion.
|
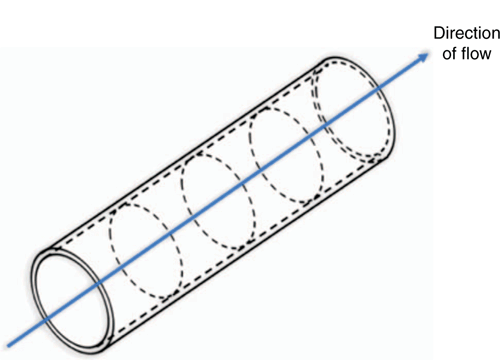
Mercury concentrations in the bulk gas and adsorbed on the pipe wall are dependent on both time and distance, therefore the mathematical model defines a set of partial differential equations (PDEs). These PDEs are solved numerically using the Aspen custom modeller (ACM) software (AspenTech, Bedford, MA, USA) after discretising the pipeline into a series of connected segments as illustrated in Fig. 8. Each segment is considered as a well mixed volume with no change in concentration in the axial direction. The PDEs are solved using the established method of lines approach as follows:
-
Time is defined as the continuous variable;
-
Distance is defined as a discrete variable;
The pipeline is discretised into a series of segments, connected in the direction of flow;
The mercury mass balance through each segment is defined and solved using the backward finite difference (BFD) method in ACM;
Approximately 500 segments are usually sufficient to describe the concentration profile smoothly at landfall during breakthrough.
Note: the concentration profile will appear to be stepped in cases were the number of segments is not sufficient, however, this does not affect the overall mass balance accuracy.
|
The results of the mercury pipeline adsorption model show the following:
-
Time to breakthrough of mercury at the exit of the pipeline;
-
Mercury concentrations with time/distance along the pipeline;
-
Mercury concentration profile at breakthrough/time available for response;
-
Mercury desorption behaviour should clean gas be introduced in later life.
Mercury partitioning in oil and gas processing
Process conditions
Mercury partitioning behaviour and the basis of design for any mercury removal facilities may be affected by the following process parameters:
-
Temperatures;
-
Pressures;
-
GOR;
-
Water cut;
-
Wellfluid compositions;
-
Process conditions.
Life of field sensitivity cases should be considered in mercury partitioning studies to ensure the impact of these parameters is thoroughly evaluated, and that mercury removal facilities are correctly positioned, sized and designed.
Through an understanding of the role of process parameters in mercury partitioning through full field life, the designer has the ability to design and operate for mercury removal efficiency and reduce requirement for or the size of MRUs.
Software
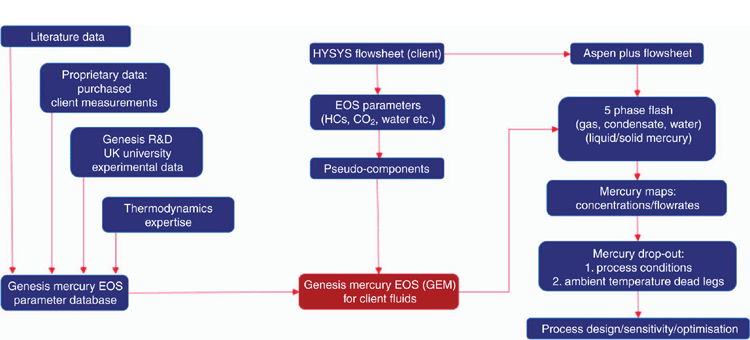
The heat and mass balance including elemental mercury is simulated using an equation of state (EOS) model as a plug in to Aspen Plus v8.8 flowsheeting software (AspenTech). Aspen Plus is selected over other process software (e.g. HYSYS, Unisim), because of the four-phase multiphase flash and flowsheet capability, which is necessary for mercury partitioning studies where liquid and solid mercury phases may occur in addition to gas, condensate and aqueous phases. The application of the EOS is illustrated in Fig. 9.
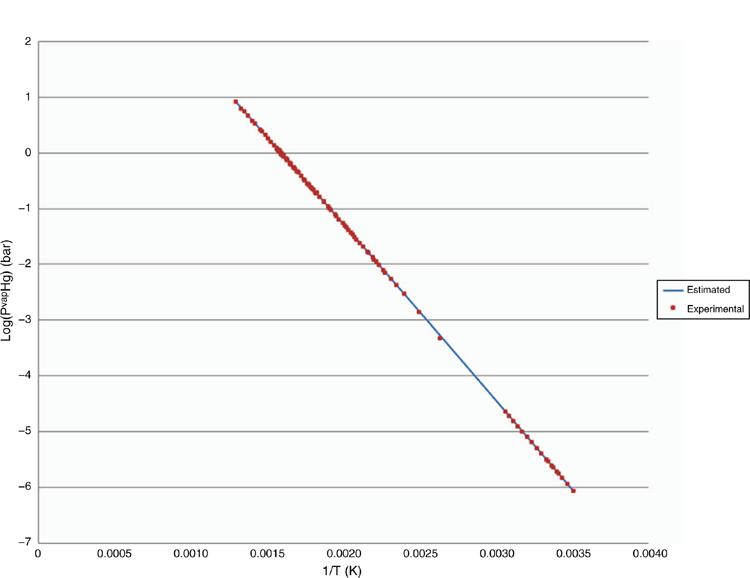
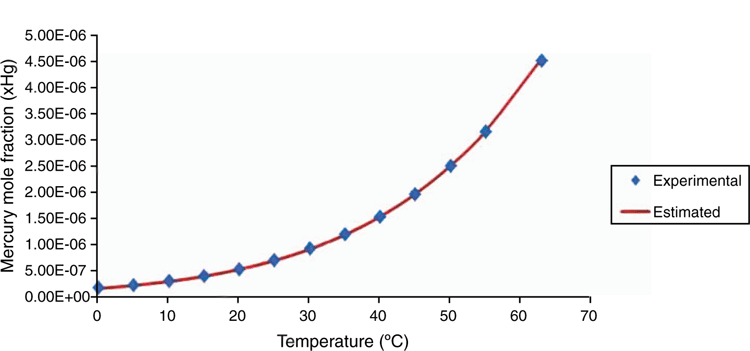
The bespoke EOS model describes the behaviour of elemental mercury in a thermodynamically consistent manner and describes the phase equilibrium behaviour of mercury with oil and gas components to a high level of accuracy, as illustrated in Figs 10–12. The customised parameter database has been developed using available literature and client specific phase equilibrium data, since 1995. A rigorous parameter regression approach is used to ensure that model parameters are statistically significant. Literature is reviewed on a regular basis to ensure that latest peer reviewed results are included in the model including results from the Gas Processors Association (GPA).
Ongoing research and development efforts with Aberdeen University are in place to measure mercury phase equilibrium data that is not currently available in the literature, and to extend the accuracy and capability of the EOS. This proprietary data includes systems with MEG, TEG and their mixtures with water.
Enhanced EOS model examples
The following figures provide some examples to compare the enhanced EOS estimated results with literature data for the following systems:
-
Pure component vapour pressure of liquid phase mercury;
-
Elemental mercury solubility in pentane;
-
Elemental mercury solubility in water.
The enhanced EOS in use is a proprietary model therefore it is not possible to disclose all experimental data used in parameter regression. It can be stated however that in most cases the model estimated results are in excellent agreement with the experimental data points and similar to the case for pentane. The results shown in Fig. 11. for water show the highest degree of uncertainty. This is most likely due to the quality of experimental data and the challenge of measuring the solubility of elemental mercury in aqueous systems, where oxidation to highly soluble ionic forms of mercury is challenging to control.
Liquid mercury dewpoint
The dewpoint of liquid mercury and the potential for liquid mercury drop-out from process streams is automatically solved by the bespoke EOS equation of state model. This model considers the effect of both liquid and vapour phase non-ideality. If a liquid mercury phase is thermodynamically stable then it is assumed to be of pure mercury, since the solubility of all other species in mercury is negligible. In this case the phase equilibria of mercury is defined by:
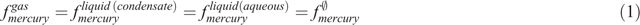
Where f is the fugacity of each component in each phase, f ∅ is the pure component fugacity, and in all cases the fugacity is at the system temperature and pressure. This relationship allows the precipitation of mercury from the gas phase or liquid phases to be predicted.
Mercury removal options
An assessment of preferred locations for mercury removal as well as MRU size and type is required to meet legislative and equipment specifications in each fluid phase. It should be noted that all the widely used mercury removal technologies are designed to treat single phase flow. Based on this technology limitation, it is not possible to implement MRUs in plant locations where the fluid is likely to be in two or three phases.
MRU sizing parameters
The sizing of the recommended MRUs is governed by the fluid phase superficial velocity constraints (diameter) and residence time (length). Therefore, the mercury load is used, not to determine bed size, but to predict frequency of bed material replacement.
MRU type summary
There are several different types of mercury removal technology used for treating hydrocarbon streams. The mercury removal can be achieved by using two types of mercury removal processes:
-
Regenerable processing
-
Non-regenerable processing
All the widely used mercury removal technologies are based on the adsorption mechanism and designed to treat single flow phase only. Adsorption occurs in vessels or beds where the stream being treated passes through it and the impurities (Hg) are removed to very low outlet levels.
This is essentially a batch process and when all the active sites for adsorption are no longer available, the bed has either to be changed out with new adsorbent material (non-regenerable) or regenerated using a regeneration stream (typically the outlet) and high temperature.
As a result, there would be typically more than one bed available for this treatment with the non-regenerable systems typically using 2 × 100% to allow for bed change-out without a shutdown and regenerable using 3 × 50% with two beds adsorbing and one bed in regeneration.
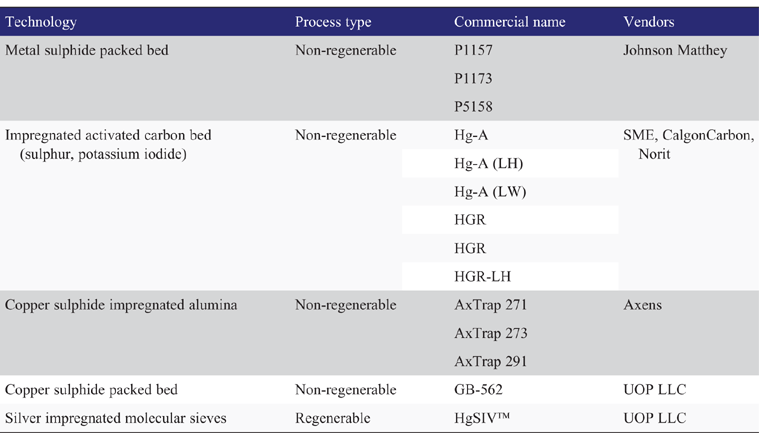
With non-regenerable systems, the bed change-out duration is typically around 4–5 days. Therefore, the bed life should be designed for a minimum of 1 year, and to align with planned shutdowns for offshore installations. In order to optimise the adsorbent usage, the bed could also be designed using a lead/lag configuration. The lag bed provides protection of the downstream process while the mercury level exiting the lead bed is allowed to increase before replacement of the spent bed is required. The most common mercury removal technologies are summarised in Table 3.
|
Gas stream
Gas stream MRUs are frequently recommended to be located offshore upstream of the dehydration units and acid gas removal unit (AGRU). This is suitable to:
-
Minimise mercury release to the environment;
-
Avoid damage of the AGRU membranes;
-
Protect the equipment and the personnel against the mercury deposition risks.
Metal catalyst beds are the most suitable technology for this application as they can be located upstream of the dehydration unit, whereas this is not recommended for other technologies such as the activated carbon beds.
Condensate
MRUs for the condensate stream should be located offshore where downstream equipment and export specifications are not met. The inlet stream should be filtered upstream of the condensate MRU. Installing the MRU on the pump suction results in reduced vessel weight (compared to pump discharge). There are several metal catalyst bed technologies which are suitable for this application.
Conclusions
Consideration of the presence of mercury in oil and gas facility design is required from testing to removal, with a range of challenges evident from wellhead to sales gate. Risks from mercury to equipment, personnel and the environment must be mitigated from all areas of subsea, offshore, onshore and transmission systems. Well testing practices require improvement to enable accurate estimation of mercury load and species present. This includes the allowance for adsorption of mercury onto production and test surfaces and the potential reactivity of mercury.
Existing technology to estimate mercury species partitioning through oil and gas processing is reasonably well established for elemental mercury in gas, hydrocarbon liquid and aqueous phases. However, this is not the case for other mercury species and fluids such as glycol mixtures. Through laboratory R&D analysis, the extension of knowledge of mercury phase equilibrium behaviour and reactivity has been improved to estimate mercury partitioning for design of mercury removal facilities and mercury risk management.
Conflicts of interest
No conflicts of interest exist.
Acknowledgements
Genesis wishes to acknowledge the contributions of J. Feldmann, E. Krupp and M. Feldman of Aberdeen University UK Trace Element Speciation Laboratory (TESLA); and E. Panteli of Equinor R&D Centre Trondheim, Norway; for collaborations and measurements of mercury phase equilibrium in oil and gas systems through a PhD project jointly funded by Genesis/TechnipFMC and Equinor.
References
ANZECC and ARMCANZ (2000), Australian and New Zealand Guidelines for Fresh and Marine Water Quality, Paper No. 4, Vol. 1, Chapters 1–7, pp. 3.4–5. (Australian and New Zealand Conservation Council and Agriculture and Resource Management Council of Australia and New Zealand) Available at https://www.waterquality.gov.au/sites/default/files/documents/anzecc-armcanz-2000-guidelines-vol1.pdf [verified 13 February 2020]Chaiyasit, N., Kositanont, C., Yeh, S., Gallup, D., and Young, L. (2010). Decontamination of Mercury Contaminated Steel (API 5L–X52) Using Iodine and Iodine Lixiviant. Modern Applied Science 4, 12–20.
| Decontamination of Mercury Contaminated Steel (API 5L–X52) Using Iodine and Iodine Lixiviant.Crossref | GoogleScholarGoogle Scholar |
Clever, H. L. (Ed.) (1987). Mercury in Liquids, Compressed Gases, Molten Salts and Other Elements. IUPAC Solubility Data Series Vol.29, p. 156. (Pergamon: Oxford) (Spencer J.N., Voigt A.F. (1968). Journal of Physical Chemistry 72, 464–470.)
Clever, H. L., and Iwamoto, M. (1987). Solubility of Mercury in Normal Alkanes. Industrial & Engineering Chemistry Research 26, 336–337.
| Solubility of Mercury in Normal Alkanes.Crossref | GoogleScholarGoogle Scholar |
Clever, H. L., Johnson, S. A., and Derrick, M. E. (1985). The Solubility of Mercury and Some Sparingly Soluble Mercury Salts in Water and Aqueous Electrolyte Solutions. Journal of Physical and Chemical Reference Data 14, 631.
| The Solubility of Mercury and Some Sparingly Soluble Mercury Salts in Water and Aqueous Electrolyte Solutions.Crossref | GoogleScholarGoogle Scholar |
NIOSH (2007). Pocket Guide to Chemical Hazards, September 2007. Available at https://www.cdc.gov/niosh/docs/2005-149/pdfs/2005-149.pdf [verified 30 APril 2020]
NOPSEMA (2019) Guidance Note, N04750–GN1344, Revision No.4 April 2019. Available at https://www.nopsema.gov.au/assets/Guidance-notes/A339814.pdf [verified 13 February 2020]
UK HSE (2018). EH40/2005 Workplace Exposure Limits, 3rd Edition. (TSO: London).
UOP (2010). UOP938-10, Total Mercury and Mercury Species in Liquid Hydrocarbons. UOP Honeywell LLC, ASTM International Standards.
Wasson A. Russ P. Asher S. 2013 Mercury Liquid Metal Embrittlement Testing of Various Alloys for Oil and Gas Production. Paper 2519, In ‘Corrosion 2013, 17–21 March, Orlando, Florida, USA.’ (NACE International).
Wilhelm, S. M., and Bloom, N. (2000). Mercury in Petroleum. Fuel Processing Technology 63, 1–27.
| Mercury in Petroleum.Crossref | GoogleScholarGoogle Scholar |
Wilhelm, S.M., and Nelson, M. (2010). Interaction of Elemental Mercury with Steel Surfaces. Journal of Corrosion Science and Engineering 13, Preprint 38..

Peter Crafts graduated with 1st class honours, Bachelor of Chemical Engineering, from University of Teesside UK, in 1992. Peter has over 28 years’ experience as a Chemical Engineer and Technical Safety Engineer, with experience gained in the Oil and Gas, Agrochemicals, and Pharmaceuticals industries, and an established track record of delivering lead roles in Concept Selection, Process Development, FEED, Detailed Design, Commissioning, and R&D for onshore and offshore projects. Peter is the Advanced Simulations Team Manager for Genesis in Aberdeen and is responsible for Genesis Global Mercury Consultancy Services and Mercury R&D Programmes. |

Mark Williams graduated with honours in both a Bachelor of Chemical Engineering and a Bachelor of Science (Inorganic and Analytical Chemistry) from the University of Melbourne, his home city. With 16 years technical and management experience in the oil and gas industry in Australia and Europe, he currently holds the role of Global Systems Manager with Genesis having previously been Australian Process and Facilities Manager. Mark was the industry supervisor for a study in the sensitivities of inlet process conditions to mercury partitioning in offshore facilities and is a focal point for mercury studies in Australia having been involved in multiple mercury processing studies and development initiatives. Mark also held the position of Chair of the Oil and Gas Group for Engineers Australia in WA and is an active member of the governing professional engineering body. |