Female mate choice in the fat-tailed dunnart (Sminthopsis crassicaudata) influences offspring sex ratio, but not fecundity
Brittney P. Gill A * , Amy M. Edwards
A * , Amy M. Edwards  A B C , Marissa L. Parrott D , Emily L. Scicluna
A B C , Marissa L. Parrott D , Emily L. Scicluna  A and Kylie A. Robert
A and Kylie A. Robert  A E
A E
A School of Agriculture, Biomedicine & Environment, La Trobe University, Melbourne, Vic. 3086, Australia.
B New South Wales National Parks and Wildlife Service, Bathurst, NSW 2795, Australia.
C University of New England, Armidale, NSW 2350, Australia.
D Wildlife Conservation and Science, Zoos Victoria, Parkville, Vic. 3052, Australia.
E Research Centre for Future Landscapes, La Trobe University, Melbourne, Vic. 3086, Australia.
Australian Journal of Zoology 70(5) 133-141 https://doi.org/10.1071/ZO22028
Submitted: 22 October 2021 Accepted: 21 March 2023 Published: 17 May 2023
© 2022 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Obtaining a suitable mate is an integral part of reproduction, with sexual selection processes such as female mate choice resulting in both direct and/or indirect benefits. Here, we investigated whether olfactory driven female mate choice influenced reproductive success in captive fat-tailed dunnarts (Sminthopsis crassicaudata). Although females spent 67% more time with a preferred male’s scent, reproductive success was not influenced by assigned mate choice. Of the 10 (of 12) litters that survived to weaning, average litter size was higher for non-preferred pairings (3 ± 0.83) compared with preferred pairings (1.6 ± 0.60), with weaning success equal for both pairings. Analyses suggested that offspring sex ratios were influenced by mate choice, with females paired with their preferred mate having more daughters (81%) in comparison to non-preferred pairings (44%) and by paternal body condition, whereby females paired with males in better body condition produced more sons. In this species, altering offspring sex ratios in favour of daughters may be achieved by pairing with preferred males, and towards sons by pairing with males in better body condition. With the increasing need for captive breeding programs, these techniques may provide opportunities to correct sex ratio biases and incorporate natural mating systems into conservation programs.
Keywords: captive breeding, conservation, dasyurid, female mate choice, life history, marsupial, olfactory cues, reproductive output, sex ratios.
Introduction
Obtaining a suitable mate is an integral part of reproduction, with favourable traits that enhance an individual’s access to mates evolving as sexual selection (Darwin 1859). Sexual selection can occur through the competition for mates via courtship displays, resource or territorial acquisition, combat, or mate choice, and is observed in both sexes (Mays and Hill 2004). However, mate choice is more often displayed by females (Mays and Hill 2004), with females in most taxa being the ‘choosier’ sex due to a relatively higher parental investment (Panhuis et al. 2001). Female mate choice may arise through direct benefits to females, for example through increased fecundity and parental care (Andersson 1994), or indirect benefits to offspring, such as the inheritance of attractiveness (e.g. ‘sexy sons’ hypothesis: Fisher 1930), or good/compatible genes (Chargé et al. 2014). An increasing number of studies show that females have consistent mating preferences for males that appear likely to generate fitness benefits to the females themselves or to their offspring (Drickamer et al. 2000; Jennions and Petrie 2000; Roberts and Gosling 2003). For example, female mate choice influences copulation success and litter size in the Columbia Basin pygmy rabbit (Brachylagus idahoensis) (Martin and Shepherdson 2012), and increases offspring survivorship in house mice (Mus musculus) (Drickamer et al. 2000).
Females are able to discriminate males based on acoustic signals (Reby et al. 2001), visual signals (Møller 1988; Sato et al. 2014), or a combination of cues (Candolin 2003; Voigt et al. 2008), but mammals most commonly use olfactory signals (Rich and Hurst 1998; Roberts and Gosling 2003; Johansson and Jones 2007). Olfactory signals have attracted considerable attention in species recognition and female mate choice, with some species being able to assess the genetic compatibility (Parrott et al. 2007), including the major histocompatibility complex (Penn and Potts 1999; Landry et al. 2001; Charpentier et al. 2008) and health status (Penn and Potts 1999) of potential mates. Using olfactory cues in marsupial mate choice studies, such as of the agile antechinus (Antechinus agilis) (Parrott et al. 2007, 2015), stripe-faced dunnart (Smithopsis macroura) (Parrott et al. 2019) and eastern barred bandicoot (Perameles gunnii) (Hartnett et al. 2018), have also demonstrated olfactory cues to be a useful method for mate choice studies. Studies of these species demonstrated that females in oestrus can distinguish between genetically similar and dissimilar males, and display more sexual behaviour towards, and mate more often with, their chosen males. Female agile antechinus that were allowed the simultaneous choice of up to four males chose to mate with males that were the most genetically dissimilar to themselves, but avoided males that were vocal or aggressive (Parrott et al. 2015). In the stripe-faced dunnart (Parrott et al. 2019) and eastern barred bandicoot (Hartnett et al. 2018), females that were paired with their preferred mate copulated and conceived faster, and had a significantly higher number of pregnancies, than females that were allocated a mate based on pedigree alone.
Due to the ongoing effects of anthropogenic change, there is an increasing need to determine species’ requirements and establish successful captive breeding programs for conservation. The incorporation of natural mating behaviours, such as female mate choice, into captive breeding programs has the potential to improve reproductive outputs and offspring fitness. This is especially relevant to the Dasyuridae (the carnivorous marsupials), in which six species are listed as endangered or critically endangered, including the Kangaroo Island dunnart (Sminthopsis aitkeni) and the eastern quoll (Dasyurus viverrinus), and 17 species are classified as near threatened or vulnerable to extinction (IUCN 2022). The fat-tailed dunnart (Sminthopsis crassicaudata) is currently listed bas near threatened in Victoria (Department of Environment, Land, Water and Planning (DELWP) 2013), with classification to Vulnerable recently accepted under the Flora and Fauna Guarantee Act 1988 (supported for listing by the FFG Scientific Advisory Committee; Flora and Fauna Guarantee - Scientific Advisory Committee (FFG-SAC) 2022; Victorian Government 2023). Extant populations are noted to be in decline, and some populations have become locally extinct (Scicluna et al. 2021; Steele and Brunner 2021; Homan 2022), including the population previously regarded as the largest in Victoria (Beardsell 1991; Scicluna et al. 2021). With the likelihood of continued decline (FFG-SAC 2022; Victorian Government 2023), as seen in other threatened dasyurids, increased focus on the species and potential conservation actions is warranted. Further, with the availability of long-term captive breeding colonies and evidence of using scent-based chemical communication (Ewer 1968), the fat-tailed dunnart is an ideal model species for female mate choice investigation using olfactory discrimination in the Dasyuridae.
Here we investigate, for the first time, whether captive fat-tailed dunnarts demonstrate female mate choice between two unrelated novel males. We then determine whether reproductive output is greater in females paired with their preferred mate, hypothesising that pairing females with their preferred mate will increase reproductive success compared with those paired with their non-preferred mate.
Methods
Study species
The fat-tailed dunnart is a small (10–20 g), nocturnal, carnivorous marsupial belonging to the family Dasyuridae (Ewer 1968). This species is generally identified by its very distinctive fat-storing tail, which can provide energy when food availability is scarce or conditions are suboptimal (Hope et al. 1997). Females are polyoestrous and seasonal breeders, with a natural breeding season between July and February (Morton 1978a). Oestrous cycles last approximately 31 days with a gestation period of 13–16 days (Morton 1978a). Females have 8–10 teats (Tyndale-Biscoe and Renfree 1987), and may successfully rear one or occasionally two litters consisting of up to 10 young in natural populations (Morton 1978a). Young are weaned 70 days after birth (Smith et al. 1978), with the female entering oestrus 2–3 days after weaning or loss of pouch young (Bennett et al. 1989). Females can reach sexual maturity as early as 115 days old and males from approximately 200 days old (Bennett et al. 1989).
Captive colony
All individuals used in this study were from a pre-existing research colony maintained at La Trobe University, Melbourne, Australia (AEC18056, DELWP10008231). The colony was established in 2001, originating from the University of Adelaide captive stock, with sporadic supplementation from other captive colonies over a 20-year period. The University of Adelaide colony was first established in the Zoology Department in 1964 and again in 1965 due to the extinction of the first colony due to poor reproductive performance (Bennett et al 1989) and a reduction in colony performance with each generation (Godfrey 1969; Godfrey and Crowcroft 1971). The colony with which this research was conducted was maintained as an ‘outbred’ colony using the Poiley rotational outbreeding system (Poiley 1960). It, however, almost became extinct in 2013 due to poor breeding performance, small litter sizes and loss of Poiley rotational pairing when moved to centralised animal care in the university rodent facility (unpubl. data). Although it is difficult to identify the factors affecting the decline in fecundity of the colony, the management of the colony centrally and failure to maintain continuous breeding appears to be the most likely explanation. The colony was recovered over a 5-year period from very low numbers (eight males and 20 females) of aging individuals and supplementation from other captive colonies by using seminatural enclosures and housing groups of females with multiple males. The colony was then maintained at approximately 100 individuals, and at the start of this research (in 2018) it consisted of 74 individuals (31 females and 43 males).
Husbandry
In this study, a total of 18 adult male and 18 adult female fat-tailed dunnarts were used, with individuals ranging from 140 to 635 days old. Individuals were weighed and had pes length measured using digital callipers (to the nearest millimetre) the week prior to the mate choice experiment. Animals were then housed separately until paired for breeding. Dunnarts were fed 40 g of a meat mix daily, consisting of dry cat food (Whiskas® Adult beef and lamb) that had been soaked in water, then mixed with wet cat food (Whiskas® Adult Jellymeat loaf), and Wombaroo small carnivore food. Three times per week they were also supplemented with five live mealworms (Tenebrio molitor) and two live crickets (Acheta domestica). Vitamins (Penta-vite® oral liquid) were added to water and provided ad libitum. Once females produced pouch young, food provisioning was doubled and mealworms increased to 20 individuals three times per week. To induce oestrous cycling and receptivity, females were housed under short light cycles (8 light: 16 dark) and cool temperatures (21 ± 5°C) for three weeks and then moved to a long light cycle (16 light: 8 dark) and warmer temperatures (23 ± 5°C). After 15 days in long light cycles, females were monitored daily for the onset of oestrus. Oestrus was identified by an increase in body weight and the presence of cornified epithelial cells in urine samples (Godfrey 1969).
Male scent stimuli
Male scent stimuli were obtained from bedding collected from the nest boxes of each male one week prior to oestrus detection in females and stored in airtight bags. Bedding was split into two equal amounts to allow for the male’s scent to be used twice if necessary, resulting in 36 samples of bedding from 18 potential males. Dunnarts readily urinate and defecate upon handling, therefore additional fresh urine and faeces were collected on tissues from each male on the day of their experiment to supplement the collected scents from bedding material. Females were presented with bedding only from males that were unrelated to the female, and that were novel, with no previous introductions or mating attempts. Thus, each female had a suite of suitable males available for scent allocation and pairing. Once a scent had been presented to a female it was discarded, with no sample presented more than once. Once a male was allocated as either preferred or unpreferred for pairing with a female (see mate choice experiment below), the bedding with his scent was excluded from future trials.
Mate choice experiment
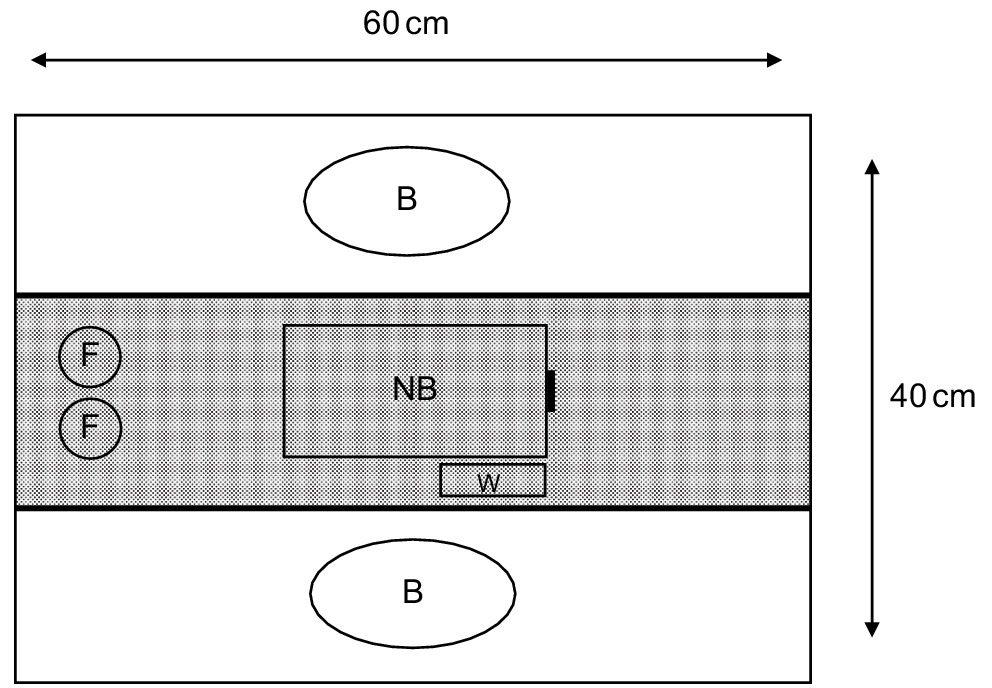
Females were assigned to the mate choice experiment on the evening of first oestrus detection (Day 0) between 2100 hours and 0500 hours, as females are most receptive in the first three days of high epithelial cell shedding (Ewer 1968). Choice experiments took place in the female’s familiar home enclosure with male scents being the only novel factors. Females were simultaneously presented with the scents of two allocated males from their suite of suitable mate options (see above). The scents were then placed at opposite sides of the enclosure. Each female was video recorded during choice experiments with infrared surveillance cameras (Super-mini Monochrome/Colour Camera, Shenzhen Vakind Technology Co. Ltd, Shenzhen, China), with recordings analysed the following day. Strips of tape were placed horizontally across the top of the enclosure to designate a central zone where the female was deemed to have not made a choice, and either side of this zone where the female was scored on the time spent with each male scent (Fig. 1). A nest box, water and food remained in the central zone of the enclosure to provide appropriate care for the female, but not influence choice scoring. Females were scored from their first emergence from their nest box until reaching a 30-min period spent cumulatively with either scent. Experiments were observed and recordings analysed in a separate room to avoid disturbance. The male whose bedding the female spent the most time with was identified as her preferred mate.
Schematic of the female fat-tailed dunnart enclosure set up during the mate choice experiment. The shaded area represents the central exclusion zone and white areas represent scoring zones for each scent. The black lines represent the strips of tape placed over the enclosure to aid discrimination between areas. The black line of the nest box represents the entrance point. F, food; W, water dispenser; B, scent on bedding from two unrelated males; NB, female nest box.
Behavioural analysis
Additional behaviours of each female during the mate choice experiment were analysed using the behavioural program BORIS v6.9.2 (Friard and Gamba 2016). As females required prompt pairing due to their short receptive period, these additional behavioural data were analysed after the females were allocated their mates and were not used to determine allocation of mates. Behaviours analysed included grooming at each scent, time spent in the exclusion zone (central area between scents), grooming in the exclusion zone and time spent inside the nest box. Grooming behaviour included rubbing the body and face and rolling in sawdust. Behaviours excluded from analysis included eating, drinking and sleeping outside the nest box.
Reproductive success
On the night following the mate choice experiment (2100 hours), females were systematically paired with either their preferred or non-preferred mate for the remainder of the study (10 weeks) or until pouch young were recorded. This ensured that nine females were allocated their preferred mate and nine were allocated their non-preferred mate. After 14 days, females were weighed and checked once per week for pouch young to decrease potential stress from handling while possibly pregnant. If pouch young were present, males were removed to avoid increased post-partum female aggression (Morton 1978a), and the number and sex of offspring recorded. Pouch young were sexed approximately 20 days after birth when the development of the pouch and testis were easily distinguished (Cook et al. 2021). Weaning success was recorded at 70 days after birth, with weaned young moved to a separate enclosure from their mother. Weaning was deemed successful if young survived to weaning age. Reproductive success was determined by production of young, the number of young produced, the number of young weaned and the sex ratio of litters.
Statistical analyses
All analyses were conducted in R v3.5.1 (RStudio Team 2018). The total time females spent with both scents was used to calculate the percentage of time females spent with each scent. Mate preference was assigned to the scent with which the female spent the most time. Additional variables examined for mate preference were mean difference between the number of visits to each scent and time spent grooming in the presence of each scent.
Body condition for individuals was calculated from the residuals of an ordinary least regression of body mass and pes length (Schulte-Hostedde et al. 2005) one week prior to the mate choice experiment. This was conducted prior to correlation tests between all measurable variables for generalised linear models (GLM). There was correlation between probable female condition, female tail width (t16 = −0.13, P = 0.90), and age (t16 = 1.36, P = 0.19), therefore only female condition was used in the model. This was also evident between male condition and male tail width (t16 = 0.51, P = 0.62) but not age of the male (t16 = 2.73, P = 0.01), therefore both probable paternal condition and age were included in the model. Our final variable included in the model was the strength of pair, which was calculated as the percentage of total time each female spent with the scent of the male with whom she was paired.
A GLM with Poisson error was run to determine if the number of offspring produced by each female, and weaning success of litters, was influenced by mate allocation (preferred versus non-preferred mate), maternal condition, paternal condition, paternal age, and strength of pair. A GLM with binomial error was used to analyse whether offspring sex ratio was influenced by mate allocation, maternal condition, paternal condition, paternal age and strength of pair.
Results
Mate choice experiment
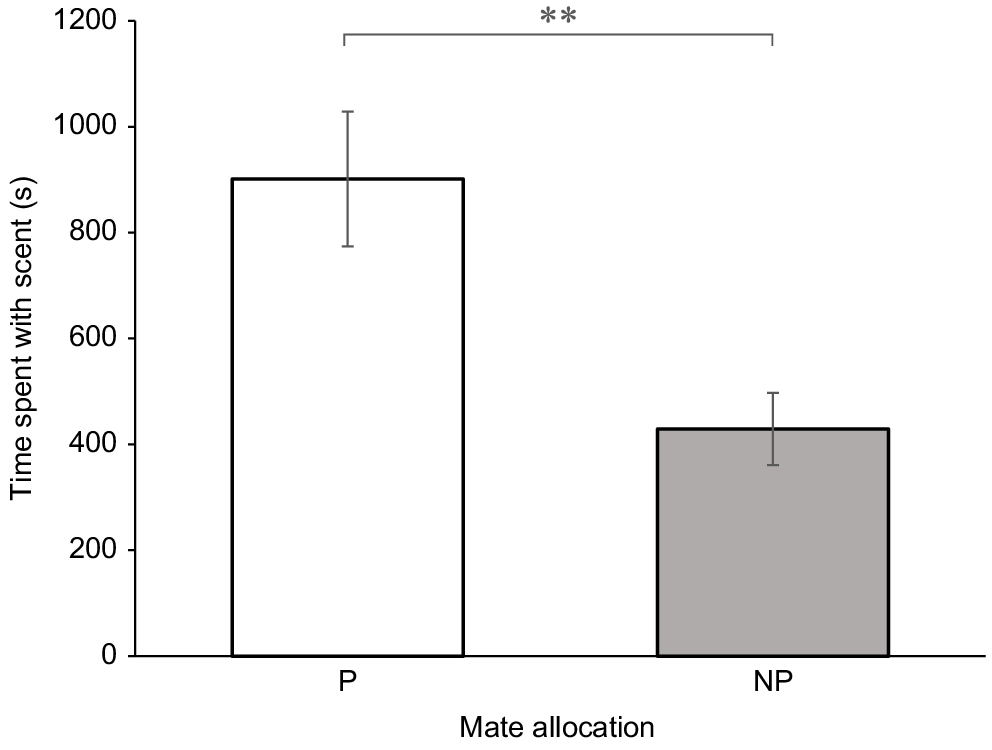
On average, females (n = 18) spent 67% of their time with the scent of one male (t17 = 4.11, P < 0.01) (Fig. 2). There was a correlation between the time spent with the females’ preferred scent and time spent grooming (t16 = 0.08, P = 0.93), and likewise for non-preferred scents (t16 = −0.01, P = 0.98). There was no correlation between the number of visits for either preferred or non-preferred scents (t16 = −1.89, P = 0.07 and t16 = −1.66, P = 0.11 respectively).
Reproductive success
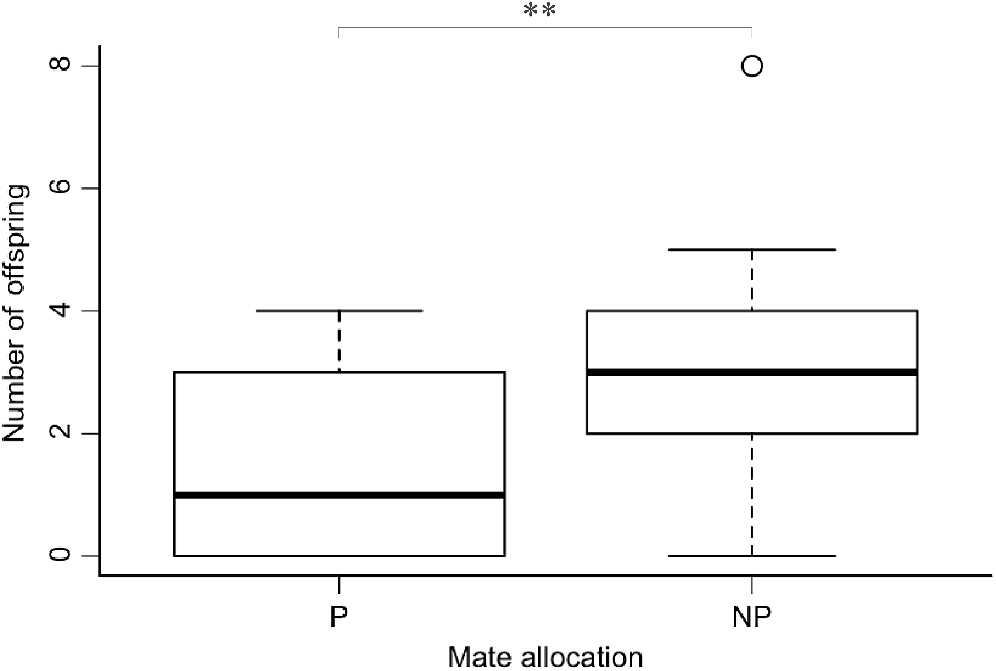
Of the females that were reproductively successful (n = 12), five out of nine females successfully produced offspring with their preferred mate compared with seven out of nine with their non-preferred mate (Fig. 3). Two litters from preferred pairings were lost during very early pouch life prior to determining litter size, resulting in a total of 15 offspring for preferred pairings and 27 for non-preferred pairings. Six females in this study failed to reproduce. Of the females that successfully raised pouch young (n = 10), the average litter size was higher for non-preferred (3 ± 0.83) than preferred pairings (1.6 ± 0.60) (Fig. 3) when considered without the inclusion of other covariates (Fig. 3). Litter size was not influenced by allocation, maternal condition, paternal condition, paternal age, or strength of pair (Table 1). With the exception of the two lost litters, all offspring survived to weaning. The number of offspring that survived to weaning was not influenced by allocation, maternal condition, paternal condition, paternal age or strength of pair (Table 2).
Mean litter size produced by female fat-tailed dunnarts in litters that survived to weaning with preferred (n = 3) and non-preferred (n = 7) mates, with one individual classed as an outlier (○). The box indicates the interquartile range around the median (black line) and vertical error bars. An asterisk (*) indicates a significant difference between values. P, preferred; NP, non-preferred.
| Variables | Estimate | s.e. | z value | Pr(>|z|) |
| (Intercept) | 1.8765 | 0.5390 | 3.4810 | 0.000499*** |
| Allocation | −0.4818 | 0.5624 | −0.8570 | 0.3916 |
| Maternal condition | 0.1565 | 0.1158 | 1.3510 | 0.1766 |
| Paternal condition | 0.2808 | 0.2280 | 1.2320 | 0.2181 |
| Paternal age | −0.0016 | 0.0012 | −1.4170 | 0.1565 |
| Strength of pair | −0.5789 | 1.4496 | −0.399 | 0.6896 |
Total offspring for preferred pairings was 15, and 27 for non-preferred pairings. Average litter size was 1.6 ± 0.60 for preferred and 3 ± 0.83 for non-preferred pairings.
An asterisk (*) indicates a significant difference between values.
| Variables | Estimate | s.e. | z value | Pr(>|z|) |
| (Intercept) | 0.4734 | 1.0059 | 0.4710 | 0.6380 |
| Allocation | 0.2364 | 1.6372 | 0.1440 | 0.8850 |
| Maternal condition | 0.1060 | 0.2199 | 0.4820 | 0.6300 |
| Paternal condition | −0.5973 | 0.9656 | −0.6190 | 0.5360 |
| Paternal age | 0.0007 | 0.0027 | 0.2530 | 0.8000 |
| Strength of pair | −2.2736 | 3.7846 | −0.601 | 0.548 |
Total offspring to survive weaning for preferred pairings was 15, and 27 for non-preferred pairings.
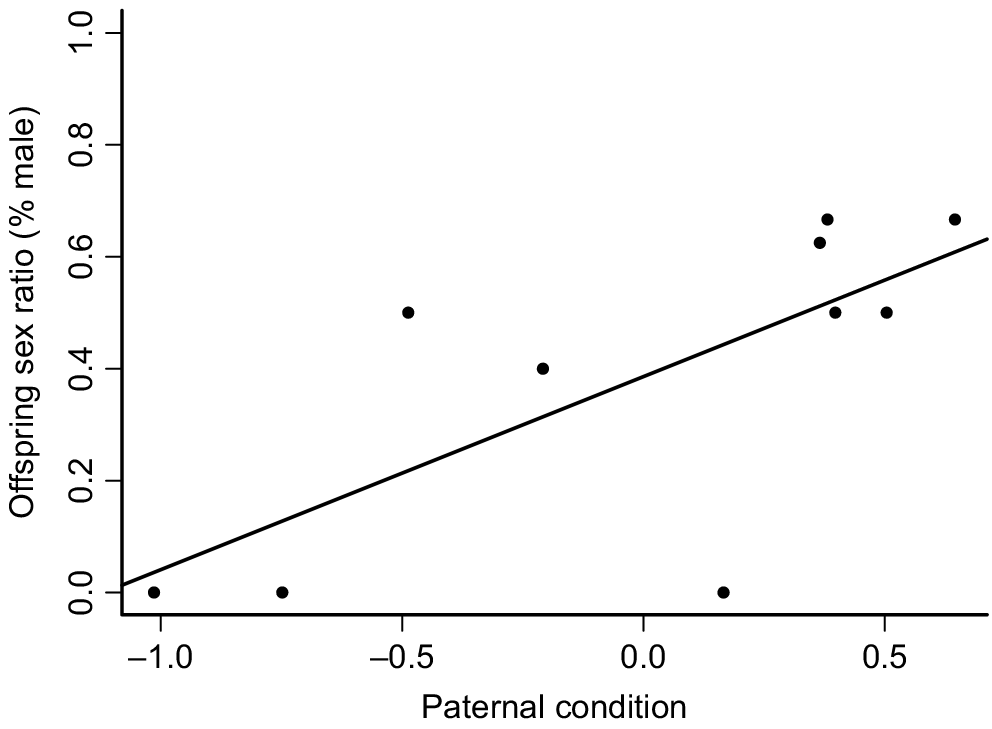
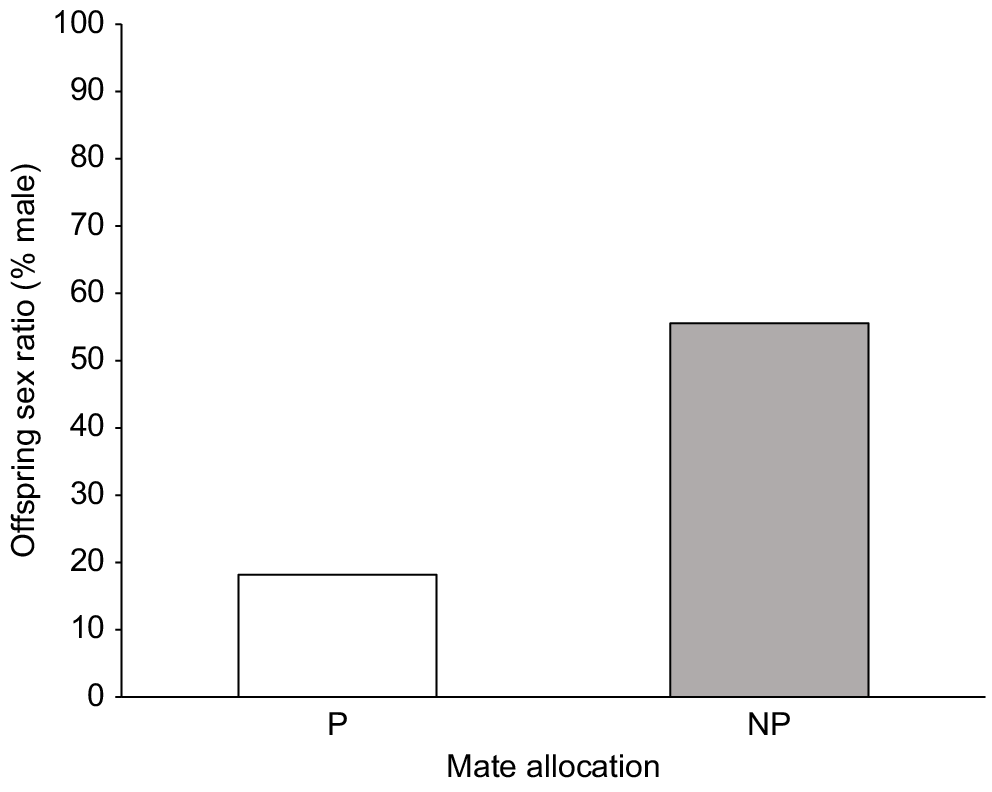
In the full model, sex ratio was observed to be influenced by paternal condition (Pr(>Chi)6 = 0.03) (Fig. 4), with more sons being sired by fathers in better body condition, but not by allocation, maternal condition, paternal age or strength of pair (Table 3). In the simplified model containing only preference, females paired with their preferred mate produced significantly more daughters (18% male, n = 2 sons, 9 daughters) than females paired with their non-preferred mate (55% male, n = 15 sons, 12 daughters) (Pr(>Chi)8 = 0.04) (Fig. 5). Irrespective of allocation, all females produced slightly more daughters, resulting in a slight bias towards females across all litters (n = 38; 44% male) (Pr(>Chi)9 = 0.33).
Relationship between paternal body condition of fat-tailed dunnarts and offspring sex ratio (% male), with females paired with a male in better condition siring more sons ((Pr(>Chi)6 = 0.03)).
| Variables | d.f. | Deviance Resid. | d.f. | Resid. Dev | Pr(>Chi) |
| NULL | 9 | 12.9116 | |||
| Allocation | 1 | 3.9039 | 8 | 9.0077 | 0.04817* |
| Maternal condition | 1 | 0.3467 | 7 | 8.661 | 0.556 |
| Paternal condition | 1 | 4.4924 | 6 | 4.1686 | 0.03405* |
| Paternal age | 1 | 0.1411 | 5 | 4.0275 | 0.70716 |
| Strength of pair | 1 | 0.0461 | 4 | 3.9814 | 0.82991 |
Females in preferred pairings produced more daughters (18% male, n = 2 sons, 9 daughters) compared to non-preferred pairings (55% male, n = 15 sons, 12 daughters).
P, preferred; NP, non-preferred.
An asterisk (*) indicates a significant difference between values.
Percentage of offspring sex ratio (% male) produced by female fat-tailed dunnarts in those litters that survived to weaning with preferred pairings producing more daughters (18% male, n = 2 sons, 9 daughters) compared with non-preferred pairings (55% male, n = 15 sons, 12 daughters). P, preferred; NP, non-preferred.
Discussion
This was the first investigation of mate preference based on chemosensory cues in the fat-tailed dunnart. Although all 18 females exhibited a strong preference for individual males by spending significantly more time with one scent, incorporating female mate choice into the fat-tailed dunnart captive breeding program did not increase immediate reproductive output (litter size or weaning success). Only 12 of the 18 females produced young, with only 10 of the 18 females successfully weaning their young; three out of nine females paired with their preferred and seven out of nine with their non-preferred mate. Surprisingly, a non-significant trend indicated that females paired with their non-preferred mate produced larger litters than those with their preferred mate. Mate allocation did not influence offspring weaning success, with all females weaning their litters, with the exception of two litters from preferred pairings that were lost soon after birth. However, loss of pouch young is not unexpected in fat-tailed dunnarts, with approximately 10% of litters lost before weaning (Bennett et al. 1989).
The lack of influence of mate allocation on offspring litter size or weaning success was unexpected as previous studies (Drickamer et al. 2000; Martin and Shepherdson 2012) showed increased reproductive output from incorporation of female mate choice. In the closely related stripe-faced dunnart, females paired with their preferred mate based on olfactory cues had a greater number of matings, greater number of pregnancies per oestrous cycle and shorter time to conception than females paired based on pedigree alone (Parrott et al. 2019). Similar results were obtained in another marsupial species, the eastern barred bandicoot, in which females paired with their preferred mate had a shorter time to conception and significantly more litters born to females paired with their preferred than non-preferred males (Hartnett et al. 2018). However, as found in our results, litter size was also not influenced in either the stripe-faced dunnart (Parrott et al. 2019) or the eastern barred bandicoot (Hartnett et al. 2018). Our results depict a lower-than-average litter size for this species in comparison to other captive studies (Ewer 1968; Bennett et al. 1989), with Tyndale-Biscoe and Renfree (1987) noting an average litter size of 7.5 young. The possible reason for this may relate to the significant population decline of the research colony prior to this study (see above in colony details). This allowed issues such as inbreeding, bottlenecks and lack of genetic diversity to occur within a population consisting of largely older individuals with restricted breeding opportunities. This could also provide an explanation for why six of the 18 females failed to reproduce.
Unlike offspring litter size or weaning success, mate allocation was observed to influence offspring sex ratios in the fat-tailed dunnart. Females paired with their preferred mate produced more female offspring (81%) compared with those paired with their non-preferred mate (44%). Irrespective of allocation, a slight bias toward female offspring was observed in this study (55% female, n = 38). However, due to the nature of this study, the small sample size and unbalanced design requires results to be taken with caution. Interestingly, our findings did not show a relationship between maternal condition and offspring sex ratio, a theory commonly suggested in mammalian sex allocation (i.e. Trivers–Willard hypothesis: Trivers and Willard 1973). The proximate explanations for how offspring sex allocation is achieved is largely unknown (see review by Robert and Schwanz (2011)) and something we did not explicitly test, although the lack of maternal influence suggests there is no evidence for the Trivers–Willard effect on fat-tailed dunnarts in this study. Our results, in which offspring ratio was skewed towards daughters when paired with a preferred mate, may relate to the fat-tailed dunnart’s life history traits. Females live up to two years and are capable of raising two litters per year, whereas males survive less than 15 months, coinciding with only one breeding season (Morton 1978a). For small marsupials with a reproductive strategy involving few breeding opportunities, heavy emphasis is placed on having successful litters in order to maximise overall reproductive success (Russell 1982). In captivity, male fat-tailed dunnarts have highly variable and unpredictable reproductive success, with Bennett et al (1989) noting that one-third of males never produced young, one-third only ever produced one litter, and only a few individuals contributed to multiple litters. Acknowledging that we do not know the reproductive success of males in the wild, a skewed sex ratio towards daughters for wild fat-tailed dunnarts could be beneficial if wild males also have the same highly variable and unpredictable reproductive success as captive males. Females reach sexual maturity more quickly and live longer than males (Morton 1978a), thus litters with more daughters will have an overall greater opportunity to produce more offspring themselves, therefore increasing the overall reproductive success for both sexes.
Females also produced more sons when paired with a male in better body condition, which was further supported by a non-significant positive trend with increasing age of the male. This strategy can be advantageous for females as they will potentially produce more litters with sons that carry traits that will enhance the offspring’s access to mates, ultimately indirectly benefiting the mother (Fisher 1930). The production of more sons is commonly associated with local resource competition theories, where mothers produce more of the dispersive sex (most often males) to avoid competition with the philopatric sex (Cockburn 1989). However, the drifting home ranges used by fat-tailed dunnarts suggest otherwise. Home ranges of fat-tailed dunnarts can cover a large area, resulting in overlap of both sexes, an advantage when hunting prey where timing of food availability and food abundance can be unpredictable (Morton 1978b). This would typically suggest an increase in territorial behaviour; however, such behaviour was observed only in mothers defending nest sites when offspring were left in the nest while she foraged (Morton 1978a). Therefore, it is possible that theories such as local resource competition do not apply given the species’ solitary nature with overlapping home ranges and weak territorial behaviour, and therefore no advantage to skewing sex ratios in order to reduce competition but instead overall lifetime reproductive fitness of an individual.
With native species numbers declining at an alarming rate (Murphy and van Leeuwen 2021), the use of ex situ conservation, such as captive breeding programs, has become increasingly important for species whose populations are no longer able to thrive in the wild. However, captive breeding programs face many potential limitations, and knowledge of the ecology of many native species and their captive husbandry requirements remain scarce. Common issues associated with captivity may relate to the colonies’ historical data and restricted sample sizes, especially in research colonies with variable funding, restricted space and enclosure availability that may inhibit breeding opportunities. This is particularly evident in species with increased longevity in captivity compared with in the wild, with individuals surviving for years past reproductive senescence (e.g. in the eastern barred bandicoot (Parrott et al. 2017) or Tasmanian devil (Farquharson et al. 2017)). Enclosure spaces may be filled by an ageing population, restricting the space available for breeding new generations (Parrott et al. 2017). If not carefully managed, such reduced breeding can result in bottlenecks and a loss of genetic variation, which has the potential to ultimately affect future reproductive output.
Skewing sex ratios towards daughters may aid in rectifying historical issues with captive colonies until populations stabilise. Biased sex ratios in captivity are most commonly associated with an overproduction of male offspring (Chargé et al. 2014), which may skew population structure and/or reduce reproductive opportunities, and ultimately affects the target size required for long-term success (Faust and Thompson 2000). Therefore, an ability to skew sex ratios towards females would assist in rectifying male biased captive populations, increase population growth due to more reproductive opportunities, and consequently decrease issues such as inbreeding depression and genetic drift (Wedekind 2002). Considering that key objectives in many captive breeding programs are to create insurance populations for declining wild populations, and/or the potential reintroduction of individuals back to the wild, introducing measures to skew sex ratios towards daughters should be explored.
Our results showed that female fat-tailed dunnarts made clear mate choices, demonstrated by the differences in time spent with a male olfactory cue. However, this choice did not affect litter size or weaning success. Despite this, offspring sex ratio was observed to be influenced by mate choice, with a skewed sex ratio towards daughters. The life history of this species suggests that producing more daughters will be advantageous to increasing the overall reproductive fitness of parents. The fat-tailed dunnart has recently been accepted for classification as Vulnerable in Victoria under the Flora and Fauna Guarantee Act 1988 (FFG-SAC 2022; Victorian Government 2023). For a species that has suffered local extinction and is likely to suffer continued decline across its wild range (Scicluna et al. 2021; Steele and Brunner 2021; FFG-SAC 2022; Homan 2022; Victorian Government 2023), such knowledge of breeding outcomes and the ability to skew sex ratios could be beneficial. Although not all mechanisms and factors of sexual selection could be explored in this study, our results provide an insight into the potential improvement of captive colonies and the importance of understanding instinctual behaviours in a captive setting.
Data availability
The datasets generated and/or analysed during the current study are available from opal.latrobe.edu.au at DOI:10.26181/21587253.
Acknowledgements
We thank the La Trobe Animal Research and Teaching Facility for assistance with animal housing and husbandry, and Alicia Dimovski for assistance with camera set up and video conversion. We also thank Steve Cooper, Lynne Selwood and one anonymous reviewer for their insightful feedback on our manuscript prior to publication. We also acknowledge the Traditional Owners of the land on which this research was conducted, the Wurundjeri and Wadawurrung people of the Kulin Nation; we pay our respects to their Elders, past and present.
References
Andersson MB (1994) ‘Sexual selection.’ (Princeton University Press: Princeton, New Jersey)Beardsell C (1991) ‘Sites of faunal significance in the Western region of Melbourne (inland of the Princes Freeway).’ (Department of Conservation and Environment: Victoria)
Bennett, JH, Breed, WG, Hayman, DL, and Hope, RM (1989). Reproductive and genetic studies with a laboratory colony of the dasyurid marsupial Sminthopsis crassicaudata. Australian Journal of Zoology 37, 207–222.
| Reproductive and genetic studies with a laboratory colony of the dasyurid marsupial Sminthopsis crassicaudata.Crossref | GoogleScholarGoogle Scholar |
Candolin, U (2003). The use of multiple cues in mate choice. Biological Reviews 78, 575–595.
| The use of multiple cues in mate choice.Crossref | GoogleScholarGoogle Scholar |
Chargé, R, Teplitsky, C, Sorci, G, and Low, M (2014). Can sexual selection theory inform genetic management of captive populations? A review. Evolutionary Applications 7, 1120–1133.
| Can sexual selection theory inform genetic management of captive populations? A review.Crossref | GoogleScholarGoogle Scholar |
Charpentier, MJE, Boulet, M, and Drea, CM (2008). Smelling right: the scent of male lemurs advertises genetic quality and relatedness. Molecular Ecology 17, 3225–3233.
| Smelling right: the scent of male lemurs advertises genetic quality and relatedness.Crossref | GoogleScholarGoogle Scholar |
Cockburn, A (1989). Sex-ratio variation in marsupials. Australian Journal of Zoology 37, 467–479.
| Sex-ratio variation in marsupials.Crossref | GoogleScholarGoogle Scholar |
Cook, LE, Newton, AH, Hipsley, CA, and Pask, AJ (2021). Postnatal development in a marsupial model, the fat-tailed dunnart (Sminthopsis crassicaudata; Dasyuromorphia: Dasyuridae). Communications Biology 4, 1028.
| Postnatal development in a marsupial model, the fat-tailed dunnart (Sminthopsis crassicaudata; Dasyuromorphia: Dasyuridae).Crossref | GoogleScholarGoogle Scholar |
Darwin C (1859) ‘On the origin of species by means of natural selection.’ (Murray: London UK)
Department of Environment, Land, Water and Planning (DELWP) (2013) Advisory list of threatened vertebrate fauna in Victoria. Available at https://www.environment.vic.gov.au/__data/assets/pdf_file/0014/50450/Advisory-List-of-Threatened-Vertebrate-Fauna_FINAL-2013.pdf [Accessed 10 October 2016]
Drickamer, LC, Gowaty, PA, and Holmes, CM (2000). Free female mate choice in house mice affects reproductive success and offspring viability and performance. Animal Behaviour 59, 371–378.
| Free female mate choice in house mice affects reproductive success and offspring viability and performance.Crossref | GoogleScholarGoogle Scholar |
Ewer, RF (1968). A preliminary survey of the behaviour in captivity of the dasyurid marsupial, Sminthopsis crassicaudata (Gould). Zeitschrift für Tierpsychologie 25, 319–365.
| A preliminary survey of the behaviour in captivity of the dasyurid marsupial, Sminthopsis crassicaudata (Gould).Crossref | GoogleScholarGoogle Scholar |
Farquharson, KA, Hogg, CJ, and Grueber, CE (2017). Pedigree analysis reveals a generational decline in reproductive success of captive Tasmanian devil (Sarcophilus harrisii): implications for captive management of threatened species. Journal of Heredity 108, 488–495.
| Pedigree analysis reveals a generational decline in reproductive success of captive Tasmanian devil (Sarcophilus harrisii): implications for captive management of threatened species.Crossref | GoogleScholarGoogle Scholar |
Faust, LJ, and Thompson, SD (2000). Birth sex ratio in captive mammals: patterns, biases, and the implications for management and conservation. Zoo Biology 19, 11–25.
| Birth sex ratio in captive mammals: patterns, biases, and the implications for management and conservation.Crossref | GoogleScholarGoogle Scholar |
Fisher RA (1930) ‘The genetical theory of natural selection.’ (Clarendon Press: Oxford)
Flora and Fauna Guarantee - Scientific Advisory Committee (FFG-SAC) (2022) Preliminary recommendation on a nomination for listing Sminthopsis crassicaudata, fat-tailed dunnart. Department of Natural Resources and Environment, Melbourne.
Friard, O, and Gamba, M (2016). BORIS: a free, versatile open-source event-logging software for video/audio coding and live observations. Methods in Ecology and Evolution 7, 1325–1330.
| BORIS: a free, versatile open-source event-logging software for video/audio coding and live observations.Crossref | GoogleScholarGoogle Scholar |
Godfrey, GK (1969). The influence of increased photoperiod on reproduction in the dasyurid marsupial, Sminthopsis crassicaudata. Journal of Mammalogy 50, 132–133.
| The influence of increased photoperiod on reproduction in the dasyurid marsupial, Sminthopsis crassicaudata.Crossref | GoogleScholarGoogle Scholar |
Godfrey, GK, and Crowcroft, P (1971). Breeding the fat-tailed marsupial mouse in captivity. International Zoo Yearbook 11, 33–38.
| Breeding the fat-tailed marsupial mouse in captivity.Crossref | GoogleScholarGoogle Scholar |
Hartnett, CM, Parrott, ML, Mulder, RA, Coulson, G, and Magrath, MJL (2018). Opportunity for female mate choice improves reproductive outcomes in the conservation breeding program of the eastern barred bandicoot (Perameles gunnii). Applied Animal Behaviour Science 199, 67–74.
| Opportunity for female mate choice improves reproductive outcomes in the conservation breeding program of the eastern barred bandicoot (Perameles gunnii).Crossref | GoogleScholarGoogle Scholar |
Homan, P (2022). Surveys of vertebrate fauna on the northern outskirts of metropolitan Melbourne, 2006–2022: (1) mammals. Victorian Naturalist 139, 173–183.
Hope, PJ, Pyle, D, Daniels, CB, Chapman, I, Horowitz, M, Morley, JE, Trayhurn, P, Kumaratilake, J, and Wittert, G (1997). Identification of brown fat and mechanisms for energy balance in the marsupial, Sminthopsis crassicaudata. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology 273, R161–R167.
| Identification of brown fat and mechanisms for energy balance in the marsupial, Sminthopsis crassicaudata.Crossref | GoogleScholarGoogle Scholar |
IUCN (2022) The IUCN red list of threatened species (version 2022-1). Available at http:9//www.iucnredlist.org. [Accessed 23 July 2022]
Jennions, MD, and Petrie, M (2000). Why do females mate multiply? A review of the genetic benefits. Biological Reviews of the Cambridge Philosophical Society 75, 21–64.
| Why do females mate multiply? A review of the genetic benefits.Crossref | GoogleScholarGoogle Scholar |
Johansson, BG, and Jones, TM (2007). The role of chemical communication in mate choice. Biological Reviews 82, 265–289.
| The role of chemical communication in mate choice.Crossref | GoogleScholarGoogle Scholar |
Landry, C, Garant, D, Duchesne, P, and Bernatchez, L (2001). ‘Good genes as heterozygosity’: the major histocompatibility complex and mate choice in Atlantic salmon (Salmo salar). Proceedings of the Royal Society of London B: Biological Sciences 268, 1279–1285.
| ‘Good genes as heterozygosity’: the major histocompatibility complex and mate choice in Atlantic salmon (Salmo salar).Crossref | GoogleScholarGoogle Scholar |
Martin, MS, and Shepherdson, DJ (2012). Role of familiarity and preference in reproductive success in ex situ breeding programs. Conservation Biology 26, 649–656.
| Role of familiarity and preference in reproductive success in ex situ breeding programs.Crossref | GoogleScholarGoogle Scholar |
Mays, HL, and Hill, GE (2004). Choosing mates: good genes versus genes that are a good fit. Trends in Ecology & Evolution 19, 554–559.
| Choosing mates: good genes versus genes that are a good fit.Crossref | GoogleScholarGoogle Scholar |
Møller, AP (1988). Female choice selects for male sexual tail ornaments in the monogamous swallow. Nature 332, 640–642.
| Female choice selects for male sexual tail ornaments in the monogamous swallow.Crossref | GoogleScholarGoogle Scholar |
Morton, SR (1978a). An ecological study of Sminthopsis crassicaudata (Marsupialia: Dasyuridae) III. Reproduction and life history. Wildlife Research 5, 183–211.
| An ecological study of Sminthopsis crassicaudata (Marsupialia: Dasyuridae) III. Reproduction and life history.Crossref | GoogleScholarGoogle Scholar |
Morton, SR (1978b). An ecological study of Sminthopsis crassicaudata (Marsupialia: Dasyuridae) II. Behaviour and social organization. Wildlife Research 5, 163–182.
| An ecological study of Sminthopsis crassicaudata (Marsupialia: Dasyuridae) II. Behaviour and social organization.Crossref | GoogleScholarGoogle Scholar |
Murphy H, van Leeuwen S (2021) Australia state of the environment 2021. Biodiversity, independent report to the Australian Government Minister for the Environment, Commonwealth of Australia, Canberra. https://doi.org/10.26194/ren9-3639
Panhuis, TM, Butlin, R, Zuk, M, and Tregenza, T (2001). Sexual selection and speciation. Trends in Ecology & Evolution 16, 364–371.
| Sexual selection and speciation.Crossref | GoogleScholarGoogle Scholar |
Parrott, ML, Ward, SJ, and Temple-Smith, PD (2007). Olfactory cues, genetic relatedness and female mate choice in the agile antechinus (Antechinus agilis). Behavioral Ecology and Sociobiology 61, 1075–1079.
| Olfactory cues, genetic relatedness and female mate choice in the agile antechinus (Antechinus agilis).Crossref | GoogleScholarGoogle Scholar |
Parrott, ML, Ward, SJ, Temple-Smith, PD, and Selwood, L (2015). Females choose mates based on genetic relatedness in a small dasyurid marsupial, the agile antechinus (Antechinus agilis). PLoS ONE 10, e0122381.
| Females choose mates based on genetic relatedness in a small dasyurid marsupial, the agile antechinus (Antechinus agilis).Crossref | GoogleScholarGoogle Scholar |
Parrott, ML, Coetsee, AL, Hartnett, CM, and Magrath, MJL (2017). New hope for the eastern barred bandicoot Perameles gunnii after 27 years of recovery effort. International Zoo Yearbook 51, 154–164.
| New hope for the eastern barred bandicoot Perameles gunnii after 27 years of recovery effort.Crossref | GoogleScholarGoogle Scholar |
Parrott, ML, Nation, A, and Selwood, L (2019). Female mate choice significantly increases captive breeding success, and scents can be frozen to determine choice, in the stripe-faced dunnart. Applied Animal Behaviour Science 214, 95–101.
| Female mate choice significantly increases captive breeding success, and scents can be frozen to determine choice, in the stripe-faced dunnart.Crossref | GoogleScholarGoogle Scholar |
Penn, DJ, and Potts, WK (1999). The evolution of mating preferences and major histocompatibility complex genes. The American Naturalist 153, 145–164.
| The evolution of mating preferences and major histocompatibility complex genes.Crossref | GoogleScholarGoogle Scholar |
Poiley, SM (1960). A systematic method of breeder rotation for non-inbred laboratory colonies. Proceedings Animal Care Panel 10, 159–166.
Reby, D, Hewison, M, Izquierdo, M, and Pépin, D (2001). Red deer (Cervus elaphus) hinds discriminate between the roars of their current harem-holder stag and those of neighbouring stags. Ethology 107, 951–959.
| Red deer (Cervus elaphus) hinds discriminate between the roars of their current harem-holder stag and those of neighbouring stags.Crossref | GoogleScholarGoogle Scholar |
Rich, TJ, and Hurst, JL (1998). Scent marks as reliable signals of the competitive ability of mates. Animal Behaviour 56, 727–735.
| Scent marks as reliable signals of the competitive ability of mates.Crossref | GoogleScholarGoogle Scholar |
Robert, KA, and Schwanz, LE (2011). Emerging sex allocation research in mammals: marsupials and the pouch advantage. Mammal Review 41, 1–22.
| Emerging sex allocation research in mammals: marsupials and the pouch advantage.Crossref | GoogleScholarGoogle Scholar |
Roberts, SC, and Gosling, LM (2003). Genetic similarity and quality interact in mate choice decisions by female mice. Nature Genetics 35, 103–106.
| Genetic similarity and quality interact in mate choice decisions by female mice.Crossref | GoogleScholarGoogle Scholar |
RStudio Team (2018) RStudio: integrated development for R. (RStudio, Inc.: Boston, MA) Available at http://www.rstudio.com/
Russell, EM (1982). Parental investment and desertion of young in marsupials. The American Naturalist 119, 744–748.
| Parental investment and desertion of young in marsupials.Crossref | GoogleScholarGoogle Scholar |
Sato, A, Ozawa, N, and Karino, K (2014). Variation in female guppy preference for male olfactory and visual traits. Journal of Ethology 32, 137–143.
| Variation in female guppy preference for male olfactory and visual traits.Crossref | GoogleScholarGoogle Scholar |
Schulte-Hostedde, AI, Zinner, B, Millar, JS, and Hickling, GJ (2005). Restitution of mass–size residuals: validating body condition indices. Ecology 86, 155–163.
| Restitution of mass–size residuals: validating body condition indices.Crossref | GoogleScholarGoogle Scholar |
Scicluna, EL, Gill, BP, Robert, KA, and Cooper, S (2021). Fat-tailed dunnarts (Sminthopsis crassicaudata) of the Werribee grasslands: a case study of a species in decline. Australian Journal of Zoology 69, 27–32.
| Fat-tailed dunnarts (Sminthopsis crassicaudata) of the Werribee grasslands: a case study of a species in decline.Crossref | GoogleScholarGoogle Scholar |
Smith, MJ, Bennett, JH, and Chesson, CM (1978). Photoperiod and some other factors affecting reproduction in female Sminthopsis crassicaudata (Gould) (Marsupialia: Dasyuridae) in captivity. Australian Journal of Zoology 26, 449–463.
| Photoperiod and some other factors affecting reproduction in female Sminthopsis crassicaudata (Gould) (Marsupialia: Dasyuridae) in captivity.Crossref | GoogleScholarGoogle Scholar |
Steele, WK, and Brunner, H (2021). Contents of eastern barn owl ‘Tyto delicatula’ regurgitation pellets at the Werribee Sewege Farm, Victoria, suggest possible decline in abundance of fat-tailed dunnart ‘Sminthopsis crassicaudata’. The Victorian Naturalist 138, 171–175.
Trivers, RL, and Willard, DE (1973). Natural selection of parental ability to vary the sex ratio of offspring. Science 179, 90–92.
| Natural selection of parental ability to vary the sex ratio of offspring.Crossref | GoogleScholarGoogle Scholar |
Tyndale-Biscoe CH, Renfree M (1987) Breeding biology of marsupials by family. In ‘Reproductive Physiology of Marsupials’. pp. 16. (Cambridge University Press: New York)
Victorian Government (2023) Victorian Government Gazette No. G 8. Victorian Government Printer, Victoria.
Voigt, CC, Behr, O, Caspers, B, von Helversen, O, Knörnschild, M, Mayer, F, and Nagy, M (2008). Songs, scents, and senses: sexual selection in the greater sac-winged bat, Saccopteryx bilineata. Journal of Mammalogy 89, 1401–1410.
| Songs, scents, and senses: sexual selection in the greater sac-winged bat, Saccopteryx bilineata.Crossref | GoogleScholarGoogle Scholar |
Wedekind, C (2002). Manipulating sex ratios for conservation: short-term risks and long-term benefits. Animal Conservation 5, 13–20.
| Manipulating sex ratios for conservation: short-term risks and long-term benefits.Crossref | GoogleScholarGoogle Scholar |