Relationship between body weight and elevation in Leadbeater’s possum (Gymnobelideus leadbeateri)
Jessica L. Williams A , Dan Harley B , Darcy Watchorn A B C , Lachlan McBurney A and David B. Lindenmayer A *
A , Dan Harley B , Darcy Watchorn A B C , Lachlan McBurney A and David B. Lindenmayer A *
A Fenner School of Environment and Society, The Australian National University, Canberra, ACT 2601, Australia.
B Wildlife Conservation and Science Department, Zoos Victoria, Healesville, Vic. 3777, Australia.
C Centre for Integrative Ecology, School of Life and Environmental Sciences, Deakin University, Burwood, Vic. 3125, Australia.
Australian Journal of Zoology 69(5) 167-174 https://doi.org/10.1071/ZO21042
Submitted: 22 October 2021 Accepted: 2 May 2022 Published: 20 June 2022
© 2021 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
The body size of mammals is influenced by several evolutionary, morphological, physiological and ecological factors. Studies of body size can provide insight into the processes underlying observed variation in patterns of mammal morphology. We sought to determine if body weight in Leadbeater’s possum (Gymnobelideus leadbeateri) is related to environmental variables and/or sex. Using linear regression modelling, we quantified the influence on body weight of broadscale geographic variables such as latitude and elevation, site-level indicators of forest productivity (forest type, slope, aspect and topographic wetness) and an individual-level variable (sex). We found that body weight was significantly associated with elevation and sex, with individuals being heavier at higher elevations and males (on average) being heavier than females. Monitoring body weight changes over time within particular forest types will be valuable, given the variations in temperature and resource productivity throughout the range of Leadbeater’s possum that are likely to arise from climate change.
Keywords: body size, body weight, elevation, Leadbeater’s possum, sex, marsupial, Bergmann’s rule, Victorian Central Highlands.
Introduction
The body size of an organism is a result of inter-relationships between evolutionary, morphological, physiological and ecological processes (Rodríguez et al. 2008; Huang et al. 2017; Roycroft et al. 2020; Weaver and Grossnickle 2020). In mammals, body size is associated with geographic factors, home range size and population density (Cooper and Purvis 2010; Monterroso et al. 2020; Pineda-Munoz et al. 2021). Generally, large-bodied mammals occupy larger home ranges to meet their energetic requirements compared to smaller-bodied animals (Brown and Nicoletto 1991; Agosta et al. 2013). The occupation of larger home ranges generally means that larger animals occur at lower densities and at lower population sizes than smaller-bodied species (Damuth 1981; Fa and Purvis 1997; Damuth 2007). Body size is linked with life traits of a species, such as reproductive rate and longevity (Blueweiss et al. 1978; Promislow and Harvey 1990; Sibly and Brown 2007; Kozłowski et al. 2020). For female placental mammals, reproduction can be energetically costly and individuals with a higher body mass may be more likely to successfully give birth and/or wean young (Lewis and Kappeler 2005; Molnár et al. 2010; Hertel et al. 2018). For males, larger body size is often associated with a higher dominance ranking and, thus, greater reproductive success (Pörschmann et al. 2010; Wright et al. 2019). Larger body size is also often correlated with reproductive success in male mammals (Newbolt et al. 2017). Body size is frequently positively correlated with gestation time, maturation time, and interbirth interval (Blueweiss et al. 1978; Oli 2004). Additionally, smaller-bodied species are less-prone to extinction (Cardillo and Bromham 2001) and have higher rates of molecular evolution due to rapid rates of reproduction, larger litter sizes, higher metabolic rates and larger population sizes (Fontanillas et al. 2007; Bromham 2009; Galtier et al. 2009).
Environmental variables such as temperature, resource seasonality, resource quality, and primary productivity may influence the body size of terrestrial mammals (McNab 2010; Kamilar et al. 2012; Hantak et al. 2021; Ryding et al. 2021). Typically, body size is negatively correlated with environmental temperature (Scholander et al. 1950, Rezende and Bacigalupe 2015; Hantak et al. 2021; Ryding et al. 2021); a relationship known as Bergmann’s rule. Bergmann (1848) proposed that endothermic species living in cooler climates (higher latitude or elevation) tend to be larger than individuals of the same or closely related species in warmer climates (lower latitude or elevation) (Bergmann 1848; Salewski and Watt 2017). Ashton et al. (2000) examined Bergmann’s rule in 110 mammal species from a diverse range of orders, and found that patterns of body weight variation in 71% of taxa fitted predictions arising from the rule. Roycroft et al. (2020) found that body size in endemic Australian rodents is strongly correlated with temperature, consistent with Bergmann’s rule. At lower latitudes (warmer areas) other environmental variables, such as elevation, resource quality and primary productivity can have a stronger effect on mammal body size than Bergmann’s rule (Rodríguez et al. 2008; Freeman 2017; Hendges et al. 2021). For example, Rodríguez et al. (2008) found that mammal body size in the Nearctic and Neotropics, at locations where the mean average temperature was above 10.9°C and 12.6°C respectively, was driven primarily by environmental changes along elevation gradients. However, at higher latitudes, where mean average temperatures fell below these thresholds, mammal body size generally followed Bergmann’s rule (Rodríguez et al. 2008).
The resource availability hypothesis suggests that in habitats where resource availability can fluctuate unpredictably, a larger body size may be advantageous – facilitating the storing of resource reserves to survive extended periods of fasting (Millar and Hickling 1990, 1992). There is discourse in the literature regarding how generalisable this is across mammals, with some species demonstrating this pattern (e.g. the Australian sandy inland mouse [Pseudomys hermannsburgensis]: Tomlinson and Withers 2008) and some other species not exhibiting this (e.g. some native European mammal species in warmer, non-glaciated areas: see Rodríguez et al. 2006). Body size is also associated with resource quality; niches characterised by low-quality food drive selection for larger body sizes with a slower metabolism and longer digestive tract (Belovsky 1997; Tomé et al. 2020). Alternatively, in habitats characterised by low primary productivity, a smaller body size may be selected as there is limited available energy (McNab 2010; Hantak et al. 2021).
In this study, we sought to determine if body weight variation in Leadbeater’s possum (Gymnobelideus leadbeateri) is related to environmental variables and/or sex. Leadbeater’s possum is a small (100–170 g) arboreal marsupial (Lindenmayer 1996) that is endemic to the state of Victoria, Australia. The species lives in matriarchal colonies of up to 12 individuals that are dominated by an adult breeding female (Smith 1984; Harley and Lill 2007). The distribution of Leadbeater’s possums is highly restricted, limited to approximately one degree of latitude and longitude (Lindenmayer et al. 2014). Leadbeater’s possum is listed as Critically Endangered under Australia’s Environmental Protection and Biodiversity Conservation Act (EPBC, Threatened Species Scientific Committee 2019). The species is found primarily in wet montane ash forests and subalpine woodlands, with one outlying, extant and genetically distinct population occupying lowland swamp forest (Lindenmayer 1989; Harley 2004; Hansen et al. 2009; Lindenmayer et al. 2014). These habitats span a 1400-m elevational gradient.
Our overarching question for this investigation was: What factors influence body weight in Leadbeater’s possum? To answer this, we quantified the influence of broad-scale geographic variables (i.e. latitude and elevation), local site-level environmental variables that can influence forest productivity (i.e. slope, aspect, and topographic wetness), and an individual-level variable (i.e. sex). At the outset of this investigation, we made four key predictions:
Prediction 1: There will be no latitude effect as almost the entire distribution of Leadbeater’s possum is currently constrained to one degree of latitude.
Prediction 2: Body weight will increase with elevation. This will be due to the lapse rate, where temperature decreases with increasing elevation (Huggett and Cheesman 2002). The lapse rate varies greatly but averages a decrease of 6.5°C for every 1000 m of elevation gain (Barry 1992).
Prediction 3: Measures of forest productivity (slope, aspect and topographic wetness) and forest type will have little to no effect on body weight in Leadbeater’s possum. We made this prediction because data on net primary productivity in the Victorian Central highlands suggest that values are relatively consistent across montane ash forests and across the region (D. B. Lindenmayer, unpubl. data). Additionally, the highly restricted distribution of Leadbeater’s possum reduces the likely environmental variability the species is subject to.
Prediction 4: There will be a small effect of sex, with males being larger than females. Long-term observations of Leadbeater’s possum colonies and genetic testing suggest that the species is primarily monogamous with limited extrapair mating despite the presence of multiple sexually mature males in some colonies (Smith 1984; Harley et al. 2005). Given that the most pronounced sexual dimorphism towards larger males is typically correlated with polygamous mating systems in mammals (Cassini 2020), we expect any differences in average female and male body weights in Leadbeater’s possum to be small.
Methods
Study species and data collection
Data were collected between 2006 and 2020 from wild Leadbeater’s possums captured at locations throughout their range in the Victorian Central Highlands. No possums included in this study used natural hollows; instead, all possums were captured from artificial nest boxes during the day. This was an important variable to control as temperatures inside nest boxes are known to fluctuate more than inside naturally occurring hollows (Rowland et al. 2017; McComb et al. 2021), potentially making it more difficult for the animals using them to remain within their thermo-neutral zone (Hardy and DuBois 1937; Rubner 1982; IUPS Thermal Commission 2001). Extended exposure to temperatures outside this zone increases an individual’s thermoregulatory energy requirements and may have significant impacts on body size (Lovegrove 2005). We restricted our analyses to body weight measurements from adult individuals of a known sex. We excluded females with pouch young (N = 19) as the additional weight of the pouch young will vary depending on developmental stage, which was unknown. Our final dataset for analysis included 135 (female = 52, male = 83) Leadbeater’s possum body weight measurements collected from 63 nest boxes distributed across the species’ range (Fig. 1). The number of individuals measured from each nest box varied from one to seven, with an average number of 2.1 individuals sampled from a given nest box.
|
|
Measurement of covariates
We included topographic measurements and forest type as potential explanatory variables in modelling body weight variation. We used LiDAR data provided by the Victorian Government Department of Environment, Land, Water and Planning (DELWP 2019) to determine the elevation (mASL), slope (degree), aspect (degree) and topographical wetness index of nest box locations (Table 1). We extracted data on elevation from a digital elevation model (DEM) generated using the Victorian Government’s LiDAR data (DELWP 2019) at a 5-m resolution. We extracted slope and aspect information from raster layers at a 5-m resolution and topographical wetness index from a raster layer at a 10-m resolution.

|
We determined forest type using the forest information portal on the DELWP interactive mapping tools website (https://www.land.vic.gov.au/maps-and-spatial/maps/interactive-mapping-tools). We plotted nest box locations onto the map provided in the portal and determined the forest type at that location using the ‘ecological vegetation class’ map layer. Three of the nest boxes where possums were captured were in rainforest, five in riparian woodlands, 14 in wet or damp forest, and 41 in subalpine woodland.
Statistical analysis
Prior to constructing regression models, we checked for correlation between sampling effort and body weight variation (without our potential predictor variables). We found a significant correlation between the number of individuals sampled from a nest box and variation in body weight (t = 8.26, d.f. = 62, P < 0.01, r = 0.72). Accordingly, we elected not to include this variable in our model. Possum body weight data were obtained from multiple projects and, consequently, sampling month varied between years. This, coupled with previously documented seasonal variations in body weight (Smith 1980), precluded examination of the effect of year on body weight. When month and forest type were included in the regression model, they were found to be collinear. We found a high level of correlation between the number of individuals sampled within a given month and variation in body weight (t = 2.16, d.f. = 9, P = 0.059, r = 0.58). However, lower levels of correlation were found between number of individuals sampled within a forest type and variation in body weight (t = 0.49, d.f. = 2, P = 0.67, r = 0.33). For this reason, we elected to include forest type rather than month as a predictor variable in the model.
We constructed linear regression models of the factors influencing body weight using the lm function in R (R Core Team 2021). We modelled latitude, elevation, slope, aspect, topographical wetness index, and sex as predictor variables in our global model (Table S1). We assessed the model residuals by plotting a histogram, scatter plot, and qq-plot. We reduced the number of covariates in the model using the stepAIC function from the MASS package (Venables and Ripley 2002), so that only the significant predictor variables were included in the best-fit model (Table S2). We completed post hoc analysis on the relationship between body weight and our best-fit predictor variables using the emmeans package (Lenth 2020).
Results
The average adult male Leadbeater’s possum weighed 137 g (95% CI = 135 g, 139 g), with 90% of weights between 122 and 153 g. The average adult female weighed 133 g (95% CI = 130 g, 135 g), with 90% of weights falling between 120 and 146 g. At our lowest elevation site (653 m above sea level), average female weight was 121.5 g and average male weight 125.5 g, and at our highest elevation site (1541 m above sea level) average weights were 138.3 g and 142.3 g, respectively.
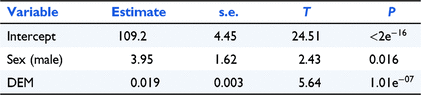
We found that variation in body weight was best explained by elevation and sex (adjusted R2 = 0.2, F = 18.21, d.f. = 2,132, P < 0.01: Table 2). There was a significant positive relationship between elevation and body weight (β = 0.02, s.e. = 0.003, P < 0.01), with average body weight increasing by 1.9 g for every 100-m increase in elevation (Fig. 2). We also found that adult males weighed significantly more than adult females (β = 3.68, s.e. = 1.63, P = 0.025). We found no effects of other potential explanatory variables (latitude and forest productivity).
|
Discussion
Numerous mammal species are characterised by marked spatial variation in body size (Huang et al. 2017). We tested four predictions associated with potential factors influencing body weight in Leadbeater’s possum. We found evidence for the effect of elevation (Prediction 2) and sex (Prediction 4) in our analysis, but no effect of latitude (Prediction 1), forest type, or measures of site productivity (slope, aspect and topographic wetness) (Prediction 3).
Environmental effects
The elevation of our sample sites varied from 653 to 1541 m above sea level, and, consistent with Bergmann’s Rule (and Prediction 2), we found that larger body weight animals occurred at higher elevations (Fig. 2). This is likely to be a physiological response to local conditions, particularly temperature. Based on the average temperature lapse rate, we estimate that the temperature difference is approximately 5.8°C between the sites with the lowest and highest elevations (which receive seasonal snow cover) (Barry 1992). In addition to behavioural strategies, such as the construction of larger nests at colder (higher elevation) sites (D. Harley, pers. obs.), Leadbeater’s possum at higher elevations may need to be bigger than individuals at lower elevations to remain primarily within their thermo-neutral zone. Larger individuals have a greater volume to surface area ratio, resulting in a reduced rate of heat loss (Scholander et al. 1950; Rezende and Bacigalupe 2015). In contrast, smaller Leadbeater’s possums at lower elevations may lose heat more rapidly, and thereby can remain cooler at warmer temperatures (Conley and Porter 1986; Guiden and Orrock 2020). As expected, we did not find an effect of latitude on body weight as stipulated by Bergmann’s Rule, likely due to the latitudinal distribution of the Leadbeater’s possum being limited to one degree (Lindenmayer et al. 2014).
We did not find a relationship between body weight and slope, aspect, or topographical wetness index – all predictors of primary productivity (Singh 2018; Dyderski and Pawlik 2020) – or forest type (Prediction 3). It is possible that this outcome is related to limited site-level variation in productivity across the sampled sites, which is quite plausible across such a restricted geographic area and consistent with observations (D. B. Lindenmayer, unpubl. data).
Effect of individual’s sex
We found adult male Leadbeater’s possums were, on average, 4 g heavier than adult females (consistent with Prediction 4). Morphological differences between males and females are driven by many factors including reproductive strategy, parental investment, habitat selection, and population density (Ralls 1977; Hedrick and Temeles 1989; Mori et al. 2017). Leadbeater’s possums live in colonies comprising a monogamous mating pair, their subadult offspring and two or more unrelated non-breeding adult males (Smith 1984; Lindenmayer 1996; Harley and Lill 2007). Our finding, that adults males are only slightly (<4%) heavier than adult females, is broadly consistent with the rule that the degree of sexual dimorphism is positively correlated with degree of polygyny in mammalian mating systems (Cassini 2020).
There are several (potentially interacting) explanations for female Leadbeater’s possums being smaller than males. One possibility is that it is part of their evolutionary history, with other species of Petauridae, including the sugar glider (P. breviceps), Krefft’s glider (P. notatus) and, to a lesser extent, the yellow-bellied glider (P. australis), being characterised by larger males than females (Quin et al. 1996; Goldingay and Jackson 2004). Males being larger could also be a result of the intense energetic costs females experience to produce up to two litters of offspring per year (Tyndale-Biscoe 2005). Reproduction is the most energetically demanding life-history process that female mammals experience, with females reaching their maximum metabolic rate and energetic cost during lactation (Gittleman and Thompson 1988; Schai-Braun et al. 2021). An extreme example of this cost occurs in harbour seals (Phoca vitulina), females of which lose more than 50% of their stored body fat while lactating (Bowen et al. 1992). Differences in body weight also could reflect selection pressures relating to male–male competition (Mori et al. 2017; Wright et al. 2019) or female mate choice (Clutton-Brock and McAuliffe 2009). However, adult female Leadbeater’s possums are highly territorial and aggressive towards breeding competitors (Smith 1984), so it is possible that selection related to the reproductive strategy is also acting on males. This explanation could account for the relatively small weight difference between the sexes.
Conclusion and future research
Our results indicate that the body weight of Leadbeater’s possums is, to some extent, driven by elevation and sex. Further research to understand the environmental drivers of Leadbeater’s possum body weight at a local scale may allow for body weight to be used as an indicator for monitoring environmental change over time. Due to variation in the months that body weight data were available across years we were unable to test for an effect of time. Body size is known to change over time in response to environmental variables. For example, Roycroft et al. (2020) found that the body size of 31 species of Australian rodents in the genus Pseudomys changed with the climate during the Pleistocene Epoch, in accordance with Bergmann’s rule. The body weight of Leadbeater’s possums varies by up to 30 g between autumn (when animals are heaviest) and spring (Smith 1980; Lindenmayer 1996). It would be interesting and informative to test whether the body weight of Leadbeater’s possums has changed between years (e.g. in response to changing climate). This would require data being collected either exclusively during a particular season or sampling spread evenly throughout the year. A prediction arising from this study is that as temperatures increase at higher elevations in response to climate change, the average body weight of adult possums at those locations would be expected to decrease over time.
Supplementary material
Supplementary material is available online.
Data avaliability
Data are available at DOI: https://doi.org/10.17632/8cydbdhkjp.1.
Conflicts of interest
The authors have no conflicts of interest to report.
Declaration of funding
This research did not receive any specific funding.
Acknowledgements
We acknowledge the Taungurung, Gunaikurnai and Wurundjeri People of the Kulin Nation, upon whose lands we conducted our study. We pay our respects to their Elders past and present. Chris Taylor assisted with accessing the LiDAR data, producing the digital elevation model and creating the map for Fig. 1.
References
Agosta, SJ, Bernardo, J, Ceballos, G, and Steele, MA (2013). A macrophysiological analysis of energetic constraints on geographic range size in mammals. PLoS ONE 8, e72731.| A macrophysiological analysis of energetic constraints on geographic range size in mammals.Crossref | GoogleScholarGoogle Scholar | 24058444PubMed |
Ashton, KG, Tracy, MC, and Queiroz, AD (2000). Is Bergmann’s rule valid for mammals? The American Naturalist 156, 390–415.
| Is Bergmann’s rule valid for mammals?Crossref | GoogleScholarGoogle Scholar | 29592141PubMed |
Barry RG (1992) ‘Mountain Weather and Climate.’ (Taylor & Francis: New York USA)
Belovsky, GE (1997). Optimal foraging and community structure: the allometry of herbivore food selection and competition. Evolutionary Ecology 11, 641–672.
| Optimal foraging and community structure: the allometry of herbivore food selection and competition.Crossref | GoogleScholarGoogle Scholar |
Bergmann C (1848) ‘Über die Verhältnisse der Wärmeökonomie der Thiere zu ihrer Grösse.’ (Göttingen University Press: Göttingen, Germany)
Blueweiss, L, Fox, H, Kudzma, V, Nakashima, D, Peters, R, and Sams, S (1978). Relationships between body size and some life history parameters. Oecologia 37, 257–272.
| Relationships between body size and some life history parameters.Crossref | GoogleScholarGoogle Scholar | 28309655PubMed |
Bowen, WD, Oftedal, OT, and Boness, DJ (1992). Mass and energy transfer during lactation in a small phocid, the harbor seal (Phoca vitulina). Physiological Zoology 65, 844–866.
| Mass and energy transfer during lactation in a small phocid, the harbor seal (Phoca vitulina).Crossref | GoogleScholarGoogle Scholar |
Bromham, L (2009). Why do species vary in their rate of molecular evolution? Biology Letters 5, 401–404.
| Why do species vary in their rate of molecular evolution?Crossref | GoogleScholarGoogle Scholar | 19364710PubMed |
Brown, JH, and Nicoletto, PF (1991). Spatial scaling of species composition: body masses of North American land mammals. The American Naturalist 138, 1478–1512.
| Spatial scaling of species composition: body masses of North American land mammals.Crossref | GoogleScholarGoogle Scholar |
Cardillo, M, and Bromham, L (2001). Body size and risk of extinction in Australian mammals. Conservation Biology 15, 1435–1440.
| Body size and risk of extinction in Australian mammals.Crossref | GoogleScholarGoogle Scholar |
Cassini, MH (2020). A mixed model of the evolution of polygyny and sexual size dimorphism in mammals. Mammal Review 50, 112–120.
| A mixed model of the evolution of polygyny and sexual size dimorphism in mammals.Crossref | GoogleScholarGoogle Scholar |
Clutton-Brock, T, and McAuliffe, K (2009). Female mate choice in mammals. The Quarterly review of Biology 84, 3–27.
| Female mate choice in mammals.Crossref | GoogleScholarGoogle Scholar | 19326786PubMed |
Conley, KE, and Porter, WP (1986). Heat loss from deer mice (Peromyscus): evaluation of seasonal limits to thermoregulation. Journal of Experimental Biology 126, 249–269.
| Heat loss from deer mice (Peromyscus): evaluation of seasonal limits to thermoregulation.Crossref | GoogleScholarGoogle Scholar | 3805994PubMed |
Cooper, N, and Purvis, A (2010). Body size evolution in mammals: complexity in tempo and mode. The American Naturalist 175, 727–738.
| Body size evolution in mammals: complexity in tempo and mode.Crossref | GoogleScholarGoogle Scholar | 20394498PubMed |
Damuth, J (1981). Population density and body size in mammals. Nature 290, 699–700.
| Population density and body size in mammals.Crossref | GoogleScholarGoogle Scholar |
Damuth, J (2007). A macroevolutionary explanation for energy equivalence in the scaling of body size and population density. The American Naturalist 169, 621–631.
| A macroevolutionary explanation for energy equivalence in the scaling of body size and population density.Crossref | GoogleScholarGoogle Scholar | 17427133PubMed |
DELWP (2019) 2015–16 Central Highlands LiDAR Project. Victorian Department of Environment, Land, Water and Planning, Melbourne, Vic., Australia.
Dyderski, MK, and Pawlik, Ł (2020). Spatial distribution of tree species in mountain national parks depends on geomorphology and climate. Forest Ecology and Management 474, 118366.
| Spatial distribution of tree species in mountain national parks depends on geomorphology and climate.Crossref | GoogleScholarGoogle Scholar |
Fa, JE, and Purvis, A (1997). Body size, diet and population density in Afrotropical forest mammals: a comparison with Neotropical species. Journal of Animal Ecology , 98–112.
| Body size, diet and population density in Afrotropical forest mammals: a comparison with Neotropical species.Crossref | GoogleScholarGoogle Scholar |
Fontanillas, E, Welch, JJ, Thomas, JA, and Bromham, L (2007). The influence of body size and net diversification rate on molecular evolution during the radiation of animal phyla. BMC Evolutionary Biology 7, 95.
| The influence of body size and net diversification rate on molecular evolution during the radiation of animal phyla.Crossref | GoogleScholarGoogle Scholar | 17592650PubMed |
Freeman, BG (2017). Little evidence for Bergmann’s rule body size clines in passerines along tropical elevational gradients. Journal of Biogeography 44, 502–510.
| Little evidence for Bergmann’s rule body size clines in passerines along tropical elevational gradients.Crossref | GoogleScholarGoogle Scholar |
Galtier, N, Jobson, RW, Nabholz, B, Glémin, S, and Blier, PU (2009). Mitochondrial whims: metabolic rate, longevity and the rate of molecular evolution. Biology Letters 5, 413–416.
| Mitochondrial whims: metabolic rate, longevity and the rate of molecular evolution.Crossref | GoogleScholarGoogle Scholar | 19324654PubMed |
Gittleman, JL, and Thompson, SD (1988). Energy allocation in mammalian reproduction. American Zoologist 28, 863–875.
| Energy allocation in mammalian reproduction.Crossref | GoogleScholarGoogle Scholar |
Goldingay R, Jackson S (2004) A review of the ecology of the Australia Petauridae. In ‘The Biology of Australian Possums and Gliders’. (Eds R Goldingay, S Jackson) pp. 376–400. (Surrey Beatty and Sons: Sydney, NSW, Australia)
Guiden, PW, and Orrock, JL (2020). Seasonal shifts in activity timing reduce heat loss of small mammals during winter. Animal Behaviour 164, 181–192.
| Seasonal shifts in activity timing reduce heat loss of small mammals during winter.Crossref | GoogleScholarGoogle Scholar |
Hansen, BD, Harley, DKP, Lindenmayer, DB, and Taylor, AC (2009). Population genetic analysis reveals a long-term decline of a threatened endemic Australian marsupial. Molecular Ecology 18, 3346–3362.
| Population genetic analysis reveals a long-term decline of a threatened endemic Australian marsupial.Crossref | GoogleScholarGoogle Scholar | 19694962PubMed |
Hantak, MM, McLean, BS, Li, D, and Guralnick, RP (2021). Mammalian body size is determined by interactions between climate, urbanization, and ecological traits. Communications Biology 4, 972.
| Mammalian body size is determined by interactions between climate, urbanization, and ecological traits.Crossref | GoogleScholarGoogle Scholar | 34400755PubMed |
Hardy, JD, and DuBois, EF (1937). Regulation of heat loss from the human body. Proceedings of the National Academy of Sciences of the United States of America 23, 624–631.
| Regulation of heat loss from the human body.Crossref | GoogleScholarGoogle Scholar | 16577831PubMed |
Harley DK (2004) A review of recent records of Leadbeater’s possum (Gymnobelideus leadbeateri). In ‘The Biology of Australian Possums and Gliders’. (Eds R Goldingay, S Jackson) pp. 330–338. (Surrey Beatty and Sons: Sydney, NSW, Australia)
Harley, DKP, and Lill, A (2007). Reproduction in a population of the endangered Leadbeater’s possum inhabiting lowland swamp forest. Journal of Zoology 272, 451–457.
| Reproduction in a population of the endangered Leadbeater’s possum inhabiting lowland swamp forest.Crossref | GoogleScholarGoogle Scholar |
Harley, DKP, Worley, MA, and Harley, TK (2005). The distribution and abundance of Leadbeater’s possum Gymnobelideus leadbeateri in lowland swamp forest at Yellingbo Nature Conservation Reserve. Australian Mammalogy 27, 7–15.
| The distribution and abundance of Leadbeater’s possum Gymnobelideus leadbeateri in lowland swamp forest at Yellingbo Nature Conservation Reserve.Crossref | GoogleScholarGoogle Scholar |
Hedrick, AV, and Temeles, EJ (1989). The evolution of sexual dimorphism in animals: hypotheses and tests. Trends in Ecology & Evolution 4, 136–138.
| The evolution of sexual dimorphism in animals: hypotheses and tests.Crossref | GoogleScholarGoogle Scholar |
Hendges, CD, Patterson, BD, and Cáceres, NC (2021). Big in the tropics: ecogeographical clines in peccary size reveal the converse of Bergmann’s rule. Journal of Biogeography 48, 1228–1239.
| Big in the tropics: ecogeographical clines in peccary size reveal the converse of Bergmann’s rule.Crossref | GoogleScholarGoogle Scholar |
Hertel, AG, Bischof, R, Langval, O, Mysterud, A, Kindberg, J, Swenson, JE, and Zedrosser, A (2018). Berry production drives bottom-up effects on body mass and reproductive success in an omnivore. Oikos 127, 197–207.
| Berry production drives bottom-up effects on body mass and reproductive success in an omnivore.Crossref | GoogleScholarGoogle Scholar |
Huang, S, Eronen, JT, Janis, CM, Saarinen, JJ, Silvestro, D, and Fritz, SA (2017). Mammal body size evolution in North America and Europe over 20 Myr: similar trends generated by different processes. Proceedings of the Royal Society B: Biological Sciences 284, 20162361.
| Mammal body size evolution in North America and Europe over 20 Myr: similar trends generated by different processes.Crossref | GoogleScholarGoogle Scholar | 28202809PubMed |
Huggett RJ, Cheesman J (2002) ‘Topography and the Environment.’ (Pearson Education: Boston, MA, USA)
(2001). Glossary of terms for thermal physiology. Japan Journal of Physiology 51, 245–280.
Kamilar, JM, Muldoon, KM, Lehman, SM, and Herrera, JP (2012). Testing Bergmann’s rule and the resource seasonality hypothesis in Malagasy primates using GIS-based climate data. American Journal of Physical Anthropology 147, 401–408.
| Testing Bergmann’s rule and the resource seasonality hypothesis in Malagasy primates using GIS-based climate data.Crossref | GoogleScholarGoogle Scholar | 22271559PubMed |
Kozłowski, J, Konarzewski, M, and Czarnoleski, M (2020). Coevolution of body size and metabolic rate in vertebrates: a life-history perspective. Biological Reviews 95, 1393–1417.
| Coevolution of body size and metabolic rate in vertebrates: a life-history perspective.Crossref | GoogleScholarGoogle Scholar | 32524739PubMed |
Lenth R (2020) emmeans: Estimated Marginal Means, aka Least-Squares Means. R package. https://cran.r-project.org/web/packages/emmeans/index.html
Lewis, RJ, and Kappeler, PM (2005). Seasonality, body condition, and timing of reproduction in Propithecus verreauxi verreauxi in the Kirindy Forest. American Journal of Primatology 67, 347–364.
| Seasonality, body condition, and timing of reproduction in Propithecus verreauxi verreauxi in the Kirindy Forest.Crossref | GoogleScholarGoogle Scholar | 16287105PubMed |
Lindenmayer DB (1989) The ecology and habitat requirements of Leadbeater’s possum. PhD Thesis, Australian National University, Canberra, ACT, Australia.
Lindenmayer DB (1996) ‘Wildlife and Woodchips: Leadbeater’s Possum: a Test Case for Sustainable Forestry.’ (UNSW Press: Sydney, NSW, Australia)
Lindenmayer, DB, Blair, D, McBurney, L, and Banks, S (2014). Preventing the extinction of an iconic globally endangered species – Leadbeater’s possum (Gymnobelideus leadbeateri). Journal of Biodiversity & Endangered Species 2, 140–147.
| Preventing the extinction of an iconic globally endangered species – Leadbeater’s possum (Gymnobelideus leadbeateri).Crossref | GoogleScholarGoogle Scholar |
Lovegrove, BG (2005). Seasonal thermoregulatory responses in mammals. Journal of Comparative Physiology Biology 175, 231–247.
| Seasonal thermoregulatory responses in mammals.Crossref | GoogleScholarGoogle Scholar |
McComb, LB, Lentini, PE, Harley, DKP, Lumsden, LF, Eyre, AC, and Briscoe, NJ (2021). Climate and behaviour influence thermal suitability of artificial hollows for a critically endangered mammal. Animal Conservation , .
| Climate and behaviour influence thermal suitability of artificial hollows for a critically endangered mammal.Crossref | GoogleScholarGoogle Scholar |
McNab, BK (2010). Geographic and temporal correlations of mammalian size reconsidered: a resource rule. Oecologia 164, 13–23.
| Geographic and temporal correlations of mammalian size reconsidered: a resource rule.Crossref | GoogleScholarGoogle Scholar | 20364270PubMed |
Millar, JS, and Hickling, GJ (1990). Fasting endurance and the evolution of mammalian body size. Functional Ecology 4, 5–12.
| Fasting endurance and the evolution of mammalian body size.Crossref | GoogleScholarGoogle Scholar |
Millar, JS, and Hickling, GJ (1992). The fasting endurance hypothesis revisited. Functional Ecology 6, 496–498.
Molnár, PK, Derocher, AE, Thiemann, GW, and Lewis, MA (2010). Predicting survival, reproduction and abundance of polar bears under climate change. Biological Conservation 143, 1612–1622.
| Predicting survival, reproduction and abundance of polar bears under climate change.Crossref | GoogleScholarGoogle Scholar |
Monterroso, P, Díaz-Ruiz, F, Lukacs, PM, Alves, PC, and Ferreras, P (2020). Ecological traits and the spatial structure of competitive coexistence among carnivores. Ecology 101, e03059.
| Ecological traits and the spatial structure of competitive coexistence among carnivores.Crossref | GoogleScholarGoogle Scholar | 32333382PubMed |
Mori E, Mazza G, Lovari S (2017) Sexual dimorphism. In ‘Encyclopedia of Animal Cognition and Behavior’. (Eds J Vonk, T Shakelford.) pp. 1–7. (Springer International Publishing, Switzerland)
Newbolt, CH, Acker, PK, Neuman, TJ, Hoffman, SI, Ditchkoff, SS, and Steury, TD (2017). Factors influencing reproductive success in male white-tailed deer. The Journal of Wildlife Management 81, 206–217.
| Factors influencing reproductive success in male white-tailed deer.Crossref | GoogleScholarGoogle Scholar |
Oli, MK (2004). The fast–slow continuum and mammalian life-history patterns: an empirical evaluation. Basic and Applied Ecology 5, 449–463.
| The fast–slow continuum and mammalian life-history patterns: an empirical evaluation.Crossref | GoogleScholarGoogle Scholar |
Pineda-Munoz, S, Jukar, AM, Tóth, AB, Fraser, D, Du, A, Barr, WA, Amatangelo, KL, Balk, MA, Behrensmeyer, AK, Blois, J, Davis, M, Eronen, JT, Gotelli, NJ, Looy, C, Miller, JH, Shupinski, AB, Soul, LC, Villaseñor, A, Wing, S, and Lyons, SK (2021). Body mass-related changes in mammal community assembly patterns during the late Quaternary of North America. Ecography 44, 56–66.
| Body mass-related changes in mammal community assembly patterns during the late Quaternary of North America.Crossref | GoogleScholarGoogle Scholar |
Pörschmann, U, Trillmich, F, Mueller, B, and Wolf, JBW (2010). Male reproductive success and its behavioural correlates in a polygynous mammal, the Galapagos sea lion (Zalophus wollebaeki). Molecular Ecology 19, 2574–2586.
| Male reproductive success and its behavioural correlates in a polygynous mammal, the Galapagos sea lion (Zalophus wollebaeki).Crossref | GoogleScholarGoogle Scholar | 20497325PubMed |
Promislow, DEL, and Harvey, PH (1990). Living fast and dying young: a comparative analysis of life-history variation among mammals. Journal of Zoology 220, 417–437.
| Living fast and dying young: a comparative analysis of life-history variation among mammals.Crossref | GoogleScholarGoogle Scholar |
Quin, DG, Smith, AP, and Norton, TW (1996). Eco-geographic variation in size and sexual dimorphism in sugar gliders and squirrel gliders (Marsupialia: Petauridae). Australian Journal of Zoology 44, 19–45.
| Eco-geographic variation in size and sexual dimorphism in sugar gliders and squirrel gliders (Marsupialia: Petauridae).Crossref | GoogleScholarGoogle Scholar |
Ralls, K (1977). Sexual dimorphism in mammals: avian models and unanswered questions. The American Naturalist 111, 917–938.
| Sexual dimorphism in mammals: avian models and unanswered questions.Crossref | GoogleScholarGoogle Scholar |
R Core Team (2021) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria.
Rezende, EL, and Bacigalupe, LD (2015). Thermoregulation in endotherms: physiological principles and ecological consequences. Journal of Comparative Physiology Biology 185, 709–727.
| Thermoregulation in endotherms: physiological principles and ecological consequences.Crossref | GoogleScholarGoogle Scholar |
Rodríguez, MÁ, López-Sañudo, IL, and Hawkins, BA (2006). The geographic distribution of mammal body size in Europe. Global Ecology and Biogeography 15, 173–181.
| The geographic distribution of mammal body size in Europe.Crossref | GoogleScholarGoogle Scholar |
Rodríguez, MÁ, Olalla-Tárraga, MÁ, and Hawkins, BA (2008). Bergmann’s rule and the geography of mammal body size in the Western Hemisphere. Global Ecology and Biogeography 17, 274–283.
| Bergmann’s rule and the geography of mammal body size in the Western Hemisphere.Crossref | GoogleScholarGoogle Scholar |
Rowland, JA, Briscoe, NJ, and Handasyde, KA (2017). Comparing the thermal suitability of nest-boxes and tree-hollows for the conservation-management of arboreal marsupials. Biological Conservation 209, 341–348.
| Comparing the thermal suitability of nest-boxes and tree-hollows for the conservation-management of arboreal marsupials.Crossref | GoogleScholarGoogle Scholar |
Roycroft, EJ, Nations, JA, and Rowe, KC (2020). Environment predicts repeated body size shifts in a recent radiation of Australian mammals. Evolution 74, 671–680.
| Environment predicts repeated body size shifts in a recent radiation of Australian mammals.Crossref | GoogleScholarGoogle Scholar | 31595503PubMed |
Rubner M (1982) ‘The Laws of Energy Consumption in Nutrition.’ (Academic Press, Inc.: London, UK)
Ryding, S, Klaassen, M, Tattersall, GJ, Gardner, JL, and Symonds, MRE (2021). Shape-shifting: changing animal morphologies as a response to climatic warming. Trends in Ecology & Evolution 36, 1036–1048.
| Shape-shifting: changing animal morphologies as a response to climatic warming.Crossref | GoogleScholarGoogle Scholar |
Salewski, V, and Watt, C (2017). Bergmann’s rule: a biophysiological rule examined in birds. Oikos 126, 161–172.
| Bergmann’s rule: a biophysiological rule examined in birds.Crossref | GoogleScholarGoogle Scholar |
Schai-Braun, SC, Steiger, P, Ruf, T, Arnold, W, and Hackländer, K (2021). Maternal effects on reproduction in the precocial European hare (Lepus europaeus). PLoS ONE 16, e0247174.
| Maternal effects on reproduction in the precocial European hare (Lepus europaeus).Crossref | GoogleScholarGoogle Scholar | 33596263PubMed |
Scholander, PF, Hock, R, Walters, V, Johnson, F, and Irving, L (1950). Heat regulation in some arctic and tropical mammals and birds. The Biological Bulletin 99, 237–258.
| Heat regulation in some arctic and tropical mammals and birds.Crossref | GoogleScholarGoogle Scholar | 14791422PubMed |
Sibly, RM, and Brown, JH (2007). Effects of body size and lifestyle on evolution of mammal life histories. Proceedings of the National Academy of Sciences of the United States of America 104, 17707–17712.
| Effects of body size and lifestyle on evolution of mammal life histories.Crossref | GoogleScholarGoogle Scholar | 17940028PubMed |
Singh, S (2018). Understanding the role of slope aspect in shaping the vegetation attributes and soil properties in montane ecosystems. Tropical Ecology 59, 417–430.
Smith A (1984) Demographic consequences of reproduction, dispersal and social interaction in a population of Leadbeater’s possum (Gymnobelideus leadbeateri). In ‘Possums and Gliders’. (Eds A Smith, I Hume) pp. 359–373. (Surrey Beatty & Sons Pty Ltd: Sydney, NSW, Australia)
Smith AP (1980) The diet and ecology of Leadbeater’s possum and the sugar glider. PhD thesis, Monash University, Melbourne, Vic., Australia.
Threatened Species Scientific Committee (2019) Conservation advice Gymnobelideus leadbeateri Leadbeater’s possum. Department of the Environment and Energy, Canberra, Australia.
Tomé, CP, Smith, EA, Lyons, SK, Newsome, SD, and Smith, FA (2020). Changes in the diet and body size of a small herbivorous mammal (hispid cotton rat, Sigmodon hispidus) following the late Pleistocene megafauna extinction. Ecography 43, 604–619.
| Changes in the diet and body size of a small herbivorous mammal (hispid cotton rat, Sigmodon hispidus) following the late Pleistocene megafauna extinction.Crossref | GoogleScholarGoogle Scholar |
Tomlinson, S, and Withers, PC (2008). Biogeographical effects on body mass of native Australian and introduced mice, Pseudomys hermannsburgensis and Mus domesticus: an inquiry into Bergmann’s Rule. Australian Journal of Zoology 56, 423–430.
| Biogeographical effects on body mass of native Australian and introduced mice, Pseudomys hermannsburgensis and Mus domesticus: an inquiry into Bergmann’s Rule.Crossref | GoogleScholarGoogle Scholar |
Tyndale-Biscoe H (2005) ‘Life of Marsupials.’ (CSIRO Publishing: Melbourne, Victoria, Australia)
Venables W, Ripley B (2002) ‘Modern Applied Statistics with S.’ 4th edn. (Springer: New York, USA)
Weaver, LN, and Grossnickle, DM (2020). Functional diversity of small-mammal postcrania is linked to both substrate preference and body size. Current Zoology 66, 539–553.
| Functional diversity of small-mammal postcrania is linked to both substrate preference and body size.Crossref | GoogleScholarGoogle Scholar | 33293932PubMed |
Wright, E, Galbany, J, McFarlin, SC, Ndayishimiye, E, Stoinski, TS, and Robbins, MM (2019). Male body size, dominance rank and strategic use of aggression in a group-living mammal. Animal Behaviour 151, 87–102.
| Male body size, dominance rank and strategic use of aggression in a group-living mammal.Crossref | GoogleScholarGoogle Scholar |


