Numerical study of a crown fire spreading toward a fuel break using a multiphase physical model
Jean-Luc Dupuy A C and Dominique Morvan BA INRA Unité de Recherches Forestiéres Méditerranéennes, Equipe de Prévention des Incendies de Forêt, 20 Avenue Antonio Vivaldi, 84000 Avignon, France.
B Université de la Méditerranée, UNIMECA 60 rue Joliot Curie Technopôle de Château Gombert, 13453 Marseille cedex 13, France.
C Corresponding author. Telephone: +33 4 90135939; fax: +33 4 90135959; email: dupuy@avignon.inra.fr
International Journal of Wildland Fire 14(2) 141-151 https://doi.org/10.1071/WF04028
Submitted: 23 June 2004 Accepted: 23 December 2004 Published: 17 May 2005
Abstract
The propagation of a wildfire through a Mediterranean pine stand was simulated using a multiphase physical model of fire behaviour. The heterogeneous character of the vegetation was taken into account using families of solid particles, i.e. the solid phases (foliage, twigs, grass). The thermal decomposition of the solid fuel by drying and pyrolysis, and the combustion of chars were considered, as well as the radiative and convective heat transfer between the gas and the vegetation. In the gaseous phase, turbulence was modelled using a two transport equations model (RNG k–ϵ) and the rate of combustion, which was assumed to be controlled by the turbulent mixing of fuel and oxygen, was calculated using an eddy dissipation concept. The radiation transfer equation, which includes absorption and emission of both the gas–soot mixture and the vegetation, was solved to calculate the contribution of radiation to the energy balance equations. Numerical solutions were calculated in a two-dimensional domain (vertical plane). Results showed the ability of this approach to simulate the propagation of a crown fire and to test the efficiency of a fuel break with success. The effects of the terrain slope were also tested. Some effects on fire behaviour of vortices resulting from the interaction of the wind flow with the canopy layer are shown.
Additional keywords: forest fire spread.
Baines PG (1990) Physical mechanisms for the propagation of surface fires. Mathematical and Computer Modelling 13, 83–94.
| Crossref | GoogleScholarGoogle Scholar |
Di Blasi C (1993) Modeling and simulation of combustion processes of charring and non-charring solid fuels. Progress in Energy and Combustion Science 19, 71–104.
| Crossref | GoogleScholarGoogle Scholar |
Haines DH (1982) Horizontal roll vortices and crown fires. Journal of Climate and Applied Meteorology 21, 751–763.
| Crossref | GoogleScholarGoogle Scholar |
Larini M, Giroud F, Porterie B , Loraud J-C (1998) A multiphase formulation for fire propagation in heterogeneous combustible media. International Journal of Heat and Mass Transfer 41, 881–897.
| Crossref | GoogleScholarGoogle Scholar |
Morvan D , Dupuy JL (2001) Modeling of fire spread through a forest fuel bed using a multiphase formulation. Combustion and Flame 127, 1981–1994.
| Crossref | GoogleScholarGoogle Scholar |
Pitts WM (1991) Wind effects on fires. Progress in Energy and Combustion Science 17, 83–134.
| Crossref | GoogleScholarGoogle Scholar |
Stocks BJ, Alexander ME , Lanoville RA (2004) Overview of the international crown fire modelling experiment (ICFME). Canadian Journal of Forest Research 34, 1543–1547.
| Crossref | GoogleScholarGoogle Scholar |

Tien CL , Lee SC (1982) Flame radiation. Progress in Energy and Combustion Science 8, 41–59.
| Crossref | GoogleScholarGoogle Scholar |

Van Wagner CE (1977) Conditions for the start and spread of crown fire. Canadian Journal of Forest Research 7, 23–34.

Weber RO (1991) Modelling fire spread through fuel beds. Progress in Energy and Combustion Science 17, 67–82.
| Crossref | GoogleScholarGoogle Scholar |

Yakhot V , Orszag SA (1986) Renormalization group analysis of turbulence. Journal of Scientific Computing 1, 3–51.
| Crossref | GoogleScholarGoogle Scholar |

Appendix 1
Nomenclature
Latin symbols
ap radiation absorption coefficient of the gas–soot mixture (m−1)
B Stefan-Boltzman constant (W m−2 K−4)
Cp,s,k specific heat of the solid phase k (J kg−1 K−1)
D mass diffusivity (kg m−1 s−1)
Fi,s drag forces due to interactions between the solid phases and the gaseous phase (component in the i-direction) (kg m−2 s−2)
gi gravity acceleration (component in the i-direction) (m s−2)
h enthalpy (J m−3)
hconv heat transfer coefficient (convection between a solid phase k and the gaseous phase) (W m−2 K−1)
I radiative intensity (W sr−1 m−2)
J irradiance (W m−2)
k turbulent kinetic energy (m2 s−2)
Ms,k,α production of the α chemical species due to thermal degradation of the solid phase k (kg m−3 s−1)
t time (s)
T temperature (K)
ui gas velocity (component in the i-direction) (m s−1)
xi cartesian coordinate in the i-direction (m)
Y mass fraction
Greek symbols
α volume fraction
ϵ dissipation rate (m2 s−3)
ρ density (kg m−3)
σi,j stress tensor (kg m−1 s−2)
σs,k surface area-to-volume ratio of the solid phase k (m−1)
ω reaction rate (kg m−3 s−1)
Subscripts
α chemical species
g gaseous phase
k solid phase
Balance equations of solid phases
The following set of balance equations governs the evolution of each solid phase (or fuel family) k:
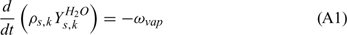
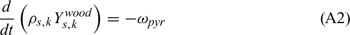
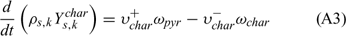
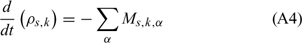
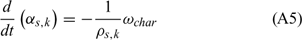
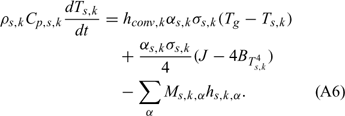
Equations (A1), (A2) and (A3) govern the evolution of the mass fractions of water, dry material and char respectively, resulting from drying, pyrolysis and char combustion processes. The terms on the right hand side of equation (A3) are written assuming that the rate of production of char is a known fraction (υchar) of the pyrolysis reaction rate and that a part of char is lost in the plume to form soot particles (υchar+ = υchar − υsoot · ωchar represents the rate of char combustion. Equation (A4) represents the mass balance of the solid phase. Equation (A5) governs the evolution of the volume fraction, assuming that the reduction of the volume of solid fuel particles results only from char combustion (when the particle undergoes drying and pyrolysis processes, its volume remains constant). The first and second terms on the right hand side of the energy balance equation (A6) represent the contributions resulting from convective exchanges with the surrounding gas and from radiation heat transfer respectively. The third term represents energy transfers due to mass transfers between the solid phase and the gaseous phase.
Transport equations of the gaseous phase
Basically, the transport equations of the gaseous phase are the Navier-Stokes equations generalised to a compressible, reactive and multiphase flow:
Mass:
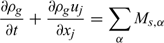
Momentum:
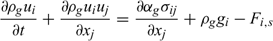
Energy:
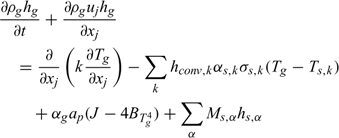
Chemical species:
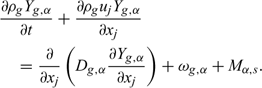
To take into account the turbulence effects, these basic transport equations are time-averaged using the Favre formalism and a turbulent diffusion model (RNG k–ϵ) is introduced.
Radiation transfer equation
The radiation intensity I is calculated solving the following radiation transfer equation:
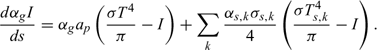
The irradiance J, which appears in the equations of energy balance, is formally calculated at each point through an integration of the radiation intensity over the space solid angle: