Understanding extractable metal species relationships with phosphorus sorption and organic carbon in soils
Bright E. Amenkhienan A B * , Feike Dijkstra A , Charles Warren
A B * , Feike Dijkstra A , Charles Warren  A and Balwant Singh
A and Balwant Singh  A
A
A
B
Abstract
Iron and aluminium oxides are important in phosphate sorption capacity of soils and preservation of soil organic carbon (SOC). However, there is a complex interplay between among Fe/Al oxides, SOC, and P in soils.
We aimed to evaluate the relationships between extractable Fe and Al, SOC concentration and P sorption capacity using generalised additive mixed models.
We compiled and analysed data from 77 published articles from Scopus and Web of Science.
Ammonium oxalate extractable aluminium (Alox) had astrong significant relationship (P < 0.0001) with P sorption capacity, but this was stronger with dithionite-citrate-bicarbonate extractable aluminium (Ald). A positive 1:1 relationship between Alox and Ald suggests that the pool of Al dissolved by ammonium oxalate and dithionite citrate bicarbonate (DCB) was nearly similar. A strong significant relationship was found between ammonium oxalate extractable iron (Feox) and Alox, and SOC concentration, but Alox had a stronger statistically significant relationship with SOC concentration. This may be due to various interactions of SOC with Al oxides, which can directly or indirectly influence P sorption capacity in soils.
From these relationships, we show that: (1) that Ald is a better predictor for P sorption capacity than Alox; and (2) Alox is a better predictor of SOC than Feox.
DCB and ammonium oxalate extractable Al (and Fe) that represent Al in crystalline and poorly crystalline, or amorphous form of Al may be used as a routine soil test, and may be able to predict P sorption capacity and SOC preservation potential, particularly in acid soils.
Keywords: aluminium, ammonium oxalate, dithionite citrate bicarbonate, extractable metals, iron, phosphorus sorption capacity, relationship, soil organic carbon.
Introduction
Soil organic matter (SOM) consists of about 50% carbon (C) and it is a critical component of soils (Banwart et al. 2019). Soil OM plays very vital roles in the functioning and productivity of ecosystems, soil quality and health, soil fertility and productivity (Reeves 1997; Watanabe 2017). Soils are the largest reservoir of C, storing twice the amount of organic C that is present in the atmosphere and vegetation combined (Batjes 1996, 2014). SOC is quite dynamic in nature, with continuous in and out fluxes that determine its net reserves in soils. It is important to increase the net storage of C in the soil to stabilise CO2 concentration in the atmosphere and to mitigate climate change, which has been the focus of scientific investigation in the last few decades (Lal 2004; Banwart et al. 2019).
The stabilisation of OM in soils is controlled by several mechanisms. Three primary mechanisms have been identified for the preservation of SOC: (1) accumulation of primary and secondary recalcitrant forms of organic molecules; (2) inaccessibility of SOC against enzymes and microbial decomposition via occlusion in aggregates; and (3 chemical interactions involving adsorption and co-precipitation, with phyllosilicates and iron (Fe) and aluminium (Al) oxides (used for brevity the term includes oxides/hydroxides/oxyhydroxides) (Sollins et al. 1996; Lützow et al. 2006; Kleber et al. 2015; Hemingway et al. 2019). The association of SOM with minerals, particularly involving Fe and Al oxides, has been identified as a key mechanism for the long-term preservation of SOC (Kaiser and Guggenberger 2000; Rasmussen et al. 2006; Tamrat et al. 2019; Hall and Thompson 2022; Ye et al. 2022) and thus, the necessity to better understand these interactions.
Due to their large specific surface area (SSA) and reactive surfaces, iron and Al oxides are common constituents of most soils and are important in the preservation of SOC via chemical interactions. During pedogenesis, Fe and Al containing primary minerals undergo chemical weathering, forming pedogenic species of Fe and Al (includes all secondary Fe and Al oxide and monomeric and polymeric species of Fe and Al in soil solution), which react with SOC. In monomeric forms, they bind with organic ligands (e.g. carboxylic functional groups) to form organo-metal complexes while in polymeric forms, they can form polynuclear complexes and secondary minerals. Secondary minerals (including oxides, hydroxides, oxyhydroxides and hydrated oxides) are often small in size, and have a large SSA and sorption capacity, and ability to bind and stabilise SOC (Kaiser and Guggenberger 2003; Wagai et al. 2013; Kleber et al. 2015; Rennert 2018; Wagai et al. 2020). This perspective has been asserted by close and strong positive relationships between SOC and ammonium oxalate extractable Fe and Al, which suggest that SOC is preferably preserved by interaction with poorly crystalline minerals (Kaiser and Guggenberger 2003; Kleber et al. 2005; Wiseman and Püttmann 2006; Rasmussen et al. 2018; Yu et al. 2021; Hall and Thompson 2022).
The preservation of SOC is important in the context of phosphorus (P) dynamics in soils. P is a major plant nutrient that plays a key role in photosynthesis, respiration, biosynthesis of nucleic acids and membranes, and regulation of several enzymes (Hawkesford et al. 2023). Total P concentrations in natural soils of terrestrial ecosystems are highly variable, ranging from 1.4 to 9636 mg kg−1, with an average concentration of 584 mg kg−1 in surface (0–30 cm) soils (He et al. 2021). Together with erosion and leaching, soil weathering depletes P that is present in soil primary minerals. Therefore, the total P concentration in soils decreases over the course of soil development (Walker and Syers 1976; Crews et al. 1995). Based on global and regional databases, He et al. (2021) reported mean total P concentration of 719, 481 and 472 mg kg−1 in slightly weathered, intermediately, and strongly weathered soils, respectively. A negligible (<0.1%) fraction of the total P in soils exists in soil solution that is in a plant-available form (Raghothama and Karthikeyan 2005). Organic soil P is the most important source of P in highly weathered soil and represents approximately 44% of total soil P in highly weathered soil (Cross and Schlesinger 1995). Phosphate associated with soil minerals is not readily bioavailable, it is either strongly adsorbed to minerals or present in insoluble P compounds formed from the reactions of phosphate with Al, Fe, and calcium (De Schrijver et al. 2012).
Similar to SOC, chemically extractable forms of Fe and Al have been identified as the key components in governing P sorption capacity of soils (Walker and Syers 1976; Borggaard et al. 1990; Singh and Gilkes 1991). P availability is widely considered to be the main constraint in limiting primary productivity in highly weathered soils because of their low P contents and propensity of phosphate to strongly adsorb onto Fe and Al oxides (Cross and Schlesinger 1995; Sanchez 2019). The effectiveness of P fertilisers in cropping soils relies primarily on P sorption capacity, which is primarily related to the concentration of different forms of Fe and Al oxides, particularly in highly weathered soils of tropic and sub-tropic regions (de Campos et al. 2016; Lin et al. 2020). Iron and Al oxides are well known to bind phosphate and limit P bioavailability in soils from both temperate and tropical systems (Walker and Syers 1976; Borggaard et al. 1990; Singh and Gilkes 1991; Vitousek et al. 2010). Herndon et al. (2019) reported that Fe oxides sequester approximately half of soil phosphate in shallow organic soils of low-lying areas from Artic and Boreal regions. Phosphate sorption is known to occur on Fe and Al oxides surfaces via ligand exchange reactions and it may be immobilised through occlusion with ageing (Torrent et al. 1992; Watanabe 2017). In addition, soil P has been linked to soil C sequestration capacity in highly weathered soils (where organic C can sorb P via cation bridging mechanism, with cations such as Fe3+, Al3+, and Ca2+ being involved in the process) because of continuous P fertilisation, which can reduce SOC mineralisation (Giardina et al. 2004; Li et al. 2006) although the relationship is not well understood under different soil environmental conditions.
There is a complex interplay among Fe/Al oxides, organic matter, and P in soils. P is a significant component of SOC, because a large amount of P, necessary for the formation of organo-mineral complexes, is stored with SOC (Spohn 2020). The OC:OP ratios of croplands indicate that ~13 and 22 kg P per ton of SOC are stored in the topsoil and subsoil of croplands, respectively (Spohn 2020). Organic amendments have been shown to increase P availability in soils by reducing phosphate adsorption or increasing phosphate desorption or by directly supplying more P contained in the organic amendments (Hunt et al. 2007; Zhang et al. 2018; Ma et al. 2019; Yang et al. 2019). Under normal soil pH conditions, OM predominantly carries negative charge and can form surface complexes with Fe and Al oxides that carry positive surface charge (Singh et al. 2016). P can be adsorbed by reversible reactions on SOM, with some bonds being much more readily and rapidly broken than others (Sposito 1989). The presence of OM on minerals can inhibit adsorption of negatively charged inorganic and organic P compounds and thus increase bioavailable P in soils (Hunt et al. 2007). Jindo et al. (2023) summarised various competitive sorption reactions that occur between SOC and bioavailable P. Firstly, SOC carrying a negative charge is adsorbed on the surfaces of metal (Fe/Al) oxides, which are positively charged thereby blocking the surface charge sites and increasing P desorption. Secondly, SOC adsorbed on the surfaces of metal oxides enhances P repulsion and, increases bioavailable P. Lastly, SOC chelating with Fe adsorbed on surfaces of metal oxides leads to formation of Fe-SOC, which can be released from the surface sites, making the surface sites positively charged and available for P sorption in soils.
The abundance of crystalline (dithionite-citrate-bicarbonate) and poorly crystalline (ammonium oxalate) Fe and Al oxides are diverse in soils and, contribute to SOC preservation and stabilisation and P sorption. Therefore, a comprehensive evaluation of the role of Fe and Al oxides in the stabilisation of SOC and sorption of P will enhance our knowledge on global soil C cycling, and P sorption and help in better soil management. Studies have synthesised large datasets of soils, showing that acid ammonium oxalate extractable Al was the strongest predictor of SOC concentration (Rasmussen et al. 2018; von Fromm et al. 2021; Yu et al. 2021; Hall and Thompson 2022; Zhao et al. 2023) while Watanabe (2017) reported that ammonium oxalate extractable Fe and Al (active Fe + Al) were the strongest predictor of P sorption. Few studies have explored relationships between extractable metals and SOC concentration but has not considered relationship with P sorption capacity within the same datasets. This is the first study to examine relationships among the different fractions of Fe and Al with SOC concentration and P sorption capacity simultaneously, thereby adding new knowledge to the existing literature. In our study, we explored published data to evaluate the relationships among the different fractions of Fe and Al with SOC and P adsorption of soils. The main objective was to identify the role of different fractions of Fe and Al oxides in the SOC preservation and P availability in soils using a generalised additive mixed model (GAMM) approach. We aimed to address two key research questions: (1) do extractable Fe and Al have any relationships with P sorption capacity and SOC concentration? and (2) does P sorption capacity and SOC concentration have any relationship?
Materials and methods
Peer-reviewed publications were searched on Scopus and Web of Science databases using the following key search terms: soil + iron oxides extract* + ‘phosph*sorpt*’; soil + iron oxides extract* + ‘organic matter’ OR ‘organic carbon’; soil + iron oxides + ‘phosph*sorpt*’; soil + iron oxides + ‘organic matter’; OR ‘organic carbon’. The key search terms did not only display chemically extractable Fe but also displayed chemically extractable Al, hence extractable Al was not included in the above key search terms. The preferred reporting items for systematic reviews and meta-analyses (PRISMA) diagram (Page et al. 2021) were used for the identification and selection of pertinent published papers for this study. A total number of 5717 publications resulted from the database searches, with 3917 publications from Scopus and 1836 from Web of Science. Publications that appeared as duplicate within the Scopus database and within the Web of Science database based on the different key search terms used were 251 and 1031; this amounted to a total of 1282 of duplicate within both databases while duplicate publications between databases (Scopus and Web, which left a total number of 3870 publications. Only publications having data for P sorption capacity (based on Langmuir model), organic C, and chemically extractable metal (Fe and Al) oxides were selected, extracted, and analysed because they adequately focused or contributed to the main objective of our study. Therefore, with the above-mentioned criteria, a total number of 3772 irrelevant publications were excluded, 18 publications were inaccessible and excluded while another three publications were also excluded because they were not in English. This resulted in a total of 77 relevant publications, which were eligible and examined for this study. A summary of the identification and selection of relevant publications used for this study is in Fig. 1.
Data analysis
The data extracted from selected journal papers were statistically analysed to examine the main drivers of P sorption capacity and SOC concentration using the gamm function within the mgcv package of generalised additive mixed models (GAMMs) (Wood 2017). The response variables were SOC concentration and P sorption capacity. The predictor variables were chemically extractable forms of Fe and Al. Ammonium oxalate extractable iron (Feox) and aluminium (Alox) consist of poorly crystalline minerals phases or short-range-ordered (SRO) mineral phases and organo-metal complexes. Dithionite-citrate-bicarbonate extractable iron (Fed) and aluminium (Ald) consist of crystalline minerals phases or pedogenic phases. All response and predictor variables were log10 transformed to conform to assumptions of residual distribution in GAMM. The GAMM accounts for non-linear relationships between response variables and the predictors by fitting penalised smooth functions to each predictor to minimise excessive ‘wiggliness’ (Wood 2017). The default K dimension of 10 (maximum degree of freedom) was used for each smooth function to dictate the flexibility of the relationship. The effective degree of freedom (edf) was used to express the plotted shape of the model. If the edf is equal to 1, then the smooth term is reduced from a flexible relationship to a simple linear relationship. We tested various smooth functions in the full model, which included the response variable, the extractable metals (Feox, Fed, Alox, Ald) and the interaction terms. However, the addition of interaction terms did not reduce the Akaike’s information criterion (AIC); hence, it was not included in the final model. The residuals approximately followed a Gaussian distribution; therefore, an identity link function was used. The F-values were used to estimate the significance (P < 0.001) of model terms. If the values of the response variables or the predictors were missing from the data, then those data were excluded from the GAMM analysis.
We initially fit two separate but single optimal GAMMs for each of the two response variables (i.e. SOC concentration and P sorption capacity), and all four predictor variables (Feox, Fed, Alox, Ald). The model assumptions were evaluated using residual plots. The single optimal GAMM showed relationships between each extractable metal and P sorption capacity, and between each extractable metal and SOC concentration. We observed that correlations between Fe and Al affected their relationships with P sorption capacity and SOC concentration, respectively (see Supplementary Figs S1 and S2). In GAMM, this is known as concurvity, which also refers to collinearity where values greater than 0.8 indicate close relationship and instability of a parameter while 0 indicates no relationship with other variables (Wood 2017). The limitation of the model is collinearity, which prevented running the single optimal GAMMs having all the predictor variables.
To avoid the problem of correlation among ammonium oxalate and dithionite-citrate-bicarbonate (DCB) extractable metals leading to concurvity, we fitted four separate additional GAMMs involving single extractable metals (Feox, Fed, Alox, Ald). The separate GAMMs were used to address questions on understanding the relationships between extractables forms of metals and P sorption capacity as well as SOC concentration. The additional GAMMs enable investigation of redundancy in various extractable metals as predictors of P sorption capacity and SOC concentration. The AIC values of each model fit by restricted maximum likelihood (REML), was used to compare the performance of models, with smaller AIC indicating better performance. The R2-values presented in the results are adjusted R2, adjusted for the number of predictors. The highest R2-values indicate better performance of the models. The R2-values of P sorption capacity is much higher compared to SOC concentration because the number of observations for P sorption capacity (n = 265) was higher than SOC (n = 249). Furthermore, we used the GAMMs to explore the non-linear relationships of extractable metals and P sorption capacity and SOC concentration, while we used the reduced major axis (RMA) regression to evaluate the linear relationships and slopes between extractable metals because assumptions were not met. The ‘lmodel2’ function was used for RMA regression. Paired t-test was used to test the differences between mean concentrations of extractable Al and Fe within depths. All analyses were performed using R studio statistical software (ver. 4.4.1; R Core Team 2024).
Results and discussion
Phosphorus sorption capacity versus extractable Fe and Al oxides
The relationship between P sorption capacity and metals extracted in acid ammonium oxalate (Feox and Alox) and citrate dithionite bicarbonate (Fed and Ald) are in Fig. S1. The single optimal GAMMs with all predictor variables; i.e. Feox, Fed, Alox and Ald, failed because of severe concurvity, i.e. the ‘concurvity’ function was close to 1; Feox = 0.99, Fed = 0.99, Alox = 0.98 and Ald = 0.98 (Fig. S1), therefore additional four separate models were fitted.
In the four separate additional GAMMs involving single extractable metals, we observed that individual extractable metals (Feox, Fed, Alox, and Ald) had statistically significant relationships with P sorption capacity (R2 = 0.91 Feox; P < 0.05, R2 = 0.91 Fed; P < 0.0001, R2 = 0.92 Alox; P < 0.0001, and R2 = Ald 0.93, P < 0.0001). P sorption capacity increased with Feox, Fed, Alox, and Ald, with curvilinear (non-linear) relationships (Fig. 2a–d). Our results show that both Ald and Alox were better predictor variables (AIC = 508 and 532, respectively) of P sorption capacity in soils than Fed and Feox (AIC = 572 and 545, respectively), and Ald was a better predictor variable of P sorption capacity than Alox (Fig. 2c and d). Several studies have reported significant correlations of P sorption capacity with Feox, Alox, Fed, and Ald contents in different soil types (Syers et al. 1971; Ping and Michaelson 1986; Peña and Torrent 1990; Singh and Gilkes 1991; Börling et al. 2001; Agbenin 2003; Wiriyakitnateekul et al. 2005).
Relationships between phosphorus sorption and individual extractable metals where each of the four metals extracted (ammonium oxalate extractable iron, Feox; dithionite-citrate-bicarbonate extractable iron, Fed; ammonium oxalate extractable aluminium, Alox; and dithionite-citrate-bicarbonate extractable aluminium, Ald) was used separately in the generalised additive mixed models (GAMMs). These results are different from the model in Fig. S1, where Feox, Fed, Alox, and Ald were included in a single GAMM model. Akaike’s information criterion values for Feox, Fed, Alox, and Ald in the GAMM model (using each extracted metal separately) were 545, 572, 532, and 508, respectively. The respective F-statistic values were 10, 2, 12, and 17; and R2-values were 0.91, 0.90, 0.92, and 0.93. The shaded red region around the smooth lines represents the 95% confidence intervals.
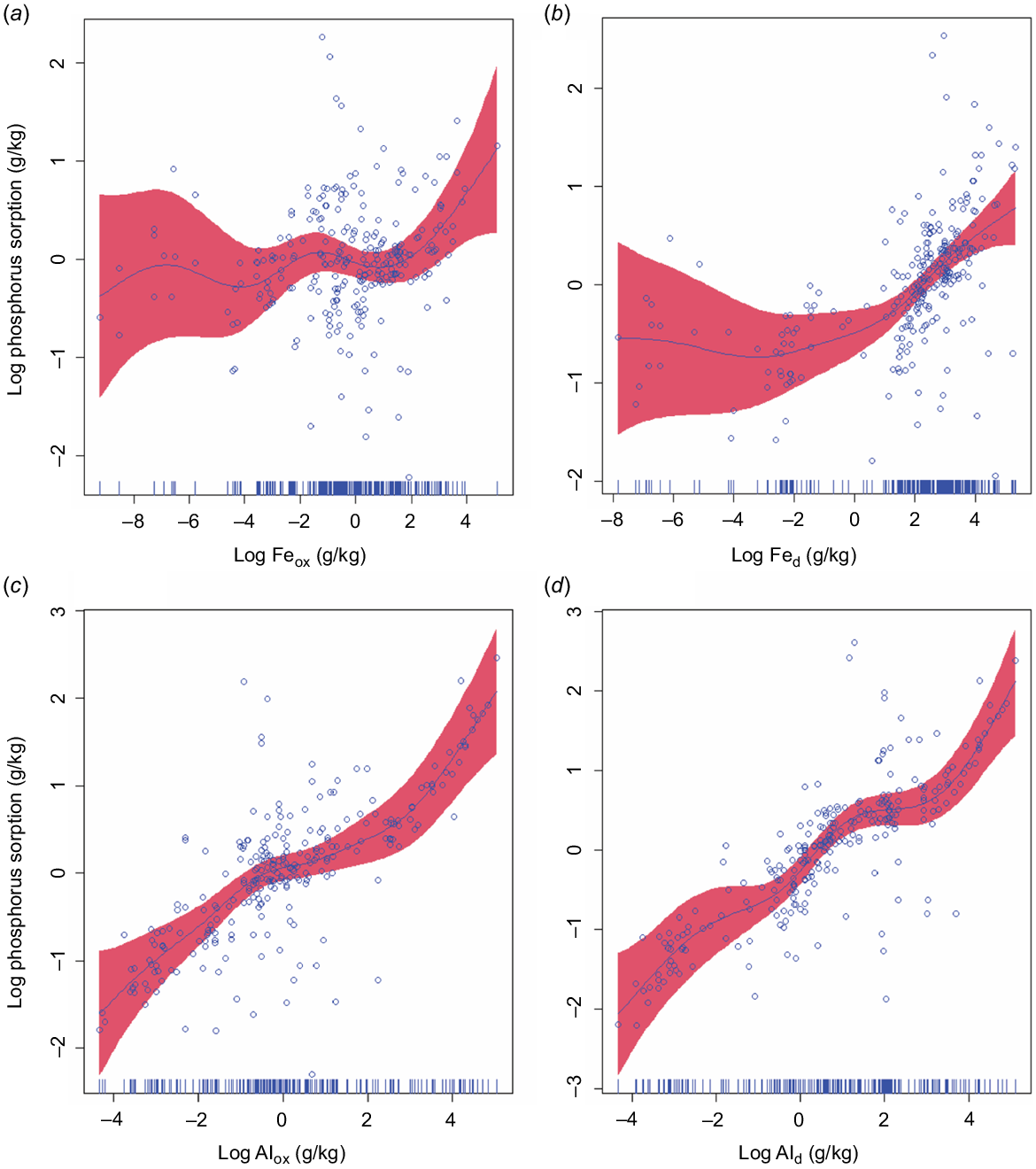
The higher P sorption capacity of extractable Al than the extractable Fe may be due to its greater SSA and, high density of reactive hydroxyl sites. Phosphate sorption on Al oxides occurs through ligand exchange reactions where singly coordinated hydroxyl (OH) groups are exchanged by phosphate anions (Borggaard et al. 1990). These results are consistent with several previous studies on different soil types (Bromfield 1965; Singh and Gilkes 1991; Börling et al. 2001; Agbenin 2003), where Alox and Ald were found to be more strongly correlated with P sorption capacity than Feox and Fed irrespective of their total contents in soils. The relative contribution of Ald in P sorption capacity has been estimated to be 3–5 times greater than for Fed in acid soils from Australia and Nigeria (Bromfield 1965; Singh and Gilkes 1991; Börling et al. 2001; Agbenin 2003). Acid ammonium oxalate extracts non-crystalline or amorphous and short-range order Al oxides and organically complexed Al (McKeague and Day 1966; Dahlgren 1994; Rennert 2018). Poorly crystalline and nano-sized crystalline goethite and haematite particles can also be dissolved during acid ammonium oxalate extraction (Acebal et al. 2000). In the DCB extraction, in addition to the Al forms extracted by acid ammonium oxalate solution, Al substituting for Fe in the structure of well crystalline Fe oxides (i.e. goethite and hematite) is also extracted. The contribution of Al released from the dissolution of Al-substituted goethite and haematite during the DCB extraction may explain the slightly better prediction of P sorption capacity with Ald than Alox. The substitution of Al for Fe in Fe oxides is common in soils, with up to one-third (Al/(Al + Fe)) = 0.33) in goethite and half of that in haematite (Singh and Gilkes 1992). The crystal size of Fe oxides has been known to decrease with increasing degree of Al substitution in their structures, thereby increasing SSA and P sorption capacity (Borggaard 1983).
Furthermore, our findings do not negate the importance of different forms of Fe oxides on P sorption capacity even though they are not the strongest predictors of P sorption capacity. As mentioned earlier, Feox and Fed showed statistically significant relationships with P sorption capacity (Fig. 2a and b). High P sorption capacity has been found in Oxisols and Alfisols, which have a high concentration of Ald and Fed (de Campos et al. 2016). Several other studies have reported significant relationships between different forms of extractable Fe and Al and P sorption capacity in soils from tropical and temperate regions of the world (Ahenkorah 1968; Loganathan and Fernando 1980; Peña and Torrent 1984, 1990; Singh and Gilkes 1991; Freese et al. 1992; Agbenin 2003; Li et al. 2007; Bortoluzzi et al. 2015; Watanabe 2017).
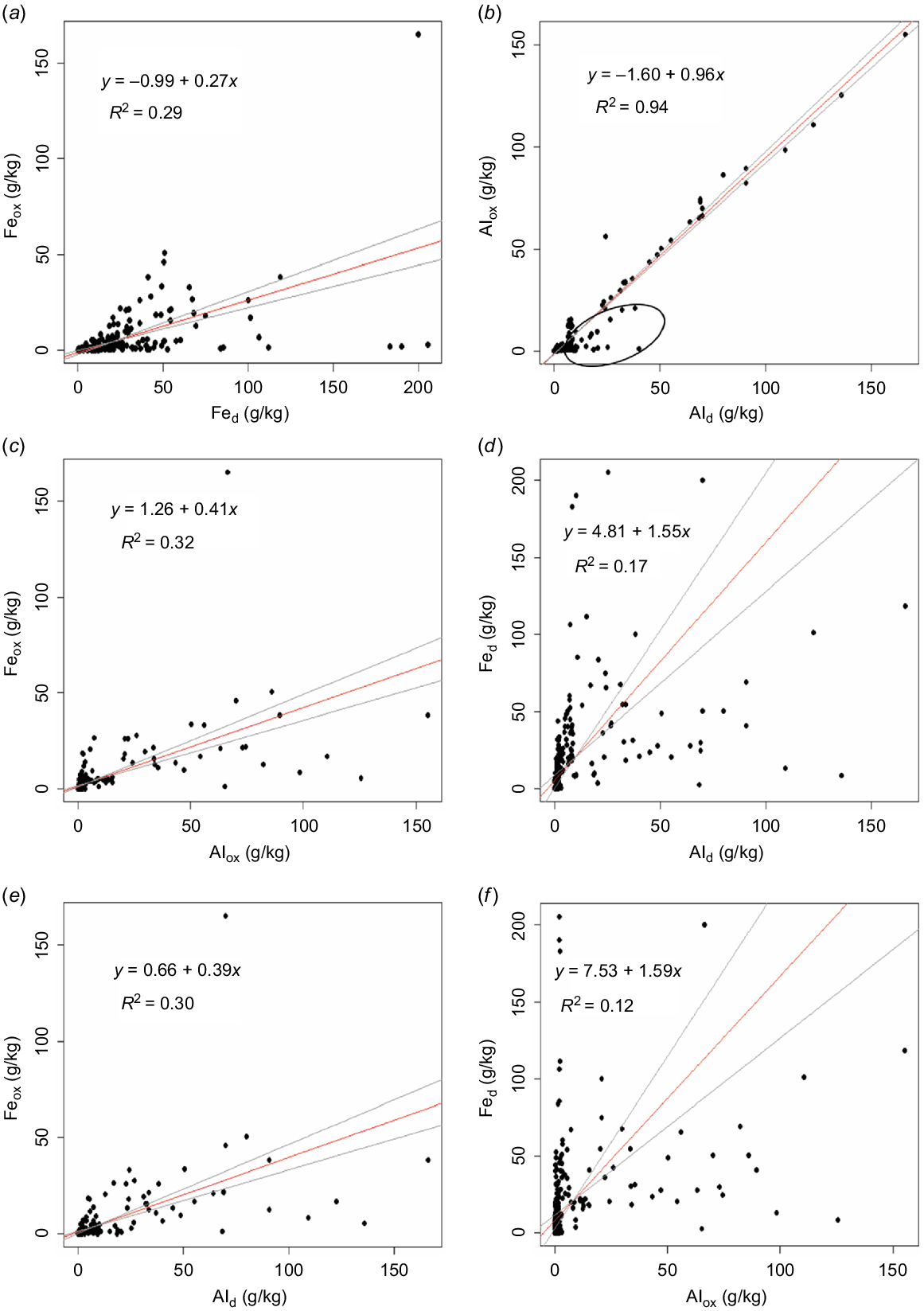
Table 1 shows the mean concentrations of Fe and Al in soils extracted by acid ammonium oxalate and DCB extractants at three soil depths. Fig. 3 shows the pairwise 1:1 relationship between extractable metals. The RMA regression of all the relationships were statistically significant (P < 0.0001) but not all the relationships were strong (Fig. 3). Ammonium oxalate Al and Ald had a nearly 1:1 relationship in the soil samples (Fig. 3b). However, a few samples (circled in Fig. 3b) appear to deviate from this relationship where additional Al (substituting for Fe) might have been released from the structure of Fe oxides during the DCB extraction. Our results are consistent with the results reported by Hall and Thompson (2022) for a large North American soil dataset. The linear relationship between Feox and Fed was weak (R2 = 0.29; Fig. 3a), and as expected, Fed was generally greater than Feox (RMA regression slope = 0.27 ± 0.02), which indicates ammonium oxalate extracted about 27% of the Fe extracted by DCB. Similar results, showing a weak relationship between Feox and Fed was reported by (Hall and Thompson 2022). The weak relationship between Fed and Ald, and between Feox and Ald (Fig. 3d and e), may indicate different environmental conditions, mineral composition, and degree of Al substitution in Fe oxides in the soils. The Fe and Al extracted in ammonium oxalate and DCB had a weak and variable relationship with each other (Fig. 3c and f).
| Soil depth (cm) A | Feox | Fed | Alox | Ald | |
|---|---|---|---|---|---|
| g/kg | |||||
| 0–20 (n = 204) | 3.09 (5.80)b | 19.89 (29.25)a | 3.29 (7.97)a | 5.57 (8.91)b | |
| 20–50 (n = 102) | 5.03 (18.04)b | 19.95 (28.46)a | 8.38 (24.87)a | 10.14 (25.85)b | |
| >50 (n = 42) | 4.97 (7.79)b | 16.10 (19.45)a | 18.36 (30.88)a | 19.28 (32.77)b | |
Different letters on the rows indicate significant (P < 0.001) differences in the mean values between ammonium oxalate Fe and Al and between dithionite-citrate-bicarbonate Fe and Al within each depth based on paired t-test.
Feox, ammonium oxalate extractable iron; Fed, dithionite-citrate-bicarbonate extractable iron; Alox, ammonium oxalate extractable aluminium; Ald, dithionite-citrate-bicarbonate extractable aluminium.
Relationships between various forms of Al and Fe extracted in ammonium oxalate and dithionite-citrate-bicarbonate solutions. Red lines, reduced major axis (RMA) regression; grey lines, confidence intervals for the RMA regression line. R2-values and RMA regression equation are given in each plot. All regression slopes were statistically significant at P < 0.0001. Feox, ammonium oxalate extractable iron; Fed, dithionite-citrate-bicarbonate extractable iron; Alox, ammonium oxalate extractable aluminium; Ald, dithionite-citrate-bicarbonate extractable aluminium.
SOC vs extractable Fe and Al oxides
Fig. S2 shows the relationships between SOC and Al and Fe extracted in acid ammonium oxalate (Alox and Feox) and dithionite citrate bicarbonate (Ald and Fed). All predictor variables (Feox, Fed, Alox and Ald) failed in the single optimal GAMM because of severe concurvity; i.e. Feox = 0.94, Fed = 0.95, Alox = 0.99, and Ald = 0.99.
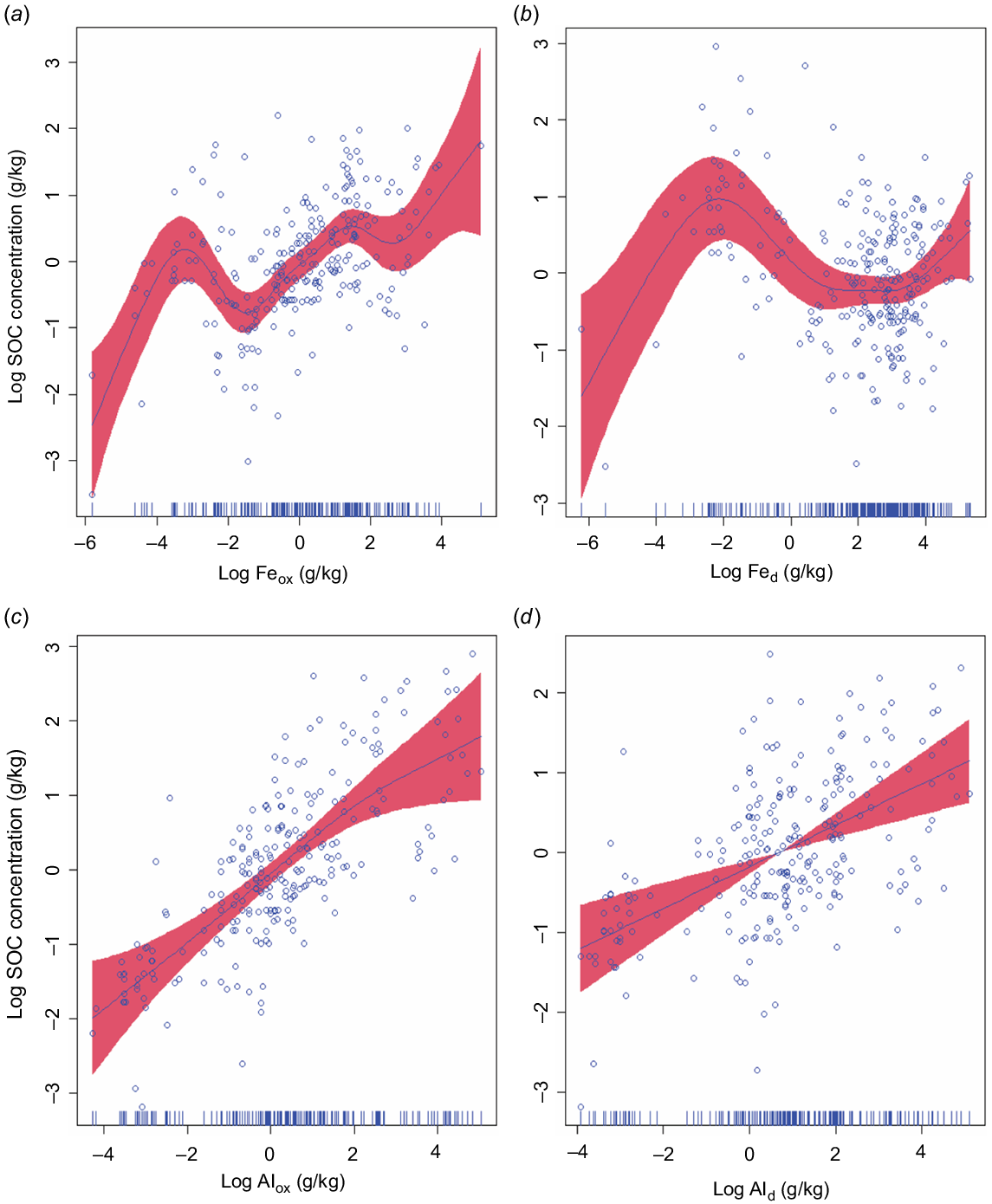
In the four separate GAMMs involving each of the four extractable metals, we observed a statistically significant relationship of each metal with SOC concentration (R2 = 0.57 Feox, 0.54 Fed, 0.58 Alox, and Ald 0.52, P < 0.0001). SOC concentration increased with Feox, Fed, and Alox, with curvilinear (non-linear) relationships (Fig. 4a–c), while Ald had a linear relationship with SOC (edf = 1) (Fig. 4d). In the GAMM plots, we observed that when these variables increased non-linearly, SOC was plateaued (i.e SOC did not show any further increase). This may suggest that many soils are not yet saturated with SOC; hence, these soils have additional capacity for SOC adsorption onto Al and Fe oxides. These results are consistent with the observations from the NEON soil dataset where an increase in the metals (Alox and ammonium acetate exchangeable calcium plus exchangeable magnesium [Caxe + Mgex) did not further increase the SOC (Yu et al. 2021). Our results show that oxalate extractable metals (Feox and Alox) are better predictor variables of SOC than DCB extracted metals (Fed and Ald) and Alox was a slightly stronger predictor variable than Feox as shown by the AIC (Alox = 645 vs Feox = 658) (Fig. 4). Recent studies from North America with larger soil datasets have consistently revealed that Alox exhibited stronger predictive power for SOC concentrations compared to Feox and Fed (Rasmussen et al. 2018; Yu et al. 2021; Hall and Thompson 2022). Watanabe (2017) reported a significant correlation between Fe + Al oxides and SOC and concluded that the major components of SOC preservation is through binding with Fe and Al oxides. The RMA regression showed statistically significant (P < 0.01) relationship between SOC concentration and Feox + Alox (Fig. S3). In this study, Feox + Alox explained 29% of the variability in the SOC concentration. Kleber et al. (2005) observed that Feox + Alox explained 78% of the variability (P < 0.001) in SOC concentration in acid soils and suggested that SOC concentration in acid soils is positively and linearly correlated to the concentration of poorly crystalline minerals. In contrast to these, Percival et al. (2000) reported a significant correlation between Al pyrophosphate (Alpp) (pyrophosphate extracts organo-Al complexes) and SOC, and observed that Alpp was the best predictor of SOC concentration.
Relationships between soil organic carbon (SOC) and individual extractable metals where each of the four metals extracted, (ammonium oxalate extractable iron, Feox; dithionite-citrate-bicarbonate extractable iron, Fed; ammonium oxalate extractable aluminium, Alox; dithionite-citrate-bicarbonate extractable aluminium, Ald) was used in the generalised additive mixed models (GAMMs). These results are different from the model in Fig. S2, where Feox, Fed, Alox, and Ald were included in a single model. Akaike’s information criterion values for separate models that included either Feox, Fed, Alox, Ald, were 658, 672, 645 and 670, respectively; F-statistic values for the individual predictors were 7, 6, 23, and 19; R2-values for the full models were 0.57, 0.54, 0.58 and 0.52. The shaded red areas around the smooth lines represent the 95% confidence intervals.
SOC stabilisation by poorly crystalline oxides (i.e. Alox and Feox) may be due to the possession of extensive SSA and increased reactive sites. The formation of stable organic-metal complexes (Al and Fe) occurs through ligand exchange reactions between carboxyl groups of SOC and singly coordinated hydroxyl (OH) groups at the metal surfaces (Kaiser and Guggenberger 2000). The interaction between SOC and Al/Fe oxides can lead to less susceptibility of SOC to desorption, oxidative degradation, biodegradation and greater long-term preservation of SOC in soils (Kaiser and Guggenberger 2003; Zimmerman et al. 2004). There was a weak relationship between Feox and Alox in soil samples (Fig. 3c), and Alox was significantly (P < 0.001) greater than Feox (Table 1). This was possibly due to greater solubility of Al than Fe at low pH in soils and the weak tendency of Al oxides to crystalise as compared to Fe (Shang and Tiessen 1998; Watanabe 2017). During acid-mediated mineral weathering, Al phases (gibbsite and aluminosilicates) are known to dissolve at higher pH values than the Fe oxides (goethite, haematite, and ferrihydrite) (Chadwick and Chorover 2001). The soluble Al3+ outperform base cations on the cation exchange sites, thereby maintaining the soil pH within the range of 4.0 to 5.5, which in turn restricts the dissolution of Fe (Chadwick and Chorover 2001). The greater abundance of Al than Fe in the parent material might also favour the higher Al concentration than Fe ions in the soil solution (Hall and Thompson 2022).
Our results do not refute the significant role of crystalline oxides (Fed or Ald) in protecting SOC. Crystalline oxides play an important role in the preservation of SOC when present in the soil in substantial amounts (Mikutta et al. 2006; Yeasmin et al. 2017). A strong positive significant correlation between SOC and crystalline Fe oxides has been observed in soils (Mikutta et al. 2006; Yeasmin et al. 2017). Contrary to this, negative relationships between Fed (crystalline Fe) and SOC concentrations have also been reported in some studies, which has been related to the smaller SSA of crystalline Fe oxides compared to SRO Fe phases (Feox) (Percival et al. 2000; Hall and Silver 2015).
The rate of SOC mineralisation is controlled by its association with extractable metals. Few studies have quantified SOC mineralisation of OC sorbed onto minerals (Mikutta et al. 2007; Schneider et al. 2010; Saidy et al. 2012). Saidy et al. (2012) studied the influence of Fe oxides (including ferrihydrite, goethite, hematite) and imogolite on C mineralisation in an illitic clay. They reported that C mineralisation was significantly reduced by ferrihydrite with increased SSA than goethite, haematite, and imogolite, and the illitic clay-ferrihydrite association provided greater stabilisation of SOC. Mikutta et al. (2007) reported that mineral associated organic matter primary held by ligand exchange exhibited resistance to mineralisation compared to organic matter bound by van der Waals forces. Likewise, SOC bound to poorly crystalline Al hydroxides reduced the rate of SOC mineralisation (Schneider et al. 2010). Soils dominated by poorly crystalline minerals have a tendency to reduce SOC mineralisation because of the stabilisation of SOC by chemical sorption of SOC while it may not be applicable to soils dominated by crystalline minerals (Parfitt et al. 2002; Rasmussen et al. 2006).
SOC and P sorption capacity
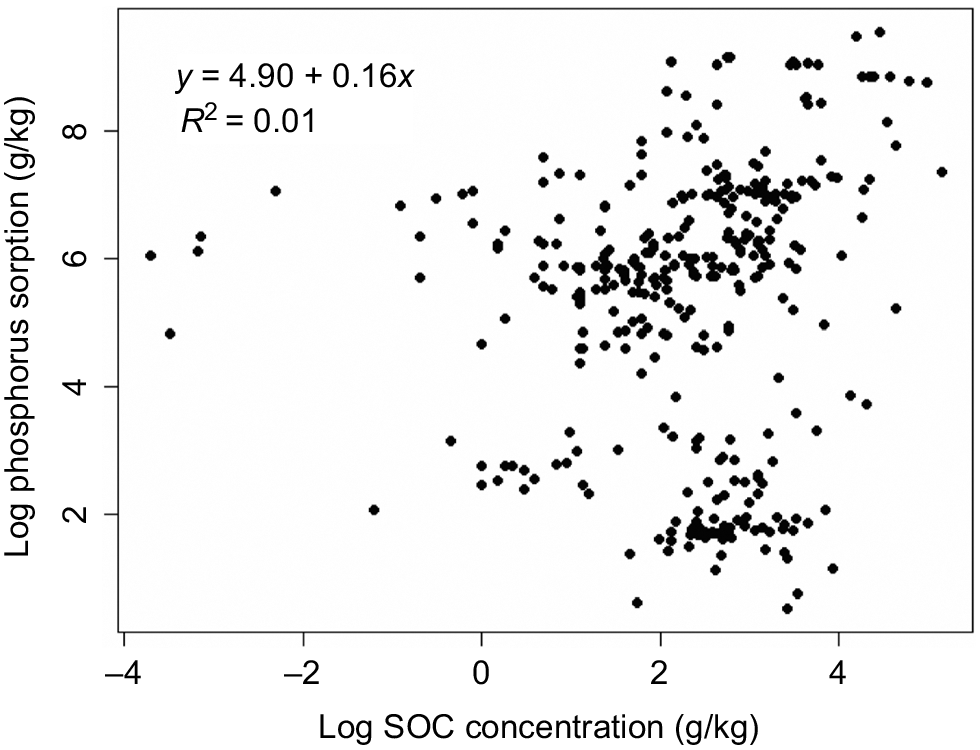
The linear relationship between SOC concentration and P sorption capacity was weak, and non-significant (R2 = 0.01, Fig. 5). This may be due to various interactions of SOC with SRO, which can directly or indirectly influence P sorption capacity in soils (Guppy et al. 2005; Wang et al. 2015; Yan et al. 2016; Hawkins et al. 2022). Studies have reported a weak and non-significant correlation between SOC and P sorption capacity (Villapando and Graetz 2001; Yan et al. 2013), and a non-significant direct effect of SOC on P sorption capacity in a path analysis (Ige et al. 2007; Kang et al. 2009).
Relationship between phosphorus sorption and soil organic carbon (SOC), R2-values and regression equation are given in plot. Regression slopes was not statistically significant.
Iron/Al oxides are important for SOC stabilisation and sorption of P (Gérard 2016). Competitive sorption occurs between SOC and phosphate for adsorption sites of Fe/Al oxides because both are negatively charged and utilise the same adsorption sites (Antelo et al. 2010; Jindo et al. 2023). The presence of high concentration of SOC in the soil can decrease P sorption capacity and increase P bioavailability in soils. The sorbed SOC can block the adsorption sites which leads to decreased phosphate sorption and repulsion, thereby increasing bioavailability P (Hunt et al. 2007; Jindo et al. 2023). The bioavailability of P in soils has been reported to be enhanced through the application of organic and inorganic fertilisersf (Hunt et al. 2007; Ma et al. 2019; Yang et al. 2019). Conversely, some studies have observed increased P sorption capacity and decreased bioavailability of P in soils with increasing SOC contents (Guppy et al. 2005; Chase et al. 2018).
Conclusions
Oxalate extractable and dithionite extractable metals are important indicators of the P sorption capacity of soils and SOC preservation. The analysis of the data from the published studies demonstrates that Alox and Ald are strong predictor variables of P sorption capacity of soils, but Ald was a better predictor variable than Alox of the P sorption capacity of soils. Further, a positive 1:1 relationship between Alox and Ald suggests that the pool of Al dissolved by ammonium oxalate and DCB was nearly the same, although small deviations from this relationship, might indicate release of additional Al substituting for Fe from the structure of Fe oxides during the DCB extraction. Similarly, we found that Feox and Alox are good predictor variables of SOC, but Alox is a better predictor variable of SOC. From the above, we deduce that extractable Fe and Al have significant relationships with P sorption capacity and SOC concentration. The DCB forms of extractable Al and ammonium oxalate extractable Al have stronger relationships with SOC concentration and P sorption capacity than extractable Fe. The concentration of ammonium oxalate extractable Al in the soil samples was significantly (P < 0.001) greater than the concentration of ammonium oxalate extractable Fe. Although, the data used from the published literature do not show a relationship between P sorption capacity and SOC concentration, further research is needed on this aspect, particularly in acid soils. The DCB and ammonium oxalate extractable Al (and Fe) represent Al in crystalline and poorly crystalline, or amorphous form of Al may be used as a routine soil test, and these may be able to predict P sorption capacity and SOC preservation potential, particularly in acid soils.
Data availability
All author’s name and published article title used in generating and analysing all data during this study are included in this published article.
Conflicts of interest
Balwant Singh is an Editor of Soil Research. To mitigate this potential conflict of interest he had no editor-level access to this manuscript during peer review. The author(s) have no further conflicts of interest to declare.
Declaration of funding
We acknowledge the Department of Agriculture, Water and the Environment, The Commonwealth of Australia, for the award of scholarship and consumables costs via the Soil Science Challenge project, A soil-plant nexus to maximise organic carbon sequestration in the soil (4-H4T0SA3). We thank the Tertiary Education Trust Fund (TETFund) for their financial support.
References
Acebal SG, Mijovilovich A, Rueda EH, Aguirre ME, Saragovi C (2000) Iron-oxide mineralogy of a mollisol from Argentina: a study by selective-dissolution techniques, X-ray diffraction, and Mössbauer spectroscopy. Clays and Clay Minerals 48, 322-330.
| Crossref | Google Scholar |
Agbenin JO (2003) Extractable iron and aluminum effects on phosphate sorption in a savanna Alfisol. Soil Science Society of America Journal 67, 589-595.
| Crossref | Google Scholar |
Ahenkorah YAW (1968) Phosphorus-retention capacities of some cocoa-growing soils of Ghana and thier relationship with soil properties. Soil Science 105, 24-30.
| Crossref | Google Scholar |
Antelo J, Fiol S, Pérez C, Mariño S, Arce F, Gondar D, López R (2010) Analysis of phosphate adsorption onto ferrihydrite using the CD-MUSIC model. Journal of Colloid and Interface Science 347, 112-119.
| Crossref | Google Scholar | PubMed |
Banwart SA, Nikolaidis NP, Zhu Y-G, Peacock CL, Sparks DL (2019) Soil functions: connecting earth’s critical zone. Annual Review of Earth and Planetary Sciences 47, 333-359.
| Crossref | Google Scholar |
Batjes NH (1996) Total carbon and nitrogen in the soils of the world. European Journal of Soil Science 47, 151-163.
| Crossref | Google Scholar |
Batjes NH (2014) Total carbon and nitrogen in the soils of the world. European Journal of Soil Science 65, 10-21.
| Crossref | Google Scholar |
Borggaard OK (1983) The influence of iron oxides on phosphate adsorption by soil. Journal of Soil Science 34, 333-341.
| Crossref | Google Scholar |
Borggaard OK, Jdrgensen SS, Moberg JP, Raben-Lange B (1990) Influence of organic matter on phosphate adsorption by aluminium and iron oxides in sandy soils. Journal of Soil Science 41, 443-449.
| Crossref | Google Scholar |
Börling K, Otabbong E, Barberis E (2001) Phosphorus sorption in relation to soil properties in some cultivated swedish soils. Nutrient Cycling in Agroecosystems 59, 39-46.
| Crossref | Google Scholar |
Bortoluzzi EC, Pérez CAS, Ardisson JD, Tiecher T, Caner L (2015) Occurrence of iron and aluminum sesquioxides and their implications for the P sorption in subtropical soils. Applied Clay Science 104, 196-204.
| Crossref | Google Scholar |
Bromfield SM (1965) Studies of the relative importance of iron and aluminium in the sorption of phosphate by some Australian soils. Australian Journal of Soil Research 3, 31-44.
| Crossref | Google Scholar |
Chadwick OA, Chorover J (2001) The chemistry of pedogenic thresholds. Geoderma 100, 321-353.
| Crossref | Google Scholar |
Chase AJ, Erich MS, Ohno T (2018) Bioavailability of phosphorus on iron (oxy)hydroxide not affected by soil amendment–derived organic matter. Agricultural and Environmental Letters 3, 170042.
| Crossref | Google Scholar |
Crews TE, Kitayama K, Fownes JH, Riley RH, Herbert DA, Mueller-Dombois D, Vitousek PM (1995) Changes in soil phosphorus fractions and ecosystem dynamics across a long chronosequence in Hawaii. Ecology 76, 1407-1424.
| Crossref | Google Scholar |
Cross AF, Schlesinger WH (1995) A literature review and evaluation of the. Hedley fractionation: applications to the biogeochemical cycle of soil phosphorus in natural ecosystems. Geoderma 64, 197-214.
| Crossref | Google Scholar |
de Campos M, Antonangelo JA, Alleoni LRF (2016) Phosphorus sorption index in humid tropical soils. Soil and Tillage Research 156, 110-118.
| Crossref | Google Scholar |
De Schrijver A, Vesterdal L, Hansen K, De Frenne P, Augusto L, Achat DL, Staelens J, Baeten L, De Keersmaeker L, De Neve S (2012) Four decades of post-agricultural forest development have caused major redistributions of soil phosphorus fractions. Oecologia 169, 221-234.
| Crossref | Google Scholar | PubMed |
Freese D, van der Zee SEATM, van Riemsdijk WH (1992) Comparison of different models for phosphate sorption as a function of the iron and aluminium oxides of soils. Journal of Soil Science 43, 729-738.
| Crossref | Google Scholar |
Gérard F (2016) Clay minerals, iron/aluminum oxides, and their contribution to phosphate sorption in soils – A myth revisited. Geoderma 262, 213-226.
| Crossref | Google Scholar |
Giardina CP, Binkley D, Ryan MG, Fownes JH, Senock RS (2004) Belowground carbon cycling in a humid tropical forest decreases with fertilization. Oecologia 139, 545-550.
| Crossref | Google Scholar | PubMed |
Guppy CN, Menzies NW, Moody PW, Blamey FPC (2005) Competitive sorption reactions between phosphorus and organic matter in soil: a review. Soil Research 43, 189-202.
| Crossref | Google Scholar |
Hall SJ, Silver WL (2015) Reducing conditions, reactive metals, and their interactions can explain spatial patterns of surface soil carbon in a humid tropical forest. Biogeochemistry 125, 149-165.
| Crossref | Google Scholar |
Hall SJ, Thompson A (2022) What do relationships between extractable metals and soil organic carbon concentrations mean? Soil Science Society of America Journal 86, 195-208.
| Crossref | Google Scholar |
Hawkins J, Vermeiren C, Blackwell M, Darch T, Granger S, Dunham S, Hernandez-Allica J, Smolders E, McGrath S (2022) The effect of soil organic matter on long-term availability of phosphorus in soil: Evaluation in a biological P mining experiment. Geoderma 423, 115965.
| Crossref | Google Scholar |
Hawkesford MJ, Cakmak I, Coskun D, De Kok LJ, Lambers H, Schjoerring JK, White PJ (2023) Chapter 6 - Functions of macronutrients. This chapter is a revision of the third edition chapter by M. Hawkesford, W. Horst, T. Kichey, H. Lambers, J. Schjoerring, I. Skrumsager Møller, and P. White, pp. 135–189. 10.1016/B978-0-12-384905-2.00006-6. In ‘Marschner’s mineral nutrition of plants’, 4th edn. (Eds Z Rengel, I Cakmak, PJ White) pp 201–281. (Academic Press)
He X, Augusto L, Goll DS, Ringeval B, Wang Y, Helfenstein J, Huang Y, Yu K, Wang Z, Yang Y, Hou E (2021) Global patterns and drivers of soil total phosphorus concentration. Earth System Science Data 13, 5831-5846.
| Crossref | Google Scholar |
Hemingway JD, Rothman DH, Grant KE, Rosengard SZ, Eglinton TI, Derry LA, Galy VV (2019) Mineral protection regulates long-term global preservation of natural organic carbon. Nature 570, 228-231.
| Crossref | Google Scholar | PubMed |
Herndon EM, Kinsman-Costello L, Duroe KA, Mills J, Kane ES, Sebestyen SD, Thompson AA, Wullschleger SD (2019) Iron (oxyhydr)oxides serve as phosphate traps in Tundra and Boreal peat soils. Journal of Geophysical Research: Biogeosciences 124, 227-246.
| Crossref | Google Scholar |
Hunt JF, Ohno T, He Z, Honeycutt CW, Dail DB (2007) Inhibition of phosphorus sorption to goethite, gibbsite, and kaolin by fresh and decomposed organic matter. Biology and Fertility of Soils 44, 277-288.
| Crossref | Google Scholar |
Ige DV, Akinremi OO, Flaten DN (2007) Direct and indirect effects of soil properties on phosphorus retention capacity. Soil Science Society of America Journal 71, 95-100.
| Crossref | Google Scholar |
Jindo K, Audette Y, Olivares FL, Canellas LP, Smith DS, Paul Voroney R (2023) Biotic and abiotic effects of soil organic matter on the phytoavailable phosphorus in soils: A review. Chemical and Biological Technologies in Agriculture 10, 29.
| Crossref | Google Scholar |
Kaiser K, Guggenberger G (2000) The role of DOM sorption to mineral surfaces in the preservation of organic matter in soils. Organic Geochemistry 31, 711-725.
| Crossref | Google Scholar |
Kaiser K, Guggenberger G (2003) Mineral surfaces and soil organic matter. European Journal of Soil Science 54, 219-236.
| Crossref | Google Scholar |
Kang J, Hesterberg D, Osmond DL (2009) Soil organic matter effects on phosphorus sorption: a path analysis. Soil Science Society of America Journal 73, 360-366.
| Crossref | Google Scholar |
Kleber M, Mikutta R, Torn MS, Jahn R (2005) Poorly crystalline mineral phases protect organic matter in acid subsoil horizons. European Journal of Soil Science 56, 717-725.
| Crossref | Google Scholar |
Kleber M, Eusterhues K, Keiluweit M, Mikutta C, Mikutta R, Nico PS (2015) Mineral–organic associations: formation, properties, and relevance in soil environments. Advances in Agronomy 130, 1-140.
| Crossref | Google Scholar |
Lal R (2004) Soil carbon sequestration impacts on global climate change and food security. Science 304, 1623-1627.
| Crossref | Google Scholar | PubMed |
Li Y, Xu M, Zou X (2006) Effects of nutrient additions on ecosystem carbon cycle in a Puerto Rican tropical wet forest. Global Change Biology 12, 284-293.
| Crossref | Google Scholar |
Li M, Hou YL, Zhu B (2007) Phosphorus sorption–desorption by purple soils of China in relation to their properties. Australian Journal of Soil Research 45, 182-189.
| Crossref | Google Scholar |
Lin Y, Gross A, O’Connell CS, Silver WL (2020) Anoxic conditions maintained high phosphorus sorption in humid tropical forest soils. Biogeosciences 17, 89-101.
| Crossref | Google Scholar |
Loganathan P, Fernando WT (1980) Phosphorus sorption by some coconut-growing acid soils of Sri Lanka and its relationship to selected soil properties. Journal of the Science of Food and Agriculture 31, 709-717.
| Crossref | Google Scholar |
Lützow MV, Kögel-Knabner I, Ekschmitt K, Matzner E, Guggenberger G, Marschner B, Flessa H (2006) Stabilization of organic matter in temperate soils: mechanisms and their relevance under different soil conditions – a review. European Journal of Soil Science 57, 426-445.
| Crossref | Google Scholar |
Ma Y, Ma J, Peng H, Weng L, Chen Y, Li Y (2019) Effects of iron, calcium, and organic matter on phosphorus behavior in fluvo-aquic soil: farmland investigation and aging experiments. Journal of Soils and Sediments 19, 3994-4004.
| Crossref | Google Scholar |
McKeague JA, Day JH (1966) Dithionite- and oxalate-extractable Fe and Al as aids in differentiating various classes of soils. Canadian Journal of Soil Science 46, 13-22.
| Crossref | Google Scholar |
Mikutta R, Kleber M, Torn MS, Jahn R (2006) Stabilization of soil organic matter: association with minerals or chemical recalcitrance? Biogeochemistry 77, 25-56.
| Crossref | Google Scholar |
Mikutta R, Mikutta C, Kalbitz K, Scheel T, Kaiser K, Jahn R (2007) Biodegradation of forest floor organic matter bound to minerals via different binding mechanisms. Geochimica et Cosmochimica Acta 71, 2569-2590.
| Crossref | Google Scholar |
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D (2021) The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 372, n71.
| Crossref | Google Scholar |
Parfitt RL, Parshotam A, Salt GJ (2002) Carbon turnover in two soils with contrasting mineralogy under long-term maize and pasture. Soil Research 40, 127-136.
| Crossref | Google Scholar |
Peña F, Torrent J (1984) Relationships between phosphate sorption and iron oxides in Alfisols from a river terrace sequence of Mediterranean Spain. Geoderma 33, 283-296.
| Crossref | Google Scholar |
Peña F, Torrent J (1990) Predicting phosphate sorption in soils of mediterranean regions. Fertilizer Research 23, 173-179.
| Crossref | Google Scholar |
Percival HJ, Parfitt RL, Scott NA (2000) Factors controlling soil carbon levels in New Zealand grasslands is clay content important? Soil Science Society of America Journal 64, 1623-1630.
| Crossref | Google Scholar |
Ping CL, Michaelson GJ (1986) Phosphorus sorption by major agricultural soils of Alaska. Communications in Soil Science and Plant Analysis 17, 299-320.
| Crossref | Google Scholar |
Raghothama KG, Karthikeyan AS (2005) Phosphate acquisition. Plant and Soil 274, 37-49.
| Crossref | Google Scholar |
R Core Team (2024) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Available at https://www.R-project.org/
Rasmussen C, Southard RJ, Horwath WR (2006) Mineral control of organic carbon mineralization in a range of temperate conifer forest soils. Global Change Biology 12, 834-847.
| Crossref | Google Scholar |
Rasmussen C, Heckman K, Wieder WR, Keiluweit M, Lawrence CR, Berhe AA, Blankinship JC, Crow SE, Druhan JL, Hicks Pries CE, Marin-Spiotta E, Plante AF, Schädel C, Schimel JP, Sierra CA, Thompson A, Wagai R (2018) Beyond clay: towards an improved set of variables for predicting soil organic matter content. Biogeochemistry 137, 297-306.
| Crossref | Google Scholar |
Reeves DW (1997) The role of soil organic matter in maintaining soil quality in continuous cropping systems. Soil and Tillage Research 43, 131-167.
| Crossref | Google Scholar |
Rennert T (2018) Wet-chemical extractions to characterise pedogenic Al and Fe species – a critical review. Soil Research 57, 1-16.
| Crossref | Google Scholar |
Saidy AR, Smernik RJ, Baldock JA, Kaiser K, Sanderman J, Macdonald LM (2012) Effects of clay mineralogy and hydrous iron oxides on labile organic carbon stabilisation. Geoderma 173-174, 104-110.
| Crossref | Google Scholar |
Schneider MPW, Scheel T, Mikutta R, van Hees P, Kaiser K, Kalbitz K (2010) Sorptive stabilization of organic matter by amorphous Al hydroxide. Geochimica et Cosmochimica Acta 74, 1606-1619.
| Crossref | Google Scholar |
Shang C, Tiessen H (1998) Organic matter stabilization in two semiarid tropical soils: size, density, and magnetic separations. Soil Science Society of America Journal 62, 1247-1257.
| Crossref | Google Scholar |
Singh B, Gilkes RJ (1991) Phosphorus sorption in relation to soil properties for the major soil types of south-western Australia. Soil Research 29, 603-618.
| Crossref | Google Scholar |
Singh B, Gilkes RJ (1992) Properties and distribution of iron oxides and their association with minor elements in the soils of south-western Australia. Journal of Soil Science 43, 77-98.
| Crossref | Google Scholar |
Singh M, Sarkar B, Biswas B, Churchman J, Bolan NS (2016) Adsorption-desorption behavior of dissolved organic carbon by soil clay fractions of varying mineralogy. Geoderma 280, 47-56.
| Crossref | Google Scholar |
Sollins P, Homann P, Caldwell BA (1996) Stabilization and destabilization of soil organic matter: mechanisms and controls. Geoderma 74, 65-105.
| Crossref | Google Scholar |
Spohn M (2020) Increasing the organic carbon stocks in mineral soils sequesters large amounts of phosphorus. Global Change Biology 26, 4169-4177.
| Crossref | Google Scholar | PubMed |
Syers JK, Evans TD, Williams JDH, Murdock JT (1971) Phosphate sorption parameters of representative soils from Rio Grande do Sul, Brazil. Soil Science 112, 267-275.
| Crossref | Google Scholar |
Tamrat WZ, Rose J, Grauby O, Doelsch E, Levard C, Chaurand P, Basile-Doelsch I (2019) Soil organo-mineral associations formed by co-precipitation of Fe, Si and Al in presence of organic ligands. Geochimica et Cosmochimica Acta 260, 15-28.
| Crossref | Google Scholar |
Torrent J, Schwertmann U, Barrón V (1992) Fast and slow phosphate sorption by goethite-rich natural materials. Clays and Clay Minerals 40, 14-21.
| Crossref | Google Scholar |
Villapando RR, Graetz DA (2001) Phosphorus sorption and desorption properties of the spodic horizon from selected Florida Spodosols. Soil Science Society of America Journal 65, 331-339.
| Crossref | Google Scholar |
Vitousek PM, Porder S, Houlton BZ, Chadwick OA (2010) Terrestrial phosphorus limitation: mechanisms, implications, and nitrogen–phosphorus interactions. Ecological applications 20, 5-15.
| Crossref | Google Scholar | PubMed |
von Fromm SF, Hoyt AM, Lange M, Acquah GE, Aynekulu E, Berhe AA, Haefele SM, McGrath SP, Shepherd KD, Sila AM, Six J, Towett EK, Trumbore SE, Vågen TG, Weullow E, Winowiecki LA, Doetterl S (2021) Continental-scale controls on soil organic carbon across sub-Saharan Africa. Soil 7, 305-332.
| Crossref | Google Scholar |
Wagai R, Mayer LM, Kitayama K, Shirato Y (2013) Association of organic matter with iron and aluminum across a range of soils determined via selective dissolution techniques coupled with dissolved nitrogen analysis. Biogeochemistry 112, 95-109.
| Crossref | Google Scholar |
Wagai R, Kajiura M, Asano M (2020) Iron and aluminum association with microbially processed organic matter via meso-density aggregate formation across soils: organo-metallic glue hypothesis. Soil 6, 597-627.
| Crossref | Google Scholar |
Walker T, Syers JK (1976) The fate of phosphorus during pedogenesis. Geoderma 15, 1-19.
| Crossref | Google Scholar |
Wang H, Zhu J, Fu QL, Xiong J-W, Hong C, Hu HQ, Violante A (2015) Adsorption of phosphate onto ferrihydrite and ferrihydrite-humic acid complexes. Pedosphere 25, 405-414.
| Crossref | Google Scholar |
Wiriyakitnateekul W, Suddhiprakarn A, Kheuruenromne I, Gilkes RJ (2005) Extractable iron and aluminium predict the P sorption capacity of Thai soils. Soil Research 43, 757-766.
| Crossref | Google Scholar |
Wiseman CLS, Püttmann W (2006) Interactions between mineral phases in the preservation of soil organic matter. Geoderma 134, 109-118.
| Crossref | Google Scholar |
Yan X, Wang D, Zhang H, Zhang G, Wei Z (2013) Organic amendments affect phosphorus sorption characteristics in a paddy soil. Agriculture, Ecosystems and Environment 175, 47-53.
| Crossref | Google Scholar |
Yan J, Jiang T, Yao Y, Lu S, Wang Q, Wei S (2016) Preliminary investigation of phosphorus adsorption onto two types of iron oxide-organic matter complexes. Journal of Environmental Sciences 42, 152-162.
| Crossref | Google Scholar | PubMed |
Yang X, Chen X, Yang X (2019) Effect of organic matter on phosphorus adsorption and desorption in a black soil from Northeast China. Soil and Tillage Research 187, 85-91.
| Crossref | Google Scholar |
Ye C, Huang W, Hall SJ, Hu S (2022) Association of organic carbon with reactive iron oxides driven by soil pH at the global scale. Global Biogeochemical Cycles 36, e2021GB007128.
| Crossref | Google Scholar |
Yeasmin S, Singh B, Johnston CT, Sparks DL (2017) Organic carbon characteristics in density fractions of soils with contrasting mineralogies. Geochimica et Cosmochimica Acta 218, 215-236.
| Crossref | Google Scholar |
Yu W, Weintraub SR, Hall SJ (2021) Climatic and geochemical controls on soil carbon at the continental scale: interactions and thresholds. Global Biogeochemical Cycles 35, e2020GB006781.
| Crossref | Google Scholar |
Zhang L, Ding X, Peng Y, George TS, Feng G (2018) Closing the loop on phosphorus loss from intensive agricultural soil: a microbial immobilization solution? Frontiers in Microbiology 9, 104.
| Crossref | Google Scholar | PubMed |
Zhao B, Dou A, Zhang Z, Chen Z, Sun W, Feng Y, Wang X, Wang Q (2023) Ecosystem-specific patterns and drivers of global reactive iron mineral-associated organic carbon. Biogeosciences Discussions 20(23), 4761-4774.
| Crossref | Google Scholar |
Zimmerman AR, Chorover J, Goyne KW, Brantley SL (2004) Protection of mesopore-adsorbed organic matter from enzymatic degradation. Environmental Science and Technology 38, 4542-4548.
| Crossref | Google Scholar | PubMed |