Behavioural thermoregulation by Australian freshwater turtles: interspecific differences and implications for responses to climate change
Bruce C. Chessman
Centre for Ecosystem Science, School of Biological, Earth and Environmental Sciences, University of New South Wales, Sydney, NSW 2052, Australia. Email: brucechessman@gmail.com
Australian Journal of Zoology 67(2) 94-105 https://doi.org/10.1071/ZO20004
Submitted: 26 January 2020 Accepted: 23 April 2020 Published: 1 May 2020
Journal Compilation © CSIRO 2019 Open Access CC BY
Abstract
The abilities of freshwater turtles to control their body temperatures by behavioural means have implications for activity, food ingestion and digestion, growth, reproduction and potential responses to climate change. I compared various forms of basking in nature, and responses to aquatic and aerial photothermal gradients in the laboratory, among three species of Australian chelid turtles: Chelodina expansa, C. longicollis and Emydura macquarii. Proclivity for behavioural thermoregulation varied substantially among these species, being highest in C. longicollis and lowest in C. expansa. However, C. expansa had a thermophilic response to feeding. For C. longicollis and E. macquarii, behavioural thermoregulation may enhance colonisation of more southerly latitudes or higher elevations as climatic warming proceeds. However, increasing air temperatures may pose a hazard to turtles dispersing or sheltering terrestrially (for example, when water bodies dry during drought). C. longicollis appears the best placed of the three species to avoid this hazard through its abilities to thermoregulate behaviourally and to aestivate in terrestrial microenvironments that are buffered against temperature extremes.
Additional keywords: basking, Chelodina expansa, Chelodina longicollis, Emydura macquarii, temperature.
Introduction
The abilities of poikilothermic animals to control their body temperatures by behavioural means have growing significance as climate change alters their thermal environments (Kearney et al. 2009; Gvoždík 2012; Woods et al. 2015). In the case of reptiles, the potential for behavioural thermoregulation to modulate responses to climate change has been explored for several squamates (e.g. Aubret and Shine 2010; Buckley et al. 2015; Caldwell et al. 2017; Rubalcaba et al. 2019), but may also be relevant to turtles (Butler 2019).
Freshwater turtle species vary in their abilities and tendencies to alter their body temperatures by behavioural means. Individuals of many species leave the water periodically to bask in sunshine on logs or other objects, a behaviour termed aerial or atmospheric basking (Moll and Legler 1971; Ewert 1976). Hypothesised functions of aerial basking include both thermoregulatory and non-thermoregulatory benefits: elevation of body temperature to speed food digestion and enhance the availability of energy for growth and reproduction, vitamin D synthesis, drying of the skin and shell to assist ecdysis, reduction of body burdens of leeches, algae, fungi and bacteria, and resting in fast-flowing streams (Cagle 1950; Neil and Allen 1954; Boyer 1965; Shealy 1976; Acierno et al. 2006; Carrière et al. 2008; Bulté and Blouin-Demers 2010). Alternatively, turtles may float at the surface of water bodies with vertical thermal stratification, a behaviour known as aquatic basking (Moll and Legler 1971; Obbard and Brooks 1979). Semiaquatic basking also occurs, whereby a turtle rests on the bottom of shallow water or on a submerged object, with its carapace exposed to air (Boyer 1965; Auth 1975). Any form of basking necessitates a trade-off between its benefits and the associated sacrifice of foraging time, which may limit basking frequency and duration (Bulté and Blouin-Demers 2010; Clavijo-Baquet and Magnone 2017).
Several species of Australian chelid turtles have been observed to bask, but opinions about the thermoregulatory significance of this behaviour differ. Webb (1978) interpreted aerial basking as serving a thermoregulatory purpose because several behaviours of aerially basking captive chelids suggested prevention of overheating of the extremities while allowing the main body mass to warm to a desired temperature. However, Manning and Grigg (1997) found that aerial basking in a riverine population of Emydura signata resulted in only occasional elevation of body temperatures above water temperatures. These authors accordingly concluded that aerial basking was not of thermoregulatory significance in their study population, and suggested that the occasional elevated body temperatures that they observed could have been due to accidental exposure to solar radiation or ‘behavioural fever’ – a response to bacterial infection (Monagas and Gatten 1983). Manning and Grigg (1997) also speculated that aerial basking of E. signata might deliver non-thermoregulatory benefits such as inhibiting algal or fungal growth and promoting synthesis of vitamin D.
The capacity or incapacity of freshwater turtles to regulate their body temperatures has growing significance as human activities drive increases in average and extreme environmental temperatures globally. In Australia, average air temperatures have increased by ~1°C over the past century (Kirono et al. 2017), and a larger increase is likely in the present one (Grose et al. 2017). Correlative bioclimatic models predict substantial shifts in the distributions of Australian freshwater turtle species in response to projected climate change (Ihlow et al. 2012; James et al. 2017; Graham et al. 2019), but such models do not incorporate mechanistic considerations such as thermoregulatory ability, which may modify responses to rising temperatures (Kearney et al. 2009).
Here, I analyse data obtained in 1972–78 as part of a Ph.D. project at Monash University, Clayton, Victoria (Chessman 1978), and in minor follow-up studies in 1979–80, to assess the capacity for behavioural thermoregulation of three Australian species of chelid turtles (Chelodina expansa, C. longicollis and Emydura macquarii), and the possible influence of recent feeding. I analyse behaviour and body temperatures in nature and in two types of photothermal gradients in the laboratory. My aim is to shed further light on the capacity and propensity of Australian freshwater turtles for behavioural thermoregulation, its potential benefits, and the implications for responses to projected climate change. Whereas observations in natural environments can reveal the frequency of basking and its relationships to biological and environmental variables, experiments in artificial thermal gradients can ensure that preferred body temperatures are always attainable, in contrast to natural situations where such temperatures may be unachievable (Angilletta et al. 2002). Thus, field and laboratory studies can provide complementary information to understand thermoregulation.
Materials and methods
Study species
Chelodina expansa Gray, 1857, the broad-shelled turtle, is a long-necked species with a maximum carapace length of ~500 mm, distributed from south-eastern Queensland through western New South Wales and northern Victoria to south-eastern South Australia (Bower and Hodges 2014). It is carnivorous (Legler 1978; Chessman 1983b), inhabits running or standing water bodies that are permanent or near to permanent water (Chessman 1988a; Ocock et al. 2018), and is not known to aestivate.
Chelodina longicollis (Shaw, 1794), the eastern long-necked turtle, has a maximum carapace length of ~280 mm, and is naturally distributed from north Queensland to southern Victoria and south-eastern South Australia (Kennett et al. 2009). It is carnivorous (Chessman 1984b; Georges et al. 1986), occupies diverse running and standing water bodies, and is particularly adapted to temporary waters by a low rate of evaporative water loss, ability to aestivate, and proclivity for overland migration (Chessman 1984a, 1988a; Roe and Georges 2007, 2008a).
Emydura macquarii (Gray, 1830), the Macquarie turtle, is a polymorphic short-necked species (or species complex) with a maximum carapace length of >400 mm (Georges et al. 2006); it is widely distributed in eastern mainland Australia and adjacent islands (Georges et al. 2018). It is omnivorous (Chessman 1986; Spencer et al. 1998), inhabits running or standing waters that are permanent or near to permanent water (Chessman 1988a; Ocock et al. 2018), and is not known to aestivate. Emydura krefftii and E. signata are considered conspecific with E. macquarii by Georges and Thomson (2010).
Thermoregulation in nature
Turtles engaged in apparent aerial, aquatic or semiaquatic basking were observed in various parts of Victoria and southern New South Wales between 1972 and 1980, mainly in the Murray Valley between the Murray-Kulkyne Park (34.7°S, 142.5°E) and Torrumbarry (35.9°S, 144.5°E), and in the La Trobe Valley in Gippsland (38.1–38.2°S, 146.5–146.8°E). Some observations were opportunistic in the course of studies of various aspects of turtle ecology and others were targeted through visits to known basking sites.
Basking turtles were often too wary to be captured but some basking fully or partly out of the water were caught by approaching them rapidly from cover and enclosing them in a hand net. Some turtles floating in the warm surface layer of water bodies with vertical thermal stratification were captured by wading slowly into the water and extending a small net, attached to a 3–5-m-long handle, beneath them. Because turtles were generally observed for only a brief time before capture, the duration of prior basking was unknown and they were likely caught at various stages of the basking process.
Body temperatures of captured turtles were mostly determined with a Yellow Springs model 46 TUC telethermometer fitted with a 402 series probe, which was inserted through the cloacal vent for a distance of ~40–70% of the turtle’s carapace length. In a few cases, rectal temperatures were taken with a small-diameter mercury thermometer. Air, water-surface and water-bottom temperatures coincident with basking observations were measured with the telethermometer or a standard mercury thermometer.
Thermoregulation in the laboratory
Turtles for experiments in laboratory photothermal gradients were obtained from the Murray Valley, ranged in mass from 100 to 3300 g, and included juveniles, adult males and non-gravid adult females. Prior to experiments, which took place in February–September (first series) or throughout the year (second series), they were housed for several months in plastic tubs of tap water (24 ± 2°C), with artificial lighting for 12 h per day (0700–1900 hours), and fed chopped meat with vitamin and calcium supplements, worms and fish. In order to test the hypothesis that basking is promoted by feeding as a means of enhancing digestion, they were tested in both series in two nutritional states: with no feeding in the week before testing (hereafter, fasted) and immediately after feeding to satiation (hereafter, fed).
Experiments were done during the daylight portion of the cycle to which the turtles had been acclimated. For the first series, a vertical photothermal gradient in water was established by suspending three or four 250-W heat lamps above a cylindrical tank of tap water 0.58 m wide and 0.87 m deep. Room temperature and lamp height were manipulated to establish thermal gradients from cooler bottom to warmer surface water of ~26–33°C or ~29–36°C. However, stirring of the water by turtle movement resulted in some deviation from these temperatures. A vertical wooden ladder with rungs 0.1 m apart, attached to one internal face of the tank, enabled turtles to rest at various depths, but they were not able to exit the water. Turtles (five C. expansa, seven C. longicollis and 16 E. macquarii) were placed individually in the tank, allowed 1 h or more to adjust to their new environment, and then removed at intervals of ~1 h (occasionally ~2–3 h) for measurement of body temperature by telethermometer as in the field. Individual turtles were tested on multiple occasions with 1–4 measurements per session. The telethermometer was also used to measure surface (50-mm depth) and bottom water temperatures in the tank at the same times as turtle body temperatures.
For the second series, a horizontal photothermal gradient in air was established in a rectangular chamber 2.6 m long, 0.8 m wide and 0.8 m deep, with a floor of asbestos sheeting. Three 250-W heat lamps were suspended at equal intervals along the central axis of the chamber at successive heights of 0.2, 0.4 and 0.6 m, and room temperature was not controlled. Turtles (seven C. expansa, eight C. longicollis and 14 E. macquarii) were placed in the chamber individually or in small groups (2–6 turtles), on multiple occasions, and deep-body temperatures were determined at intervals as for the first series, with 1–6 measurements per turtle per session. The air temperature at the coldest point in the chamber was measured with a mercury thermometer at the same time as measurements of turtle body temperatures.
Statistical analysis
Linear mixed models with restricted maximum-likelihood estimation were used to relate turtle body temperatures to environmental temperature (random effect), species and nutritional status (fixed effects), and interactions of these predictors. Separate analyses were conducted for aerial basking in the field, aquatic basking in the field, and the two series of laboratory experiments. Relationships to air, water surface and water bottom temperatures were also analysed separately, because these temperatures were highly correlated with one another. No analysis was done for semiaquatic basking in the field because sample sizes were low, and information on nutritional status was available only for the experiments. Turtle identity, nested within species, was included in the models for the experiments because multiple measurements were made on the same individuals. Linear regressions were also calculated for relationships between body and environmental temperatures. All tests were performed with XLSTAT 2020.1 (Addinsoft 2020), and F and P values were based on Type III tests.
Results
Thermoregulation in nature
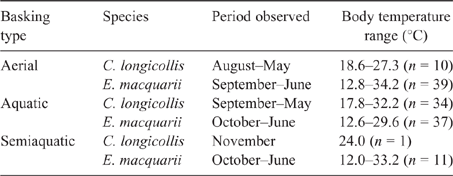
No basking was observed for C. expansa, but apparent aerial, aquatic and semiaquatic basking were observed for both C. longicollis and E. macquarii. Although lotic waters were studied extensively, 91% of aerial and 100% of aquatic and semiaquatic basking observations were in lentic waters, especially oxbow lakes and farm ponds in the La Trobe Valley for C. longicollis and Lake Boga in the Murray Valley for E. macquarii. Basking was observed at all times of year except mid-winter (Table 1), and all three forms of basking were associated with maximum body temperatures in the range 32–34°C (Table 1).
|
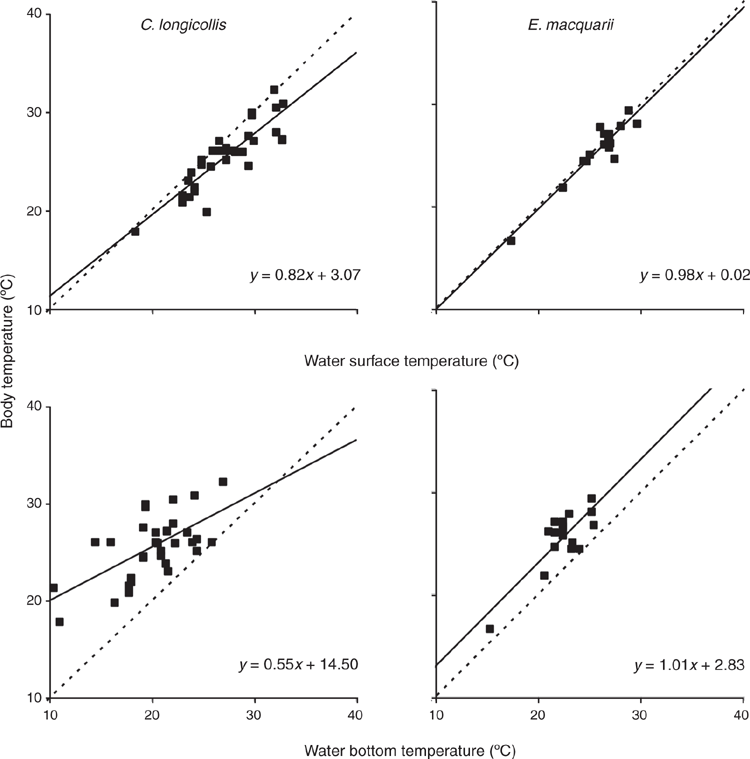
Body temperature during aerial basking was significantly related to simultaneous air and water surface temperatures, and there was a significant difference between species and a significant interaction between species and water surface temperature (Table 2). In C. longicollis, body temperature during aerial basking was essentially independent of water surface temperature, whereas in E. macquarii the two had a strong positive correlation (Fig. 1). Body temperature during aquatic basking was significantly related to both water surface and water bottom temperatures, being below or about equal to the former and above or about equal to the latter, and there was no significant difference between species (Table 2; Fig. 2). Semiaquatic basking produced body temperatures similar to simultaneous water surface temperatures and above simultaneous water bottom temperatures.
Thermoregulation in the laboratory
Experiments with C. expansa in the vertical photothermal gradient in water were discontinued after initial testing because this species seldom left the bottom of the tank. However, C. longicollis and E. macquarii frequently swam or rested in the tank’s upper or middle parts, achieving body temperatures that were often well above water bottom temperatures and sometimes about equal to water surface temperatures (Figs 3 and 4). Body temperatures of these species were significantly related to surface and bottom water temperatures, and there was a significant difference between species (Table 2), with body temperatures of E. macquarii tending to be more elevated above water bottom temperatures than those of C. longicollis (Fig. 4). The main effect of nutritional status was not significant but there were significant interactions between species and nutritional status (Table 2), due to a slight tendency for C. longicollis to have higher body temperatures when fed than when fasted, and for E. macquarii to have higher body temperatures when fasted than when fed (Figs 3 and 4). Slopes of regressions of body on environmental temperatures were always substantially <1, especially for C. longicollis (Figs 3 and 4).
In the horizontal photothermal gradient in air, minimum air temperatures ranged from 6.4 to 29.5°C. The highest body temperature was 34.4°C and only four of 562 body temperatures exceeded 32.0°C, even though turtles could probably have achieved higher temperatures by basking beneath the lowest lamp for extended periods. C. longicollis and E. macquarii sometimes assumed an apparent basking posture under one of the lamps, but this behaviour was never observed in C. expansa. The main effects of minimum air temperature, species and nutritional status on body temperature were all significant, as was the interaction between species and nutritional status (Table 2). All three species achieved body temperatures well above minimum air temperatures when the latter was low, but similar to or slightly below minimum air temperatures when the latter was high (Fig. 5). The notable difference among the species was that fed C. expansa achieved higher body temperatures than fasted C. expansa when minimum air temperature was low, whereas for the other two species the relationship between body temperature and minimum air temperature was independent of nutritional status (Fig. 5). Slopes of regressions of body on environmental temperatures were always appreciably <1 (Fig. 5).
Discussion
All three species considered in this study showed some capacity to buffer their body temperatures against ambient temperature variation in environments that enabled them to do so. This capacity was demonstrated by slopes substantially <1 for regressions of body on environmental temperature both in nature and in the laboratory, particularly for C. longicollis (Figs 1–5). For example, in the vertical photothermal gradient in water in the laboratory, mean body temperatures of C. longicollis and E. macquarii were quite stable as surface and bottom water temperatures varied over a range of ~8°C (Figs 3 and 4). The turtles could have achieved this stability only by tending to select shallower water when ambient temperatures across the gradient were lower and deeper water when ambient temperatures across the gradient were higher.
The high thermoregulatory ability of C. longicollis may relate to its proclivity for terrestrial dispersal (Roe and Georges 2007, 2008a), because terrestrial activity exposes turtles to a greater range of environmental temperatures than those experienced in the water, including potentially lethal temperatures. However, terrestrial activity of C. longicollis often coincides with rainfall (Roe and Georges 2008b; Santori et al. 2018), which likely reduces risks of both overheating and dehydration.
C. expansa was never observed to bask, either aerially or aquatically. Unlike the other two species, C. expansa is an ambush predator (Legler 1978; Chessman 1983b), and this feeding mode may require it to spend large amounts of time resting on the bottom of water bodies, or even buried in sediment with only the head exposed, waiting for prey to approach. Such a necessity could preclude devoting time to basking, which can be rare in other aquatic turtles that are ambush feeders, such as North American chelydrids (Ewert 1976; Brown et al. 1990; Brown and Brooks 1991; Harrel et al. 1996).
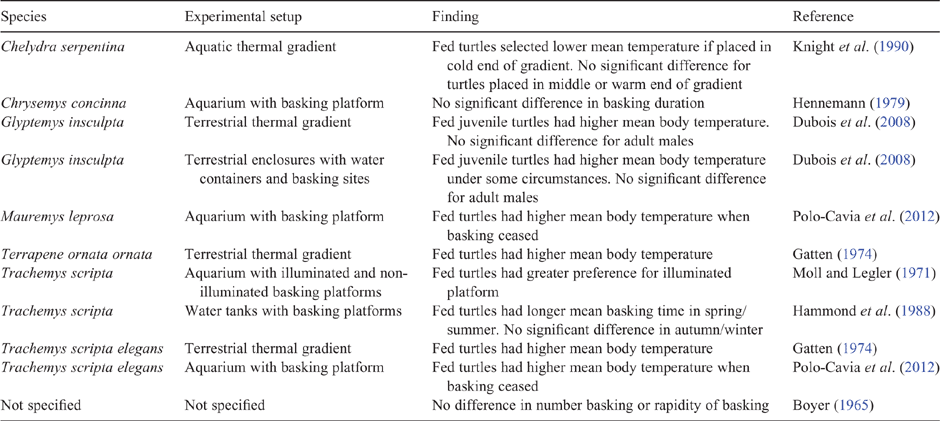
The effect of nutritional status varied among the three species and between the two experimental series. Previous research has also shown that the effect of nutritional status on basking behaviour, environmental temperature selection and body temperatures of turtles varies among both species and experimental conditions (Table 3). It may seem surprising that the effect of feeding was most evident in C. expansa, given its apparent reluctance to bask. However, the principal distinction was that when air temperature was low, fasted C. expansa tended to have lower body temperatures than fed C. expansa and both fed and fasted individuals of the other two species (Fig. 5). Thus a combination of fasting and low ambient temperature apparently reduced the thermophily of C. expansa, possibly by inducing a degree of dormancy given that this species appears to be the least cold adapted of the three (Chessman 1988b).
|
The observation that fasted individuals of C. longicollis and E. macquarii often selected warm and irradiated microenvironments when offered the opportunity to do so suggests that basking in those species may serve purposes other than enhancing food digestion, at least in part. For example, aerial basking, and perhaps even aquatic basking at the surface, might be explained by a requirement for periodic exposure to solar radiation to promote synthesis of vitamin D. This function could account for aerial basking of freshwater turtles often being an occasional activity (Manning and Grigg 1997; Singh 2018) that may occur especially on the first sunny day after a period of cloudy weather (Moll and Legler 1971; Auth 1975), or upon being released after a stay in captivity without basking opportunities (Shealy 1976).
In some cases, aerial basking by freshwater turtles may be motivated by ill health. For example, Dodd (1988) found that 20 of 32 aerially basking Sternotherus depressus that were examined for disease had advanced symptoms, and Ibáñez et al. (2014) reported that male Mauremys leprosa that spent more time aerially basking tended to have lower white blood cell counts and a higher frequency of infection with Hepatozoon spp. Leech removal has been suggested as an advantage of aerial basking (McAuliffe 1977), and detachment of leeches and harvesting by birds from aerially basking turtles have been reported (Vogt 1980; Selman and Qualls 2008, 2009). However, Readel et al. (2008) questioned this benefit because leeches can be very tolerant of desiccation. Chessman (1987) found that E. macquarii captured during aerial basking at Lake Boga had a higher incidence of obvious ailments (e.g. ulcers, lesions and eye infections) and parasitism by leeches than non-basking conspecifics. However, the difference was statistically significant only for a burden of more than five leeches.
C. expansa, C. longicollis and E. macquarii all appeared to consistently avoid raising body temperatures above ~34°C, a maximum close to that found for E. signata by Manning and Grigg (1997), and one that leaves a narrow safety margin to thresholds for thermal stress. The onset of uncoordinated movements has been observed at head and posterior body temperatures of ~38–39°C and ~31–36°C respectively in C. longicollis, and ~37–38°C and ~36–40°C respectively in E. macquarii (Webb and Johnson 1972). Heat-induced muscular spasms have been found to commence at head temperatures of ~42–44°C in C. longicollis and body temperatures of ~39°C in C. expansa, ~39–42°C in C. longicollis and ~40°C in Emydura krefftii (Burbidge 1967; Webb and Johnson 1972; Webb and Witten 1973). However, the physiological performance of ectotherms typically peaks close to their upper thermal tolerances (Kearney et al. 2009), and aerially basking aquatic cryptodires may leave an even slimmer safety margin, with reported body temperatures as high as 41.5°C (Rowe and Dalgarn 2009).
Average air and water temperatures in south-eastern Australia are projected to increase by ~2°C by the late 21st century (van Vliet et al. 2013; Olson et al. 2016). Such environmental warming could present both opportunities and threats to freshwater turtles: enhanced activity, feeding and growth when in the water, subject to availability of aquatic habitat and food, but increased risk of overheating and dehydration when on land (Chessman 2018). The current results suggest that, of the three species studied, C. longicollis will have the greatest capacity to behaviourally exploit the opportunities and avoid the threats, and C. expansa the least. For the latter, the response to warming is likely to be passive, with some range expansion where its distribution is limited by its apparently low level of adaptation to cold conditions (Chessman 1988b). For C. longicollis and E. macquarii, aerial or aquatic basking may enhance the potential to colonise more southerly latitudes or higher elevations, including where translocation by humans overcomes biogeographic barriers to dispersal, as in the introduction of C. longicollis to northern Tasmania (Fearn 2013). Aerial basking may also be of some advantage in rivers affected by releases of cold, hypolimnetic water from dams, although Singh (2018) inferred little such benefit for E. macquarii in the Murray River downstream of the Hume Dam. Even if the motivation for basking of C. longicollis and E. macquarii is primarily non-thermoregulatory, aquatic basking has the effect of intermittently raising body temperatures above bottom water temperatures, and aerial basking of raising body temperatures above surface water temperatures. Consequently, both forms of basking are likely to amplify food ingestion and digestion to some degree, because both of these processes are enhanced at higher body temperatures in various freshwater turtle species (Kepenis and McManus 1974; Parmenter 1981; Avery et al. 1993; Spencer et al. 1998; Mitchell et al. 2012).
Increases in water temperatures are unlikely to be sufficient to threaten C. expansa, C. longicollis and E. macquarii at the warmer and more arid extremes of their ranges (Chessman 2018). However, given their critical thermal maxima of only ~40°C, higher air temperatures may be hazardous to turtles dispersing or sheltering terrestrially, for example as a result of water bodies drying under projected increases in the frequency and intensity of drought (Dai 2013; Feng et al. 2019). C. longicollis appears best placed to avoid this risk through its ability to thermoregulate behaviourally as well as its capacity for aestivation.
C. longicollis cannot swallow food out of water (author’s obs.), and the duration of its aestivation in terrestrial environments could be limited by either starvation (Roe et al. 2008) or dehydration (Chessman 1978, 1984a). Chessman (1978) estimated that without access to water, a C. longicollis with a mass of 1 kg and could withstand evaporative water loss for a period of only 40 days at 34°C and 25% relative humidity, but 200 days at 8°C and 60% relative humidity, the lowest temperature and highest humidity considered. Roe and Georges (2007, 2008a) documented terrestrial aestivation of C. longicollis for apparently continuous periods of up to 480 days, although the turtles were monitored only monthly from April to August. These observations do not conflict with Chessman’s (1978) predictions because temperature and humidity adjacent to the aestivating turtles were not reported, and they probably had intermittent opportunities to drink pooled rainwater (Roe et al. 2008). Since both metabolism and evaporative water loss of C. longicollis are positively related to temperature (Chessman 1984a, 2018), its apparent ability to select cooler microenvironments for aestivation (Chessman 1983a; Beck 1991), and the availability of such microenvironments, could substantially affect its survival of prolonged drought.
Conflicts of interest
The author declares no conflicts of interest.
Acknowledgements
The Ph.D. project at Monash University in 1972–78 was supervised by Dr P. S. Lake, Associate Professor A. K. Lee and Professor J. W. Warren, and permits for research in 1972–80 were issued by the NSW National Parks and Wildlife Service, NSW State Fisheries and the Victorian Fisheries and Wildlife Division. This research did not receive any specific funding.
References
Acierno, M. J., Mitchell, M. A., Roundtree, M. K., and Zachariah, T. T. (2006). Effects of ultraviolet radiation on 25-hydroxyvitamin D3 synthesis in red-eared slider turtles (Trachemys scripta elegans). American Journal of Veterinary Research 67, 2046–2049.| Effects of ultraviolet radiation on 25-hydroxyvitamin D3 synthesis in red-eared slider turtles (Trachemys scripta elegans).Crossref | GoogleScholarGoogle Scholar | 17144809PubMed |
Addinsoft (2020). XLSTAT statistical and data analysis solution. Addinsoft, Long Island, NY. Available at: https://www.xlstat.com
Angilletta, M. J., Niewiarowski, P. H., and Navas, C. A. (2002). The evolution of thermal physiology in ectotherms. Journal of Thermal Biology 27, 249–268.
| The evolution of thermal physiology in ectotherms.Crossref | GoogleScholarGoogle Scholar |
Aubret, F., and Shine, R. (2010). Thermal plasticity in young snakes: how will climate change affect the thermoregulatory tactics of ectotherms? The Journal of Experimental Biology 213, 242–248.
| Thermal plasticity in young snakes: how will climate change affect the thermoregulatory tactics of ectotherms?Crossref | GoogleScholarGoogle Scholar | 20038657PubMed |
Auth, D. L. (1975). Behavioral ecology of basking in the yellow-bellied turtle, Chrysemys scripta scripta (Schoepff). Bulletin of the Florida State Museum Biological Sciences 20, 1–45.
Avery, H. W., Spotila, J. R., Congdon, J. D., Fischer, R. U., Standora, E. A., and Avery, S. B. (1993). Roles of diet protein and temperature in the growth and nutritional energetics of juvenile slider turtles, Trachemys scripta. Physiological Zoology 66, 902–925.
| Roles of diet protein and temperature in the growth and nutritional energetics of juvenile slider turtles, Trachemys scripta.Crossref | GoogleScholarGoogle Scholar |
Beck, R. G. (1991). The common longnecked tortoise Chelodina longicollis (Shaw 1802) (Testudinata: Chelidae): a comparative study of the morphology and field behaviour of disjunct populations. South Australian Naturalist 66, 4–22.
Bower, D. S., and Hodges, K. M. (2014). Chelodina expansa Gray 1857 – broad-shelled turtle, giant snake-necked turtle. In ‘Conservation Biology of Freshwater Turtles and Tortoises: a Compilation Project of the IUCN/SSC Tortoise and Freshwater Turtle Specialist Group’. (Eds A. G. J. Rhodin, P. C. H. Pritchard, P. P. van Dijk, R. A. Saumure, K. A. Buhlmann, J. B. Iverson, and R. A. Mittermeier.) Chelonian Research Monographs 5, 071.1–071.5.
Boyer, D. R. (1965). Ecology of the basking habit in turtles. Ecology 46, 99–118.
| Ecology of the basking habit in turtles.Crossref | GoogleScholarGoogle Scholar |
Brown, G. P., and Brooks, R. J. (1991). Thermal and behavioral responses to feeding in free-ranging turtles, Chelydra serpentina. Journal of Herpetology 25, 273–278.
| Thermal and behavioral responses to feeding in free-ranging turtles, Chelydra serpentina.Crossref | GoogleScholarGoogle Scholar |
Brown, G. P., Brooks, R. J., and Layfield, J. A. (1990). Radiotelemetry of body temperatures of free-ranging snapping turtles (Chelydra serpentina) during summer. Canadian Journal of Zoology 68, 1659–1663.
| Radiotelemetry of body temperatures of free-ranging snapping turtles (Chelydra serpentina) during summer.Crossref | GoogleScholarGoogle Scholar |
Buckley, L. B., Ehrenberger, J. C., and Angilletta, M. J. (2015). Thermoregulatory behaviour limits local adaptation of thermal niches and confers sensitivity to climate change. Functional Ecology 29, 1038–1047.
| Thermoregulatory behaviour limits local adaptation of thermal niches and confers sensitivity to climate change.Crossref | GoogleScholarGoogle Scholar |
Bulté, G., and Blouin-Demers, G. (2010). Estimating the energetic significance of basking behaviour in a temperate-zone turtle. Ecoscience 17, 387–393.
| Estimating the energetic significance of basking behaviour in a temperate-zone turtle.Crossref | GoogleScholarGoogle Scholar |
Burbidge, A. A. (1967). The biology of south-western Australian tortoises. Ph.D. Thesis, University of Western Australia, Perth.
Butler, C. J. (2019). A review of the effects of climate change on chelonians. Diversity 11, 138.
| A review of the effects of climate change on chelonians.Crossref | GoogleScholarGoogle Scholar |
Cagle, F. R. (1950). The life history of the slider turtle, Pseudemys scripta troostii (Holbrook). Ecological Monographs 20, 31–54.
| The life history of the slider turtle, Pseudemys scripta troostii (Holbrook).Crossref | GoogleScholarGoogle Scholar |
Caldwell, A. J., While, G. M., and Wapstra, E. (2017). Plasticity of thermoregulatory behaviour in response to the thermal environment by widespread and alpine reptile species. Animal Behaviour 132, 217–227.
| Plasticity of thermoregulatory behaviour in response to the thermal environment by widespread and alpine reptile species.Crossref | GoogleScholarGoogle Scholar |
Carrière, M.-A., Rollinson, N., Suley, A. N., and Brooks, R. J. (2008). Thermoregulation when the growing season is short: sex-biased basking patterns in a northern population of painted turtles (Chrysemys picta). Journal of Herpetology 42, 206–209.
| Thermoregulation when the growing season is short: sex-biased basking patterns in a northern population of painted turtles (Chrysemys picta).Crossref | GoogleScholarGoogle Scholar |
Chessman, B. C. (1978). Ecological studies of freshwater turtles in south-eastern Australia. Ph.D. Thesis, Monash University, Melbourne.
Chessman, B. C. (1983a). A note on aestivation in the snake-necked turtle, Chelodina longicollis (Shaw) (Testudines: Chelidae). Herpetofauna 14, 96–97.
Chessman, B. C. (1983b). Observations on the diet of the broad-shelled turtle, Chelodina expansa Gray (Testudines: Chelidae). Australian Wildlife Research 10, 169–172.
| Observations on the diet of the broad-shelled turtle, Chelodina expansa Gray (Testudines: Chelidae).Crossref | GoogleScholarGoogle Scholar |
Chessman, B. C. (1984a). Evaporative water loss from three south-eastern Australian species of freshwater turtle. Australian Journal of Zoology 32, 649–655.
| Evaporative water loss from three south-eastern Australian species of freshwater turtle.Crossref | GoogleScholarGoogle Scholar |
Chessman, B. C. (1984b). Food of the snake-necked turtle, Chelodina longicollis (Shaw) (Testudines: Chelidae) in the Murray Valley, Victoria and New South Wales. Australian Wildlife Research 11, 573–578.
| Food of the snake-necked turtle, Chelodina longicollis (Shaw) (Testudines: Chelidae) in the Murray Valley, Victoria and New South Wales.Crossref | GoogleScholarGoogle Scholar |
Chessman, B. C. (1986). Diet of the Murray turtle, Emydura macquarii (Gray) (Testudines: Chelidae). Australian Wildlife Research 13, 65–69.
| Diet of the Murray turtle, Emydura macquarii (Gray) (Testudines: Chelidae).Crossref | GoogleScholarGoogle Scholar |
Chessman, B. C. (1987). Atmospheric and aquatic basking of the Australian freshwater turtle Emydura macquarii (Gray) (Testudines: Chelidae). Herpetologica 43, 301–306.
Chessman, B. C. (1988a). Habitat preferences of freshwater turtles in the Murray Valley, Victoria and New South Wales. Australian Wildlife Research 15, 485–491.
| Habitat preferences of freshwater turtles in the Murray Valley, Victoria and New South Wales.Crossref | GoogleScholarGoogle Scholar |
Chessman, B. C. (1988b). Seasonal and diel activity of freshwater turtles in the Murray Valley, Victoria and New South Wales. Australian Wildlife Research 15, 267–276.
| Seasonal and diel activity of freshwater turtles in the Murray Valley, Victoria and New South Wales.Crossref | GoogleScholarGoogle Scholar |
Chessman, B. C. (2018). Effects of temperature and exercise on metabolism of three species of Australian freshwater turtles: implications for responses to climate change. Australian Journal of Zoology 66, 317–325.
| Effects of temperature and exercise on metabolism of three species of Australian freshwater turtles: implications for responses to climate change.Crossref | GoogleScholarGoogle Scholar |
Clavijo-Baquet, S., and Magnone, L. (2017). Daily and seasonal basking behavior in two South American freshwater turtles, Trachemys dorbigni and Phrynops hilarii. Chelonian Conservation and Biology 16, 62–69.
| Daily and seasonal basking behavior in two South American freshwater turtles, Trachemys dorbigni and Phrynops hilarii.Crossref | GoogleScholarGoogle Scholar |
Dai, A. (2013). Increasing drought under global warming in observations and models. Nature Climate Change 3, 52–58.
| Increasing drought under global warming in observations and models.Crossref | GoogleScholarGoogle Scholar |
Dodd, C. K. (1988). Disease and population declines in the flattened musk turtle Sternotherus depressus. American Midland Naturalist 119, 394–401.
| Disease and population declines in the flattened musk turtle Sternotherus depressus.Crossref | GoogleScholarGoogle Scholar |
Dubois, Y., Blouin-Demers, G., and Thomas, D. (2008). Temperature selection in wood turtles (Glyptemys insculpta) and its implications for energetics. Ecoscience 15, 398–406.
| Temperature selection in wood turtles (Glyptemys insculpta) and its implications for energetics.Crossref | GoogleScholarGoogle Scholar |
Ewert, M. A. (1976). Nests, nesting and aerial basking of Macroclemys under natural conditions, and comparisons with Chelydra (Testudines: Chelydridae). Herpetologica 32, 150–156.
Fearn, S. (2013). Successful incubation of eggs for a free-ranging invasive turtle (Testudines: Chelidae: Chelodina longicollis) in Tasmania. Tasmanian Naturalist 135, 2–8.
Feng, P., Liu, D. L., Wang, B., Waters, C., Zhang, M., and Yu, Q. (2019). Projected changes in drought across the wheat belt of southeastern Australia using a downscaled climate ensemble. International Journal of Climatology 39, 1041–1053.
| Projected changes in drought across the wheat belt of southeastern Australia using a downscaled climate ensemble.Crossref | GoogleScholarGoogle Scholar |
Gatten, R. E. (1974). Effect of nutritional status on the preferred body temperature of the turtles Pseudemys scripta and Terrapene ornata. Copeia 1974, 912–917.
| Effect of nutritional status on the preferred body temperature of the turtles Pseudemys scripta and Terrapene ornata.Crossref | GoogleScholarGoogle Scholar |
Georges, A., and Thomson, S. (2010). Diversity of Australasian freshwater turtles, with an annotated synonymy and keys to species. Zootaxa 2496, 1–37.
| Diversity of Australasian freshwater turtles, with an annotated synonymy and keys to species.Crossref | GoogleScholarGoogle Scholar |
Georges, A., Norris, R. H., and Wensing, L. (1986). Diet of the freshwater turtle Chelodina longicollis (Testudines: Chelidae) from the coastal dune lakes of the Jervis Bay Territory. Australian Wildlife Research 13, 301–308.
| Diet of the freshwater turtle Chelodina longicollis (Testudines: Chelidae) from the coastal dune lakes of the Jervis Bay Territory.Crossref | GoogleScholarGoogle Scholar |
Georges, A., Guarino, F., and White, M. (2006). Sex-ratio bias across populations of a freshwater turtle (Testudines: Chelidae) with genotypic sex determination. Wildlife Research 33, 475–480.
| Sex-ratio bias across populations of a freshwater turtle (Testudines: Chelidae) with genotypic sex determination.Crossref | GoogleScholarGoogle Scholar |
Georges, A., Gruber, B., Pauly, G. B., White, D., Adams, M., Young, M. J., Kilian, A., Zhang, X., Shaffer, H. B., and Unmack, P. J. (2018). Genomewide SNP markers breathe new life into phylogeography and species delimitation for the problematic short-necked turtles (Chelidae: Emydura) of eastern Australia. Molecular Ecology 27, 5195–5213.
| Genomewide SNP markers breathe new life into phylogeography and species delimitation for the problematic short-necked turtles (Chelidae: Emydura) of eastern Australia.Crossref | GoogleScholarGoogle Scholar | 30403418PubMed |
Graham, E. M., Reside, A. E., Atkinson, I., Baird, D., Hodgson, L., James, C. S., and Van Der Wal, J. J. (2019). Climate change and biodiversity in Australia: a systematic modelling approach to nationwide species distributions. Australasian Journal of Environmental Management 26, 112–123.
| Climate change and biodiversity in Australia: a systematic modelling approach to nationwide species distributions.Crossref | GoogleScholarGoogle Scholar |
Grose, M. R., Colman, R., Bhend, J., and Moise, A. F. (2017). Limits to global and Australian temperature change this century based on expert judgment of climate sensitivity. Climate Dynamics 48, 3325–3339.
| Limits to global and Australian temperature change this century based on expert judgment of climate sensitivity.Crossref | GoogleScholarGoogle Scholar |
Gvoždík, L. (2012). Plasticity of preferred body temperatures as means of coping with climate change? Biology Letters 8, 262–265.
| Plasticity of preferred body temperatures as means of coping with climate change?Crossref | GoogleScholarGoogle Scholar | 22072284PubMed |
Hammond, K. A., Spotila, J. R., and Standora, E. A. (1988). Basking behavior of the turtle Pseudemys scripta: effects of digestive state, acclimation temperature, sex, and season. Physiological Zoology 61, 69–77.
| Basking behavior of the turtle Pseudemys scripta: effects of digestive state, acclimation temperature, sex, and season.Crossref | GoogleScholarGoogle Scholar |
Harrel, J. B., Allen, C. M., and Hebert, S. J. (1996). Movements and habitat use of subadult alligator snapping turtles (Macroclemys temminckii) in Louisiana. American Midland Naturalist 135, 60–67.
| Movements and habitat use of subadult alligator snapping turtles (Macroclemys temminckii) in Louisiana.Crossref | GoogleScholarGoogle Scholar |
Hennemann, W. W. (1979). The influence of environmental cues and nutritional status on frequency of basking in juvenal Suwannee terrapins (Chrysemys concinna). Herpetologica 35, 129–131.
Ibáñez, A., Marzal, A., González-Blázquez, M., López, P., and Martín, J. (2014). Basking activity is modulated by health state but is constrained by conspicuousness to predators in male Spanish terrapins. Ethology 120, 1–10.
| Basking activity is modulated by health state but is constrained by conspicuousness to predators in male Spanish terrapins.Crossref | GoogleScholarGoogle Scholar |
Ihlow, F., Dambach, J., Engler, J. O., Flecks, M., Hartmann, T., Nekum, S., Rajaei, H., and Rödder, D. (2012). On the brink of extinction? How climate change may affect global chelonian species richness and distribution. Global Change Biology 18, 1520–1530.
| On the brink of extinction? How climate change may affect global chelonian species richness and distribution.Crossref | GoogleScholarGoogle Scholar |
James, C. S., Reside, A. E., Van Der Wal, J., Pearson, R. G., Burrows, D., Capon, S. J., Harwood, T. D., Hodgson, L., and Waltham, N. J. (2017). Sink or swim? Potential for high faunal turnover in Australian rivers under climate change. Journal of Biogeography 44, 489–501.
| Sink or swim? Potential for high faunal turnover in Australian rivers under climate change.Crossref | GoogleScholarGoogle Scholar |
Kearney, M., Shine, R., and Porter, W. P. (2009). The potential for behavioral thermoregulation to buffer “cold-blooded” animals against climate warming. Proceedings of the National Academy of Sciences of the United States of America 106, 3835–3840.
| The potential for behavioral thermoregulation to buffer “cold-blooded” animals against climate warming.Crossref | GoogleScholarGoogle Scholar | 19234117PubMed |
Kennett, R., Roe, J., Hodges, K., and Georges, A. (2009). Chelodina longicollis (Shaw 1794) – eastern long-necked turtle, common long-necked turtle, common snake-necked turtle. In ‘Conservation Biology of Freshwater Turtles and Tortoises: a Compilation Project of the IUCN/SSC Tortoise and Freshwater Turtle Specialist Group’. (Eds A. G. J. Rhodin, P. C. H. Pritchard, P. P. van Dijk, R. A. Saumure, K. A. Buhlmann, J. B. Iverson, and R. A. Mittermeier.) Chelonian Research Monographs 5, 031.1–031.8.
Kepenis, V., and McManus, J. J. (1974). Bioenergetics of young painted turtles, Chrysemys picta. Comparative Biochemistry and Physiology Part A: Physiology 48, 309–317.
| Bioenergetics of young painted turtles, Chrysemys picta.Crossref | GoogleScholarGoogle Scholar |
Kirono, D. G. C., Hennessy, K. J., and Grose, M. R. (2017). Increasing risk of months with low rainfall and high temperature in southeast Australia for the past 150 years. Climate Risk Management 16, 10–21.
| Increasing risk of months with low rainfall and high temperature in southeast Australia for the past 150 years.Crossref | GoogleScholarGoogle Scholar |
Knight, T. W., Layfield, J. A., and Brooks, R. J. (1990). Nutritional status and mean selected temperature of hatchling snapping turtles (Chelydra serpentina): is there a thermophilic response to feeding? Copeia 1990, 1067–1072.
| Nutritional status and mean selected temperature of hatchling snapping turtles (Chelydra serpentina): is there a thermophilic response to feeding?Crossref | GoogleScholarGoogle Scholar |
Legler, J. M. (1978). Observations on behavior and ecology in an Australian turtle, Chelodina expansa (Testudines: Chelidae). Canadian Journal of Zoology 56, 2449–2453.
| Observations on behavior and ecology in an Australian turtle, Chelodina expansa (Testudines: Chelidae).Crossref | GoogleScholarGoogle Scholar |
Manning, B., and Grigg, G. C. (1997). Basking is not of thermoregulatory significance in the “basking” freshwater turtle Emydura signata. Copeia 1997, 579–584.
| Basking is not of thermoregulatory significance in the “basking” freshwater turtle Emydura signata.Crossref | GoogleScholarGoogle Scholar |
McAuliffe, J. R. (1977). An hypothesis explaining variations of haemogregarine parasitemia in different aquatic turtle species. The Journal of Parasitology 63, 580–581.
| An hypothesis explaining variations of haemogregarine parasitemia in different aquatic turtle species.Crossref | GoogleScholarGoogle Scholar | 405469PubMed |
Mitchell, N. J., Jones, T. V., and Kuchling, G. (2012). Simulated climate change increases juvenile growth in a critically endangered tortoise. Endangered Species Research 17, 73–82.
| Simulated climate change increases juvenile growth in a critically endangered tortoise.Crossref | GoogleScholarGoogle Scholar |
Moll, E. O., and Legler, J. M. (1971). The life history of a neotropical slider turtle, Pseudemys scripta (Schoepff), in Panama. Bulletin of the Los Angeles County Museum of Natural History Science, No. 11, 1–102.
Monagas, W. R., and Gatten, R. E. (1983). Behavioural fever in the turtles Terrapene carolina and Chrysemys picta. Journal of Thermal Biology 8, 285–288.
| Behavioural fever in the turtles Terrapene carolina and Chrysemys picta.Crossref | GoogleScholarGoogle Scholar |
Neil, W. T., and Allen, E. R. (1954). Algae on turtles: some additional considerations. Ecology 35, 581–584.
| Algae on turtles: some additional considerations.Crossref | GoogleScholarGoogle Scholar |
Obbard, M. E., and Brooks, R. J. (1979). Factors affecting basking in a northern population of the common snapping turtle, Chelydra serpentina. Canadian Journal of Zoology 57, 435–440.
| Factors affecting basking in a northern population of the common snapping turtle, Chelydra serpentina.Crossref | GoogleScholarGoogle Scholar |
Ocock, J. F., Bino, G., Wassens, S., Spencer, J., Thomas, R. F., and Kingsford, R. T. (2018). Identifying critical habitat for Australian freshwater turtles in a large regulated floodplain: implications for environmental water management. Environmental Management 61, 375–389.
| Identifying critical habitat for Australian freshwater turtles in a large regulated floodplain: implications for environmental water management.Crossref | GoogleScholarGoogle Scholar | 28280912PubMed |
Olson, R., Evans, J. P., Di Luca, A., and Argüeso, D. (2016). The NARCliM project: model agreement and significance of climate projections. Climate Research 69, 209–227.
| The NARCliM project: model agreement and significance of climate projections.Crossref | GoogleScholarGoogle Scholar |
Parmenter, R. R. (1981). Digestive turnover rates in freshwater turtles: the influence of temperature and body size. Comparative Biochemistry and Physiology Part A: Physiology 70, 235–238.
| Digestive turnover rates in freshwater turtles: the influence of temperature and body size.Crossref | GoogleScholarGoogle Scholar |
Polo-Cavia, N., López, P., and Martín, J. (2012). Feeding status and basking requirements of freshwater turtles in an invasion context. Physiology & Behavior 105, 1208–1213.
| Feeding status and basking requirements of freshwater turtles in an invasion context.Crossref | GoogleScholarGoogle Scholar |
Readel, A. M., Phillips, C. A., and Wetzel, M. J. (2008). Leech parasitism in a turtle assemblage: effects of host and environmental characteristics. Copeia 2008, 227–233.
| Leech parasitism in a turtle assemblage: effects of host and environmental characteristics.Crossref | GoogleScholarGoogle Scholar |
Roe, J. H., and Georges, A. (2007). Heterogeneous wetland complexes, buffer zones, and travel corridors: landscape management for freshwater reptiles. Biological Conservation 135, 67–76.
| Heterogeneous wetland complexes, buffer zones, and travel corridors: landscape management for freshwater reptiles.Crossref | GoogleScholarGoogle Scholar |
Roe, J. H., and Georges, A. (2008a). Maintenance of variable responses for coping with wetland drying in freshwater turtles. Ecology 89, 485–494.
| Maintenance of variable responses for coping with wetland drying in freshwater turtles.Crossref | GoogleScholarGoogle Scholar | 18409437PubMed |
Roe, J. H., and Georges, A. (2008b). Terrestrial activity, movements and spatial ecology of an Australian freshwater turtle, Chelodina longicollis, in a temporally dynamic wetland system. Austral Ecology 33, 1045–1056.
| Terrestrial activity, movements and spatial ecology of an Australian freshwater turtle, Chelodina longicollis, in a temporally dynamic wetland system.Crossref | GoogleScholarGoogle Scholar |
Roe, J. H., Georges, A., and Green, B. (2008). Energy and water flux during terrestrial estivation and overland movement in a freshwater turtle. Physiological and Biochemical Zoology 81, 570–583.
| Energy and water flux during terrestrial estivation and overland movement in a freshwater turtle.Crossref | GoogleScholarGoogle Scholar | 18717626PubMed |
Rowe, J. W., and Dalgarn, S. F. (2009). Effects of sex and microhabitat use on diel body temperature variation in midland painted turtles (Chrysemys picta marginata). Copeia 2009, 85–92.
| Effects of sex and microhabitat use on diel body temperature variation in midland painted turtles (Chrysemys picta marginata).Crossref | GoogleScholarGoogle Scholar |
Rubalcaba, J. G., Gouveia, S. F., and Olalla-Tárraga, M. A. (2019). Upscaling microclimatic conditions into body temperature distributions of ectotherms. American Naturalist 193, 677–687.
| Upscaling microclimatic conditions into body temperature distributions of ectotherms.Crossref | GoogleScholarGoogle Scholar | 31002566PubMed |
Santori, C., Spencer, R.-J., Van Dyke, J. U., and Thompson, M. B. (2018). Road mortality of the eastern long-necked turtle (Chelodina longicollis) along the Murray River, Australia: an assessment using citizen science. Australian Journal of Zoology 66, 41–49.
| Road mortality of the eastern long-necked turtle (Chelodina longicollis) along the Murray River, Australia: an assessment using citizen science.Crossref | GoogleScholarGoogle Scholar |
Selman, W., and Qualls, C. (2008). Graptemys gibbonsi (Pascagoula map turtle). Basking and parasite removal. Herpetological Review 39, 216.
Selman, W., and Qualls, C. (2009). Graptemys flavimaculata (yellow‐blotched map turtle). Basking and parasite removal. Herpetological Review 40, 78–79.
Shealy, R. M. (1976). The natural history of the Alabama map turtle, Graptemys pulchra Baur, in Alabama. Bulletin of the Florida State Museum Biological Sciences 21, 47–111.
Singh, K. (2018). Ecology of the Macquarie turtle (Emydura macquarii macquarii) downstream of a large hypolimnetic-releasing impoundment in Australia’s southern Murray–Darling Basin. Ph.D. Thesis, Charles Sturt University, Albury–Wodonga.
Spencer, R.-J., Thompson, M. B., and Hume, I. D. (1998). The diet and digestive energetics of an Australian short-necked turtle, Emydura macquarii. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology 121, 341–349.
| The diet and digestive energetics of an Australian short-necked turtle, Emydura macquarii.Crossref | GoogleScholarGoogle Scholar |
van Vliet, M. T. H., Franssen, W. H. P., Yearsley, J. R., Ludwig, F., Haddeland, I., Lettenmaier, D. P., and Kabat, P. (2013). Global river discharge and water temperature under climate change. Global Environmental Change 23, 450–464.
| Global river discharge and water temperature under climate change.Crossref | GoogleScholarGoogle Scholar |
Vogt, R. C. (1980). Natural history of the map turtles Graptemys pseudogeographica and G. ouachitensis in Wisconsin. Tulane Studies in Zoology and Botany 22, 17–48.
Webb, G. J. W. (1978). Observations on basking in some Australian turtles (Reptilia: Testudines: Chelidae). Herpetologica 34, 39–42.
Webb, G. J. W., and Johnson, C. R. (1972). Head–body temperature differences in turtles. Comparative Biochemistry and Physiology Part A: Physiology 43, 593–611.
| Head–body temperature differences in turtles.Crossref | GoogleScholarGoogle Scholar |
Webb, G. J. W., and Witten, G. J. (1973). Critical thermal maxima of turtles: validity of body temperature. Comparative Biochemistry and Physiology Part A: Physiology 45, 829–832.
| Critical thermal maxima of turtles: validity of body temperature.Crossref | GoogleScholarGoogle Scholar |
Woods, H. A., Dillon, M. E., and Pincebourde, S. (2015). The roles of microclimatic diversity and of behavior in mediating the responses of ectotherms to climate change. Journal of Thermal Biology 54, 86–97.
| The roles of microclimatic diversity and of behavior in mediating the responses of ectotherms to climate change.Crossref | GoogleScholarGoogle Scholar | 26615730PubMed |