Habitat suitability correlates with mean population fitness of a threatened marsupial predator
Harry A. Moore A B * , Judy A. Dunlop
A B * , Judy A. Dunlop  B C and Dale G. Nimmo
B C and Dale G. Nimmo  C
C
A Department of Biodiversity, Conservation and Attractions, Bentley Delivery Centre, Locked Bag 104, Perth, WA, Australia.
B School of Agriculture and Environmental Sciences, University of Western Australia, Crawley, WA 6009, Australia.
C Gulbali Institute, School of Agricultural, Environmental and Veterinary Sciences, Charles Sturt University, Albury, NSW, 2640, Australia.
Abstract
Measuring the quality of habitats necessary for the survival of threatened species is a priority for conservation management, but traditional metrics are often too costly to implement. As a result, many practitioners rely on proxies such as habitat suitability, which are measured by relating environmental variables to species occurrence data using habitat suitability models. However, little research has examined how these proxies relate to actual measures of habitat quality, such as body condition.
By testing the relationship between habitat suitability and habitat quality – as characterised by mean population fitness – the aim of this study was to improve our understanding of ways in which we can reliably map habitat of high importance for a particular species, as well as habitats where populations are most susceptible to local extinction.
We used data from a large-scale monitoring program on the northern quoll (Dasyurus hallucatus), a threatened marsupial predator, which collected data on three measures of population mean fitness (measured as body mass accounting for size, tail circumference, and body mass). We correlated these measures with habitat suitability derived from a habitat suitability model.
We found quoll mean population fitness increased with increasing habitat suitability. In addition, we found mean population fitness increased with increasing topographic ruggedness, annual rainfall, rainfall variability, and decreasing distance to water, consistent with previous studies that suggest quolls are able to persist better in habitat where resource availability (shelter, food) is higher.
Our findings demonstrate the usefulness of habitat suitability models for predicting habitat quality for a threatened predator at a large scale, and that predictions of habitat suitability can correspond with measures of fitness. In addition, they support previous studies in highlighting the importance of topographically complex habitat for this species.
These findings have important implications for identifying both source populations, where species recruitment is likely to exceed mortality, and more vulnerable populations that may require targeted conservation interventions to ensure their long-term persistence and stability.
Keywords: body condition, conservation management, fitness, habitat quality, habitat suitability, northern quoll, Pilbara, threatened species.
Introduction
Spatial variation in environmental conditions influences the distribution of resources, and, in doing so, shapes habitat quality for species (Pulliam 2000). Habitat quality can be defined as the extent to which an environment provides the conditions necessary for individual and population persistence (Hall et al. 1997). Indicators of habitat quality include population-based metrics such as abundance or population growth, as well as individual based fitness metrics such as body condition, survival, or reproduction (Johnson 2007). Identifying high quality habitat for target species has long been a priority for conservation managers (Van Horne 1983), but the process of mapping its occurrence at scale poses substantial logistical challenges. Directly measuring indicators of high quality habitat (i.e. population growth, individual fitness) requires extensive field work that is prohibitively costly for many conservation organisations, particularly if the target species is not charismatic (Fleming and Bateman 2016; Bellon 2019) or funding is scarce (Wintle et al. 2019).
In lieu of directly measuring indicators of habitat quality, conservationists are increasingly relying on proxies (Stephens et al. 2015) such as habitat suitability (Bean et al. 2014). Habitat suitability models combine species presence (and sometimes absence) records with environmental data to produce a gridded surface, where each cell is ranked on a scale of 0–1 in an attempt to estimate the probability or relative likelihood that a species is present given the environment (Elith et al. 2011; Guillera-Arroita et al. 2015). Although ready access to data and software required to run habitat suitability models has made them a popular tool, it remains unclear as to what extent habitat suitability correlates with indicators of habitat quality (Falcucci et al. 2009; Bean et al. 2014).
Previous research has shown habitat suitability can be an important predictor of species abundance metrics (VanDerWal et al. 2009; Fancourt et al. 2015); however, less attention has been directed toward the impact of habitat suitability on measures of individual fitness, defined as attributes that increase the likelihood of an individual to pass on its genes (Ellis et al. 2012; Lee-Yaw et al. 2022). Mean population fitness refers to measures of individual fitness averaged across all individuals in a population (Lee-Yaw et al. 2022). Although population growth is the most rigorous measure of population fitness, a host of fitness components have been used to measure mean population fitness when such data are not available, including body size (Bean et al. 2014; Mammola et al. 2019), growth rate (Wittmann et al. 2016), and body condition (Lunghi et al. 2018). Measures of body condition are often associated with individual persistence (i.e. survival) (Romero and Wikelski 2001; Johnson et al. 2006) and population persistence (Suorsa et al. 2003; Hinam and Clair 2008; Dunlop and Morris 2018), and can be obtained when animals are captured to provide an indicator of fitness (Jakob et al. 1996; Stevenson and Woods 2006). Measures of body condition in animals often comprise a measure of fat content relative to body size, given fat reserves are critical for energetically demanding activities such as dispersal and reproduction, as well as survival, particularly in resource-limited environments (Stevenson and Woods 2006).
Here, we make use of data from a large-scale monitoring program to examine the relationship between habitat suitability and mean population fitness using a threatened species of marsupial predator, the northern quoll (Dasyurus hallucatus). Once widespread across northern Australia (Braithwaite and Griffiths 1994), northern quolls have recently suffered large scale declines in both range and niche (Moore et al. 2019), and are now listed as endangered in Australia (DCCEEW 2022) and by the ICUN (Oakwood et al. 2016). Northern quolls are an ideal candidate species for testing the influence of habitat suitability on mean population fitness for several reasons. First, models mapping northern quoll habitat suitability across their contemporary range exist and are easily accessible (Moore et al. 2019). Second, metrics of northern quoll body condition and their relationship to fitness have been studied for many years and are now relatively well understood (Heiniger et al. 2020; Rew-Duffy et al. 2020; Thomas et al. 2021). Finally, longitudinal monitoring data exists that captures northern quoll body condition across a suite of sites where habitat suitability estimates are available (Cramer et al. 2016; Dunlop 2016). By testing the relationship between habitat suitability and habitat quality as characterised by mean population fitness, we hope to improve our understanding of ways in which we can reliably map habitat of high importance for the species, as well as habitats where populations are most susceptible to local extinction.
Methods
The northern quoll
The northern quoll is a medium sized marsupial predator (300–1000 g) native to northern Australia. It is currently listed as endangered internationally (Oakwood et al. 2016), nationally (Department of the Environment 2016), and at the state level (Western Australian Government 2017). The Pilbara bioregion in Western Australia is regarded as a last remaining stronghold for the northern quoll, given it is the only major mainland population yet to be invaded by cane toads (Cramer et al. 2016; Indigo et al. 2023).
Study area
The Pilbara bioregion covers a 178 060-km2 section of north-west Western Australia, bounded by the Indian Ocean in the west and sand deserts in the east and north (McKenzie et al. 2009) (Fig. 1). Climate in the region is characterised by hot summers and mild winters, especially in the northern section where average daily maximums exceed 35°C in summer (Sudmeyer 2016). Average annual rainfall is typically less than 350 mm and falls mostly between January and March when cyclone activity is at its peak (Sudmeyer 2016). Inter-annual rainfall variability is extremely high (BOM 2022). Surface geology is dominated by banded ironstone formations separated by rocky sand plains scattered with granite outcrops (McKenzie et al. 2009). Vegetation varies among subregions, but mostly comprises spinifex grasslands (Tridodia spp.) and open shrublands (Acacia spp.). Riparian zones support eucalypt (Eucalyptus victrix, E. camaldulensis) and paperbark (Melaleuca argentea) woodlands, and figs (Ficus brachypoda) and kurrajong (Brachychiton acuminatus) are common within and around breakaways and granite outcrops.
Northern quoll (Dasyurus hallucatus) trapping sites (dark circles) used as part of the Pilbara northern quoll monitoring program, superimposed over northern quoll habitat suitability, as derived from Moore et al. (2019). Dark triangles are towns.

Trapping data
Live trapping data was collected as part of the Pilbara Northern Quoll Monitoring (PNQM) program – a program designed to track the status of northern quoll populations across the Pilbara bioregion (Cramer et al. 2016; Dunlop 2016). Here, we use data collected from 11 monitoring sites that were trapped at least once between 2014 and 2019. Either the entire set of 11 sites, or a subset thereof, were sampled annually using a trapping protocol developed specifically for northern quolls (Dunlop et al. 2014) (Supplementary Table S1). A larger number of sites were surveyed between 2014 and 2019, but some were excluded from this analysis due to low sample size. The protocol consisted of two transects of 25 traps, with each trap separated by 50 m and each transect separated by 200 m (Dunlop 2016). Traps were opened for four consecutive nights, and baited with a mixture of peanut butter, oats, and sardines. Northern quolls are regarded as semelparous, with males typically living for less than 1 year (Oakwood et al. 2001). To avoid times when males are either dead or in a deteriorated state, live trap surveys were conducted between March and September.
Data collected from trapped individuals included mass (g), head length (mm), pes (foot) length (mm), tail circumference (mm) and sex (Dunlop 2016). In addition, all individuals captured were implanted with subcutaneous Passive Integrated Transponders (PIT) for individual identification. Data were first filtered by removing capture records that were missing information related to site, year, sex, mass, tail circumference, or head length. We also removed individuals that were not sexually mature (estimated using teeth condition and reproductive status). Age was not included as a predictor, given very few individuals were recorded living more than a year. Further, the age of adult individuals (1 year, 2 years etc.) could not always be assigned with a high level of confidence. Next, average body condition, tail circumference, and mass (Table 1) were calculated for each site within each year, for both males and females.
| Measure | Justification | |
|---|---|---|
| Body condition | Measures of an animal’s mass while accounting for size. Such measures are commonly used as proxies for habitat quality (Johnson 2007). A body condition index was calculated for the northern quoll by dividing body mass (g) by head length, following Jones (1996) who found head length was a more accurate gauge of quoll body size. Strong links between body condition and survival have previously been demonstrated in Dasyurids (Wolfe et al. 2004; Rew-Duffy et al. 2020). Northern quolls that occupy high quality rocky habitat exhibit higher body condition, as well as greater reproductive capacity and survival (Braithwaite and Griffiths 1994). | |
| Tail circumference | Quolls store fat reserves in their tails (Thomson et al. 1985), and thus tail circumference has commonly been used as a metric for measuring northern quoll body condition (Schmitt et al. 1989; Oakwood 1994; Rankmore et al. 2008; Hernandez-Santin et al. 2019; Heiniger et al. 2020), as well as for other quoll species (Serena and Soderquist 1988). Female northern quolls are more likely to survive to at least 21 months of age with increased tail circumference (relative to body mass) (Rew-Duffy et al. 2020). | |
| Mass | Body mass can be a useful indicator of habitat quality given it can be indicative of individual fat reserves (Wells et al. 2019), breeding activity (Rieger 1996), adult and juvenile survival (Sæther 1989; Plard et al. 2015), including for northern quolls (Griffiths et al. 2017). Variation in individual body mass has also been linked to resource availability (McNab 2010). In addition, body mass of northern quolls tends to peak during breeding season, indicating that it likely plays an important role in their reproductive success (Braithwaite and Griffiths 1994; Heiniger et al. 2020). |
Habitat suitability and predictor variables
A measure of habitat suitability for all study sites was derived from a habitat suitability model developed as part of a previous study (Moore et al. 2019). The model was fit using the MaxEnt algorithm (Phillips et al. 2006), and covered the entire extent of the current study area (Fig. 1). The model output consisted of a spatial grid, with each cell measuring 1 km by 1 km. The value of each cell ranged from 0 to 1 and denoted the relative suitability of that location as a habitat for northern quolls. Model predictor variables included topographic ruggedness, distance to permanent water, elevation, precipitation seasonality, and annual precipitation. Model discrimination performance was high (AUC > 0.9), and the most important predictors of habitat suitability in the Pilbara were topographic ruggedness and precipitation seasonality (Moore et al. 2019). Values for predictor variables included in the habitat suitability model (ruggedness, annual rainfall etc.) were also recorded for each site used in this study. The data utilised for the creation of this model, as well as the raw model outputs, can be found in an online repository, which is referenced in Moore et al. (2019).
In order to account for inter-annual variation in resource availability, we measured the rainfall recorded at each site during the wet season (January to March) that preceded the trapping period. High rainfall during this period potentially affects quoll body condition by triggering a surge in resources, thereby increasing the abundance of key prey species such as insects and small vertebrates in the subsequent dry season. Although we explored the influence of dry season rainfall (April–December) in our preliminary analysis, the results indicated it was not as influential as the rainfall during the wet season, probably because inter-annual variation in rainfall is greater in the wet season.
Data analysis
All analysis was carried out using R ver. 4.1.2 (R Core Team 2021). The association between habitat suitability and mean population fitness (measured as body condition, tail circumference and body mass) was examined using Generalised Linear Mixed-Effects Models fit using the statistical package ‘lme4’ (Bates et al. 2015).
Two sets of models were used in this analysis. The first set was designed to examine the effect of habitat suitability derived from the habitat suitability model (Moore et al. 2019) on northern quoll mean population fitness. As such, this set comprised global models that included average body condition, average tail circumference, and average mass as response variables. Habitat suitability (derived from the habitat suitability model; Moore et al. 2019), wet season rainfall, sex, and capture month (accounting for annual cycles in growth and resource availability) were set as fixed effects. Capture year was included as a random effect to account for any correlations in the data relating to inter-annual variations in climate that were not directly explained by the fixed effect of wet season rainfall.
The second set of models was designed to examine the effect of environmental factors on northern mean population fitness. This set of models again fit average body condition, average tail circumference (mm), and average mass as response variables, but fixed effects included elevation, topographic ruggedness, annual rainfall, rainfall seasonality, and distance to nearest permanent water source (Table S2) – all of which were used by Moore et al. (2019) to derive the habitat suitability layer used above. Sex and capture month were also included as fixed effects, and year was again included as random effect. Interactive effects between sex and other predictor variables were explored as part of preliminary analyses but received limited support and were therefore not included within final models.
Top models were ranked by AIC scores, corrected for small sample size (AICc), using the ‘dredge’ function in R package ‘MuMIn’. Predictor variables were regarded as being strongly influential if the estimated 95% confidence intervals did not overlap zero (Nakagawa and Cuthill 2007). Model estimates were generated using the ‘fixef’ function from the package ‘lme4’ (Bates et al. 2015). Confidence intervals were generated used using the ‘confint’ function in the R stats package (R Core Team 2021), and the ‘ggpredict’ function in the ‘ggeffects’ package (Lüdecke 2018). Model performance was assessed using marginal and conditional pseudo-R-squared values, calculated using the R package ‘MuMIn’ (Barton 2022). Marginal R2 values represent the variance explained by the fixed effects, and conditional R2 values represent the variance explained by the fixed and random effects.
Results
The final trapping data set comprised 185 northern quoll individuals (n = 95 females, 90 males) captured over 6 years. A further 29 individuals were excluded because they were missing data or were not sexually mature. For female northern quolls, average body mass across all trapping sessions was 387.0 g (s.e. = 10.3 g), and average tail circumference was 49.8 mm (s.e. = 1.3 mm). Average male mass was 640.0 g (s.e. = 21.4 g), and average male tail circumference was 57.6 mm (s.e. = 1.3 mm).
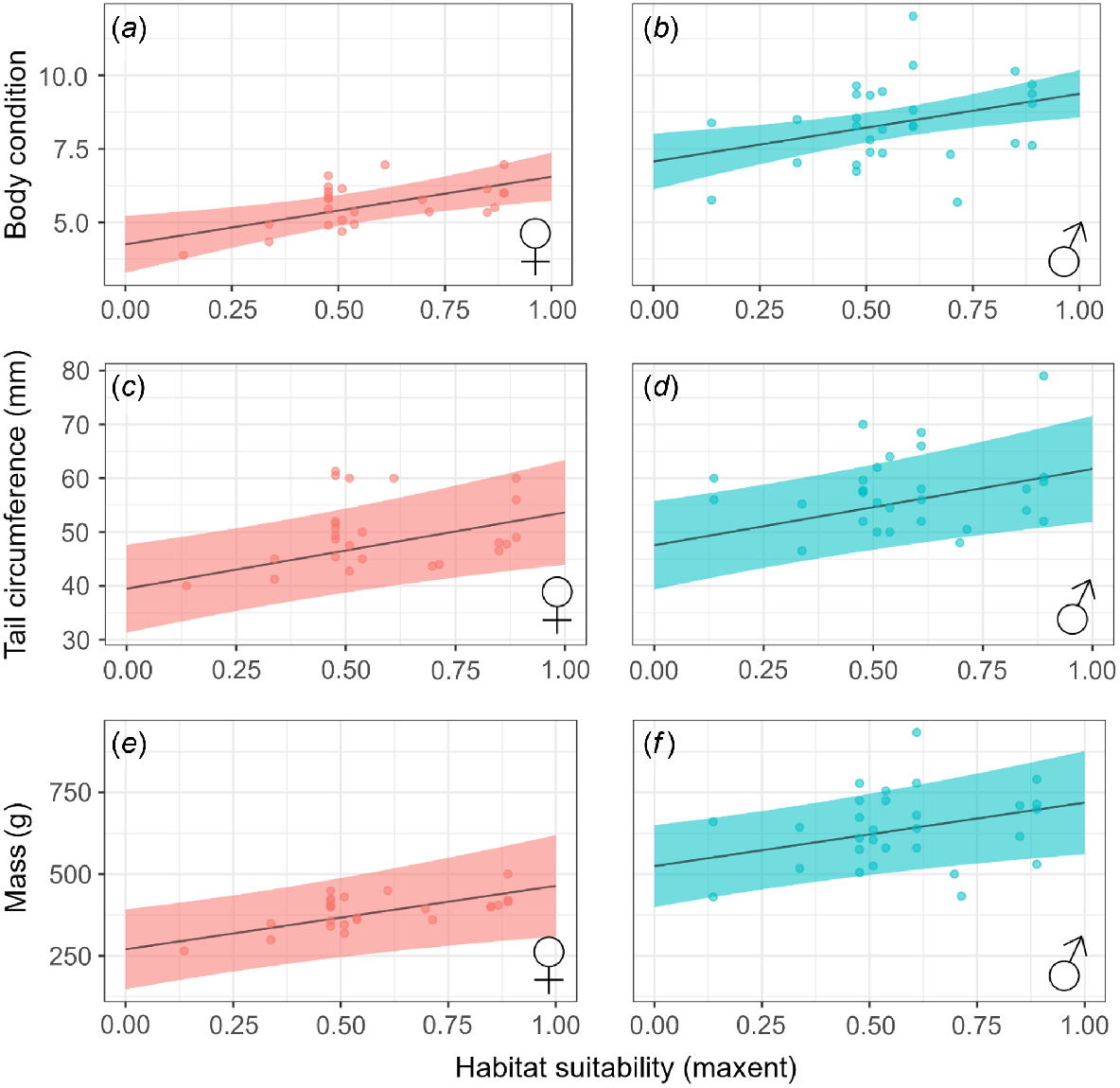
The most parsimonious models of those examining the effect of habitat suitability on quoll body condition, tail circumference, and mass all included habitat suitability and sex as predictors (Table S3). The models for body mass and tail circumference also included previous wet season rainfall and capture month. Variance explained by the most parsimonious model was high for all three measures: body condition (Marginal R2 = 69.8%); tail circumference (R2 = 49.9%); and body mass (R2 = 71.2%).
Predicted body condition, tail circumference, and body mass all increased with increasing habitat suitability (Table 2, Fig. 2). Female and male body condition was 27.4% and 18.9% higher respectively, at sites with maximum habitat suitability (0.89) compared to sites with minimum habitat suitability (0.14). Similarly, predicted quoll tail circumference increased by 10.63 mm between minimum and maximum habitat suitability values, translating to a 20.4% increase for females, and a 17.7% increase for males. Body mass increased by 170 g (F = 39.2%, M = 24.5%). Body mass also increased significantly within increasing previous wet-season rainfall.
| Variable | Estimate | Lower 85% CI | Upper 95% CI | |
|---|---|---|---|---|
| Body condition | ||||
| (Intercept) | 5.6 | 5.0 | 6.1 | |
| Habitat suitability | 0.5 | 0.2 | 0.7 | |
| Sex (male) | 2.8 | 2.3 | 3.4 | |
| Tail circumference | ||||
| (Intercept) | 47.6 | 40.5 | 54.9 | |
| Month 5 | 6.6 | −1.0 | 13.8 | |
| Month 6 | 5.6 | −1.9 | 12.9 | |
| Month 7 | 4.7 | −3.3 | 12.4 | |
| Month 8 | −2.5 | −10.5 | 5.1 | |
| Month 9 | −5.6 | −14.7 | 4.1 | |
| Month 10 | −4.1 | −17.5 | 8.6 | |
| Habitat suitability | 2.8 | 1.2 | 4.5 | |
| Previous wet season rainfall | 1.8 | −0.4 | 4.2 | |
| Sex (male) | 8.1 | 5.4 | 10.8 | |
| Mass | ||||
| (Intercept) | 364.0 | 259.4 | 468.6 | |
| Month 5 | 17.1 | −99.1 | 133.2 | |
| Month 6 | 50.1 | −66.7 | 166.9 | |
| Month 7 | 35.3 | −79.1 | 149.8 | |
| Month 8 | −9.7 | −122.0 | 102.7 | |
| Month 9 | 0.0 | −132.7 | 132.6 | |
| Month 10 | −42.4 | −236.9 | 152.0 | |
| Habitat suitability | 45.5 | 19.6 | 71.5 | |
| Previous wet season rainfall | 40.2 | 13.5 | 67.0 | |
| Sex (male) | 261.1 | 217.9 | 304.4 | |
The effect of habitat suitability and sex on metrics of northern quoll (Dasyurus hallucatus) population mean fitness, including body condition (a, b), tail circumference (c, d) and mass (e, f). Shading represents 95% confidence intervals.
The most parsimonious models from those examining the effect of environmental factors on quoll condition all included topographic ruggedness and sex (Table S4). Elevation, annual rainfall, and month were also included in the top model for predicting quoll tail circumference and body mass (Table 3). Variance explained was high for body condition (Marginal R2 = 63.6%), tail circumference (R2 = 50.3%), and body mass (R2 = 75.8%) (Fig. 3).
| Variable | Estimate | Lower 95% CI | Upper 95% CI | |
|---|---|---|---|---|
| Body condition | ||||
| (Intercept) | 5.6 | 5.1 | 6.1 | |
| Ruggedness | 0.4 | 0.1 | 0.6 | |
| Sex (male) | 2.7 | 2.1 | 3.3 | |
| Tail circumference | ||||
| (Intercept) | 45.3 | 38.6 | 52.1 | |
| Month 5 | 9.6 | 2.8 | 16.1 | |
| Month 6 | 9.6 | 2.5 | 16.4 | |
| Month 7 | 6.9 | −0.5 | 14.1 | |
| Month 8 | 0.3 | −6.8 | 7.3 | |
| Month 9 | −4.2 | −12.6 | 4.2 | |
| Month 10 | 1.3 | −10.6 | 13.1 | |
| Annual rainfall | 1.8 | 0.0 | 3.6 | |
| Elevation | −1.3 | −3.1 | 0.4 | |
| Ruggedness | 3.5 | 2.0 | 5.0 | |
| Sex (male) | 7.5 | 5.0 | 10.0 | |
| Mass | ||||
| (Intercept) | 361.5 | 271.6 | 454.1 | |
| Month 5 | 12.7 | −98.7 | 112.4 | |
| Month 6 | 93.6 | −19.7 | 197.3 | |
| Month 7 | 32.5 | −67.4 | 130.6 | |
| Month 8 | −15.5 | −115.0 | 82.5 | |
| Month 9 | −9.0 | −131.2 | 133.4 | |
| Month 10 | −41.7 | −227.3 | 139.0 | |
| Annual rainfall | 50.7 | 25.1 | 77.4 | |
| Elevation | −29.7 | −57.1 | −3.2 | |
| Distance to water | 1.9 | −30.1 | 31.0 | |
| Rainfall variability | 40.5 | 8.5 | 74.6 | |
| Ruggedness | 78.0 | 45.0 | 113.0 | |
| Sex (male) | 254.6 | 216.0 | 292.6 | |
Statistically significant results are indicated in bold.
The effect of topographic ruggedness and sex on metrics of northern quoll (Dasyurus hallucatus) population mean fitness, including body condition (a, b), tail circumference (c, d) and mass (e, f). Statistically significant results are indicated in bold. Shading represents 95% confidence intervals.

Both female and male predicted body condition increased by 17.8% and 12.4% respectively when topographic ruggedness increased from the minimum recorded value to the maximum. Similarly, predicted tail circumference increased by 10.9 mm (F = 21.0%, M = 18.4%), and predicted body mass increased by 241.18 g (F = 47.7%, M = 31.7%). Predicted quoll tail circumference was higher earlier in the year (May, June) when compared with later months (August, September), and increased with increasing average annual rainfall (Table 3, Fig. 4). Quoll body mass also increased in response to increasing annual rainfall – body mass was 201.8 g (F = 42.3%, M = 27.6%) higher at the wettest site (annual rainfall = 595 mm) when compared with the driest site (annual rainfall = 114 mm).
Discussion
Our results suggest habitat suitability models are useful for estimating quoll mean population fitness: all three measures of mean population fitness were positively related to habitat suitability predicted by a habitat suitability model. These results are partially supported by a recent review of how species population mean fitness responds to habitat suitability. Lee-Yaw et al. (2022) systematically reviewed 42 studies that compared habitat suitability predicted by habitat suitability models with measures of mean population fitness and found that only 15 studies supported the hypothesis that more suitable sites would have higher measures of mean population fitness, and 12 studies offered mixed support. However, they note that studies like ours that use measures of fitness components other than population growth rate tended to find more consistent relationships, as did studies focusing on individual species. For instance, Brambilla and Ficetola (2012) found that territories of red-backed shrikes (Lanius collurio) were smaller (indicative of higher quality habitat), Wittmann et al. (2016) found growth rates of grass carp (Ctenopharyngodon idella) were higher, and Lunghi et al. (2018) found that a salamander species (Hydromantes flavus) had better body condition in areas with high habitat suitability predicted from habitat suitability models. Studies that offered mixed support often found that a relationship existed for one measure of fitness but not for others, with Lee-Yaw et al. (2022) suggesting that such variability may arise due to variable relationships between fitness measures and environmental gradients, and the negative relationships between demographic measures (i.e. demographic compensation). Our three measures are all conceptually related and would not be expected to negatively co-vary, potentially explaining the consistency of our results. Overall, this study is another example of habitat suitability models correctly identifying areas with high mean population fitness for an individual species.
Topographic ruggedness has been demonstrated to be an important predictor of species habitat quality globally (Esquerré et al. 2019; Dilts et al. 2023), largely due to the role of rugged habitats in mitigating predator–prey interactions (Fox et al. 1992; Forshee et al. 2022), providing protection from extreme temperatures (Chytrý et al. 2022) and fire (Krawchuk et al. 2016), and fostering a diversity of microclimates (Dobrowski 2011). Our study further confirms the importance of topographically rugged areas for northern quolls. Braithwaite and Griffiths (1994) found that northern quolls in topographically complex rocky habitat in the Northern Territory and Kimberley were in better condition than quolls in topographically simple savanna habitat, and survived longer. In this study, we showed that northern quoll body condition, tail circumference, and body mass were positively associated with topographic ruggedness. Given that our measures of mean population fitness all relate to fat reserves, an obvious explanation of our findings would be that rugged areas have a greater abundance of food for northern quolls; however, a recent study suggests this may not be the case: Hernandez-Santin et al. (2022) did not find any difference in prey availability when comparing rugged rocky habitats with simple sandplain areas. However, they did find that rocky areas had a greater abundance of both dens and temporary shelters that are used to evade predation. This increased density of resources in rugged rocky habitat has been demonstrated to allow northern quolls occurring here to use smaller home ranges when compared with open sand plain country (Cowan et al. 2022), likely reducing overall energy costs. Predators are also less common in rocky areas when compared with adjacent sandplains (Hernandez-Santin et al. 2016). Given that predation risk can compromise the ability of animals to acquire and maintain energy reserves by increasing stress and interrupting foraging activity (Pérez-Tris et al. 2004; Sabino-Marques and Mira 2011; DeWitt et al. 2019), it would be expected that quolls forage more efficiently in rocky habitat than in open sand plain country. This has been demonstrated in the Pilbara – Murphy et al. (2021) found northern quolls were more likely to visit a foraging site, and visit a foraging site more often, when the site was in structurally complex habitat compared with habitat that was less structurally complex. Our results build on the findings of these studies by providing evidence that rocky habitats increase body condition, supporting the idea that increased foraging opportunities and reduced predation risk can improve northern quoll mean population fitness in the Pilbara.
In addition to habitat suitability and topographic ruggedness, we found mean individual northern quoll mass and tail circumference increased with increasing average annual rainfall, and mass and tail circumference increased with increasing previous wet season rainfall. These trends are consistent with previous studies that have found habitats that receive higher rainfall are preferable for northern quolls (Hohnen et al. 2016; Moore et al. 2019; von Takach et al. 2020). Northern quolls are likely able to accrue greater body mass in areas where water availability is higher due to increased primary productivity, resulting in increased availability of prey such as insects, small rodents and reptiles (Braithwaite and Muller 1997; Letnic and Dickman 2005, 2010). This theory is supported by previous studies that have demonstrated that northern quoll body mass declines following years of unseasonably low rainfall (Heiniger et al. 2020), and northern quoll declines and local extinctions have been most prevalent within the arid parts of their range (Braithwaite and Griffiths 1994; Ziembicki et al. 2013; Moore et al. 2019). Similarly, quoll body mass may be lower at higher elevations given availability of potential prey such as insects may be lower here, due to cooler and drier conditions (Hodkinson 2005).
Northern quoll condition is known to fluctuate with season (Oakwood 1994; Hernandez-Santin et al. 2019), and we found evidence of this in our study; tail circumference measurements were significantly higher in May and June, before declining to their lowest point in September. Although month did not have a significant effect on other indices of population mean fitness, boxplots of the raw data suggested similar trends may exist for mass and body condition but were too weak to be detected in our analysis. Observed declines in tail circumference are likely attributable to two primary factors. Firstly, the semelparous reproductive strategy of male quolls, in which they invest heavily in a single breeding season, causes a considerable physical toll on the animals (Oakwood 2004). As the males engage in exhaustive mating behaviour and competition for limited opportunities, their energy reserves and overall body condition tend to deteriorate over time, potentially contributing to the decline in tail circumference (Gaschk et al. 2023). Secondly, the decline in tail circumference might also be linked to the seasonal changes in resource availability, particularly during the dry season in northern Australia (Oakwood 1994). As food resources become scarcer, the northern quolls face increased challenges in maintaining their energy balance and overall body condition. This could result in decreased tail circumference as the animals utilize their fat reserves for survival during this resource-scarce period.
In addition to highlighting the utility of habitat suitability models in predicting habitat quality for northern quolls, these results have important implications for the management of this species, specifically in the identification of critical habitat where ‘source’ populations may occur. Source populations are defined as habitats where species recruitment outweighs mortality, and are generally considered important from a conservation perspective given they play a critical role in supplying excess individuals to ‘sink’ populations, where mortality outweighs recruitment (Furrer and Pasinelli 2016). Identifying and protecting source populations is of particular importance for northern quolls, given their semelparous life history (males live for only 1 year) predisposes them to naturally high rates of local extinction (Oakwood 2000), even from small (5% per annum) declines in recruitment (Moro et al. 2019). Building on these results, we suggest future research should examine the influence of northern quoll body condition on other population attributes, such as growth patterns, spatial use, population density, and reproductive timing, similar to previous work on other threatened fauna (Heithaus et al. 2007; Williams et al. 2013; Dunlop and Morris 2018). Better understanding these interactions will likely improve our ability to predict population consequences of subtle decreases in available resources that are effected through anthropogenic disturbances such as mining. Overall, our results support previous studies that suggest habitat suitability model outputs can indeed be useful in predicting mean population fitness, as well as those that suggest conservation efforts aimed at protecting northern quolls should focus on preserving and restoring habitats that are topographically rugged, lower in elevation, receive higher rainfall, and are closer to permanent water.
Declaration of funding
This project was supported by the Western Australian Department of Biodiversity, Conservation and Attractions. Environmental offsets and public good funding was provided by BHP, Rio Tinto, Atlas Iron, Fortescue Metals Group, Roy Hill, Process Minerals International, Metals X and Main Roads Western Australia.
Acknowledgements
We acknowledge the Traditional Owners of the land on which this study occurred, which includes the Kuruma, Yindjibarndi, Bajima, Palyku, Kariyarra, Nymal, Thalanyji, and Ngarla people. We are grateful to those who assisted with data collection, in particular Neal Birch and the many volunteers that were involved in live trapping. We are also grateful to pastoralists who assisted us by providing access to sites.
References
Barton K (2022) MuMIn: multi-model inference. R Package Version 1.47.1. Available at https://CRAN.R-project.org/package=MuMIn
Bates D, Maechler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. Journal of Statistical Software 67, 1-48.
| Crossref | Google Scholar |
Bean WT, Prugh LR, Stafford R, Butterfield HS, Westphal M, Brashares JS (2014) Species distribution models of an endangered rodent offer conflicting measures of habitat quality at multiple scales. Journal of Applied Ecology 51(4), 1116-1125.
| Crossref | Google Scholar |
Bellon AM (2019) Does animal charisma influence conservation funding for vertebrate species under the US Endangered Species Act? Environmental Economics and Policy Studies 21(3), 399-411.
| Crossref | Google Scholar |
BOM (2022) Average annual, seasonal and monthly rainfall [map]. Available at http://www.bom.gov.au/jsp/ncc/climate_averages/rainfall/index.jsp
Braithwaite RW, Griffiths AD (1994) Demographic variation and range contraction in the northern quoll, Dasyurus hallucatus (MArsupialia: Dasyuridae). Wildlife Research 21(2), 203-217.
| Crossref | Google Scholar |
Braithwaite RW, Muller WJ (1997) Rainfall, groundwater and refuges: predicting extinctions of Australian tropical mammal species. Australian Journal of Ecology 22(1), 57-67.
| Crossref | Google Scholar |
Brambilla M, Ficetola GF (2012) Species distribution models as a tool to estimate reproductive parameters: a case study with a passerine bird species. Journal of Animal Ecology 81(4), 781-787.
| Crossref | Google Scholar |
Chytrý K, Willner W, Chytrý M, Divíšek J, Dullinger S (2022) Central European forest–steppe: an ecosystem shaped by climate, topography and disturbances. Journal of Biogeography 49(6), 1006-1020.
| Crossref | Google Scholar |
Cowan MA, Moore HA, Hradsky BA, Jolly CJ, Dunlop JA, Wysong ML, Hernandez-Santin L, Davis RA, Fisher DO, Michael DR, Turner JM, Gibson LA, Knuckey CG, Henderson M, Nimmo DG, Vernes K (2022) Non-preferred habitat increases the activity area of the endangered northern quoll (Dasyurus hallucatus) in a semi-arid landscape. Australian Mammalogy 45, 138-150.
| Crossref | Google Scholar |
Cramer VA, Dunlop J, Davis R, Ellis R, Barnett B, Cook A, Morris K, van Leeuwen S (2016) Research priorities for the northern quoll (Dasyurus hallucatus) in the Pilbara region of Western Australia. Australian Mammalogy 38(2), 135.
| Crossref | Google Scholar |
DCCEEW (2022) EPBC Act list of threatened fauna. Australian Government. Available at http://www.environment.gov.au/cgi-bin/sprat/public/publicthreatenedlist.pl?wanted=fauna
DeWitt PD, Visscher DR, Schuler MS, Thiel RP (2019) Predation risks suppress lifetime fitness in a wild mammal. Oikos 128(6), 790-797.
| Crossref | Google Scholar |
Díaz S, Settele J, Brondízio ES, Ngo HT, Agard J, Arneth A, Balvanera P, Brauman KA, Butchart SHM, Chan KMA, Garibaldi LA, Ichii K, Liu J, Subramanian SM, Midgley GF, Miloslavich P, Molnár Z, Obura D, Pfaff A, Polasky S, Purvis A, Razzaque J, Reyers B, Chowdhury RR, Shin Y-J, Visseren-Hamakers I, Willis KJ, Zayas CN (2019) Pervasive human-driven decline of life on Earth points to the need for transformative change. Science 366(6471), eaax3100.
| Crossref | Google Scholar |
Dilts TE, Blum ME, Shoemaker KT, Weisberg PJ, Stewart KM (2023) Improved topographic ruggedness indices more accurately model fine-scale ecological patterns. Landscape Ecology 38, 1395-1410.
| Crossref | Google Scholar |
Dobrowski SZ (2011) A climatic basis for microrefugia: the influence of terrain on climate. Global Change Biology 17(2), 1022-1035.
| Crossref | Google Scholar |
Dunlop J, Morris K (2018) Environmental determination of body size in mammals: rethinking ‘island dwarfism’ in the golden bandicoot. Austral Ecology 43(7), 817-827.
| Crossref | Google Scholar |
Elith J, Phillips SJ, Hastie T, Dudík M, Chee YE, Yates CJ (2011) A statistical explanation of MaxEnt for ecologists. Diversity and Distributions 17(1), 43-57.
| Crossref | Google Scholar |
Ellis RD, McWhorter TJ, Maron M (2012) Integrating landscape ecology and conservation physiology. Landscape Ecology 27(1), 1-12.
| Crossref | Google Scholar |
Esquerré D, Brennan IG, Catullo RA, Torres-Pérez F, Keogh JS (2019) How mountains shape biodiversity: the role of the Andes in biogeography, diversification, and reproductive biology in South America’s most species-rich lizard radiation (Squamata: Liolaemidae). Evolution 73(2), 214-230.
| Crossref | Google Scholar |
Falcucci A, Ciucci P, Maiorano L, Gentile L, Boitani L (2009) Assessing habitat quality for conservation using an integrated occurrence-mortality model. Journal of Applied Ecology 46(3), 600-609.
| Crossref | Google Scholar |
Fancourt BA, Bateman BL, VanDerWal J, Nicol SC, Hawkins CE, Jones ME, Johnson CN (2015) Testing the role of climate change in species decline: is the eastern quoll a victim of a change in the weather? PLoS ONE 10(6), e0129420.
| Crossref | Google Scholar |
Fleming PA, Bateman PW (2016) The good, the bad, and the ugly: which Australian terrestrial mammal species attract most research? Mammal Review 46(4), 241-254.
| Crossref | Google Scholar |
Forshee SC, Mitchell MS, Stephenson TR (2022) Predator avoidance influences selection of neonatal lambing habitat by Sierra Nevada bighorn sheep. The Journal of Wildlife Management 86(8), e22311.
| Crossref | Google Scholar |
Fox JL, Sinha SP, Chundawat RS (1992) Activity patterns and habitat use of Ibex in the Himalaya Mountains of India. Journal of Mammalogy 73(3), 527-534.
| Crossref | Google Scholar |
Furrer RD, Pasinelli G (2016) Empirical evidence for source–sink populations: a review on occurrence, assessments and implications. Biological Reviews 91(3), 782-795.
| Crossref | Google Scholar |
Gaschk JL, Del Simone K, Wilson RS, Clemente CJ (2023) Resting disparity in quoll semelparity: examining the sex-linked behaviours of wild roaming northern quolls (Dasyurus hallucatus) during breeding season. Royal Society Open Science 10(2), 221180.
| Crossref | Google Scholar |
Griffiths AD, Rankmore B, Brennan K, Woinarski JCZ (2017) Demographic evaluation of translocating the threatened northern quoll to two Australian islands. Wildlife Research 44(3), 238-247.
| Crossref | Google Scholar |
Guillera-Arroita G, Lahoz-Monfort JJ, Elith J, Gordon A, Kujala H, Lentini PE, McCarthy MA, Tingley R, Wintle BA (2015) Is my species distribution model fit for purpose? Matching data and models to applications. Global Ecology and Biogeography 24(3), 276-292.
| Crossref | Google Scholar |
Hall LS, Krausman PR, Morrison ML (1997) The habitat concept and a plea for standard terminology. Wildlife Society Bulletin (1973-2006) 25(1), 173-182.
| Google Scholar |
Heiniger J, Cameron SF, Madsen T, Niehaus AC, Wilson RS (2020) Demography and spatial requirements of the endangered northern quoll on Groote Eylandt. Wildlife Research 47(3), 224-238.
| Crossref | Google Scholar |
Heithaus MR, Frid A, Wirsing AJ, Dill LM, Fourqurean JW, Burkholder D, Thomson J, Bejder L (2007) State-dependent risk-taking by green sea turtles mediates top-down effects of tiger shark intimidation in a marine ecosystem. Journal of Animal Ecology 76(5), 837-844.
| Crossref | Google Scholar |
Hernandez-Santin L, Goldizen AW, Fisher DO (2016) Introduced predators and habitat structure influence range contraction of an endangered native predator, the northern quoll. Biological Conservation 203, 160-167.
| Crossref | Google Scholar |
Hernandez-Santin L, Dunlop JA, Goldizen AW, Fisher DO (2019) Demography of the northern quoll (Dasyurus hallucatus) in the most arid part of its range. Journal of Mammalogy 100(4), 1191-1198.
| Crossref | Google Scholar |
Hernandez-Santin L, Goldizen AW, Fisher DO (2022) Northern quolls in the Pilbara persist in high-quality habitat, despite a decline trajectory consistent with range eclipse by feral cats. Conservation Science and Practice 4(8), e12733.
| Crossref | Google Scholar |
Hinam HL, Clair CCS (2008) High levels of habitat loss and fragmentation limit reproductive success by reducing home range size and provisioning rates of Northern saw-whet owls. Biological Conservation 141(2), 524-535.
| Crossref | Google Scholar |
Hirzel AH, Le Lay G (2008) Habitat suitability modelling and niche theory. Journal of Applied Ecology 45(5), 1372-1381.
| Crossref | Google Scholar |
Hodkinson ID (2005) Terrestrial insects along elevation gradients: species and community responses to altitude. Biological Reviews 80(3), 489-513.
| Crossref | Google Scholar |
Hohnen R, Tuft KD, Legge S, Hillyer M, Spencer PBS, Radford IA, Johnson CN, Burridge CP (2016) Rainfall and topography predict gene flow among populations of the declining northern quoll (Dasyurus hallucatus). Conservation Genetics 17(5), 1213-1228.
| Crossref | Google Scholar |
Indigo NL, Kelly E, Smith J, Webb JK, Phillips BL (2023) Can conditioned taste aversion be deployed at a landscape level to mitigate the impact of invasive cane toads on northern quolls? Wildlife Research
| Google Scholar |
Jakob EM, Marshall SD, Uetz GW (1996) Estimating fitness: a comparison of body condition indices. Oikos 77, 61-67.
| Crossref | Google Scholar |
Johnson MD (2007) Measuring habitat quality: a review. The Condor 109(3), 489-504.
| Crossref | Google Scholar |
Johnson MD, Sherry TW, Holmes RT, Marra PP (2006) Assessing habitat quality for a migratory songbird wintering in natural and agricultural habitats. Conservation Biology 20(5), 1433-1444.
| Crossref | Google Scholar |
Krawchuk MA, Haire SL, Coop J, Parisien M-A, Whitman E, Chong G, Miller C (2016) Topographic and fire weather controls of fire refugia in forested ecosystems of northwestern North America. Ecosphere 7(12), e01632.
| Crossref | Google Scholar |
Lee-Yaw JA, McCune JL, Pironon S, Sheth SN (2022) Species distribution models rarely predict the biology of real populations. Ecography 6, e05877.
| Google Scholar |
Letnic M, Dickman CR (2005) The responses of small mammals to patches regenerating after fire and rainfall in the Simpson Desert, central Australia. Austral Ecology 30(1), 24-39.
| Crossref | Google Scholar |
Letnic M, Dickman CR (2010) Resource pulses and mammalian dynamics: conceptual models for hummock grasslands and other Australian desert habitats. Biological Reviews 85(3), 501-521.
| Crossref | Google Scholar |
Lunghi E, Manenti R, Mulargia M, Veith M, Corti C, Ficetola GF (2018) Environmental suitability models predict population density, performance and body condition for microendemic salamanders. Scientific Reports 8(1), 7527.
| Crossref | Google Scholar |
Lüdecke D (2018) ggeffects: tidy data frames of marginal effects from regression models. Journal of Open Source Software 3, 772.
| Crossref | Google Scholar |
Mammola S, Milano F, Vignal M, Andrieu J, Isaia M (2019) Associations between habitat quality, body size and reproductive fitness in the alpine endemic spider Vesubia jugorum. Global Ecology and Biogeography 28(9), 1325-1335.
| Crossref | Google Scholar |
McKenzie NL, van Leeuwen S, Pinder AM (2009) Introduction to the Pilbara Biodiversity Survey, 2002–2007. Records of the Western Australian Museum Supplement 78(1), 3.
| Crossref | Google Scholar |
McNab BK (2010) Geographic and temporal correlations of mammalian size reconsidered: a resource rule. Oecologia 164(1), 13-23.
| Crossref | Google Scholar |
Moore HA, Dunlop JA, Valentine LE, Woinarski JCZ, Ritchie EG, Watson DM, Nimmo DG (2019) Topographic ruggedness and rainfall mediate geographic range contraction of a threatened marsupial predator. Diversity and Distributions 25(12), 1818-1831.
| Crossref | Google Scholar |
Moro D, Dunlop J, Williams MR (2019) Northern quoll persistence is most sensitive to survivorship of juveniles. Wildlife Research 46(2), 165-175.
| Crossref | Google Scholar |
Nakagawa S, Cuthill IC (2007) Effect size, confidence interval and statistical significance: a practical guide for biologists. Biological Reviews 82(4), 591-605.
| Crossref | Google Scholar |
Oakwood M (2000) Reproduction and demography of the northern quoll, Dasyurus hallucatus, in the lowland savanna of northern Australia. Australian Journal of Zoology 48(5), 519-539.
| Crossref | Google Scholar |
Oakwood M (2004) Death after sex. Biologist 51(1), 5-8.
| Google Scholar |
Oakwood M, Bradley AJ, Cockburn A (2001) Semelparity in a large marsupial. Proceedings of the Royal Society of London. Series B: Biological Sciences 268(1465), 407-411.
| Crossref | Google Scholar |
Oakwood M, Woinarski J, Burnett S (2016) The IUCN red list of threatened species. IUCN Red List of Threatened Species. Available at https://www.iucnredlist.org/en
Phillips SJ, Anderson RP, Schapire RE (2006) Maximum entropy modeling of species geographic distributions. Ecological Modelling 190(3), 231-259.
| Crossref | Google Scholar |
Pimm SL, Jenkins CN, Abell R, Brooks TM, Gittleman JL, Joppa LN, Raven PH, Roberts CM, Sexton JO (2014) The biodiversity of species and their rates of extinction, distribution, and protection. Science 344(6187), 1246752.
| Crossref | Google Scholar |
Plard F, Gaillard J-M, Coulson T, Hewison AJM, Douhard M, Klein F, Delorme D, Warnant C, Bonenfant C (2015) The influence of birth date via body mass on individual fitness in a long-lived mammal. Ecology 96(6), 1516-1528.
| Crossref | Google Scholar |
Pulliam HR (2000) On the relationship between niche and distribution. Ecology Letters 3(4), 349-361.
| Crossref | Google Scholar |
Pérez-Tris J, Dı́az JA, Tellerı́a JL (2004) Loss of body mass under predation risk: cost of antipredatory behaviour or adaptive fit-for-escape? Animal Behaviour 67(3), 511-521.
| Crossref | Google Scholar |
Rankmore RP, Griffiths AD, Woinarski JCZ, Ganambarr BL, Taylor R, Brennan K, Firestone K, Cardoso M (2008) Island translocation of the northern quoll Dasyurus hallucatus as a conservation response to the spread of the cane toad Chaunus (Bufo) marinus in the Northern Territory, Australia. Report to the Natural Heritage Trust. p. 37. Department of Natural Resources, Environment and The Arts.
Rew-Duffy M, Cameron SF, Freeman NJ, Wheatley R, Latimer JM, Wilson RS (2020) Greater agility increases probability of survival in the endangered northern quoll. Journal of Experimental Biology 223(15), jeb218503.
| Crossref | Google Scholar |
Rieger JF (1996) Body size, litter size, timing of reproduction, and juvenile survival in the Unita ground squirrel, Spermophilus armatus. Oecologia 107(4), 463-468.
| Crossref | Google Scholar |
Romero LM, Wikelski M (2001) Corticosterone levels predict survival probabilities of Galápagos marine iguanas during El Niño events. Proceedings of the National Academy of Sciences 98(13), 7366-7370.
| Crossref | Google Scholar |
Sabino-Marques H, Mira A (2011) Living on the verge: are roads a more suitable refuge for small mammals than streams in Mediterranean pastureland? Ecological Research 26(2), 277-287.
| Crossref | Google Scholar |
Schmitt LH, Bradley AJ, Kemper CM, Kitchener DJ, Humphreys WF, How RA (1989) Ecology and physiology of the northern quoll, Dasyurus hallucatus (Marsupialia, Dasyuridae), at Mitchell Plateau, Kimberley, Western Australia. Journal of Zoology 217(4), 539-558.
| Crossref | Google Scholar |
Serena M, Soderquist TR (1988) Growth and development of pouch young of wild and captive Dasyurus-Geoffroii (Marsupialia, Dasyuridae). Australian Journal of Zoology 36(5), 533-543.
| Crossref | Google Scholar |
Stephens PA, Pettorelli N, Barlow J, Whittingham MJ, Cadotte MW (2015) Management by proxy? The use of indices in applied ecology. Journal of Applied Ecology 52(1), 1-6.
| Crossref | Google Scholar |
Stevenson RD, Woods WA, Jr (2006) Condition indices for conservation: new uses for evolving tools. Integrative and Comparative Biology 46(6), 1169-1190.
| Crossref | Google Scholar |
Suorsa P, Huhta E, Nikula A, Nikinmaa M, Jäntti A, Helle H, Hakkarainen H (2003) Forest management is associated with physiological stress in an old–growth forest passerine. Proceedings of the Royal Society of London. Series B: Biological Sciences 270(1518), 963-969.
| Crossref | Google Scholar |
Sæther B-E (1989) Survival rates in relation to body weight in European birds. Ornis Scandinavica (Scandinavian Journal of Ornithology) 20(1), 13-21.
| Crossref | Google Scholar |
Thomas H, Cameron SF, Campbell HA, Micheli-Campbell MA, Kirke EC, Wheatley R, Wilson RS (2021) Rocky escarpment versus savanna woodlands: comparing diet and body condition as indicators of habitat quality for the endangered northern quoll (Dasyurus hallucatus). Wildlife Research 48(5), 434.
| Crossref | Google Scholar |
Thomson DF, Dixon JM, Huxley L (1985) ‘Donald Thomson’s mammals and fishes of northern Australia.’ (Nelson) Available at https://scholar.google.com/scholar_lookup?title=Donald+Thomson%27s+Mammals+and+fishes+of+northern+Australia&author=Thomson%2C+Donald+F.+%28Donald+Ferguson%29&publication_year=1985
VanDerWal J, Shoo LP, Johnson CN, Williams SE (2009) Abundance and the environmental niche: environmental suitability estimated from niche models predicts the upper limit of local abundance. The American Naturalist 174(2), 282-291.
| Crossref | Google Scholar |
Van Horne B (1983) Density as a misleading indicator of habitat quality. The Journal of Wildlife Management 47(4), 893-901.
| Crossref | Google Scholar |
von Takach B, Scheele BC, Moore H, Murphy BP, Banks SC (2020) Patterns of niche contraction identify vital refuge areas for declining mammals. Diversity and Distributions 26(11), 1467-1482.
| Crossref | Google Scholar |
Wells CP, Wilson JA, Kelt DA, Van Vuren DH (2019) Body mass as an estimate of female body condition in a hibernating small mammal. The Canadian Field-Naturalist 133(1), 34-42.
| Crossref | Google Scholar |
Williams R, Vikingsson GA, Gislason A, Lockyer C, New L, Thomas L, Hammond PS (2013) Evidence for density-dependent changes in body condition and pregnancy rate of North Atlantic fin whales over four decades of varying environmental conditions. ICES Journal of Marine Science 70(6), 1273-1280.
| Crossref | Google Scholar |
Wintle BA, Cadenhead NCR, Morgain RA, Legge SM, Bekessy SA, Cantele M, Possingham HP, Watson JEM, Maron M, Keith DA, Garnett ST, Woinarski JCZ, Lindenmayer DB (2019) Spending to save: what will it cost to halt Australia’s extinction crisis? Conservation Letters 12(6), e12682.
| Crossref | Google Scholar |
Wittmann ME, Barnes MA, Jerde CL, Jones LA, Lodge DM (2016) Confronting species distribution model predictions with species functional traits. Ecology and Evolution 6(4), 873-879.
| Crossref | Google Scholar |
Wolfe KM, Mills HR, Garkaklis MJ, Bencini R (2004) Post-mating survival in a small marsupial is associated with nutrient inputs from seabirds. Ecology 85(6), 1740-1746.
| Crossref | Google Scholar |
Ziembicki Mr, Woinarski Jcz, Mackey B (2013) Evaluating the status of species using Indigenous knowledge: novel evidence for major native mammal declines in northern Australia. Biological Conservation 157, 78-92.
| Crossref | Google Scholar |



